Green Tea Induces the Browning of Adipose Tissue—Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Selection and Study Eligibility
2.3. Data Extraction and Analysis
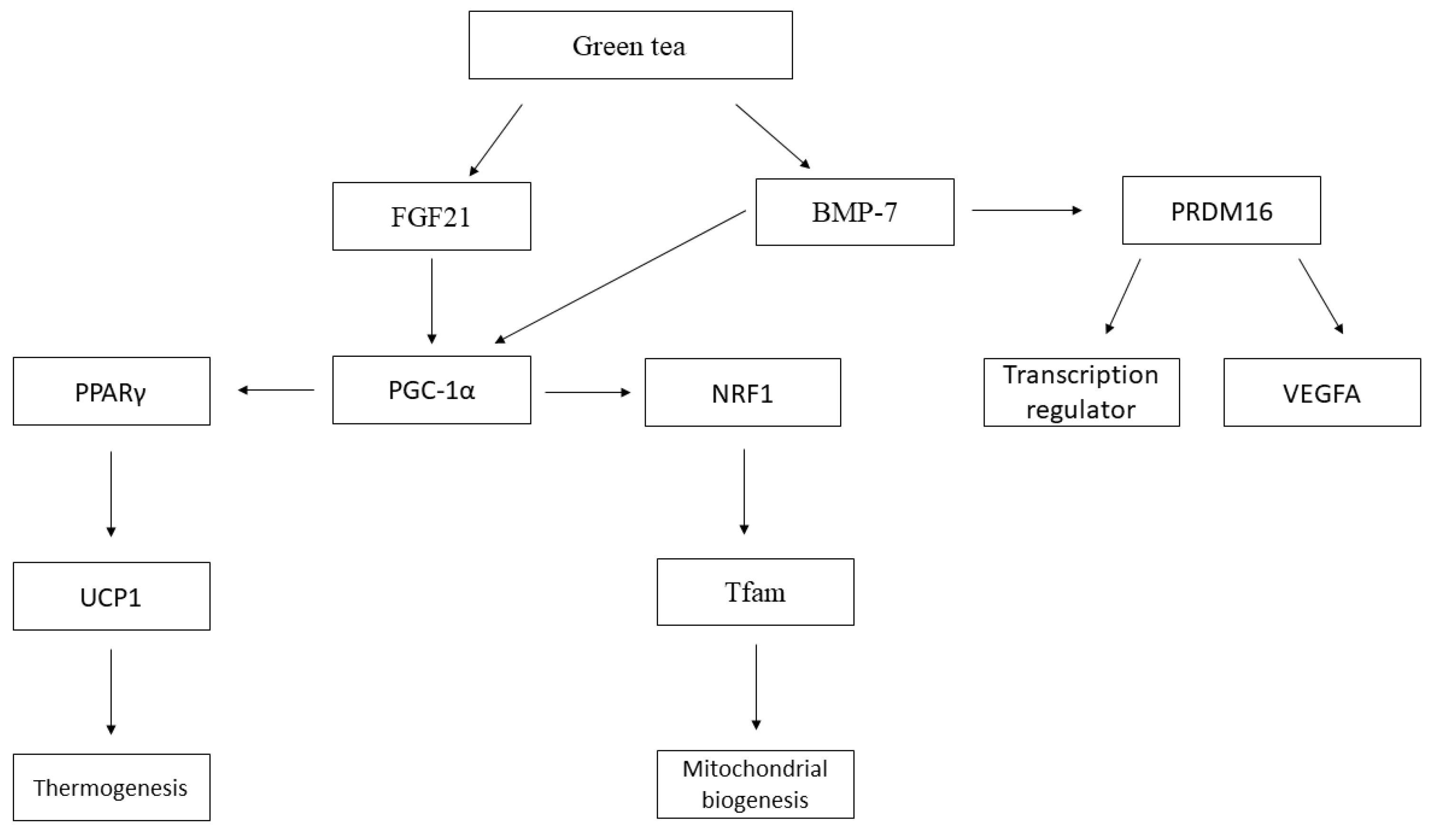
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC2 | Acetyl-CoA Carboxylase 2 |
| AGCL | Long-Chain Fatty Acids |
| BAT | Brown Adipose Tissue |
| BMP-7 | Bone Morphogenetic Protein 7 |
| cAMP | Cyclic Adenosine Monophosphate |
| COMT | Catechol-O-Methyl Transferase |
| CPT-1 | Carnitine Palmitoyltransferase I |
| Epicatechin | ECG |
| EGCG | Epigallocatechin 3-gallate |
| EGC | Epigallocatechin |
| Epicatechin | PPE |
| FASN | Fatty Acid Synthase |
| FGF-21 | Fibroblast Growth Factor 21 |
| GTE | Green Tea Extract |
| HFD | High-fat Diet |
| IR | Insulin Resistance |
| MUFA | Monounsaturated Fatty Acids |
| NAD | Nicotinamide Adenine Dinucleotide |
| NASH | Non-alcoholic Steatohepatitis |
| NRF1 | Nuclear Respiratory Factor 1 |
| PPARγ | Peroxisome Proliferator Type Gamma |
| PGC-1α | Peroxisome proliferator Co-activator 1-alpha |
| SBCAL | Brazilian Society of Science in Laboratory Animals |
| SIRT1 | Sirtuin 1 |
| TFAM | Mitochondrial Transcription Factor A |
| TLE-3 | Transducin-like Enhancer Protein-3 |
| T2DM | Type 2 diabetes |
| UCP1 | Uncoupling Protein-1 |
| UCP2 | Uncoupling Protein 2 |
| WAT | White Adipose Tissue |
References
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology 2011, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Chansaenpak, K.; Liu, Y.; Yuan, H.; Xie, J.; Yin, H.; Branca, R.T.; Li, Z.; Wu, Z. A Novel PET Probe for Brown Adipose Tissue Imaging in Rodents. Mol. Imaging Biol. 2020, 22, 675–684. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanari, T.; Pošćić, N.; Colitti, M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: A review. Obes Rev. 2017, 18, 495–513. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20160051. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.; Geoghegan, G.; Maschek, J.A.; Bensard, C.L.; Pasquali, M.; Miao, R.; Lee, S.; Jiang, L.; Huck, I.; Kershaw, E.E.; et al. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab. 2017, 26, 509–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Matsuo, T.; Suzuki, M.; Tokuyama, K. Effect of dietary fat type on β-oxidation of brown adipose tissue and Na+ channel density of brain nerve membrane in rats. J. Nutr. Sci. Vitaminol. 1996, 42, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Vázquez-Cabral, B.D.; González-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 2020, 18, e300041. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Delzenne, N.M. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef]
- Lee, M.-S.; Shin, Y.; Jung, S.; Kim, Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr. Res. 2017, 61, 1325307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1681. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-H.; Chien, Y.-W.; Liang, C.-T.; Chan, C.-H.; Fan, M.-H.; Huang, H.-Y. Green tea extract induces genes related to browning of white adipose tissue and limits weight-gain in high energy diet-fed rat. Food Nutr. Res. 2017, 61, 1347480. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Ichinose, T.; Jinde, M.; Kawashima, Y.; Tachiyashiki, K.; Imaizumi, K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J. Nutr. Biochem. 2008, 19, 840–847. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Zhao, B.L. Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Sci. China Life Sci. 2013, 56, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Santana, A.; Santamarina, A.; Souza, G.; Mennitti, L.; Okuda, M.; Venancio, D.; Seelaender, M.; Nascimento, C.O.D.; Ribeiro, E.; Lira, F.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate improves insulin resistance and metabolic profiles in normolipidic diet—But not high-fat diet-fed mice. J. Nutr. Biochem. 2015, 26, 893–902. [Google Scholar] [CrossRef]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.; Loureiro, M.D.P.; Macedo, L.E.; Nocca, D.; Nedelcu, M.; Costa-Casagrande, T.A. Animal models in metabolic syndrome. Rev. Col. Bras. Cir. 2018, 45, 1–10. [Google Scholar] [CrossRef] [Green Version]
- White, P.A.; Cercato, L.M.; Araújo, J.; Souza, L.A.; Soares, A.F.; Barbosa, A.P.O.; RNeto, J.M.D.; Marçal, A.C.; Machado, U.F.; Camargo, E.A.; et al. Modelo de obesidade induzida por dieta hiperlipídica e associada à resistência à ação da insulina e intolerância à glicose. Arq. Bras. Endocrinol. Metabol. 2013, 57, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Titov, A.A.; Morel, L. An update on lupus animal models. Curr. Opin. Rheumatol. 2017, 29, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Hojna, S.; Jordan, M.D.; Kollias, H.; Pausova, Z. High-fat diet induces emergence of brown-like adipocytes in white adipose tissue of spontaneously hypertensive rats. Hypertens. Res. 2012, 35, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Jin, H.; Chei, S.; Lee, J.-Y.; Oh, H.-J.; Lee, B.-Y. Dietary Silk Peptide Prevents High-Fat Diet-Induced Obesity and Promotes Adipose Browning by Activating AMP-Activated Protein Kinase in Mice. Nutrients 2020, 12, 201. [Google Scholar] [CrossRef] [Green Version]
- García-Ruiz, E.; Reynés, B.; Díaz-Rúa, R.; Ceresi, E.; Oliver, P.; Palou, A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int. J. Obes. 2015, 39, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef] [Green Version]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kiuchi, S.; Murase, T. Synergistic activation of thermogenic adipocytes by a combination of PPARγ activation, SMAD3 inhibition and adrenergic receptor activation ameliorates metabolic abnormalities in rodents. Diabetologia 2019, 62, 1915–1927. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, J.; Wu, Z.; Choi, C.H.J.; Nguyen, L.; Tegegne, S.; Ackerman, S.E.; Crane, A.; Marchildon, F.; Tessier-Lavigne, M.; Cohen, P. Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab. 2018, 27, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Fatima, L.A.; Campello, R.S.; Santos, R.D.S.; Freitas, H.S.; Frank, A.P.; Machado, U.F.; Clegg, D.J. Estrogen receptor 1 (ESR1) regulates VEGFA in adipose tissue. Sci. Rep. 2017, 7, 16716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.F.; Miao, L.J.; Wang, X.N.; Huang, C.C.; Qian, Y.S.; Huang, X.; Wang, X.L.; Jin, W.Z.; Ji, G.J.; Fu, M.; et al. CD38 deficiency suppresses adipogenesis and lipogenesis in adipose tissues through activating Sirt1/PPARγ signaling pathway. J. Cell Mol. Med. 2018, 22, 101–110. [Google Scholar] [CrossRef]
- Cristina, L.; Cisneros, V.; López-uriarte, P.; López-Espinoza, A.; Espinoza-Gallardo, A.C.; Aburto, G. Effects of green tea and its epigallocatechin (EGCG) content on body weight and fat mass in humans: A systematic review. Nutr. Hosp. 2017, 34, 731–737. [Google Scholar]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Edmondson, A.C.; Salant, J.; Ierardi-Curto, L.A.; Ficicioglu, C. Missed Newborn Screening Case of Carnitine Palmitoyltransferase-II Deficiency. JIMD Rep. 2016, 33, 93–97, Erratum in JIMD Rep. 2017, 33, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Guilherme, A.; Pedersen, D.J.; Henchey, E.; Henriques, F.S.; Danai, L.V.; Shen, Y.; Yenilmez, B.; Jung, D.; Kim, J.K.; Lodhi, I.J.; et al. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol. Metab. 2017, 6, 781–796. [Google Scholar] [CrossRef]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of oleic acid in the gut-liver axis: From diet to the regulation of its synthesis via Stearoyl-CoA desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gougoula, C.; Bielfeld, A.P.; Pour, S.J.; Sager, M.; Krüssel, J.-S.; Benten, W.P.M.; Baston-Büst, D.M. Metabolic and behavioral parameters of mice with reduced expression of Syndecan-1. PLoS ONE 2019, 14, e0219604. [Google Scholar] [CrossRef] [Green Version]
- Rosini, T.C.; Ramos da Silva, A.S.; de Moraes, C. Diet-induced obesity: Rodent model for the study of obesity-related disorders. Rev. Assoc. Med. Bras. 2012, 58, 383–387. [Google Scholar] [PubMed]
- Johnson, C.; Drummer, C.; Virtue, A.; Gao, T.; Wu, S.; Hernandez, M.; Singh, L.; Wang, H.; Yang, X.-F. Increased Expression of Resistin in MicroRNA-155-Deficient White Adipose Tissues May Be a Possible Driver of Metabolically Healthy Obesity Transition to Classical Obesity. Front. Physiol. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerson, A.; Traurig, M.; Ossowski, V.; Fleming, J.M.; Mullins, M.; Baier, L.J. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia 2013, 56, 1971–1979. [Google Scholar] [CrossRef] [Green Version]
- Kornfeld, J.-W.; Baitzel, C.; Könner, A.C.; Nicholls, H.T.; Vogt, M.C.; Herrmanns, K.; Scheja, L.; Haumaitre, C.; Wolf, A.M.; Knippschild, U.; et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 2013, 494, 111–115. [Google Scholar] [CrossRef]
- Nirengi, S.; Amagasa, S.; Homma, T.; Yoneshiro, T.; Matsumiya, S.; Kurosawa, Y.; Sakane, N.; Ebi, K.; Saito, M.; Hamaoka, T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. SpringerPlus 2016, 5, 1363. [Google Scholar] [CrossRef] [Green Version]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef] [Green Version]

| Author/Year | Specie | Type of Diet | Method of Administration | Administered Dose | Catechin Content | Duration | Results |
|---|---|---|---|---|---|---|---|
| Chen et al. 2017 [15] | Rats (Sprague Dawley) induced obesity by diet | High-energy diet | Green Tea Extract | 77.5 mg·kg−1 per day of extract of green tea 155 mg·kg−1 per day of extract of green tea | 83.5% of catechins | 8 weeks | eWAT → adipocytes were much smaller in the supplemented group compared to the high calorie diet; Significant increase in the expression of PPAR-γ, PRDM-16, BMP-7, FGF-21 and PGC-1α and reduction in TLE-3 in supplemented groups. |
| Klaus et al. 2005 [19] | Mice (New Zealand black–NZB) induced obesity by diet | High-fat diet (17% protein, 15% lipids and 42% carbohydrates) | EGCG | 0.5% e 1% de TEAVIGO ™ | 94% of EGCG | 4 weeks (induction obesity) + 4 weeks (treatment) | There were no changes in gene expression in BAT; WAT → SCD1 expression was reduced in groups supplemented with EGCG. |
| Lee et al. 2017 [13] | Mice (C57BL/6J) induced obesity by diet | High-fat diet (60% lipids) | EGCG | 0.2% added in the diet | - | 8 weeks (induction obesity) + 8 weeks (treatment) | The weights of WAT and BAT were decreased by 45% and 34%, respectively, in the group supplemented with EGCG compared to the high-fat group; BAT → EGCG in the diet significantly increased the expression of UCP1, UCP2, PRDM16, PGC-1α, NRF1, TFAM and CPT-1β and decreased of ACC2, compared to the high-fat group. |
| Mi et al. 2017 [10] | Mice (C57BL/6J) induced obesity by diet | High-fat and high fructose diet (45% lipids and 10% fructose) | EGCG | 2 g EGCG per liter of water | - | 16 weeks | BAT → EGCG intake restored average cell size and distribution; increased Sirt1 and Cpt2 and reduced Fasn; WAT → EGCG prevented HFFD-induced adipocyte hypertrophy and the uneven size distribution common to iWAT and eWAT; EGCG increased Sirt, PGC-1α and Cpt2 and reduced PPARγ and Fasn. |
| Neyrinck et al. 2017 [11] | Mice (C57BL/6J) induced obesity by diet | High-fat diet (60% lipids) | Green Tea Extract | 0.5% extract of green tea added in the diet | 60% of catechins 30% of EGCG | 8 weeks | sWAT → GTE supplementation reduced the weight, the size of the adipocytes, significantly increased the expression of PPARγ, PGC-1α, Prdm16 and Cited1; BAT → GTE supplementation promoted normalization of weight and reduced size of lipid droplets in cells; significant reduction in the expression of C/EBPα and aP2; up-regulation of PGC-1α, Vegfa165; Beige adipocytes were defined by their multilocular lipid droplet morphology. |
| Nomura et al. 2008 [16] | Rats (Sprague Dawley) induced obesity by diet | High-fat diet (60% fat) | Green Tea catechin | 5 g catechins per kilo of feed | 81.5% of catechins (EGCG–40.6%, ECG–23.1%, EGC–12.4%, EPI–9.2%) | 8 weeks | BAT–weight reduction in supplemented animals Control group supplemented with GTC showed UCP1 mRNA expression 70% higher than animals fed a control diet; High-fat group showed similar mRNA expressions from the three UCPs. |
| Otton et al. 2018 [12] | Mice (C57BL/6) induced to obesity by diet | High-fat diet (20% protein, 36% carbohydrates and 34% lipids) | Green Tea Extract | 500 mg·kg−1 of body weight per day | 30% of catechins | 4 weeks (inducation obesity) + 12 weeks (treatment) | BAT → weight and adipocyte reduction; sWAT and eWAT → weight and adipocyte reduction; eWAT → In the OB + GT group, only miR-802 was increased and 3 miRNAs were reduced (miR-335, miR-221, miR-155). |
| Santana et al. 2015 [18] | Swiss mice induced obesity by diet | High-fat diet | EGCG | 50 mg·kg−1 of body weight per day | - | 8 weeks | It did not promote changes in the hyperlipidic group supplemented with EGCG. |
| Yan et al. 2013 [17] | Rats (Sprague Dawley) induced obesity by diet | High fat diet (15% saturated fat and 1% cholesterol) | Green Tea Catechin | 100 mg·kg−1 of body weight per day | 50%–EGCG, 22%–ECG, 18%–EGC and 10%–EPI | 6 weeks | sWAT and vWAT →GTCs increased the PPARγ and UCP-1; BAT → PPAR level increased, significantly increased the expression of CPT1, AOX, and UCP-1. |
| Zhou et al. 2018 [14] | Mice (C57BL/6) induced by diet to obesity | High-fat diet (60% lipids) | EGCG | 1% EGCG of the diet composed | - | 4 weeks | BAT→ increased expression of UCP1, PGC-1α and PRDM16 in the HFD + EGCG group. |
| Author/Year | Final Score (20 Points) | Percentage of Adequacy by ARRIVE |
|---|---|---|
| Chen et al. 2017 [15] | 14/21 | 67% |
| Klaus et al. 2005 [19] | 14/21 | 67% |
| Lee et al. 2017 [13] | 11/21 | 52% |
| Mi et al. 2017 [10] | 15/21 | 71% |
| Neyrinck et al. 2017 [11] | 12/21 | 57% |
| Nomura et al. 2008 [16] | 14/21 | 67% |
| Otton et al. 2018 [12] | 17/21 | 80% |
| Santana et al. 2015 [18] | 13/21 | 62% |
| Yan et al. 2013 [17] | 13/21 | 62% |
| Zhou et al. 2018 [14] | 15/21 | 71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macêdo, A.P.A.; Gonçalves, M.d.S.; Barreto-Medeiros, J.M.; da Silva Neto, O.C.; David, J.M.; Villarreal, C.F.; Macambira, S.G.; Pereira Soares, M.B.; Couto, R.D. Green Tea Induces the Browning of Adipose Tissue—Systematic Review. Obesities 2023, 3, 193-206. https://doi.org/10.3390/obesities3030016
Macêdo APA, Gonçalves MdS, Barreto-Medeiros JM, da Silva Neto OC, David JM, Villarreal CF, Macambira SG, Pereira Soares MB, Couto RD. Green Tea Induces the Browning of Adipose Tissue—Systematic Review. Obesities. 2023; 3(3):193-206. https://doi.org/10.3390/obesities3030016
Chicago/Turabian StyleMacêdo, Ana Paula Azevêdo, Mariane dos Santos Gonçalves, Jairza Maria Barreto-Medeiros, Oscar Caetano da Silva Neto, Jorge Mauricio David, Cristiane Flora Villarreal, Simone Garcia Macambira, Milena Botelho Pereira Soares, and Ricardo David Couto. 2023. "Green Tea Induces the Browning of Adipose Tissue—Systematic Review" Obesities 3, no. 3: 193-206. https://doi.org/10.3390/obesities3030016
APA StyleMacêdo, A. P. A., Gonçalves, M. d. S., Barreto-Medeiros, J. M., da Silva Neto, O. C., David, J. M., Villarreal, C. F., Macambira, S. G., Pereira Soares, M. B., & Couto, R. D. (2023). Green Tea Induces the Browning of Adipose Tissue—Systematic Review. Obesities, 3(3), 193-206. https://doi.org/10.3390/obesities3030016








