Abstract
(1) Background: Few studies focus on the development of obesity as a chronic disease as opposed to an acute condition. The “general purpose” C3H/HeJ (C3H) mouse strain is an alternative model for obesity development with regards to sex disparities and non-predisposed populations over time. (2) Methods: In this study, 64 female and 64 male C3H mice were separated into two groups (n = 32) and maintained on a control or high-fat diet (HFD) for up to 18 months. At 6-month intervals, a cross-sectional cohort (n = ~8) was censored for evaluation. The mice were monitored for change in total, lean and fat mass, survivability, and tumor incidence. (3) Results: Both sexes in the C3H mouse strain developed diet-induced obesity (DIO). An increase in total mass consistent with a HF diet was observed in both female and male C3H mice. Survivorship at 18 months was the highest in the HF-diet-fed males (~62%) and lowest in the males fed the control diet (~19%). Females showed survivability at ~40%, regardless of diet. Cancer development increased more notably in the males with the HF diet and showed sex bias for liver cancer (males) and ovarian cancer (females) incidence with age. (4) Conclusions: This study establishes a baseline for future use of C3H mice as a strong model for studying obesity as a chronic disease, in both sexes, and as long-term model for age-related diet-induced obesity and cancer development.
1. Introduction
Animal models are used to provide researchers with a physiological and behavioral platform to predict experimental outcomes in human subjects [1]. Rodent models are the most commonly used animal models for conducting biomedical research, accounting for ~99.3% of mammals used [2]. One survey estimates that approximately 111.5 million rodents were used in 2017 for research in the United States [2]. It is important to note, that of this estimate, more than 95% of the rodents used were mice, a trend supported by other surveys [1,2].
The most prolific animal model and gold standard for in vivo scientific research is Mus musculus [3]. The most common strain of mouse for studying obesity is the inbred strain C57BL/6, (C57) [3]. These mice are highly susceptible to diet-induced obesity (DIO), type 2 diabetes, atherosclerosis, and alcohol consumption [4,5,6]. When C57 mice are maintained for 12 weeks on an 11% kcal fat diet from 4 weeks old, the C57 mice weigh an average of ~25 g for males and 23 g for females [7]. When maintained on a high-fat diet (HFD) (46% kcal fat), this strain reaches a maximum of ~35 g for males, but only ~23 g for females at 12 weeks, showing no difference in weight gain from those on a low-fat diet [7]. Similar trends in mass can be observed at other age ranges and substrains of C57s using HFD [8,9]. This makes them an excellent model for studying acute etiology and pathogenesis of obesity over a short time in males but not in females. However, since this model is highly susceptible to developing obesity in males, the current studies using C57 mice to examine population-based changes and/or long-term pathogenesis may be less reflective of the progression and effects in the general human population and certainly for females. Thus, the use of alternative, utilitarian, rodent models should be considered to better represent the pathogenesis of obesity, its co-morbidities, and how its development may be altered in different demographics.
To this end, a long-term study on DIO was performed in C3H/HeJ (C3H) mice. Previous studies have reported that this strain lacks the exogenous mouse mammary tumor virus, but females still develop mammary tumors later in life [10]. There are also reported incidences of alopecia areata in up to 20% of mice between 12 and 18 months of age. Among C3H mice, male mice live for ~25 months and females live for ~28 months [11]. They possess a spontaneous mutation in Tlr4 at the lipopolysaccharide response locus (Tlr4Lps-d), resulting in resistance to endotoxin [12].
With regards to studies in obesity or metabolic syndrome, Poggi et al. indicated that C3H mice with Tlr4Lps-d are protected against insulin resistance when maintained on an HFD [10]. These mice also exhibited reductions in liver size attributable to lower hepatic triacylglycerol content [12]. C3H mice demonstrated lower levels of triglyceride (TG) content, after only 12 weeks on an HFD, when compared to C57 mice hepatic lipid accumulation [13]. The findings indicated that the TG content of wildtype C3H is comparable to CD44 knockouts, a gene identified for its association with adiposity/obesity, in both C57 and C3H [13]. This makes C3H a unique contrast to the C57 strain, providing alternatives for obesity and inflammaging research to reflect a population with low susceptibility to obesity (“obesity-resistant”), and over a longer duration [14]. Other markers for inflammation, associated with obesity, such as TNFα, IL-6, CCL2, have also been measured in C3H [13].
A recent study on young adult (2 months) and aged (14 months) C57 mice fed an HFD (60% kcal fat) for up to 20 weeks showed that there were sexual disparities between male and female mice in developing obesity, both in early and late adulthood [15]. Moreover, their results showed that obesity developed in the older (~19 months at the termination of this study) individuals due to HFDs [15]. However, they do not investigate the effects of continued HFD intake long-term. It is important to note that most studies report results after ≤16 weeks, providing insight into arguably acute development of obesity, potentially missing subtle physiological changes that occur when the obese condition is allowed to progress as a chronic condition [7,8,12,16]. Thus, the differences in rapidly induced obesity may differ in its effects when studied as a long-term, chronically progressing disease in a mouse strain with no reported susceptibility to obesity.
In this study, a long-term investigation of the pathogenesis of obesity in the C3H mouse strain was performed to report sex and aging-based responses. This strain provides an alternative perspective for modeling the growing obesity epidemic in Western culture. Using a strain that is not genetically predisposed to develop obesity as a longevity model may provide insight into the challenges associated with diet-induced lifestyle factors relating to obesity not observed in transgenic or obesity-susceptible strains.
2. Methods
2.1. Animal Study and Diets
Selection of the C3H mouse strain was based on disease susceptibility, lifespan, and less-aggressive social behavior [17]. Mouse handling followed regulatory compliance at all times under the approved Texas Tech University IACUC protocol number 19021-02. For this study, 72 male and 72 female C3H mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) at 4 weeks old. After one week of acclimatization, the mice were randomly separated by cage into baseline, control (CC) (380 kcal/100 g, 11% kcal fat), and HF-diet groups (460 kcal/100 g, 46% kcal fat) (Research Diets, New Brunswick, NJ, USA). Dietary components are listed in detail in Supplementary Table S1. Females and males were housed in separate rooms, 4 per cage, under temperature-, humidity- and 12 h light and 12 h dark cycle-controlled rooms. Animals had access to water and their assigned dietary group in pellet form, ad libitum. At collection time points and at termination of this study, mice were euthanized after a 1 h fast, using CO2 followed by cervical separation. The baseline group consisted of 8 male and 8 female mice, which were euthanized after the one-week adjustment period. The long-term study groups (consisting of 32 mice each) were maintained on control (11% kcal fat) and HF (46% kcal fat) diets, respectively, for up to 18 months. Weekly measurements of individual weights, food consumption per cage, and photographic records of individuals were performed. Monthly measurements for assessing changes in fat and lean mass were performed using EchoMRI (EchoMRI, Houston, TX, USA). Terminal cross-sectional tissue collections (censoring) were performed every 6 months on ~8 mice (~1/4 of each group) where the mice were euthanized using CO2.
2.2. Histology
Immediately following euthanasia, tissues from the brain, heart, lung, liver, kidneys, visceral fat, stomach, small intestines, reproductive organs, and skeletal muscle were collected. A portion of the tissue of interest and any observed tumors were fixed in 10% formalin and processed for embedding. The tissues were embedded in paraffin using a Leica EG1160 tissue embedding station (Leica, Nussloch, Germany) and 5 µm sections were cut. Upon obtaining 3 clean serial sections, one section from each tissue was stained with Mayer’s hematoxylin (Electron Microscopy Sciences, Hatfield, PA, USA) and alcoholic eosin Y (Recca Chemical Company, Arlington, TX, USA) Hemotoxylin and eosin (H&E) staining followed standard practice [18]. After standard dewaxing and dehydration of sections specific staining procedures included initial staining with hematoxylin (15 min), rinsing in both bluing solution (1 min in 0.01% NaCO3) and running tap water (15 min), then a wash in 95% ethanol (1 min), followed by eosin staining (1 min), and a final 80% ethanol wash (1 min) before completing with standard steps from dehydration to cover-slipping.
2.3. Statistical Analysis
The full code and raw data can be found at https://github.com/BenjaminBarr/CC-HFC/releases/tag/v1.0 (accessed on 8 August 2024). A modified Kaplan–Meier graph was generated to show differences in longevity between collection points in R (v4.3.1) [19], using the survival package (v1.1.0) and ggsurvfit (v3.6-4) [20,21]. Significance was assessed using the log-rank test (Mantel–Haenszel) [21]. Post hoc analysis was not performed for the Kaplan-Meier analysis. ANOVA was performed with R to assess mass and body composition, using a fixed-effects linear model with mass type (total, lean or fat) as the response variable and sex and diet at each time point as terms via lme4 (v1.1-35.3) [22]. To detect the differences between dietary fat content, sex, and their combination, emmeans (v1.10.2) was used with Sidak pairwise comparisons (post hoc) applied and a family-wise error rate set at 0.05 [23]. The data used for ANOVA and the post hoc test as well as T0 comparisons, come from measurements made with the Echo MRI. All results are mean ± SEM (standard error of mean), with statistical significance considered at p < 0.05.
3. Results
3.1. Weekly Change in Total Mass
The average weekly mass from each diet and sex was locally estimated using scatterplot smoothing (LOESS) (Figure 1). The points indicate the actual weekly mean total mass from the diet group with the sample size dependent on censorship, ranging from 32 at T0 to 7 at week 72. The gray bands indicate SE calculated in R for the LOESS. Figure 1 shows a progressive increase in total mass until ~20 weeks for all diets, at which point CC mouse weights begin to plateau while HF mouse weights continued to increase. Notably, the HF female mass increased more rapidly than other groups until ~48 weeks. In contrast, males, regardless of diet, maintained the largest mass until ~week 28, at which time the HF females attained a mass greater than the CC males. This phenomenon was observed for the remainder of this study. It is of note that the CC females had markedly lower total body mass than the other groups for the duration of this study.
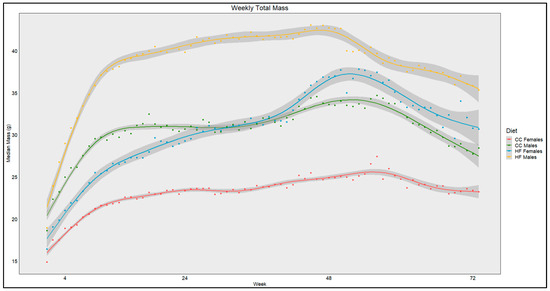
Figure 1.
LOESS representations of weekly trends in mass over 72 weeks (18 months). Colors red, green, blue, and yellow represent CC females, CC males, HF females, and HF males, respectively. Line shadows represent 0.95 CI for the estimation (n = 7–32, based on age/censorship).
3.2. Kaplan–Meier Analysis of Male and Female C3H Mice Maintained on Control and High-Fat Diets over Time
Kaplan–Meier analysis was used to assess differences in survivability between groups over the course of this study. Figure 2 shows survival differences based on diet (C or HF) and sex (M or F). Over time, the HF males have the highest survivability ratio followed by CC females and HF females, then finally CC males. When analyzed using the log-rank test for statistical significance, survival rates were significantly different between the HF males and the CC males (Chi-square = 6.3 on three degrees of freedom and p-value = 0.1, α = 0.1). Similar significance was found using a Cox proportional-hazards model, though it is not reported here.
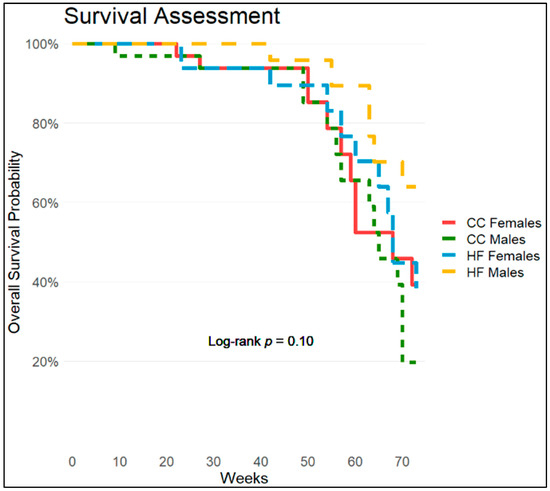
Figure 2.
Kaplan–Meier survival assessment over study duration for male and female mice maintained on control and HFD. Colors red, green, blue, and yellow represent CC females, CC males, HF females, and HF males, respectively (n ≤ 32). The log-rank test for significance was calculated. Results showed Chi-square = 6.3 on 3 degrees of freedom and p-value = 0.1, α = 0.1.
3.3. Analysis of Mass Composition Compared with T0
When compared to time 0 baseline individuals, the CC diet, regardless of sex, had the highest fat mass percentage of total body mass at 12 months. For the HF mice, females demonstrated the same. But the males showed the highest fat mass percentage of total body mass at 6 months. This was also the time point at which females on CC diets demonstrated the highest lean mass percentage of total mass. Females maintained on the HF and CC males had the highest lean mass percentage at 18 months. Additionally, males maintained on HF diets had the highest lean mass percent of total mass at 12 months. The change in percent fat mass composition for females at 12 months (Table 1), when compared with the T0 control, showed an increase of 90.76% in CC-fed mice and 1063.87% in HF-fed mice. At that same time point, female lean mass differed from the T0 control by 13.72% and 53.17% in the CC and HF groups, respectively. For the males at 12 months (Table 2), the change in fat mass when compared to the T0 control was 127.78% (CC) and 549.31% (HF). When compared to the T0 control, male lean mass decreased by 1.83% in the CC males and increased by 54.64% in the HF males. Interestingly, the only decrease in any mass type compared with the T0 controls is observed in the lean mass of the CC males at 12 months. HFDs induced greater than 500% increase in fat mass at 52 weeks (12 months) in both sexes, when compared to the T0 mice. In contrast, CC females and CC males showed maximal fat accumulation at 12 months of ≤150% when compared with T0 controls.

Table 1.
Female mass composition compared with 4-week-old controls.

Table 2.
Male mass composition compared with 4-week-old controls.
3.4. Quarter Rule (Fat-Free Mass (FFM) Lost)
The quarter rule is a general concept used to describe the amount of fat-free mass (lean mass) lost as healthy individuals undergo weight loss (1/4 fat-free mass for every 3/4 fat mass lost) [24]. Adherence to the quarter rule is summarized in Table 3. The data are determined from a calculation of lean mass lost compared with fat mass lost for each group as a function of aging (between 12 and 18 months) and expressed as a ratio (lean:fat mass). The HF females’ results reflect the quarter rule most closely. For the females, the HF mice only lost ~0.23 g of lean mass per gram of fat mass while the CC mice lost ~0.83 g of lean mass for every gram of fat mass lost, demonstrating they still lost more fat mass than lean mass. The CC males appear to gain ~5.97 g of lean mass for every gram of fat mass lost, while the HF males lost ~1.23 g of lean mass for each gram of fat lost. The noticeable difference in mass trends identified in the CC males is likely in part due to the loss of moribund individuals between these two time points as identified by the sample sizes in Table 2 and the Kaplan–Meier curve in Figure 2. In contrast to the CC males and the HF females, the HF males appeared to remain in an obese state (unhealthy) before the quarter rule could be accurately applied. Interestingly, as mice aged from 12 to 18 months, both lean and fat mass began to decline in all groups except the CC males.

Table 3.
The quarter rule demonstrating the use of fat mass in protecting lean mass degradation during the aging process.
3.5. Analysis of Mass Composition
When assessing total mass, it is crucial to consider both the lean and fat mass components. Each type of mass plays a vital role in the body. Lean or fat-free mass makes up muscles as well as most of the denser tissues like those of the liver, heart, and kidneys. Fat mass comprises adipose tissue as well as any fat which has infiltrated tissues as a result of obesity. In this study, mass composition was assessed at each collection point using two-way ANOVA. Statistical significance is reported in Table 4. The F-statistic is reported to express the variance ratio between groups:within groups. Larger F-stats correspond with more variance between groups or more significance identified. In females, total mass, lean mass, and fat mass was significantly increased by HFD at all collection points when compared with the CC mice (6, 12, and 18 months) (Table 4 and Figure 3). Males demonstrated a similar pattern at all time points (6, 12, and 18 months) (Table 4 and Figure 3). Total mass, lean mass, and fat mass were significantly increased for HFD mice when compared with CC diets. One exception for the total mass was observed in males at 12 months (Table 4 and Figure 3). Although the CC males and HF males at 12 months did not significantly differ in total mass, this appears to be an intersection between the declining total mass for the HF males and the peak mass point for the CC males. With respect to sexual dimorphism in the same diet groups, CC-fed males had significantly increased total mass at 6 and 12 months, and significantly increased lean mass at 18 months when compared with the CC females. In the HFD groups, total mass was significantly increased in males at 6 months only, when compared with females. However, lean mass was significantly increased for males at 6, 12, and 18 months against females. Interestingly, fat mass was significantly increased in the HF males at 6 months, but significantly increased at 12 months in females (Table 4 and Figure 3).

Table 4.
Effects of fat content and sex on total mass and mass composition in C3H mice.
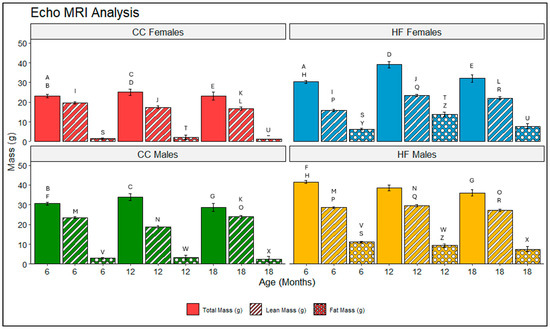
Figure 3.
Two-way ANOVA showing differences between each diet and sex at specific time points. Significance is indicated by matching letters (A is significantly different from A) and only with p-values ≤ 0.05. Pairwise comparisons were only made within sexes or within diets. The colors red, green, blue, and yellow represent CC females, CC males, HF females, and HF males, respectively. Solid bars indicate total mass, striped bars indicate lean mass, and dotted bars indicate fat mass.
3.6. Tumor Incidence and Type
Over the course of this study, a total of 41 tumors with various origins were identified between all groups (Figure 4). The HF mice scored the highest tumor incidence with 11 tumors in the females and 14 in the males. Among the CC mice, eight tumors were found in the females and seven tumors were found in the males. Tumor development was first observed around the 12-month collection point, and the highest incidences were observed at the 18-month collection point. All tumors developed within this timespan (12–18). Tumors from the censored groups are included in the totals.
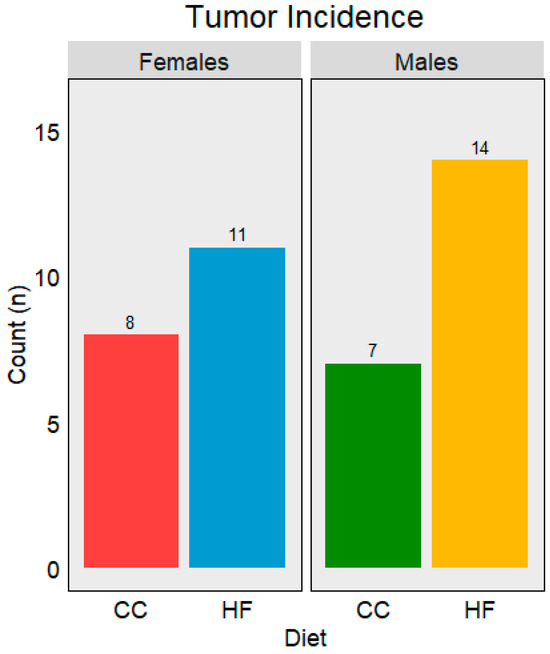
Figure 4.
The total number of tumors observed at the censorship points for each diet. Red = CC females, green = CC males, blue = HF females, and yellow = HF males. The number above each bar is the total number of tumors identified over the course of this study.
Tumor development as a function of diet and sex is summarized in Figure 4. Interestingly, there was a strong sex-based difference in the tumor type which developed as a function of diet. The most abundant tumor types for each group were the following: ovarian tumors for the CC females (4) and HF females (5), lung tumors for the CC males (4), and liver tumors for the HF males (13). Tumor tissue incidence is summarized in Table 5. Male C3H mice developed either lung tumors or liver tumors. In contrast, the females demonstrated the development of ovarian tumors in addition to lung and liver tumors across both diets. Most tumors were observed during the 18-month collection of tissues, whereas none were identified prior to 12 months (Table 5).

Table 5.
Tumor distributions across each diet by sex.
Figure 5 shows representative liver tissue and tumors collected from necropsied individuals, along with corresponding H&E-stained livers and liver tumors from 18-month-old individuals. The tumors are identified histologically by abnormal tissue density, cell shape, and atypical nuclei.
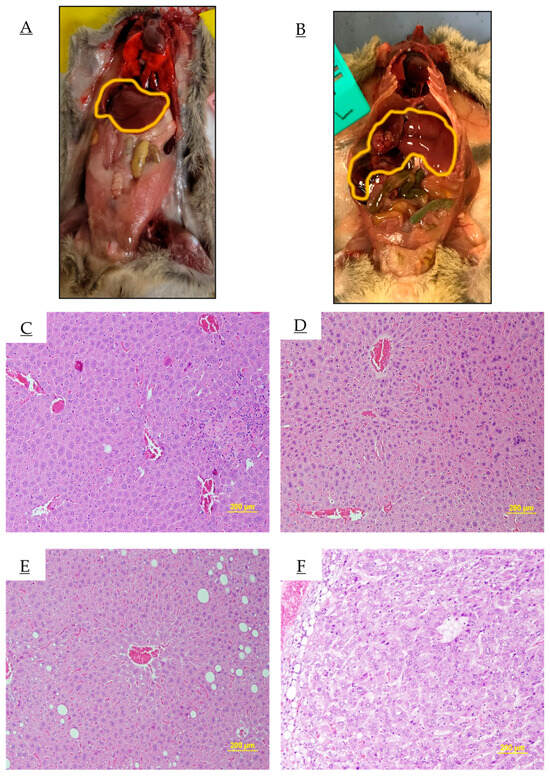
Figure 5.
Sample images of normal and cancerous livers from different diets. Skin and muscles layers reflected to expose (A) normal liver and (B) cancerous liver. Livers outlined in yellow. (C–F) are histological sections of livers stained with H&E from male C3H mice at 18 months. (C) Normal liver from CC mice, (D) liver tumor from CC mice, (E) normal Liver from HF mice, and (F) liver tumor from HF mice.
3.7. Mouse Appearance and Behavior
As this was a long-term study and the mice were housed four to a cage, a section on appearance and behavior has been included to provide insight on differences between sexes and diet types. The diet formulations had identical amounts of protein both in mass (179.0 g/kg) and caloric composition of the whole diet (18% kcal protein). For the CC mice, the remaining 71% kcal was supplied by carbohydrates, while the HF mice only had 36% from carbohydrates (Supplementary Table S1). This distribution of macromolecules results in slightly different overall caloric densities of 3.8 and 4.6 kcal/g for the CC and HF mice, respectively. Surprisingly, even with this difference, there was no significant difference in food consumption by mass. The average weekly consumption per individual, ad libitum, was consistently between 15 and 22 g (57–95 kcal for CC mice and 69–115 kcal for the HF mice). Female mice consumed quantities ranging from 15 to 18 g, while males consumed ~17–22 g regardless of diet type. Though maintained ad libitum, the mice never consumed more food than was provided (40 g/week/mouse). The individuals were examined weekly. For the mice that demonstrated excessive weight loss, injuries (usually fighting males), or extreme barbering or barbering-induced alopecia (primarily females) (Figure 6), these individuals were placed in separate cages and monitored [25,26]. While these mice were never reintroduced into their original cage, they maintained average mass and food consumption comparable to those housed in groups. Another notable behavior of the C3H mice throughout this study was their docile nature when handled, with no reported incidents of aggression towards handlers over the 18-month period.
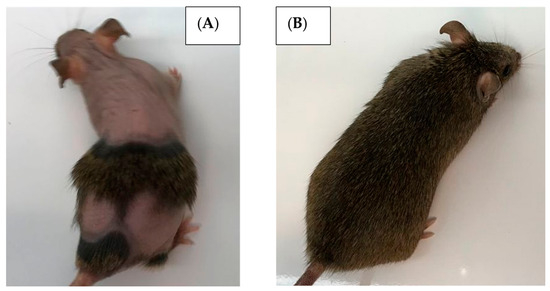
Figure 6.
Example of extreme barbering behavior in C3HheJ female mice at 12 months. (A) Over-barbered (potentially barbering-induced alopecia) mouse separated into a different cage. (B) Mouse from the same cage exhibiting no barbering.
The HF males were more active and upon visual inspection and exhibited a healthy pelage when compared with the CC males. Based on Animal Care and Use observational criteria, the CC males, with body weights in the lower range, presented with greater incidence of penile prolapse and loss of motor function with aging. In contrast, the HF males were more robust during the aging process; ten CC males were lost prior to censorship, compared with the HF males, of which only five died pre-censorship. In contrast, the CC and HF females lost a comparable number of individuals during this time (seven and eight, respectively).
4. Discussion
Current studies focus on recreating disease conditions with the most rapid methods possible. With respect to obesity, this often means using mouse models highly susceptible to fat accumulation and total mass increase. The mice are then maintained on HFD to replicate obese conditions as quickly as possible, potentially missing critical steps in the etiology of obesity. Previously, the C3H strain has been identified as “obesity-resistant”, and it was used as a negative control for DIO against strains like C57 [14]. However, these comparisons lacked assessments of C3H with the same reference diets (low fat or control diets). Thus, it is arguable that the exclusion of these alternative diet assessments limited the accuracy of DIO assessment in C3H as a result of the HFD [12]. A later study by Rendina-Ruedy et al. showed that obesity can be induced in C3H mice using HFD compared with controls [14]. The findings from our study align with those of Rendina-Ruedy et al. and demonstrate the progression of obesity and development of cancer induced by a long-term, HFD for C3H mice compared with their counterparts on a reference diet. The strengths of this study include the demonstration of a novel mouse strain that develops DIO in both sexes and analysis of the gradual and long-term effects of DIO and aging body composition on liver lipogenesis and cancer development. These investigations include both sexes and discuss the sexual dimorphisms identified. The limitations of this study are as follows: No blood and serum analysis was conducted. An intermediate-sized antenna was not available for the EchoMRI equipment; thus, analysis for a few animals was underestimated because of size constraints; they were too large for the smaller antenna and undersized to generate a truly accurate reading in the larger antenna.
Due to the availability of obesity- and cancer-susceptible mouse strains [3,27], researchers conduct studies of obesity and obesity-related conditions (i.e., metabolic syndrome and cancer) over relatively short time frames (e.g., 12–16 weeks instead of 12–16 months). However, obesity is a chronic disease in humans, not an acute condition [28], and cancer is a disease of aging [29]. Therefore, we investigated DIO and aging changes in a “general-purpose” mouse strain to determine if there were subtle differences in results that may be more reflective of obesity and cancer progression in humans, as a function of diet, sex, and aging [28,29]. When comparing C3H with C57 mice, differences in total mass at 12 weeks for control and HFDs are observed [Table 6]. It is notable that the C57 males demonstrate high susceptibility to obesity, while the females do not; a phenomenon that is also observed in aged C57 cohorts for both sexes [30]. In contrast, our results show that both sexes for the C3H mice gain weight and become obese over time. Furthermore, the control diet weight data align with data reported by Jackson Laboratory (Bar Harbor, ME, USA, and the source of the strain used in this study) for total mass at 12 weeks (27.9 g and 21.3 g for males and females, respectively) [31]. The HFD provided an average increase in total mass of C3H mice by ~5 g (~26.6 g total mass) in females and ~7 g (~37.8 g total mass) in males at 12 weeks, respectively.

Table 6.
Comparison of mean total mass in C57 obtained at 4 weeks using a similar diet [7] and C3H mice from this study.
This study is also the longest reported study of DIO in C3H mice, investigating the effects of DIO and aging over 72 weeks (18 months). As this study is three times longer than most mouse studies, it is critical to discuss the differences identified in the survival probabilities as a result of each diet [7,15,32]. C3H males maintained on the HFD had markedly elevated survival probability compared with C3H males on the CC diet. This is a strong possible confounding variable for tumor incidence with the crucial role of age in the development of cancer. Notably, the females, regardless of diet, had similar survival probabilities at 18 months. Further compared with the CC males, the CC females had a higher incidence of tumors which could also be due to the influence of age.
This study can provide two unique perspectives as a model for chronic DIO as cancer development over time, in relation to diet and sex was investigated.First, this study provides a gradual analysis of the development of obesity. Obesity is defined as a complex, multifactorial chronic disease [28]. Although it can be rapidly induced (<24 weeks), the nuances of a chronic condition may continue to unfold over the lifespan of the subject. With shorter studies, subtle changes or conditions which have slower progression, such as altered food consumption, altered muscular function, and tissue scarring as lipid infiltration recedes from organs, may not be as readily observed [14]. The second perspective is that this study allowed for standard fluctuation of mass composition associated with age, increasing during adolescence and decreasing during old age. In many strains which are currently used, elevated dietary fat content leads to overconsumption [33]. Although this certainly induces obesity, it may not be as reflective as chronic consumption of a poor diet. Differences in lean mass and fat mass accumulation as C3H mice progress through adolescence more closely model obesity in humans at the same life stage and this holds true for older mice studies using this approach. Similarly, as shown by this study, changes in FFM and fat mass (females) and increases in cancer incidence associated with aging more accurately represent human trends in inflammaging and obesity [34,35,36]. These results indicate that this model may provide further insight into the intricacies of human aging than those provided by previous strains [28]. High tumor incidence rates in the HF groups strongly correlate with the findings connecting obesity with tumor development. The liver tumor incidence in the CC diet is much lower than that of the HF diet; however, this may partially be due to the influence of age. Since the HF males had more individuals alive at old age vs. CC males, this could be a potential reason for higher tumor incidence. Additionally, the HFD for males likely induced some liver tumor formation as metabolic associated fatty liver disease progressed. Studies investigating at these possibilities are underway in our lab.
For C3H mice in this study, lean mass was observed to increase rapidly through adolescence (0–16 weeks), and for most diets, lean mass continued to slowly increase until middle age (~48 weeks). From this point forward, lean mass was observed to decline until the termination of this study. These results more closely reflect trends reported in humans when compared with other rodent models [37]. Similarly, C3H mice have demonstrated a susceptibility to spontaneous development of cancer, a disease associated with aging [29] and obesity [38]. Prior studies report that these mice begin to develop cancers during the middle of their lifespan, at ~48 weeks, and incidence progressively increases as they near the end of life [39,40]. This strain also reflects the sex-dependent dimorphism displayed in humans with respect to liver cancer, with higher percentage rates developing in males than in females [39]. Future studies will need to be performed that address the impact each diet type on the development of more organ-specific diseases, like metabolic syndrome or metabolic associated fatty liver disease and even specific cancers. Further studies using this long-term model for HFD consumption and chronic obesity are crucial for advancing the understanding of one of the most prevalent conditions worldwide.
5. Conclusions
C3H mice, although generally smaller than their C57 counterparts, consume a similar amount of food per day: ~3.5 g for standard chow diets. In this study, this was true regardless of dietary fat content, but it has been shown that C57 mice tend to increase daily food consumption when maintained on HFD [33], this likely being the major contributor to their high susceptibility to developing obesity. By utilizing both strains in a longitudinal study of obesity, it would be possible to model for individuals who continue to increase dietary consumption, and those who do not. Improvements to survivability in the HF C3H males and the lack of change in survivability found between the CC and HF females shown by this study necessitates further investigations into the role of dietary fat content and its ability to protect against aging for individuals who are not prone to over consumption, especially with regards to sex.
As the obesity epidemic becomes increasingly problematic throughout Western culture, it is important to understand its chronic progression as well as co-morbidities and pathologic outcomes. Results from this study suggest that using mouse strains with low susceptibility to developing obesity, can demonstrate a more gradual increase in fat accumulation, and thus, different questions in the etiology of obesity and cancer development can be addressed. Although further assessment of obesity-related markers such as insulin levels, blood glucose levels, and inflammation need to be performed, trends in body mass composition clearly demonstrate typical fat mass accumulation associated with obese conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/obesities4030025/s1, Table S1: Total dietary components and formulations; Table S2: Food consumed per week per mouse.
Author Contributions
Conceptualization, B.B. and L.G.; methodology, B.B. and L.G.; software, B.B.; validation, B.B.; formal analysis, B.B. and L.G.; investigation, B.B.; resources, L.G.; data curation, B.B. and L.G.; writing—original draft preparation, B.B.; writing—review and editing, B.B. and L.G.; visualization, B.B.; supervision, L.G.; project administration, B.B. and L.G.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Empirical Foods, Incorporated, Grant # A18-0187-001, and TTUAB Grants-in-Aid.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The full code and raw data are available at https://github.com/BenjaminBarr/CC-HFC/releases/tag/v1.0 (accessed on 13 August 2024). Other data are available upon reasonable request to the corresponding author.
Acknowledgments
This project would not be possible without the efforts of the Gollahon Lab personnel Nicholas Wolpert, Noshin Mubtasim, Caleb Boren, Michael Thomas, Morgan Williamson, Emily Garrison, Caroline Schuster, Belinda D’Costa, Jordan Greer, Yusuff Olayiwola, Jiaqi Niu, and the numerous undergraduates who participated. Special thanks to Naima Moustaid-Moussa and Mindy Brashears for sharing their equipment and expertise.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ericsson, A.C.; Crim, M.J.; Franklin, C.L. A brief history of animal modeling. Mo. Med. 2013, 110, 201–205. [Google Scholar]
- Carbone, L. Estimating mouse and rat use in American laboratories by extrapolation from Animal Welfare Act-regulated species. Sci. Rep. 2021, 11, 493. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly Used Animal Models. Principles of Animal Research for Graduate and Undergraduate Students 2017. pp. 117–175. Available online: https://www.sciencedirect.com/science/article/pii/B9780128021514000074?via%3Dihub (accessed on 8 August 2024). [CrossRef]
- Surwit, R.S.; Seldin, M.F.; Kuhn, C.M.; Cochrane, C.; Feinglos, M.N. Control of Expression of Insulin Resistance and Hyperglycemia by Different Genetic Factors in Diabetic C57BL/6J Mice. Diabetes 1991, 40, 82–87. [Google Scholar] [CrossRef]
- Paigen, B.; Morrow, A.; Brandon, C.; Mitchell, D.; Holmes, P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 1985, 57, 65–73. [Google Scholar] [CrossRef]
- Lê, A.; Ko, J.; Chow, S.; Quan, B. Alcohol consumption by C57BL/6, BALB/c, and DBA/2 mice in a limited access paradigm. Pharmacol. Biochem. Behav. 1994, 47, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Menikdiwela, K.R.; Guimarães, J.P.T.; Scoggin, S.; Gollahon, L.S.; Moustaid-Moussa, N. Dietary pH Enhancement Improves Metabolic Outcomes in Diet-Induced Obese Male and Female Mice: Effects of Beef vs. Casein Proteins. Nutrients 2022, 14, 2583. [Google Scholar] [CrossRef]
- Cooper, M.A.; O’Meara, B.; Jack, M.M.; Elliot, D.; Lamb, B.; Khan, Z.W.; Menta, B.W.; Ryals, J.M.; Winter, M.K.; Wright, D.E. Intrinsic Activity of C57BL/6 Substrains Associates with High-Fat Diet-Induced Mechanical Sensitivity in Mice. J. Pain 2018, 19, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.; Welch, G.; DiBerardo, L.; Freeman, L.R. Sex differences in a mouse model of diet-induced obesity: The role of the gut microbiome. Biol. Sex Differ. 2024, 15, 5. [Google Scholar] [CrossRef]
- Outzen, H.C.; Corrow, D.; Shultz, L.D. Attenuation of Exogenous Murine Mammary Tumor Virus Virulence in the C3H/HeJ Mouse Substrain Bearing the Lps Mutation2. JNCI J. Natl. Cancer Inst. 1985, 75, 917–923. [Google Scholar] [CrossRef]
- Yuan, R.; Tsaih, S.; Petkova, S.B.; De Evsikova, C.M.; Xing, S.; Marion, M.A.; Bogue, M.A.; Mills, K.D.; Peters, L.L.; Bult, C.J.; et al. Aging in inbred strains of mice: Study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 2009, 8, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Poggi, M.; Bastelica, D.; Gual, P.; Iglesias, M.A.; Gremeaux, T.; Knauf, C.; Peiretti, F.; Verdier, M.; Juhan-Vague, I.; Tanti, J.F.; et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 2007, 50, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- VerHague, M.; Albright, J.; Barron, K.; Kim, M.; Bennett, B.J. Obesogenic and diabetic effects of CD44 in mice are sexually dimorphic and dependent on genetic background. Biol. Sex Differ. 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Rendina-Ruedy, E.; Hembree, K.D.; Sasaki, A.; Davis, M.R.; Lightfoot, S.A.; Clarke, S.L.; Lucas, E.A.; Smith, B.J. A Comparative Study of the Metabolic and Skeletal Response of C57BL/6J and C57BL/6N Mice in a Diet-Induced Model of Type 2 Diabetes. J. Nutr. Metab. 2015, 2015, 758080. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, M.E.; Sharma, V.; Stankiewicz, T.E.; Oates, J.R.; Doll, J.R.; Damen, M.S.M.A.; Almanan, M.A.T.A.; Chougnet, C.A.; Hildeman, D.A.; Divanovic, S. Aging mitigates the severity of obesity-associated metabolic sequelae in a gender independent manner. Nutr. Diabetes 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci. Rep. 2020, 10, 14052. [Google Scholar] [CrossRef] [PubMed]
- Wahlsten, D.; Metten, P.; Crabbe, J.C. A rating scale for wildness and ease of handling laboratory mice: Results for 21 inbred strains tested in two laboratories. Genes Brain Behav. 2003, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sampias, C.; Rolls, G. An Intro to H&E Staining: Protocol, Best Practices, Steps & More. Available online: https://www.leicabiosystems.com/us/knowledge-pathway/he-staining-overview-a-guide-to-best-practices/ (accessed on 8 August 2024).
- Team, R.C. R: A Language and Environment for Statistical Computing. (v4.3.1) 2023. Available online: https://www.R-project.org/ (accessed on 8 August 2024).
- Sjoberg, D.D.; Baillie, M.; Fruechtenicht, C.; Haesendonckx, S.; Treis, T. ggsurvfit: Flexible Time-to-Event Figures. (v1.1.0). 2024. Available online: https://CRAN.R-project.org/package=ggsurvfit (accessed on 8 August 2024).
- Therneau, T.M. A Package for Survival Analysis in R. (v3.6-4) 2024. Available online: https://CRAN.R-project.org/package=survival (accessed on 8 August 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. (v1.10.2) 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 8 August 2024).
- Heymsfield, S.B.; Gonzalez, M.C.C.; Shen, W.; Redman, L.; Thomas, D. Weight loss composition is one-fourth fat-free mass: A critical review and critique of this widely cited rule. Obes. Rev. 2014, 15, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.; Minasyan, A.; Keisala, T.; Shah, Z.; Tuohimaa, P. Hair barbering in mice: Implications for neurobehavioural research. Behav. Process. 2006, 71, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bechard, A.; Meagher, R.; Mason, G. Environmental enrichment reduces the likelihood of alopecia in adult C57BL/6J mice. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 171–174. [Google Scholar]
- Reed, D.R.; Bachmanov, A.A.; Tordoff, M.G. Forty mouse strain survey of body composition. Physiol. Behav. 2007, 91, 593–600. [Google Scholar] [CrossRef]
- Lempesis, I.G.; Tsilingiris, D.; Liu, J.; Dalamaga, M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metab. Open 2022, 15, 100208. [Google Scholar] [CrossRef]
- Berben, L.; Floris, G.; Wildiers, H.; Hatse, S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef]
- Reynolds, T.H.; Dalton, A.; Calzini, L.; Tuluca, A.; Hoyte, D.; Ives, S.J. The impact of age and sex on body composition and glucose sensitivity in C57BL/6J mice. Physiol. Rep. 2019, 7, e13995. [Google Scholar] [CrossRef] [PubMed]
- The Jackson Laboratory C3H/HeJ—000659. 2024. Available online: https://www.jax.org/strain/000659 (accessed on 21 May 2024).
- Jackson, E.E.; Rendina-Ruedy, E.; Smith, B.J.; Lacombe, V.A. Loss of Toll-Like Receptor 4 Function Partially Protects against Peripheral and Cardiac Glucose Metabolic Derangements During a Long-Term High-Fat Diet. PLoS ONE 2015, 10, e0142077. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, P.N.; Cornejo, M.P.; Barrile, F.; Romero, G.G.; Valdivia, S.; Andreoli, M.F.; Perello, M. Inter-individual Variability for High Fat Diet Consumption in Inbred C57BL/6 Mice. Front. Nutr. 2019, 6, 67. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Dziubek, W.; Wolny-Rokicka, E.; Dabrowska, G.; Wozniewski, M. The Relation of Inflammaging with Skeletal Muscle Properties in Elderly Men. Am. J. Men’s Health 2019, 13, 1557988319841934. [Google Scholar] [CrossRef]
- Santos, C.A.F.; Amirato, G.R.; Paixão, V.; Almeida, E.B.; Amaral, J.B.D.; Monteiro, F.R.; Roseira, T.; Juliano, Y.; Novo, N.F.; Rossi, M.; et al. Association among inflammaging, body composition, physical activity, and physical function tests in physically active women. Front. Med. 2023, 10, 1206989. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017, 8, 1745. [Google Scholar] [CrossRef]
- Pappas, L.E.; Nagy, T.R. The translation of age-related body composition findings from rodents to humans. Eur. J. Clin. Nutr. 2019, 73, 172–178. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, M.; Lei, L.; Yang, F.; Li, H.; Yan, X.; He, S.; Zhang, S.; Teng, Y.; Xia, C.; et al. Burden of liver cancer: From epidemiology to prevention. Chin. J. Cancer Res. 2022, 34, 554–566. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).