Silymarin: A Natural Compound for Obesity Management
Abstract
1. Introduction
2. Obesity and Metabolic Diseases
3. Silybum marianum (L.): An Overview
4. Evidence of the Efficacy of Silybum marianum (L.) in Obesity and Metabolic Diseases
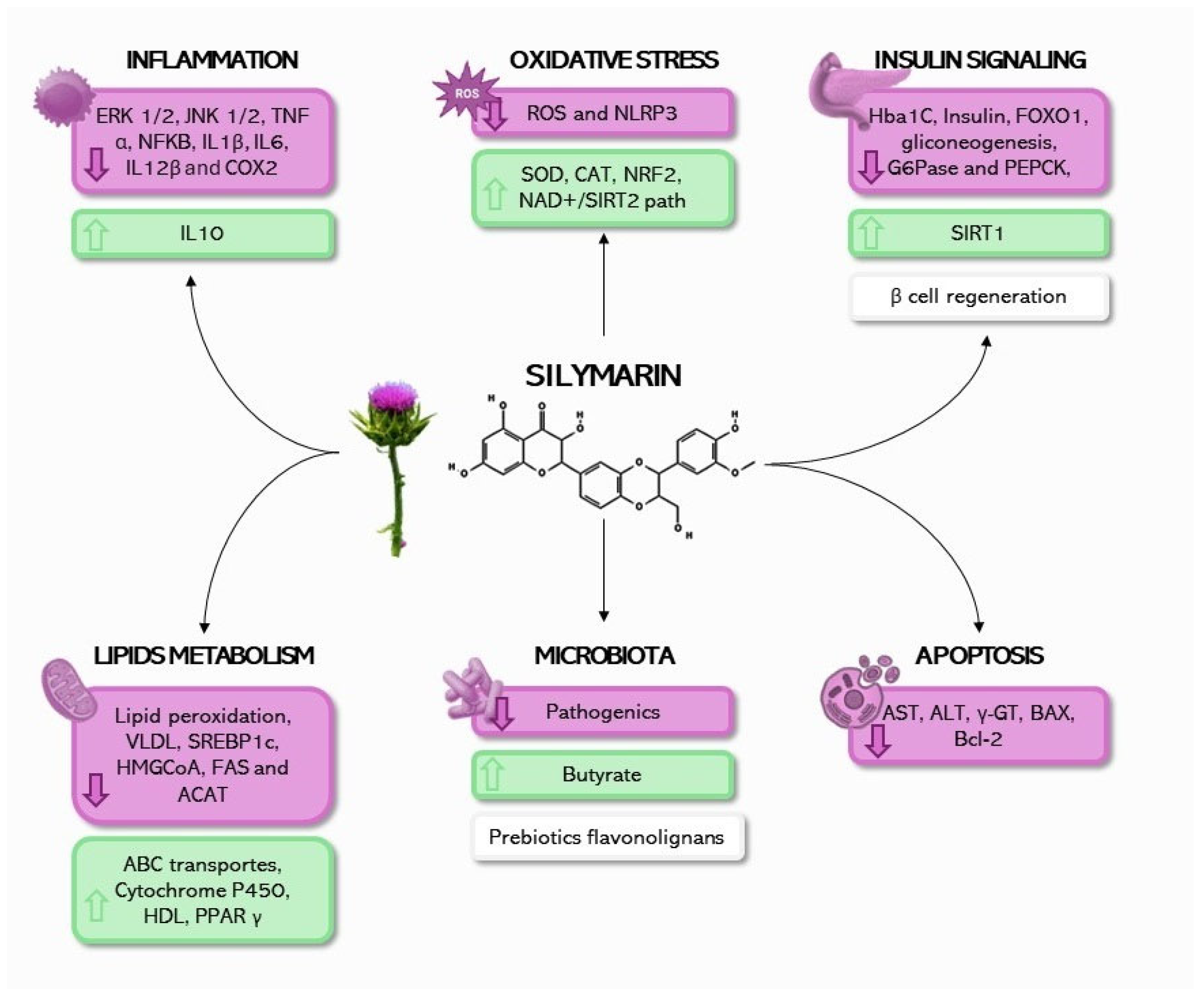
5. Mechanisms of Action of Silybum marianum (L.) in Obesity and Metabolic Diseases
6. Contraindications and Drug Interaction of Silybum marianum (L.)
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bahmani, M.; Eftekhari, Z.; Saki, K.; Fazeli-Moghadam, E.; Jelodari, M.; Rafieian-Kopaei, M. Obesity Phytotherapy: Review of Native Herbs Used in Traditional Medicine for Obesity. J. Evid. Based Complement. Altern. Med. 2016, 21, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Mayén, A.-L.; Marques-Vidal, P.; Paccaud, F.; Bovet, P.; Stringhini, S. Socioeconomic Determinants of Dietary Patterns in Low- and Middle-Income Countries: A Systematic Review. Am. J. Clin. Nutr. 2014, 100, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.; Frazão, E. Food Costs, Diet Quality and Energy Balance in the United States. Physiol. Behav. 2014, 134, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Deguchi, Y.; Nozaki, Y.; Higami, Y. Contribution of Pgc-1α to Obesity- and Caloric Restriction-related Physiological Changes in White Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 6025. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X. The Obesity Epidemic: Pathophysiology and Consequences of Obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Power of Cities: Tackling Noncommicable Diseases and Road Traffic Injuries; WHO: Geneva, Switzerland, 2019; p. 83. [Google Scholar]
- Makhoul, E.; Aklinski, J.L.; Miller, J.; Leonard, C.; Backer, S.; Kahar, P.; Parmar, M.S.; Khanna, D. A Review of COVID-19 in Relation to Metabolic Syndrome: Obesity, Hypertension, Diabetes, and Dyslipidemia. Cureus 2022, 14, e27438. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Medical Treatment of Obesity: The Past, the Present and the Future. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Liu, T.T.; Teia, F.K.F.; Xie, M.Z. Exploring the Underlying Mechanisms of Obesity and Diabetes and the Potential of Traditional Chinese Medicine: An Overview of the Literature. Front. Endocrinol. 2023, 14, 1218880. [Google Scholar] [CrossRef] [PubMed]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum marianum (Milk Thistle) and Its Main Constituent, Silymarin, as a Potential Therapeutic Plant in Metabolic Syndrome: A Review. Phytother. Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological Potential and Mechanisms of Tea’s Bioactive Compounds: An Updated Review. J. Adv. Res. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia Cambogia for Weight Loss. Am. J. Health Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Wu, S.C. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Huang, B.; Jing, Y.; Shen, S.; Feng, W.; Wang, W.; Meng, R.; Zhu, D. Silymarin Ameliorates the Disordered Glucose Metabolism of Mice with Diet-Induced Obesity by Activating the Hepatic SIRT1 Pathway. Cell. Signal. 2021, 84, 110023. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Moraes, R.C.M.; Nehmi Filho, V.; Murata, G.M.; de Freitas, J.A.; de Miranda, D.A.; Cerqueira, A.R.A.; Costa, S.K.P.; Ferreira, A.F.F.; Britto, L.R.; et al. The Symbiotic Effect of a New Nutraceutical with Yeast β-Glucan, Prebiotics, Minerals, and Silybum marianum (Silymarin) for Recovering Metabolic Homeostasis via Pgc-1α, Il-6, and Il-10 Gene Expression in a Type-2 Diabetes Obesity Model. Antioxidants 2022, 11, 447. [Google Scholar] [CrossRef]
- Nehmi-Filho, V.; de Freitas, J.A.; Franco, L.A.; da Silva Fonseca, J.V.; Martins, R.C.; Santamarina, A.B.; Murata, G.M.; Sabino, E.C.; Souza, E.; Ferreira, M.T.; et al. Novel Nutraceutical (Silymarin, Yeast β-Glucan, Prebiotics, and Minerals) Shifts Gut Microbiota and Restores Large Intestine Histology of Diet-Induced Metabolic Syndrome Mice. J. Funct. Foods 2023, 107, 105671. [Google Scholar] [CrossRef]
- Atarodi, H.; Pazouki, A.; Gholizadeh, B.; Karami, R.; Kabir, A.; Sadri, G.; Kassir, R.; Kermansaravi, M. Effect of Silymarin on Liver Size and Nonalcoholic Fatty Liver Disease in Morbidly Obese Patients: A Randomized Double-Blind Clinical Trial. J. Res. Med. Sci. 2022, 27, 76. [Google Scholar] [CrossRef] [PubMed]
- Nehmi-Filho, V.; Santamarina, A.B.; de Freitas, J.A.; Trarbach, E.B.; de Oliveira, D.R.; Palace-Berl, F.; de Souza, E.; de Miranda, D.A.; Escamilla-Garcia, A.; Otoch, J.P.; et al. Novel Nutraceutical Supplements with Yeast β-Glucan, Prebiotics, Minerals, and Silybum marianum (Silymarin) Ameliorate Obesity-Related Metabolic and Clinical Parameters: A Double-Blind Randomized Trial. Front. Endocrinol. 2023, 13, 1089938. [Google Scholar] [CrossRef] [PubMed]
- Nehmi-Filho, V.; de Freitas, J.A.; Franco, L.A.; Martins, R.C.; Turri, J.A.O.; Santamarina, A.B.; da Fonseca, J.V.S.; Sabino, E.C.; Moraes, B.C.; Souza, E.; et al. Modulation of the Gut Microbiome and Firmicutes Phylum Reduction by a Nutraceutical Blend in the Obesity Mouse Model and Overweight Humans: A Double-Blind Clinical Trial. Food Sci. Nutr. 2024, 12, 2436–2454. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- World Health Organization. Acceleration Plan to Stop Obesity; World Health Organization: Geneva, Switzerland, 2018; p. 20. [Google Scholar]
- Bahia, L.; Araújo, D.V. Impacto econômico da obesidade no Brasil. Rev. Hosp. Univ. Pedro Ernesto 2014, 13, 13–17. [Google Scholar] [CrossRef]
- Algoblan, A.; Alalfi, M.; Khan, M. Mechanism Linking Diabetes Mellitus and Obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in Obesity, Insulin Resistance and Diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, K.J.P.; Bolzon, C.M.; Li, C.; Allard, J.P. Non-Alcoholic Fatty Liver Disease and Obesity: The Role of the Gut Bacteria. Eur. J. Nutr. 2019, 58, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Jin, Y.; Wang, T.Y.; Zheng, K.I.; Rios, R.S.; Zhang, H.Y.; Targher, G.; Byrne, C.D.; Yuan, W.J.; Zheng, M.H. MAFLD and Risk of CKD. Metab. Clin. Exp. 2021, 115, 154433. [Google Scholar] [CrossRef] [PubMed]
- Rohla, M.; Weiss, T.W. Metabolic Syndrome, Inflammation and Atherothrombosis. Hamostaseologie 2013, 33, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.; Zimmet, P.; Shaw, J. IDF Epidemiology Task Force Consensus Group. The Metabolic Syndrome: A New Worldwide Definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Di Somma, C.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and Sleep Disturbance: The Chicken or the Egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.J.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 March 2024).
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Diabetes Atlas 2021, 10th ed.; IDF: Brussels, Belgium, 2021. [Google Scholar]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 8 January 2024).
- Muka, T.; Imo, D.; Jaspers, L.; Colpani, V.; Chaker, L.; van der Lee, S.J.; Mendis, S.; Chowdhury, R.; Bramer, W.M.; Falla, A.; et al. The Global Impact of Non-Communicable Diseases on Healthcare Spending and National Income: A Systematic Review. Eur. J. Epidemiol. 2015, 30, 251–277. [Google Scholar] [CrossRef]
- Amarchand, R.; Krishnan, A.; Saraf, D.; Mathur, P.; Shukla, D.; Nath, L. Lessons for Addressing Noncommunicable Diseases within a Primary Health-Care System from the Ballabgarh Project, India. WHO South East Asia J. Public Health 2015, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and Tissue Injury: Agents of Defense or Destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.; Calder, P. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and Adipocyte Physiology: Implications for Adipose Tissue Dysfunction in Obesity. Annu. Rev. Nutr. 2014, 34, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Jornayvaz, F.R. Inflammation as a Potential Link between Nonalcoholic Fatty Liver Disease and Insulin Resistance. J. Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Erbay, E. Nutrient Sensing and Inflammation in Metabolic Diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Sundaram, S.; Johnson, A. Obesity, Metabolism and the Microenvironment: Links to Cancer. J. Carcinog. 2013, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- van Greevenbroek, M.M.J.; Schalkwijk, C.G.; Stehouwer, C.D. Obesity-Associated Low-Grade Inflammation in Type 2 Diabetes Mellitus: Causes and Consequences. Neth. J. Med. 2013, 71, 174–187. [Google Scholar] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Bianco, F.; Falcone, I.; Prisco, M.; Liverini, G.; Iossa, S. Alterations in Hepatic Mitochondrial Compartment in a Model of Obesity and Insulin Resistance. Obesity 2008, 16, 958–964. [Google Scholar] [CrossRef]
- Mantena, S.K.; Vaughn, D.P., Jr.; Andringa, K.K.; Eccleston, H.B.; King, A.L.; Abrams, G.A.; Doeller, J.E.; Kraus, D.W.; Darley-Usmar, V.M.; Bailey, S.M. High Fat Diet Induces Dysregulation of Hepatic Oxygen Gradients and Mitochondrial Function In Vivo. Biochem. J. 2009, 417, 183–193. [Google Scholar] [CrossRef]
- Iossa, S.; Lionetti, L.; Mollica, M.P.; Crescenzo, R.; Botta, M.; Barletta, A.; Liverini, G. Effect of High-Fat Feeding on Metabolic Efficiency and Mitochondrial Oxidative Capacity in Adult Rats. Br. J. Nutr. 2003, 90, 953–960. [Google Scholar] [CrossRef]
- Hoeks, J.; van Herpen, N.A.; Mensink, M.; Moonen-Kornips, E.; van Beurden, D.; Hesselink, M.K.C.; Schrauwen, P. Mitochondrial Dysfunction as Consequence Rather Than Cause of Human Insulin Resistance. Diabetes 2010, 59, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK Regulation of Fatty Acid Metabolism and Mitochondrial Biogenesis: Implications for Obesity. Mol. Cell. Endocrinol. 2013, 366, 135–151. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Van Denderen, B.J.W.; Howlett, K.F.; Mollica, J.; Schertzer, J.D.; Kemp, B.E.; Hargreaves, M. AMP-Activated Protein Kinase Regulates GLUT4 Transcription by Phosphorylating Histone Deacetylase 5. Diabetes 2008, 57, 860–867. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Curtis, J.M.; Grimsrud, P.A.; Wright, W.S.; Xu, X.; Foncea, R.E.; Graham, D.W.; Brestoff, J.R.; Wiczer, B.M.; Ilkayeva, O.; Cianflone, K.; et al. Downregulation of Adipose Glutathione S-Tansferase A4 Leads to Increased Protein Carbonylation, Oxidative Stress, and Mitochondrial Dysfunction. Diabetes 2010, 59, 1132–1142. [Google Scholar] [CrossRef]
- Le Lay, S.; Simard, G.; Martinez, M.C.; Andriantsitohaina, R. Oxidative Stress and Metabolic Pathologies: From an Adipocentric Point of View. Oxidative Med. Cell. Longev. 2014, 2014, 908539. [Google Scholar] [CrossRef]
- Valenzuela, R.; Echeverria, F.; Ortiz, M.; Rincón-Cervera, M.Á.; Espinosa, A.; Hernandez-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol Prevents Reduction in Liver Activity of Δ-5 and Δ-6 Desaturases, Oxidative Stress, and Depletion in Long Chain Polyunsaturated Fatty Acid Content in Different Tissues of High-Fat Diet Fed Mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Ehrenshaft, M.; Cleland, E.; Stadler, K. High-Fat Diet Induces an Initial Adaptation of Mitochondrial Bioenergetics in the Kidney despite Evident Oxidative Stress and Mitochondrial ROS Production. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1047–E1058. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk Thistle (Silybum marianum): A Concise Overview on Its Chemistry, Pharmacological, and Nutraceutical Uses in Liver Diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The Food Plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and Clinical Evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Hong, S.Y.; Han, J.-H.; Yu, Y.; Yoo, E.; Sung, J.; Chin, J.H.; Lee, O.N. A Genomic Evaluation of Six Selected Inbred Lines of the Naturalized Plants of Milk Thistle (Silybum marianum L. Gaertn.) in Korea. Plants 2023, 12, 2702. [Google Scholar] [CrossRef]
- Navarro, V.J.; Belle, S.H.; D’Amato, M.; Adfhal, N.; Brunt, E.M.; Fried, M.W.; Reddy, K.R.; Wahed, A.S.; Harrison, S.; on behalf of the Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Silymarin in Non-Cirrhotics with Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo Controlled Trial. PLoS ONE 2019, 14, e0221683. [Google Scholar] [CrossRef]
- Okiljević, B.; Martić, N.; Govedarica, S.; Andrejić Višnjić, B.; Bosanac, M.; Baljak, J.; Pavlić, B.; Milanović, I.; Rašković, A. Cardioprotective and Hepatoprotective Potential of Silymarin in Paracetamol-Induced Oxidative Stress. Pharmaceutics 2024, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Gür, F.M.; Bilgiç, S. Silymarin, an Antioxidant Flavonoid, Protects the Liver from the Toxicity of the Anticancer Drug Paclitaxel. Tissue Cell 2023, 83, 102158. [Google Scholar] [CrossRef] [PubMed]
- DuBreuil, D.M.; Lai, X.; Zhu, K.; Chahyadinata, G.; Perner, C.; Chiang, B.M.; Battenberg, A.; Sokol, C.L.; Wainger, B.J. Phenotypic Screen Identifies the Natural Product Silymarin as a Novel Anti-Inflammatory Analgesic. Mol. Pain 2023, 19, 174480692211483. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk Thistle in Liver Diseases: Past, Present, Future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Carrier, D.J.; Crowe, T.; Sokhansanj, S.; Wahab, J.; Barl, B. Milk Thistle, Silybum marianum (L.) Gaertn., Flower Head Development and Associated Marker Compound Profile. J. Herbs Spices Med. Plants 2003, 10, 65–74. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef] [PubMed]
- Doğan, G.; Kara, N.; Gür, S.; Bagci, E. Chemical Composition and Biological Activity of Milk Thistle Seeds (Silybum marianum (L.) Gaertn.). Int. J. Nat. Life Sci. 2022, 6, 90–98. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Tutor, A.W.; Lavie, C.J.; Kachur, S.; Milani, R.V.; Ventura, H.O. Updates on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2023, 78, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Nelson, D.R.; Mitroi, J.; Heaton, P.C.; Hincapie, A.L.; Brouwers, B. The Roles of Type 2 Diabetes and Obesity in Disease Activity and Progression of Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis. Curr. Med. Res. Opin. 2024, 40, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.-M.; Tong, Q.; et al. Silymarin Ameliorates Metabolic Dysfunction Associated with Diet-Induced Obesity via Activation of Farnesyl X Receptor. Front. Pharmacol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- MacDonald-Ramos, K.; Monroy, A.; Bobadilla-Bravo, M.; Cerbón, M. Silymarin Reduced Insulin Resistance in Non-Diabetic Women with Obesity. Int. J. Mol. Sci. 2024, 25, 2050. [Google Scholar] [CrossRef] [PubMed]
- Nehmi, V.A.; Murata, G.M.; Moraes, R.C.; Lima, G.C.; Miranda, D.A.; Radloff, K.; Costa, R.G.; Jesus, J.D.; Freitas, J.A.; Viana, N.I.; et al. A Novel Supplement with Yeast β-Glucan, Prebiotic, Minerals and Silybum marianum Synergistically Modulates Metabolic and Inflammatory Pathways and Improves Steatosis in Obese Mice. J. Integr. Med. 2021, 19, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Aghemo, A.; Alekseeva, O.P.; Angelico, F.; Bakulin, I.G.; Bakulina, N.V.; Bordin, D.; Bueverov, A.O.; Drapkina, O.M.; Gillessen, A.; Kagarmanova, E.M.; et al. Role of Silymarin as Antioxidant in Clinical Management of Chronic Liver Diseases: A Narrative Review. Ann. Med. 2022, 54, 1548–1560. [Google Scholar] [CrossRef]
- Hüttl, M.; Markova, I.; Miklankova, D.; Zapletalova, I.; Poruba, M.; Racova, Z.; Vecera, R.; Malinska, H. The Beneficial Additive Effect of Silymarin in Metformin Therapy of Liver Steatosis in a Pre-Diabetic Model. Pharmaceutics 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, S.; Wang, Y.; Zhu, T. Silymarin Improved Diet-Induced Liver Damage and Insulin Resistance by Decreasing Inflammation in Mice. Pharm. Biol. 2016, 54, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Poruba, M.; Kazdová, L.; Oliyarnyk, O.; Malinská, H.; Matusková, Z.; Tozzi Di Angelo, I.; Skop, V.; Vecera, R. Improvement Bioavailability of Silymarin Ameliorates Severe Dyslipidemia Associated with Metabolic Syndrome. Xenobiotica 2015, 45, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Alsaggar, M.; Bdour, S.; Ababneh, Q.; El-Elimat, T.; Qinna, N.; Alzoubi, K.H. Silibinin Attenuates Adipose Tissue Inflammation and Reverses Obesity and Its Complications in Diet-Induced Obesity Model in Mice. BMC Pharmacol. Toxicol. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Butanda-Nuñez, A.; Rodríguez-Cortés, O.; Ramos-Martínez, E.; Cerbón, M.A.; Escobedo, G.; Chavarría, A. Silybin Restores Glucose Uptake after Tumour Necrosis Factor-Alpha and Lipopolysaccharide Stimulation in 3T3-L1 Adipocytes. Adipocyte 2024, 13, 2374062. [Google Scholar] [CrossRef] [PubMed]
- Akheratdoost, V.; Panahi, N.; Safi, S.; Mojab, F.; Akbari, G. Protective Effects of Silymarin-Loaded Chitosan Nanoparticles in the Diet-Induced Hyperlipidemia Rat Model. Iran. J. Basic Med. Sci. 2024, 27. [Google Scholar] [CrossRef]
- Schrieber, S.J.; Hawke, R.L.; Wen, Z.; Smith, P.C.; Reddy, K.R.; Wahed, A.S.; Belle, S.H.; Afdhal, N.H.; Navarro, V.J.; Meyers, C.M.; et al. Differences in the Disposition of Silymarin between Patients with Nonalcoholic Fatty Liver Disease and Chronic Hepatitis C. Drug Metab. Dispos. 2011, 39, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Formisano, E.; Pasta, A.; Cremonini, A.L.; Favari, E.; Ronca, A.; Carbone, F.; Semino, T.; Di Pierro, F.; Sukkar, S.G.; Pisciotta, L. Efficacy of Nutraceutical Combination of Monacolin K, Berberine, and Silymarin on Lipid Profile and PCSK9 Plasma Level in a Cohort of Hypercholesterolemic Patients. J. Med. Food 2020, 23, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Mazzotti, A.; Caletti, M.T.; Brodosi, L.; Di Domizio, S.; Forchielli, M.L.; Petta, S.; Bugianesi, E.; Bianchi, G.; Marchesini, G. An Internet-Based Approach for Lifestyle Changes in Patients with NAFLD: Two-Year Effects on Weight Loss and Surrogate Markers. J. Hepatol. 2018, 69, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Fu, J.; Liu, W.; Hayashi, T.; Nie, Y.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin Inhibits Migration and Invasion of Breast Cancer MDA-MB-231 Cells through Induction of Mitochondrial Fusion. Mol. Cell Biochem. 2020, 463, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, Y.; Li, X.; Deng, G.; Han, Y.; Yuan, F.; Yi, G.; Xia, X. Liposomal Silybin Improves Glucose and Lipid Metabolisms in Type 2 Diabetes Mellitus Complicated with Non-Alcoholic Fatty Liver Disease via AMPK/TGF-β1/Smad Signaling. Tohoku J. Exp. Med. 2023, 261, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.F.; Ferreira, F.M.; Dias, B.V.; Azevedo, D.C.D.; De Souza, G.H.B.; Milagre, M.M.; De Lana, M.; Vieira, P.M.D.A.; Carneiro, C.M.; Paula-Gomes, S.D.; et al. Silymarin Inhibits the Lipogenic Pathway and Reduces Worsening of Non-Alcoholic Fatty Liver Disease (NAFLD) in Mice. Arch. Physiol. Biochem. 2022, 130, 460–474. [Google Scholar] [CrossRef]
- Cui, C.-X.; Deng, J.-N.; Yan, L.; Liu, Y.-Y.; Fan, J.-Y.; Mu, H.-N.; Sun, H.-Y.; Wang, Y.-H.; Han, J.-Y. Silibinin Capsules Improves High Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Hamsters through Modifying Hepatic de Novo Lipogenesis and Fatty Acid Oxidation. J. Ethnopharmacol. 2017, 208, 24–35. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, M.; Gao, X.; Hu, P.; Li, C.; Yang, X. Effect and the Probable Mechanisms of Silibinin in Regulating Insulin Resistance in the Liver of Rats with Non-Alcoholic Fatty Liver. Braz. J. Med. Biol. Res. 2013, 46, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.J.; Nunes, P.R.; Matias, M.L.; Ribeiro, V.R.; Devides, A.C.; Bannwart-Castro, C.F.; Romagnoli, G.G.; Peraçoli, J.C.; Peraçoli, M.T.S.; Romao-Veiga, M. Silibinin Induces in Vitro M2-like Phenotype Polarization in Monocytes from Preeclamptic Women. Int. Immunopharmacol. 2020, 89, 107062. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Valsala Gopalakrishnan, A. The Interplay of Arsenic, Silymarin, and NF-ĸB Pathway in Male Reproductive Toxicity: A Review. Ecotoxicol. Environ. Saf. 2023, 252, 114614. [Google Scholar] [CrossRef]
- Soleymani, S.; Ayati, M.H.; Mansourzadeh, M.J.; Namazi, N.; Zargaran, A. The Effects of Silymarin on the Features of Cardiometabolic Syndrome in Adults: A Systematic Review and Meta-analysis. Phytother. Res. 2022, 36, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Chlopčíková, Š.; Psotová, J.; Miketová, P.; Šimánek, V. Chemoprotective Effect of Plant Phenolics against Anthracycline-induced Toxicity on Rat Cardiomyocytes. Part I. Silymarin and Its Flavonolignans. Phytother. Res. 2004, 18, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.K.; Park, S.J.; Lee, M.Y.; Cha, M.J.; Kim, O.H.; You, H.J.; Chang, I.Y.; Yoon, S.P.; Jeon, Y.J. Silibinin Inhibits LPS-Induced Macrophage Activation by Blocking P38 MAPK in RAW 264.7 Cells. Biomol. Ther. 2013, 21, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Agarwal, C.; Agarwal, R. Effect of Silibinin in Human Colorectal Cancer Cells: Targeting the Activation of NF-κB Signaling. Mol. Carcinog. 2013, 52, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, B.; Cao, S.; Wang, Y.; Wu, D. Silybin Attenuates LPS-Induced Lung Injury in Mice by Inhibiting NF-κB Signaling and NLRP3 Activation. Int. J. Mol. Med. 2017, 39, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Salamone, F.; Galvano, F.; Cappello, F.; Mangiameli, A.; Barbagallo, I.; Li Volti, G. Silibinin Modulates Lipid Homeostasis and Inhibits Nuclear Factor Kappa B Activation in Experimental Nonalcoholic Steatohepatitis. Transl. Res. 2012, 159, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Andleeb, A.; Ashraf, H.; Meer, B.; Mehmood, A.; Jan, H.; Zaman, G.; Nadeem, M.; Drouet, S.; Fazal, H.; et al. Potential Antimicrobial, Antidiabetic, Catalytic, Antioxidant and ROS/RNS Inhibitory Activities of Silybum marianum Mediated Biosynthesized Copper Oxide Nanoparticles. RSC Adv. 2022, 12, 14069–14083. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; She, L.; Wang, Z.; Yang, N.; Sun, R.; Zhang, Y.; Yan, C.; Wei, Q.; Aa, J.; et al. Silybin Inhibits NLRP3 Inflammasome Assembly through the NAD+/SIRT2 Pathway in Mice with Nonalcoholic Fatty Liver Disease. FASEB J. 2018, 32, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Haddad, Y.; Vallerand, D.; Brault, A.; Haddad, P.S. Antioxidant and Hepatoprotective Effects of Silibinin in a Rat Model of Nonalcoholic Steatohepatitis. Evid. Based Complement. Altern. Med. 2011, 2011, nep164. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The Efficacy of Silybum marianum (L.) Gaertn. (Silymarin) in the Treatment of Type II Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake. In Blood Glucose Levels; Szablewski, L., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-525-8. [Google Scholar]
- Metz, H.E.; McGarry Houghton, A. Insulin Receptor Substrate Regulation of Phosphoinositide 3-Kinase. Clin. Cancer Res. 2011, 17, 206–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Hai, J.; Cao, M.; Zhang, Y.; Pei, S.; Wang, J.; Zhang, Q. Silibinin Ameliorates Steatosis and Insulin Resistance during Non-Alcoholic Fatty Liver Disease Development Partly through Targeting IRS-1/PI3K/Akt Pathway. Int. Immunopharmacol. 2013, 17, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Xu, Y.; Zhang, X.; Wu, B.; Wang, C. Role of FXR in the Development of NAFLD and Intervention Strategies of Small Molecules. Arch. Biochem. Biophys. 2024, 757, 110024. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Wang, H. Silymarin Attenuated Hepatic Steatosis through Regulation of Lipid Metabolism and Oxidative Stress in a Mouse Model of Nonalcoholic Fatty Liver Disease (NAFLD). Am. J. Transl. Res. 2016, 8, 1073–1081. [Google Scholar] [PubMed]
- Mengesha, T.; Gnanasekaran, N.; Mehare, T. Hepatoprotective Effect of Silymarin on Fructose Induced Nonalcoholic Fatty Liver Disease in Male Albino Wistar Rats. BMC Complement. Med. Ther. 2021, 21, 104. [Google Scholar] [CrossRef]
- Poruba, M.; Matušková, Z.; Kazdová, L.; Oliyarnyk, O.; Malínská, H.; Tozzi Di Angelo, I.; Večeřa, R. Positive Effects of Different Drug Forms of Silybin in the Treatment of Metabolic Syndrome. Physiol. Res. 2015, 64, S507–S512. [Google Scholar] [CrossRef] [PubMed]
- Krečman, V.; Škottová, N.; Walterová, D.; Ulrichová, J.; Šimanek, V. Silymarin Inhibits the Development of Diet-Induced Hypercholesterolemia in Rats. Planta Med. 1998, 64, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S.; Gay, H.C. Statins for the Primary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2013, 1, CD004816. [Google Scholar] [CrossRef] [PubMed]
- Kottova, N. Phenolics-Rich Extracts from Silybum marianum and Prunella vulgaris Reduce a High-Sucrose Diet Induced Oxidative Stress in Hereditary Hypertriglyceridemic Rats. Pharmacol. Res. 2004, 50, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Uchitel, S.; Denniston, K.; Chopra, D.; Mills, P.J.; Peterson, S.N. Prebiotic Potential of Herbal Medicines Used in Digestive Health and Disease. J. Altern. Complement. Med. 2018, 24, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-L.; Hua, S.; Li, X.-Y.; Shen, L.; Wu, H.; Ji, H.-F. Microbially Produced Vitamin B12 Contributes to the Lipid-Lowering Effect of Silymarin. Nat. Commun. 2023, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Song, Q.; Ouyang, Z.; Zheng, M.; Zhang, X.; Zhang, C. Efficacy of Silymarin in Treatment of COPD via P47phox Signaling Pathway. Food Sci. Technol. 2022, 42, e52821. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Y.; Liu, S.; Yang, M. Anti-Viral Activity of Bioactive Molecules of Silymarin against COVID-19 via In Silico Studies. Pharmaceuticals 2023, 16, 1479. [Google Scholar] [CrossRef]
- Wu, C.-P.; Calcagno, A.M.; Hladky, S.B.; Ambudkar, S.V.; Barrand, M.A. Modulatory Effects of Plant Polyphenols on Human Multidrug Resistance Proteins 1, 4, and 5 (ABCC1, 4, and 5). FEBS J. 2005, 272, 4725–4740. [Google Scholar] [CrossRef]
- Koltai, T.; Fliegel, L. Role of Silymarin in Cancer Treatment: Facts, Hypotheses, and Questions. J. Evid. Based Complement. Altern. Med. 2022, 27, 2515690X2110688. [Google Scholar] [CrossRef]
- Barbosa, C.C.; Nishimura, A.N.; Santos, M.L.D.; Junior, W.D.; Andersen, M.L.; Mazaro-Costa, R. Silymarin Administration during Pregnancy and Breastfeeding: Evaluation of Initial Development and Adult Behavior of Mice. Neurotoxicology 2020, 78, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Milić, N.; Milošević, N.; Suvajdžić, L.; Žarkov, M.; Abenavoli, L. New Therapeutic Potentials of Milk Thistle (Silybum marianum). Nat. Prod. Commun. 2013, 8, 1934578X1300801. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and Their Bioactive Phytochemicals for Women’s Health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef] [PubMed]
- Malewicz, B. Enhancement of Mammary Carcinogenesis in Two Rodent Models by Silymarin Dietary Supplements. Carcinogenesis 2006, 27, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Emadi, E.; Ghasemzadeh Rahbardar, M.; Mehri, S.; Hosseinzadeh, H. A Review of Therapeutic Potentials of Milk Thistle (Silybum marianum L.) and Its Main Constituent, Silymarin, on Cancer, and Their Related Patents. Iran. J. Basic Med. Sci. 2022, 25, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Wojas, O.; Krzych-Fałta, E.; Samel-Kowalik, P.; Żalikowska-Gardocka, M.; Majsiak, E.; Mari, A.; Samoliński, B. A Case of Allergy to Silybum marianum (Milk Thistle) and Eragrostis Tef (Teff). Allergy Asthma Clin. Immunol. 2020, 16, 23. [Google Scholar] [CrossRef]
- Ramírez-Santos, A.; Pérez-Bustillo, A.; González-Sixto, B.; Suárez-Amor, O.; Rodríguez-Prieto, M.A. cute Generalized Exanthematous Pustulosis Due to Milk Thistle (Silybum marianum) Tea. Actas Dermo Sifiliográficas 2011, 102, 744–745. [Google Scholar] [CrossRef]
- Gamissans, M.; Expósito-Serrano, V.; López-Llunell, C.; Valdivieso, L.; Garbayo-Salmons, P. Bullous Pemphigoid Triggered by Silybum Marianum: An Unexpected Side Effect of an Herbal Remedy. Int. J. Dermatol. 2022, 61, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Post-White, J.; Ladas, E.J.; Kelly, K.M. Advances in the Use of Milk Thistle (Silybum marianum). Integr. Cancer Ther. 2007, 6, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Illuri, R.; Venkataramana, S.H.; Daguet, D.; Kodimule, S. Sub-Acute and Acute Toxicity of Ferula Asafoetida and Silybum Marianum Formulation and Effect of the Formulation on Delaying Gastric Emptying. BMC Complement. Altern. Med. 2019, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Lin, L.C.; Tsai, T.H. Drug-Drug Interactions of Silymarin on the Perspective of Pharmacokinetics. J. Ethnopharmacol. 2009, 121, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Faisal, Z.; Mohos, V.; Fliszár-Nyúl, E.; Valentová, K.; Káňová, K.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Interaction of Silymarin Components and Their Sulfate Metabolites with Human Serum Albumin and Cytochrome P450 (2C9, 2C19, 2D6, and 3A4) Enzymes. Biomed. Pharmacother. 2021, 138, 111459. [Google Scholar] [CrossRef] [PubMed]
- Doehmer, J.; Weiss, G.; McGregor, G.P.; Appel, K. Assessment of a Dry Extract from Milk Thistle (Silybum marianum) for Interference with Human Liver Cytochrome-P450 Activities. Toxicol. Vitr. 2011, 25, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, X.; Ho, P.C.L.; Chan, S.Y.; Heng, P.W.S.; Chan, E.; Duan, W.; Koh, H.L.; Zhou, S. Herb-Drug Interactions: A Literature Review. Drugs 2005, 65, 1239–1282. [Google Scholar] [CrossRef] [PubMed]
- Moltó, J.; Valle, M.; Miranda, C.; Cedeño, S.; Negredo, E.; Clotet, B. Effect of Milk Thistle on the Pharmacokinetics of Darunavir-Ritonavir in HIV-Infected Patients. Antimicrob. Agents Chemother. 2012, 56, 2837–2841. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.J.; Oberlies, N.H.; Kroll, D.J.; Paine, M.F. Two Flavonolignans from Milk Thistle (Silybum marianum) Inhibit CYP2C9-Mediated Warfarin Metabolism at Clinically Achievable Concentrations. J. Pharmacol. Exp. Ther. 2010, 332, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi-Suzuki, M.; Frye, R.F.; Zhu, H.-J.; Brinda, B.J.; Chavin, K.D.; Bernstein, H.J.; Markowitz, J.S. The Effects of Milk Thistle (Silybum marianum) on Human Cytochrome P450 Activity. Drug Metab. Dispos. 2014, 42, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Mooiman, K.D.; Maas-Bakker, R.F.; Hendrikx, J.J.M.A.; Bank, P.C.D.; Rosing, H.; Beijnen, J.H.; Schellens, J.H.M.; Meijerman, I. The Effect of Complementary and Alternative Medicines on CYP3A4-Mediated Metabolism of Three Different Substrates: 7-Benzyloxy-4-Trifluoromethyl-Coumarin, Midazolam and Docetaxel. J. Pharm. Pharmacol. 2014, 66, 865–874. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, J.A.; Santamarina, A.B.; Otoch, J.P.; Pessoa, A.F.M. Silymarin: A Natural Compound for Obesity Management. Obesities 2024, 4, 292-313. https://doi.org/10.3390/obesities4030024
de Freitas JA, Santamarina AB, Otoch JP, Pessoa AFM. Silymarin: A Natural Compound for Obesity Management. Obesities. 2024; 4(3):292-313. https://doi.org/10.3390/obesities4030024
Chicago/Turabian Stylede Freitas, Jessica Alves, Aline Boveto Santamarina, José Pinhata Otoch, and Ana Flávia Marçal Pessoa. 2024. "Silymarin: A Natural Compound for Obesity Management" Obesities 4, no. 3: 292-313. https://doi.org/10.3390/obesities4030024
APA Stylede Freitas, J. A., Santamarina, A. B., Otoch, J. P., & Pessoa, A. F. M. (2024). Silymarin: A Natural Compound for Obesity Management. Obesities, 4(3), 292-313. https://doi.org/10.3390/obesities4030024




