Obesity Rodent Models Applied to Research with Food Products and Natural Compounds
Abstract
:1. Introduction
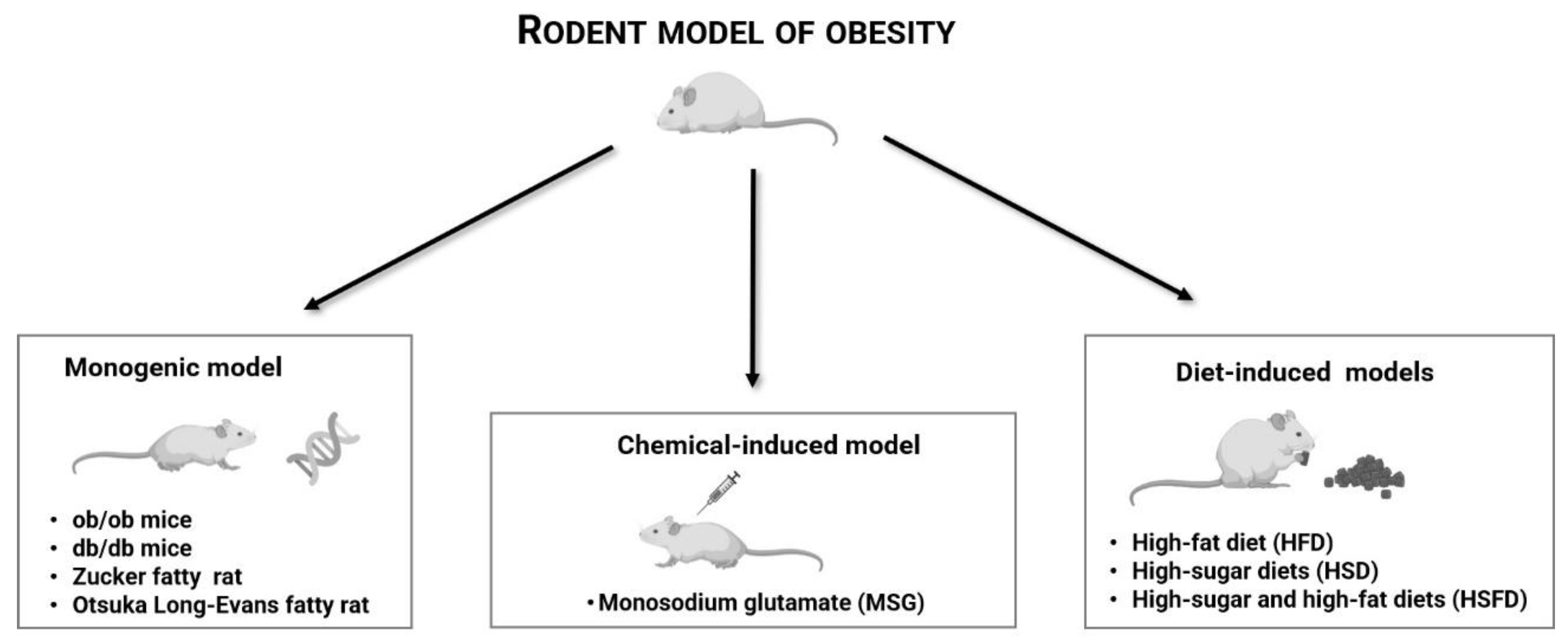
2. Rodent Models of Obesity Used in Natural Compounds Research
2.1. db/db Mice
2.2. ob/ob Mice
2.3. Zucker Fatty Rat
2.4. Otsuka Long-Evans Tokushima Fatty Rat
2.5. Monosodium Glutamate (MSG)-Induced Obesity Model
| Food Product/Plant | Bioactive Compounds | Strain/Obesity Model | Dose and Treatment | Observed Effects | References |
|---|---|---|---|---|---|
| Artemisia extract | Artemether | C57BL/KsJ db/db mice ♂ | 200 mg/kg (oral gavage), for 2 weeks | ↓ Food intake and weight increase rate | [21] |
| ↓ Fasting blood glucose levels | |||||
| ↑ Tolerance to glucose | |||||
| ↑ Insulin sensitivity | |||||
| ↑ Insulin secretion | |||||
| Improved hyperinsulinemia | |||||
| Ameliorated islet vacuolar degeneration and hepatic steatosis | |||||
| ↓ Apoptosis of pancreatic beta cells | |||||
| Barley | N.A. | db/db mice (BKS.Cg-+Leprdb/+Leprdb/OlaHsd—fat, black, homozygous) ♂ | 88% (w/w; mixed with the diet), for 8 weeks | ↓ Plasma insulin and resistin levels | [22] |
| ↓ TC levels in the liver | |||||
| Bilberries (Vaccinium myrtillus) | Nonacylated anthocyanin extract | Zucker (fa/fa) rats ♂, fed with HFD | 25 mg/kg/day (oral gavage), for 8 weeks | ↓ Fasting plasma glucose level | [38] |
| ↓ Levels of branched-chain amino acids | |||||
| Improved lipid profiles | |||||
| Cannabis sativa | Cannabinoid Δ9-tetrahydrocannabivarin | C57BL/6 ob/ob mice ♀ | 0.1, 0.5, 2.5 and 12.5 mg/kg/day (oral gavage), for 30 days | ↓ Liver TG concentration (only for 12.5 mg/kg) | [27] |
| Cinnamon extract (Cinnamomum zeylanicum) | N.A. | B6.V-Lepob/J mice [on a C57BL/6J background (ob/ob)] ♂ | 4.5 mL/kg (equates to 0.8 g/kg) (in drinking water), for 6 weeks | ↑ Insulin sensitivity and glucose tolerance | [28] |
| ↓ Hepatic levels of TG | |||||
| ↓ Fat accumulation in the liver | |||||
| ↑ Liver glycogen content | |||||
| Improvement of insulin-stimulated locomotor activity | |||||
| Celastraceae family members (including Tripterygium wilfordii) | Celastrol (tripterine) | C57BL/6J ob/ob mice ♂, fed with HFD | 3 mg/kg/day (mixed with the HFD), for 6 weeks | ↓ B.w. | [30] |
| ↓ Liver weight | |||||
| ↓ TG levels in the liver | |||||
| ↑ Glucose clearance | |||||
| Downregulation of intestinal lipid transporters | |||||
| ↑ Lipid excretion in feces | |||||
| Green tea | Polyphenols | Zucker (fa/fa) rats ♂, fed with HFD | 200 mg/kg/day (oral gavage), for 8 weeks | ↓ B.w. gain | [37] |
| ↓ Visceral fat | |||||
| ↓ Fasting serum insulin, glucose and lipids levels | |||||
| Liriope platyphylla (dry roots) | Aqueous extract | OLETF rats | 5 or 10% (15 mL/g b.w./day; oral gavage), for 2 weeks | ↓ Abdominal fat mass | [51] |
| ↓ Glucose concentration | |||||
| ↑ Insulin production (only for 10% concentration) | |||||
| ↓ Expression of Glut-1 | |||||
| Mix of Curcuma longa L., Alnus japonica and Massa Medicata Fermentata | Gambigyeongsinhwan | OLETF rats ♂ | 250 or 500 mg/kg/day (oral gavage), for 8 weeks | ↓ B.w. gain | [41] |
| ↓ Adipose tissue weight and visceral adipocyte size | |||||
| ↑ mRNA levels PPARα in adipose tissue | |||||
| Mix of edible mushrooms (Lentinus edodes, Ganoderma lucidum, Pleurotus ostreatus and Flammulina velutipes) in fermented milk | N.A. | OLETF rats ♂ | 10 and 20% (v/w; mixed with the diet), for 6 weeks | ↓ B.w., | [43] |
| ↓ Perirenal fat, visceral and epididymal fat (only for 20% concentration), | |||||
| ↓TG and FFA levels | |||||
| Mix of Liriope platyphylla, Platycodon grandiflorum, Schisandra chinensis, and Ephedra sinica | Gyeongshingangjeehwan | OLETF rats ♂ | 121.7 mg/kg/day (oral gavage), for 8 weeks | ↓ Visceral WAT weight | [42] |
| ↓ Size adipocytes in mesenteric WAT | |||||
| ↓ mRNA expression levels of adipocyte marker genes (PPARγ, aP2 and leptin) in visceral WAT | |||||
| ↑ mRNA expression levels of PPARα target genes in visceral WAT | |||||
| ↓ Plasma levels of FFA, TG, insulin and glucose | |||||
| Purple Potato (Solanum tuberosum) | Acylated anthocyanin extract | Zucker (fa/fa) rats ♂, fed with HFD | 25 mg/kg/day (oral gavage), for 8 weeks | ↓ Levels of branched-chain amino acids | [38] |
| improved lipid profiles | |||||
| ↑ Glutamine/glutamate ratio | |||||
| ↓ Glycerol levels and metabolites involved in glycolysis | |||||
| Red Wine (ProvinolsTM) | Polyphenol extract (70% Polyphenols) | Zucker (fa/fa) rats | 20 mg/kg/day (mixed with the diet), for 8 weeks | ↓ Plasma levels of glucose, fructosamine, TG, TC and LDL-cholesterol | [35] |
| ↑ NO | |||||
| ↑ eNOS activity | |||||
| ↓Superoxide anion | |||||
| Roselle (Hibiscus sabdariffa L.) aqueous extract | Anthocyanins | Swiss Webster (CFW) mice ♂ induced by MSG | 120 mg/kg/day (60 mg/kg/day by oral gavage plus 60 mg/kg/day dissolved in tap water given ad libitum), for 60 days | ↓ B.w. gain | [50] |
| ↓ Glycemia | |||||
| ↑ ALT levels | |||||
| Rosemary (Rosmarinus officinalis L.) extract | Carnosic acid and carnosol | Zucker (fa/fa) rats ♀ | 0.5% (w/w; mixed with the diet), for 64 days | ↓ B.w. gain, | [36] |
| ↓ Serum TG, TC and insulin levels | |||||
| Lipase activity inhibition in the stomach | |||||
| Soy products, grains and legumes | Genistein | ob/ob mice ♀ | 0.06% (w/w; mixed with the diet), for 4 weeks | ↓B.w. gain | [25] |
| Downregulation of SOD activity | |||||
| ↑ iNOS expression in mesenteric artery perivascular adipose tissue |
2.6. Diet-Induced Obesity Models
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Speakman, J.; Hambly, C.; Mitchell, S.; Król, E. The Contribution of Animal Models to the Study of Obesity. Lab. Anim. 2008, 42, 413–432. [Google Scholar] [CrossRef] [PubMed]
- WHO Overweight and Obesity—Children and Adolescents Aged 5–19. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 January 2021).
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical Parameters and Markers of Obesity in Rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaila, B.; Raman, M. Obesity: A Review of Pathogenesis and Management Strategies. Can. J. Gastroenterol. 2008, 22, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittwede, P.N.; Clemmer, J.S.; Bergin, P.F.; Xiang, L. Obesity and Critical Illness: Insights from Animal Models. Shock 2016, 45, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, D.C.W.; Douketis, J.D.; Morrison, K.M.; Hramiak, I.M.; Sharma, A.M.; Ur, E.; for members of the Obesity Canada Clinical Practice Guidelines Expert Panel. 2006 Canadian Clinical Practice Guidelines on the Management and Prevention of Obesity in Adults and Children [Summary]. Can. Med. Assoc. J. 2007, 176, S1–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural Anti-Obesity Agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Wu, L.; Chen, X.; Huang, Z.; Hu, W. Effects of Food-Additive-Information on Consumers’ Willingness to Accept Food with Additives. Int. J. Environ. Res. Public Health 2018, 15, 2394. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic Compounds as Natural and Multifunctional Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Ferreira, T.; Nascimento-Gonçalves, E.; Gil Da Costa, R.M.; Rosa, E.; Alexandra Oliveira, P. Therapeutic and Toxicological Effects of Natural Compounds: Data from HPV16-Transgenic and ICR Mice (Review). World Acad. Sci. J. 2020, 2, 9. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Blachier, F.; Tomé, D.; Blais, A. Animal Models for the Study of the Relationships between Diet and Obesity: A Focus on Dietary Protein and Estrogen Deficiency. Front. Nutr. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speakman, J.; Hambly, C.; Mitchell, S.; Król, E. Animal Models of Obesity. Obes. Rev. 2007, 8, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Woods, S.C. Overview of Animal Models of Obesity. Curr. Protoc. Pharmacol. 2012, 58, 5.61.1–5.61.18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, P.; Mercer, J.G.; Morgan, P.J. Preclinical Models for Obesity Research. Dis. Model. Mech. 2016, 9, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Preguiça, I.; Alves, A.; Nunes, S.; Fernandes, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-induced Rodent Models of Obesity-related Metabolic Disorders—A Guide to a Translational Perspective. Obes. Rev. 2020, 21, e13081. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The Impact of Rodents on Advances in Biomedical Research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Kanasaki, K.; Koya, D. Biology of Obesity: Lessons from Animal Models of Obesity. J. Biomed. Biotechnol. 2011, 2011, 197636. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, J.B.; Mohamed, M.; Bakar, A.B.A. A Systematic Review on Different Models of Inducing Obesity in Animals: Advantages and Limitations. J. Adv. Vet. Anim. Res. 2020, 7, 103–114. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal Models of Obesity and Diabetes Mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Fu, W.; Xin, Y.; Bai, J.; Peng, H.; Fu, L.; Liu, J.; Li, L.; Ma, Y.; Jiang, H. Antidiabetic and Antiobesity Effects of Artemether in Db/Db Mice. BioMed Res. Int. 2018, 2018, 8639523. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Mills, D.A.; Murphy, K.; Noratto, G. Effect of Barley Supplementation on the Fecal Microbiota, Caecal Biochemistry, and Key Biomarkers of Obesity and Inflammation in Obese Db/Db Mice. Eur. J. Nutr. 2018, 57, 2513–2528. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Carreira, M.C.; Cabia, B.; Andrade, S.; Amil, M.; Casanueva, F.F. Leptin Resistance in Obesity: An Epigenetic Landscape. Life Sci. 2015, 140, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Andres, M.; Hernandez, S.; Mazitschek, R.; Ozcan, U.; Hospital, G. Treatment of Obesity with Celastrol. Treatment 2016, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 Neurones Are Direct Targets for Leptin in the Ob/Ob Mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef]
- Ritskes-Hoitinga, M.; Tobin, G.; Jensen, T.L.; Mikkelsen, L.F. Nutrition of the Laboratory Mouse. In The Laboratory Mouse; Elsevier: Amsterdam, The Netherlands, 2012; pp. 567–599. ISBN 978-0-12-382008-2. [Google Scholar]
- Wargent, E.T.; Zaibi, M.S.; Silvestri, C.; Hislop, D.C.; Stocker, C.J.; Stott, C.G.; Guy, G.W.; Duncan, M.; Di Marzo, V.; Cawthorne, M.A. The Cannabinoid Δ9-Tetrahydrocannabivarin (THCV) Ameliorates Insulin Sensitivity in Two Mouse Models of Obesity. Nutr. Diabetes 2013, 3, e68. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, T.; Peter, A.; Schulz, N.; Drescher, A.; Bergheim, I.; Machann, J.; Schick, F.; Siegel-Axel, D.; Schürmann, A.; Weigert, C.; et al. Cinnamon Extract Improves Insulin Sensitivity in the Brain and Lowers Liver Fat in Mouse Models of Obesity. PLoS ONE 2014, 9, e92358. [Google Scholar] [CrossRef] [Green Version]
- Simperova, A.; Al-Nakkash, L.; Faust, J.J.; Sweazea, K.L. Genistein Supplementation Prevents Weight Gain but Promotes Oxidative Stress and Inflammation in the Vasculature of Female Obese ob/ob Mice. Nutr. Res. 2016, 36, 789–797. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, Y.; Zhao, F.; Chen, K.; Wu, T.; Liu, Q.; Huang, S.; Zhang, A.; Jia, Z. Celastrol Inhibits Intestinal Lipid Absorption by Reprofiling the Gut Microbiota to Attenuate High-Fat Diet-Induced Obesity. iScience 2021, 24, 102077. [Google Scholar] [CrossRef]
- van der Spek, R.; Kreier, F.; Fliers, E.; Kalsbeek, A. Circadian Rhythms in White Adipose Tissue. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 199, pp. 183–201. ISBN 978-0-444-59427-3. [Google Scholar]
- Kitada, M.; Ogura, Y.; Koya, D. Rodent Models of Diabetic Nephropathy: Their Utility and Limitations. Int. J. Nephrol. Renov. Dis. 2016, 9, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Yorek, M.A. Alternatives to the Streptozotocin-Diabetic Rodent, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 127, ISBN 9780128039151. [Google Scholar]
- Owens, D.R. Spontaneous, Surgically and Chemically Induced Models of Disease. In The laboratory Rat; Academic Press: Cambridge, MA, USA, 2006; pp. 711–732. [Google Scholar] [CrossRef]
- Agouni, A.; Lagrue-Lak-Hal, A.-H.; Mostefai, H.A.; Tesse, A.; Mulder, P.; Rouet, P.; Desmoulin, F.; Heymes, C.; Martínez, M.C.; Andriantsitohaina, R. Red Wine Polyphenols Prevent Metabolic and Cardiovascular Alterations Associated with Obesity in Zucker Fatty Rats (Fa/Fa). PLoS ONE 2009, 4, e5557. [Google Scholar] [CrossRef] [Green Version]
- Romo Vaquero, M.; Yáñez-Gascón, M.-J.; García Villalba, R.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; Espín de Gea, J.C.; García-Conesa, M.-T. Inhibition of Gastric Lipase as a Mechanism for Body Weight and Plasma Lipids Reduction in Zucker Rats Fed a Rosemary Extract Rich in Carnosic Acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Kim, J.; Cheng, J.; Ong, M.; Lao, W.-G.; Jin, X.-L.; Lin, Y.-G.; Xiao, L.; Zhu, X.-Q.; Qu, X.-Q. Green Tea Polyphenols Ameliorate Non-Alcoholic Fatty Liver Disease through Upregulating AMPK Activation in High Fat Fed Zucker Fatty Rats. World J. Gastroenterol. 2017, 23, 3805. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of Anthocyanin Extracts from Bilberry (Vaccinium Myrtillus L.) and Purple Potato (Solanum Tuberosum L. Var. ‘Synkeä Sakari’) on the Plasma Metabolomic Profile of Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Moran, T.H. Actions of CCK in the Controls of Food Intake and Body Weight: Lessons from the CCK-A Receptor Deficient OLETF Rat. Neuropeptides 2002, 36, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M. Models to Investigate Cardiac Metabolism Michael. In The Scientist’s Guide to Cardiac Metabolism; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 45, p. 114. ISBN 9780128023945. [Google Scholar]
- Sung Roh, J.; Lee, H.; Woo, S.; Yoon, M.; Kim, J.; Dong Park, S.; Shik Shin, S.; Yoon, M. Herbal Composition Gambigyeongsinhwan (4) from Curcuma Longa, Alnus Japonica, and Massa Medicata Fermentata Inhibits Lipid Accumulation in 3T3-L1 Cells and Regulates Obesity in Otsuka Long-Evans Tokushima Fatty Rats. J. Ethnopharmacol. 2015, 171, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Jung, Y.S.; Yoon, K.H.; Choi, S.; Hong, Y.; Park, D.; Lee, H.; Seo, B., II; Lee, H.Y.; Yoon, M. The Korean Traditional Medicine Gyeongshingangjeehwan Inhibits Adipocyte Hypertrophy and Visceral Adipose Tissue Accumulation by Activating PPARα Actions in Rat White Adipose Tissues. J. Ethnopharmacol. 2010, 127, 47–54. [Google Scholar] [CrossRef]
- Jeon, B.S.; Park, J.W.; Kim, B.K.; Kim, H.K.; Jung, T.S.; Hahm, J.R.; Kim, D.R.; Cho, Y.S.; Cha, J.Y. Fermented Mushroom Milk-Supplemented Dietary Fibre Prevents the Onset of Obesity and Hypertriglyceridaemia in Otsuka Long-Evans Tokushima Fatty Rats. Diabetes Obes. Metab. 2005, 7, 709–715. [Google Scholar] [CrossRef]
- Wanezaki, S.; Tachibana, N.; Nagata, M.; Saito, S.; Nagao, K.; Yanagita, T.; Kohno, M. Soy β-Conglycinin Improves Obesity-Induced Metabolic Abnormalities in a Rat Model of Nonalcoholic Fatty Liver Disease. Obes. Res. Clin. Pract. 2015, 9, 168–174. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, P.; Liu, W.; Li, L.; Ai, H.; Ma, X. Ventromedial Hypothalamic Nucleus in Regulation of Stress-Induced Gastric Mucosal Injury in Rats. Sci. Rep. 2018, 8, 10170. [Google Scholar] [CrossRef]
- Kiba, T.; Tanaka, K.; Numata, K.; Hoshino, M.; Misugi, K.; Inoue, S. Ventromedial Hypothalamic Lesion-Induced Vagal Hyperactivity Stimulates Rat Pancreatic Cell Proliferation. Gastroenterology 1996, 110, 885–893. [Google Scholar] [CrossRef]
- Parasuraman, S.; Wen, L.E. Animal Model for Obesity-An Overview. Syst. Rev. Pharm. 2016, 6, 9–12. [Google Scholar] [CrossRef]
- Von Diemen, V.; Trindade, E.N.; Trindade, M.R.M. Experimental Model to Induce Obesity in Rats. Acta Cir. Bras. 2006, 21, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Miśkowiak, B.; Partyka, M. Effects of Neonatal Treatment with MSG (Monosodium Glutamate) on Hypothalamo-Pituitary-Thyroid Axis in Adult Male Rats. Histol. Histopathol. 1993, 8, 731–734. [Google Scholar] [PubMed]
- Alarcon-Aguilar, F.J.; Zamilpa, A.; Perez-Garcia, M.D.; Almanza-Perez, J.C.; Romero-Nuñez, E.; Campos-Sepulveda, E.A.; Vazquez-Carrillo, L.I.; Roman-Ramos, R. Effect of Hibiscus Sabdariffa on Obesity in MSG Mice. J. Ethnopharmacol. 2007, 114, 66–71. [Google Scholar] [CrossRef]
- Kim, J.-E.; Hwang, I.-S.; Choi, S.-I.; Hye-Ryun, L.; Young-Ju, L.; Jun-Seo, G.; Hee-Seob, L.; Son, H.-J.; Jang, M.-J.; Lee, S.-H.; et al. Aqueous Extract of Liriope Platyphylla, a Traditional Chinese Medicine, Significantly Inhibits Abdominal Fat Accumulation and Improves Glucose Regulation in OLETF Type II Diabetes Model Rats. Lab Anim. Res 2012, 28, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Granados, M.J.; Ramírez-Emiliano, J.; Franco-Robles, E. Rodent Models of Obesity and Diabetes. In Experimental Animal Models of Human Diseases—An Effective Therapeutic Strategy; Bartholomew, I., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-164-9. [Google Scholar]
- Geiger, B.M.; Pothos, E.N. Translating Animal Models of Obesity and Diabetes to the Clinic. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29, pp. 1–16. ISBN 978-0-12-803161-2. [Google Scholar]
- Panchal, S.K.; Brown, L. Rodent Models for Metabolic Syndrome Research. J. Biomed. Biotechnol. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Smith, D.L.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in Body Weight, Food Intake and Body Composition after Long-Term High-Fat Diet Feeding in C57BL/6J Mice: Variations in Diet-Induced Obese C57BL/6J Mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef]
- Glastras, S.J.; Chen, H.; Teh, R.; McGrath, R.T.; Chen, J.; Pollock, C.A.; Wong, M.G.; Saad, S. Mouse Models of Diabetes, Obesity and Related Kidney Disease. PLoS ONE 2016, 11, e0162131. [Google Scholar] [CrossRef]
- Eom, J.; Thomas, S.S.; Sung, N.-Y.; Kim, D.-S.; Cha, Y.-S.; Kim, K.-A. Abeliophyllum Distichum Ameliorates High-Fat Diet-Induced Obesity in C57BL/6J Mice by Upregulating the AMPK Pathway. Nutrients 2020, 12, 3320. [Google Scholar] [CrossRef]
- Cha, Y.-S.; Rhee, S.-J.; Heo, Y.-R. Acanthopanax Senticosus Extract Prepared from Cultured Cells Decreases Adiposity and Obesity Indices in C57BL/6J Mice Fed a High Fat Diet. J. Med. Food 2004, 7, 422–429. [Google Scholar] [CrossRef]
- Yoshizumi, K.; Hirano, K.; Ando, H.; Hirai, Y.; Ida, Y.; Tsuji, T.; Tanaka, T.; Satouchi, K.; Terao, J. Lupane-Type Saponins from Leaves of Acanthopanax Sessiliflorus and Their Inhibitory Activity on Pancreatic Lipase. J. Agric. Food Chem. 2006, 54, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, Y.; Han, L.; Wang, H.; Saito, M.; Ling, M.; Kimura, Y.; Feng, Y. Saponins (Ginsenosides) from Stems and Leaves of Panax Quinquefolium Prevented High-Fat Diet-Induced Obesity in Mice. Phytomedicine 2008, 15, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S. Platycodon Grandiflorum> Extract Represses Up-Regulated Adipocyte Fatty Acid Binding Protein Triggered by a High Fat Feeding in Obese Rats. World J. Gastroenterol. 2007, 13, 3493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ae Yoon, N.; Park, J.; Yeon Jeong, J.; Rashidova, N.; Ryu, J.; Seob Roh, G.; Joon Kim, H.; Jae Cho, G.; Sung Choi, W.; Hoon Lee, D.; et al. Anti-Obesity Activity of Ethanol Extract from Bitter Melon in Mice Fed High-Fat Diet. Dev. Reprod. 2019, 23, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Benn, T.; Kim, B.; Park, Y.-K.; Wegner, C.J.; Harness, E.; Nam, T.-G.; Kim, D.-O.; Lee, J.S.; Lee, J.-Y. Polyphenol-Rich Blackcurrant Extract Prevents Inflammation in Diet-Induced Obese Mice. J. Nutr. Biochem. 2014, 25, 1019–1025. [Google Scholar] [CrossRef]
- Uchiyama, S.; Taniguchi, Y.; Saka, A.; Yoshida, A.; Yajima, H. Prevention of Diet-Induced Obesity by Dietary Black Tea Polyphenols Extract in Vitro and in Vivo. Nutrition 2011, 27, 287–292. [Google Scholar] [CrossRef]
- Ikarashi, N.; Toda, T.; Okaniwa, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Anti-Obesity and Anti-Diabetic Effects of Acacia Polyphenol in Obese Diabetic KKAy Mice Fed High-Fat Diet. Evid. Based Complement. Alternat. Med. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aborehab, N.M.; El Bishbishy, M.H.; Waly, N.E. Resistin Mediates Tomato and Broccoli Extract Effects on Glucose Homeostasis in High Fat Diet-Induced Obesity in Rats. BMC Complement. Altern. Med. 2016, 16, 225. [Google Scholar] [CrossRef] [Green Version]
- Zandani, G.; Anavi-Cohen, S.; Tsybina-Shimshilashvili, N.; Sela, N.; Nyska, A.; Madar, Z. Broccoli Florets Supplementation Improves Insulin Sensitivity and Alters Gut Microbiome Population—A Steatosis Mice Model Induced by High-Fat Diet. Front. Nutr. 2021, 8, 680241. [Google Scholar] [CrossRef]
- Li, X.; Tian, S.; Wang, Y.; Liu, J.; Wang, J.; Lu, Y. Broccoli Microgreens Juice Reduces Body Weight by Enhancing Insulin Sensitivity and Modulating Gut Microbiota in High-Fat Diet-Induced C57BL/6J Obese Mice. Eur. J. Nutr. 2021, 60, 3829–3839. [Google Scholar] [CrossRef]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.-C.; Lee, H.-G.; Kim, H.-S.; Song, K.-M.; Chun, Y.-G.; Lee, M.H.; Kim, B.-K.; Jeon, Y.-J. Anti-Obesity Effects of Sargassum Thunbergii via Downregulation of Adipogenesis Gene and Upregulation of Thermogenic Genes in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 3325. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Choi, J.; Lee, M.; Kwon, C.; Nam, T. Anti-Obesity Effects of Pectinase and Cellulase Enzyme-treated Eckloniaïcava Extract in High-fat Diet-fed C57BL/6N Mice. Int. J. Mol. Med. 2017, 41, 924–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, J.; Lee, Y. Anti-Inflammatory and Anti-Diabetic Effects of Brown Seaweeds in High-Fat Diet-Induced Obese Mice. Nutr. Res. Pract. 2016, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Hou, J.G.; Begum, S.; Wang, Y.B.; Oh, D.S.; Wi, A.J.; Yoon, B.-S.; Sung, C.-G. Anti-Obesity Effects of Sparassis Crispa on High-Fat Diet-Induced Obese Mice. J. Life Sci. 2014, 24, 952–958. [Google Scholar] [CrossRef] [Green Version]
- Han, L.-K.; Sumiyoshi, M.; Zhang, J.; Liu, M.-X.; Zhang, X.-F.; Zheng, Y.-N.; Okuda, H.; Kimura, Y. Anti-Obesity Action Of Salix Matsudana Leaves (Part 1). Anti-Obesity Action by Polyphenols of Salix Matsudana in High Fat-Diet Treated Rodent Animals. Phytother. Res. 2003, 17, 1188–1194. [Google Scholar] [CrossRef]
- Choi, E.H.; Yang, H.P.; Chun, H.S. Chitooligosaccharide Ameliorates Diet-Induced Obesity in Mice and Affects Adipose Gene Expression Involved in Adipogenesis and Inflammation. Nutr. Res. 2012, 32, 218–228. [Google Scholar] [CrossRef]
- Pan, H.; Yang, Q.; Huang, G.; Ding, C.; Cao, P.; Huang, L.; Xiao, T.; Guo, J.; Su, Z. Hypolipidemic Effects of Chitosan and Its Derivatives in Hyperlipidemic Rats Induced by a High-Fat Diet. Food Nutr. Res. 2016, 60, 31137. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Zhang, H.-L.; Hu, Y.-M.; Wan, S.; Su, Z.-Q. Preparation of Chitosan and Water-Soluble Chitosan Microspheres via Spray-Drying Method to Lower Blood Lipids in Rats Fed with High-Fat Diets. Int. J. Mol. Sci. 2013, 14, 4174–4184. [Google Scholar] [CrossRef]
- de Melo, C.L.; Queiroz, M.G.R.; Arruda Filho, A.C.V.; Rodrigues, A.M.; de Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic Acid, a Natural Pentacyclic Triterpenoid, Prevents Abdominal Fat Accumulation in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yang, H.; Seo, S.G.; Shin, S.H.; Chung, M.-Y.; Kim, J.; Lee, S.J.; Lee, H.J.; Lee, K.W. Cocoa Polyphenols Suppress Adipogenesis In Vitro and Obesity In Vivo by Targeting Insulin Receptor. Int. J. Obes. 2013, 37, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Jiang, W.; Thompson, H.J. Edible Dry Bean Consumption (Phaseolus Vulgaris L.) Modulates Cardiovascular Risk Factors and Diet-Induced Obesity in Rats and Mice. Br. J. Nutr. 2012, 108, S66–S73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.-H.; Nair, M.G. Amelioration of Obesity and Glucose Intolerance in High-Fat-Fed C57BL/6 Mice by Anthocyanins and Ursolic Acid in Cornelian Cherry (Cornus Mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, K.-M.; Liu, Q.; Kim, S.; Ji, H.-J.; Kim, M.; Shin, S.-K.; Do, S.-G.; Shin, E.; Jung, G.; et al. Anti-Obesity Effect of 6,8-Diprenylgenistein, an Isoflavonoid of Cudrania Tricuspidata Fruits in High-Fat Diet-Induced Obese Mice. Nutrients 2015, 7, 10480–10490. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Dietary Curcuminoids Prevent High-Fat Diet–Induced Lipid Accumulation in Rat Liver and Epididymal Adipose Tissue. J. Nutr. 2001, 131, 2932–2935. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Kwon, C.-S.; Sohn, H.Y.; Kim, S.H.; Kim, J.H.; Son, K.H.; Lee, J.S.; Lim, J.K.; Kim, J.-S. Anti-Obesity Effect of Dioscorea Nipponica Makino with Lipase-Inhibitory Activity in Rodents. Biosci. Biotechnol. Biochem. 2003, 67, 1451–1456. [Google Scholar] [CrossRef] [Green Version]
- Jeong, E.J.; Jegal, J.; Ahn, J.; Kim, J.; Yang, M.H. Anti-Obesity Effect of Dioscorea Oppositifolia Extract in High-Fat Diet-Induced Obese Mice and Its Chemical Characterization. Biol. Pharm. Bull. 2016, 39, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Belguith-Hadriche, O.; Ammar, S.; del Mar Contreras, M.; Turki, M.; Segura-Carretero, A.; El Feki, A.; Makni-Ayedi, F.; Bouaziz, M. Antihyperlipidemic and Antioxidant Activities of Edible Tunisian Ficus carica L. Fruits in High Fat Diet-Induced Hyperlipidemic Rats. Plant Foods Hum. Nutr. 2016, 71, 183–189. [Google Scholar] [CrossRef]
- Ibarra, A.; Bai, N.; He, K.; Bily, A.; Cases, J.; Roller, M.; Sang, S. Fraxinus Excelsior Seed Extract FraxiPureTM Limits Weight Gains and Hyperglycemia in High-Fat Diet-Induced Obese Mice. Phytomedicine 2011, 18, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.-Z.; Yu, X.-F.; Wang, H.-M.; Ren, Q.-Y.; Chen, B.-M. Anti-Obesity and Hypolipidemic Effects of Ethanolic Extract from Alpinia Officinarum Hance (Zingiberaceae) in Rats Fed High-Fat Diet. J. Med. Food 2010, 13, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, H.-R.; Kim, J.-H.; Om, A.-S. Beneficial Effects of Allium sativum L. Stem Extract on Lipid Metabolism and Antioxidant Status in Obese Mice Fed a High-Fat Diet: Beneficial Effects of Allium sativum L. Stem Extract in Obese Mice. J. Sci. Food Agric. 2013, 93, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger Prevents Obesity through Regulation of Energy Metabolism and Activation of Browning in High-Fat Diet-Induced Obese Mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef]
- Karu, N.; Reifen, R.; Kerem, Z. Weight Gain Reduction in Mice Fed Panax Ginseng Saponin, a Pancreatic Lipase Inhibitor. J. Agric. Food Chem. 2007, 55, 2824–2828. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Anandh Babu, P.V.; Zhang, W.; Gilbert, E.; Cline, M.; McMillan, R.; Hulver, M.; Alkhalidy, H.; Zhen, W.; et al. Dietary Supplementation of Chinese Ginseng Prevents Obesity and Metabolic Syndrome in High-Fat Diet-Fed Mice. J. Med. Food 2014, 17, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and Hypolipidemic Effect of Water Extract from Pleurotus Citrinopileatus in C57 BL /6J Mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Kadouh, H.; Zhou, K. Dietary Supplementation of Grape Skin Extract Improves Glycemia and Inflammation in Diet-Induced Obese Mice Fed a Western High Fat Diet. J. Agric. Food Chem. 2011, 59, 3035–3041. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Kim, M.S.; Park, C.M.; Rhyu, D.Y. Caulerpa Okamurae Extract Inhibits Adipogenesis in 3T3-L1 Adipocytes and Prevents High-Fat Diet–Induced Obesity in C57BL/6 Mice. Nutr. Res. 2017, 47, 44–52. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.H.; Oh, T.; Ahn, B.; Unno, T. Codium Fragile Ameliorates High-Fat Diet-Induced Metabolism by Modulating the Gut Microbiota in Mice. Nutrients 2020, 12, 1848. [Google Scholar] [CrossRef]
- Kim, H.-J.; Hong, S.-H.; Chang, S.-H.; Kim, S.; Lee, A.Y.; Jang, Y.; Davaadamdin, O.; Yu, K.-N.; Kim, J.-E.; Cho, M.-H. Effects of Feeding a Diet Containing Gymnema Sylvestre Extract: Attenuating Progression of Obesity in C57BL/6J Mice. Asian Pac. J. Trop. Med. 2016, 9, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zar Kalai, F.; Han, J.; Ksouri, R.; El Omri, A.; Abdelly, C.; Isoda, H. Antiobesity Effects of an Edible Halophyte Nitraria Retusa Forssk in 3T3-L1 Preadipocyte Differentiation and in C57B6J/L Mice Fed a High Fat Diet-Induced Obesity. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-Obesity Effect of Nelumbo Nucifera Leaves Extract in Mice and Rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, J.; Park, H.J.; Kim, H. Anti-Obesity Effects of a Prunus Persica and Nelumbo Nucifera Mixture in Mice Fed a High-Fat Diet. Nutrients 2020, 12, 3392. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-N.; Zhu, X.-M.; Han, L.-K.; Saito, M.; Sun, Y.-S.; Yoshikawa, M.; Kimura, Y.; Zheng, Y.-N. Anti-Obesity Effects of Escins Extracted from the Seeds of Aesculus Turbinata BLUME (Hippocastanaceae). Chem. Pharm. Bull. 2008, 56, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H.; Ogawa, S.; Katsube, T.; Jisaka, M.; Yokota, K. Antiobese Effects of Novel Saponins from Edible Seeds of Japanese Horse Chestnut (Aesculus Turbinata BLUME) after Treatment with Wood Ashes. J. Agric. Food Chem. 2008, 56, 4783–4788. [Google Scholar] [CrossRef]
- Aoe, S.; Kudo, H.; Sakurai, S. Effects of Liquid Konjac on Parameters Related to Obesity in Diet-Induced Obese Mice. Biosci. Biotechnol. Biochem. 2015, 79, 1141–1146. [Google Scholar] [CrossRef]
- Kim, J.H.; Hahm, D.H.; Yang, D.C.; Kim, J.H.; Lee, H.J.; Shim, I. Effect of Crude Saponin of Korean Red Ginseng on High-Fat Diet-Induced Obesity in the Rat. J. Pharmacol. Sci. 2005, 97, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Suruga, K.; Tomita, T.; Kadokura, K.; Arai, T. Rhus Verniciflua Leaf Extract Suppresses Obesity in High-Fat Diet-Induced Obese Mice. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Weidner, C.; Wowro, S.J.; Freiwald, A.; Kodelja, V.; Abdel-Aziz, H.; Kelber, O.; Sauer, S. Lemon Balm Extract Causes Potent Antihyperglycemic and Antihyperlipidemic Effects in Insulin-Resistant Obese Mice. Mol. Nutr. Food Res. 2014, 58, 903–907. [Google Scholar] [CrossRef]
- Alsaggar, M.; Bdour, S.; Ababneh, Q.; El-Elimat, T.; Qinna, N.; Alzoubi, K.H. Silibinin Attenuates Adipose Tissue Inflammation and Reverses Obesity and Its Complications in Diet-Induced Obesity Model in Mice. BMC Pharmacol. Toxicol. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.; Kim, B.; Park, H.; Lee, K.; Kim, H.-H.; Sim, H.-C.; Do, H.-J.; Hyun, C.-K.; Do, M.-S. Fermented Moringa Oleifera Decreases Hepatic Adiposity and Ameliorates Glucose Intolerance in High-Fat Diet-Induced Obese Mice. J. Med. Food 2017, 20, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Metwally, F.M.; Rashad, H.M.; Ahmed, H.H.; Mahmoud, A.A.; Abdol Raouf, E.R.; Abdalla, A.M. Molecular Mechanisms of the Anti-Obesity Potential Effect of Moringa Oleifera in the Experimental Model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221. [Google Scholar] [CrossRef]
- Gourineni, V.; Shay, N.F.; Chung, S.; Sandhu, A.K.; Gu, L. Muscadine Grape (Vitis Rotundifolia) and Wine Phytochemicals Prevented Obesity-Associated Metabolic Complications in C57BL/6J Mice. J. Agric. Food Chem. 2012, 60, 7674–7681. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.A.; Cho, J.-H.; Afinanisa, Q.; An, G.-H.; Han, J.-G.; Kang, H.J.; Choi, S.H.; Seong, H.-A. Ganoderma Lucidum Extract Reduces Insulin Resistance by Enhancing AMPK Activation in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 3338. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma Lucidum Reduces Obesity in Mice by Modulating the Composition of the Gut Microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Begum, S.; Oh, D.S.; Wee, A.J.; Yun, B.S.; Sung, C.K. Ameliorating Effect of Mycoleptodonoides Aitchisonii on High-Fat Diet-Induced Obese Mice. Prev. Nutr. Food Sci. 2014, 19, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Chi, Q.; Wang, G.; Sheng, Y.; Xu, W.; Shi, P.; Zhao, C.; Huang, K. Ethanolic Extract of the Golden Oyster Mushroom, Pleurotus Citrinopileatus (Agaricomycetes), Alleviates Metabolic Syndrome in Diet-Induced Obese Mice. Int. J. Med. Mushrooms 2017, 19, 1001–1008. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, X.; Liu, L.; Shen, J. Polyphenol-Rich Extracts from Oiltea Camellia Prevent Weight Gain in Obese Mice Fed a High-Fat Diet and Slowed the Accumulation of Triacylglycerols in 3T3-L1 Adipocytes. J. Funct. Foods 2014, 9, 148–155. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive Leaf Extract Attenuates Obesity in High-Fat Diet-Fed Mice by Modulating the Expression of Molecules Involved in Adipogenesis and Thermogenesis. Evid. Based Complement. Alternat. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Han, L.-K.; Takaku, T.; Li, J.; Kimura, Y.; Okuda, H. Anti-Obesity Action of Oolong Tea. Int. J. Obes. 1999, 23, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.-K.; Zheng, Y.-N.; Yoshikawa, M.; Okuda, H.; Kimura, Y. Anti-Obesity Effects of Chikusetsusaponins Isolated from Panax Japonicus Rhizomes. BMC Complement. Altern. Med. 2005, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-J.; Kim, H.K. Perilla Leaf Extract Ameliorates Obesity and Dyslipidemia Induced by High-Fat Diet: ANTIOBESITY EFFECT OF PERILLA LEAF EXTRACT. Phytother. Res. 2009, 23, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-K.; Xu, B.-J.; Kimura, Y.; Zheng, Y.; Okuda, H. Platycodi Radix Affects Lipid Metabolism in Mice with High Fat Diet–Induced Obesity. J. Nutr. 2000, 130, 2760–2764. [Google Scholar] [CrossRef] [Green Version]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of Anti-Obesity Effects of the Pomegranate Leaf Extract in High-Fat Diet Induced Obese Mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.H.; An, M.; Kim, J.-K.; Lim, Y.-H. Antiobesity Effect of Ethanolic Extract of Ramulus Mori in Differentiated 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kang, N.; Kim, S.-Y.; Lima, I.S.; Ko, S.-C.; Kim, Y.-T.; Kim, Y.-B.; Jeung, H.-D.; Choi, K.-S.; Jeon, Y.-J. Popular Edible Seaweed, Gelidium Amansii Prevents against Diet-Induced Obesity. Food Chem. Toxicol. 2016, 90, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Lee, H.-A.; Kim, H.-J.; Han, J.-S. Gelidium Amansii Extract Ameliorates Obesity by Down-Regulating Adipogenic Transcription Factors in Diet-Induced Obese Mice. Nutr. Res. Pract. 2017, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Bargallo, M.; Grau, A.; Fernandezlarrea, J.; Anguiano, G.; Segarra, M.; Rovira, M.; Ferre, L.; Olive, M. Moderate Red-Wine Consumption Partially Prevents Body Weight Gain in Rats Fed a Hyperlipidic Diet. J. Nutr. Biochem. 2006, 17, 139–142. [Google Scholar] [CrossRef]
- Villalpando-Arteaga, E.V.; Mendieta-Condado, E.; Esquivel-Solís, H.; Canales-Aguirre, A.A.; Gálvez-Gastélum, F.J.; Mateos-Díaz, J.C.; Rodríguez-González, J.A.; Márquez-Aguirre, A.L. Hibiscus sabdariffa L. Aqueous Extract Attenuates Hepatic Steatosis through down-Regulation of PPAR-γ and SREBP-1c in Diet-Induced Obese Mice. Food Funct. 2013, 4, 618. [Google Scholar] [CrossRef]
- Yan, K.; Wang, X.; Pan, H.; Wang, L.; Yang, H.; Liu, M.; Zhu, H.; Gong, F. Safflower Yellow and Its Main Component HSYA Alleviate Diet-Induced Obesity in Mice: Possible Involvement of the Increased Antioxidant Enzymes in Liver and Adipose Tissue. Front. Pharmacol. 2020, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Cho, I.-H.; Han, S.H.; Jeon, Y.-J.; Choi, J.-G.; Kim, J.-S.; Lee, J.-H. Antiobesity Effects of Salvia Plebeia R. Br. Extract in High-Fat Diet-Induced Obese Mice. J. Med. Food 2016, 19, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Kim, S.H.; Son, S.W.; Seo, J.; Jeong, T.B.; Kim, K.-M.; Jung, J.-C.; Jung, M.S.; Lee, Y.-H.; Jung, Y.-S. Germinated Soybean Embryo Extract Ameliorates Fatty Liver Injury in High-Fat Diet-Fed Obese Mice. Pharmaceuticals 2020, 13, 380. [Google Scholar] [CrossRef]
- Yamasaki, M.; Ogawa, T.; Wang, L.; Katsube, T.; Yamasaki, Y.; Sun, X.; Shiwaku, K. Anti-Obesity Effects of Hot Water Extract from Wasabi (Wasabia Japonica Matsum.) Leaves in Mice Fed High-Fat Diets. Nutr. Res. Pract. 2013, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Neyrinck, A.M.; Bindels, L.B.; De Backer, F.; Pachikian, B.D.; Cani, P.D.; Delzenne, N.M. Dietary Supplementation with Chitosan Derived from Mushrooms Changes Adipocytokine Profile in Diet-Induced Obese Mice, a Phenomenon Linked to Its Lipid-Lowering Action. Int. Immunopharmacol. 2009, 9, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-M.; Lee, S.-H.; Lee, D.-S.; You, M.-J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.-S.; Lee, Y.-J.; Ku, S.-K. Fermented Garlic Protects Diabetic, Obese Mice When Fed a High-Fat Diet by Antioxidant Effects. Nutr. Res. 2011, 31, 387–396. [Google Scholar] [CrossRef]
- Arçari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; Funck, A.; Pedrazzoli, J.; de Souza, M.F.F.; Saad, M.J.; Bastos, D.H.M.; Gambero, A.; et al. Antiobesity Effects of Yerba Maté Extract (Ilex Paraguariensis) in High-Fat Diet-Induced Obese Mice. Obesity 2009, 17, 2127–2133. [Google Scholar] [CrossRef] [Green Version]

| Food Product/Plant | Bioactive Compounds/Extraction Method | Strain | Dose and Treatment | Observed Effects | Reference |
|---|---|---|---|---|---|
| Abeliophyllum distichum | N.A. | C57BL/6 mice ♂ | 100 and 300 mg/kg/day (oral administration), for 8 weeks | ↓ B.w., | [57] |
| ↓ Lipid accumulation (liver and BAT) | |||||
| ↓ Expression of lipogenic genes (PPARγ, C/EBPα, ACC, and FAS) (at 300 mg/kg). | |||||
| ↑ Expression of p-AMPK and p-ACC (at 300 mg/kg) | |||||
| Acanthopanax senticosus | N.A. | C57BL/6J mice ♂ | 0.5 g/kg (oral administration), for 12 weeks | ↓ B.w. gain | [58] |
| ↓ Abdominal fat accumulation | |||||
| ↓ Liver TG accumulation | |||||
| ↓ LDL-cholesterol serum levels | |||||
| Acanthopanax sessiliflorus | Saponins | ICR mice ♀ | 1% and 0.5% (w/w; mixed with the diet), for 4 weeks | ↓ B.w. | [59] |
| American ginseng (Panax quinquefolium), stems and leaves | Saponins | ICR mice ♀ | 1% and 3% (w/w; mixed with the diet), for 8 weeks | ↓ Adipose tissue weight | [60] |
| ↓TG plasma levels | |||||
| Balloon flower (Platycodon grandiflorum) | Water extract | Sprague Dawley rats ♂ | 150 mg/kg/day (oral administration), for 7 weeks | ↓ B.w. gain | [61] |
| ↓ Food intake | |||||
| ↓ Subcutaneous adipose tissue weight | |||||
| ↓ Subcutaneous adipocytes size | |||||
| ↓ TC and TG plasma levels | |||||
| Bitter melon (Momordica charantia L.) | Ethanol extract | C57BL/6 mice ♂ | 250 and 500 mg/kg/day (mixed with the diet), for 12 weeks | ↓ B.w. | [62] |
| ↓ WAT weight | |||||
| ↑ Expression of SIRT-1 | |||||
| Suppressed PPARγ and SREBP-1 expressions of WAT | |||||
| Blackcurrant (Ribes nigrum) | 25% anthocyanins and 40% polyphenols | C57BL/6J mice ♂ | 0.1% (w/w; mixed with the diet), for 12 weeks | ↓ B.w., | [63] |
| ↓ Adipocyte size of the epididymal fat | |||||
| ↓ Inflammatory gene expression in the splenocytes (TNFα, IL-6, IL-1β) | |||||
| ↑ Gene expression in skeletal muscle (ACOX-1, PPARα, PPARδ, UCP-2, UCP-3, PGC-1α, TFAM) | |||||
| Black tea extract | Polyphenol fraction | C57BL/6N mice ♀ | 5% of extract (w/w; mixed with the diet), for 8 weeks | ↓ B.w., | [64] |
| ↓ Parametrial adipose tissue | |||||
| ↓ Liver lipid content | |||||
| Black wattle tree (Acacia meansii) | Acacia polyphenol | KKAy mice ♂ | 2.5% and 5% (w/w; mixed with the diet), for 7 weeks | ↓ B.w. | [65] |
| ↓ Glucose and insulin plasma levels | |||||
| ↓ mRNA expression of fat acid synthesis-related genes (SREBP-1c, ACC and FAS) in the liver | |||||
| ↓ mRNA expression of TNF-α in WAT | |||||
| ↑ mRNA expression of adiponectin in WAT | |||||
| ↑ mRNA expression of energy expenditure-related genes (PPARα, PPARδ, CPT1, ACOX and UCP-3) in skeletal muscle | |||||
| ↑ Protein expression of CPT1, ACOX and UCP-3 | |||||
| Broccoli florets and stalks (Brassica oleracea L. var. italica) | Aqueous extracts | Albino rats ♂ | 200 and 400 mg/kg/day (oral administration), for 1 month | ↓ B.w. gain | [66] |
| ↓ Adipose tissue index | |||||
| ↓ Serum levels of insulin, glucose, leptin, resistin and HOMA-IR index | |||||
| ↑ Adiponectin serum levels | |||||
| ↓ Serum levels of TC, TG and LDL-cholesterol | |||||
| ↑ HDL-cholesterol serum levels | |||||
| N.A. | C57BL/6J mice ♂ | 10% florets or 10% stalks (w/w; mixed with the diet), for 17 weeks | ↓ Serum insulin levels and HOMA-IR index (only florets) | [67] | |
| ↑ Adiponectin receptors 1 and 2 mRNA expression (only florets) | |||||
| Broccoli microgreens juice (Brassica oleracea L. var. italica) | N.A. | C57BL/6J mice ♂ | 20 g/kg/day (oral gavage), for 10 weeks | ↓ B.w. | [68] |
| ↓ WAT mass | |||||
| ↓ Liver fat | |||||
| ↓ Adipocyte size | |||||
| ↑ Water intake | |||||
| ↑ Glucose tolerance and insulin sensitivity | |||||
| ↓ Serum insulin levels, HOMA-IR index | |||||
| ↓ Serum levels of TG and LDL-cholesterol | |||||
| ↓ Serum levels of inflammatory cytokines (IL-1β, IL-6 and TNFα) | |||||
| Broccoli sprouts extract (Brassica oleracea L. var. italica) | Glucoraphanin-rich extract | C57BL/6JSlc mice ♂ | 0.3% (w/w; 2.2% extract powder mixed with the diet), for 14 weeks | ↓ B.w. gain | [69] |
| ↓ Fat mass | |||||
| ↓ Liver weight | |||||
| ↓ Hepatic steatosis | |||||
| ↓ Hepatic oxidative stress | |||||
| ↑ Energy expenditure and core body temperature | |||||
| ↑ Glucose tolerance and insulin sensitivity | |||||
| ↓ Plasma insulin levels and HOMA-IR index | |||||
| ↑ Insulin stimulated Akt phosphorylation on Ser473 in the liver, quadriceps muscle and epididymal WAT | |||||
| ↑ UCP-1 protein levels in epididymal and inguinal WAT | |||||
| Brown alga (Sargassum thunbergii) | Ethanol Extract | C57BL/6 mice ♂ | 100 and 300 mg/kg/day (oral administration), for 7 weeks | ↓ B.w. | [70] |
| ↓ WAT mass | |||||
| ↓ Occurrence of fatty liver | |||||
| ↓ Insulin, TG and TC serum levels | |||||
| ↓ Gene expression of PPARγ in WAT | |||||
| ↑ Expression of thermogenic genes (UCP-1 and UCP-3) in BAT | |||||
| Brown alga (Ecklonia cava) | N.A. | C57BL/6 mice ♂ | 5, 25 or 150 mg/kg/day (mixed with the diet), for 10 weeks | ↓ B.w. | [71] |
| ↓ Liver weight, | |||||
| ↓ Epididymal, perirenal and mesenteric WAT | |||||
| ↓ Insulin, leptin and glutamate pyruvate transaminase serum levels | |||||
| ↓ Serum and hepatic levels of TG | |||||
| ↓ Hepatic protein expression levels of C/ERPα, PPARy, SREBP-1c, A-FABP, FAS and leptin | |||||
| ↑ Hepatic protein expression levels of GLUT4 | |||||
| Brown alga Wakame (Undaria pinnatifida) | Fucoxanthin | C57BL/6J mice ♂ | 1.06% and 2.22% % (w/w; mixed with the diet), for 5 weeks | ↓ B.w. | [72] |
| ↓ WAT | |||||
| ↓ Plasma levels of insulin, glucose, leptin and LDL-cholesterol | |||||
| ↑ Plasma levels of TC | |||||
| ↓ mRNA expression of MCP-1 and leptin in WAT | |||||
| ↑ mRNA expression of Adrb3 in WAT | |||||
| ↑ mRNA expression of GLUT4 in skeletal muscle | |||||
| Brown alga: Undaria Pinnatifida (UP), Laminaria Japonica (LJ), Sargassum Fulvellum (SF), Hizikia Fusiforme (HF) | N.A. | C57BL/6N mice ♂ | 5% of freeze-dried UP, LJ, SF, or HF (w/w; mixed with the diet), for 16 weeks | ↓ Plasma levels of leptin | [73] |
| ↓ Plasma levels of adiponectin (for UP supplementation) | |||||
| ↓ Formation of CLS in gonadal adipose tissue | |||||
| ↓ Insulin resistance (for LJ supplementation) | |||||
| Cannabis sativa | Cannabinoid Δ9-tetrahydrocannabivarin | C57BL/6 mice ♀ | 0.3, 1, 2.5, 5 and 12.5 mg/kg twice daily (oral gavage), for 30 days | ↓ Glucose and insulin plasma levels for highest doses | [27] |
| Improvement of insulin sensitivity index for highest doses. | |||||
| Cauliflower mushroom (Sparassis crispa) | Lupane-type saponins | C57BL/6 mice ♂ | 1%, 3% and 5% (w/w; mixed with the diet), for 12 weeks | ↓ B.w. gain | [74] |
| ↓ Food intake | |||||
| ↓ FER | |||||
| ↓ Serum levels of TC and TG | |||||
| ↓ Liver lipids | |||||
| ↓ Occurrence of fatty liver deposits and steatosis | |||||
| Chinese willow dry leaves (Salix matsudana) | Polyphenol fraction | ICR mice ♂ | 2% and 5% (w/w; mixed with the diet), for 9 weeks | ↓ B.w. | [75] |
| ↓ Parametrial adipose tissue | |||||
| ↓ Adipocyte size | |||||
| ↓ Hepatic TC (at 5% concentration) | |||||
| Chitooligosaccharide | N.A. | C57BL/6N mice ♂ | 1% or 3% (w/w; mixed with the diet), for 5 months | ↓ B.w. gain (at 3% concentration) | [76] |
| ↓ Epididymal adipocyte size (at 3% concentration) | |||||
| ↓ Serum levels of TG and TC | |||||
| ↓ Hepatic levels of total lipid and TG (at 3% concentration) | |||||
| ↓ Serum levels of AST and ALT (at 3% concentration) | |||||
| Chitosan and water-soluble derivatives chitosan oligosaccharides | N.A. | Sprague Dawley rat ♂ | 250, 500 and 1000 mg/kg × d (w/w; mixed with the diet), for 6 weeks | ↓ B.w. gain | [77] |
| ↓ Serum levels of LDL-cholesterol, TC and TG | |||||
| ↓ Hepatic levels of TC, TG and TBA | |||||
| ↑ Fecal levels of TC, TG and TBA | |||||
| ↓ ALT and AST levels in serum and liver | |||||
| ↑ SOD levels in serum and liver | |||||
| ↓ Growth inhibition of subcutaneous and mesenteric WAT | |||||
| Relieved fatty liver | |||||
| ↑ Liver mRNA expression of PPARα and HL | |||||
| Chitosan (CTS) and water-soluble chitosan microspheres | N.A. | Sprague Dawley ♂ | 225 and 450 mg/kg/day (oral administration), for 4 weeks | ↓ B.w. gain | [78] |
| ↓ Blood lipids and plasma viscosity | |||||
| ↑ Serum levels of SOD | |||||
| Clusia nemorosa L. | Betulinic acid | Swiss mice ♂ | 50 mg/L (in drinking water), for 15 weeks | ↓ B.w. | [79] |
| ↓ Abdominal fat accumulation | |||||
| ↓ Plasma levels of glucose, TG and TC | |||||
| ↑ Plasma levels of insulin and leptin | |||||
| ↓ Plasma levels of amylase and ghrelin | |||||
| Cocoa powder (Theobroma cacao L.) | Cocoa polyphenol extract | C57BL/6N mice ♂ | 40 and 200 mg/kg (mixed with the diet), for 5 weeks | ↓ B.w. gain | [80] |
| ↓ Fat accumulation | |||||
| Common bean dried (Phaseolus vulgaris L.) | N.A. | C57BL/6J mice ♂ | 30% and 46.5% (w/w; mixed with the diet), for 7 or 12 days, respectively | ↓ B.w. and Lee Index | [81] |
| ↓ Plasma levels of TG, LDL-cholesterol | |||||
| Cornelian cherries (Cornus mas) | Anthocyanins | C57BL/6 mice ♂ | 1 g/kg (w/w; mixed with the diet), for 8 weeks | ↓ B.w., | [82] |
| Improvement of glucose tolerance | |||||
| ↓ Lipid accumulation in liver | |||||
| ↑ Plasma insulin levels | |||||
| Ursolic acid | C57BL/6 mice ♂ | 500 mg/kg (w/w; mixed with the diet), for 8 weeks | Improvement of glucose tolerance | [82] | |
| ↓ Lipid accumulation in liver | |||||
| ↑ Plasma insulin levels | |||||
| Cudrania tricuspidata fruits | 6,8-diprenylgenistein (DPG) (a major isoflavonoid) | C57BL/6J mice ♂ | 10 and 30 mg/mL (in distilled water) (oral administration of 10 mL/kg), for 6 weeks | ↓ B.w. gain | [83] |
| ↓ FER | |||||
| ↓ Epididymal fat weight | |||||
| ↓ Epididymal adipocyte size | |||||
| ↓ Liver fat accumulation and weight | |||||
| ↓ Serum levels of TC, TG, HDL-cholesterol, LDL-cholesterol, ALT and AST | |||||
| ↓ Protein levels of PPARγ, C/EBPα and leptin in adipose tissue | |||||
| ↑ Protein levels of adiponectin in adipose tissue | |||||
| ↑ Phosphorylation of AMPK and ACC | |||||
| Curcumin (Curcuma longa) | Curcuminoids | Sprague Dawley rat ♂ | 0.2% and 1% (w/w; mixed with the diet), for 2 weeks | ↓ Epididymal adipose tissue weight | [84] |
| ↓ Hepatic levels of TC and TG (at 1% concentration) | |||||
| ↑ Hepatic ACOX activity | |||||
| N.A. | C57BL/6 mice ♂ | 500 mg/kg (w/w; mixed with the diet), for 12 weeks | ↓ B.w. gain and body fat | [85] | |
| ↓ Liver weight and hepatic steatosis | |||||
| ↓ TC serum levels | |||||
| ↓ mRNA expression of VEGF and VEGFR-2 in adipose tissue | |||||
| ↓ Microvessel density in adipose tissue | |||||
| ↑ Phosphorylation of AMPK and ACC in adipose tissue | |||||
| ↑ mRNA expression of CPT-1 in adipose tissue | |||||
| ↓ mRNA expression of GPAT-1 in adipose tissue | |||||
| ↓ mRNA expression of PPARγ and C/EBPα in adipose tissue | |||||
| Dioscorea nipponica Makino | Methanol extract | Sprague Dawley ♂ | 2% or 5% (w/w; mixed with the diet), for 8 weeks | ↓ B.w. gain | [86] |
| ↓ Subcutaneous, perirenal and epididymal fat | |||||
| ↓ Plasma levels of TG, TC, VLDL-cholesterol and atherogenic index | |||||
| ↑ HDL-cholesterol plasma levels | |||||
| ↑ Fecal fat excretion | |||||
| Dioscorea oppositifolia | n-BuOH extract of D. oppositifolia | ICR mice ♀ | 100 mg/kg (oral administration), for 8 weeks | ↓ B.w. gain | [87] |
| ↓ FER | |||||
| ↓ Parametrial adipose tissue weight | |||||
| ↓ Liver weight | |||||
| ↓ Serum levels of TG, TC LDL-cholesterol and atherogenic index | |||||
| ↑ HDL (in serum) | |||||
| ↓ Hepatic levels of total lipids, TG, TC, AST and ALT | |||||
| ↑ Fecal excretion of TG, TC and total lipids | |||||
| Fig fruit (Ficus carica L.) | Aqueous-ethanolic extract | Wistar male ♂ | 400 mg/kg (w/w; mixed with the diet), for 8 weeks | ↓ Plasma levels of TC, TG and LDL-cholesterol | [88] |
| ↑ HDL-cholesterol plasma levels | |||||
| ↓ TBARS levels in liver, kidney and heart | |||||
| ↑ Antioxidant enzymes (GPx, SOD and CAT) in liver, kidney and heart | |||||
| Fraxinus excelsior L. seed extract (FraxiPureTM) | 1 phenolic compound, and 9 secoiridoid glucosides | C57BL/6J mice ♂ | 0.5% (w/w; mixed with the diet), for 16 weeks | ↓ B.w. gain | [89] |
| ↓ omental and retroperitoneal fat | |||||
| ↓ fasting blood glucose levels and plasma insulin levels | |||||
| ↓ Liver weight gain and incidence of fatty liver | |||||
| Galangal (Alpinia officinarum Hance) | Ethanolic extract | Sprague Dawley ♂ | 3% and 5% (w/w; mixed with the diet), for 6 weeks | ↓ B.w. gain | [90] |
| ↓ FER | |||||
| ↓ Adipose tissue weight | |||||
| ↓ Serum levels of TC, TG, LDL-cholesterol, atherogenic index, leptin and ALT | |||||
| ↑ HDL-cholesterol serum levels | |||||
| ↓ Hepatic levels of TC and TG | |||||
| Garlic (Allium sativum L.) | Chlorophyll, carotenoids and vitamin C | C57BL/6J mice ♂ | 100, 250 and 500 mg/kg/day (oral administration), for 4 weeks | ↓ B.w. gain | [91] |
| ↓ FER | |||||
| ↓ WAT weight and adipocyte size | |||||
| ↓ Serum levels of TC, TG and leptin | |||||
| ↓ Serum levels of fasting glucose, insulin and HOMA-IR | |||||
| ↑ Serum levels of high-molecular-weight adiponectin | |||||
| ↓ Hepatic levels of TC and TG | |||||
| ↑ Fecal TG excretion | |||||
| ↓ Hepatic FAS levels | |||||
| ↑ Hepatic CPT-1A levels | |||||
| ↓ HMG-CoA reductase activity | |||||
| ↑ Hepatic antioxidant enzyme activities (SOD, GST, GSH, GPx and GR) | |||||
| ↓ Hepatic MDA activity | |||||
| Ginger rhizomes (Zingiber officinale Roscoe) | N.A. | C57BL/6J mice ♂ | 500 mg/kg/day (oral gavage), for 16 weeks | ↓ B.w. | [92] |
| ↓ Fat accumulation | |||||
| ↓ Serum levels of glucose, TG and TC | |||||
| Enhancement of BAT function and activation of WAT browning | |||||
| Ginseng (Panax ginseng) | Saponins | Balb/c ♂ mice | 3% (w/w; mixed with the diet), for 3 weeks | ↓ B.w. | [93] |
| ↓ FER | |||||
| ↓ TG plasma levels | |||||
| N.A. | C57BL/6J mice ♂ | 0.5 g/kg (w/w; mixed with the diet), for 15 weeks | ↓ Body fat mass, | [94] | |
| ↑ Glucose tolerance and insulin sensitivity | |||||
| ↓ Plasma levels of TG, HDL-cholesterol, insulin and leptin | |||||
| ↑ Body temperature | |||||
| Prevented hypertension | |||||
| ↑ Fatty acid oxidation in liver | |||||
| ↑ mRNA expression of C/EBPa, PPARγ and FAS in adipose tissue | |||||
| Golden mushroom (Pleurotus citrinopileatus) | Water extract | C57BL/6J mice ♂ | 400 and 800 mg/kg/day (oral gavage), for 12 weeks | ↓ B.w. gain | [95] |
| ↓ Food intake | |||||
| ↓ Fat accumulation | |||||
| ↑ Glucose tolerance | |||||
| ↓ Serum levels of TG, TC, LDL-cholesterol, AST, nonesterified fatty acid and creatinine | |||||
| ↑ HDL-cholesterol serum levels | |||||
| Grape skin extract (Vitis aestivalis) | Phenolic compounds | C57BLK/6J mice ♂ | 250 mg/kg/day (mixed with the diet), for 12 weeks | ↑ B.w. | [96] |
| ↓ Fasting blood glucose and plasma CRP levels | |||||
| Green alga (Caulerpa okamurae) | Ethanolic extract | C57BL/6 mice ♂ | 250 mg/kg (oral gavage), for 10 weeks | ↓ B.w., | [97] |
| ↓ Fat weight | |||||
| ↓ Liver weight | |||||
| ↓ Plasma levels of FFA, TG, TC, glucose and insulin | |||||
| ↓ Hepatic levels of FFA, TG, TC, and total lipid | |||||
| ↓ PPARγ and C/EBPα protein levels in adipose tissue | |||||
| ↓ mRNA expression of FAS, SREBP-1c, ACC, and CD36 in adipose tissue | |||||
| Green alga (Codium fragile) | Ethanol Extract | C57BL/6 mice ♂ | 600 mg/kg/day (intragastric administration), for 12 weeks | ↓ B.w., | [98] |
| ↓ Size of adipocytes | |||||
| ↓ Serum levels of TC and glucose | |||||
| ↑ Abundance of Bacteroidetes species in the gut | |||||
| ↓ Abundance of Verrucomicrobia species in the gut | |||||
| Gymnema sylvestre | Methanol extract | C57BL/6 mice ♂ | 1 g/kg (w/w; mixed with the diet), for 4 weeks | ↓ B.w. | [99] |
| ↓ Abdominal and epididymal fat weight | |||||
| ↓ Serum levels of TC, TG, LDL-cholesterol, VLDL-cholesterol, leptin, AST and ALT | |||||
| ↓ Occurrence of hepatic steatosis | |||||
| Halophyte (Nitraria retusa) | Ethanol extract | C57BL/6 mice ♂ | 50 and 100 mg/kg/day (oral administration), for 4 weeks | ↓ B.w. gain | [100] |
| ↓ FER | |||||
| ↓ Adipose tissue weight | |||||
| ↓ Serum levels of TG and glucose | |||||
| ↑ HDL-cholesterol | |||||
| ↑ Hepatic mRNA expression of PPARγ1, PPARα, ACC-1, CPT1 and LPL | |||||
| ↓ Hepatic mRNA expression of FAS | |||||
| Indian lotus leaves extract (Nelumbo nucifera Gaertn.) | Alcoholic extract | ICR mice ♀ | 5% (w/w; mixed with the diet), for 5 weeks | ↓ B.w. | [101] |
| ↓ Parametrial adipose tissue weight | |||||
| ↓ Hepatic TG levels | |||||
| Indian lotus (Nelumbo nucifera) and Peach tree (Prunus persica) mixture | N.A. | C57BL/6 mice ♂ | 0.1%, 0.2% and 0.4% (w/w; mixed with the diet), for 12 weeks | ↓ B.w. gain | [102] |
| ↓ Abdominal fat weight | |||||
| ↓ Liver weight | |||||
| ↓ Hepatic levels of TG and TC | |||||
| ↓ Serum levels of glucose, TC, ALT, AST and leptin | |||||
| ↑ Adiponectin serum levels | |||||
| ↑ AST/ALT and adiponectin/leptin ratios | |||||
| ↓ mRNA levels of FAS and SCD-1 in adipose tissue (at 0.4% concentration) | |||||
| ↑ mRNA levels of PGC-1a and PPARα in adipose tissue (at 0.4% concentration) | |||||
| Japanese Horse Chestnut (Aesculus turbinata BLUME) | Escins (saponin) | ICR mice ♀ | 0.35%, 1% and 2% (w/w; mixed with the diet), for 11 weeks | ↓ Parametrial adipose tissue (at 2% concentration) | [103] |
| ↓ Hepatic levels of TG | |||||
| ↑ TG fecal excretion (at 2% concentration) | |||||
| Saponins | ICR mice ♀ | 0.1% and 0.5% (w/w; mixed with the diet), for 8 weeks | ↓ B.w. | [104] | |
| ↓ Peritoneal adipose tissues | |||||
| ↓ TG plasma levels | |||||
| ↓ GOT activity | |||||
| ↑ TG fecal excretion | |||||
| Konjac (Amorphophallus konjac) | Liquid konjac | C57BL/6J mice ♂ | 2.5% and 5% (w/w; mixed with the diet), for 80 days | ↓ B.w. gain | [105] |
| ↓ FER | |||||
| ↓ Abdominal fat accumulation | |||||
| ↓ Liver weight | |||||
| ↓ Serum levels of TC, leptin, insulin and HOMA-IR | |||||
| ↓ Hepatic levels of TC and TG | |||||
| ↑ Fecal fat excretion | |||||
| Korean red ginseng (Ginseng Radix Rubra) | Crude saponin | Sprague Dawley rats ♂ | 200 mg/kg/day (intraperitoneal administration), for 3 weeks | ↓ B.w. | [106] |
| ↓ Food intake | |||||
| ↓ Fat weight | |||||
| ↓ Serum leptin levels | |||||
| ↓ Expression of NPY neurons in the hypothalamus | |||||
| Lacquer tree leaf extract (Rhus verniciflua) | Quercetin | C57BL/6 mice ♂ | 1% and 2% (w/w; mixed with the diet), for 56 days | ↓ B.w. | [107] |
| ↓ Intra-abdominal fat | |||||
| ↓ Plasma leptin levels | |||||
| Lemon balm (Melissa officinalis) | Ethanol extract | C57BL/6J mice ♂ | 200 mg/kg/day (in drinking water), for 6 weeks | ↓ Hyperglycemia and insulin resistance | [108] |
| ↓ Plasma levels of TG, nonesterified fatty acids and LDL/VLDL cholesterol ratio | |||||
| ↑ HDL/LDL cholesterol ratio | |||||
| hyperglycemia | |||||
| and insulin resistance, | |||||
| hyperglycemia | |||||
| and insulin resistance, | |||||
| hyperglycemia | |||||
| and insulin resistance | |||||
| Milk thistle seeds extract (Silybum marianum) | Silibinin | C57BL/6 mice ♂ | 50 mg/kg (intraperitoneal injection), for 8 weeks | ↓ B.w. gain | [109] |
| ↓ Fat accumulation in liver | |||||
| ↓ Fat accumulation and adipose tissue hypertrophy | |||||
| Reversed gene expression profile from pro-inflammatory to anti-inflammatory profile | |||||
| Moringa oleifera L. | N.A. | C57BL/6J mice ♂ | 250 mg/kg/day (oral administration), for 10 weeks | ↓ Liver weight and hepatic lipid accumulation | [110] |
| ↑ Glucose tolerance | |||||
| ↓ Oxidative stress, endoplasmic reticulum stress and lipotoxicity in quadriceps muscles | |||||
| ↓ Hepatic expression of genes involved in lipid synthesis (ACC, FAS, LPL and SREBP-1c) | |||||
| ↑ Hepatic genes involved in lipid oxidation (CD36 and ATGL) | |||||
| ↓ Proinflammatory cytokine mRNA expression (TNF-α, IL-1β, IL-6, IL-12 and MCP-1) in the liver, epididymal adipose tissue, and quadriceps | |||||
| Ethanolic extract | Wistar rats ♀ | 600 mg/kg/day (oral administration), for 12 weeks | ↓ B.w., | [111] | |
| Improvement of the atherogenic index and coronary artery index | |||||
| ↓ Serum glucose levels and insulin resistance | |||||
| ↓ mRNA expression of leptin and resistin in adipose tissue | |||||
| ↑ mRNA expression of adiponectin in adipose tissue | |||||
| ↓ Hepatic levels of AST and ALT | |||||
| Muscadine wine phytochemical and muscadine grape phytochemical (Vitis rotundifolia) | Anthocyanins | C57BL/6J mice ♂ | 0.4% (w/w; mixed with the diet) of each phytochemical, for 15 weeks | ↓ B.w. | [112] |
| ↑ Glucose tolerance | |||||
| ↓ Plasma levels of FFA, TG, TC, CRP | |||||
| Mushroom (Ganoderma lucidum) | Ethanolic extract | Specific pathogen free (SPF) C57BL/6 mice ♂ | 1%, 3% or 5% (w/w; mixed with the diet), for 12 weeks | ↓ B.w. gain | [113] |
| ↓ FER | |||||
| ↓ WAT weight | |||||
| ↓ Size of adipocytes in epidydimal WAT | |||||
| ↓ Liver weight | |||||
| ↓ Serum levels of TG, TC, HDL-cholesterol, LDL-cholesterol and FFA | |||||
| ↓ Serum levels of glucose, insulin and leptin | |||||
| ↑ Serum adiponectin levels | |||||
| ↑ Glucose tolerance and insulin sensitivity | |||||
| ↓ mRNA expression of lipogenic genes (FAS, SCD1 and SREBP-1c) in liver and WAT | |||||
| Water extract | C57BL/6NCrlBltw ♂ | 2%, 4% and 8% (w/v; oral gavage of 100 µL, daily), for 8 weeks | ↓ B.w., | [114] | |
| ↓ Epididymal and subcutaneous fat | |||||
| ↓ Liver weight | |||||
| ↓ mRNA expression levels pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and PAI-1) in the liver and adipose tissues | |||||
| ↑ mRNA expression levels of IL-10 in the liver and adipose tissues | |||||
| Mushroom (Mycoleptodonoides aitchisonii) | N.A. | C57BL/6 mice ♂ | 1%, 3% and 5% (w/w; mixed with the diet), for 12 weeks | ↓ B.w. gain | [115] |
| ↓ Food intake | |||||
| ↓ FER | |||||
| ↓ Adipose tissue weight | |||||
| ↓ Serum levels of TC and TG | |||||
| ↓ Hepatic lipid and TC levels | |||||
| ↓ Occurrence of fatty liver deposits and steatosis | |||||
| ↓ Epididymal adipocyte size | |||||
| Mushroom (Pleurotus citrinopileatus) | Ethanolic extract | C57BL/6J mice ♂ | 200 and 500 mg/kg, for 12 weeks | ↓ B.w. gain | [116] |
| ↓ Fat accumulation | |||||
| ↑ Glucose tolerance | |||||
| Oiltea camellia | Ethanolic extract | ICR mice ♀ | 100, 200 and 300 mg/kg (oral administration), for 4 weeks | ↓ B.w. | [117] |
| ↓ Fat accumulation | |||||
| ↓ Serum levels of TC and TG | |||||
| ↑ HDL-cholesterol serum levels | |||||
| ↓ Hepatic FAS activity | |||||
| Olive leaf extract (Olea europaea L.) | Ethanolic extract | C57BL/6N mice ♂ | 0.15% (w/w; mixed with the diet), for 8 weeks | ↓ B.w. gain | [118] |
| ↓ Food intake | |||||
| ↓ FER | |||||
| ↓ Visceral fat-pad weights | |||||
| ↓ Plasma levels of glucose and leptin | |||||
| ↓ Plasma levels of TG, TC, LDL + VLDL cholesterol and FFA | |||||
| ↓ Gene expression of PPARγ, C/EBPα, CD36, FAS, and leptin in the epididymal adipose tissue | |||||
| Oolong tea dry leaf (Thea sinensis L.) | Caffeine | ICR mice ♀ | 5% (w/w; mixed with the diet), for 10 weeks | ↓ B.w. | [119] |
| ↓ Parametrial adipose tissue | |||||
| ↓ Accumulation of liver TG | |||||
| Panax japonicus rhizomes | Chikusetsusaponins | ICR mice ♀ | 1% and 3% (w/w; mixed with the diet), for 9 weeks | ↓ B.w. | [120] |
| ↓ Parametrial adipose tissue weight | |||||
| ↓ Liver weight | |||||
| ↓ Hepatic TG levels | |||||
| ↑ Feces weight and TG fecal excretion | |||||
| Perilla leaf extract (Perilla frutescens L.) | Ethanolic extract | C57BL/6 mice ♀ | 1% and 3% (w/w; mixed with the diet), for 4 weeks | ↓ B.w. gain | [121] |
| ↓ FER | |||||
| ↓ Epididymal fat mass | |||||
| ↓ Liver weight | |||||
| ↓ Occurrence of hepatic steatosis | |||||
| ↓ Plasma levels of TG, TC and LDL-cholesterol | |||||
| ↑ HDL-cholesterol plasma levels | |||||
| ↓ Gene expression of ACC, GPDH and PPARγ in epididymal adipose tissue | |||||
| Platycodi radix (Platycodon grandiflorum) | Saponins | ICR mice ♀ | 5% (w/w; mixed with the diet), for 8 weeks | ↓ B.w., | [122] |
| ↓ Parametrial adipose tissue weight | |||||
| ↓ Hepatic TG levels | |||||
| Pomegranate leaf extract (Punica granatum) | 10.6% ellagic acid | ICR mice ♀ and ♂ | 400 and 800 mg/kg/day (oral gavage), for 5 weeks | ↓ B.w. | [123] |
| ↓ Energy intake | |||||
| ↓ Adipose pad weight percents and Lee index | |||||
| ↓ Serum levels of glucose, TC, TG and TC/HDL-cholesterol ratio | |||||
| Ramulus mori (the twig of Morus alba L.) | Ethanolic extract | C57BL/6 mice ♂ | 20, 50 and 100 mg/kg/day (oral administration), for 7 weeks | ↓ B.w. | [124] |
| ↓ Epididymal adipose tissue weight | |||||
| ↓ Liver weight | |||||
| ↓ Lipid accumulation in the liver | |||||
| ↓ Serum levels of TC and TG | |||||
| ↓ mRNA expression and protein levels of PPARγ, C/EBPα, SREBP-1, ACC, FAS and SCD-1 | |||||
| ↑ mRNA expression and protein levels of lipolytic genes (ATGL and HSL) | |||||
| Red alga (Gelidium amansii) | 70% ethanol extract | C57BL/6 mice ♂ | 1% and 3% (w/w; mixed with the diet), for 12 weeks | ↓ B.w. | [125] |
| ↑ Food and water intake | |||||
| ↓ Epididymal fat weight | |||||
| ↓ Serum levels of TC, TG, glucose and insulin | |||||
| Ethanolic extract | C57BL/6J mice ♂ | 0.5%, 1% and 2% (w/w; mixed with the diet), for 8 weeks | ↓ B.w. | [126] | |
| ↓ Epididymal and mesenteric adipose tissue weight | |||||
| ↓ Liver weight | |||||
| ↓ Plasma levels of TC, TG, LDL-cholesterol, FFA, and leptin | |||||
| ↑ Plasma levels of HDL-cholesterol and adiponectin | |||||
| ↓ Hepatic TC and TG levels | |||||
| ↓ Protein expression of FAS, SREBP-1c, PPARγ, and C/EBPα | |||||
| ↑ Protein expression of HSL and p-AMPK | |||||
| Red wine | 2.09 g/L total polyphenol | Zucker lean rats ♂ | Free access to red table wine (average consumption of 1.70 ± 0.38 mL/day per animal, corresponding to a dose of 3.4 ± 0.79 mg/day per animal of total polyphenols) | ↓ B.w. gain | [127] |
| ↓ Energy intake | |||||
| ↓ Epididymal fat weight | |||||
| Roselle (Hibiscus sabdariffa L.) | Anthocyanins (delphinidin-3-sambubioside and cyanidin-3-sambubioside) | C57BL/6NHsd mice ♂ | 33 mg/kg (oral gavage) three times a week, for 8 weeks | ↓ B.w. gain | [128] |
| ↓ WAT accumulation | |||||
| ↓ Serum levels of TG, LDL-cholesterol and glucose | |||||
| ↑ HDL-cholesterol serum levels | |||||
| ↓ Hepatic steatosis | |||||
| ↓ Hepatic mRNA levels of SREBP-1c, PPARγ, TNF-α and IL-1 | |||||
| ↑ Hepatic CAT mRNA expression | |||||
| Safflower (Carthamus tinctorius L.) | Safflower yellow (SY) and hydroxysafflor yellow A (HSYA) | C57BL/6 mice ♂ | 200 mg/kg/day SY or HSYA (intraperitoneal injection), for 10 weeks | ↓ B.w. | [129] |
| ↓ WAT mass | |||||
| ↓ Blood glucose levels and HOMA-IR | |||||
| ↓ Serum ALT levels | |||||
| ↑ Hepatic SOD activity | |||||
| ↑ mRNA levels of antioxidant enzymes in liver and epididymal adipose tissues | |||||
| Salvia plebeian | Ethanolic extract | C57BL/6 mice ♂ | 200 and 400 mg/kg/day (oral administration), for 8 weeks | ↓ B.w. | [130] |
| ↓ Adipose tissue weight | |||||
| ↓ Liver weight | |||||
| ↓ Hepatic steatosis, TG accumulation and inflammatory cells infiltration | |||||
| ↓ Serum levels of TG, HDL-cholesterol, leptin, adiponectin and glucose | |||||
| ↓ Adipocytes size in adipose tissue | |||||
| ↓ Expression of adipogenesis transcription factors and lipogenesis-related target genes in adipose tissue | |||||
| Soybean (Glycine max (L.) Merrill) | Ethanolic extract (soyasaponin Ab) | C57BL/6 mice ♂ | 15 and 45 mg/10 mL/kg/day (oral administration), for 10 weeks | ↓ B.w. | [131] |
| ↓ Adipose tissue weight | |||||
| ↓ Liver weight and steatosis | |||||
| ↓ Serum levels of TC, TG, FFA, LDL-cholesterol, AST and ALT | |||||
| ↓ Hepatic lipid synthesis (SREBP1c) | |||||
| ↑ Hepatic fatty acid oxidation (p-AMPKα, PPARα, PGC1α, and ACOX) and lipid export (MTTP and ApoB) | |||||
| ↓ Expression of inflammatory genes (TNFα, IL-6, IL-1β, iNOS, COX2, CD14 and F4/80) in liver | |||||
| ↓ WAT differentiation and lipogenesis (PPARγ, C/EBPα, and FAS) | |||||
| ↑ Browning genes (PGC1α, PRDM16, CIDEA, and UCP1) in adipose tissue | |||||
| Wasabi leaf (Wasabia japonica Matsum.) | Water extract | C57J/BL mice ♂ | 5% (w/w; mixed with the diet), for 163 days | ↓ B.w. gain | [132] |
| ↓ Liver weight | |||||
| ↓ Epididymal WAT | |||||
| ↓ Plasma levels of TC, leptin and γ-GTP | |||||
| ↓ Gene expression of PPARγ, leptin and C/EBPα in WAT | |||||
| ↑ Gene expression of adiponectin and ACOX1 in WAT | |||||
| ↓ Gene expression of PPARγ, SREBP-1c, ACC1, FAS and HMG-CoA reductase in liver | |||||
| ↑ PPARα gene expression in liver | |||||
| White mushroom exoskeleton (Agaricus bisporus) | Chitosan | C57BL/6J mice ♂ | 5% (w/w; mixed with the diet), for 10 weeks | ↓ B.w. gain | [133] |
| ↓ FER | |||||
| ↓ Adipose tissue weight | |||||
| ↓ Fat accumulation in liver and muscle | |||||
| ↓ TG levels in serum, liver and muscle | |||||
| ↓ TC levels in serum and muscle | |||||
| ↓ Serum levels of IL-6, leptin, resistin, insulin | |||||
| ↓ FIAF mRNA expression in visceral adipose tissue | |||||
| ↑ Caecal tissue weight and content weight | |||||
| ↑ Caecal total lipids and nonesterified fatty acid levels | |||||
| ↑ β-hydroxybutyrate plasma levels in postprandial state | |||||
| Yeast (Saccharomyces cerevisiae)-fermented aged black garlic | N.A. | ICR mice ♀ | 100, 200 and 400 mg/kg (10 mL/kg; oral administration), for 63 days | ↓ B.w. | [134] |
| ↓ Adipose tissue accumulation and adipocytes diameter | |||||
| ↓ Serum levels of TC, TG, LDL-cholesterol, AST, ALT, BUN and creatinine | |||||
| ↑ HDL-cholesterol serum levels | |||||
| ↓ Hepatic steatosis and hepatocyte hypertrophy | |||||
| ↓ Number of abnormal kidney tubules | |||||
| Yerba maté extract (Ilex paraguariensis) | Water extract | Swiss mice ♂ | 1 g/kg (oral gavage), for 8 weeks | ↓ B.w. | [135] |
| ↓ Epididymal fat weight | |||||
| ↓ Serum levels of TC, TG, LDL-cholesterol and glucose | |||||
| ↓ Expression levels of cytokines (TNF-α, IL-6 and leptin) and chemoattractant proteins (CCR2 and CCL2) in WAT | |||||
| ↓ Expression of genes involved in the regulation of blood pressure, vascular homeostasis or angiogenesis (angiotensinogen and PAI-1) in WAT | |||||
| ↑ Expression of genes involved in adipogenesis (PPARγ) and glucose and lipid metabolism (adiponectin) in WAT | |||||
| ↑ Expression of genes implicated in thermogenesis (PGC-1α and UCP-1) in BAT | |||||
| ↓ Macrophage infiltration marker (F4/80) in epididymal fat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, T.; Ferreira, T.; Nascimento-Gonçalves, E.; Castro-Ribeiro, C.; Lemos, S.; Rosa, E.; Antunes, L.M.; Oliveira, P.A. Obesity Rodent Models Applied to Research with Food Products and Natural Compounds. Obesities 2022, 2, 171-204. https://doi.org/10.3390/obesities2020015
Martins T, Ferreira T, Nascimento-Gonçalves E, Castro-Ribeiro C, Lemos S, Rosa E, Antunes LM, Oliveira PA. Obesity Rodent Models Applied to Research with Food Products and Natural Compounds. Obesities. 2022; 2(2):171-204. https://doi.org/10.3390/obesities2020015
Chicago/Turabian StyleMartins, Tânia, Tiago Ferreira, Elisabete Nascimento-Gonçalves, Catarina Castro-Ribeiro, Sílvia Lemos, Eduardo Rosa, Luís Miguel Antunes, and Paula Alexandra Oliveira. 2022. "Obesity Rodent Models Applied to Research with Food Products and Natural Compounds" Obesities 2, no. 2: 171-204. https://doi.org/10.3390/obesities2020015
APA StyleMartins, T., Ferreira, T., Nascimento-Gonçalves, E., Castro-Ribeiro, C., Lemos, S., Rosa, E., Antunes, L. M., & Oliveira, P. A. (2022). Obesity Rodent Models Applied to Research with Food Products and Natural Compounds. Obesities, 2(2), 171-204. https://doi.org/10.3390/obesities2020015







