Theoretical Thermal Management Concepts of Recovery Heat Waste in Solid Oxide Fuel Cell System
Abstract
1. Introduction
- A set of three mathematical theoretical models based on thermal heat equation that incorporates the concept of specific heat capacity developed for calculation of the thermal heat and cool in a SOEC system is proposed. In particular, a general thermal model excludes the waste heat recovery and it is focused just on heat transfer processes within a SOEC system. In the second thermal model is integrated in the SOEC system the high, middle, and low-temperature heat exchangers to recover waste heat of the hydrogen-water mixture. In the third model is integrated in the SOEC system the high and low-temperature heat exchangers to recover waste heat of the hydrogen-water mixture, oxygen and hydrogen.
- Three block diagram models in Simulink for a SOEC system integrated with and without high, middle, and low-temperature heat exchanger are proposed to enhance system efficiency and utilize the waste heat from the SOEC system.
2. Materials and Methods
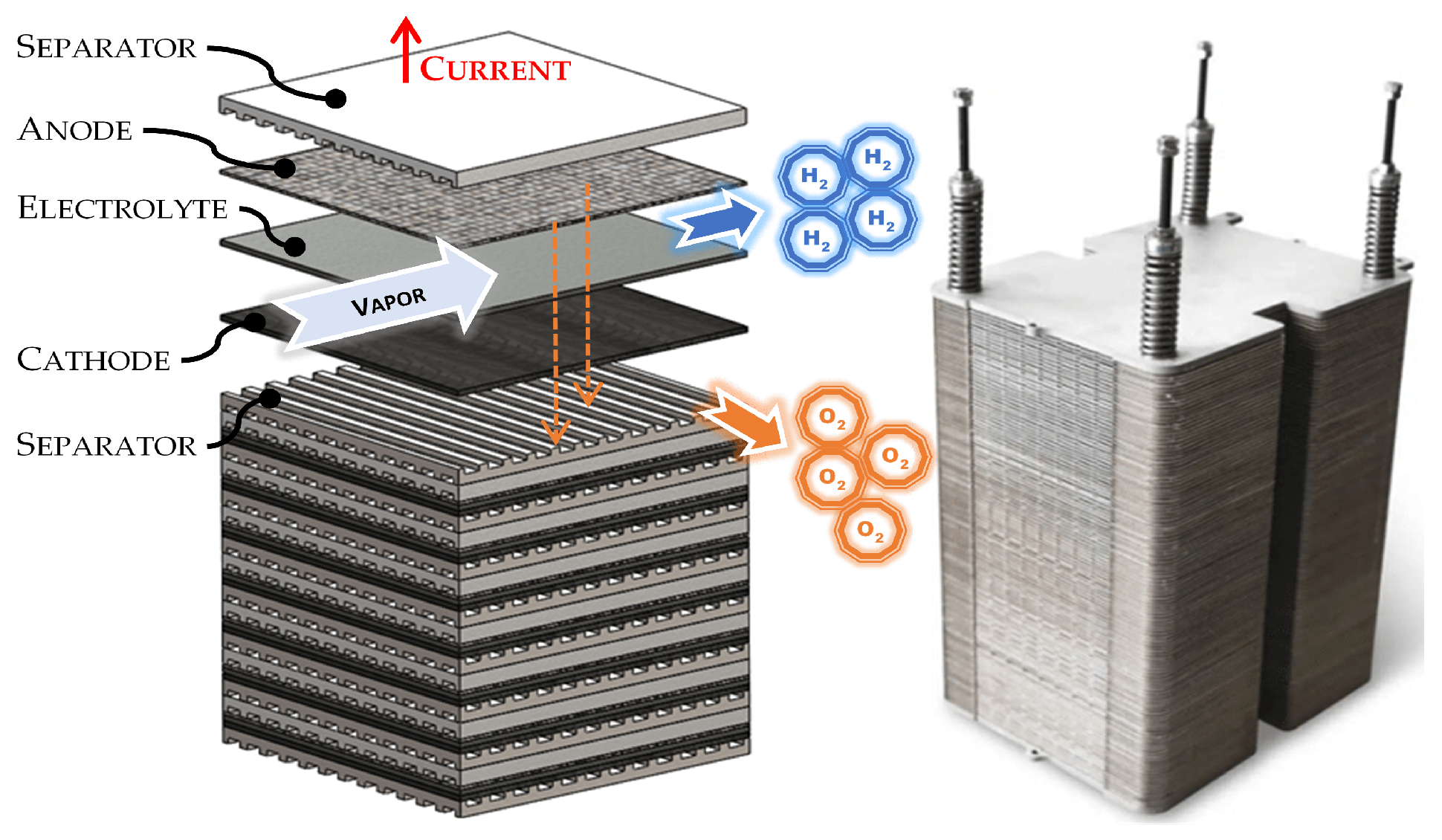
Description of Solid Oxide Electrolyzer Cell System
- deionization module is used to purify and deionize water by applying functional current to remove ionized substances from water.
- water heating module transforms the incoming water into steam vapor, requiring a significant high amount of energy for the phase change in the next steam module [29,30,31,32,33,34,35]. The heating module can transform incoming water into steam vapor. The vaporization involves additional thermal energy to the water in order to changes into steam vapor.
- SOEC subsystem for electrolysis at high-temperature (700–1000 °C) electrochemical device that utilizes a solid oxide electrolyte to convert steam into hydrogen
- cooler module is used to cool down the mixture of hydrogen and steam water at high temperature in order to separate the hydrogen gas.
- separator module provides the physical separation of the hydrogen gas from the resultant steam water and oxygen gases. The membrane unit used to separate hydrogen gas from steam is made of solid polymer, Nafion or ceramic [36]. This module is the key for conducting protons of hydrogen gas with high purity to pass from the anode to the cathode. It is considered a “black box” constrained for its input and output characteristics in the thermal models proposed in the next sections.
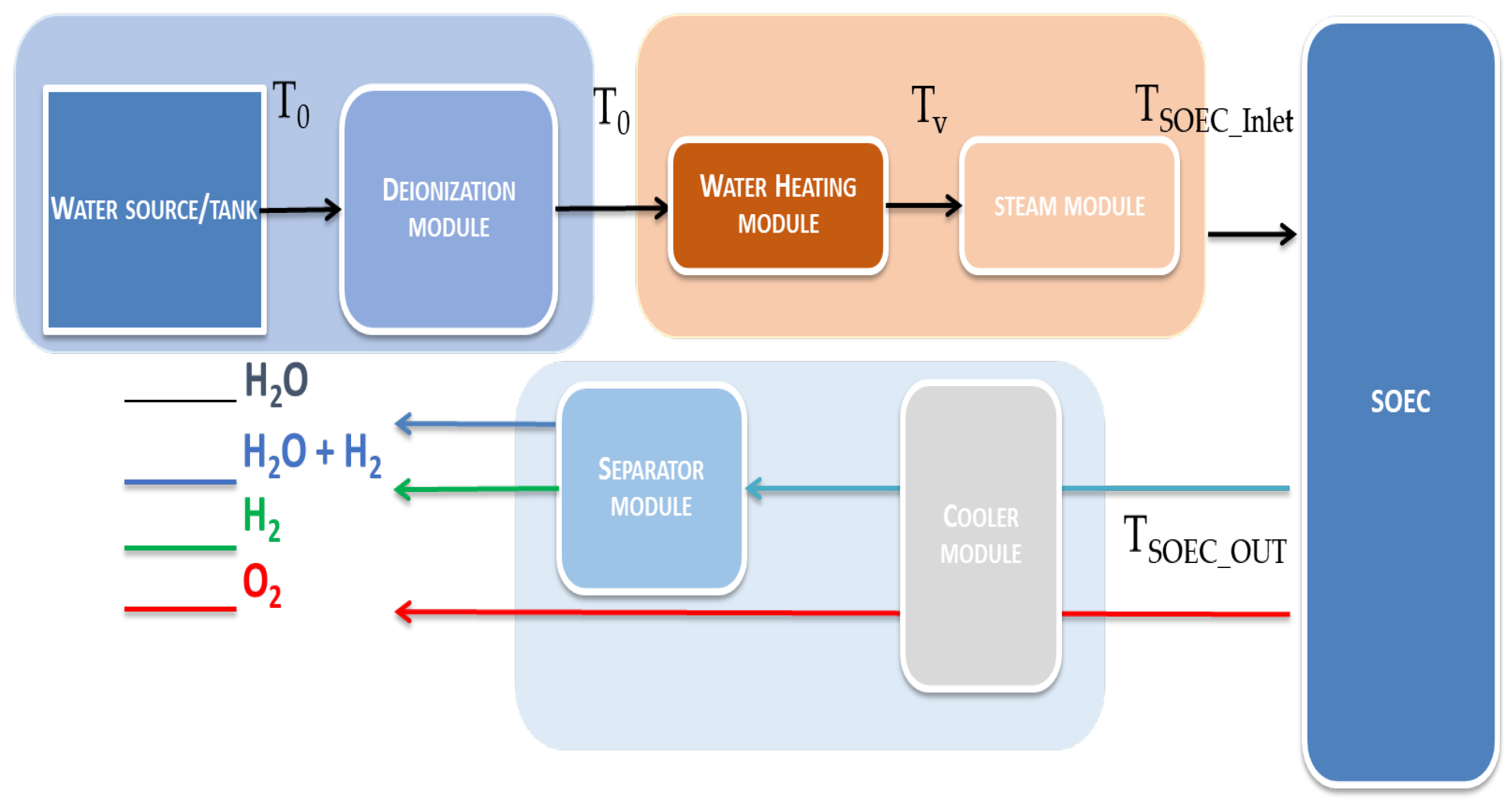
3. Base Model Assessment: General Conventional Thermal Heat Transfer in SOEC System ()
- In SOEC inlet the mass is 1.67 kg of H2O
- In SOEC outlet the mass is 1.67 kg equal to sum of 0.67 kg of H2O, 0.125 kg of H2 and 0.875 kg of O2.
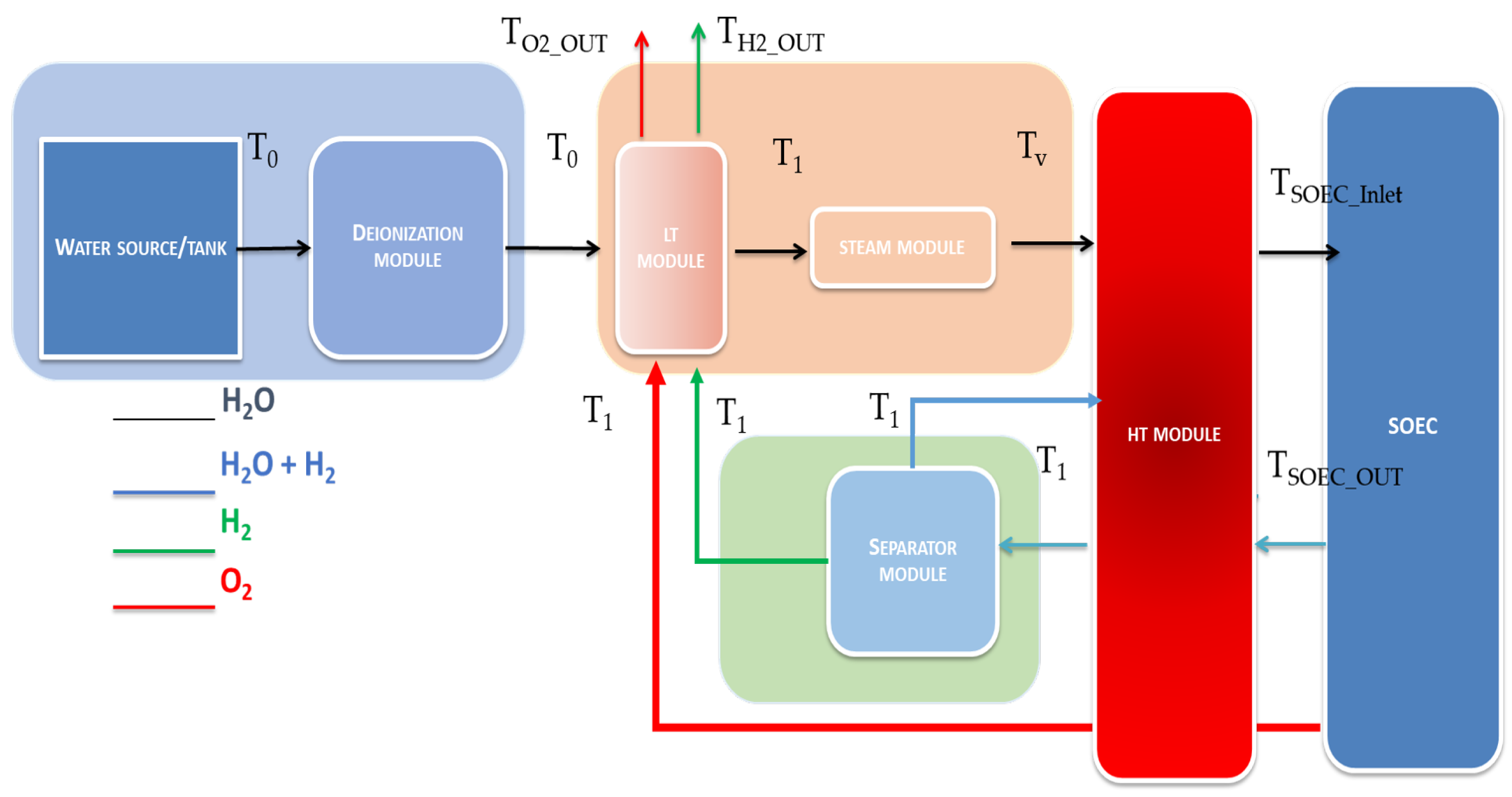
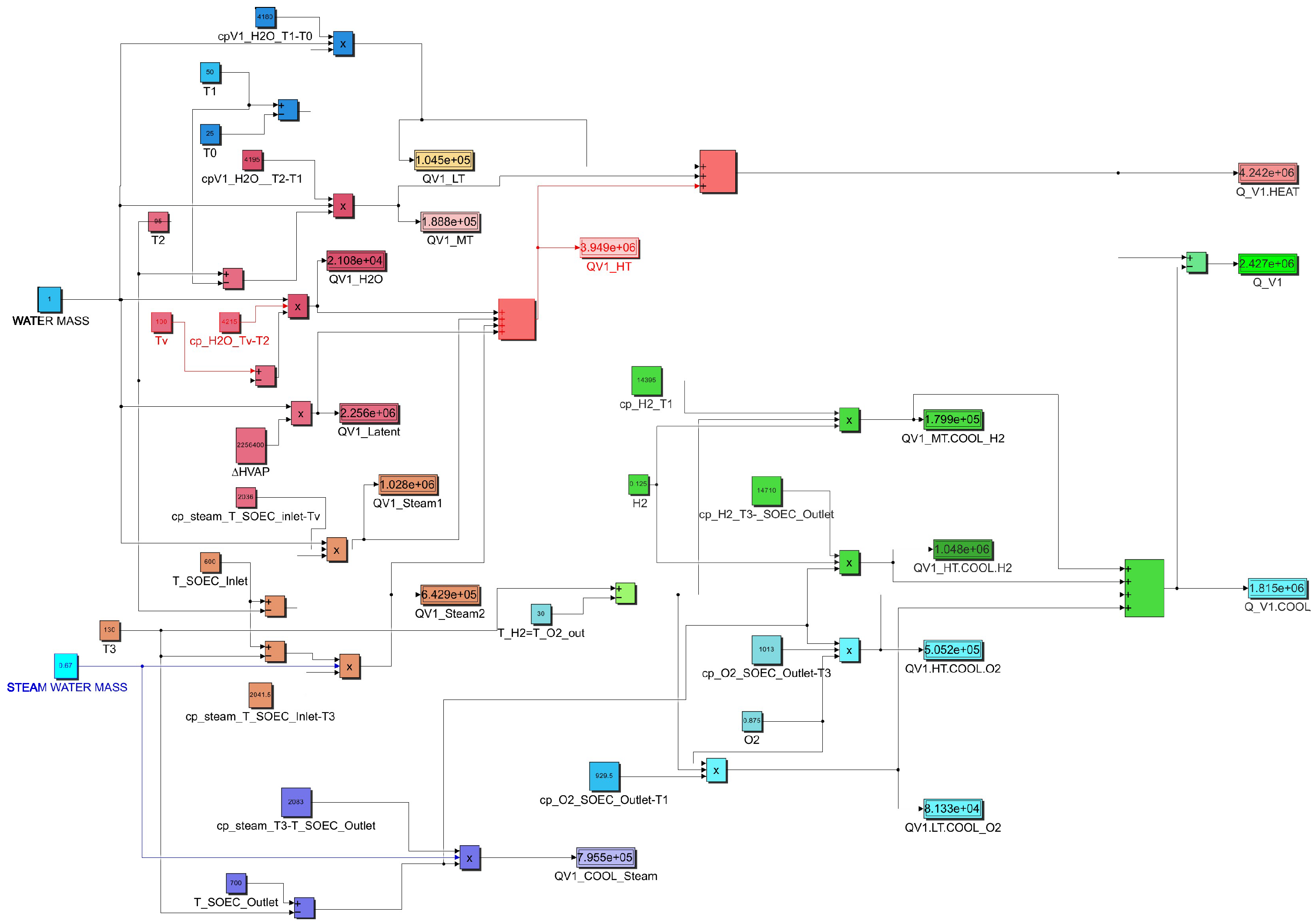
3.1. Thermal Heat Transfer in SOEC System with Low, Middle and High Module Heat Exchangers ()
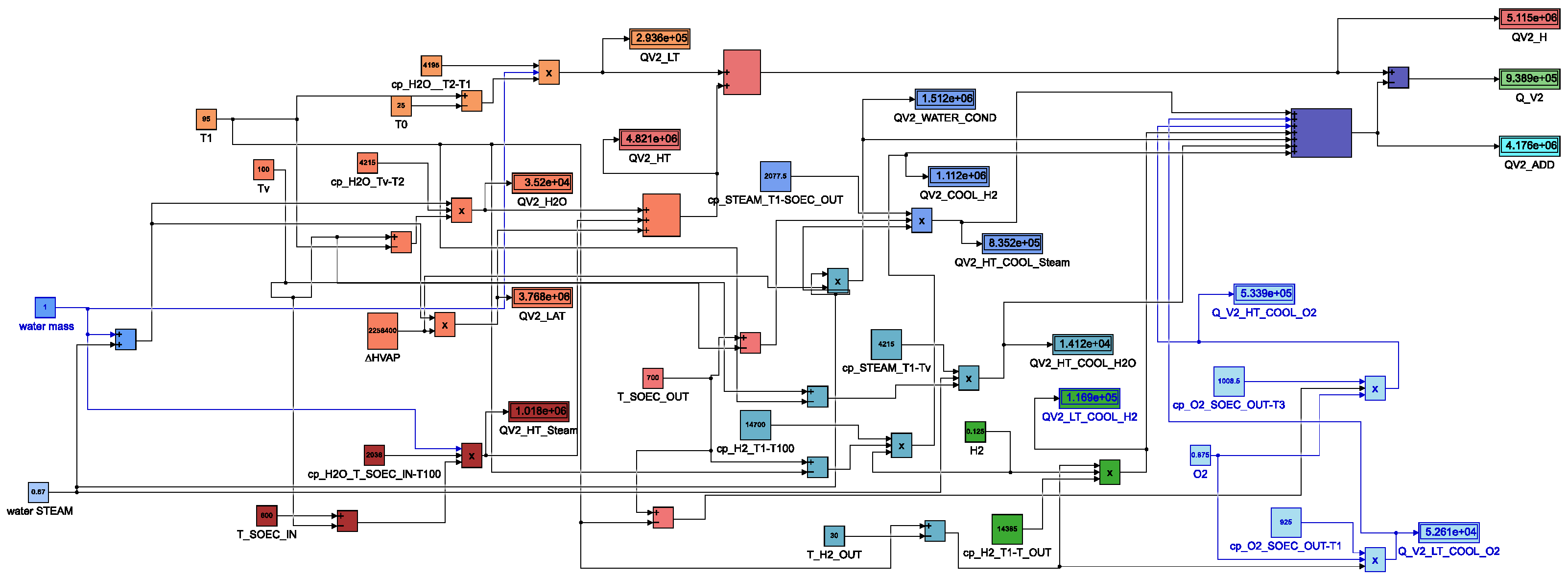
3.2. Thermal Heat Transfer in SOEC System with Low and High Module Heat Exchangers ()
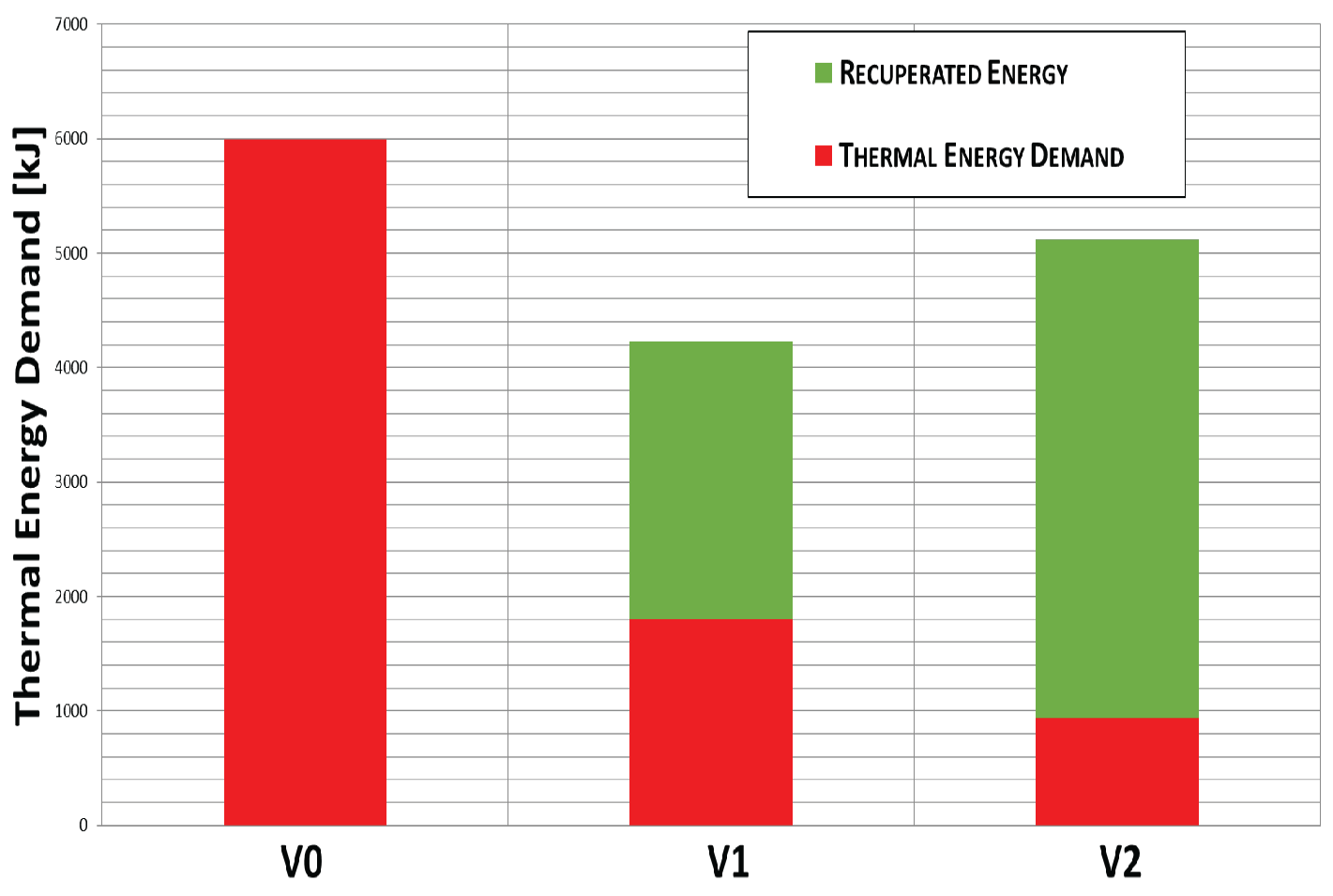
4. Results
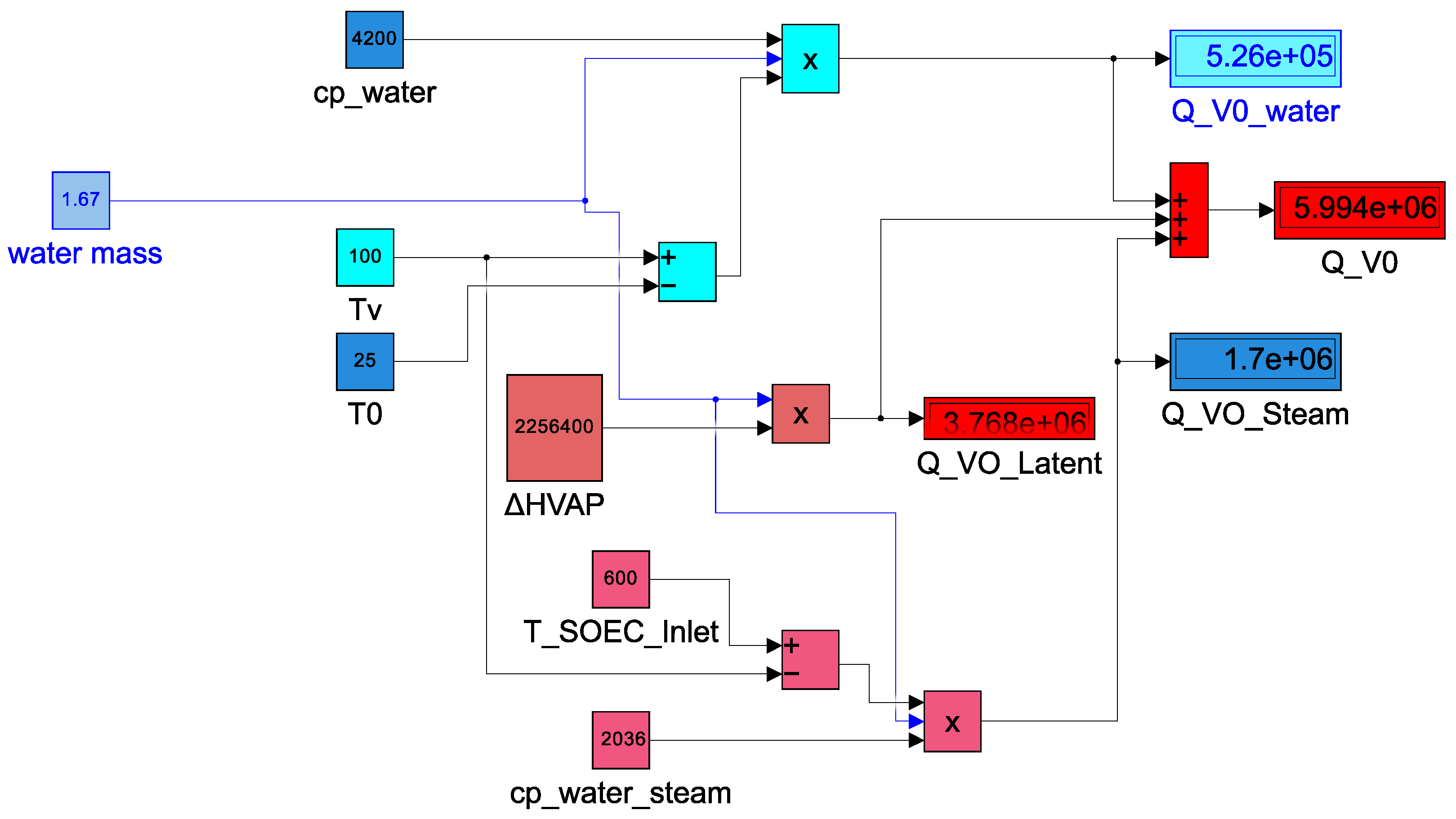
4.1. First Thermal Heat Model ()
- the water mass is 1.67 kg used as an input for the SOEC module
- the ambient temperature () is 25 °C, the vaporization temperature () is 100 °C and is 600 °C indicated by the manufacturer of Elcogen E350 stack
- the specific heat capacity of water () is described in the Figure 5 as equal to 4200 J/(kg·C) in the range temperature of to and the specific heat capacity of steam water from to
- is 2256.4 kJ/kg
4.2. Second Thermal Heat Transfer Model with Low, Middle and High Module Heat Exchangers ()
4.3. Third Thermal Heat Transfer Model with Low and High Module Heat Exchangers ()
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AWE | Alkaline Water Electrolyzers |
| AEMWE | Anion Exchange Membrane Water Electrolyzers |
| HT | High Temperature (module) |
| LT | Low Temperature (module) |
| MT | Mid Temperature (module) |
| PCCEL | Proton Conducting Ceramic Electrolyzers |
| PEMWE | Proton Ex-change Membrane Water Electrolyzers |
| RSOFC | Reversible Solid Oxide Fuel Cell |
| SOEC | Solid Oxide Electrolysis Cell |
| SOFC | Solid Oxide Fuel Cell |
References
- Yan, Z.; Hitt, J.L.; Turner, J.A.; Mallouk, T.E. Renewable electricity storage using electrolysis. Proc. Natl. Acad. Sci. USA 2020, 117, 12558–12563. [Google Scholar] [CrossRef]
- Van Der Roest, E.; Bol, R.; Fens, T.; van Wijk, A. Utilisation of waste heat from PEM electrolysers—Unlocking local optimisation. Int. J. Hydrogen Energy 2023, 48, 27872–27891. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of renewable energy sources in tandem with electrolysis: A technology review for green hydrogen production. Int. J. Hydrogen Energy 2025, 107, 218–240. [Google Scholar] [CrossRef]
- Panigrahy, B.; Narayan, K.; Rao, B.R. Green hydrogen production by water electrolysis: A renewable energy perspective. Mater. Today Proc. 2022, 67, 1310–1314. [Google Scholar] [CrossRef]
- Hassan, Q.; Tabar, V.S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. A review of green hydrogen production based on solar energy; techniques and methods. Energy Harvest. Syst. 2024, 11, 20220134. [Google Scholar]
- Zhao, H.; Yuan, Z.Y. Progress and perspectives for solar-driven water electrolysis to produce green hydrogen. Adv. Energy Mater. 2023, 13, 2300254. [Google Scholar]
- Vidas, L.; Castro, R. Recent developments on hydrogen production technologies: State-of-the-art review with a focus on green-electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Cooper, N.; van’t Noordende, H.; Shah, N. Operational strategies and integrated design for producing green hydrogen from wind electricity. Int. J. Hydrogen Energy 2024, 64, 650–675. [Google Scholar] [CrossRef]
- Bonanno, M.; Müller, K.; Bensmann, B.; Hanke-Rauschenbach, R.; Aili, D.; Franken, T.; Chromik, A.; Peach, R.; Freiberg, A.T.; Thiele, S. Review and prospects of PEM water electrolysis at elevated temperature operation. Adv. Mater. Technol. 2024, 9, 2300281. [Google Scholar] [CrossRef]
- Hauch, A.; Ebbesen, S.D.; Jensen, S.H.; Mogensen, M. Highly efficient high temperature electrolysis. J. Mater. Chem. 2008, 18, 2331–2340. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Ni, M.; Zhan, R.; Du, Q.; Jiao, K. Three-dimensional modeling of flow field optimization for co-electrolysis solid oxide electrolysis cell. Appl. Therm. Eng. 2020, 172, 114959. [Google Scholar] [CrossRef]
- Moser, M.; Gils, H.C.; Pivaro, G. A sensitivity analysis on large-scale electrical energy storage requirements in Europe under consideration of innovative storage technologies. J. Clean. Prod. 2020, 269, 122261. [Google Scholar] [CrossRef]
- Gómez, S.Y.; Hotza, D. Current developments in reversible solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 61, 155–174. [Google Scholar] [CrossRef]
- Shen, M.; Ai, F.; Ma, H.; Xu, H.; Zhang, Y. Progress and prospects of reversible solid oxide fuel cell materials. iScience 2021, 24, 103464. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xu, Y.; Cai, S.; Chi, B.; Tu, Z. Thermal dynamic analysis and multi-objective optimization of a 10 kW dual preheating solid oxide fuel cell system. Int. J. Hydrogen Energy 2024, 137, 799–805. [Google Scholar]
- Xiao, G.; Sun, A.; Liu, H.; Ni, M.; Xu, H. Thermal management of reversible solid oxide cells in the dynamic mode switching. Appl. Energy 2023, 331, 120383. [Google Scholar]
- Mottaghizadeh, P.; Fardadi, M.; Jabbari, F.; Brouwer, J. Thermal management of a reversible solid oxide system for long-term renewable energy storage. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers: New York, NY, USA, 2020; Volume 84560, p. V008T08A046. [Google Scholar]
- Kim, J.Y.; Mastropasqua, L.; Saeedmanesh, A.; Brouwer, J. Development of thermal control strategies for solid oxide electrolysis cell systems under dynamic operating conditions-Hot-standby and cold-start scenarios. Energy 2025, 317, 134679. [Google Scholar]
- Petipas, F.; Brisse, A.; Bouallou, C. Thermal management of solid oxide electrolysis cell systems through air flow regulation. Chem. Eng. Trans. 2017, 61, 1069–1074. [Google Scholar]
- Angelico, R.; Giametta, F.; Bianchi, B.; Catalano, P. Green hydrogen for energy transition: A critical perspective. Energies 2025, 18, 404. [Google Scholar] [CrossRef]
- Shash, A.Y.; Abdeltawab, N.M.; Hassan, D.M.; Darweesh, M.; Hegazy, Y. Computational methods, artificial intelligence, modeling, and simulation applications in green hydrogen production through water electrolysis: A review. Hydrogen 2025, 6, 21. [Google Scholar] [CrossRef]
- Niknam, P.H. Low-Carbon Industrial Heating in the EU and UK: Integrating Waste Heat Recovery, High-Temperature Heat Pumps, and Hydrogen Technologies. Energies 2025, 18, 4313. [Google Scholar] [CrossRef]
- Lange, H.; Klose, A.; Beisswenger, L.; Erdmann, D.; Urbas, L. Modularization approach for large-scale electrolysis systems: A review. Sustain. Energy Fuels 2024, 8, 1208–1224. [Google Scholar] [CrossRef]
- Maddaloni, M.; Marchionni, M.; Abbá, A.; Mascia, M.; Tola, V.; Carpanese, M.P.; Bertanza, G.; Artioli, N. Exploring the Via-bility of Utilizing Treated Wastewater as a Sustainable Water Resource for Green Hydrogen Generation Using Solid Oxide Electrolysis Cells (SOECs). Water 2023, 15, 2569. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, Q.; Dou, B.; Fu, Z.; Wang, J.; Mao, S. Thermodynamic analysis of a solid oxide electrolysis cell system in thermoneutral mode integrated with industrial waste heat for hydrogen production. Energy 2024, 301, 131678. [Google Scholar] [CrossRef]
- Choi, S.; Hong, J. Thermal Integration of Solid Oxide Electrolysis Cell Systems with External Heat Sources to Maximize Heat Recuperation and Exergetic Efficiency. ECS Trans. 2023, 111, 1203. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, K.; Salihi, H.; Heo, S.; Ju, H. The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells. Energies 2024, 17, 125. [Google Scholar] [CrossRef]
- Chen, K.; Peng, H.; Zhang, J.; Xu, X.; Zhou, S.; Ruan, J.; Li, B.; Wang, Y. Analysis of performance optimization of high-temperature solid oxide electrolytic cell based on the coupling of flow, heat, and mass transfer and electrochemistry. Energy Sci. Eng. 2022, 10, 3918–3927. [Google Scholar]
- Wang, H.; Xiao, L.; Liu, Y.; Zhang, X.; Zhou, R.; Liu, F.; Yuan, J. Performance and Thermal Stress Evaluation of Full-Scale SOEC Stack Using Multi-Physics Modeling Method. Energies 2023, 16, 7720. [Google Scholar] [CrossRef]
- Hasbi, S.; Amber, I.; Hossain, M.; Saharudin, M.S. Performance optimisation of solid oxide electrolyser cell (SOEC) using response surface method (RSM) for thermal gradient reduction. Int. J. Sustain. Energy 2025, 44, 2482837. [Google Scholar] [CrossRef]
- Rosati, G.; Baiguini, M.; Di Marcoberardino, G.; Invernizzi, C.M.; Iora, P.G. Integrated ORC-SOEC system for green hy-drogen production from incineration of solid fuels. J. Phy. Conf. Ser. 2022, 2385, 012108. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Lim, B. Hydrogen separation membranes: A material perspective. Molecules 2024, 29, 4676. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087. [Google Scholar] [CrossRef]
- Luo, K.; Dong, R.; Li, X.; Peng, Z.; Lu, L.; Xu, J.; Jin, H.; Guo, L. Thermodynamic and safety analysis for hydrogen production in a supercritical water gasification system of coal by multi-oxidizers distribution. Energy 2025, 331, 137105. [Google Scholar] [CrossRef]
- Tallgren, J.; Himanen, O.; Noponen, M. Experimental characterization of low temperature solid oxide cell stack. ECS Trans. 2017, 78, 3103. [Google Scholar] [CrossRef]
- Kyle, B.G. Chemical and Process Thermodynamics; Chemical Engineering Thermodynamics; Prentice-Hall: Wilmington, DE, USA, 1984. [Google Scholar]
- Keating, E.L. Applied Combustion; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kreith, F.; Black, W.Z. Basic Heat Transfer; Harper & Row: New York, NY, USA, 1980. [Google Scholar]
- Oh, D.-K.; Lee, K.-Y.; Park, J.-S. Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology. Membranes 2020, 10, 338. [Google Scholar] [CrossRef]
- Marino, F.; Ferrario, A.M.; Santoni, F.; Alfano, A.; Noponen, M.; Neubauer, R.; Cigolotti, V.; Jannelli, E. Performance Evaluation of an Anode-Supported SOFC Short-Stack Operating with Different Fuel Blends as Stationary-CHP System. J. Electrochem. Soc. 2024, 171, 054511. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Q.; Yang, L.; Huang, H.; Wei, K.; Li, D.; Zhao, C.; Liu, J.; Li, Z. Energy analysis of hydrogen production via fuel-assisted high-temperature solid oxide electrolysis cell via system modelling. Sustain. Energy Res. 2025, 12, 31. [Google Scholar] [CrossRef]
- Ferrete, F.; Molina, A.; Cabello González, G.M.; Moreno-Racero, Á.; Olmedo, H.; Iranzo, A. Solid Oxide Electrolyzers Process Integration: A Comprehensive Review. Processes 2025, 13, 2656. [Google Scholar] [CrossRef]

| Specific Heat Capacity [J/(kg·°C)] | Values |
|---|---|
| 4180 | |
| 4195 | |
| 4215 | |
| 2036 | |
| 2041.5 | |
| 2083 | |
| 14,710 | |
| 14,395 | |
| 1013 | |
| 929.5 |
| Specific Heat Capacity [J/(kg·°C)] | Values |
|---|---|
| 4195 | |
| 4215 | |
| 2036 | |
| 2041.5 | |
| 2077.5 | |
| 14,700 | |
| 14,395 | |
| 1008.5 | |
| 925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, G.D.; Todorov, T.; Kamberov, K.; Lo Sciuto, G. Theoretical Thermal Management Concepts of Recovery Heat Waste in Solid Oxide Fuel Cell System. Hydrogen 2025, 6, 82. https://doi.org/10.3390/hydrogen6040082
Todorov GD, Todorov T, Kamberov K, Lo Sciuto G. Theoretical Thermal Management Concepts of Recovery Heat Waste in Solid Oxide Fuel Cell System. Hydrogen. 2025; 6(4):82. https://doi.org/10.3390/hydrogen6040082
Chicago/Turabian StyleTodorov, Georgi D., Todor Todorov, Konstantin Kamberov, and Grazia Lo Sciuto. 2025. "Theoretical Thermal Management Concepts of Recovery Heat Waste in Solid Oxide Fuel Cell System" Hydrogen 6, no. 4: 82. https://doi.org/10.3390/hydrogen6040082
APA StyleTodorov, G. D., Todorov, T., Kamberov, K., & Lo Sciuto, G. (2025). Theoretical Thermal Management Concepts of Recovery Heat Waste in Solid Oxide Fuel Cell System. Hydrogen, 6(4), 82. https://doi.org/10.3390/hydrogen6040082









