Feasibility of Using Rainwater for Hydrogen Production via Electrolysis: Experimental Evaluation and Ionic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Water Sources
2.1.1. Research Methodology
2.1.2. Deionized Water
2.1.3. Rainwater
- Nitrate Standard for IC, 1000 mg/L, 100 mL, catalog no. 74246—from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), used for nitrate identification.
- Sulfate Standard for IC, 1000 mg/L, 100 mL, catalog no. 90071—from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), used for sulfate identification.
- Fluoride Standard for IC, 1000 mg/L, 100 mL, catalog no. 77365—from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), used for fluoride identification.
- Chloride Standard for IC, 1000 mg/L, 100 mL, catalog no. 39883—from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), used for chloride identification.
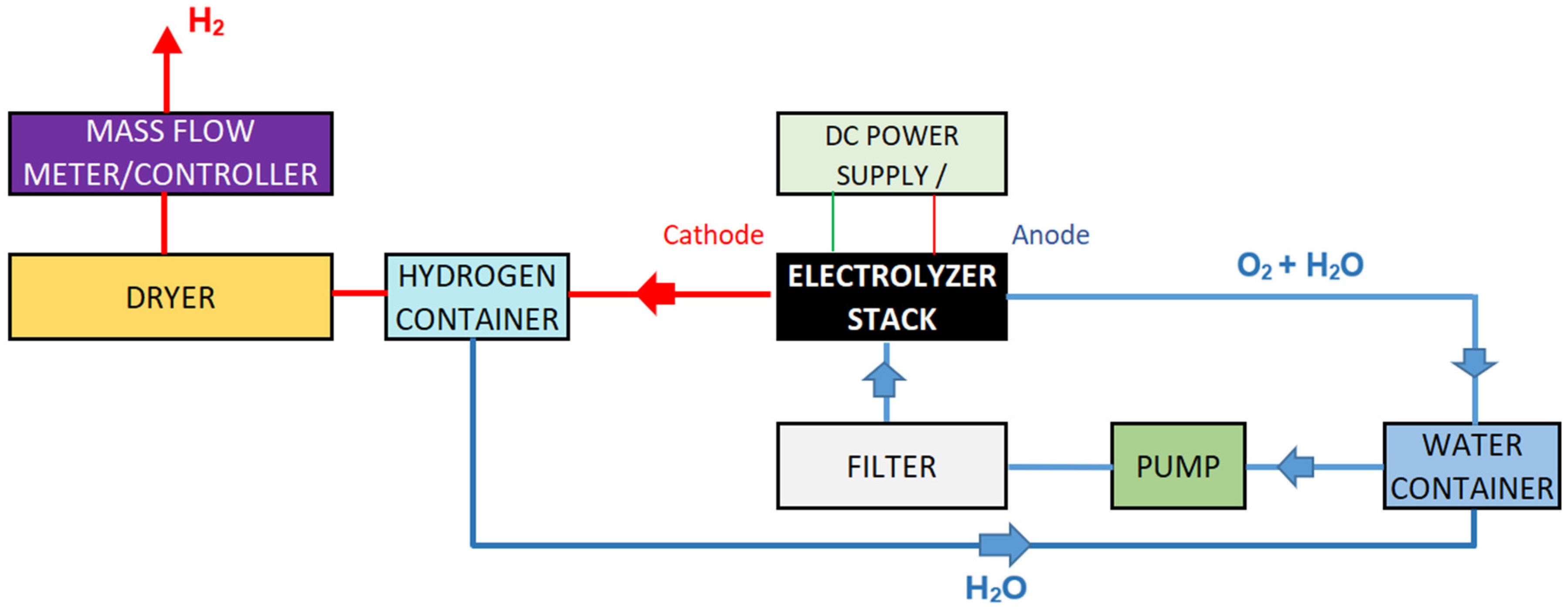
2.2. The Electrolyzer System
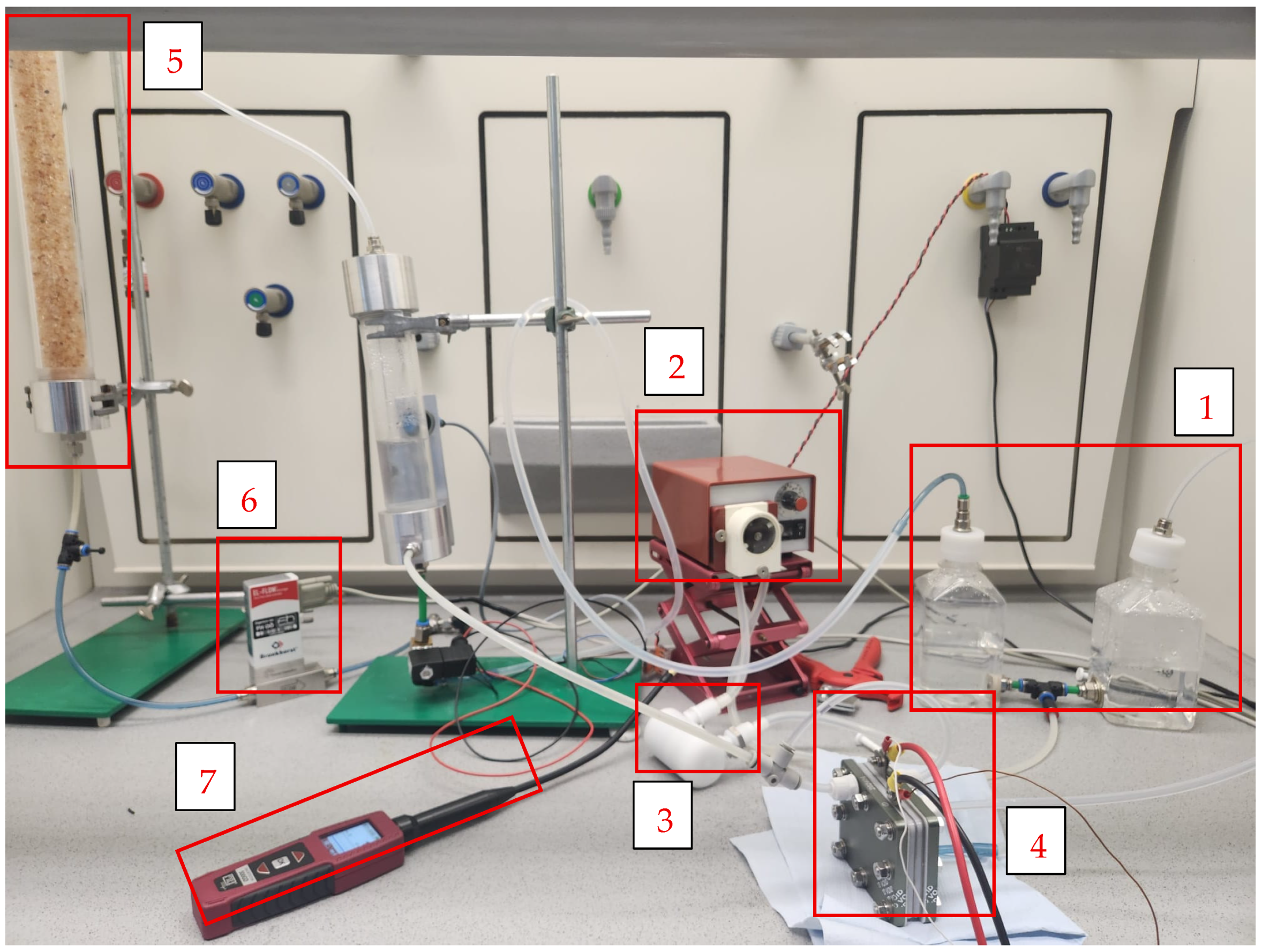
- Water Tank (1): The water tank serves as the primary reservoir for the electrolysis process, storing either deionized or rainwater depending on the test. It ensures a consistent water supply to the PEM electrolyzer, acting as the initial stage of the system’s circulation loop. Made of polyethylene, the tank offers durability and chemical resistance necessary for maintaining water purity throughout the process. Its role is not limited to storage; it also supports the return of unused or condensed water from the recirculation line, promoting efficiency and reducing water consumption during repeated testing cycles.
- Pump (2): The pump is responsible for maintaining a steady and controlled flow of water from the tank through the filter and into the PEM stack. It plays a crucial role in maintaining system performance, especially during variations in load or water quality. In this setup, with the aim of guaranteed hydration, heat dissipation, and recirculation needs of the system, a peristaltic pump with a rated flow of 300 mL/min was used.
- Filter (3): Positioned between the water tank and the electrolyzer, the filter safeguards the PEM stack by removing particulates and potential ionic contaminants. The filter used in this setup is made of Acetal Homopolymer with NBR (nitrile butadiene rubber) seals, possessing an internal volume of 30 cm3, a maximum flow capacity of 2 L/min and has a particulate retention of less than 10 microns. Operating effectively between 5 °C and 50 °C, it ensures that no damaging impurities reach the sensitive membrane and catalyst layers of the electrolyzer. This is particularly important when testing with rainwater, which may contain residual organic or inorganic matter even after pre-filtration.
- PEM Stack (Electrolyzer): The PEM stack (4) is the central component where the electrochemical reaction occurs, splitting water into hydrogen and oxygen gases. The system comprises two electrolysis cells, each requiring 1.8–2.2 V for operation, and is equipped with Nafion 115 membrane (DuPont, Wilmington, DE, USA). This configuration allows for hydrogen production rates between 300 and 400 mL/min. Regarding electrocatalysts, the configuration utilize platinum-based cathodes and iridium-based anodes—optimized for HER and OER, respectively. The PEM stack must remain free from contamination to preserve high proton conductivity and membrane integrity, making the upstream water purification stages critically important.
- Recirculation Line: The recirculation line is designed to return unused water from the electrolyzer back to the tank, minimizing waste and improving overall system efficiency. It also recovers water vapor that is transported along with the generated hydrogen gas. This closed-loop setup allows the same volume of water to be used repeatedly, significantly lowering consumption and ensuring consistent input quality. In rainwater scenarios, where water conservation is part of the sustainable objective, the recirculation line is especially valuable.
- Dryer (5): After electrolysis, the produced hydrogen gas may carry residual moisture. The dryer removes this moisture to ensure the hydrogen output is dry and of higher purity. It functions by passing the humid hydrogen through a bed of silica gel (desiccant material), which absorbs water molecules onto its porous surface. This absorption process reduces the water vapor content in the gas stream, achieving lower dew point. This step is vital for protecting downstream measurement equipment and ensuring accurate quantification of hydrogen production. Additionally, dry hydrogen is preferable in real-world applications like fuel cells, where water content can interfere with performance and safety.
- DC Power Supply: The DC power unit delivers the necessary electrical input to initiate and sustain the electrolysis reaction. Operating in a controlled mode, it delivers up to 4.2 V while the current is gradually increased to a maximum of 26 A during testing. Voltage and current regulation are essential to manage the electrolyzer’s performance curve and efficiency. The stability of this power source ensures reproducible testing conditions across all water types and operating scenarios.
- Control Unit: The control unit acts as the central management system for the electrolyzer setup. FlowSuite software, version 2.91 (Bronkhorst High-Tech B.V., Ruurlo, The Netherlands) was used to monitor operational parameters such as voltage, current, flow rates, and safety conditions. By monitoring these parameters, the control unit maintains efficient operation and safeguards the system from potential harm. It also contributes significantly to data collection, transmitting performance indicators to the FlowSuite software responsible for analyzing results and generating efficiency curves. The complementary activities of the control system were carried out manually, setting the pump and the input power manually.
- Mass Flow Controller (MFC): The selected MFC (6), EL-FLOW Prestige FG-111BP (Bronkhorst High-Tech B.V., Ruurlo, The Netherlands), Bronkhorst monitors and controls the hydrogen gas output rate during operation. It allows real-time monitoring of gas output and ensures that hydrogen production data is consistent and reliable. By integrating directly with the control unit and FlowSuite data logging software, version 2.91, the MFC enables precise calculation of electrolyzer efficiency, correlating gas output to electrical input during performance testing.
- Gas Leak Detector (7): Given the flammable nature of hydrogen, the system includes a gas leak detector as a safety mechanism, 500GD Multi-Gas Detector (MRU Instruments, Neckarsulm, Germany), which was installed in the experimental room near the electrolyzer. The detector monitors any unintended hydrogen release and if a leak is detected, it allows operators to shut down the system immediately, preventing possible hazards. Its inclusion was particularly important since the experiments were conducted in confined indoor spaces.
2.3. Overview of Conducted Tests
- How do the physical and chemical characteristics of rainwater compare to those of deionized water?
- Does rainwater influence the electrolysis system efficiency and hydrogen output differently than deionized water?
Electrolyzer Performance Evaluation
3. Results
3.1. Water Quality and Composition
Ionic Composition, Organic Content and pH
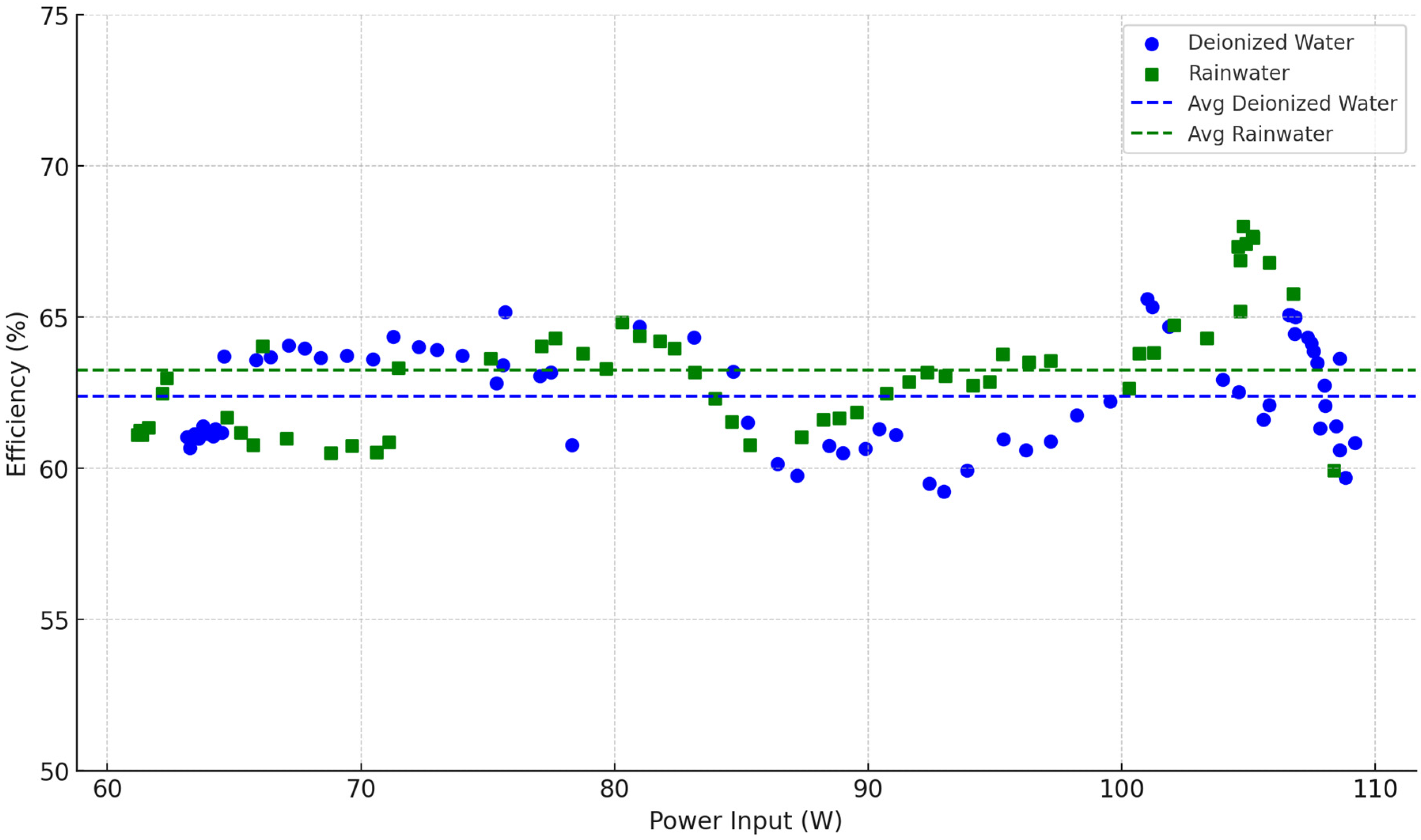
3.2. Electrolyzer Performance
3.2.1. Efficiency Calculations and Comparative Results
3.2.2. Performance Interpretation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEM | Proton Exchange Membrane |
| DI | Deionized Water |
| DC | Direct Current |
| TOC | Total Organic Carbon |
| IC | Ion Chromatography |
| ICP | Inductively Coupled Plasma |
| HER | Hydrogen Evolution Reaction |
| OER | Oxygen Evolution Reaction |
| ppb | Parts per billion |
| ppm | Parts per million |
| pH | Potential of Hydrogen |
| µS/cm | Microsiemens per centimeter |
References
- Roy, S. Underground Hydrogen Storage; Technische Universität: Darmstadt, Germany, 2023. [Google Scholar]
- IEA. The Future of Hydrogen. International Energy Agency. 2020. Available online: https://iea.blob.core.windows.net/assets/9e3a3493-b9a6-4b7d-b499-7ca48e357561/The_Future_of_Hydrogen.pdf (accessed on 10 September 2025).
- Ball, M.; Weeda, M. The hydrogen economy–Vision or reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Wei, D.; Shi, X.; Qu, R.; Junge, K.; Junge, H.; Beller, M. Toward a Hydrogen Economy: Development of Heterogeneous Catalysts for Chemical Hydrogen Storage and Release Reactions. ACS Energy Lett. 2022, 7, 3734–3752. [Google Scholar] [CrossRef]
- Jeje, S.O.; Marazani, T.; Obiko, J.O.; Shongwe, M.B. Advancing the hydrogen production economy: A comprehensive review of technologies, sustainability, and future prospects. Int. J. Hydrogen Energy 2024, 78, 642–661. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Recent progress in hydrogen production from formic acid decomposition. Int. J. Hydrogen Energy 2018, 43, 7055–7071. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-Nanoparticle-Catalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Geng, J.; Zong, S.; Wang, P. Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules 2025, 30, 630. [Google Scholar] [CrossRef]
- Impemba, S.; Provinciali, G.; Filippi, J.; Salvatici, C.; Berretti, E.; Caporali, S.; Banchelli, M.; Caporali, M. Engineering the heterojunction between TiO2 and In2O3 for improving the solar-driven hydrogen production. Int. J. Hydrogen Energy 2024, 63, 896–904. [Google Scholar] [CrossRef]
- Maccarrone, D.; Italiano, C.; Giorgianni, G.; Centi, G.; Perathoner, S.; Vita, A.; Abate, S. A Comprehensive Review on Hydrogen Production via Catalytic Ammonia Decomposition. Catalysts 2025, 15, 811. [Google Scholar] [CrossRef]
- Bulushev, D.A. Progress in Catalytic Hydrogen Production from Formic Acid over Supported Metal Complexes. Energies 2021, 14, 1334. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. A review of water electrolysis–based systems for hydrogen production using hybrid/solar/wind energy systems. Environ. Sci. Pollut. Res. 2022, 29, 86994–87018. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis: A techno-economic and environmental assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Zhao, Y.; Wei, T.; Fu, J.; Ren, Z.; Xu, X.; Zhou, L.; Shao, Z. Energy-efficient and cost-effective ammonia electrolysis for converting ammonia to green hydrogen. Cell Rep. Phys. Sci. 2024, 5, 102171. [Google Scholar] [CrossRef]
- Rashid, M.; Mesfer, M.A.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 80–93. [Google Scholar]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen-A Guide to Policy Making; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Snoeyink, V.; Jenkins, D. Water Chemistry; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Schoonderwoerd, J.C.T.; Bianchi, A.B.; Zonjee, T.; Chen, W.-S.; Torbaghan, S.S. Hydrogen production from non-potable water resources: A techno-economic investment and operation planning approach. J. Clean. Prod. 2024, 473, 143501. [Google Scholar] [CrossRef]
- Becker, H.; Murawski, J.; Shinde, D.V.; Stephens, I.E.L.; Hinds, G.; Smith, G. Impact of impurities on water electrolysis: A review. Sustain. Energy Fuels 2023, 7, 1565–1603. [Google Scholar] [CrossRef]
- Binhazzaa, Z.; Almutairi, Z. Impact of Water Properties on the Performance of PEM Electrolyzer. Preprints 2023. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; Di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.M.; Ponce De Leão, T. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Mishra, A.; Park, H.; El-Mellouhi, F.; Han, D.S. Seawater electrolysis for hydrogen production: Technological advancements and future perspectives. Fuel 2024, 361, 130636. [Google Scholar] [CrossRef]
- Qureshi, F.; Asif, M.; Khan, A.; Aldawsari, H.; Yusuf, M.; Khan, M.Y. Green Hydrogen Production From Non-Traditional Water Sources: A Sustainable Energy Solution With Hydrogen Storage and Distribution. Chem. Rec. 2024, 24, e202400080. [Google Scholar] [CrossRef]
- Puretec. What is Deionized Water? Puretec Industrial Water. Available online: https://puretecwater.com/resources/what-is-deionized-water/ (accessed on 9 July 2025).
- ISO 3696:1987; Water for Analytical Laboratory Use-Specification and Test Methods. ISO: Geneva, Switzerland, 1987.
- Potter, B.; Wimsatt, J. Determination of Total Organic Carbon and Specific UV Absorbance at 254 nm in Source Water and Drinking Water; Environmental Protection Agency: Washington, DC, USA, 2009. [Google Scholar]
- Cleaning Laboratory Glassware. Available online: https://www.txst.edu/chemistry/student-resources/chemistry-biochemistry-stockroom.html (accessed on 1 October 2025).
- ELGA. Sub-Micron Filtration. ELGA LabWater. Available online: https://www.elgalabwater.com/technologies/sub-micron-filtration#:~:text=Pre-treatment%20may%20include%20one%20or%20more%20filters%20with,to%200.22%20micron%29%20or%20ultrafilters%20%280.001%20to%200.01micron%29 (accessed on 10 September 2025).
- ISO 14687:2019; Hydrogen Fuel Quality-Product Specification. ISO: Geneva, Switzerland, 2019.
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Yodwong, B.; Guilbert, D.; Phattanasak, M.; Kaewmanee, W.; Hinaje, M.; Vitale, G. Faraday’s Efficiency Modeling of a Proton Exchange Membrane Electrolyzer Based on Experimental Data. Energies 2020, 13, 4792. [Google Scholar] [CrossRef]
- Kassamba-Diaby, M.L.; Galy-Lacaux, C.; Yoboué, V.; Hickman, J.E.; Mouchel-Vallon, C.; Jaars, K.; Gnamien, S.; Konan, R.; Gardrat, E.; Silué, S. The Chemical Characteristics of Rainwater and Wet Atmospheric Deposition Fluxes at Two Urban Sites and One Rural Site in Côte d’Ivoire. Atmosphere 2023, 14, 809. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Rainwater Chemistry Reveals Air Pollution in a Karst Forest: Temporal Variations, Source Apportionment, and Implications for the Forest. Atmosphere 2020, 11, 1315. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Rainwater chemistry observation in a karst city: Variations, influence factors, sources and potential environmental effects. PeerJ 2021, 9, e11167. [Google Scholar] [CrossRef]
- Open University. What Chemical Compounds Might Be Present in Drinking Water? Available online: https://www.open.edu/openlearn/science-maths-technology/what-chemical-compounds-might-be-present-drinking-water/content-section-1.1 (accessed on 27 July 2025).
- Global Seafood Alliance. Typical Chemical Characteristics of Full-Strength Seawater. Available online: https://www.globalseafood.org/advocate/typical-chemical-characteristics-of-full-strength-seawater (accessed on 25 July 2025).
- Svalbardi. The Key Indicators and Parameters of Water Quality. Available online: https://svalbardi.com/blogs/water/quality-indicator (accessed on 25 July 2025).
- Visco, G.; Campanella, L.; Nobili, V. Organic carbons and TOC in waters: An overview of the international norm for its measurements. Microchem. J. 2005, 79, 185–191. [Google Scholar] [CrossRef]
- Lenntech. Water Conductivity–Lenntech Water Treatment Solutions. Available online: https://www.lenntech.com/applications/ultrapure/conductivity/water-conductivity.htm (accessed on 29 July 2025).
- United States Environmental Protection Agency. National Secondary Drinking Water Regulations (NSDWRs). Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants#List (accessed on 30 July 2025).
- Galway Water Supply. What’s in Your Water?–AGWS Guide to the Parameters in the European Communities (Drinking Water) (No.2) Regulations 2007 (S.I. No. 278 of 2007); Association for Galway Water Supply (AGWS): Dublin, Ireland, 2012. [Google Scholar]
- Terhaar, J.; Frölicher, T.L.; Joos, F. Ocean acidification in emission-driven temperature stabilization scenarios: The role of TCRE and non-CO2 greenhouse gases. Environ. Res. Lett. 2023, 18, 024033. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Begum, H.; Ibrahim, F.A.; Hamdy, M.S.; Oyshi, T.A.; Khatun, N.; Hasnat, M.A. Electrocatalytic Hydrogen Evolution Reaction from Acetic Acid over Gold Immobilized Glassy Carbon Surface. Catalysts 2023, 13, 744. [Google Scholar] [CrossRef]
- Guo, W.L.; Li, L.; Li, L.L.; Tian, S.; Liu, S.L.; Wu, Y.P. Hydrogen production via electrolysis of aqueous formic acid solutions. Int. J. Hydrogen Energy 2011, 36, 9415–9419. [Google Scholar] [CrossRef]
- Karatok, M.; Ngan, H.T.; Jia, X.; O’Connor, C.R.; Boscoboinik, J.A.; Stacchiola, D.J.; Sautet, P.; Madix, R.J. Achieving Ultra-High Selectivity to Hydrogen Production from Formic Acid on Pd–Ag Alloys. J. Am. Chem. Soc. 2023, 145, 5114–5124. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Shi, S.; Hu, J.; Zeng, Y.; Xiao, W.; Wang, S.; Chen, C. Photoelectrochemical dual hydrogen production via formaldehyde oxidation and neutral water reduction. Int. J. Hydrogen Energy 2025, 111, 546–554. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Singh, S.K. Ruthenium catalyzed hydrogen production from formaldehyde–water solution. Sustain. Energy Fuels 2021, 5, 549–555. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Zhao, Y.; Wang, H.; Liu, H.; Zhang, Q. A mini review on recent progress of steam reforming of ethanol. RSC Adv. 2023, 13, 23991–24002. [Google Scholar] [CrossRef]
- Diglio, M.; Contento, I.; Impemba, S.; Berretti, E.; Della Sala, P.; Oliva, G.; Naddeo, V.; Caporali, S.; Primo, A.; Talotta, C.; et al. Hydrogen Production from Formic Acid Decomposition Promoted by Gold Nanoparticles Supported on a Porous Polymer Matrix. Energy Fuels 2025, 39, 14320–14329. [Google Scholar] [CrossRef] [PubMed]
- Deionization and High Purity DI Tank Systems. US Water Systems. Available online: https://uswatersystems.com/collections/for-business-deionization-and-high-purity-di-tank-systems (accessed on 10 September 2025).

| Ion | Sample A | Sample B | Sample C | Rainwater STD | Deionized Water | Tap Water (Lit.) | Seawater (Lit.) |
|---|---|---|---|---|---|---|---|
| Cl− (mg/L) | 9.816 | 6.975 | 6.558 | 1.773 | - | 73.9 [39] 79 [23] | 19,162 [40] 200 [23] |
| F− (mg/L) | 0.042 | 0.012 | 0.036 | 0.016 | - | 0.000 [23] | 1.3 [40] 1 [23] |
| SO42− (mg/L) | 3.304 | 2.750 | 3.070 | 0.278 | - | 120 [39] 47 [23] | 2680 [40] 2800 [23] |
| NO3− (mg/L) | 1.310 | 2.242 | 0.940 | 0.671 | - | 20.6 [39] 0.82 [23] | 0.000 [23] |
| Na+ (mg/L) | 1.197 | 1.607 | 3.535 | 1.248 | 0.008 | 49.1 [39] 62 [23] | 10,679 [40] 11,000 [23] |
| Ca2+ (mg/L) | 0.851 | 0.561 | 0.455 | 0.205 | 0.000 | 102 [39] 51 [23] | 410 [40] 400 [23] |
| Mg2+ (mg/L) | 0.309 | 0.235 | 0.164 | 0.073 | 0.000 | 8.81 [39] 7 [23] | 1278 [40] 130 [23] |
| Al3+ (mg/L) | 0.007 | 0.011 | 0.033 | 0.014 | 0.000 | 0.004 [23] | 0.000 [23] |
| K+ (mg/L) | 5.936 | 1.514 | 1.336 | 2.606 | 0.009 | 0.000 [39] 0.000 [23] | 395 [40] 400 [23] |
| Organic Matter (mg/L) | 7.130 | 7.680 | 5.980 | 0.867 | 0.318 | 0.1–10 [41] | <1 [42] |
| Conductivity (μS/cm) | 16.260 | 25.500 | 14.480 | 5.916 | 1.160 | 50–500 [43] | 50,000 [43] 44,000–58,000 [40] |
| pH (-) | 6.755 | 6.563 | 6.068 | 0.354 | 5.539 | 6.5–8.5 [44] 6.5–8.0 [45] | 8.05–8.15 [46] 7.6–8.4 [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres A. F. Dutra, J.V.; Kroeppl, M.; Toigo, C. Feasibility of Using Rainwater for Hydrogen Production via Electrolysis: Experimental Evaluation and Ionic Analysis. Hydrogen 2025, 6, 83. https://doi.org/10.3390/hydrogen6040083
Torres A. F. Dutra JV, Kroeppl M, Toigo C. Feasibility of Using Rainwater for Hydrogen Production via Electrolysis: Experimental Evaluation and Ionic Analysis. Hydrogen. 2025; 6(4):83. https://doi.org/10.3390/hydrogen6040083
Chicago/Turabian StyleTorres A. F. Dutra, João Victor, Michaela Kroeppl, and Christina Toigo. 2025. "Feasibility of Using Rainwater for Hydrogen Production via Electrolysis: Experimental Evaluation and Ionic Analysis" Hydrogen 6, no. 4: 83. https://doi.org/10.3390/hydrogen6040083
APA StyleTorres A. F. Dutra, J. V., Kroeppl, M., & Toigo, C. (2025). Feasibility of Using Rainwater for Hydrogen Production via Electrolysis: Experimental Evaluation and Ionic Analysis. Hydrogen, 6(4), 83. https://doi.org/10.3390/hydrogen6040083








