Microbial Biogas Production from Pork Gelatine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Measuring
2.2. Equipment and Analysis
3. Results and Discussion
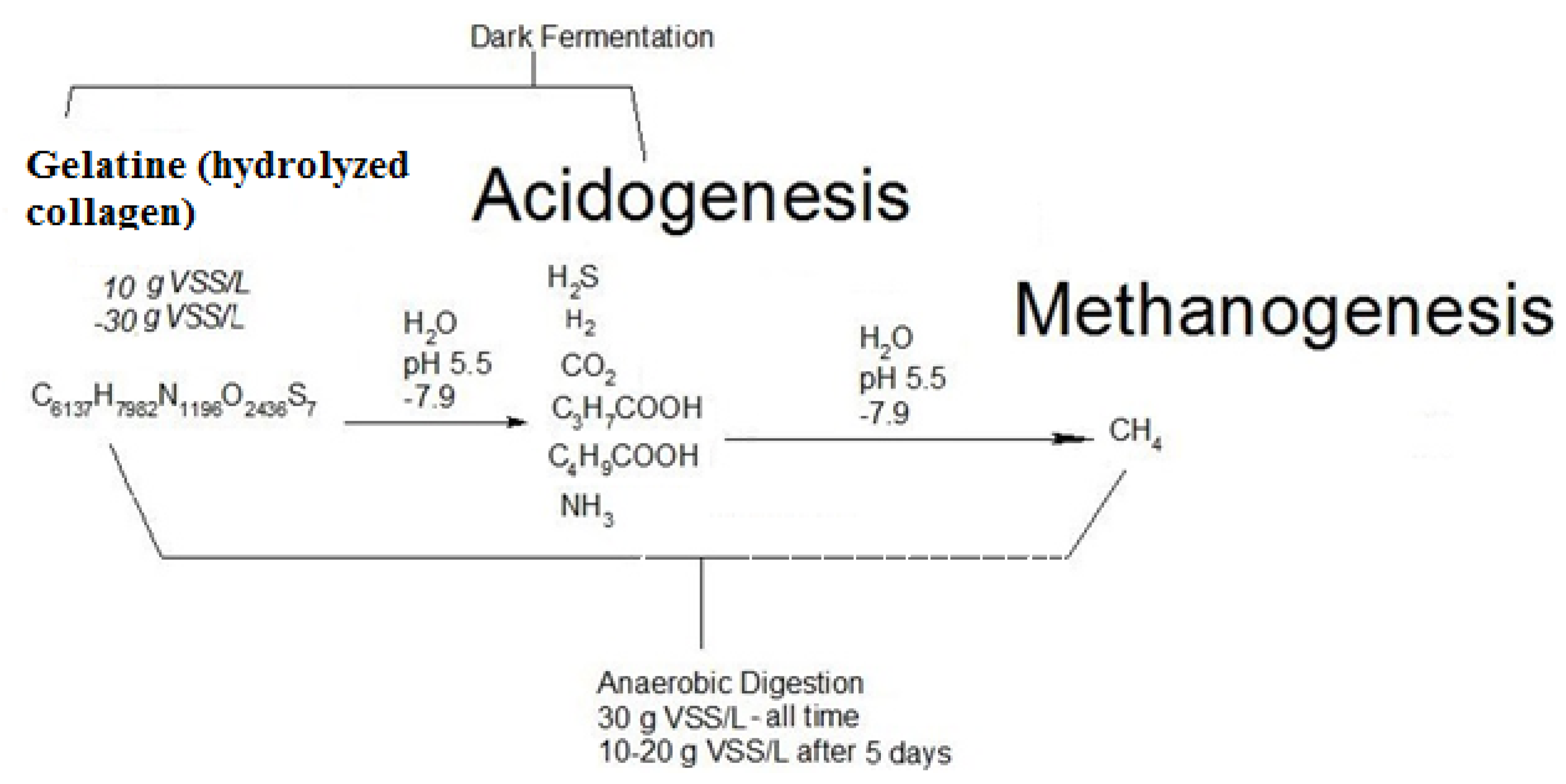
3.1. Biogas Pathways
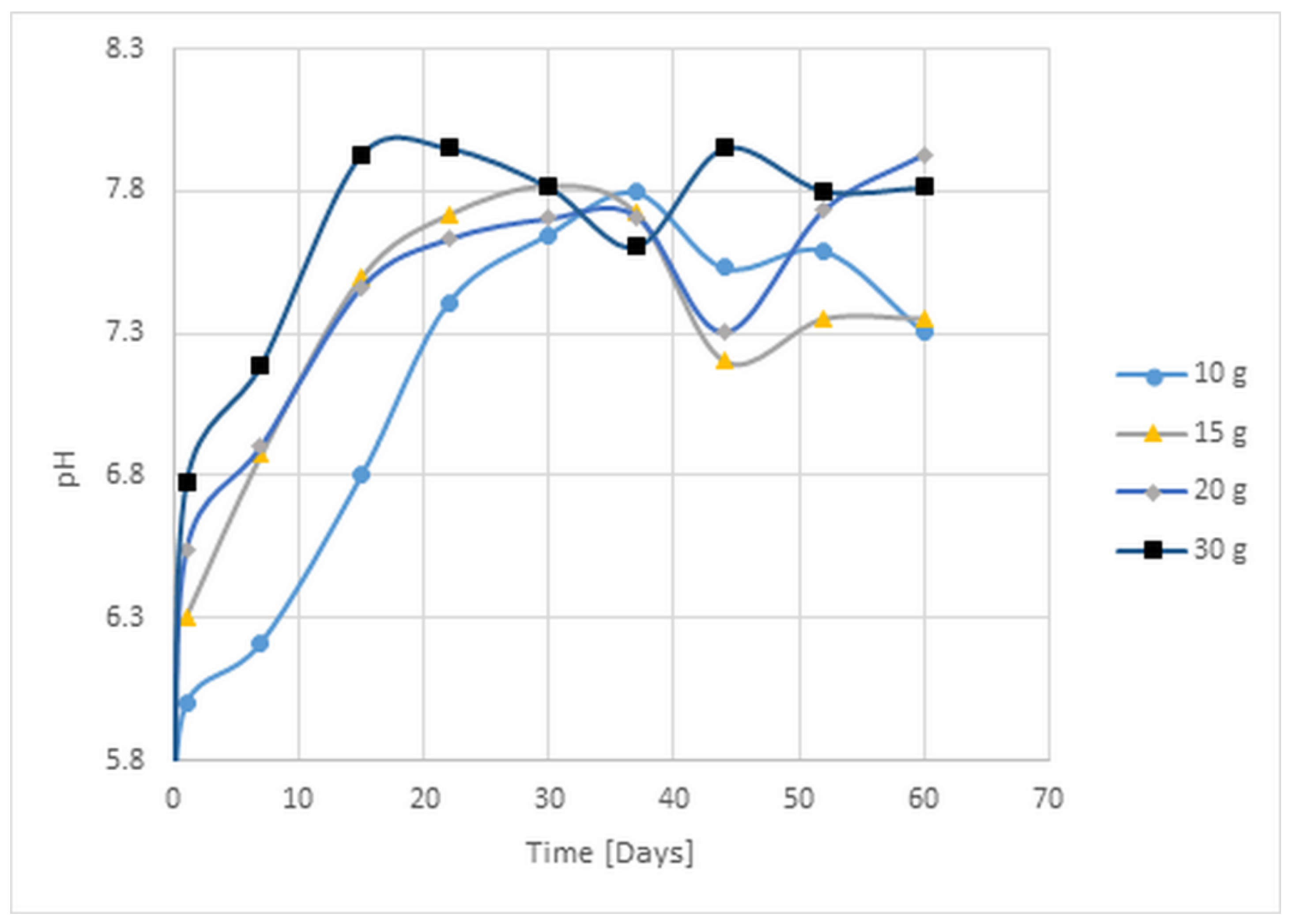
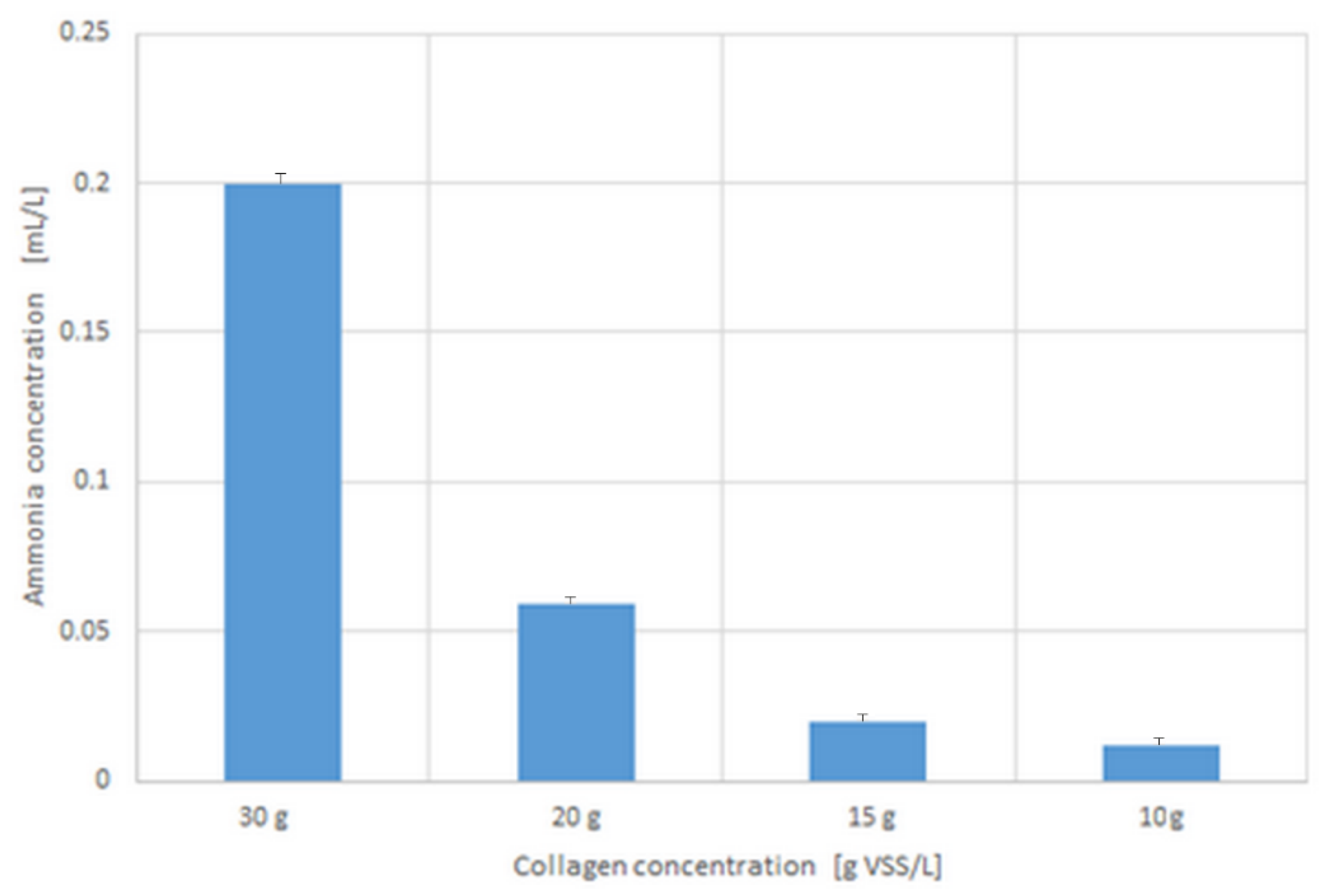
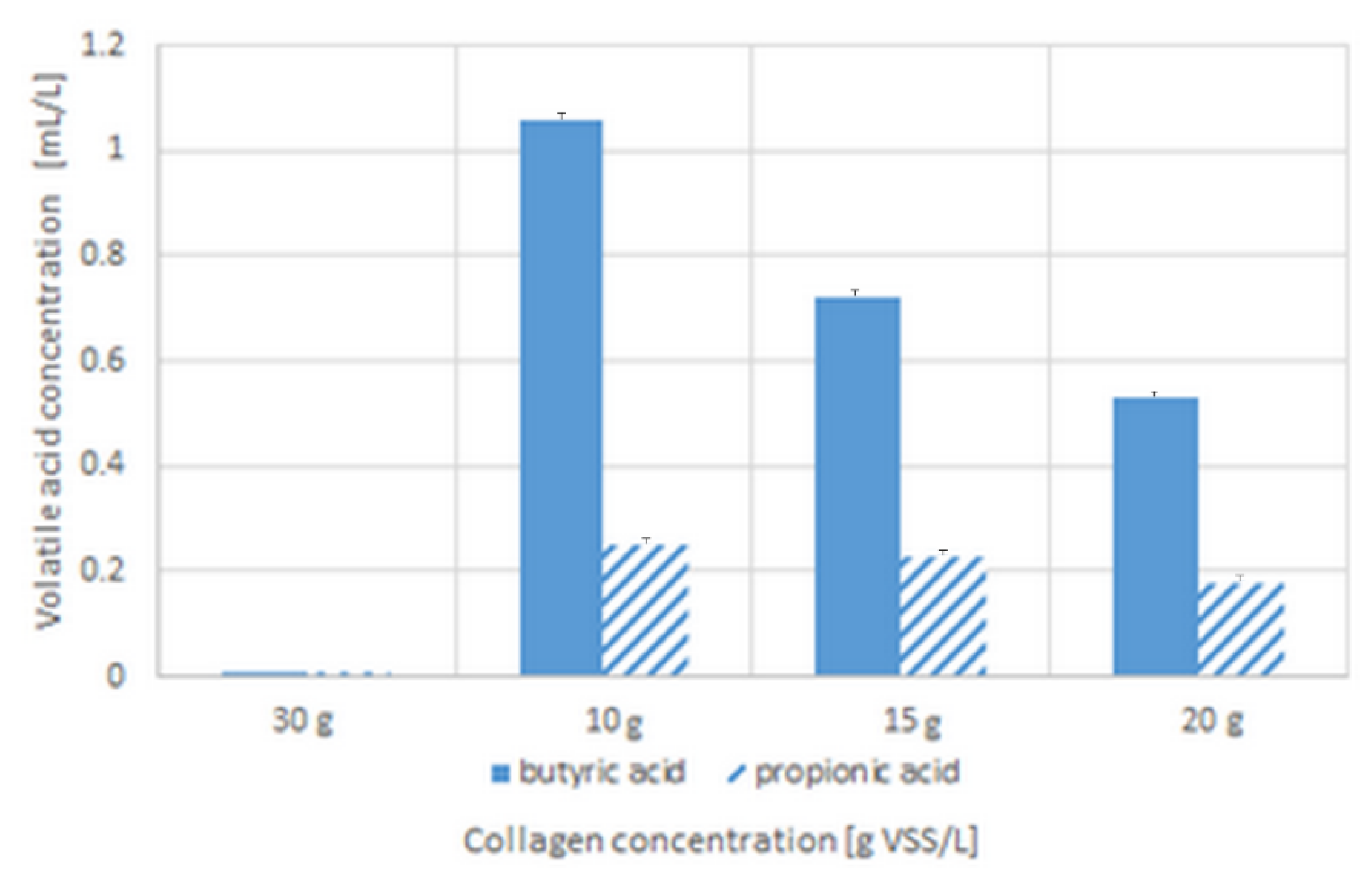
3.2. pH Change, Ammonia, and Volatile Organic Acids Concentrations
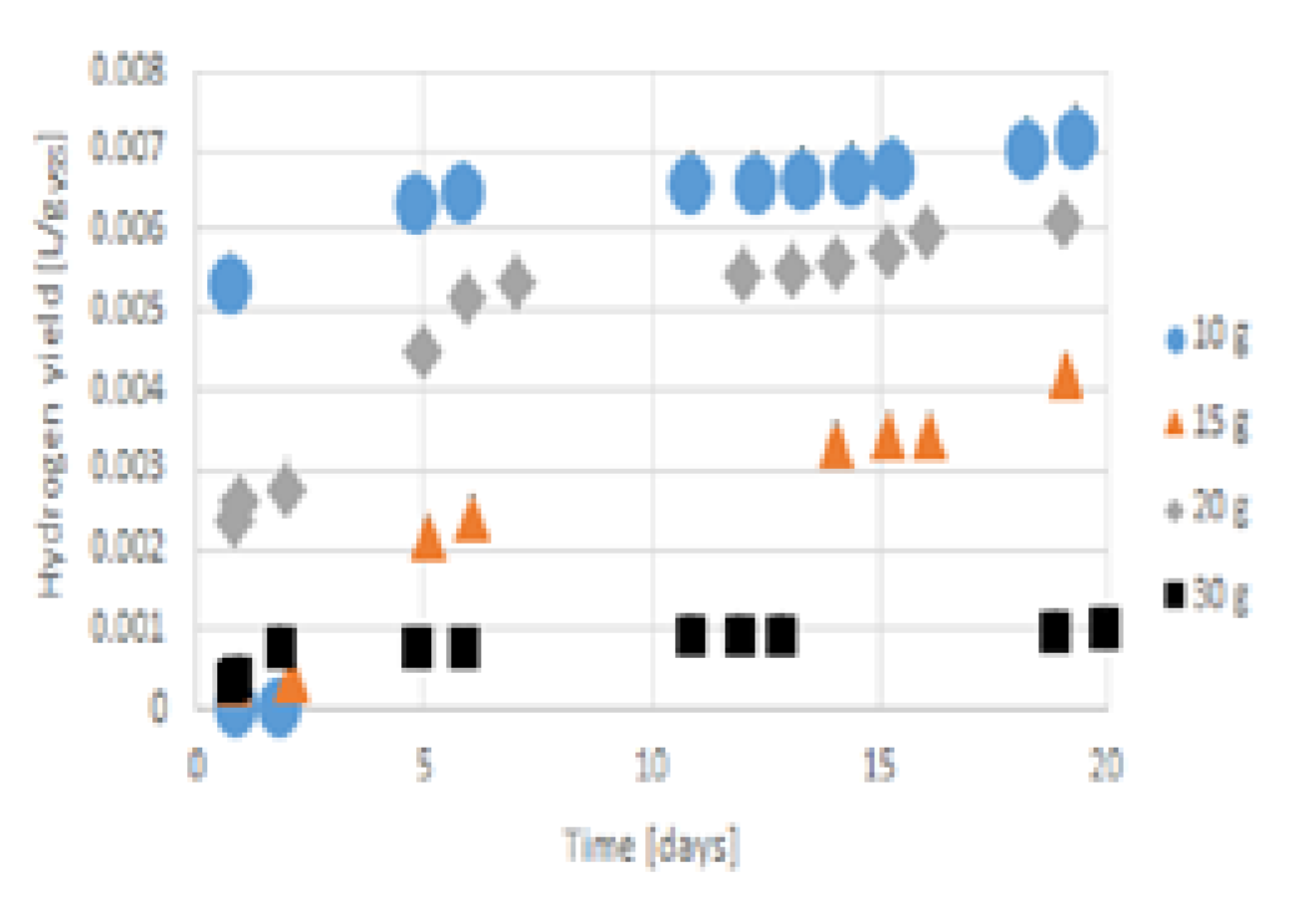
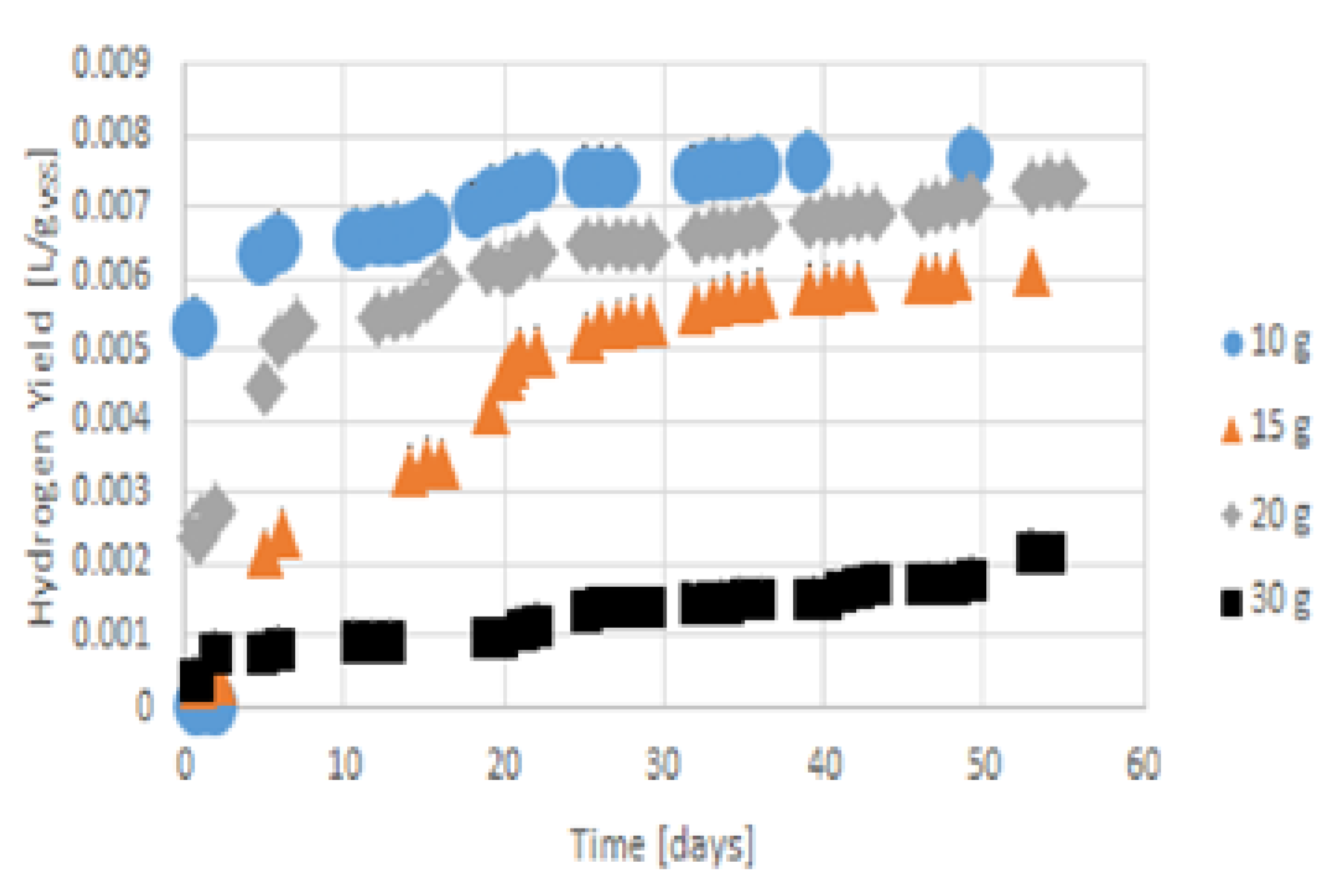
3.3. Hydrogen Production
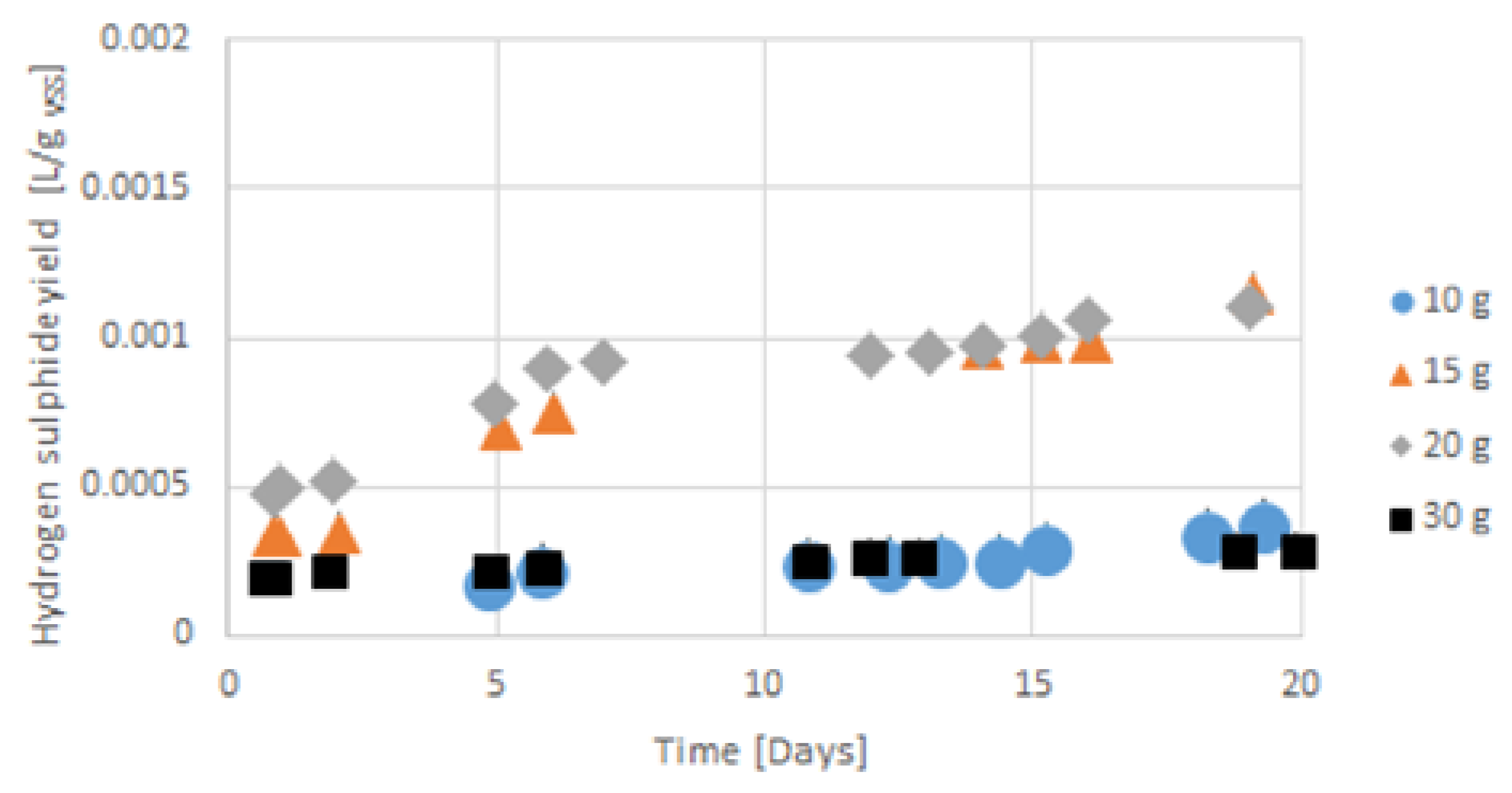
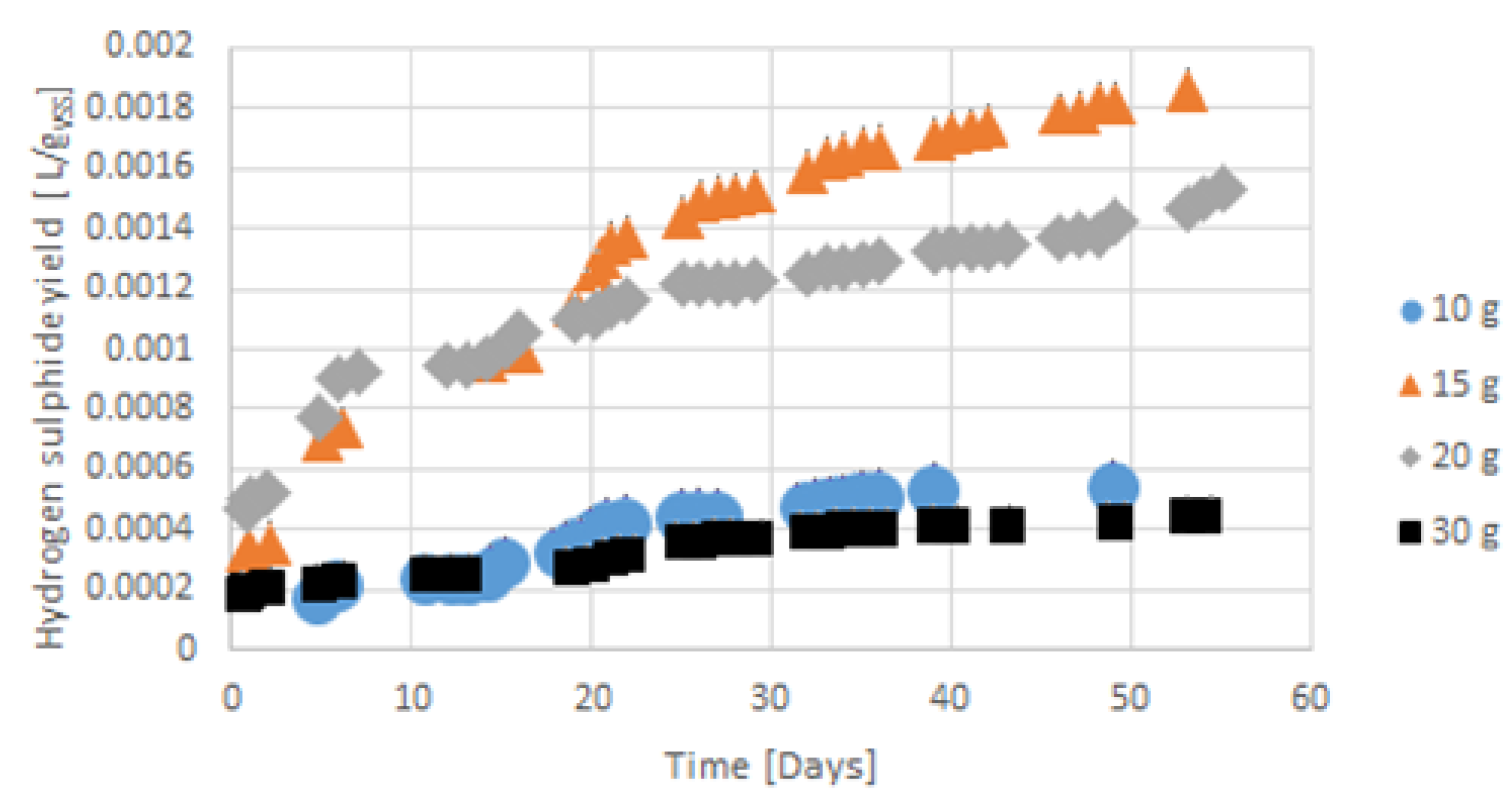
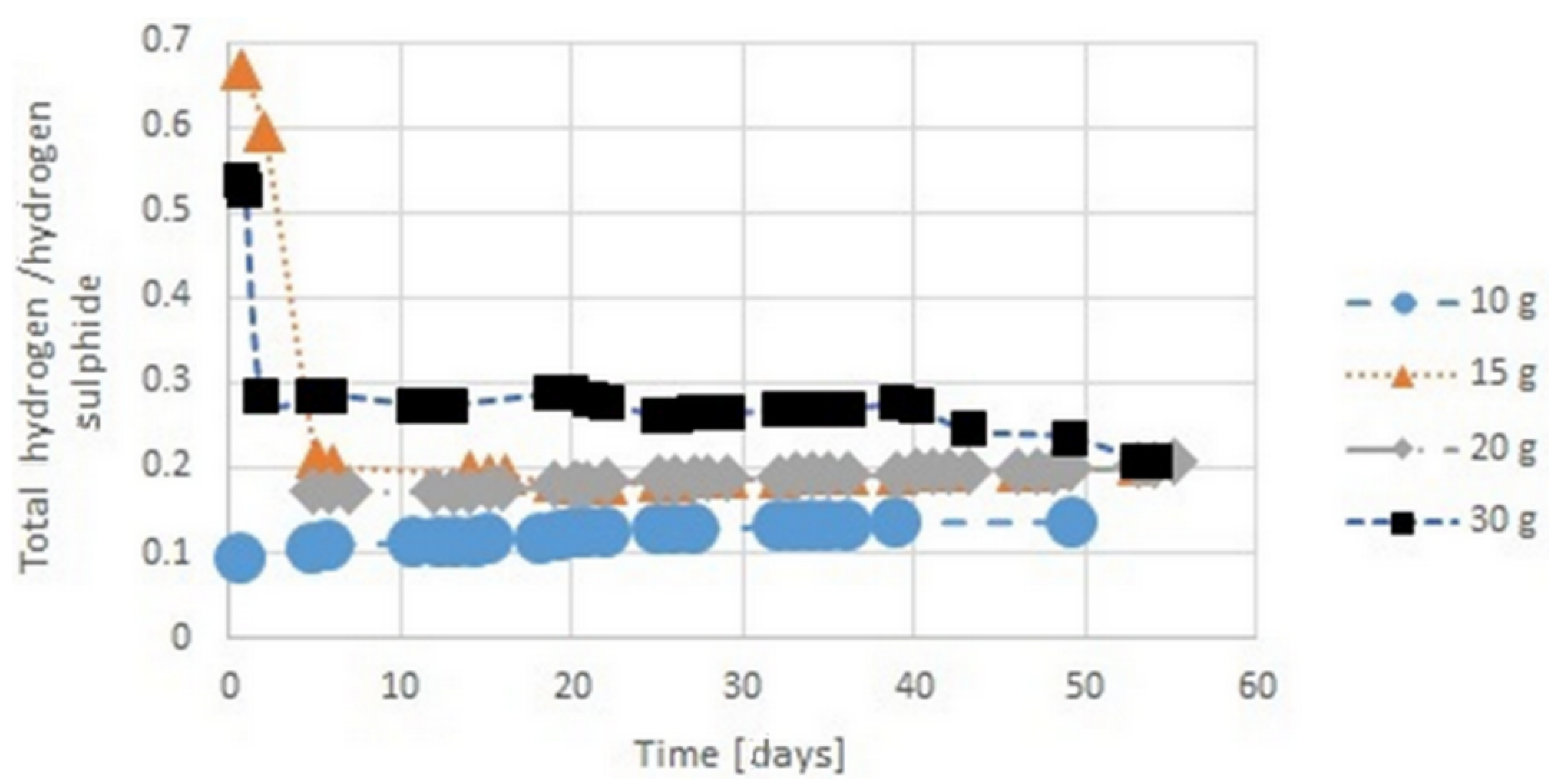
3.4. Hydrogen Sulphide Emission
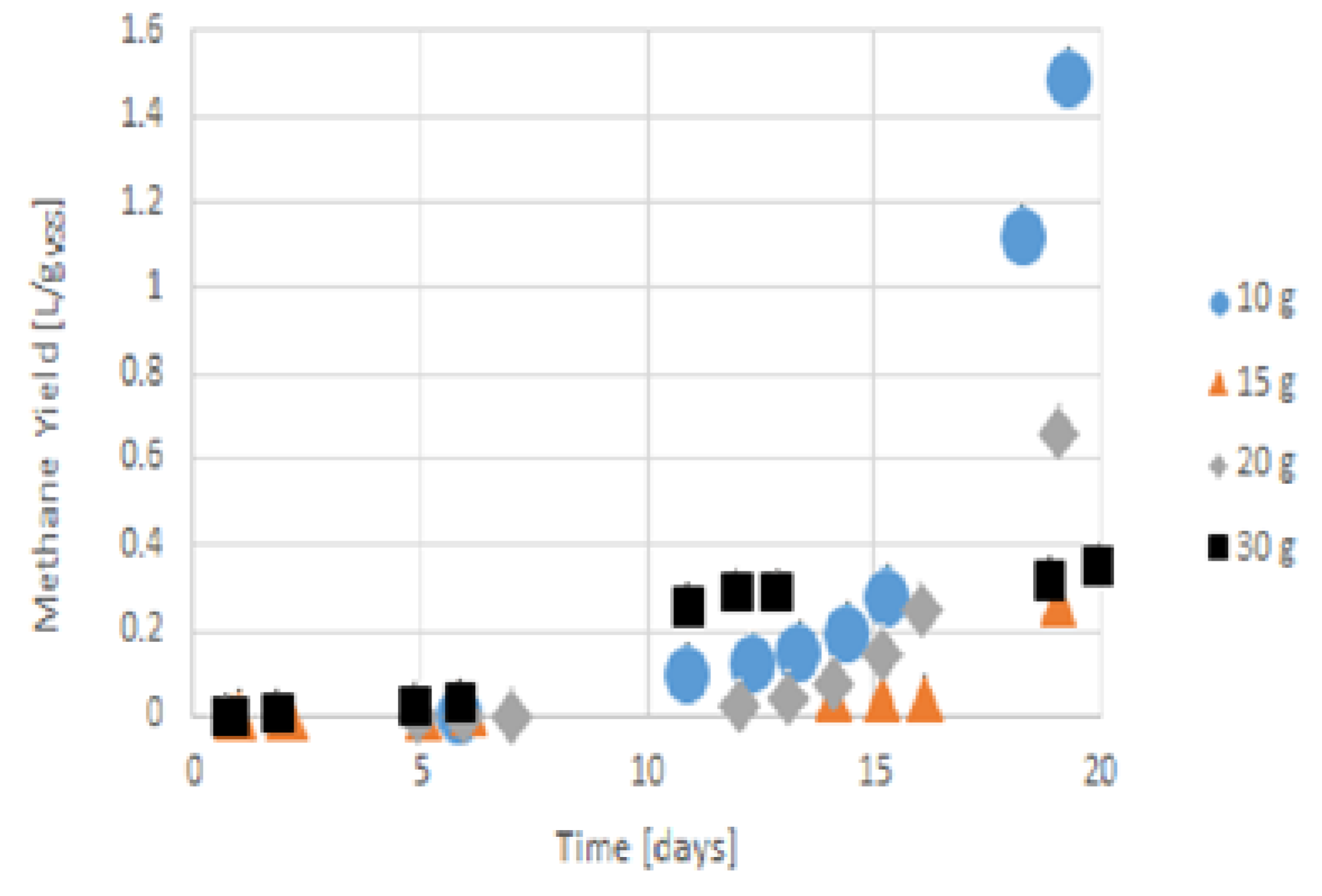
3.5. Methane Production and Overall Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; de Montoya-Rosales, J.J.; Razo-Flores, E. Advances towards the understanding of microbial communities in dark fermentation of enzymatic hydrolysates: Diversity, structure and hydrogen production performance. Int. J. Hydrogen Energy 2021, 46, 27459–27472. [Google Scholar] [CrossRef]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.P.; Escudié, R. Temperature and inoculum origin influence the performance of ex-situ biological hydrogen methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, Ö.; Cetecioglu, Z. Bioconversion of food waste to volatile fatty acids: Impact of microbial community, pH and retention time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef]
- Cho, S.C.E.; Deok, J.L.; Moon, H. Synergistic benefits for hydrogen production through CO2—Cofeeding catalytic pyrolysis of cellulosic biomass waste. Cellulose 2021, 28, 4781–4792. [Google Scholar] [CrossRef]
- Elreedy, A.; Fujii, M.; Tawfik, A. Psychrophilic hydrogen production from petrochemical wastewater via anaerobic sequencing batch reactor: Techno-economic assessment and kinetic modelling. Int. J. Hydrogen Energy 2019, 44, 5189–5202. [Google Scholar] [CrossRef]
- Rojas, J.C.; Ramírez, K.G.; Velasquez, P.E.; Acevedo, P.; Santis, A. Evaluation of bio-hydrogen production by dark fermentation from Cocoa waste mucilage. Chem. Eng. Trans. 2020, 79, 283–288. [Google Scholar] [CrossRef]
- Masilela, P.; Pradhan, A. Application of thermophilic temperatures, low hydraulic retention times and high recycling of de-gassed effluent for higher biohydrogen production. Int. J. Hydrogen Energy 2020, 46, 7176–7182. [Google Scholar] [CrossRef]
- Urbaniec, K.; Grabarczyk, R. Raw materials for fermentative hydrogen production. J. Clean. Prod. 2009, 17, 959–962. [Google Scholar] [CrossRef]
- Panagiotopoulos, J.A.; Bakker, R.R.; De Vrije, T.; Urbaniec, K.; Koukios, E.G.; Claassen, P.A.M. Prospects of utilization of sugar beet carbohydrates for biological hydrogen production in the EU. J. Clean. Prod. 2010, 18, S9–S14. [Google Scholar] [CrossRef]
- Detman, A.; Mielecki, D.; Chojnacka, A.; Salamon, A.; Błaszczyk, M.K.; Sikora, A. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors. Microb. Cell Fact. 2019, 18, 36. [Google Scholar] [CrossRef]
- Abreu, C.; Rabelo, B.S.; Soares, A.L.; Kimiko, I.; Luiz, E.; Bernadete, M.; Varesche, A. Optimization of hydrogen and organic acids productions with autochthonous and allochthonous bacteria from sugarcane bagasse in batch reactors Optimization of hydrogen and organic acids productions with autochthonous and allochthonous bacteria from sugarca. J. Environ. Manag. 2018, 223, 952–963. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Shalaby, M.; Cenian, A. Rapid hydrogen generation from cotton wastes by mean of dark fermentation. SN Appl. Sci. 2020, 2, 1438. [Google Scholar] [CrossRef]
- Silva, J.S.; Mendes, J.S.; Correia, J.A.C.; Rocha, M.V.P.; Micoli, L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J. Biotechnol. 2018, 286, 71–78. [Google Scholar] [CrossRef]
- Mıynat, M.E.; Argun, H. Prevention of substrate and product inhibitions by using a dilution strategy during dark fermentative hydrogen production from molasses. Int. J. Hydrogen Energy 2020, 45, 34695–34706. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Ramprakash, B.; Ramadoss, G.; Suresh, S.; Pugazhendhi, A.; Incharoensakdi, A. High potential of Rhizopus treated rice bran waste for the nutrient-free anaerobic fermentative biohydrogen production. Bioresour. Technol. 2021, 319, 124193. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ayeni, A.O.; Daramola, M.O. Parametric optimization of biohydrogen production from potato waste and scale-up study using immobilized anaerobic mixed sludge. Waste Biomass Valoriz. 2019, 10, 1177–1189. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- François, E.; Dumas, C.; Gougeon, R.D.; Alexandre, H.; Vuilleumier, S.; Ernst, B. Unexpected high production of biohydrogen from the endogenous fermentation of grape must deposits. Bioresour. Technol. 2020, 320, 124334. [Google Scholar] [CrossRef]
- Pecorini, I.; Baldi, F.; Iannelli, R. Biochemical hydrogen potential tests using different inocula. Sustainability 2019, 11, 622. [Google Scholar] [CrossRef] [Green Version]
- Keskin, T.; Abubackar, H.N.; Yazgin, O.; Gunay, B.; Azbar, N. Effect of percolation frequency on biohydrogen production from fruit and vegetable wastes by dry fermentation. Int. J. Hydrogen Energy 2019, 44, 18767–18775. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Rupani, P.F.; Krishnan, S.; Ahmad, F.; Rezania, S.; Rashid, M.A.; Sha, C.; Md Din, M.F. Biohydrogen production via thermophilic fermentation: A prospective application of Thermotoga species. Energy 2020, 197, 117199. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.P. Potentialities of dark fermentation effluents as substrates for microalgae growth: A review. Process Biochem. 2015, 51, 1843–1854. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Abd Rahman, N. Food waste and food processing waste for biohydrogen production: A review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Iannelli, R.; Pecorini, I.; Polettini, A.; Pomi, R.; Rossi, A. Influence of the pH control strategy and reactor volume on batch fermentative hydrogen production from the organic fraction of municipal solid waste. Waste Manag. Res. 2019, 37, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Devos, P.; Haddad, M.; Carrère, H. Thermal hydrolysis of municipal sludge: Finding the temperature sweet spot: A review. Waste Biomass Valoriz. 2020, 12, 2187–2205. [Google Scholar] [CrossRef]
- Gómez, X.; Fernández, C.; Fierro, J.; Sánchez, M.E.; Escapa, A.; Morán, A. Hydrogen production: Two stage processes for waste degradation. Bioresour. Technol. 2011, 102, 8621–8627. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberan, A.; Chen, Y.; Blaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- García Depraect, O.; Muñoz, R.; van Lier, J.B.; Rene, E.B.; Diaz-Cruces, V.F.; León Becerril, E. Three-stage process for tequila vinasse valorization through sequential lactate, biohydrogen and methane production. Bioresour. Technol. 2020, 307, 123160. [Google Scholar] [CrossRef]
- Panin, S.; Setthapun, W.; Elizabeth Sinsuw, A.A.; Sintuya, H.; Chu, C.Y. Biohydrogen and biogas production from mashed and powdered vegetable residues by an enriched microflora in dark fermentation. Int. J. Hydrogen Energy 2020, 46, 14073–14082. [Google Scholar] [CrossRef]
- Dai, L.; He, C.; Wang, Y.; Liu, Y.; Ruan, R.; Zhou, Y.; Duan, D.; Fan, L.; Zhao, Y.; Yu, Z. Hydrothermal pretreatment of bamboo sawdust using microwave irradiation. Bioresour. Technol. 2018, 247, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Dauptain, K.; Schneider, A.; Noguer, M.; Fontanille, P.; Escudie, R.; Carrère, H.; Trably, E. Impact of microbial inoculum storage on dark fermentative H2 production. Bioresour. Technol. 2020, 319, 124234. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J. Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: Energy conversion and microbial community. Energy Convers. Manag. 2020, 225, 113436. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Fuentes, L.; Etchebehere, C.; Bernet, N.; Trably, E. Glucose electro-fermentation with mixed cultures: A key role of the Clostridiaceae family. Int. J. Hydrogen Energy 2020, 46, 1694–1704. [Google Scholar] [CrossRef]
- Gomes, S.D.; Fuess, L.T.; Penteado, E.D.; Lucas, S.D.M.; Gotardo, J.; Zaiat, M. The application of an innovative continuous multiple tube reactor as a strategy to control the specific organic loading rate for biohydrogen production by dark fermentation. Bioresour. Technol. 2015, 197, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Penteado, E.D.; Lazaro, C.Z.; Sakamoto, I.K.; Zaiat, M. Influence of seed sludge and pretreatment method on hydrogen production in packed-bed anaerobic reactors. Int. J. Hydrogen Energy 2013, 38, 6137–6145. [Google Scholar] [CrossRef]
- Prabakar, D.; Suvetha, K.S.; Manimudi, V.T.; Mathimani, T.; Kumar, G.; Rene, A.D.; Pugazhendhi, A. Pretreatment technologies for industrial effluents: Critical review on bioenergy production and environmental concerns. J. Environ. Manag. 2018, 218, 165–180. [Google Scholar] [CrossRef]
- Arun, J.; Panchamoorthy, K.; Sivaramakrishnan, R.; Sundarrajan, P.; Malolan, R.; Pugazhendhi, A. Technical insights into the production of green fuel from CO 2 sequestered algal biomass: A conceptual review on green energy. Sci. Total Environ. 2020, 755, 142636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mathimani, T.; Rene, E.R.; Pugazhendhi, A. Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. Int. J. Hydrogen Energy 2019, 44, 13106–13113. [Google Scholar] [CrossRef]
- Azbar, N.; Dokgöz, F.T.; Keskin, T.; Eltem, R.; Korkmaz, K.S.; Gezgin, Y.; Akbal, Z.; Öncel, S.; Dalay, M.C.; Gönen, Ç.; et al. Comparative evaluation of bio-hydrogen production from cheese whey wastewater under thermophilic and mesophilic anaerobic conditions. Int. J. Green Energy 2009, 6, 192–200. [Google Scholar] [CrossRef]
- Wang, J.L.; Wan, W. Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrogen Energy 2008, 33, 2934–2941. [Google Scholar] [CrossRef]
- Mahata, C.; Ray, S.; Das, D. Optimization of dark fermentative hydrogen production from organic wastes using acidogenic mixed consortia. Energy Convers. Manag. 2020, 219, 113047. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Kucharska, K.; Cieśliński, H.; Rybarczyk, P.; Słupek, E.; Łukajtis, R.; Wychodnik, K.; Kamiński, M. Fermentative conversion of two-step pre-treated lignocellulosic biomass to hydrogen. Catalysts 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Kalamaras, S.D.; Vasileiadis, S.; Karas, P.; Angelidaki, I.; Kotsopoulos, T.A. Microbial adaptation to high ammonia concentrations during anaerobic digestion of manure-based feedstock: Biomethanation and 16S rRNA gene sequencing. J. Chem. Technol. Biotechnol. 2020, 95, 1970–1979. [Google Scholar] [CrossRef]
- Poirier, S.; Steyer, J.P.; Bernet, N.; Trably, E. Mitigating the variability of hydrogen production in mixed culture through bioaugmentation with exogenous pure strains. Int. J. Hydrogen Energy 2019, 45, 2617–2626. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Yanxin, W.; Qiuxiang, X.; Wang, D.; Yang, Q.; Liu, Y.; Bing-Jie, N.; Wang, Q.X. Freezing in the presence of nitrite pretreatment enhances hydrogen production from dark fermentation of waste activated sludge. J. Clean. Prod. 2020, 248, 119305. [Google Scholar] [CrossRef]
- Gallipoli, A.; Braguglia, C.M.; Gianico, A.; Montecchio, D.; Pagliaccia, P. Kitchen waste valorization through a mild-temperature pretreatment to enhance biogas production and fermentability: Kinetics study in mesophilic and thermophilic regimen. J. Environ. Sci. 2020, 89, 167–179. [Google Scholar] [CrossRef]
- Hovorukha, V.; Havryliuk, O.; Gladka, G.; Tashyrev, O.; Kalinichenko, A.; Sporek, M.; Dołhańczuk-Śródka, A. Hydrogen dark fermentation for degradation of solid and liquid food waste. Energies 2021, 14, 1831. [Google Scholar] [CrossRef]
- Masilamani, D.; Madhan, B.; Shanmugam, G.; Palanivel, S.; Narayan, B. Extraction of collagen from raw trimming wastes of tannery: A waste to wealth approach. J. Clean. Prod. 2016, 113, 338–344. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process disturbances in agricultural biogas production—Causes, mechanisms and effects on the biogas microbiome: A review. Energies 2019, 12, 365. [Google Scholar] [CrossRef] [Green Version]
- Andreides, M.; Pokorná-Krayzelová, L.; Říhová Ambrožová, J.; Volcke, E.I.P.; Bartáček, J. Key parameters influencing hydrogen sulfide removal in microaerobic sequencing batch reactor. Biochem. Eng. J. 2021, 168, 107951. [Google Scholar] [CrossRef]
- Ramos, I.; Peña, M.; Fdz-Polanco, M. Where does the removal of H2S from biogas occur in microaerobic reactors? Bioresour. Technol. 2014, 166, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sołowski, G.; Konkol, I.; Cenian, A. Methane and Hydrogen Production from Cotton Wastes in Dark Fermentation Process Under Anaerobic and Microaerobic Conditions. In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability; Naddeo, V., Balakrishnan, M., Choo, K.-H., Eds.; Springer: Cham, Switzerland, 2020; pp. 285–287. [Google Scholar]
- Rangel, C.; Sastoque, J.; Calderon, J.; Mosquera, J.; Velasquez, P.; Cabezab, I.; Acevedob, P. Hydrogen production by dark fermentation process: Effect of initial organic load. Chem. Eng. Trans. 2020, 79, 133–138. [Google Scholar] [CrossRef]
- Seifert, K.; Zagrodnik, R.; Stodolny, M.; Łaniecki, M. Biohydrogen production from chewing gum manufacturing residue in a two-step process of dark fermentation and photofermentation. Renew. Energy 2018, 122, 526–532. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Mechanical pretreatment for increased biogas production from lignocellulosic biomass; predicting the methane yield from structural plant components. Waste Manag. 2018, 78, 903–910. [Google Scholar] [CrossRef]
- Bertalero, G.; Addebito, P.; Bancario, C.C.; Cliente, C.A.L. Proteinaceous methanotrophs for feed additive using biowaste as carbon and nutrients source. Bioresour. Technol. 2020, 313, 123646. [Google Scholar] [CrossRef]
- Hitit, Z.Y.; Zampol Lazaro, C.; Hallenbeck, P.C. Increased hydrogen yield and COD removal from starch/glucose based medium by sequential dark and photo-fermentation using Clostridium butyricum and Rhodopseudomonas palustris. Int. J. Hydrogen Energy 2017, 42, 18832–18843. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; Da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from fresh spring and summer grass: Effect of the harvesting period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Meneses, Y.E.; Stratton, J.; Lau, S.K.; Subbiah, J. Integration of ozone with co-immobilized microalgae-activated sludge bacterial symbiosis for efficient on-site treatment of meat processing wastewater. J. Environ. Manag. 2021, 285, 112152. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic digestion for bioenergy production: Global status, environmental and techno-economic implications, and government policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Akoma, O.N.; Ononugbo, C.M.; Eze, C.C.; Chukwudozie, K.I.; Ogwu, J.E. Microbial Assessment of Selected, Locally- Fermented and Ready-to-eat Cassava Products Sold in Lokoja, Nigeria. Asian Food Sci. J. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Dreschke, G.; Papirio, S.; Sisinni, D.M.G.; Lens, P.N.L.; Esposito, G. Effect of feed glucose and acetic acid on continuous biohydrogen production by Thermotoga neapolitana. Bioresour. Technol. 2019, 273, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Łochyńska, M.; Frankowski, J. The biogas production potential from silkworm waste. Waste Manag. 2018, 79, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.M.; Fan, Y.T.; Zhao, P.; Hou, H.W. Fermentative hydrogen production by the newly isolated Clostridium beijerinckii Fanp3. Int. J. Hydrogen Energy 2008, 33, 5383–5391. [Google Scholar] [CrossRef]
- Moodley, P.; Gueguim Kana, E.B. Comparative study of three optimized acid-based pretreatments for sugar recovery from sugarcane leaf waste: A sustainable feedstock for biohydrogen production. Eng. Sci. Technol. Int. J. 2018, 21, 107–116. [Google Scholar] [CrossRef]
- Moriarty, K. Feasibility Study of Anaerobic Digestion of Food Waste in St. Bernard, Louisiana. A Study Prepared in Partnership with the Environmental Protection Agency for the RE-Powering America’s Land Initiative: Siting Renewable Energy on Potentially Contaminated Land and Mine Sites; National Renewable Energy Laboratory: Golden, CO, USA, 2013. Available online: https://www.nrel.gov/docs/fy13osti/57082.pdf (accessed on 20 March 2020).
- Marks, S.; Dach, J.; Garcia-Morales, J.L.; Fernandez-Morales, F.J. Bio-energy generation from synthetic winery wastewaters. Appl. Sci. 2020, 10, 8360. [Google Scholar] [CrossRef]
- Janczak, D.; Malinska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, Y.; Qi, B.; Li, S.; Luo, J.; Wan, J. Structure-property-performance relationships of lactic acid-based deep eutectic solvents with different hydrogen bond acceptors for corn stover pretreatment. Bioresour. Technol. 2021, 336, 125312. [Google Scholar] [CrossRef]
- Pineda-Muñoz, C.F.; Conde-Baez, L.; Lucho-Constantino, C.; Medina-Moreno, S.A.; Jiménez-González, A. Ultrasonic energy effect on dark fermentation by ultrasound application alone and in combination with heat shock. Bioenergy Res. 2020, 13, 334–348. [Google Scholar] [CrossRef]
- Hernández, C.; Alamilla-Ortiz, Z.L.; Escalante, A.E.; Navarro-Díaz, M.; Carrillo-Reyes, J.; Moreno-Andrade, I.; Valdez-Vazquez, I. Heat-shock treatment applied to inocula for H 2 production decreases microbial diversities, interspecific interactions and performance using cellulose as substrate. Int. J. Hydrogen Energy 2019, 44, 13126–13134. [Google Scholar] [CrossRef]
- Dauptain, K.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Carrère, H. Role of indigenous bacteria in dark fermentation of organic substrates. Bioresour. Technol. 2020, 313, 123665. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, N.; Almassi, M.; Minaei, S.; Widmann, R. Development of a method for biohydrogen production from wheat straw by dark fermentation. Int. J. Hydrogen Energy 2011, 36, 411–420. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Cabrol, L.; Jeison, D.; Trably, E.; Steyer, J.-P.; Tapia-Venegas, E. Impact of the microbial inoculum source on pre-treatment efficiency for fermentative H2 production from glycerol. Int. J. Hydrogen Energy 2020, 45, 1597–1607. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Capson-Tojo, G.; Marone, A.; Paillet, F. Basics of Bio-Hydrogen Production by Dark Fermentation. In Bioreactors for Microbial Biomass and Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2017; pp. 199–220. [Google Scholar]
- Sołowski, G.; Ziminski, T.; Cenian, A. A shift from anaerobic digestion to dark fermentation in glycol ethylene fermentation. Environ. Sci. Pollut. Res. 2021, 28, 15556–15564. [Google Scholar] [CrossRef]
- Hitit, Z.Y.; Hallenbeck, P.C. Analytical procedures, data reporting and selected reference values for biological hydrogen production. Biomass Bioenergy 2021, 147, 106014. [Google Scholar] [CrossRef]
- Rafieenia, R.; Girotto, F.; Peng, W.; Cossu, R.; Pivato, A.; Raga, R.; Lavagnolo, M.C. Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manag. 2017, 59, 194–199. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Perspectives of hydrogen production from corn wastes in Poland by means of dark fermentation. Ecol. Chem. Eng. S 2019, 26, 255–263. [Google Scholar] [CrossRef]
- Detman, A.; Mielecki, D.; Pleśniak, Ł.; Bucha, M.; Janiga, M.; Matyasik, I.; Chojnacka, A.; Jȩdrysek, M.O.; Błaszczyk, M.K.; Sikora, A. Methane-yielding microbial communities processing lactate-rich substrates: A piece of the anaerobic digestion puzzle. Biotechnol. Biofuels 2018, 11, 116. [Google Scholar] [CrossRef]
- Wu, Y.; Kovalovszki, A.; Pan, J.; Lin, C.; Liu, H.; Duan, N.; Angelidaki, I. Early warning indicators for mesophilic anaerobic digestion of corn stalk: A combined experimental and simulation approach. Biotechnol. Biofuels 2019, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Zhao, L.; Wang, R.; Ning, J.; Pan, X.; Li, C.; Cai, G.; Zhu, G. Novel strategy for relieving acid accumulation by enriching syntrophic associations of syntrophic fatty acid-oxidation bacteria and H2/formate-scavenging methanogens in anaerobic digestion. Bioresour. Technol. 2020, 313, 123702. [Google Scholar] [CrossRef] [PubMed]
- Bartacek, J.; Zabranska, J.; Lens, P.N.L. Developments and constraints in fermentative hydrogen production. Biofuels Bioprod. Biorefin. 2007, 1, 201–214. [Google Scholar] [CrossRef]
- Lee, K.S.; Lo, Y.C.; Lin, P.J.; Chang, J.S. Improving biohydrogen production in a carrier-induced granular sludge bed by altering physical configuration and agitation pattern of the bioreactor. Int. J. Hydrogen Energy 2006, 31, 1648–1657. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.J.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Sołowski, G.; Pastuszak, K. Modelling of dark fermentation of glucose and sour cabbage. Heliyon 2021, 7, e07690. [Google Scholar] [CrossRef]
- D’ippolito, G.; Squadrito, G.; Tucci, M.; Esercizio, N.; Sardo, A.; Vastano, M.; Lanzilli, M.; Fontana, A.; Cristiani, P. Electrostimulation of hyperthermophile Thermotoga neapolitana cultures. Bioresour. Technol. 2021, 319, 124078. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Teleken, J.G.; Weiser Meier, T.R.; Alves, H.J. Two-Stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2020, 281, 111854. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of initial uncontrolled pH on acidogenic fermentation of brewery spent grains to biohydrogen and volatile fatty acids production: Optimization and scale-up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Adish Kumar, S.; Bhatia, S.H.; Kumar, G. Integrated biorefinery routes of biohydrogen: Possible utilization of acidogenic fermentative effluent. Bioresour. Technol. 2021, 319, 124241. [Google Scholar] [CrossRef]
- Domański, J.; Marchut-Mikołajczyk, O.; Cieciura-Włoch, W.; Patelski, P.; Dziekońska-Kubczak, U.; Januszewicz, B.; Zhang, B.; Dziugan, P. Production of methane, hydrogen and ethanol from Secale cereale L. straw pretreated with sulfuric acid. Molecules 2020, 25, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, A.; Szczęsny, P.; Błaszczyk, M.K.; Zielenkiewicz, U.; Detman, A.; Salamon, A.; Sikora, A. Noteworthy facts about a methane-producing microbial community processing acidic effluent from sugar beet molasses fermentation. PLoS ONE 2015, 10, e0128008. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Luo, G.; Liu, H.; Yang, Z.; Angelidaki, I.; O-Thong, S.; Wang, W. CO as electron donor for efficient medium chain carboxylate production by chain elongation: Microbial and thermodynamic insights. Chem. Eng. J. 2020, 390, 124577. [Google Scholar] [CrossRef]
- Hassan, G.K.; Hemdan, B.A.; El-Gohary, F.A. Utilization of food waste for bio-hydrogen and bio-methane production: Influences of temperature, OLR, and in situ aeration. J. Mater. Cycles Waste Manag. 2020, 22, 1218–1226. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Shalaby, M.; Cenian, A. Methane and hydrogen production from potato wastes and wheat straw under dark fermentation. Chem. Process Eng. 2021, 42, 3–13. [Google Scholar] [CrossRef]
- She, Y.; Hong, J.; Zhang, Q.; Chen, B.Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef]
- Murarka, A.; Dharmadi, Y.; Yazdani, S.S.; Gonzalez, R. Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl. Environ. Microbiol. 2008, 74, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Sandriaty, R.; Priadi, C.; Kurnianingsih, S.; Abdillah, A. Potential of biogas production from anaerobic co-digestion of fat, oil and grease waste and food waste. E3S Web Conf. 2008, 67, 02047. [Google Scholar] [CrossRef] [Green Version]
- Sołowski, G.; Konkol, I.; Shalaby, M. Effect of rotten butter shock load on anaerobic digestion of chicken manure. agriTECH 2021, 41, 362. [Google Scholar] [CrossRef]
- Rafieenia, R.; Lavagnolo, M.C.; Pivato, A. Pre-treatment technologies for dark fermentative hydrogen production: Current advances and future directions. Waste Manag. 2018, 71, 734–748. [Google Scholar] [CrossRef]
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ponsá, S.; Gea, T.; Sánchez, A. Anaerobic co-digestion of the organic fraction of municipal solid waste with several pure organic co-substrates. Biosyst. Eng. 2011, 108, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, Q.; Deng, L.; Liu, Z.; Jiang, H.; Wang, F. Biohydrogen production from fermentation of cotton stalk hydrolysate by Klebsiella sp. WL1316 newly isolated from wild carp (Cyprinus carpio L.) of the Tarim River basin. Appl. Microbiol. Biotechnol. 2018, 102, 4231–4242. [Google Scholar] [CrossRef] [PubMed]


| Material | pH | C:N Ratio | TS [%FM] | VSS [%TS] |
|---|---|---|---|---|
| Inoculum | 7.6 | 10.53 | 1.09 ± 0.03 | 36.35 ± 1.02 |
| Collagen (pork gelatine) | - | 12.8 | 89 ± 0.03 | 96.5 ± 1.06 |
| Technique and method: | Volatile acids contamination in sample using (GC-FID) |
| Equipment: | Gas chromatograph Thermo Scientific Trace 1300 |
| Analysis conditions: | Column: Rxi 5MS 60 m Gas carrier: helium Flow: 1.0 mL/min The temperature of injection: 250 °C Stream separation: 1:10 Detector FID: 300 °C Temperature program: from 40 °C (3 min)—20 °C/min to 300 °C −300 °C (5 min) |
| Sample preparation: | To sample (6 mL) sulphuric acid (VI) (drop 0.25 mL) and sodium chloride (100 mg), then extracted with tert-butyl-methyl ether (2 mL) |
| Technique and method: | Determination of compound in gas chromatograph with a mass spectrometer (GC-MS) |
| Equipment: | Gas chromatograph of firm Shimadzu GC-2010Plus |
| Analysis conditions: | Column: Rxi 5MS 60 m Gas carrier: helium Flow: 1.0 mL/min The temperature of injection: 250 °C Stream separation: 1:20 Detector MS: 210 °C Temperature program: from 50 °C (4 min)—20 °C/min to 300 °C–300 °C (5 min) |
| Sample preparation: | To sample (6 mL) sulphuric acid (VI) (drop 0.25 mL) and sodium chloride (100 mg), then extracted with tert-butyl-methyl ether (2 mL) |
| Mass of Gelatine Added [g VSS/L] | Mass of Sulphur in Added Gelatine [g VSS/L] | The Percentage of Sulphur Converted in Emitted H2S% | Mass Converted to Gas% |
|---|---|---|---|
| 10 | 0.0203 | 46.402 | 3.2 |
| 15 | 0.031 | 107.59 | 4.66 |
| 20 | 0.041 | 96.41 | 5.15 |
| 30 | 0.06 | 26.1 | 8 |
| Substrate | Hydrogen Yield [mL/gVSS] | Accumulated Hydrogen Production [mL] | Methane Yield [L/gVSS] | Accumulated Methane Production [L] | Hydrogen Sulphide Yield [mL/gVSS] | Accumulated Hydrogen Sulphide Emission [mL] | Reference |
|---|---|---|---|---|---|---|---|
| Pork gelatine 30 g | 2.14 | 64.21 | 1.63 | 48.97 | 0.44 | 12.53 | This study |
| Pork gelatine 20 g | 7.36 | 147.2 | 2.86 | 57.23 | 1.53 | 30.6 | This study |
| Pork gelatine 15 g | 6.12 | 91.77 | 3.47 | 52 | 1.2 | 18.5 | This study |
| Pork gelatine 10 g | 7.65 | 76.47 | 3.49 | 34.88 | 1.03 | 10.3 | This study |
| Rapeseed oil 15 g VSS/L | 0.007 | 0.11 | [99] | ||||
| Glycerol 10 g VSS/L | 0.08 | 0.8 | [100] | ||||
| Cow manure with food wastes (butter mixture, palm oil, meat, and margarine) of ratio 1:8 | 0.31 | 3.1 | [101] | ||||
| Chicken manure with rotten butter Oxygen Flow Rate 1.4 mL/h butter 30 g VSS/L | 2.6 | 8 | 0.83 | 25 | 0.05 | 1.5 | [102] |
| Lipid waste 1.67 g VSS/L (tuna 7.5% butter 22.3%, apple 27%, banana 27%, chicken breast 7.5%, bread 1.5%, pasta 1.5%, minestrone soup 5.5%) | 27.93 | 46.64 | 0.26 | 0.43 | [103] | ||
| Protein waste 1.67 g VSS/L (tuna 31.1% butter 5.5%, apple 7.85%, banana 7.85%, chicken breast 31.1%, bread 3.2%, pasta 3.2%, minestrone soup 10.2%) | 8.02 | 13.4 | 0.35 | 0.58 | [103] | ||
| Switchgrass 5 g VSS/L | 0.26 | 1.5 | [104] | ||||
| The organic fraction of municipal solid waste 5 g VSS/L | 0.69 | 3.45 | [105] | ||||
| Cotton stalk hydrolysate 40 g VSS/L | 179 | 7160 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołowski, G. Microbial Biogas Production from Pork Gelatine. Hydrogen 2022, 3, 179-196. https://doi.org/10.3390/hydrogen3020012
Sołowski G. Microbial Biogas Production from Pork Gelatine. Hydrogen. 2022; 3(2):179-196. https://doi.org/10.3390/hydrogen3020012
Chicago/Turabian StyleSołowski, Gaweł. 2022. "Microbial Biogas Production from Pork Gelatine" Hydrogen 3, no. 2: 179-196. https://doi.org/10.3390/hydrogen3020012
APA StyleSołowski, G. (2022). Microbial Biogas Production from Pork Gelatine. Hydrogen, 3(2), 179-196. https://doi.org/10.3390/hydrogen3020012






