First-Principles Investigation of Structural, Electronic, Thermoelectric, and Hydrogen Storage Properties of MgXH3 (X = Cr, Mn, Fe, Co, Ni, Cu) Perovskite Hydrides
Abstract
1. Introduction
2. Calculation Method
3. Interpretation of Results
3.1. Structural Properties
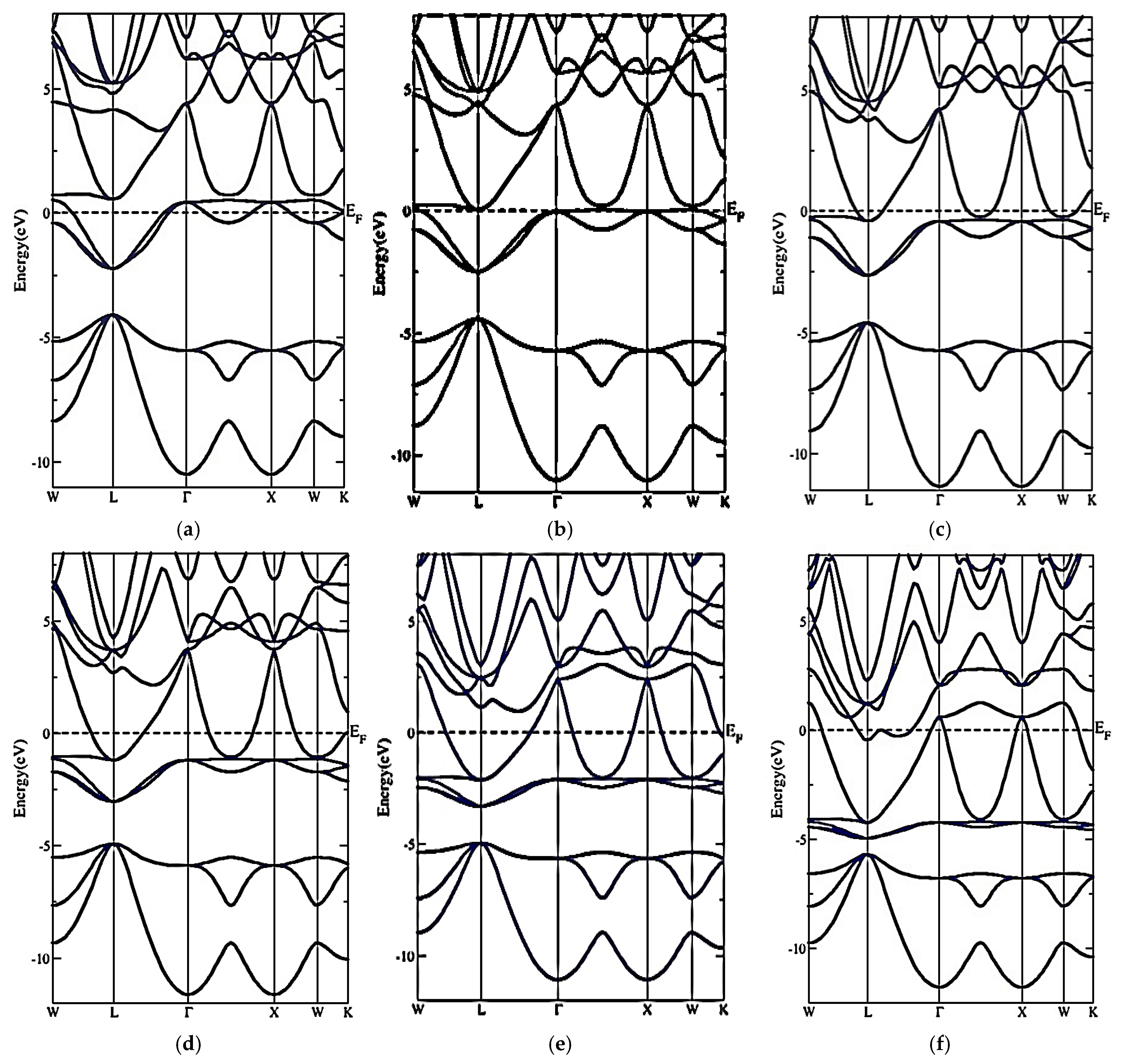
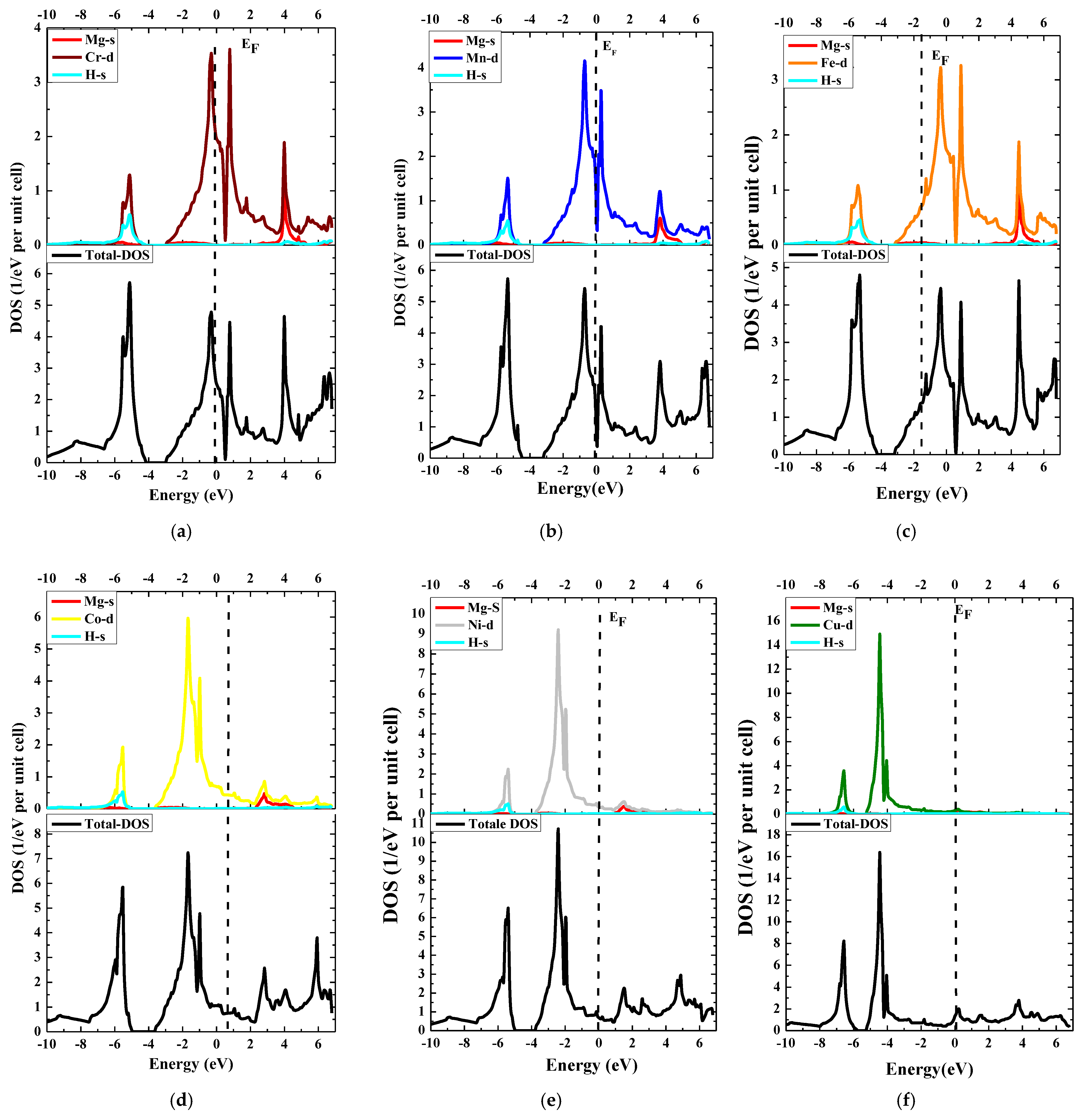
3.2. Electronic Properties
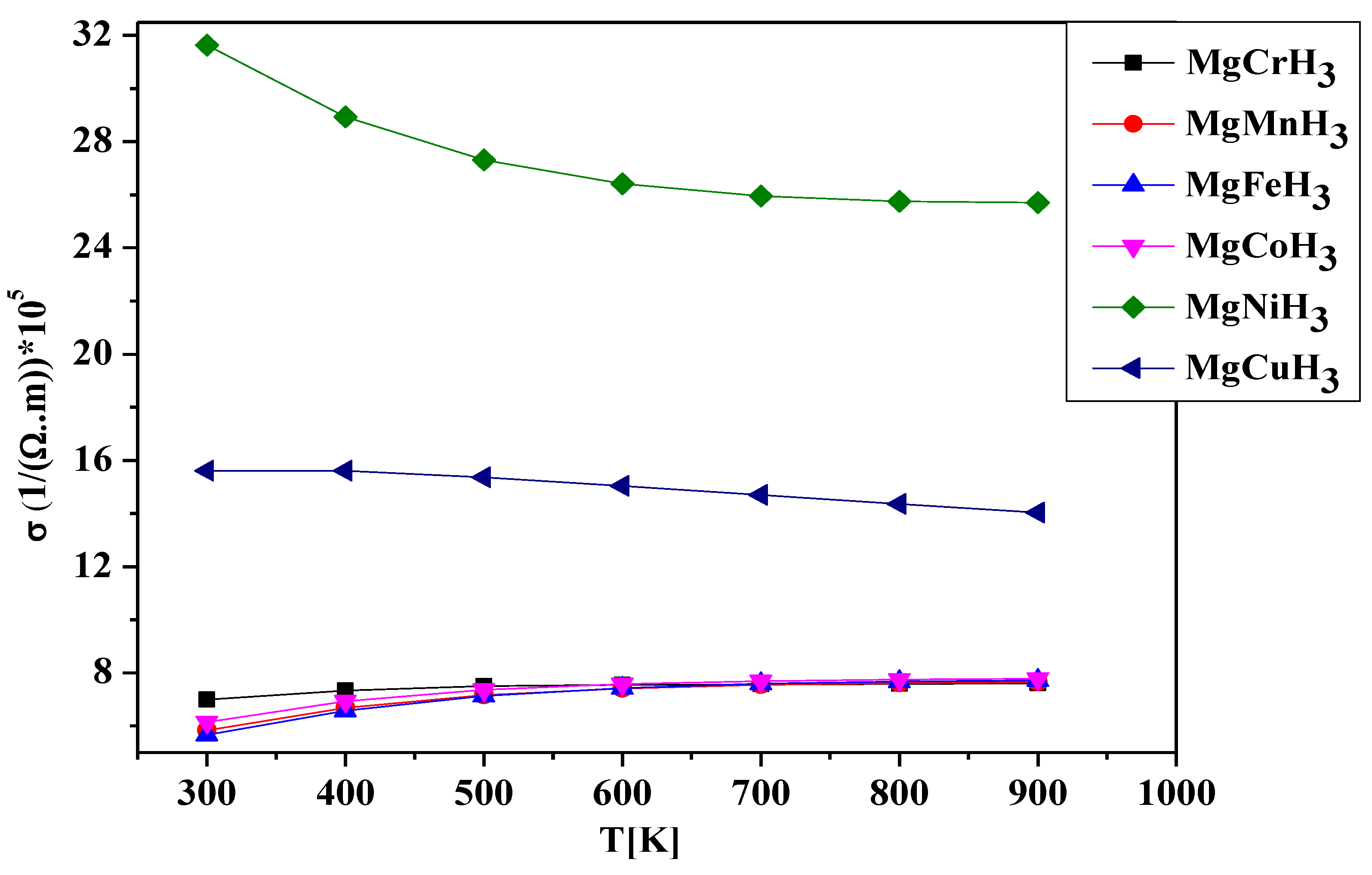
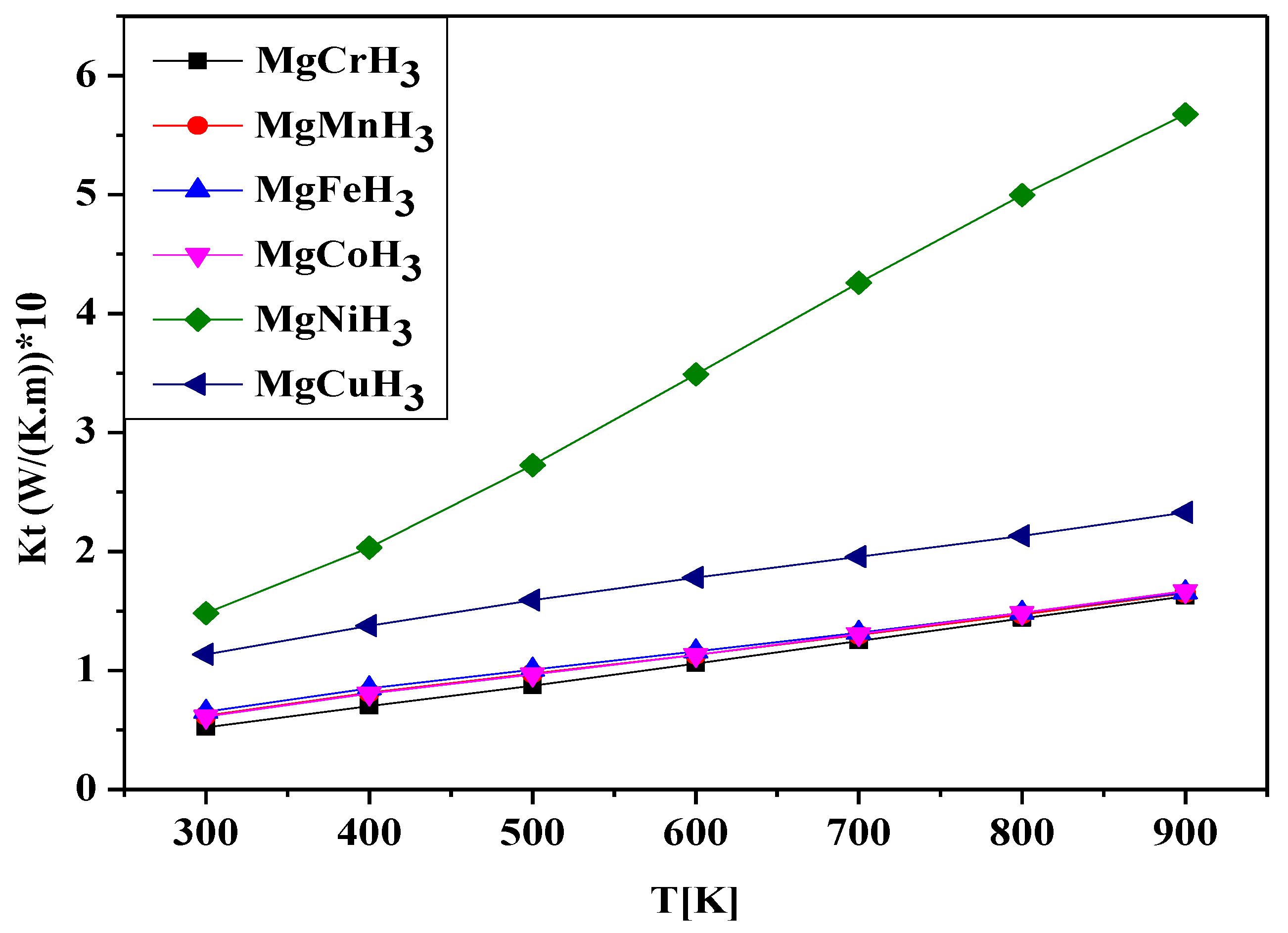
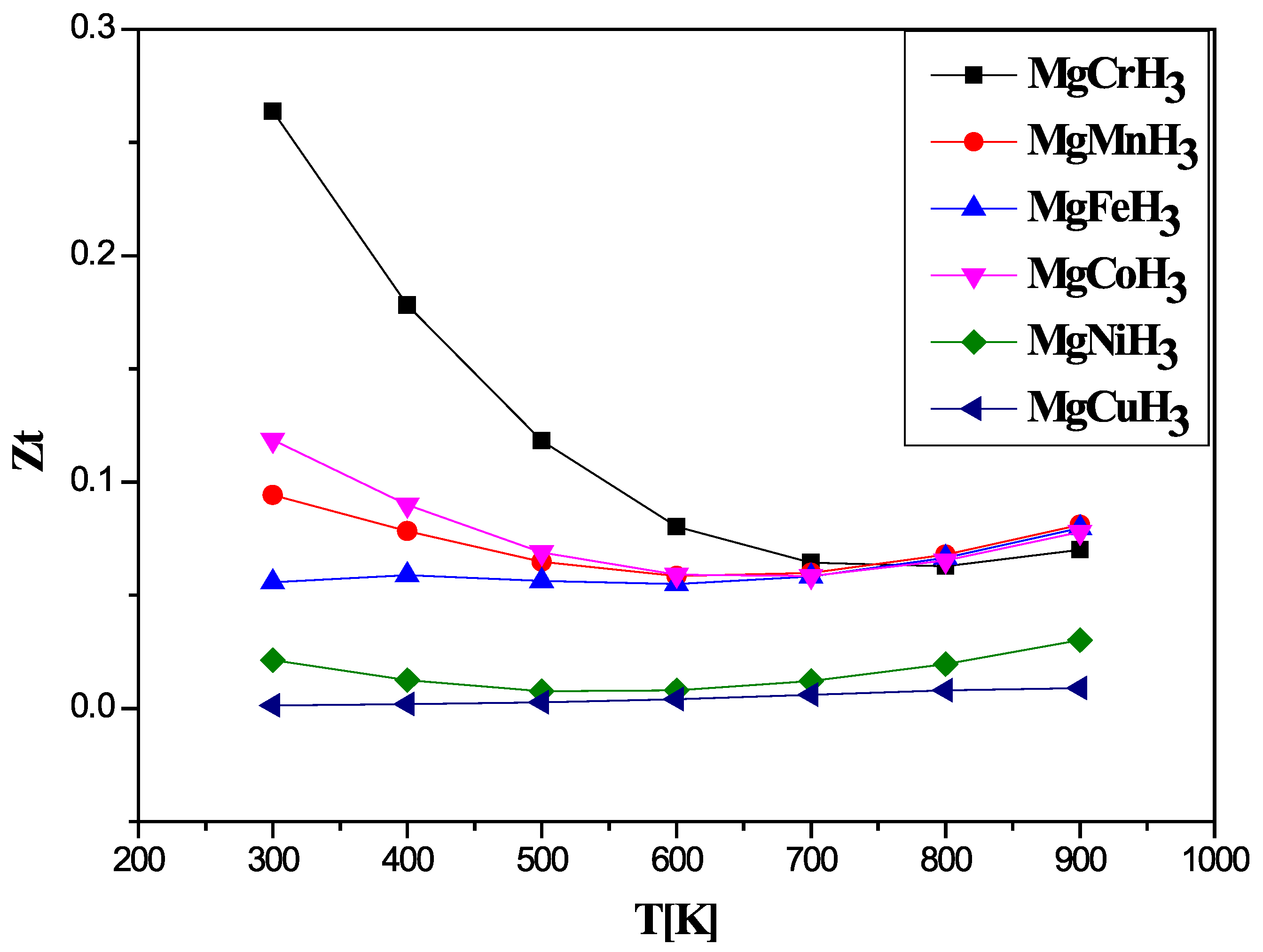
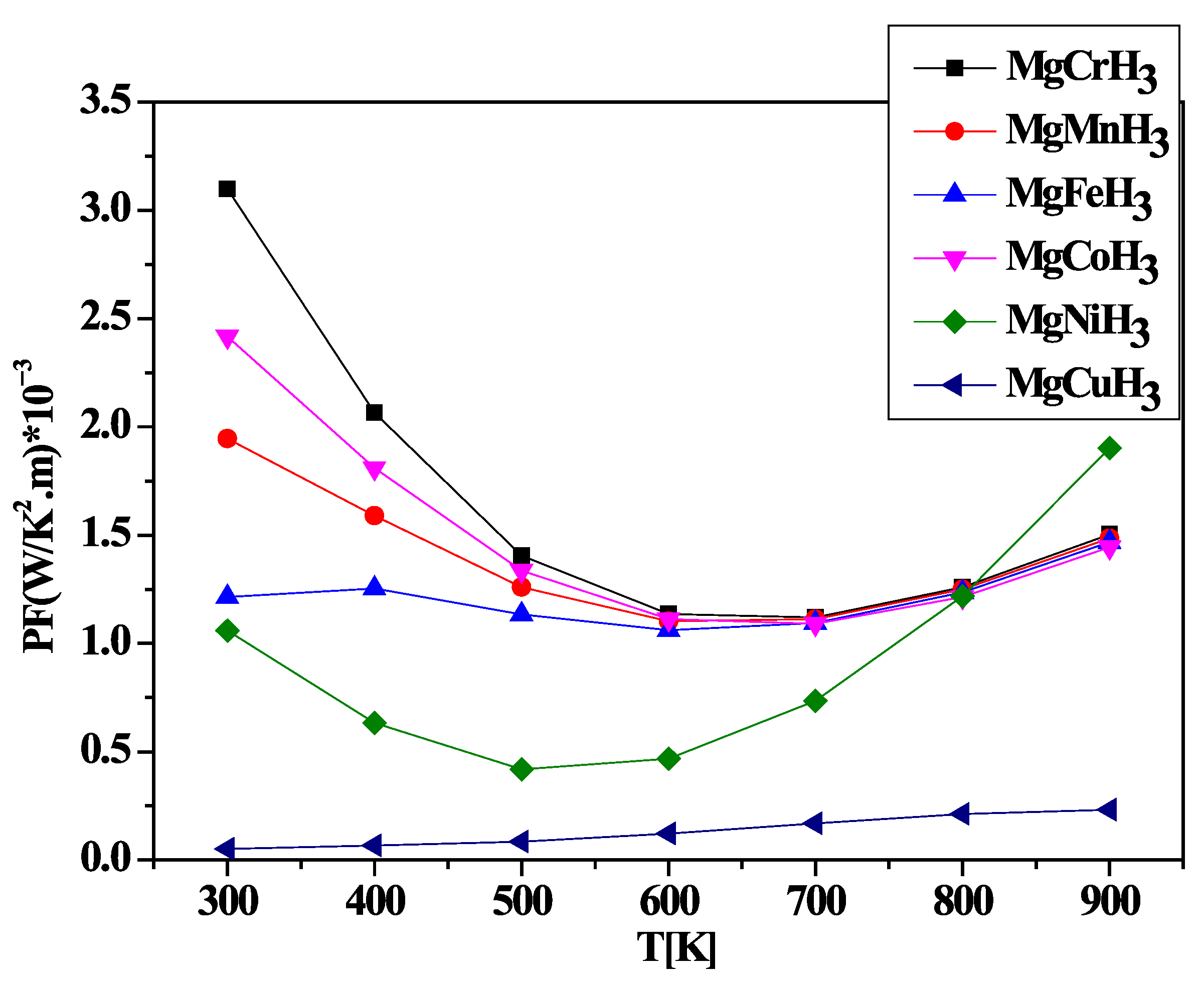
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Ende, Y. New insight into the structural and physical properties of AlH3. Energy Res. 2022, 46, 19678–19685. [Google Scholar] [CrossRef]
- Alonso, J.; Cabria, I.; Lopez, M. Simulation of hydrogen storage in porous carbons. J. Mater. Res. 2013, 28, 589–604. [Google Scholar] [CrossRef]
- Ende, Y.; Pan, Y. Catalytic properties of borophene/MoS2 heterojunctions for hydrogen evolution reaction under different stacking conditions. J. Mater. Chem. A 2022, 46, 24866–24876. [Google Scholar]
- Pandey, P.; Shukla, S. Pandey Mesoporous silica beads encapsulated with functionalized palladium nanocrystallites: Novel catalyst for selective hydrogen evolution. J. Mater. Res. 2017, 32, 3574–3584. [Google Scholar] [CrossRef]
- Sosa, A.N.; de Santiago, F.; Miranda, Á.; Trejo, A.; Salazar, F.; Pérez, L.A.; Cruz-Irisson, M. Alkali andtransition metal atom-functionalized germanene for hydrogen storage: A DFT investigation. Int. J. Hydrogen Energy 2020, 46, 20245–20256. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Pan, Y.; Ende, Y. Theoretical prediction of structure, electronic and optical properties of VH2 hydrogen storage material. Int. J. Hydrogen Energy 2022, 47, 27608–27616. [Google Scholar] [CrossRef]
- Laghlimi, C.; Moutcine, A.; Ziat, Y.; Belkhanchi, H.; Koufi, A.; Bouyassan, S. Hydrogen, Chronology and Electrochemical Production. Sol. Energy Sustain. Dev. 2024, 14, 22–37. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Y. Mechanism of interlayer spacing on catalytic properties of MoS2 from ab-initio calculation. Appl. Surf. Sci. 2022, 599, 154041. [Google Scholar] [CrossRef]
- Adaikalam, K.; Vikraman, D.; Karuppasamy, K.; Kim, H.S. Solar hydrogen production and storage in solid form: Prospects for materials and methods. Nanomaterials 2024, 14, 1560. [Google Scholar] [CrossRef]
- Nagpal, M.; Kakkar, R. An evolving energy solution: Intermediate hydrogen storage. Hydrog. Energy 2018, 43, 12168–12188. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Lim, K.; Kazemian, H.; Daud, Z.Y. Solid-state materials and methods for hydrogen storage: A critical review. Chem. Eng. Technol. 2010, 33, 213–226. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, L.; Yang, F.; Nyamsi, S.; Porpatha, E. Toward the design of interstitial nonmetals co-doping for Mg-based hydrides as hydrogen storage material. J. Mater. Res. 2018, 33, 4080–4091. [Google Scholar] [CrossRef]
- Batalovi, K.; Radakovi, C.; Belo, C.; Kotesk, V. Transition metal doping of Mg2FeH6—A DFT insight into synthesis and electronic structure. Phys. Chem. Chem. Phys. 2014, 24, 12356–12361. [Google Scholar] [CrossRef] [PubMed]
- Baro, M.D.; Surinach, S.; Rossinyol, E.; Marini, A.; Girella, A.; Milanese, C.; Pellicer, E.; Garroni, S. Hydrogen sorption performance of MgH2 doped with mesoporous nickeland cobalt-based oxides. Int. J. Hydrogen Energy 2011, 36, 540–5410. [Google Scholar]
- Haider, S.; Haider, A.; Ud-Din Khan, S. (Eds.) Density Functional Theory: New Perspectives and Applications; IntechOpen: London, UK, 2024. [Google Scholar]
- Khatun, S.; Bhagat, R.P.; Amin, S.A.; Jha, T.; Gayen, S. Density functional theory (DFT) studies in HDAC-based chemotherapeutics: Current findings, case studies and future perspectives. Comput. Biol. Med. 2024, 175, 108468. [Google Scholar] [CrossRef]
- Feng, J.; Wan, L.; Li, J.; Jiao, S.; Cui, X.; Hu, W.; Yang, J. Massively parallel implementation of iterative eigensolvers in large-scale plane-wave density functional theory. Comput. Phys. Commun. 2024, 299, 109135. [Google Scholar] [CrossRef]
- Raghavan, V. Materials Science and Engineering: A First Course; PHI Learning Pvt. Ltd.: Delhi, India, 2015. [Google Scholar]
- Zhang, S.; Makoś, Z.; Jadrich, R.B.; Kraka, E.; Barros, K.; Nebgen, B.T.; Tretiak, S.; Isayev, O.; Lubbers, N.; Messerly, R.A.; et al. Exploring the frontiers of condensed-phase chemistry with a general reactive machine learning potential. Nat. Chem. 2024, 16, 727–734. [Google Scholar] [CrossRef]
- Butera, V. Density functional theory methods applied to homogeneous and heterogeneous catalysis: A short review and a practical user guide. Phys. Chem. Chem. Phys. 2024, 26, 7950–7970. [Google Scholar] [CrossRef]
- Cernatic, F.; Loos, P.F.; Senjean, B.; Fromager, E. Neutral electronic excitations and derivative discontinuities: An extended N-centered ensemble density functional theory perspective. Phys. Rev. B 2024, 109, 235113. [Google Scholar] [CrossRef]
- Alvertis, A.M.; Williams-Young, D.B.; Bruneval, F.; Neaton, J.B. Influence of Electronic Correlations on Electron–Phonon Interactions of Molecular Systems with the GW and Coupled Cluster Methods. J. Chem. Theory Comput. 2024, 20, 6175–6183. [Google Scholar] [CrossRef] [PubMed]
- Bosoni, E.; Beal, L.; Bercx, M.; Blaha, P.; Blügel, S.; Bröder, J.; Callsen, M.; Cottenier, S.; Degomme, A.; Dikan, V.; et al. How to verify the precision of density-functional-theory implementations via reproducible and universal workflows. Nat. Rev. Phys. 2024, 6, 45–58. [Google Scholar] [CrossRef]
- Gusarov, S. Advances in Computational Methods for Modeling Photocatalytic Reactions: A Review of Recent Developments. Materials 2024, 17, 2119. [Google Scholar] [CrossRef]
- Ribaldone, C.; Casassa, S. Born–Oppenheimer Molecular Dynamics with a Linear Combination of Atomic Orbitals and Hybrid Functionals for Condensed Matter Simulations Made Possible. Theory and Performance for the Microcanonical and Canonical, Ensembles. J. Chem. Theory Comput. 2024, 20, 3954–3975. [Google Scholar] [CrossRef] [PubMed]
- Bouzaid, A.; Ziat, Y.; Belkhanchi, H.; Hamdani, H.; Koufi, A.; Miri, M.; Laghlimi, C.; Zarhri, Z. Ab initio study of the structural, electronic, and optical properties of MgTiO3 perovskite materials doped with N and P. E3S Web Conf. 2024, 582, 02006. [Google Scholar] [CrossRef]
- Koufi, A.; Ziat, Y.; Belkhanchi, H.; Bouzaid, A. DFT and BoltzTrap investigations on the thermal and structural characteristics of the perovskite MgCuH3 and MgCoH3. Comput. Condens. Matter 2025, 42, e01010. [Google Scholar] [CrossRef]
- Koufi, A.; Ziat, Y.; Belkhanchi. Study of the Gravimetric, Electronic and Thermoelectric Properties of XAlH3 (X = Be, Na, K) as hydrogen storage perovskite using DFT and the BoltzTrap Software Package. Sol. Energy Sustain. Dev. J. 2024, 14, 53–66. [Google Scholar] [CrossRef]
- Ikeda, K.; Kogure, Y.; Nakamori, Y.; Orimo, S. Reversible hydriding and dehydriding reactions of perovskite-type hydride NaMgH3. Scripta Mater. 2005, 53, 319–322. [Google Scholar] [CrossRef]
- Nunez, J.A. High-Pressure and High-Temperature Synthesis of Light Perovskite Hydrides for Hydrogen Storage. Ph.D. Thesis, University Grenoble Alpes, Saint-Martin-d’Hères, France, 2022. [Google Scholar]
- Sato, T.; Noréus, D.; Takeshita, H.; Häussermann, U. Hydrides with the perovskite structure: General bonding and stability considerations and the new representative CaNiH3. J. Solid State Chem. 2005, 178, 3381–3388. [Google Scholar] [CrossRef]
- Ikeda, K.; Kato, S.; Ohoyama, K.; Nakamori, Y.; Takeshita, H.T.; Orimo, S. Formation of perovskite-type hydrides and thermal desorption processes in Ca–T–H (T = 3d transition metals). Scripta Mater. 2006, 55, 827–830. [Google Scholar] [CrossRef]
- Sato, R.; Saitoh, H.; Endo, N.; Takagi, S.; Matsuo, M.; Aoki, K.; Orimo, S.I. Formation process of perovskite-type hydride LiNiH3: In situ synchrotron radiation X-ray diffraction study. Appl. Phys. Lett. 2013, 102, 091901. [Google Scholar] [CrossRef]
- Koufi, A.; Ziat, Y.; Belkhanchi, H.; Miri, M.; Lakouari, N.; Bougayr, E.H.; Baghli, F.Z. A computational study of the structural and thermal conduct of MgCrH3 and MgFeH3 perovskite-type hydrides: FP-LAPW and BoltzTraP insight. E3S Web Conf. 2024, 582, 02003. [Google Scholar] [CrossRef]
- Ikeda, K.; Sato, T.; Orimo, S.I. Perovskite-type hydrides—Synthesis, structures and properties. Int. J. Mater. Res. 2008, 99, 471–479. [Google Scholar] [CrossRef]
- Yalcin, B.G.; Salmankurt, B.; Duman, S. Investigation of structural, mechanical, electronic, optical, and dynamical properties of cubic BaLiF3, BaLiH3, and SrLiH3. Mater. Res. Express 2016, 3, 036301. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.; Kvasnicka, D.; Luitz, J. wien2k. In An Augmented Plane Wave+ Local Orbitals Program for Calculating Crystal Properties; Vienna University of Technology: Vienna, Austria, 2001. [Google Scholar]
- Rehman, Z.U.; Rehman, M.A.; Rehman, B.; Sikiru, S.; Qureshi, S.; Ali, E.M.; Awais, M.; Amjad, M.; Iqbal, I.; Rafique, A.; et al. Ab initio insight into the physical properties of MgXH3 (X = Co, Cu, Ni) lead-free perovskite for hydrogen storage application. Environ. Sci. Pollut. Res. 2023, 30, 113889–113902. [Google Scholar] [CrossRef]
- ur Rehman, Z.; Rehman, M.A.; Rehman, B.; Amjad, M.; Awais, M.; Iqbal, I.; Rafique, A. A DFT study of structural, electronic, mechanical, phonon, thermodynamic, and H2 storage properties of lead-free perovskite hydride MgXH3 (X = Cr, Fe, Mn). J. Phys. Chem. Solids 2024, 186, 111801. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Lakhal, M.; Benzidi, H.; Mounkachi, O.; Benyoussef, A.; El Kenz, A.; Loulidi, M. The hydrogen storage properties of Mg-intermetallic-hydrides by ab initio calculations and kinetic Monte Carlo simulations. Int. J. Hydrogen Energy 2020, 45, 11158–11166. [Google Scholar] [CrossRef]
- Garara, M.; Benzidi, H.; Abdellaoui, M.; Lakhal, M.; Benyoussef, A.; Mounkachi, O.; Loulidi, M. Hydrogen storage properties of perovskite-type MgCoH3 under strain effect. Mater. Chem. Phys. 2020, 254, 123417. [Google Scholar] [CrossRef]
- Matara, S.F.; Al Alamc, A.F.; Ouainic, N. Hydrogen Insertion Effects on the Electronic Structure of Equiatomic MgNi Traced by ab initio Calculations. Z. Für Naturforschung B 2013, 68, 44–50. [Google Scholar] [CrossRef][Green Version]
- Selgin, A.; Iyigor, A. Structural, electronic, elastic and thermodynamic properties of hydrogen storage magnesium-based ternary hydrides. Chem. Phys. Lett. 2020, 743, 137184. [Google Scholar] [CrossRef]
- Murnaghan, F.D. On the theory of the tension of an elastic cylinder. Proc. Natl. Acad. Sci. USA 1944, 30, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.; Ziat, Y.; Belkhanchi, H.; Koufi, A.; El Kadi, Y.A. Modulating the Electronic and Optical Properties of InGeF3 Perovskite under Pressure: A Computational Approach. Eur. Phys. J. B 2025, 98, 47. [Google Scholar] [CrossRef]
- Vala, M.; Bhatt, M.; Pajtler, M.V.; Kansara, S.; Sonvane, Y. DFT Insights into 2D Yttrium Arsenide: Pioneering New Avenues in Optoelectronic Materials. Nano 2025, 10, 2650001. [Google Scholar] [CrossRef]
- Raza, H.; Hamid, G. Murtaza Optoelectronic and thermal properties of LiXH3 (X = Ba, Sr and Cs) for hydrogen storage materials: A first principle study. Solid State Commun. 2019, 299, 113659. [Google Scholar] [CrossRef]
- Surucu, G.; Candan, A.; Gencer, A.; Isik, M. First-principle investigation for the hydrogen storage properties of NaXH3 (X = Mn, Fe, Co) perovskite type hydrides. Int. J. Hydrogen Energy 2019, 44, 30218–30225. [Google Scholar] [CrossRef]
- Ededet, E.; Louis, H.; Chukwu, U.G.; Magu, T.O.; Udo, A.E.; Adalikwu, S.A.; Adeyinka, A.S. Ab Initio Study of the Effects of d-Block Metal (Mn, Re, Tc) Encapsulation on the Electronic, Phonon, Thermodynamic, and Gravimetric, Hydrogen Capacity of BaXH4 Hydride Perovskites. J. Electron. Mater. 2024, 53, 250–264. [Google Scholar] [CrossRef]
- Raza, H.H.; Murtaza, G.; Razzaq, S.; Azam, A. Improving thermodynamic properties and desorption temperature in MgH2 by doping Be: DFT study. Mol. Simul. 2023, 49, 497–508. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Zhang, J.; Hou, Q. Hydrogen storage performance of Mg/MgH2 and its improvement measures: Research progress and trends. Materials 2023, 16, 1587. [Google Scholar] [CrossRef]
- Cargioli, N. Standard Model Physics and Beyond in Low Energy Neutrino Scattering and Parity Violating Electron Interactions with Nuclei. Ph.D. Thesis, University of Cagliari, Cagliari, Italy, 2024. [Google Scholar]
- Takabe, H. Physical of Warm Dense Matters. In The Physics of Laser Plasmas and Applications-Volume 2: Fluid Models and Atomic Physics of Plasmas; Springer International Publishing: Cham, Switzerland, 2024; pp. 397–450. [Google Scholar]
- Diao, X. A Computational Study of Mixed Metal Oxides. Ph.D. Thesis, UCL (University College London), London, UK, 2024. [Google Scholar]
- Pan, Y. First-principles investigation of structural stability, electronic and optical properties of suboxide (Zr3O). Mater. Sci. Eng. B 2022, 281, 115746. [Google Scholar] [CrossRef]
- Pan, Y. The influence of Ag and Cu on the electronic and optical properties of ZrO from first-principles calculations. Mater. Sci. Semicond. Process. 2021, 135, 106084. [Google Scholar] [CrossRef]
- Candan, A.; Kurban, M. Electronic structure, elastic and phonon properties of perovskite-type hydrides MgXH3 (X = Fe, Co) for hydrogen storage. Solid State Commun. 2018, 281, 38–43. [Google Scholar] [CrossRef]
- Du, Y.; Xu, N.; Chen, S.; Chen, Y.; Song, R.; Luo, W.; Zhang, W. First-principles study of the hydrogen storage properties of hydride perovskites XCuH3 (X= K, Rb) for hydrogen storage applications. Int. J. Hydrogen Energy 2024, 78, 713–720. [Google Scholar] [CrossRef]
- Rehman, M.A.; Rehman, Z.U.; Usman, M.; Hamad, A. The DFT study of the structural, hydrogen, electronic, mechanical, thermal, and optical properties of KXH3 (X = Ca, Sc, Ti, Ni) perovskites for H2 storage applications. Struct. Chem. 2024, 36, 235–248. [Google Scholar] [CrossRef]
- Tang, T.; Tang, Y. First-principles investigations for the structural, optoelectronic and hydrogen storage properties of double perovskite KNaMg2F6-xHx and KNaAe2H6 (Ae = Be, Mg, Ca). Int. J. Hydrogen Energy 2024, 61, 13–24. [Google Scholar] [CrossRef]
- Halais, W.T.; Doghmane, M.; Bouhlala, A.; Chettibi, S. Structural, magnetic, and optoelectronic properties of rock salt MgO co-doped with Eu-TM (TM = Cu, Ag, and Au) for spintronic and UV detector applications: SP-DFT investigations. Mod. Phys. Lett. B 2024, 38, 2450180. [Google Scholar] [CrossRef]
- Pan, Y. Influence of N-vacancy on the electronic and optical properties of bulk GaN from first-principles investigations. Int. J. Energy Res. 2021, 45, 15512–15520. [Google Scholar] [CrossRef]
- Yaseen, M.; Ambreen, H.; Mehmood, R.; Munawar, I.; Nessrin, A.; Alshahrani, T.; Noreen, S.; Laref, A. Investigation of optical and thermoelectric properties of PbTiO3 under pressure. Phys. B Condens. Matter 2021, 615, 412857. [Google Scholar] [CrossRef]
- Ding, C.-H.; Duan, Z.-F.; Ding, Z.-K.; Pan, H.; Wang, J.; Xiao, W.-H.; Liu, W.-P.; Li, Q.-Q.; Luo, N.-N.; Zeng, J. XMoSiN2 (X = S, Se, Te): A novel 2D Janus semiconductor with ultra-high carrier mobility and excellent thermoelectric performance. Europhys. Lett. 2023, 143, 1. [Google Scholar] [CrossRef]
- Azadparvar, M.; Rahnamaye Aliabad, H.A.; Özdemir, E.G. Optoelectronic and thermoelectric properties of Sb2S3 under hydrostatic pressure for energy conversion. AIP Adv. 2023, 13, 065218. [Google Scholar] [CrossRef]
- Li, J.C.; Li, D.; Qin, X.Y.; Zhang, J. Enhanced thermoelectric performance of p-type SnSe doped with Zn. Scr. Mater. 2017, 126, 6–10. [Google Scholar] [CrossRef]
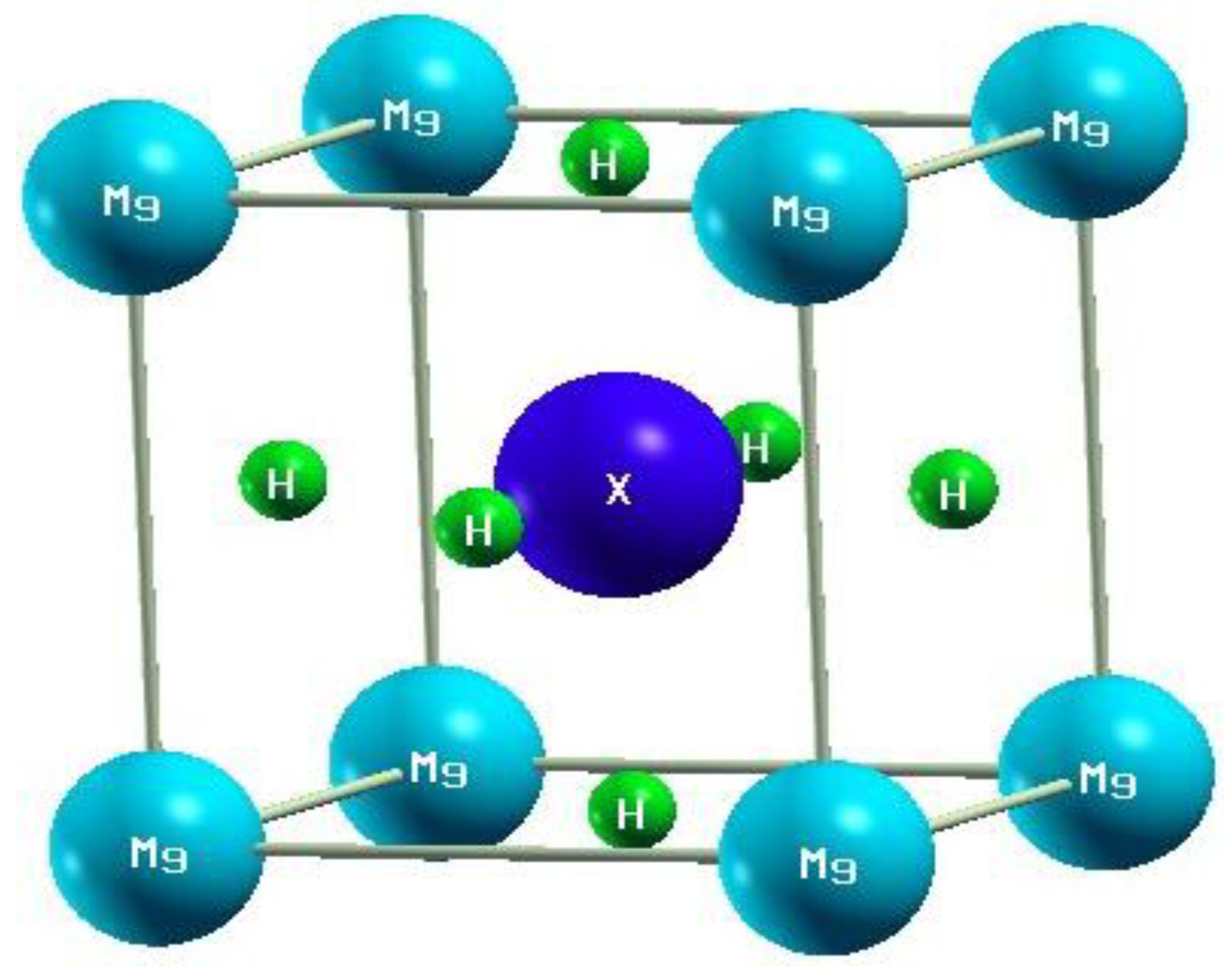
| Parameter (a0 = b0 = c0) | |||
|---|---|---|---|
| Exp. Values (Å) | Theoretical Values (Å) | Lattice Parameters Optimize (Å) | |
| MgCrH3 | 3.45 [41] | 3.4590 [41] | 3.3807 |
| MgMnH3 | 3.34 [41] | 3.3485 [41] | 3.3199 |
| MgFeH3 | 3.02 [41] | 3.0284 [41] | 3.2958 |
| MgCoH3 | 3.31 [42] | 3.278 [43] | 3.2997 |
| MgNiH3 | 3.32 [44] | 3.370 [42] | 3.3572 |
| MgCuH3 | 3.32 [44] | 3.456 [45] | 3.4817 |
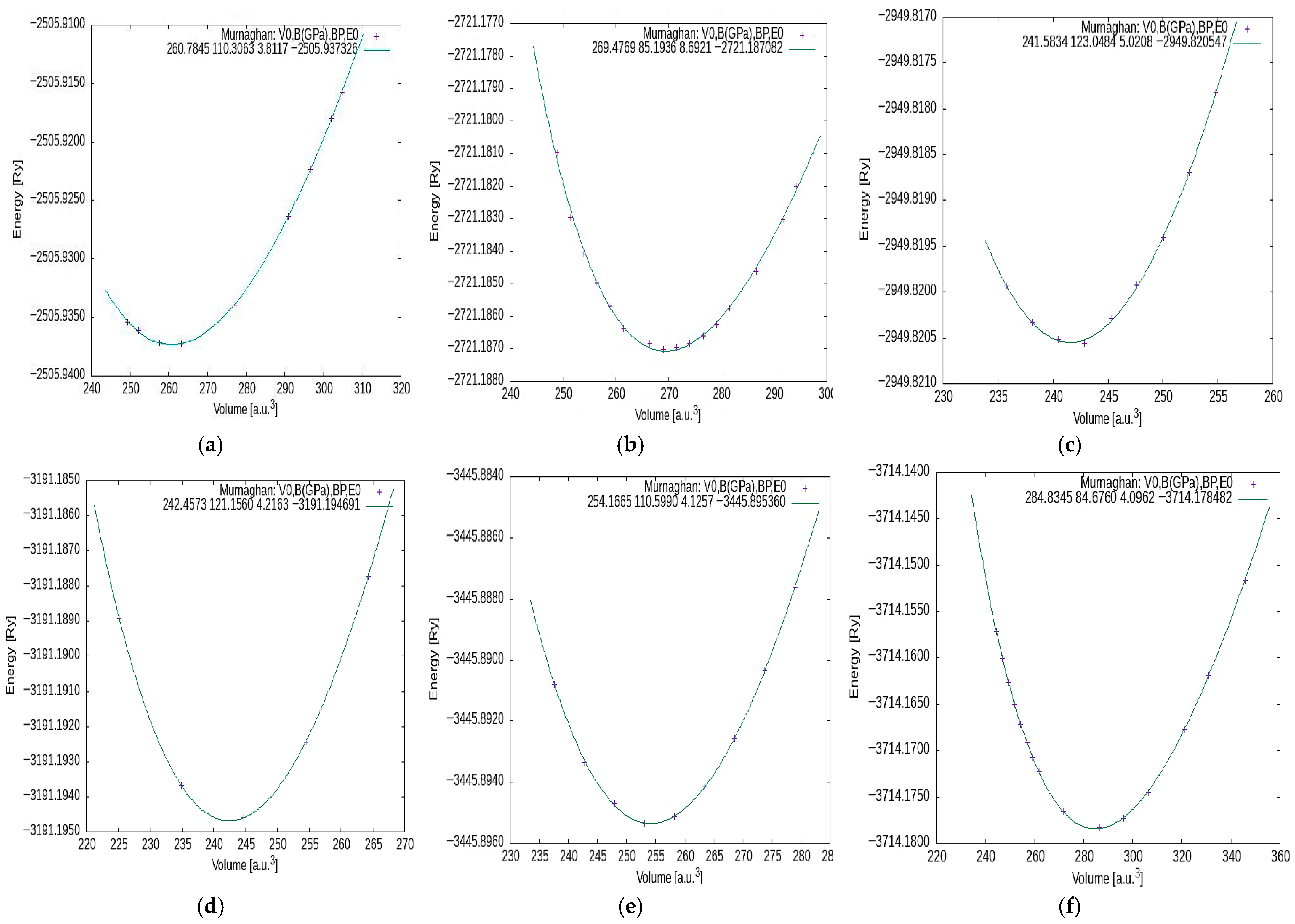
| Compound | Lattice Parameters Optimize (Å) | Volume V (Å3) | Total Energy E (Ry) | Bulk Modulus B (GPa) | Pressure Derivative B′ |
|---|---|---|---|---|---|
| MgCrH3 | 3.3807 | 38.67 | −2505.93726 | 110.3063 | 3.8117 |
| MgMnH3 | 3.3199 | 36.58 | −2721.18708 | 119.2684 | 3.6840 |
| MgFeH3 | 3.2958 | 35.78 | −2949.82054 | 123.0484 | 5.0208 |
| MgCoH3 | 3.2997 | 35.93 | −3191.19469 | 121.1560 | 4.2163 |
| MgNiH3 | 3.3572 | 37.68 | −3445.89536 | 113.8785 | 4.2121 |
| MgCuH3 | 3.4817 | 42.20 | −3714.17848 | 84.6760 | 4.0962 |
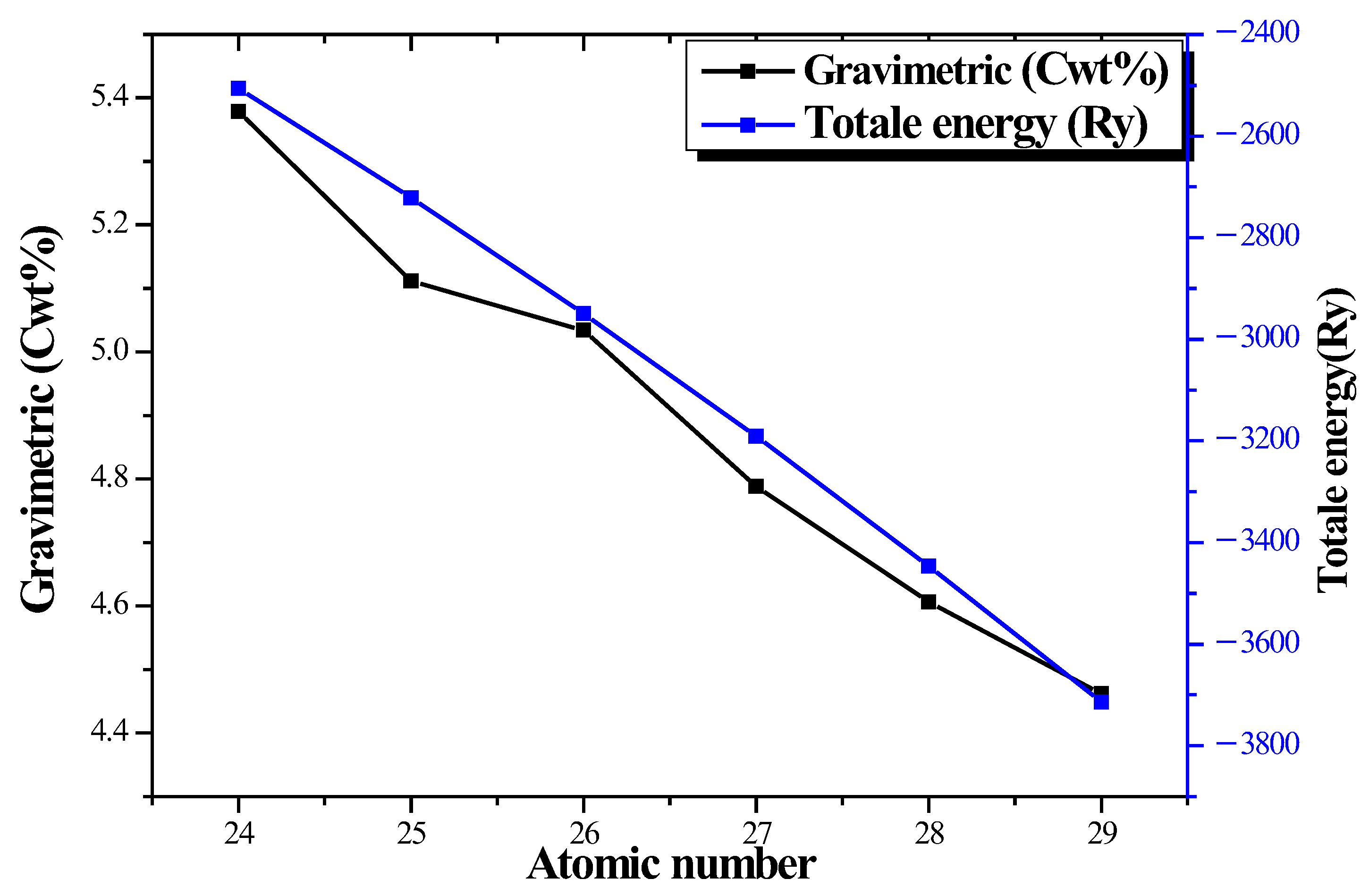
| Element | Atomic Number | Gravimetric (Cwt%) | Total Energy E (Ry) |
|---|---|---|---|
| Cr | 24 | 5.378 | −2505.93726 |
| Mn | 25 | 5.111 | −2721.18708 |
| Fe | 26 | 5.034 | −2949.82054 |
| Co | 27 | 4.788 | −3191.19469 |
| Ni | 28 | 4.607 | −3445.89536 |
| Cu | 29 | 4.462 | −3714.17848 |
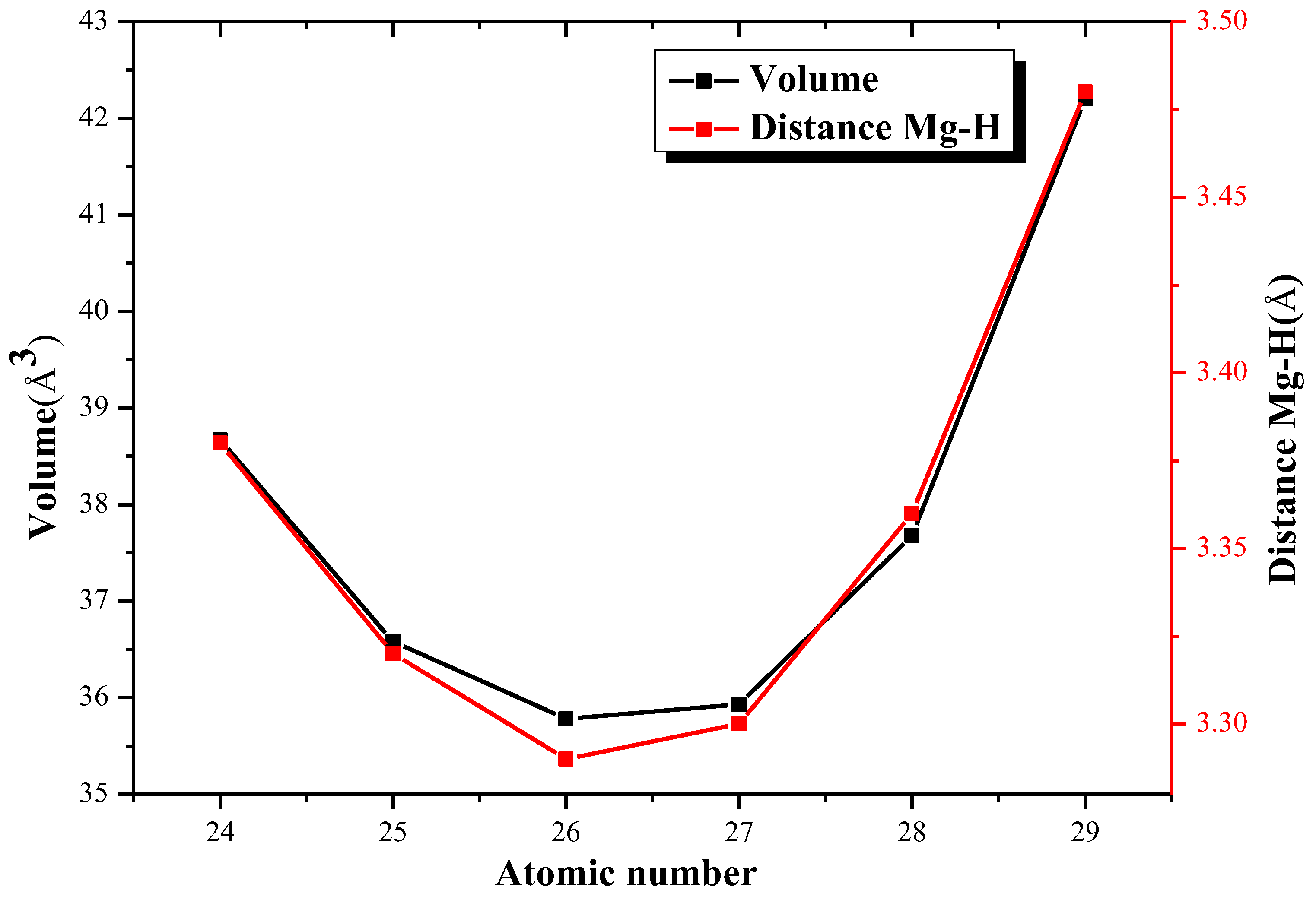
| Element | Atomic Number | Volume (Å3) | Distance (Å) |
|---|---|---|---|
| Cr | 24 | 38.67 | 3.38 |
| Mn | 25 | 36.58 | 3.32 |
| Fe | 26 | 35.78 | 3.29 |
| Co | 27 | 35.93 | 3.30 |
| Ni | 28 | 37.68 | 3.36 |
| Cu | 29 | 42.20 | 3.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koufi, A.; Ziat, Y.; Belkhanchi, H. First-Principles Investigation of Structural, Electronic, Thermoelectric, and Hydrogen Storage Properties of MgXH3 (X = Cr, Mn, Fe, Co, Ni, Cu) Perovskite Hydrides. Hydrogen 2025, 6, 106. https://doi.org/10.3390/hydrogen6040106
Koufi A, Ziat Y, Belkhanchi H. First-Principles Investigation of Structural, Electronic, Thermoelectric, and Hydrogen Storage Properties of MgXH3 (X = Cr, Mn, Fe, Co, Ni, Cu) Perovskite Hydrides. Hydrogen. 2025; 6(4):106. https://doi.org/10.3390/hydrogen6040106
Chicago/Turabian StyleKoufi, Ayoub, Younes Ziat, and Hamza Belkhanchi. 2025. "First-Principles Investigation of Structural, Electronic, Thermoelectric, and Hydrogen Storage Properties of MgXH3 (X = Cr, Mn, Fe, Co, Ni, Cu) Perovskite Hydrides" Hydrogen 6, no. 4: 106. https://doi.org/10.3390/hydrogen6040106
APA StyleKoufi, A., Ziat, Y., & Belkhanchi, H. (2025). First-Principles Investigation of Structural, Electronic, Thermoelectric, and Hydrogen Storage Properties of MgXH3 (X = Cr, Mn, Fe, Co, Ni, Cu) Perovskite Hydrides. Hydrogen, 6(4), 106. https://doi.org/10.3390/hydrogen6040106







