Abstract
Ketone metabolism is currently being spotlighted for its health benefits. Strict dietary carbohydrate restriction is required to increase plasma ketone levels, which can be achieved with D-β-hydroxybutyric acid (D-BHB) supplementation as well. Although 2.9 g/day of D-BHB may reduce body fat without dieting or exercise interventions, the lower effective intake limit of exogenous D-BHB remains unknown. In this randomized, double-blind, placebo-controlled study (UMIN000054231), we aimed to assess the safety and fat-reduction effects of a 12-week intake of D-BHB in healthy Japanese adults (low-dose [1.5 g/day, n = 33], normal-dose [2.9 g/day, n = 33], and placebo [n = 34] groups). Blood samples were collected pre- and post-intervention. Participants’ blood chemistry, anthropometric, and body composition parameters were investigated. The low-dose group had a lower visceral fat area and body mass index (BMI) and higher plasma ketone levels than the placebo group. The normal-dose group had a significantly lower visceral fat area than the placebo group. Significant between-group (normal-dose vs. placebo) differences were observed in body weight, BMI, body fat percentage, fat mass, and plasma ketone levels. Participants reported no D-BHB-related adverse effects or discomfort. In conclusion, 1.5 or 2.9 g/day of D-BHB may reduce body fat without dieting or exercise interventions.
1. Introduction
Overweight and obesity are key risk factors for lifestyle-related diseases [1]. Body fat is classified as subcutaneous and visceral fat, with visceral fat having a stronger correlation with lifestyle-related diseases [2]. Therefore, visceral fat reduction in particular is essential for preventing lifestyle-related diseases. Various types of diets, such as low-carbohydrate (e.g., ketogenic diet [KD]), paleo-, plant-forward, traditional regional (e.g., Mediterranean diet), and specifically designed (e.g., the dietary approaches to stop hypertension [DASH] and Mayo Clinic diets) diets, have been developed, and along with intermittent fasting and clean eating, recommended for weight reduction [3].
Among these, the KD is a high-fat, low-carbohydrate, and adequate-protein diet [4,5,6]. It has been clinically used since the early 1920s to control seizures in patients with epilepsy, especially those not responding adequately to anti-epileptics [6,7,8]. Recently, the KD has garnered much interest both for its effectiveness in weight reduction and beneficial effects on several health conditions, including neurological, intestinal, and respiratory disorders, obesity, type 2 diabetes mellitus, and cancer [9,10,11,12,13,14,15,16,17,18,19]. The KD induces a metabolic switch from glycolysis to ketosis by producing natural ketone bodies in the liver, namely D-β-hydroxybutyric acid (D-BHB), acetoacetate, and acetone from fat, with D-BHB accounting for >70% of the total ketones produced [20]. Ketone production in the liver requires strict dietary restrictions, and many patients are unable to continue with the KD; hence, exogenous ketones, such as 1,3-butanediol, medium-chain triglycerides (MCT), ketone salts, and ketone esters, may be more successful in inducing ketosis [21].
We had recently reported human studies on the effects of exogenous D-BHB in reducing visceral fat and improving sleep quality [22,23]. Recent advances in D-BHB research have further updated the knowledge on this topic. D-BHB is a signaling molecule that can activate G-protein-coupled receptors (GPCRs) [24,25], post-translationally modify proteins [26], or regulate epigenetic phenomena, such as histone methylation, acetylation, and β-hydroxybutyrylation and linking gene transcription [27,28,29]. Furthermore, D-BHB-derived metabolites, known as BHB-amino acids, include N-β-hydroxybutyryl amino acids (BHB-Phe) predominantly. BHB-Phe is a structural and functional analog of N-lactoyl-phenylalanine (Lac-Phe) and reduces food intake and body weight [30].
However, evidence on the role of exogenous ketones in weight reduction and other clinical benefits remains limited. To the best of our knowledge, the lower effective intake limit of exogenous D-BHB is currently unknown. Our previous study demonstrated that an intake of 2.9 g/day of D-BHB exhibited significant effects [22]. However, considering the potential influence of epigenetic phenomena and secondary metabolites [27,30], D-BHB may be effective at even lower doses. In the present study, we defined 1.5 g/day as a lowdose, which is half the amount previously shown to be effective. This dose was selected not only to investigate whether a lower dose could maintain efficacy, but also to improve safety and tolerability, and to explore the minimum effective dose in healthy adults. Therefore, this randomized controlled trial was conducted to verify the effects of consumption of a low-dose (1.5 g/day) D-BHB-supplemented test beverage on visceral fat area and body mass index (BMI) in healthy Japanese adults.
2. Materials and Methods
2.1. Participants and Clinical Study
Participants were recruited between April and May 2024. This randomized, double-blind, placebo-controlled trial was conducted from August 2024 to December 2024 at the Medical Corporation Seishinkai, Takara Clinic, Tokyo, Japan.
Potential participants included healthy Japanese adults of both sexes, with a BMI of 23–30 kg/m2 and a large visceral fat area at the time of screening.
This study adhered to the CONSORT (Supplementary materials) guidelines.
Exclusion Criteria
- Receiving treatment for or having a history of malignancy, heart failure, or myocardial infarction
- Pacemaker or cardioverter defibrillator implants
- Receiving treatment for the following: cardiac arrhythmia, liver disorder, chronic kidney disease, cerebrovascular disorder, rheumatic disease, diabetes mellitus, dyslipidemia, hypertension, or any other chronic diseases
- Regular consumption of “Foods for Specified Health Uses” or “Foods with Functional Claims”
- Regularly using pharmaceutical preparations (including herbal medicines) or supplements
- Allergies (to pharmaceuticals and food related to the test beverage)
- Thrice-a-week exercise routine
- Irregular lifestyle such as night-shifts
- Extreme dietary restrictions, such as a zero-carbohydrate diet
- On a diet
- Pregnancy, lactating, or intending to become pregnant during the study period
- Participated in another clinical trial in the 28 days before the date of consent or will participate in another trial during the study period
- Deemed ineligible for study participation by the physician
2.2. Randomization and Blinding
After screening for study eligibility and enrollment, the participants were randomized to the low-dose, normal-dose, and placebo groups in a 1:1:1 ratio. This allocation was determined using a stratified block randomization table based on the visceral fat area (≥median, <median).
Double-blinding was accomplished by labeling the test beverage with only an identification number. The identification numbers of the active and placebo beverages were withheld, maintaining strict confidentiality, and were not disclosed until the allocation manager released the allocation list after study completion. The allocation manager generated an allocation order based on the identification numbers and created an allocation list and an emergency key. For each study participant, the emergency key was placed in an envelope, stamped with an allotment seal, and sealed. After study completion and data fixation, the identification numbers of the test beverages were released once the allocation manager confirmed that the allocation list and the emergency key had not been unsealed.
2.3. D-BHB and Placebo Beverage Preparation
D-BHB (OKETOA®) was provided by Osaka Gas Chemicals Co., Ltd. (Osaka, Japan). The D-BHB amount was based on that mentioned in previous studies [22,23]. The low-dose beverage contained 1.5 g D-BHB, 0.22 g acidulant (sodium DL-malate), 90 mg sweetener (sucralose, acesulfame potassium, or stevia), and 0.20 g flavorings per 100 mL. The normal-dose beverage contained 2.9 g D-BHB, 0.40 g acidulant, 90 mg sweetener, and 0.20 g flavorings per 100 mL. The placebo beverage contained 0.11 g acidulant, 90 mg sweetener, and 0.20 g flavorings per 100 mL. The beverages were prepared by API Co., Ltd. (Gifu, Japan).
2.4. Intervention Characteristics
The intervention period comprised 12 weeks, the first day of which was defined as week zero. Pre-intervention, the enrolled participants were randomized to the normal-dose D-BHB (2.9 g/day, n = 36), low-dose D-BHB (1.5 g/day, n = 36), or placebo (n = 36) groups according to the median visceral fat area (Figure 1). All participants were to consume the test beverage 2 h before dinner. If the test beverage had not been consumed as prescribed, participants should have consumed it as soon as they had realized they were missing the consumption, or as early as possible. However, the total daily dosage must have been consumed within the same day and not deferred to the following day. All participants completed the intervention tests.
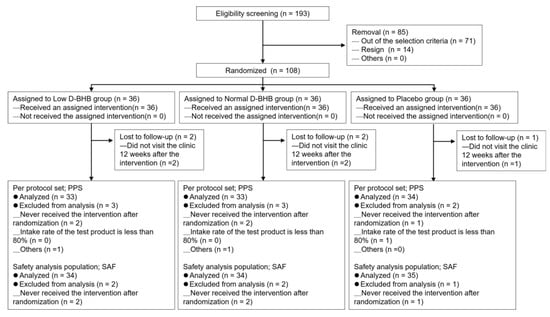
Figure 1.
Flowchart of participant selection and randomization. D-BHB, D-β-hydroxybutyric acid.
The test beverage was administered 2 h before dinner for three reasons: (i) to minimize interference from postprandial metabolism; (ii) to align exposure with the nocturnal shift toward increased lipid oxidation and ketogenesis reported during sleep [31,32]; and (iii) based on pharmacokinetics indicating that after a 3 g oral dose, blood BHB levels return near baseline by ~120 min [33], reducing the likelihood of residual exogenous BHB at bedtime.
Compliance Parameters of the Participants
- Consume the test beverage according to the prescribed dosage and usage
- Ensure an intake of ≥80% of the test beverage
- Avoid excessive food and beverage consumption and maintain previous lifestyle habits, from the date of trial consent until the final examination
- Avoid consuming alcohol or engaging in excessive exercise from the day before each examination to until the completion of the examination
- Food and beverage consumption, including the test beverage, prohibited for 10 h before blood collection. However, water was permitted, albeit not functional water and tea.
- Contact the contracted clinical trial institution immediately and seek guidance on subsequent actions if any health changes occurred during the trial period
- Refrain from consuming specific health foods, functional foods, or any other foods/beverages with functional properties, during the trial period
- Measure and record the daily step count using a pedometer during the trial period.
- Measure and record the daily basal body temperature using a thermometer from 1 week before the intervention to until the end of the intervention period
- Avoid engaging in dieting behaviors (such as gym workouts or dietary restrictions); however, continue maintaining pre-trial exercise and eating habits throughout the trial period
2.5. Primary and Secondary Endpoints
The primary endpoints were the measured values of visceral fat area and BMI at 12 weeks post-intake (low-dose vs. placebo groups). The secondary endpoints included the following: the measured value of visceral fat area at 12 weeks (normal-dose vs. placebo groups); amount and rate of change in the visceral fat area from that at screening (normal-dose vs. placebo groups and low-dose vs. placebo groups); measured values of total fat area and subcutaneous fat area at 12 weeks, as well as amount and rate of change from that at screening (normal-dose vs. placebo groups and low-dose vs. placebo groups); measured value of BMI at 12 weeks (normal-dose vs. placebo groups); amount and rate of change in BMI from that at screening (normal-dose vs. placebo groups and low-dose vs. placebo groups); measured values of body weight, body fat percentage, and waist circumference at 12 weeks, as well as amount and rate of change from that at screening (normal-dose vs. placebo groups; low-dose vs. placebo groups); measured values of each item in an original questionnaire, scored using a Likert scale, at 12 weeks (normal-dose vs. placebo groups and low-dose vs. placebo groups); measured values of the basal body temperature at 12 weeks and amount and rate of changes in these from those at baseline (normal-dose vs. placebo groups and low-dose vs. placebo groups), with the average value of basal body temperature over the 7 days from 7 days before intervention initiation to the day before the intervention’s initiation being defined as the baseline; and measured values of total ketone bodies, acetoacetate, and BHB at 12 weeks, as well as amount and rate of change from those at screening (normal-dose vs. placebo groups and low-dose vs. placebo groups). Blood samples were collected from the participants 12 weeks pre- and post-intervention in the mornings. Before blood sampling, the participants fasted for 10 h.
2.6. Participants’ Blood Chemistry, Urine Analysis, and Anthropometric Variables
White and red blood cell counts, hemoglobin and hematocrit levels, and platelet count were measured using a Sysmex XN-9100 automated hematology analyzer (Sysmex, Kobe, Japan). Aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), total bilirubin, total protein, urea nitrogen, creatinine, uric acid, sodium, potassium, chlorine, serum amylase, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides (TG) were measured using the LABOSPECT 008α (Hitachi High-Tech, Tokyo, Japan) and JCA-BM8060 (JEOL Ltd., Tokyo, Japan), and regardless of either equipment, similar results were obtained. Blood glucose and glycated hemoglobin (HbA1c) levels were measured using the JCA-BM8060. Total ketone bodies, acetoacetate, and BHB levels were measured using the JCA-BM9130 (JEOL Ltd., Tokyo, Japan).
Urinary protein, glucose, occult blood, and pH levels were measured using the US-3500MS (TERAMECS, Kyoto, Japan).
Systolic and diastolic blood pressures were measured using the HEM-6022 (OMRON, Kyoto, Japan).
Body height was measured using a stadiometer (Matsuyoshi, Tokyo, Japan). Bodyweight, body fat percentage, and body mass (fat, lean body, and muscle mass) were measured using a Multi-frequency Body Composition Analyzer MC-780A-N (Tanita, Tokyo, Japan). Body temperature was measured using a MC-652LC-PK basal thermometer (OMRON, Kyoto, Japan).
2.7. Original Questionnaire
Subjective symptoms were assessed using an original questionnaire that included 12 questions, and each item was graded on a 6-point Likert scale ranging from 1 (strongly disagree) to 6 (strongly agree). The questionnaire consisted of the following items: concerns about abdominal fat, feeling tightness around the waist, experiencing fatigue and heaviness in performing daily activities, shortness of breath during light exercise, difficulty sweating, poor sleep onset, feeling tired upon waking, frequent waking up during sleep, lack of sensation of fullness, easily feeling hungry, tendency to feel down or depressed, and inability to control appetite.
2.8. Computed Tomography
Computed tomography (CT) scans were used to assess changes in the visceral, subcutaneous, and total fat areas with weight loss. The CTs were performed at the Medical Scanning (Tokyo, Japan) using a NAEOTOM Alpha (SIEMENS, Tokyo, Japan) scanner.
2.9. Statistical Analyses
The sample size was calculated based on the hypothesis that visceral fat would be reduced after a 12-week of D-BHB intake, assuming the example of the pilot study on MCT, which has a similar mechanism of action [34]. The actual measured between-group difference in visceral fat reduction after 12 weeks of MCT intake was −10.6 cm2 (test beverage group: N = 33, −12.2 ± 11.2; placebo group: N = 31, −1.6 ± 12.8). Accordingly, the Cohen’s d was estimated to be 0.897. The statistical significance level (α) was 5% two-sided, the statistical power (1-β) was 80%, and the sample size for the t-test was estimated to be 21 participants per group, and 36 participants per group were chosen assuming dropout.
Per-protocol analysis set (PPAS) was used for the efficacy analysis. Between-group comparisons were performed using the analysis of covariance (ANCOVA). Data were expressed as the means, standard deviation (SD), and standard error (SE), with baseline (at screening) as the covariate. p < 0.05 was considered statistically significant. A safety analysis set (SAF) was used for the data analysis. The adverse events that occurred were aggregated by each trial participant. Additionally, the incidence rates of adverse events were compiled group-wise, and the 95% confidence intervals (CI) for group-wise incidence rates and the between-group differences in incidence rates were calculated. Furthermore, between-group differences in the incidence rates of adverse events were analyzed using the chi-square test. All statistical analyses were two-sided, with the significance level set at 5%. The SPSS Statistics software version 23 was used for the analyses. The primary outcome was considered for multiplicity, and the significance probability was adjusted using the Bonferroni correction.
Multiplicity was controlled for the primary endpoint using Bonferroni correction. Secondary endpoints were analyzed without multiplicity adjustment and are considered exploratory; p-values should be interpreted cautiously.
2.10. Ethical Considerations
This study strictly conformed to the Declaration of Helsinki (2013 revision) guidelines and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects (2023 revision). All participants provided written informed consent after being briefed on the study’s nature and purpose and the possible adverse effects of the supplements. This study was approved by the Seishinkai Medical Corporation Takara Clinic Ethics Committee (Approval No.: 2404-05240-0032-0D-TC) and registered with the UMIN Clinical Trial Registry (http://www.umin.ac.jp (11 November 2025)) (UMIN000054231).
3. Results
3.1. Baseline Participant Characteristics
Of the 193 potential participants, 108 met the participation criteria and were randomly assigned to one of the three groups (Figure 1). Of these, five participants did not attend the last examination (n = 2, low-dose group; n = 2, normal-dose group; and n = 1, placebo group). Hence, 103 participants completed the study. The PPAS excluded the aforementioned five participants, as well as another participant from the placebo group who had an intake of <80% for the test beverage. Additionally, two more participants (one each from the normal-dose and low-dose groups) were excluded at the discretion of the principal investigator. For the SAF, 103 participants were included after excluding the aforementioned participants (n = 34, low-dose group; n = 34, normal-dose group; and n = 35, placebo group). Consequently, 100 participants were included in the analysis (n = 33, low-dose group; n = 33, normal-dose group; and n = 34, placebo group).
The participants’ characteristics are presented in Table 1. No significant between-group differences were observed. The mean (SD) percentages of the participants in the low-dose, placebo, and normal-dose groups who consumed the test beverage were 95.0% (23.4%), 97.1% (17.1%), and 94.6% (23.3%), respectively.

Table 1.
Baseline participant characteristics.
3.2. Anthropometric Variables and Body Composition
Anthropometric data are listed in Table 2. All test data were compared for the degrees of change post-intervention for each individual item between the low-dose and placebo groups and normal-dose and placebo groups.

Table 2.
Anthropometric data of the participants.
ANCOVA results of the comparison between the low-dose and placebo revealed the following:
- Regarding visceral fat area, the mean difference in the change in its measured value (cm2) from baseline was −10.6 cm2 (SE 3.3; 95% CI− −18.1, 95% CI+ −3.0; p = 0.004) and the mean difference in change in the amount of change (cm2) and rate of change (%) from baseline were −10.6 cm2 (SE 3.3; 95% CI− −17.1, 95% CI+ −4.0; p = 0.002) and −10.8% (SE 3.4; 95% CI− −17.6, 95% CI+ −4.1; p = 0.002), respectively.
- Regarding BMI, the mean difference in change in its measured value (kg/m2) from baseline was −0.2 kg/m2 (SE 0.2; 95% CI− −0.6, 95% CI+ 0.3; p = 0.741) and the mean difference in change in the amount of change (kg/m2) and rate of change (%) from baseline were −0.2 kg/m2 (SE 0.2; 95% CI− −0.6, 95% CI+ 0.2; p = 0.370) and −0.6% (SE 0.7; 95% CI− −2.1, 95% CI+ 0.8; p = 0.389), respectively.
ANCOVA results of the comparison between the normal-dose and placebo groups revealed the following:
- Regarding visceral fat area, the mean difference in change in its measured value (cm2) from baseline was −10.9 cm2 (SE 3.3; 95% CI− −17.5, 95% CI+ −4.2; p = 0.002) and the mean difference in change in the amount of change (cm2) and rate of change (%) from baseline were −10.9 cm2 (SE 3.3; 95% CI− −17.5, 95% CI+ −4.2; p = 0.002) and −11.1% (SE 3.6; 95% CI− −18.2, 95% CI+ −4.0; p = 0.003), respectively.
- Regarding body weight, the mean difference in change in its measured value (kg) from baseline was −1.4 kg (SE 0.7; 95% CI− −2.7, 95% CI+ 0.0; p = 0.045) and the mean differences in change in the amount of change (kg) and rate of change (%) from baseline were −1.4 kg (SE 0.7; 95% CI− −2.7, 95% CI+ 0.0; p = 0.045) and −2.0% (SE 1.0; 95% CI− −4.0, 95% CI+ 0.0; p = 0.046), respectively.
- Regarding BMI, the mean difference in change in its measured value (kg/m2) from baseline was −0.5 kg/m2 (SE 0.3; 95% CI− −1.0, 95% CI+ 0.0; p = 0.041) and the mean difference in change in the amount of change (kg/m2) and rate of change (%) from baseline were −0.5 kg/m2 (SE 0.3; 95% CI− −1.0, 95% CI+ 0.0; p = 0.041) and −2.0% (SE 1.0; 95% CI− −4.0, 95% CI+ 0.0; p = 0.045), respectively.
- Regarding body fat percentage, the mean difference in change its measured value (%) from baseline was −1.3% (SE 0.6, 95% CI− −2.5, 95% CI+ 0.0, p = 0.044) and the mean difference in change in the amount of change (%) and rate of change (%) from baseline were −1.3% (SE 0.6; 95% CI− −2.5, 95% CI+ 0.0; p = 0.044) and −4.0% (SE 2.4; 95% CI− −8.8, 95% CI+ 0.8; p = 0.103), respectively.
3.3. Peripheral Blood Characteristics
Baseline and week 12 white and red blood cell counts, hemoglobin and hematocrit levels, platelet count, AST, ALT, γ-GT, total bilirubin, total protein, urea nitrogen, creatinine, uric acid, sodium, potassium, chloride, serum amylase, total cholesterol, HDL cholesterol, LDL cholesterol, TG, glucose, and HbA1c (NGSP) analyses results are listed in Table 3. Total ketone bodies, acetoacetate, and BHB analyses results are listed in Table 4. The degrees of change post-intervention for each individual item between the low-dose and placebo groups and between the normal-dose and placebo groups were compared.

Table 3.
Baseline clinical characteristics of the study participants.

Table 4.
Ketone body levels.
ANCOVA results of the comparison between the low-dose and placebo revealed the following:
- Regarding total ketone bodies, the mean difference in change in its measured value (μmol/L) from baseline was 78.3 μmol/L (SE 36.9; 95% CI− 4.6, 95% CI+ 152.0; p = 0.038) and the mean difference in change in the amount of change (μmol/L) and rate of change (%) from baseline were 78.3 μmol/L (SE 36.9; 95% CI− 4.6, 95% CI+ 152.0; p = 0.038) and 49.6% (SE 26.9; 95% CI− −4.2, 95% CI+ 103.4; p = 0.070), respectively.
- Regarding acetoacetate, the mean difference in change in its measured value (μmol/L) from baseline was 14.9 μmol/L (SE 7.3; 95% CI− 0.3, 95% CI+ 29.5; p = 0.046) and the mean differences in change in the amount of change (μmol/L) and rate of change (%) from baseline were 14.9 μmol/L (SE 7.3; 95% CI− 0.3, 95% CI+ 29.5; p = 0.046) and 33.2% (SE 20.2; 95% CI− −7.1, 95% CI+ 73.5; p = 0.105), respectively.
- Regarding BHB, the mean difference in change in its measured value (μmol/L) from baseline was 63.4 μmol/L (SE 30.7; 95% CI− 2.0, 95% CI+ 124.9; p = 0.043) and the mean difference in change in the amount of change (μmol/L) and rate of change (%) were 63.4 μmol/L (SE 30.7; 95% CI− 2.0, 95% CI+ 124.9; p = 0.043) and 59.6% (SE 31.2; 95% CI− −2.7, 95% CI+ 121.8; p = 0.060), respectively.
ANCOVA results of the comparison between the normal-dose and placebo groups revealed the following:
- Regarding total ketone bodies, the mean difference in change in its measured value (μmol/L) from baseline was 132.3 μmol/L (SE 53.1; 95% CI− 26.3, 95% CI+ 238.3; p = 0.015) and the mean difference in change in the amount of change (μmol/L) and rate of change (%) from baseline were 132.3 μmol/L (SE 53.1; 95% CI− 26.3, 95% CI+ 238.3; p = 0.015) and 93.6% (SE 37.1; 95% CI− 19.5, 95% CI+ 167.6; p = 0.014), respectively.
- Regarding acetoacetate, the mean difference in change in its measured value (μmol/L) from baseline was 29.0 μmol/L (SE 10.2; 95% CI− 8.6, 95% CI+ 49.3; p = 0.006) and the mean difference in change in the amount of change (μmol/L) and rate of change (%) from baseline were 29.0 μmol/L (SE 10.2; 95% CI− 8.6, 95% CI+ 49.3; p = 0.006) and 58.9% (SE 23.6; 95% CI− 11.8, 95% CI+ 106.1; p = 0.015), respectively.
- Regarding BHB, the mean difference in change in its measured value (μmol/L) from baseline was 102.9 μmol/L (SE 43.6; 95% CI− 15.7, 95% CI+ 190.1; p = 0.021), the mean difference in change in the amount of change (μmol/L) and the rate of change (%) from baseline were 102.9 μmol/L (SE 43.6; 95% CI− 15.7, 95% CI+ 190.1; p = 0.021) and 119.3% (SE 48.3; 95% CI− 22.8, 95% CI+ 215.8; p = 0.016), respectively.
3.4. Baseline Clinical Characteristics
Systolic and diastolic blood pressure data are presented in Table 5. Basal body temperature data is presented in Table 6. Urinary protein, glucose, occult blood, and pH data are presented in Table 7. The investigator confirmed that no medically concerning variations related to the intake of the test beverage occurred.

Table 5.
Blood pressure.

Table 6.
Body temperature.

Table 7.
Urinary test results.
3.5. Original Questionnaire
The results of the original questionnaire are summarized in Table 8. In the normal-dose group, the difficulty in falling asleep was improved by more than one stage from week 0 to week 12 (n = 1), which was significantly better than that in the placebo group.

Table 8.
Original questionnaire.
3.6. Adverse Events
None of participants withdrew from the trial owing to adverse effects or beverage-related discomfort. Therefore, the trial physician concluded that no trial-related adverse events had occurred.
4. Discussion
In this study, the low-dose and normal-dose groups exhibited a significant reduction in the mean change from baseline in visceral fat area (cm2) compared to the placebo group. The normal-dose group exhibited a significant reduction in visceral fat, body weight, BMI, body fat percentage, and fat mass when compared to the placebo group. These results were similar to those reported in our previous study with intake amount same as the normal dose [22]. While the previous study involved a pre-breakfast intervention, the present study used a pre-dinner intervention. Although several secondary outcomes did not differ significantly between groups, the primary outcome demonstrated a consistent and dose-dependent reduction in visceral fat area. Specifically, the adjusted mean difference in visceral fat area from baseline to week 12 was −10.6 cm2 (SE 3.3, p = 0.004) in the low-dose group and −10.9 cm2 (SE 3.3, p = 0.002) in the normal-dose group, compared to placebo, corresponding to a relative reduction of approximately 10–11% from baseline. These findings reinforce the reliability of the primary outcome. Notably, the extent of visceral fat reduction observed in this study is comparable to previous MCT-based interventions of similar duration, which reported a reduction of approximately −12.2 cm2 (about 11% relative decrease from baseline) over 12 weeks [34]. While the effects on secondary anthropometric measures such as body weight and BMI were modest and more pronounced at the normal-dose (body weight: −1.4 kg, SE 0.7, p = 0.045; BMI: −0.5 kg/m2, SE 0.3, p = 0.041), the consistent reductions in visceral fat observed at both doses suggest a qualitative dose–response relationship, with broader effects apparent at the higher dose. Adipose tissue can be classified as either subcutaneous (underneath the skin) or visceral (around internal organs) [35], with the majority (80–90%) being subcutaneous [36]. Visceral adipocytes are known to be more metabolically active and have higher lipolytic activity than subcutaneous adipocytes [37,38]. They are also more sensitive to catecholamine-induced lipolysis and less sensitive to insulin-induced lipolysis. Furthermore, visceral adipose tissue is generally more responsive to weight reduction than subcutaneous adipose tissue [39,40]. As a result, weight loss tends to affect visceral fat more than subcutaneous fat. In line with these characteristics, the present study found that low-dose D-BHB was effective in reducing visceral fat.
According to previous studies, the average respiratory quotient (RQ) in non-obese adults is reported to be 0.88 during wakefulness and 0.83 during sleep [31]. A decrease in RQ indicates enhanced fatty acid oxidation and a shift in energy substrate utilization from carbohydrates to fats [41]. Regarding breath acetone concentration, previous studies have established a correlation between RQ and breath acetone levels, estimating that at an RQ of 0.88, breath acetone concentration is approximately 432.8 ppb, and at an RQ of 0.83, it increases to 663.0 ppb [42]. However, these values are model estimates based on published literature and were not directly measured in the present study. In this study, RQ and breath acetone concentration were not measured. Instead, blood ketone body concentrations were assessed, and their validity was confirmed by comparison with previously reported values. Utilizing validated relationships between breath acetone levels and blood ketone body concentrations, the corresponding blood ketone body concentrations were estimated to be 43.3 μM at RQ 0.88 and 66.3 μM at RQ 0.83. This quantitative estimation indicates an approximate increase of 23.0 μM in blood ketone body concentration during sleep, reflecting a metabolic shift toward greater lipid oxidation and ketogenesis. However, as these are indirect estimations, the interpretation should be made with caution.
Previous studies have reported that human energy substrate utilization exhibits a circadian rhythm, with carbohydrate oxidation predominating upon awakening, whereas lipid oxidation becomes the main energy source during sleep [31]. Due to this circadian rhythm, free fatty acids are released from adipose tissue during sleep and are converted to ketone bodies in the liver. Therefore, the promotion of lipid oxidation during sleep is likely to contribute to the reduction in visceral fat and the elevation of blood ketone body concentrations observed the following morning.
A pharmacokinetic study revealed that after an intake of 3 g of D-BHB, blood BHB levels reached a maximum of 0.28 mM at 45 min and returned to baseline after 120 min [33]. In contrast, blood BHB levels in the present study were measured the following morning, approximately 16–20 h after D-BHB intake. Therefore, it is unlikely that exogenous BHB remained in the bloodstream at the time of measurement. The significantly increased blood BHB levels observed in the low-dose and normal-dose groups are thus likely attributable to endogenous ketone body production resulting from fat breakdown, rather than the direct effect of exogenous BHB; however, other factors such as dietary intake, physical activity, and individual metabolic differences may also have contributed, and these possibilities cannot be excluded. When comparing endogenous ketone body production during sleep to the increase in blood ketone body concentration following exogenous ketone intake, the increase was 78.3 μM for the low-dose group and 132.3 μM for the normal-dose group. The productivity enhancement rate, calculated as the ratio of endogenous ketone body production during sleep to the increase from exogenous intake, was 22.7% for the low-dose group and 14.8% for the normal-dose group (productivity enhancement rate = 23.0 μM/[increase in intake group + 23.0 μM]). In summary, these findings suggest that the elevated blood BHB levels observed after D-BHB intake may be largely attributable to endogenous ketone body production, rather than the persistence of exogenous BHB. However, variability in ketone responses was considerable, as reflected by large SDs in week 12 fasting BHB (e.g., 174.5 μM in the low-dose and 256.1 μM in the normal-dose groups; Table 4). Such interindividual variability potentially influenced by habitual diet, fasting interval, sex, and metabolic phenotype may attenuate power for secondary outcomes. Future trials could incorporate stratification by baseline metabolic markers or adjust for relevant covariates to better resolve efficacy signals. Furthermore, a human study reported that a long-term ketogenic formula containing MCT exhibited increased plasma BHB concentrations [43]. Another human study reported that during the sixth week of a KD, participants’ urine and blood ketone concentrations measured at regular intervals over a 24 h period revealed an increase in blood and urine BHB levels in the 3 am samples [32]. Therefore, continuous daily intake of ketone bodies may promote lipid oxidation and ketone body production, potentially contributing to improvements in metabolic health and reductions in visceral fat.
Several reports have described mechanisms related to this process. In cellular studies, BHB has been shown to increase the expression of lipolysis-related genes in adipocytes and promote the activation of lipolytic enzymes via β-hydroxybutyrylation [44]. In addition to its role in promoting lipolysis, β-hydroxybutyrylation has been implicated in various other physiological processes, including amino acid catabolism, circadian rhythms, redox balance, PPAR signaling pathways, and oxidative phosphorylation [26]. Furthermore, BHB directly regulates lipolysis in adipocytes via the GPR109A receptor, thereby participating in the molecular mechanisms of fat breakdown [45]. Animal studies have demonstrated that administration of exogenous ketone bodies promotes lipolysis and reduces body fat. In mice who were fed a ketone ester diet, mitochondrial biogenesis and increased expression of uncoupling protein 1 in brown adipose tissue were observed [46]. However, in the present human study, no statistically significant differences in basal body temperature were observed among the placebo, normal-dose, and low-dose groups, suggesting that the thermogenic effects observed in animal models may not directly translate to changes in basal body temperature in humans under the tested conditions, or that other factors may mask such effects in humans. Human studies have also demonstrated that dietary medium- and long-chain triacylglycerols significantly enhance diet-induced thermogenesis, promoting postprandial energy expenditure and fat oxidation [47].
Previous studies have reported that BHB administration can lower blood cholesterol levels [48]. However, in this study, no significant changes in cholesterol levels were observed in either the low-dose or normal-dose group compared to those in the placebo group. This discrepancy may be attributable to differences in study design, duration, dosage, or participant characteristics. Further investigation is required to clarify the conditions under which BHB exerts cholesterol-lowering effects in humans.
Some studies have shown reduced levels of ghrelin following the administration of ketone esters, which increase the concentration of ketone bodies in the blood, to healthy individuals. The exact mechanism underlying this effect remains unclear; however, the concomitant reduction in GLP-1 and PPY, or the stability of these hormone levels, suggests that the decrease in ghrelin is likely the primary factor responsible for changes in appetite [49,50]. Recent studies have also revealed that BHB-Phe (β-hydroxybutyrylated phenylalanine), a secondary metabolite of BHB, possesses appetite-suppressing effects. BHB-Phe, a condensation product of BHB and amino acids, has been shown to reduce food intake and body weight in animal models [30]. However, in the present study, no significant differences in food intake were observed among the groups, suggesting that the appetite-suppressing effect of BHB-Phe may not manifest in humans, or that such effects were not apparent under the conditions of this study. Notably, GLP-1, PPY, and BHB-Phe were not measured in this trial, and further studies are warranted to clarify their roles in appetite regulation in humans.
Notably, in cases where the difficulty in falling asleep at week 12 improved by more than one stage compared to that at week 0, the normal-dose group showed greater improvement than the placebo group. This finding is consistent with those of previous reports [23]. Further investigation is warranted to clarify the physiological significance and the conditions required for the manifestation of these effects in humans.
This study has some limitations. First, the participants were limited to healthy Japanese adults with a BMI of 23–30 kg/m2, which may restrict the generalizability of the findings to populations with different ethnic backgrounds, dietary habits, lifestyle factors, or higher levels of obesity and comorbidities. Further research is warranted to evaluate the efficacy of D-BHB in more diverse and clinically obese groups. Second, adherence to D-BHB intake was partially self-reported, potentially introducing reporting bias. Third, the intervention period was relatively short, leaving the long-term effects and safety undetermined. Fourth, body fat was assessed using bioelectrical impedance analysis (BIA), which is less precise than dual-energy X-ray absorptiometry (DEXA) and may have introduced measurement bias. Despite its lower accuracy, BIA was selected due to its widespread use in clinical and consumer settings, supporting the study’s real-world applicability. Fifth, detailed dietary records were not collected, which may have limited the ability to account for dietary variations that could influence metabolic outcomes. Finally, the modest sample size may have limited the detection of rare adverse events or small effect sizes. In addition, several physiological and metabolic parameters, such as GLP-1, PPY, BHB-Phe, and other appetite or fat metabolism related biomarkers, were not measured in this study. Interpretations regarding these mechanisms are therefore based on previous literature and should be considered as hypotheses rather than definitive conclusions. Future studies should include comprehensive assessments of these biomarkers to elucidate the detailed physiological effects and mechanisms of D-BHB supplementation.
5. Conclusions
Our study indicated that body fat was significantly reduced after a daily oral intake of 1.5 g or 2.9 g of D-BHB, regardless of diet and exercise. Body fat reduction was observed in this study; however, the mechanisms underlying this phenomenon were not directly investigated and require further research. Exogenous D-BHB may serve as a superior dietary supplement for weight loss without the need for additional dieting or exercise interventions. The study results may benefit in this field and provide a foundation for future studies on ketone metabolism and supplementation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/obesities5040082/s1: File S1: Consort checklist.
Author Contributions
Conceptualization, J.T., S.K., J.K., T.G., Y.K., and T.T.; investigation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, J.T., S.K., J.K., T.G., Y.K., and T.T.; supervision, J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Seishinkai Medical Corporation Takara Clinic Ethics Committee (Approval No.: 2404-05240-0032-0D-TC and date of approval: 10 April 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
We wish to thank ORTHOMEDICO Inc. (Tokyo, Japan) for the test planning and analyses. We also thank LSI Medience Corporation for the analyses.
Conflicts of Interest
S.K., Y.K., and J.T. are employees of Osaka Gas Co., Ltd. (Osaka, Japan). J.K. is an employee of Osaka Gas Chemicals Co., Ltd. (Osaka, Japan). T.G. and T.T. declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| KD | Ketogenic diet |
| DASH | Dietary approaches to stop hypertension |
| MCT | Medium-chain triglycerides |
| D-BHB | D-β-hydroxybutyric acid |
| GPCRs | G-protein-coupled receptors |
| BHB-Phe | N-β-hydroxybutyryl amino acids |
| Lac-Phe | N-lactoyl-phenylalanine |
| BMI | Body mass index |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| γ-GTP | γ-glutamyl transpeptidase |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| TG | Triglycerides |
| CT | Computed tomography |
| HbA1c | Glycated hemoglobin |
| ANCOVA | Analysis of covariance |
| SD | Standard deviation |
| SE | Standard error |
| PPAS | Per-protocol analysis set |
| SAF | Safety analysis set |
| CI | Confidence interval |
| GLP-1 | Glucagon-like peptide |
| HPTMs | Histone post-translational modifications |
| Kbhb | Lysine β-hydroxybutyrylation |
References
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Therapy insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307–308. [Google Scholar]
- Peterman, M.G. The ketogenic diet. JAMA 1928, 90, 1427–1429. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic diet and epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef]
- Yuen, A.W.C.; Sander, J.W. Rationale for using intermittent calorie restriction as a dietary treatment for drug resistant epilepsy. Epilepsy Behav. 2014, 33, 110–114. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic diet in Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef]
- Westman, E.C.; Tondt, J.; Maguire, E.; Yancy, W.S., Jr. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert. Rev. Endocrinol. Metab. 2018, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic diet and microbiota: Friends or enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M.F.; Goel, A.; Qin, H.; et al. Ketogenic diet alleviates colitis by reduction of colonic Group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct. Target. Ther. 2021, 6, 154. [Google Scholar] [CrossRef]
- Gangitano, E.; Tozzi, R.; Gandini, O.; Watanabe, M.; Basciani, S.; Mariani, S.; Lenzi, A.; Gnessi, L.; Lubrano, C. Ketogenic diet as a preventive and supportive care for COVID-19 patients. Nutrients 2021, 13, 1004. [Google Scholar] [CrossRef]
- Li, R.J.; Liu, Y.; Liu, H.Q.; Li, J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J. Food Biochem. 2020, 44, e13140. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA 2018, 319, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Balasse, E.O.; Féry, F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes Metab. Rev. 1989, 5, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef]
- Katsuya, S.; Kawata, Y.; Goto, T.; Tsubota, J. Daily intake of D-ß-Hydroxybutyric acid (D-BHB) reduces body fat in Japanese adult participants: A randomized, double-blind, placebo-controlled study. J. Nutr. Sci. Vitaminol. 2023, 69, 121–128. [Google Scholar] [CrossRef]
- Katsuya, S.; Kawata, Y.; Kawamura, Y.; Kawamura, J.; Tsubota, J. Effect of D-β-Hydroxybutyrate on sleep quality in healthy participants: A randomized, double-blind, placebo-controlled study. Biosci. Biotechnol. Biochem. 2025, 89, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R.; et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 2016, 62, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Dobosy, J.R.; Selker, E.U. Emerging connections between DNA methylation and histone acetylation. Cell. Mol. Life Sci. 2001, 58, 721–727. [Google Scholar] [CrossRef]
- Moya-Garzon, M.D.; Wang, M.; Li, V.L.; Lyu, X.; Wei, W.; Tung, A.S.H.; Raun, S.H.; Zhao, M.; Coassolo, L.; Islam, H.; et al. A β-hydroxybutyrate shunt pathway generates anti-obesity ketone metabolites. Cell 2025, 188, 175–186.e20. [Google Scholar] [CrossRef]
- Zhang, S.; Tanaka, Y.; Ishihara, A.; Uchizawa, A.; Park, I.; Iwayama, K.; Ogata, H.; Yajima, K.; Omi, N.; Satoh, M.; et al. Metabolic flexibility during sleep. Sci. Rep. 2021, 11, 17849. [Google Scholar] [CrossRef]
- Urbain, P.; Bertz, H. Monitoring for compliance with a ketogenic diet: What is the best time of day to test for urinary ketosis? Nutr. Metab. 2016, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Ishimoto, T.; Sato, M.; Yasuda, R.; Nakamura, Y.; Watanabe, H.; Suzuki, T.; Araragi, Y.; Kato, Y.; Yoshida, K.I.; et al. Optimizing oral 3-hydroxybutyrate dosage using pharmacokinetic model to improve cognitive function and mood in healthy subjects. Front. Nutr. 2024, 11, 1470331. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, N.; Maki, H.; Suzuki, Y.; Haruna, H.; Ohara, A.; Kasai, M.; Tsuji, H.; Aoyama, T.; Okazaki, M.; Igarashi, O.; et al. Effects of margarine containing medium-chain triacylglycerols on body fat reduction in humans. J. Atheroscler. Thromb. 2003, 10, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Arner, P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann. Med. 1995, 27, 435–438. [Google Scholar] [CrossRef]
- Lemieux, S.; Després, J.P. Metabolic complications of visceral obesity: Contribution to the aetiology of type 2 diabetes and implications for prevention and treatment. Diabetes Metab. 1994, 20, 375–393. [Google Scholar]
- Bjorntorp, P. Regional obesity. In Obesity; Bjorntorp, P., Brodoff, B.N., Eds.; J. B. Lippincott Publishers: Philadelphia, PA, USA, 1992; pp. 579–586. [Google Scholar]
- Armellini, F.; Zamboni, M.; Rigo, L.; Bergamo-Andreis, I.A.; Robbi, R.; De Marchi, M.; Bosello, O. Sonography detection of small intra-abdominal fat variations. Int. J. Obes. 1991, 15, 847–852. [Google Scholar]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Prabhakar, A.; Quach, A.; Wang, D.; Zhang, H.; Terrera, M.; Jackemeyer, D.; Xian, X.; Tsow, F.; Tao, N.; Forzani, E.S. Breath acetone as biomarker for lipid oxidation and early ketone detection. Glob. J. Obes. Diabetes Metab. Syndr. 2014, 1, 12–19. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Mell, B.; Manandhar, I.; Yeoh, B.S.; Mei, X.; Akinola, O.M.; Ahlidja, W.; Joe, B. Ketone body β-hydroxybutyrate-mediated histone β-hydroxybutyrylation upregulates lipolysis and attenuates metabolic syndrome. Am. J. Physiol.-Cell Physiol. 2025, 329, C726–C743. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-Beta-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Srivastava, S.; Kashiwaya, Y.; King, M.T.; Baxa, U.; Tam, J.; Niu, G.; Chen, X.; Clarke, K.; Veech, R.L. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 2012, 26, 2351–2362. [Google Scholar] [CrossRef]
- Ogawa, A.; Nosaka, N.; Kasai, M.; Aoyama, T.; Okazaki, M.; Igarashi, O.; Kondo, K. Dietary medium- and long-chain triacylglycerols accelerate diet-induced thermogenesis in humans. J. Oleo Sci. 2007, 56, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Fulghum, K.; Salathe, S.F.; Davis, X.; Thyfault, J.P.; Puchalska, P.; Crawford, P.A. Ketone body metabolism and cardiometabolic implications for cognitive health. NPJ Metab. Health Dis. 2024, 2, 29. [Google Scholar] [CrossRef]
- Vestergaard, E.T.; Zubanovic, N.B.; Rittig, N.; Møller, N.; Kuhre, R.E.; Holst, J.J.; Rehfeld, J.F.; Thomsen, H.H. Acute ketosis inhibits appetite and decreases plasma concentrations of acyl ghrelin in healthy young men. Diabetes Obes. Metab. 2021, 23, 1834–1842. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Cyranka, M.; Clarke, K.; de Wet, H. A ketone ester drink lowers human ghrelin and appetite. Obesity 2018, 26, 269–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).