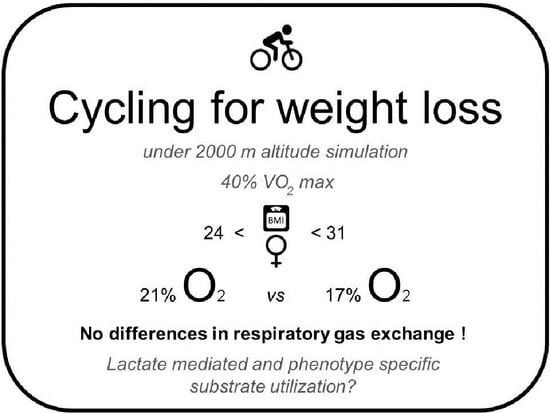
Cycling for Weight Loss May Clear Carbohydrates Rather Than Fat, Irrespective of Normal or Mildly Reduced Normobaric Oxygen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects, Inclusion and Exclusion Criteria
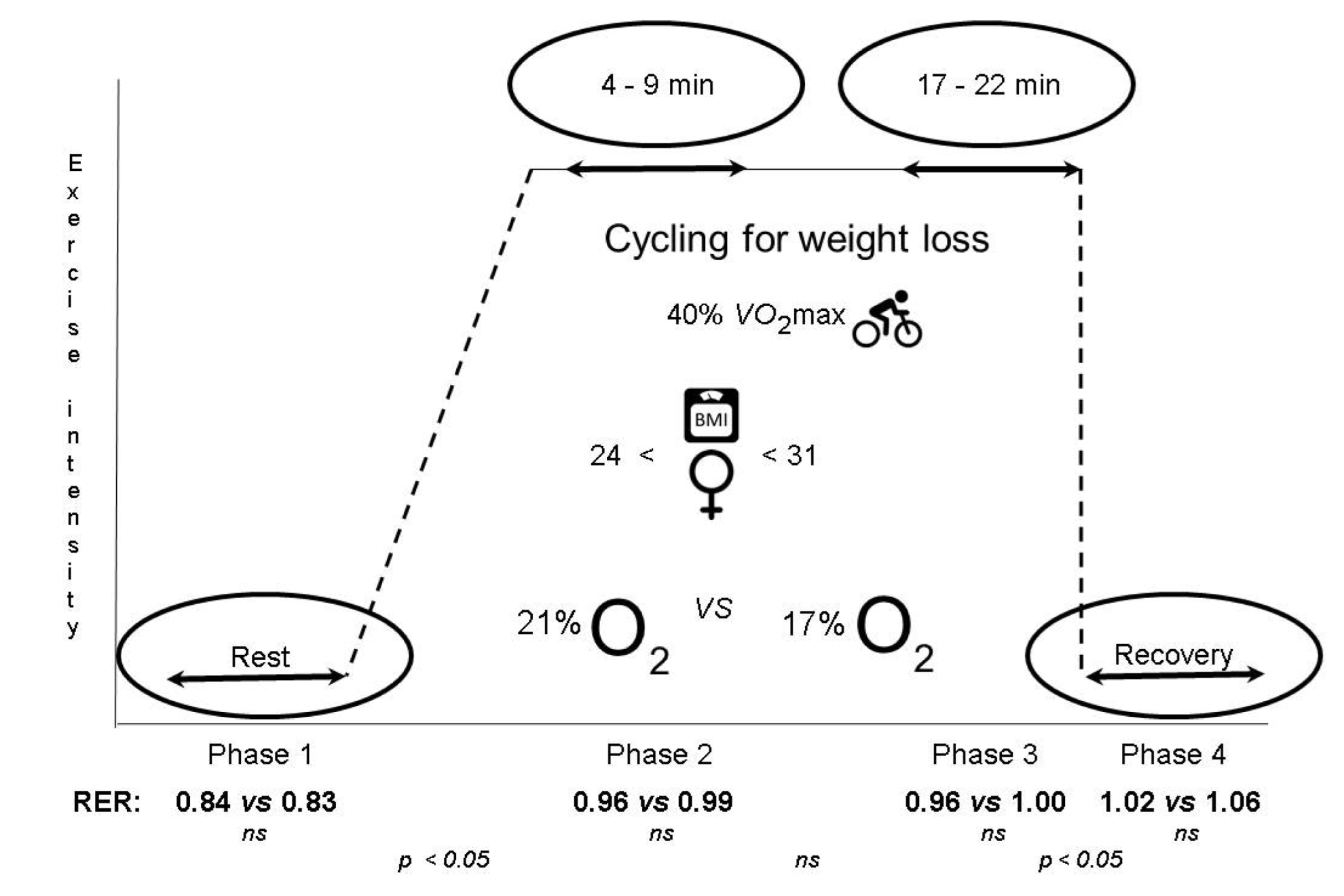
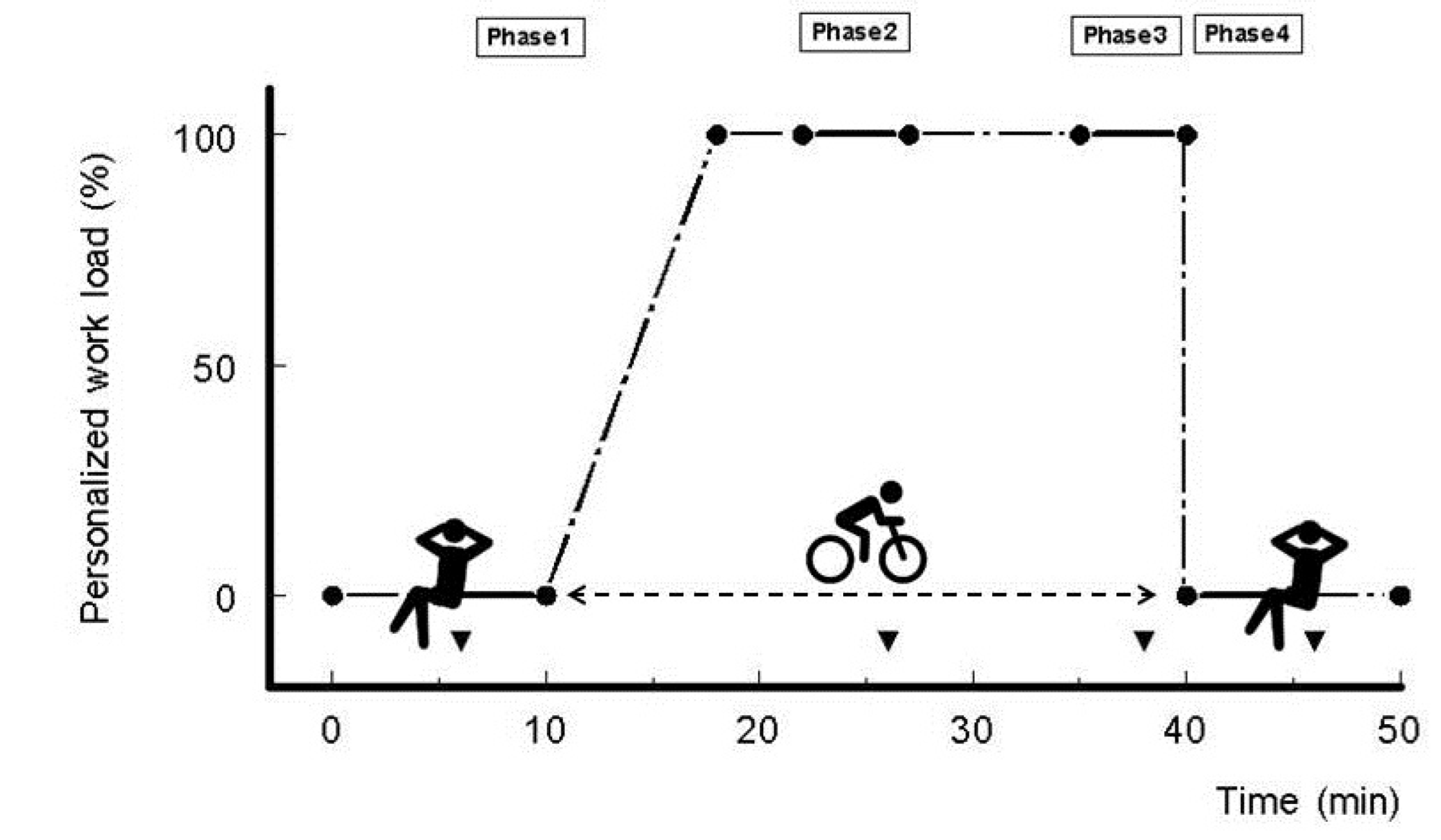
2.2. Experimental Cycling Protocol
2.3. Measurements
2.4. Personalized Cycling Workload
2.5. Facilities and Equipment
2.6. Statistical Analysis
3. Results
3.1. Subjects and General Aspects
3.2. Blood Oxygen Saturation
3.3. Oxygen Consumption
3.4. Respiratory Exchange Ratio (RER)
| Treatment | p-Value | ||||
|---|---|---|---|---|---|
| Phase | N-Ox | R-Ox | Time | Treatment | Time × Treatment |
| SaO2 (%) | |||||
| 1—Rest | 98.1 ± 0.1 | 95.4 ± 0.4 | <0.001 a,b,e,f | <0.001 | <0.001 |
| 2—Cycling (4–9 min) | 97.1 ± 0.3 | 93.8 ± 0.4 | |||
| 3—Cycling (17–22 min) | 97.8 ± 0.2 | 93.8 ± 0.4 | |||
| 4—Recovery | 98.2 ± 0.1 | 96.4 ± 0.3 | |||
| O2 (mL/min) | |||||
| 1—Rest | 239 ± 6 | 257 ± 28 | <0.001 a,b,e,f | 0.313 | 0.178 |
| 2—Cycling (4–9 min) | 1011 ± 34 | 974 ± 37 | |||
| 3—Cycling (17–22 min) | 1010 ± 41 | 982 ± 37 | |||
| 4—Recovery | 261 ± 8 | 250 ± 11 | |||
| RER (VCO2/VO2) | |||||
| 1—Rest | 0.84 ± 0.02 | 0.83 ± 0.02 | <0.001 a,b,c,e | 0.291 | 0.407 |
| 2—Cycling (4–9 min) | 0.96 ± 0.01 | 0.99 ± 0.04 | |||
| 3—Cycling (17–22 min) | 0.96 ± 0.01 | 1.00 ± 0.04 | |||
| 4—Recovery | 1.02 ± 0.02 | 1.06 ± 0.03 | |||
| Glucose (mM) | |||||
| 1—Rest | 4.8 ± 0.1 | 5.0 ± 0.2 | 0.032 | 0.205 | 0.450 |
| 2—Cycling (4–9 min) | 4.5 ± 0.1 | 4.7 ± 0.1 | |||
| 3—Cycling (17–22 min) | 4.6 ± 0.2 | 4.5 ± 0.1 | |||
| 4—Recovery | 4.5 ± 0.1 | 4.7 ± 0.1 | |||
| Lactate (mM) | |||||
| 1—Rest | 1.7 ± 0.2 | 1.8 ± 0.3 | 0.052 | 0.962 | 0.833 |
| 2—Cycling (4–9 min) | 2.0 ± 0.3 | 2.1 ± 0.2 | |||
| 3—Cycling (17–22 min) | 2.4 ± 0.3 | 2.5 ± 0.5 | |||
| 4—Recovery | 2.0 ± 0.4 | 1.8 ± 0.2 | |||
3.5. Blood Glucose and Lactate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series; WHO: Geneva Switzerland, 2003; Volume 916, Available online: http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf;jsessionid=F7DDF915029799B7E07FCFDB1432CB28?sequence=1 (accessed on 20 February 2022).
- WHO. Obesity and Overweight; WHO Fact Sheet; WHO: Geneva, Swizterland, 2011; Volume 311, Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 February 2022).
- Houmard, J.A.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; E Kraus, W. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 2004, 96, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpey, S.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; Hickner, R.C.; Kraus, W.E.; Houmard, J.A. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J. Appl. Physiol. 2009, 106, 1079–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Gao, X.; Chen, M.; van Dam, R.M. Long term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J. Obes. 2011, 2011, 868305. [Google Scholar] [CrossRef] [Green Version]
- Faghihnia, N.; Siri-Tarino, P.W.; Krauss, R.M.; Brooks, G.A. Energy substrate partitioning and efficiency in individuals with atherogenic lipoprotein phenotype. Obesity 2011, 19, 1360–1365. [Google Scholar] [CrossRef] [Green Version]
- Barwell, N.D.; Malkova, D.; Leggate, M.; Gill, J.M.R. Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metab. Clin. Exp. 2009, 58, 1320–1328. [Google Scholar] [CrossRef]
- Jobson, S.A.; Hopker, J.G.; Korff, T.; Passfield, L. Gross efficiency and cycling performance: A brief review. J. Sci. Cycl. 2012, 1, 3–8. [Google Scholar]
- Palmer, B.F.; Clegg, D.J. Ascent to altitude as a weight loss method: The good and bad of hypoxia inducible factor activation. Obesity 2014, 22, 311–317. [Google Scholar] [CrossRef]
- Johnson, A.O.C. Chronic obstructive pulmonary disease• 11: Fitness to fly with COPD. Thorax 2003, 58, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Jéquier, E. Indirect calorimetry: A method to assess energy and substrate balances. In Current Topics in Diabetes Research; Belfiore, F., Brgman, R.N., Molinatti, G.M., Eds.; Karger: Basel, Switzerland, 1993; Volume 12, pp. 32–38. [Google Scholar]
- Elia, M.; Livesey, G. Theory and validity of indirect calorimetry during net lipid synthesis. Am. J. Clin. Nutr. 1988, 47, 591–607. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl. S1), S28–S37. [Google Scholar] [CrossRef]
- Bakker, J.; Nijsten, M.W.; Jansen, T.C. Clinical use of lactate monitoring in critically ill patients. Ann. Intensive Care 2013, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Van Aggel-Leijsen, D.P.; Saris, W.; Wagenmakers, A.J.; Hul, G.B.; van Beek, M.A. The effect of low-intensity exercise training on fat metabolism of obese women. Obes. Res. 2001, 9, 86–96. [Google Scholar] [CrossRef]
- Braun, B.; Sharoff, C.; Chipkin, S.R.; Beaudoin, F. Effects of insulin resistance on substrate utilization during exercise in overweight. J. Appl. Physiol. 2004, 97, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Braun, B.; Mawson, J.T.; Muza, S.R.; Dominick, S.B.; Brooks, G.A.; Horning, M.A.; Rock, P.B.; Moore, L.G.; Mazzeo, R.S.; Ezeji-Okoye, S.C.; et al. Women at altitude: Carbohydrate utilization during exercise at 4300 m. J. Appl. Physiol. 2000, 88, 246–256. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Beatty, C.L.; Burelle, Y.; Trump, M.E.; McKenzie, D.C.; Matheson, G.O. The lactate paradox in human high-altitude physiological performance. News Physiol. Sci. 2002, 17, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Katayama, K.; Goto, K.; Ishida, K.; Ogita, F. Substrate utilization during exercise and recovery at moderate altitude. Metabolism 2010, 59, 959–966. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [Green Version]
- Malin, S.K.; Viskochil, R.; Oliver, C.; Braun, B. Mild fasting hyperglycemia shifts fuel reliance toward fat during exercise in adults with impaired glucose tolerance. J. Appl. Physiol. 2013, 115, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Bender, D.A. Introduction to Nutrition and Metabolism, 4th ed.; Taylor & Francis Group: London, UK, 2008; ISBN 1-4200-4312-9. [Google Scholar]
- Braun, B. Effects of high altitude on substrate use and metabolic economy: Cause and effect? Med. Sci. Sports Exerc. 2008, 40, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology, Energy, Nutrition & Human Performance, 6th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2007; ISBN 0-7817-4990-5. [Google Scholar]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichol, A.D.; Egi, M.; Pettila, V.; Bellomo, R.; French, C.; Hart, G.; Cooper, D.J.; Stachowski, E.; Reade, M.C.; Bailey, M.; et al. Relative hyperlactemia and hospital mortality in critically ill patients: A retrospective multi-centre study. Crit. Care 2010, 14, R25. Available online: http://ccforum.com/content/14/1/R25 (accessed on 20 February 2022). [CrossRef] [PubMed] [Green Version]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.; Morscher, R.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Redant, S.; Hussein, H.; Mugisha, A.; Attou, R.; De Bels, D.; Honore, P.M.; De Laet, C.C. Differentiating hyperlactatemia type A from B: How does the lactate/pyruvate ratio help? J. Transl. Int. Med. 2019, 7, 43–45. [Google Scholar] [CrossRef] [Green Version]
- Leverve, X. Metabolic and nutritional consequences of chronic hypoxia. Clin. Nutr. 1998, 17, 241–251. [Google Scholar] [CrossRef]
- Le Stunff, C.; Bougnères, P.F. Alterations of plasma lactate and glucose metabolism in obese children. Am. J. Physiol. 1996, 271, E814–E820. [Google Scholar] [CrossRef]
- Basu, R.; Schwenk, W.F.; Rizza, R.A. Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am. J. Physiol. 2004, 287, E55–E62. [Google Scholar] [CrossRef]
- Consoli, A.; Nurjhan, N.; Reilly, J.J.; Bier, D.M.; E Gerich, J. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1999, 86, 2038–2045. [Google Scholar] [CrossRef]
- Choi, C.S.; Kim, Y.B.; Lee, F.N.; Zabolotny, J.M.; Kahn, B.B.; Youn, J.H. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am. J. Physiol. Endocrinol. Metab. 2002, 283, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Vettor, R.; Lombardi, A.M.; Fabris, R.; Pagano, C.; Cusin, I.; Rohner-Jeanrenaud, F.; Federspil, G.; Jeanrenaud, B. Lactate infusion in anesthetized rats produces insulin resistance in heart and skeletal muscles. Metabolism 1997, 46, 684–690. [Google Scholar] [CrossRef]
- Feneberg, R.; Sparber, M.; Veldhuis, J.D.; Mehls, O.; Ritz, E.; Schaefer, F. Synchronous fluctuations of blood insulin and lactate concentrations in humans. J. Clin. Endocrinol. Metab. 1999, 84, 220–227. [Google Scholar] [CrossRef]
- Dankner, R.; Chetrit, A.; Shanik, M.H.; Raz, I.; Roth, J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over 24-year follow-up. Diabetes Care 2009, 32, 1464–1466. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, A.M.; Fabris, R.; Bassetto, F.; Serra, R.; Leturque, A.; Federspil, G.; Girard, J.; Vettor, R. Hyperlactemia reduces muscle glucose uptake and GLUT-4 mRNA while increasing (E1α)PDH gene expression in rat. Am. J. Physiol. 1999, 276, E922–E929. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [Green Version]
- DiGirolamo, M.; Newby, F.D.; Lovejoy, J. Lactate production in adipose tissue: A regulated function with extra-adipose implications. FASEB J. 1992, 6, 2405–2412. [Google Scholar] [CrossRef]
- Woerle, H.J.; Meyer, C.; Dostou, J.M.; Gosmanov, N.R.; Islam, N.; Popa, E.; Wittlin, S.D.; Welle, S.L.; Gerich, J.E. Pathways for glucose disposal after meal ingestion in humans. Am. J. Physiol. 2003, 284, E716–E725. [Google Scholar] [CrossRef] [Green Version]
- Alemany, M. Utilization of dietary glucose in the metabolic syndrome. Nutr. Metab. 2011, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B.J. Metabolic response to the stress of critically illness. Br. J. Anaest. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, J.J.E.; Lagerwaard, B.; Porbahaie, M.; Nieuwenhuizen, A.G.; Savelkoul, H.F.J.; van Neerven, R.J.J.; Keijer, J.; de Boer, V.C.J. Extracellular flux analyses reveal differences in mitochondrial PBMC metabolism between high-fit and low-fit females. Am. J. Physiol.—Endocrinol. Metab. 2022, 322, E141–E153. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 29.3 ± 6.5 | 20–40 |
| Height (cm) | 167 ± 4.9 | 157–175 |
| Body mass (kg) | 76.8 ± 7.2 | 65.4–89.0 |
| BMI (kg·m−2) | 27.4 ± 2.1 | 24.0–31.2 |
| VO2max (mL·kg−1·min−1) HRmax RPEmax (screening) | 33.9 ± 5.6 185 ± 9.6 14.1 ± 2.5 | 22.6–43.4 162–203 10–20 |
| Personalized workload (watt) | 54.2 ± 11.7 | 40–70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreurs, V.V.A.M.; Maas, T.; van den Borne, J.J.G.C.; Keijer, J. Cycling for Weight Loss May Clear Carbohydrates Rather Than Fat, Irrespective of Normal or Mildly Reduced Normobaric Oxygen. Obesities 2022, 2, 205-214. https://doi.org/10.3390/obesities2020016
Schreurs VVAM, Maas T, van den Borne JJGC, Keijer J. Cycling for Weight Loss May Clear Carbohydrates Rather Than Fat, Irrespective of Normal or Mildly Reduced Normobaric Oxygen. Obesities. 2022; 2(2):205-214. https://doi.org/10.3390/obesities2020016
Chicago/Turabian StyleSchreurs, Victor V. A. M., Tjieu Maas, Joost J. G. C. van den Borne, and Jaap Keijer. 2022. "Cycling for Weight Loss May Clear Carbohydrates Rather Than Fat, Irrespective of Normal or Mildly Reduced Normobaric Oxygen" Obesities 2, no. 2: 205-214. https://doi.org/10.3390/obesities2020016
APA StyleSchreurs, V. V. A. M., Maas, T., van den Borne, J. J. G. C., & Keijer, J. (2022). Cycling for Weight Loss May Clear Carbohydrates Rather Than Fat, Irrespective of Normal or Mildly Reduced Normobaric Oxygen. Obesities, 2(2), 205-214. https://doi.org/10.3390/obesities2020016