Analysis of Hydrogen in Inorganic Materials and Coatings: A Critical Review
Abstract
:1. Introduction
2. Bulk Analysis of Hydrogen
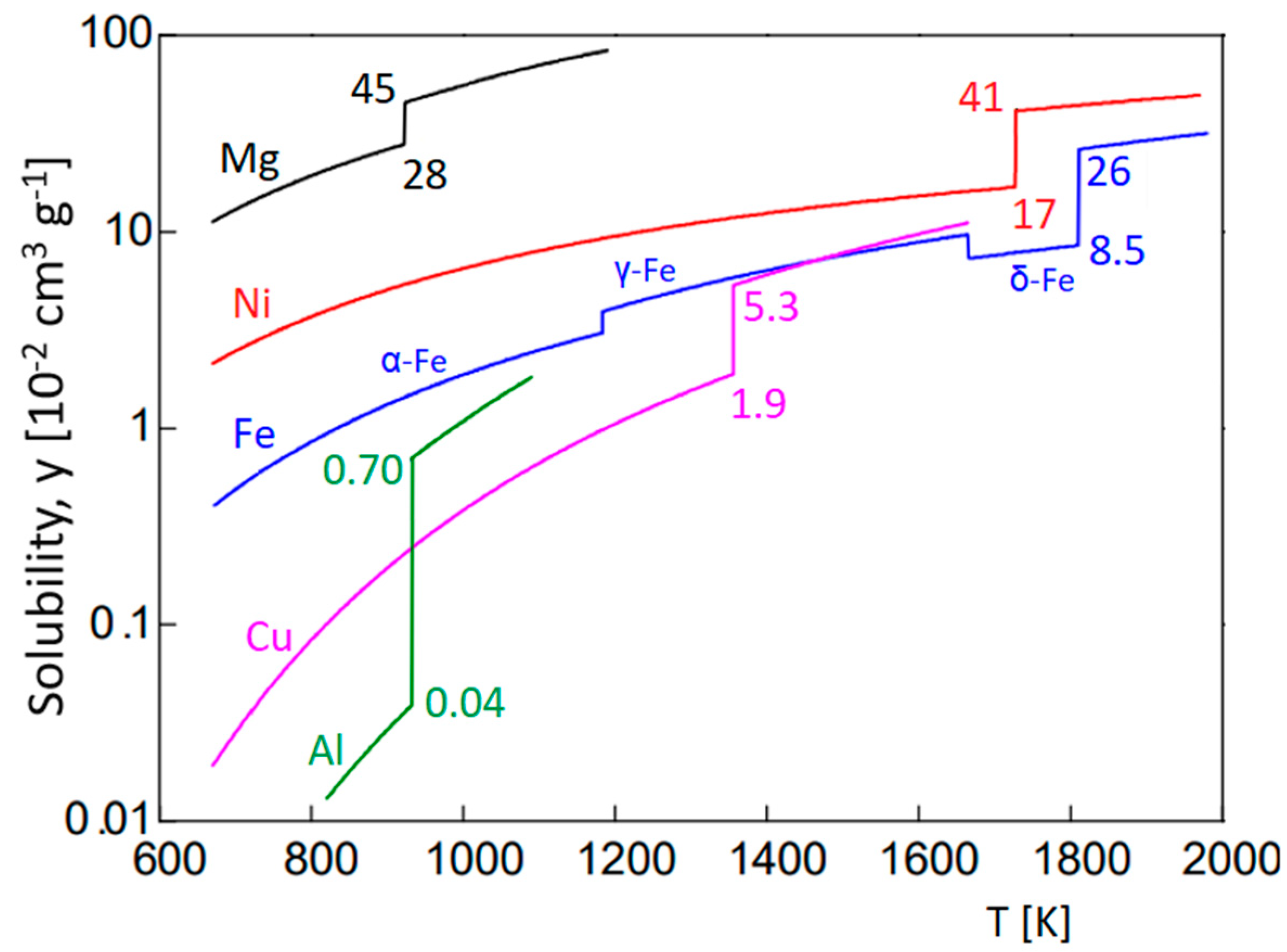
2.1. Inert Gas Fusion
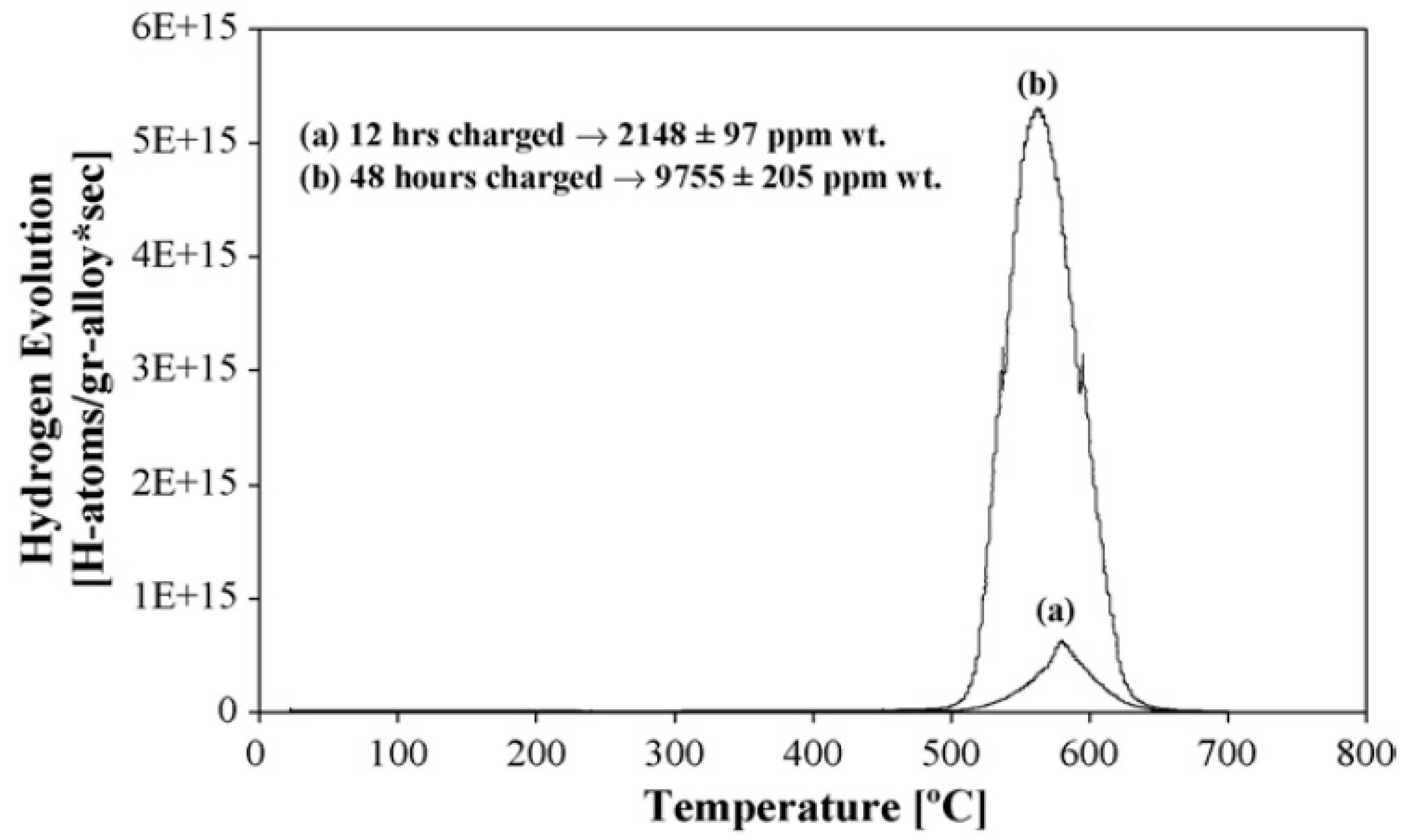
2.2. Thermal Desorption Analysis
3. Analysis of Hydrogen in Coatings and Thin Films
3.1. Emission Spectroscopy and Mass Spectrometry
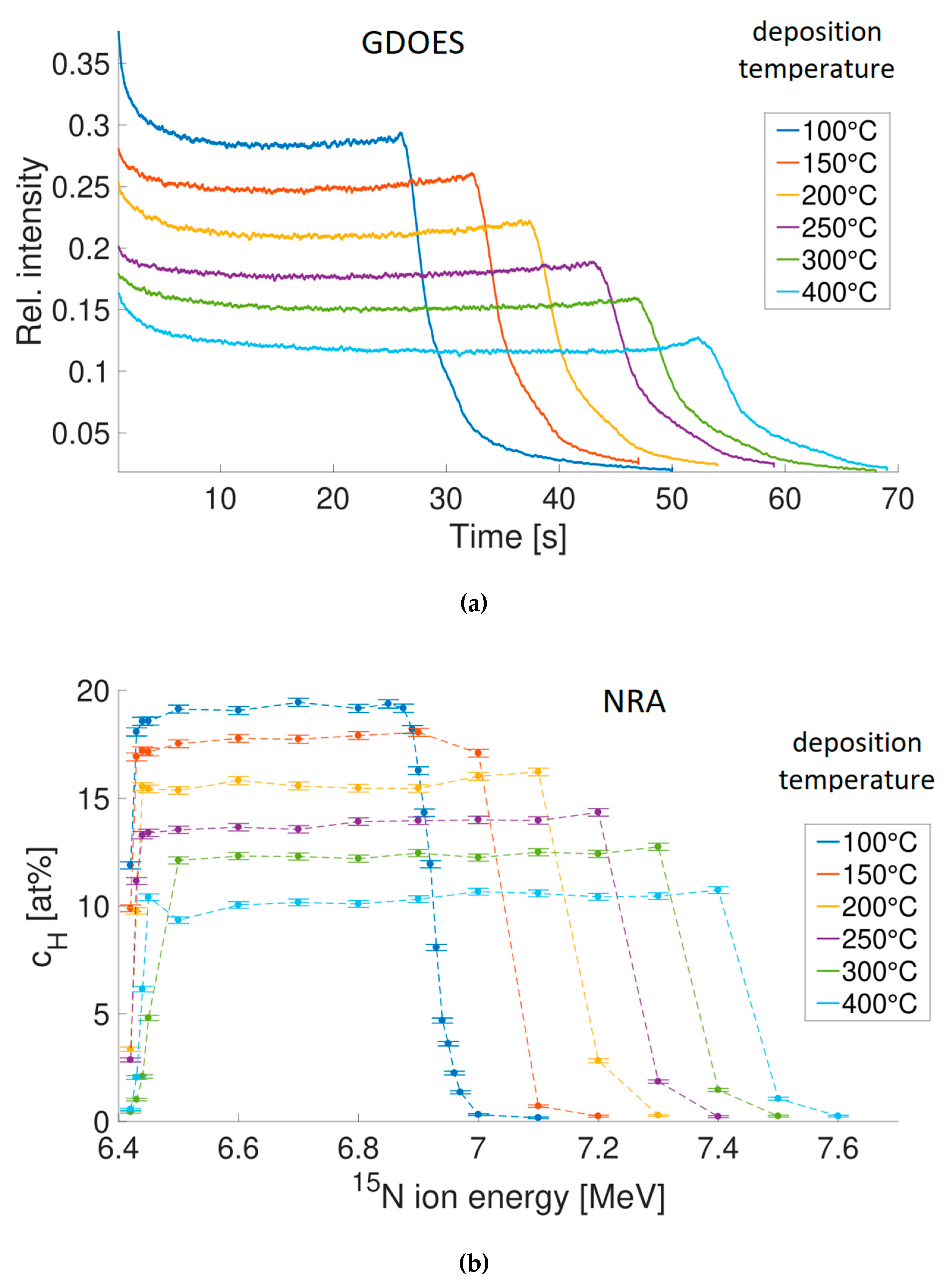
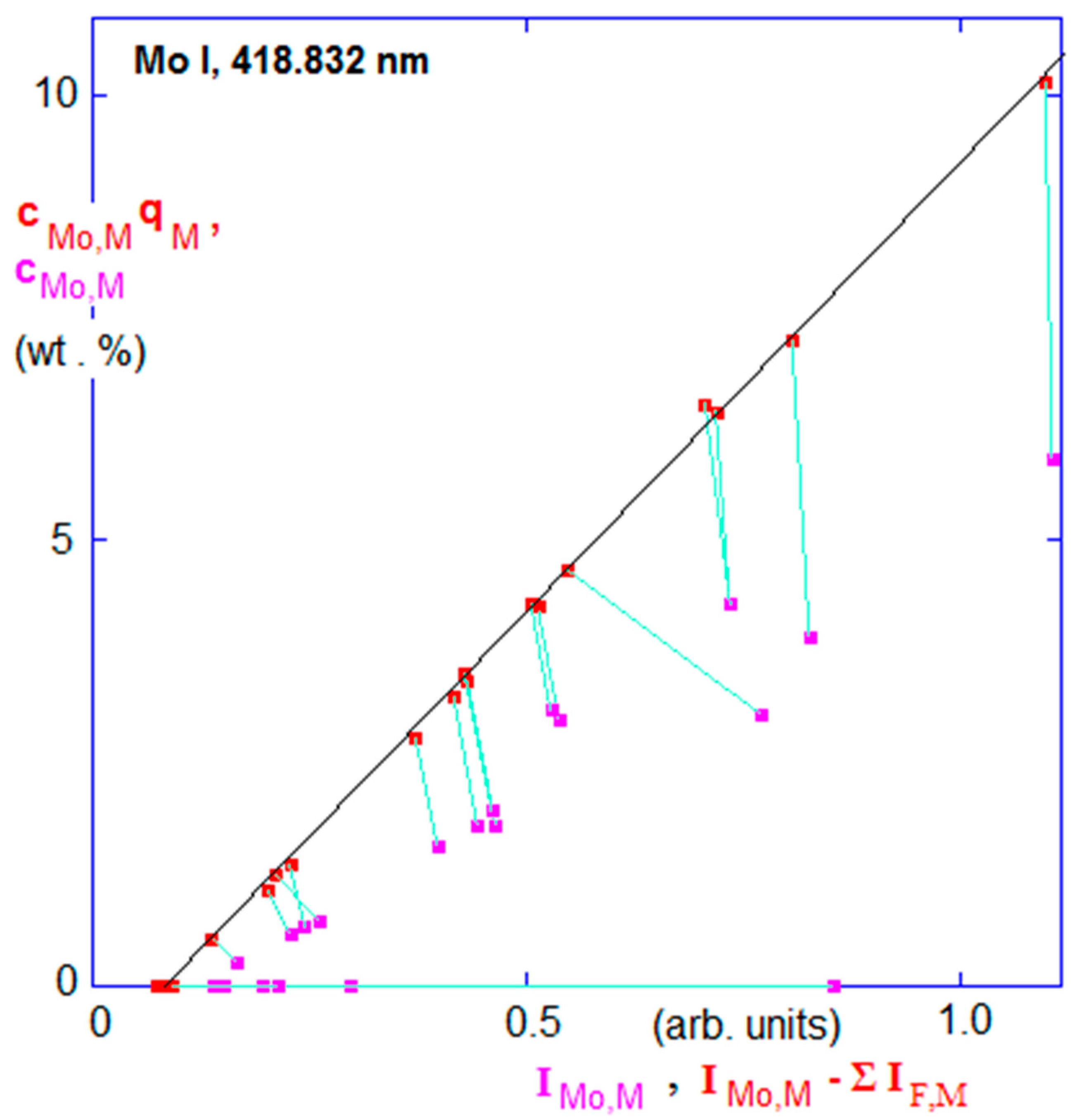
3.1.1. Glow Discharge Optical Emission Spectroscopy (GDOES) and Glow Discharge Mass Spectrometry (GDMS)
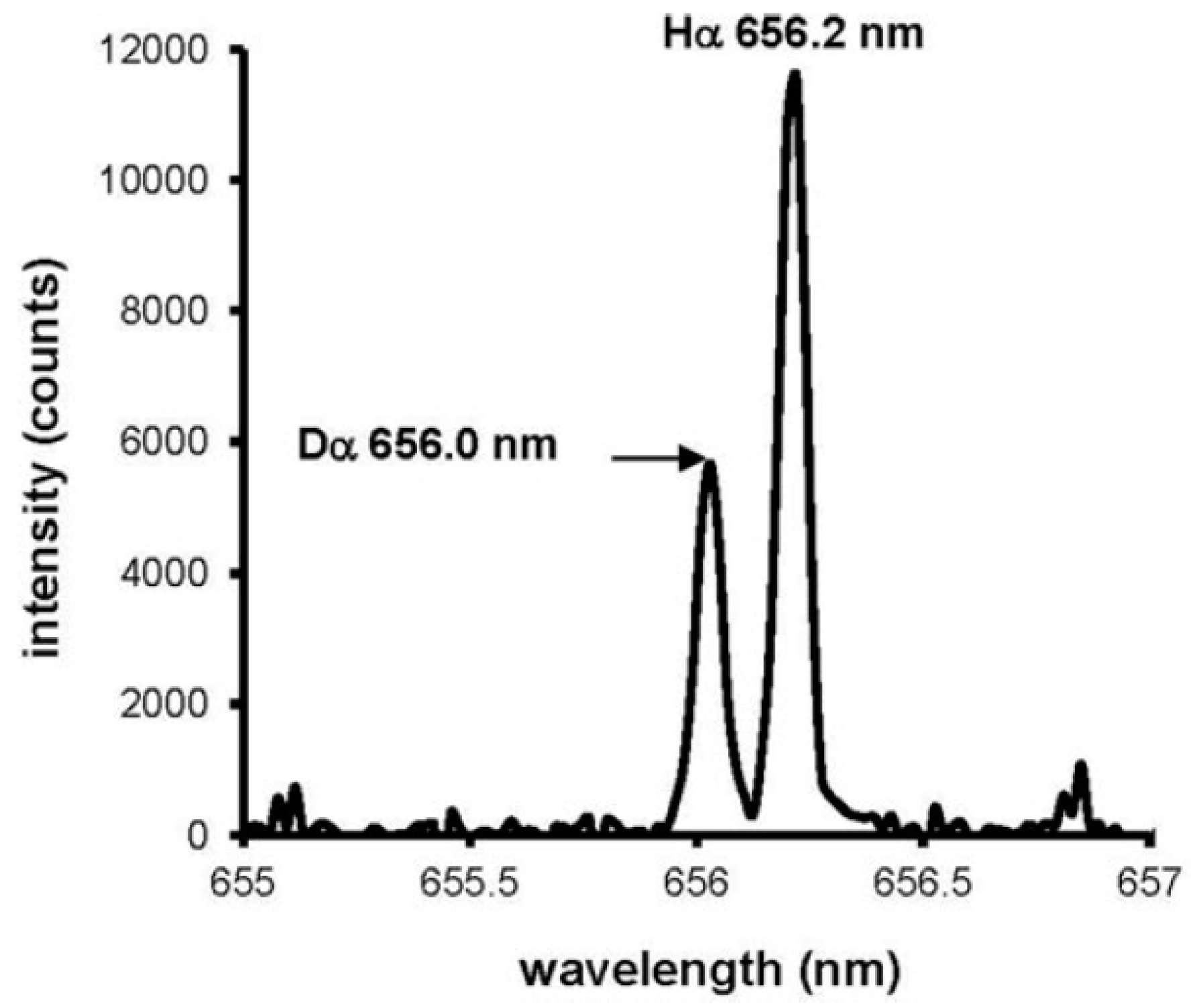
3.1.2. Laser-Induced Breakdown Spectrometry (LIBS)
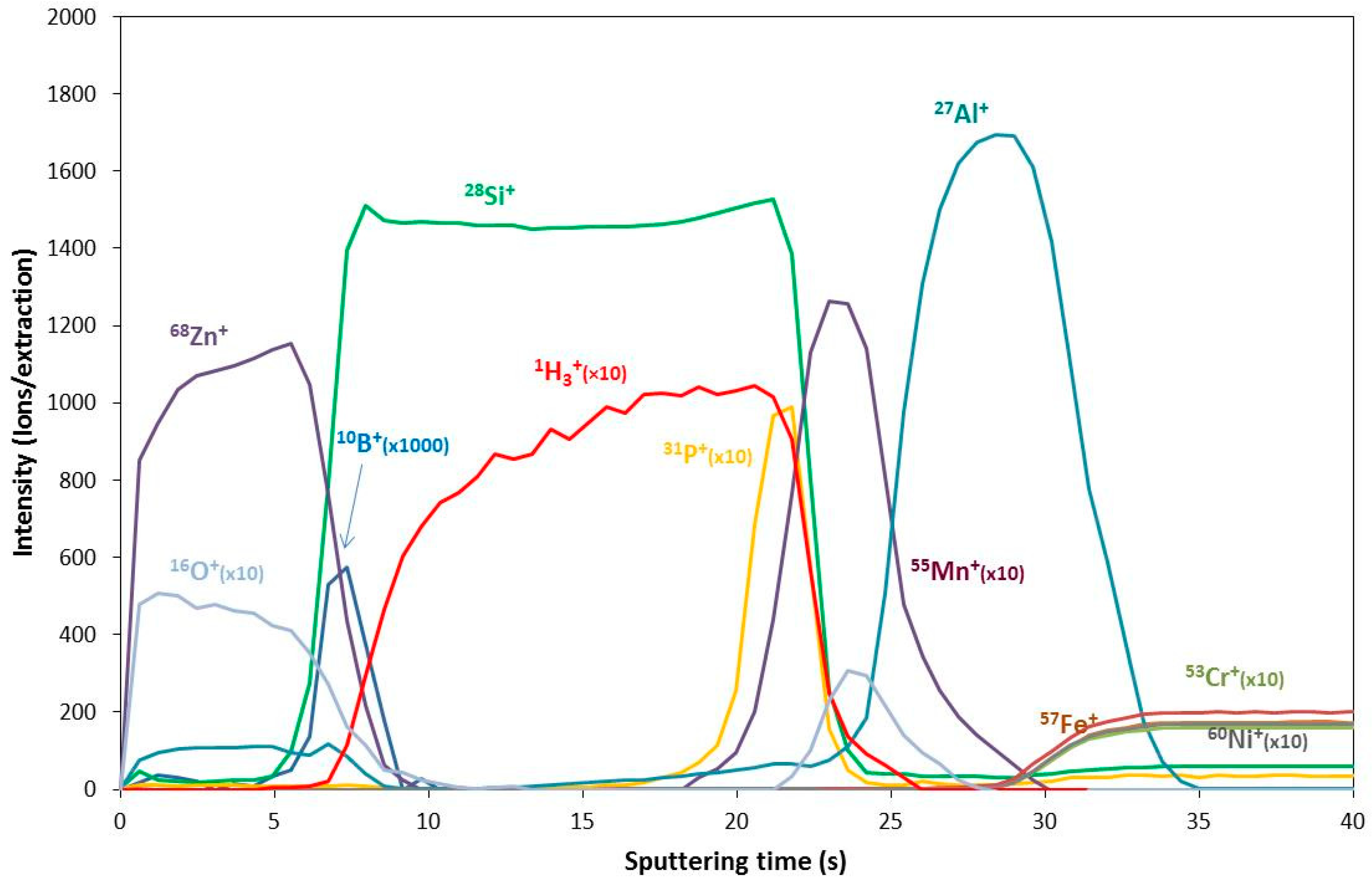
3.1.3. Secondary Ion Mass Spectrometry (SIMS)
3.2. Nuclear Methods
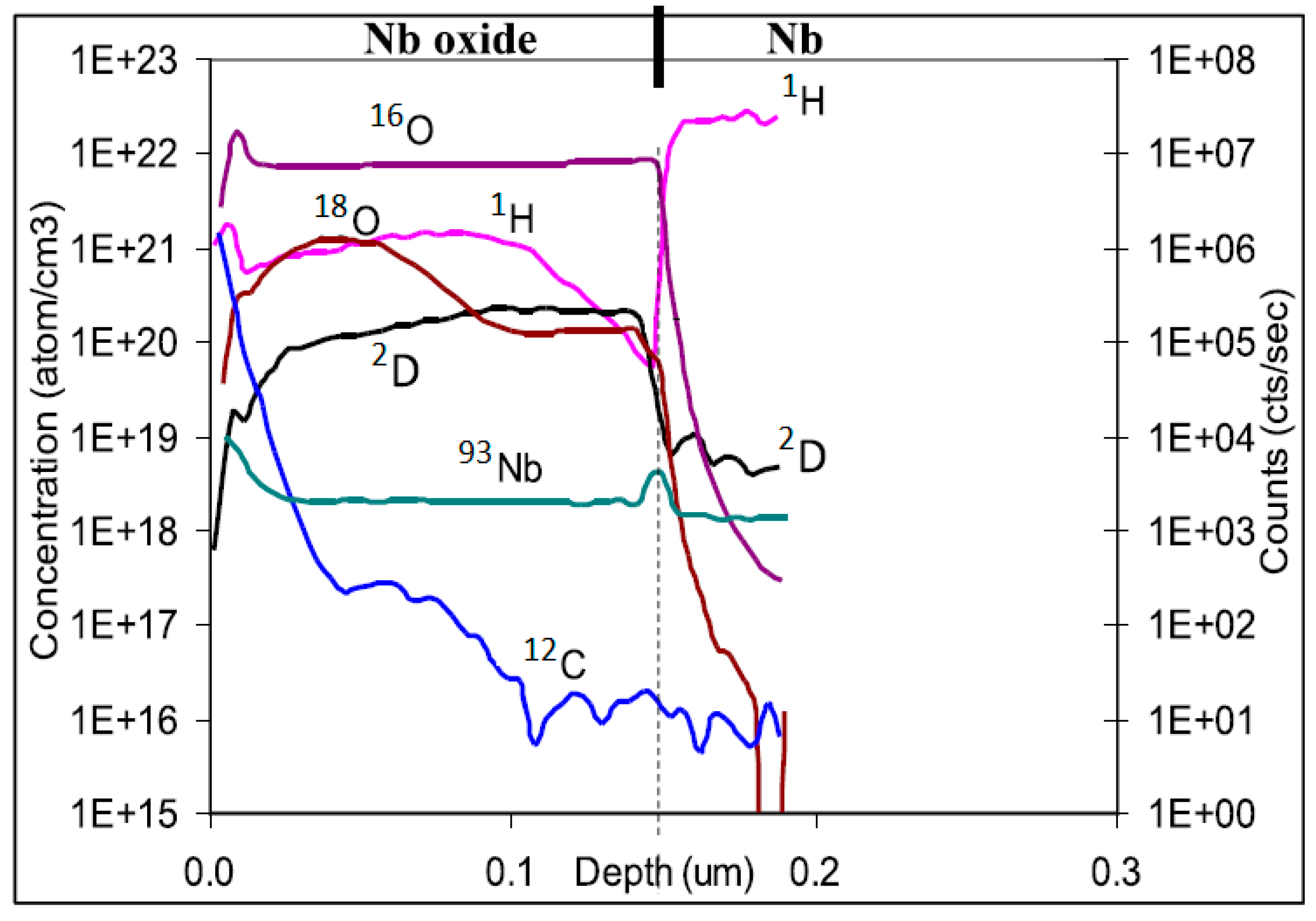
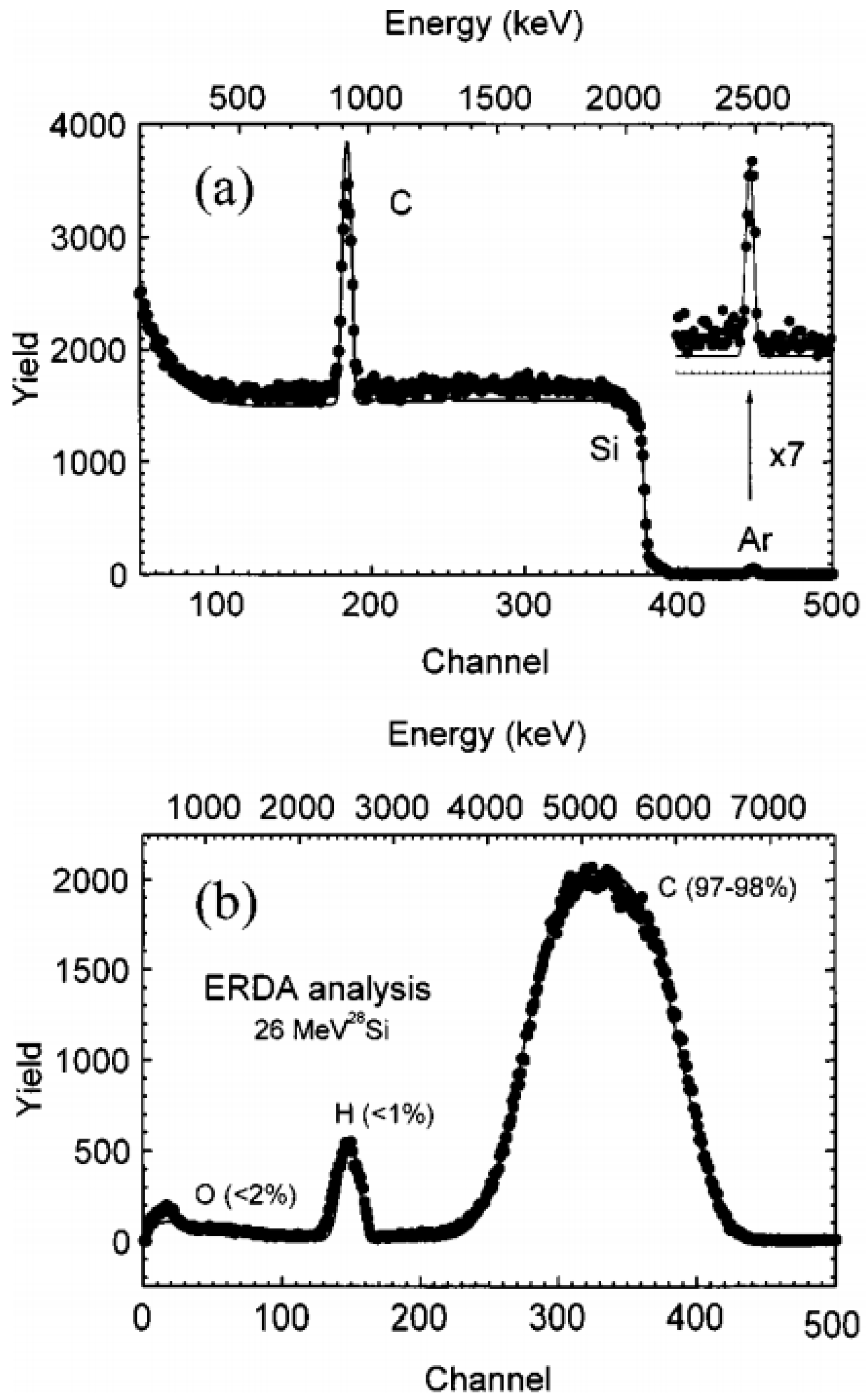
3.2.1. Elastic Recoil Detection Analysis (ERDA)
3.2.2. Nuclear Reaction Analysis (NRA)
4. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Alefeld, G.; Völkl, J. (Eds.) Hydrogen in Metals II: Application-Oriented Properties; Springer: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Fukai, Y. The Metal-Hydrogen System; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Smialowski, M. Hydrogen in Steel: Effect of Hydrogen on Iron and Steel during Production, Fabrication and Use; Pergamon: Oxford, UK, 1962. [Google Scholar]
- Hydrogen Embrittlement. Available online: https://en.wikipedia.org/wiki/Hydrogen_embrittlement (accessed on 3 February 2021).
- Świerczyńska, A.; Fydrych, D.; Landowski, M.; Rogalski, G.; Łabanowski, J. Hydrogen embrittlement of X2CrNiMoCuN25-6-3 super duplex stainless steel welded joints under cathodic protection. Constr. Build. Mater. 2020, 238, 117697. [Google Scholar] [CrossRef]
- Fan, J.; Chen, H.; Zhao, W.; Yan, L. Study on flake formation behavior and its influence factors in Cr5 steel. Materials 2018, 11, 690. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Su, Y.J.; Chu, W.Y.; Li, Z.B.; Liang, D.T.; Qiao, L.J. Hydrogen embrittlement of weld metal of austenitic stainless steels. Corros. Sci. 2002, 44, 1983–1993. [Google Scholar] [CrossRef]
- Padhy, G.K.; Komizo, Y.I. Diffusible hydrogen in steel weldments: A status review. Trans. JWRI 2013, 42, 39–62. [Google Scholar]
- Fydrych, D.; Łabanowski, J. Determining diffusible hydrogen amounts using the mercury method. Weld. Int. 2012, 26, 697–702. [Google Scholar] [CrossRef]
- Enomoto, M.; Cheng, L.; Mizuno, H.; Watanabe, Y.; Omura, T.; Sakai, J.I.; Yokoyama, K.I.; Suzuki, H.; Okuma, R. Hydrogen Absorption into Austenitic Stainless Steels Under High-Pressure Gaseous Hydrogen and Cathodic Charge in Aqueous Solution. Metall. Mater. Trans. E 2014, 1, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Fournier, L.; Delafosse, D.; Magnin, T. Cathodic hydrogen embrittlement in alloy 718. Mater. Sci. Eng. 1999, 269, 111–119. [Google Scholar] [CrossRef]
- Ambat, R.; Dwarakadasa, E.S. Effect of hydrogen in aluminium and aluminium alloys: A review. Bull. Mater. Sci. 1996, 19, 103–114. [Google Scholar] [CrossRef]
- Anyalebechi, P.N. Techniques for determination of the Hydrogen Content in Aluminium and its Alloys—A Review. Cast Met. 1990, 3, 182–201. [Google Scholar] [CrossRef]
- Froes, F.H.; Eliezer, D.; Moody, N.R. Hydrogen Effects in Titanium, a Chapter in Hydrogen Effects in Materials; Thompson, A.W., Moody, N.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Kamilyan, M.; Silverstein, R.; Eliezer, D. Hydrogen trapping and hydrogen embrittlement of Mg alloys. J. Mater. Sci. 2017, 52, 11091–11100. [Google Scholar] [CrossRef]
- Talbot, D.E.J. Effects of Hydrogen in Aluminium, Magnesium, Copper, and Their Alloys. Int. Metall. Rev. 1975, 20, 166–184. [Google Scholar] [CrossRef]
- Dekura, S.; Kobayashi, H.; Kusada, K.; Kitagawa, H. Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal-Organic Framework Coating Effects. ChemPhysChem 2019, 20, 1158–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alloyed Steel No. 1.4546.9 with Certified Hydrogen Content. Available online: https://rrr.bam.de/RRR/Navigation/EN/Reference-Materials/reference-materials.html (accessed on 11 March 2021).
- Xie, Z.K.; Ikeda, T.; Okuda, Y.; Nakajima, H. Characteristics of Sound Absorption in Lotus-Type Porous Magnesium. Jpn. J. Appl. Phys. 2004, 43, 7315–7319. [Google Scholar] [CrossRef]
- Lawrenz, D.; Mitchell, J. Thermal Evolution Methods for Carbon, Sulfur, Oxygen, Nitrogen and Hydrogen in Iron and Steel Analysis. In Encyclopedia of Analytical Chemistry, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 8991–9008. [Google Scholar]
- Thermal Conductivity Detector. Available online: https://en.wikipedia.org/wiki/Thermal_conductivity_detector (accessed on 13 March 2021).
- Littlewood, A.B. Sensitivity of Catharometers in Gas Chromatography and the Thermal Conductivity of Binary Gas Mixtures. Nature 1959, 184, 1631–1632. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D.; Tal-Gutelmacher, E. Hydrogen trapping in alloys studied by thermal desorption spectrometry. J. Alloys Compd. 2018, 747, 511–522. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D.; Abramov, E. Thermal desorption spectroscopy (TDS)—Application in quantitative study of hydrogen evolution and trapping in crystalline and non-crystalline materials. Mater. Sci. Eng. A 2007, 445–446, 625–631. [Google Scholar] [CrossRef]
- Rhode, M.; Schaupp, T.; Muenster, C.; Mente, T.; Boellinghaus, T.; Kannengiesser, T. Hydrogen determination in welded specimens by carrier gas hot extraction—A review on the main parameters and their effects on hydrogen measurement. Weld. World 2019, 63, 511–526. [Google Scholar] [CrossRef]
- Bergers, K.; de Souza, E.C.; Thomas, I.; Mabho, N.; Flock, J. Determination of Hydrogen in Steel by Thermal Desorption Mass Spectrometry. Steel Res. Int. 2010, 81, 499–507. [Google Scholar] [CrossRef]
- Georges, C.; Sturel, T.; Drillet, P.; Mataigne, J.-M. Absorption/Desorption of Diffusible Hydrogen in Aluminized Boron Steel. ISIJ Int. 2013, 53, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, M.; Mitzschke, N.; Jüttner, S. Determination of Hydrogen Transport Behaviour in Boron-Manganese Steels Using Different Methods and Boundary Conditions. Metals 2019, 9, 1007. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element-based systems—A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Babikhina, M.N.; Kudiiarov, V.N.; Mostovshchikov, A.V.; Lider, A.M. Quantitative and Qualitative Analysis of Hydrogen Accumulation in Hydrogen-Storage Materials Using Hydrogen Extraction in an Inert Atmosphere. Metals 2018, 8, 672. [Google Scholar] [CrossRef] [Green Version]
- Titanium Hydride, Wikipedia. Available online: https://en.wikipedia.org/wiki/Titanium_hydride (accessed on 20 March 2021).
- Lisowski, W.; Keim, E.G.; Kaszkur, Z.; Smithers, M.A. Decomposition of thin titanium deuteride films; thermal desorption kinetics studies combined with microstructure analysis. Appl. Surf. Sci. 2008, 254, 2629–2637. [Google Scholar] [CrossRef]
- Ashcheulov, P.; Taylor, A.; Vlčková-Živcová, Z.; Hubík, P.; Honolka, J.; Vondráček, M.; Remzová, M.; Kopeček, J.; Klimša, L.; Lorinčik, J.; et al. Low temperature synthesis of transparent conductive boron doped diamond films for optoelectronic applications: Role of hydrogen on the electrical properties. Appl. Mater. Today 2020, 19, 10063. [Google Scholar]
- Marcus, R.K.; Broekaert, J.A.C. (Eds.) Glow Discharge Plasmas in Analytical Spectroscopy; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Lobo, L.; Fernandez, B.; Pereiro, R. Depth profile analysis with glow discharge spectrometry. J. Anal. At. Spectrom. 2017, 32, 920–930. [Google Scholar] [CrossRef]
- Steffens, J.; Becker, H.-W.; Gerke, S.; Joos, S.; Hahn, G.; Terheiden, B. Replacing NRA by fast GD-OES measurements as input to a model-based prediction of hydrogen diffusion in a-Si. Energy Procedia 2017, 124, 180–187. [Google Scholar] [CrossRef]
- Alvarez-Toral, A.; Sanchez, P.; Menéndez, A.; Pereiro, R.; Sanz-Medel, A.; Fernández, B. Depth Profile Analysis of Amorphous Silicon Thin Film Solar Cells by Pulsed Radiofrequency Glow Discharge Time of Flight Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 305–314. [Google Scholar] [CrossRef]
- Weiss, Z. Calibration methods in glow discharge optical emission spectroscopy: A tutorial review. J. Anal. At. Spectrom. 2015, 30, 1038–1049. [Google Scholar] [CrossRef]
- Gonzalez-Gago, C.; Bordel, N.; Pisonero, J. Glow Discharge Mass Spectrometry. In ASM Handbook, Materials Characterization; ASM International: Novelty, OH, USA, 2019; Volume 10, pp. 153–161. [Google Scholar]
- Gonzalez-Gago, C.; Šmíd, P.; Hofmann, T.; Venzago, C.; Hoffmann, V.; Gruner, W.; Pfeifer, J.; Richter, S.; Kipphardt, H. Investigations of matrix independent calibration approaches in fast flow glow discharge mass spectrometry. J. Anal. At. Spectrom. 2019, 34, 1109–1124. [Google Scholar] [CrossRef]
- Schubert, C.; Hoffmann, V.; Kümmel, A.; Sinn, J.; Härtel, M.; Reuther, A.; Thomalla, M.; Gemming, T.; Eckert, J.; Leyens, C. Compositional depth profiling of diamond-like carbon layers by glow discharge optical emission spectroscopy. J. Anal. At. Spectrom. 2016, 31, 2207–2212. [Google Scholar] [CrossRef] [Green Version]
- Bengtson, A.; Hänström, S. The influence of hydrogen on emission intensities in GD-OES, consequences for quantitative depth profile analysis. In Proceedings of the 5th International Conference on Progress in Analytical Chemistry in the Steel and Metals (CETAS), Luxembourg, 12–14 May 1998; pp. 47–54. [Google Scholar]
- Šmíd, P.; Steers, E.B.M.; Weiss, Z.; Pickering, J.C.; Hoffmann, V. The effect of hydrogen and nitrogen on emission spectra of iron and titanium atomic lines in analytical glow discharges. J. Anal. At. Spectrom. 2008, 23, 1223–1233. [Google Scholar] [CrossRef]
- Steers, E.B.M.; Smid, P.; Weiss, Z. Asymmetric charge transfer with hydrogen ions—An important factor in the “hydrogen effect” in glow discharge optical emission spectroscopy. Spectrochim. Acta Part B 2006, 61, 414–420. [Google Scholar] [CrossRef]
- Mushtaq, S.; Steers, E.B.M.; Whitby, J.A.; Horvath, P.; Michler, J.; Pickering, J.C. A glow discharge time-of flight mass spectrometry (GD-TOFMS) study of the ‘hydrogen effect’ using copper, iron and titanium cathodes. J. Anal. At. Spectrom. 2015, 30, 1774–1781. [Google Scholar] [CrossRef]
- Weiss, Z.; Vlcak, P. Analysis of shallow depth profiles of titanium nitride and N-implanted titanium by GD-OES: The ‘hydrogen effect’ after the discharge startup and a correction thereof. J. Anal. At. Spectrom. 2017, 32, 2476–2484. [Google Scholar] [CrossRef]
- Hoffmann, V.; Uhlemann, M.; Richter, S.; Pfeifer, J. Calibration capacity of hot-pressed hydrogen standards for glow discharge optical emission and mass spectrometry. Spectrochim. Acta Part B 2021, 176, 106039. [Google Scholar] [CrossRef]
- Priamusho, T.S.; Mikhaylov, A.A.; Babikhina, M.N.; Kudiiarov, V.N.; Laptev, R.S. Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals 2018, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Hodoroaba, V.-D.; Klemm, D.; Reinholz, U.; Strub, E.; Röhrich, J.; Bohne, W.; Hoffmann, V.; Wetzig, K. Potential candidates of certified reference material for determination of hydrogen concentration with glow discharge optical emission spectrometry (GD-OES)—A feasibility study. J. Anal. At. Spectrom. 2008, 23, 460–462. [Google Scholar] [CrossRef]
- Wilke, M.; Teichert, G.; Gemma, R.; Pundt, A.; Kirchheim, R.; Romanus, H.; Schaaf, P. Glow discharge optical emission spectroscopy for accurate and well resolved analysis of coatings and thin films. Thin Solid Film 2011, 520, 1660–1667. [Google Scholar] [CrossRef]
- Takahara, H.; Ishigami, R.; Kodama, K.; Kojyo, A.; Nakamura, T.; Oka, Y. Hydrogen analysis in diamond-like carbon by glow discharge optical emission spectroscopy. J. Anal. At. Spectrom. 2016, 31, 940–947. [Google Scholar] [CrossRef]
- Richter, K.; Waldmann, T.; Kasper, M.; Pfeifer, C.; Memm, M.; Axmann, P.; Wohlfahrt-Mehrens, M. Surface Film Formation and Dissolution in Si/C Anodes of Li-Ion Batteries: A Glow Discharge Optical Emission Spectroscopy Depth Profiling Study. J. Phys. Chem. C 2019, 123, 18795–18803. [Google Scholar] [CrossRef]
- Lang, E.; Taylor, C.N.; Allain, J.P. GD-OES study of the influence of second phase particles on the deuterium depth distribution in dispersion-strengthened tungsten. J. Nucl. Mater. 2020, 532, 152047. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, X.; He, R.; Zhang, H.; Fu, E.; Wang, P. Experimental measurement of deuterium concentration and depth profiling in tungsten by radio frequency glow discharge optical emission spectroscopy. Spectrochim. Acta Part B 2020, 173, 105975. [Google Scholar] [CrossRef]
- Efimov, A.; Kasik, M.; Putyera, K.; Moreau, O. Measurement of Hydrogen and Deuterium Concentration in Gold Electroplated Layer by Glow Discharge Mass Spectrometry. Electrochem. Solid State Lett. 2000, 3, 477–478. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Joliveta, L.; Leprince, M.; Moncayo, S.; Sorbier, L.; Lienemann, C.-P.; Motto-Ros, V. Review of the recent advances and applications of LIBS-based imaging. Spectrochim. Acta Part B 2019, 151, 41–53. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G.; Legnaioli, S.; Palleschi, V. Calibration-free laser-induced breakdown spectroscopy: State of the art. Spectrochim. Acta Part B 2010, 65, 1–14. [Google Scholar] [CrossRef]
- Roldán, A.M.; Pisarčík, M.; Veis, M.; Držík, M.; Veis, P. Calibration-free analysis of a tungsten-based target for diagnostics of relevant fusion materials comparing picosecond and nanosecond LIBS. Spectrochim. Acta Part B 2021, 177, 106055. [Google Scholar] [CrossRef]
- Paris, P.; Jõgi, I.; Piip, K.; Passoni, M.; Dellasega, D.; Grigore, E.; Arnoldbik, W.M.; van der Meiden, H. In-situ LIBS and NRA deuterium retention study in porous W-O and compact W coatings loaded by Magnum-PSI. Fusion Eng. Des. 2021, 168, 112403. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A. Quantitative analysis by laser-induced breakdown spectroscopy based on generalized curves of growth. Spectrochim. Acta Part B 2015, 110, 124–133. [Google Scholar] [CrossRef]
- Ashikawa, N.; Zhao, D.; Li, C.; Ding, H.; LHD Experimental Group. Hydrogen Depth Profiles Using Laser-Induced Breakdown Spectroscopy (LIBS) on Graphite Target of Divertor in LHD, Proc. of A3 Foresight Program Seminar on Critical Physics Issues Specific to Steady State Sustainment of High-Performance Plasmas 2015. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/49/089/49089674.pdf?r=1 (accessed on 28 March 2021).
- Dwivedi, V.; Marín-Roldán, A.; Karhunen, J.; Paris, P.; Jõgi, I.; Porosnicu, C.; Lungu, C.P.; van der Meiden, H.; Hakola, A.; Veis, P. CF-LIBS quantification and depth profile analysis of Be coating mixed layers. Nucl. Mater. Energy 2021, 27, 100990. [Google Scholar] [CrossRef]
- Kautz, E.J.; Devaraj, A.; Senor, D.J.; Harilal, S.S. Hydrogen isotopic analysis of nuclear reactor materials using ultrafast laser-induced breakdown spectroscopy. Opt. Express 2001, 29, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Landis, G.P.; Maroef, I.; Olson, D.L.; Wildeman, T.R. The determination of hydrogen distribution in high-strength steel weldments Part 1: Laser ablation methods. Weld. J. 2001, 80, 115s–121s. [Google Scholar]
- Imashuku, S.; Kamimura, T.; Kashiwakura, S.; Wagatsuma, K. Quantitative Analysis of Hydrogen in High-Hydrogen-Content Material of Magnesium Hydride via Laser-Induced Breakdown Spectroscopy. Anal. Chem. 2020, 92, 11171–11176. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yang, R.; Li, Q.; Ye, X.; Wu, J.; Chen, C.; Wang, X.; Chen, X. Quantitative measurement of hydrogen isotopes in titanium using laser-induced breakdown spectroscopy. Appl. Opt. 2020, 59, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Q.; Yang, R.; Cui, X. Quantitative determination of hydrogen isotope in titanium using LIBS. In AOPC 2019: Optical Spectroscopy and Imaging, Proceedings of the Applied Optics and Photonics China (AOPC2019), Beijing, China, 7–9 July 2019; Proc. SPIE—International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 11337, p. 12. [Google Scholar] [CrossRef]
- Thomas, N.H.; Ehlmann, B.L.; Anderson, D.E.; Clegg, S.M.; Forni, O.; Schröder, S.; Rapin, W.; Meslin, P.-Y.; Lasue, J.; Delapp, D.M.; et al. Characterization of Hydrogen in Basaltic Materials With Laser-Induced Breakdown Spectroscopy (LIBS) for Application to MSL ChemCam Data. J. Geophys. Res. Planets 2018, 123, 1996–2021. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, K.H.; Tjia, M.O.; Kagawa, K. Review of Laser-Induced Plasma, Its Mechanism, and Application to Quantitative Analysis of Hydrogen and Deuterium. Appl. Spectr. Rev. 2014, 49, 323–434. [Google Scholar] [CrossRef]
- Kurniawan, K.H.; Lie, T.J.; Suliyanti, M.M.; Hedwig, R.; Pardede, M.; Kurniawan, D.P.; Kusumoto, Y.; Kagawa, K. Quantitative analysis of deuterium using laser-induced plasma at low pressure of helium. Anal. Chem. 2006, 78, 5768–5773. [Google Scholar] [CrossRef]
- Pardede, M.; Lie, T.J.; Iqbal, J.; Bilal, M.; Hedwig, R.; Ramli, M.; Khumaeni, A.; Budi, W.S.; Idris, N.; Abdulmadjid, S.N. H–D analysis employing energy transfer from metastable excited-state He in double-pulse LIBS with low-pressure He gas. Anal. Chem. 2019, 91, 1571–1577. [Google Scholar] [CrossRef]
- Walker, A.V. Secondary Ion Mass Spectrometry, chapter in Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 44–49. [Google Scholar]
- Van der Heide, P. Secondary Ion Mass Spectrometry: An Introduction to Principles and Practices; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Magee, C.W.; Botnick, E.M. Hydrogen depth profiling using SIMS—Problems and their solutions. J. Vac. Sci. Technol. 1981, 19, 47–52. [Google Scholar] [CrossRef]
- Zhu, Z.; Shutthanandan, V.; Engelhard, M. An investigation of hydrogen depth profiling using ToF-SIMS. Surf. Interface Anal. 2012, 44, 232–237. [Google Scholar] [CrossRef]
- Chakraborty, P. Secondary ion mass spectrometry for quantitative surface and in-depth analysis of materials. Pramana J. Phys. 1998, 50, 617–640. [Google Scholar] [CrossRef]
- Wilson, R.G.; Stevie, F.A.; Magee, C.W. Secondary Ion Mass Spectrometry: A Practical Handbook for Depth Profiling and Bulk Impurity Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1989. [Google Scholar]
- Williams, P.; Stika, K.M.; Davies, J.A.; Jackman, T.E. Quantitative SIMS analysis of hydrogenated amorphous silicon using superimposed deuterium implant standards. Nucl. Instrum. Methods 1983, 218, 299–302. [Google Scholar] [CrossRef]
- Lehmanna, M.; Valle, N.; Horzel, J.; Pshenova, A.; Wyss, P.; Döbeli, M.; Despeisse, M.; Eswara, S.; Wirtz, T.; Jeangros, Q.; et al. Analysis of hydrogen distribution and migration in fired passivating contacts (FPC). Sol. Energy Mater. Solar Cells 2019, 200, 110018. [Google Scholar] [CrossRef] [Green Version]
- Röhsler, A.; Sobol, O.; Hänninen, H.; Böllinghaus, T. In-situ ToF-SIMS analyses of deuterium re-distribution in austenitic steel AISI 304L under mechanical load. Sci. Rep. 2020, 10, 3611. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Seyeux, A.; Vickridge, I.; Voyshnis, S.; Marcus, P. ToF-SIMS and ERDA study of hydrogen and deuterium in nickel-base alloys oxidized in water. Corros. Sci. 2018, 140, 151–158. [Google Scholar] [CrossRef]
- Stevie, F.A. Analysis of hydrogen in materials with and without high hydrogen mobility. Surf. Interface Anal. 2016, 48, 310–314. [Google Scholar] [CrossRef]
- Xu, Y.; Hirooka, Y.; Luo, L.M.; Wu, Y.C. Deuterium concentration depth profiling in sputter-deposited tungsten coated F82H using secondary ion mass spectrometry. Nucl. Mater. Energy 2019, 21, 100708. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Sayers, J.; Aarholt, T.; He, G.; Hulme, H.; Garner, A.; Preuss, M.; Nordin, H.; Partezana, J.M.; et al. Characterisation of deuterium distributions in corroded zirconium alloys using high-resolution SIMS imaging. Acta Mater. 2020, 200, 581–596. [Google Scholar] [CrossRef]
- Hauri, E.; Wang, J.; Dixon, J.E.; King, P.L.; Mandeville, C.; Newman, S. SIMS analysis of volatiles in silicate glasses 1. Calibration, matrix effects and comparisons with FTIR. Chem. Geol. 2002, 183, 99–114. [Google Scholar] [CrossRef]
- Koga, K.; Hauri, E.H.; Hirschmann, M.M.; Bell, D. Hydrogen concentration analyses using SIMS and FTIR: Comparison and calibration for nominally anhydrous minerals. Geochem. Geophys. Geosyst. 2003, 4, 1019. [Google Scholar] [CrossRef] [Green Version]
- Mosenfelder, J.L.; le Voyer, M.; Rossman, G.R.; Guan, Y.; Bell, D.R.; Asimow, P.D.; Eiler, J.M. Analysis of hydrogen in olivine by SIMS: Evaluation of standards and protocol. Am. Mineral. 2011, 96, 1725–1741. [Google Scholar] [CrossRef]
- Shimizu, N.; Hart, S.R. Application of the ion microprobe to geochemistry and cosmochemistry. Annu. Rev. Earth Planet. Sci. 1982, 10, 483–526. [Google Scholar] [CrossRef]
- Yurimoto, H.; Kurosawa, M.; Sueno, S. Hydrogen analysis in quartz crystals and quartz glasses by secondary ion mass spectrometry. Geochim. Cosmochim. Acta 1989, 53, 751–755. [Google Scholar] [CrossRef]
- Maheshwari, P.; Stevie, F.A.; Myneni, G.; Ciovati, G.; Rigsbee, J.M.; Griffis, D.P. Analysis of Interstitial Elements in Niobium with Secondary Ion Mass Spectrometry (SIMS). In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2011; Volume 1352, pp. 151–160. [Google Scholar]
- Cohen, D.D.; Bird, R.; Dytlewski, N.; Siegele, R. Ion Beams for Material Analysis. In Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 55–63. [Google Scholar]
- Jain, I.P.; Jain, A.; Jain, P. ERDA: Technique for Hydrogen Content and Depth Profile in Thin Film Metal Hydride. In Proceedings of the 18th World Hydrogen Energy Conference 2010, Essen, Germany, 16–21 May 2010; Available online: https://www.osti.gov/etdeweb/servlets/purl/21400976 (accessed on 5 April 2021).
- Siketić, Z.; Radović, I.B.; Jakšić, M. Quantitative analysis of hydrogen in thin films using time-of-flight recoil detection analysis. Thin Solid Film 2010, 518, 2617–2622. [Google Scholar] [CrossRef]
- Tunmee, S.; Supruangnet, R.; Nakajima, H.; Zhou, X.; Arakawa, S.; Suzuki, T.; Kanda, K.; Ito, H.; Komatsu, K.; Saitoh, H. Study of Synchrotron Radiation Near-Edge X-Ray Absorption Fine-Structure of Amorphous Hydrogenated Carbon Films at Various Thicknesses. J. Nanomater. 2015, 2015, 276790. [Google Scholar] [CrossRef]
- Gago, R.; Jiménez, I.; Albella, J.M.; Climent-Font, A.; Banks, J.C.; Doyle, B.L.; Terminello, J.L. Bonding and hardness in nonhydrogenated carbon films with moderate sp3 content. J. Appl. Phys. 2000, 87, 8174–8180. [Google Scholar] [CrossRef] [Green Version]
- Ouchabane, M.; Salah, H.; Herrmann, M.; Tabet, N.; Henda, K.; Touchrift, B.; Kechouane, M. Influence of bias voltage on the structure and deposition mechanism of diamond-like carbon films produced by RF (13.56 MHz) CH4 plasma. Phys. Status Solidi A 2010, 207, 2311–2318. [Google Scholar] [CrossRef]
- Čekada, M.; Kahn, M.; Pelicon, P.; Siketić, Z.; Radović, I.B.; Waldhauser, W.; Paskvale, S. Analysis of nitrogen-doped ion-beam-deposited hydrogenated diamond-like carbon films using ERDA/RBS, TOF-ERDA and Raman spectroscopy. Surf. Coat. Technol. 2012, 211, 72–75. [Google Scholar] [CrossRef]
- Khánh, N.Q.; Serényi, M.; Csik, A.; Frigeri, C. Determination of hydrogen concentration in a-Si and a-Ge layers by elastic recoil detection analysis. Vacuum 2012, 86, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Wielunski, L.S.; Grambole, D.; Kreissig, U.; Grötzschel, R.; Harding, G.; Szilágyi, E. Hydrogen depth resolution in multilayer metal structures, comparison of elastic recoil detection and resonant nuclear reaction method. Nucl. Instrum. Methods Phys. Res. B 2002, 190, 693–698. [Google Scholar] [CrossRef]
- Pranevicius, L.; Wirth, E.; Milcius, D.; Lelis, M.; Pranevicius, L.L.; Bacianskas, A. Structure transformations and hydrogen storage properties of co-sputtered MgNi films. Appl. Surf. Sci. 2009, 255, 5971–5974. [Google Scholar] [CrossRef]
- Wirth, E.; Munnik, F.; Pranevicius, L.L.; Milcius, D. Dynamic surface barrier effects on hydrogen storage capacity in Mg–Ni films. J. Alloys Compd. 2009, 475, 917–922. [Google Scholar] [CrossRef]
- Jain, I.P.; Devi, B.; Sharma, P.; Williamson, A.; Vijay, Y.K.; Avasthi, D.K.; Tripathi, A. Hydrogen in FeTi thin films by ERDA with Ag107 ions. Int. J. Hydrog. Energy 2000, 25, 517–521. [Google Scholar] [CrossRef]
- Wilde, M.; Fukutani, K. Hydrogen detection near surfaces and shallow interfaces with resonant nuclear reaction analysis. Surf. Sci. Rep. 2014, 69, 196–295. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.F.; Biersack, J.P.; Littmark, U. Stopping and Ranges of Ions in Solids; Pergamon: New York, NY, USA, 1985. [Google Scholar]
- Hjörvarsson, B.; Rydén, J.; Ericsson, T.; Karlsson, E. Hydrogenated tantalum: A convenient calibration substance for hydrogen profile analysis using nuclear resonance reactions. Nucl. Instrum. Methods Phys. Res. B 1989, 42, 257–263. [Google Scholar] [CrossRef]
- Sekiba, D.; Yonemura, H.; Ogura, S.; Matsumoto, M.; Kitaoka, Y.; Yokoyama, Y.; Matsuzaki, H.; Narusawa, T.; Fukutani, K. Development of micro-beam NRA for hydrogen mapping: Observation of fatigue-fractured surface of glassy alloys. Nucl. Instrum. Methods Phys. Res. B 2011, 269, 627–631. [Google Scholar] [CrossRef]
- Dieumegard, D.; Dubreuil, D.; Amsel, G. Analysis and depth profiling of deuterium with the D(3He, p)4He reaction by detecting the protons at backward angles. Nucl. Instrum. Methods 1979, 166, 431–445. [Google Scholar] [CrossRef]
- Wampler, W.R. Trapping of deuterium in beryllium. J. Nucl. Mater. 1992, 196–198, 981–985. [Google Scholar] [CrossRef]
- Danesh, P.; Pantchev, B.; Schmidt, B. Infrared absorption strengths of ion-implanted hydrogenated amorphous silicon. Thin Solid Film 2008, 516, 3383–3386. [Google Scholar] [CrossRef]
- Portmann, J.; Haug, C.; Brenn, R.; Schneider, J.; Rottner, K.; Helbig, R. Determination of hydrogen in 6H–SiC epitaxial layers by the 15N nuclear reaction analysis technique. Nucl. Instrum. Methods Phys. Res. B 1999, 155, 132–136. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Ito, S.; Wilde, M.; Fukutani, K.; Hirozawa, I.; Koganezawa, T. A hydrogen storage layer on the surface of silicon nitride films. Appl. Phys. Lett. 2008, 92, 192115. [Google Scholar] [CrossRef]
- Bernard, E.; Khodja, H.; Chêne, J.; Pégourié, B.; Martin, C.; Pardanaud, C. Simultaneous deuterium implantation and ion beam microanalyses in CFC NB31: Understanding the in-bulk migration. J. Nucl. Mater. 2013, 438, S975–S978. [Google Scholar] [CrossRef]
- Kulisch, W.; Sasaki, T.; Rossi, F.; Popov, C.; Sippel, C.; Grambole, D. Hydrogen incorporation in ultrananocrystalline diamond/amorphous carbon films. Phys. Status Solidi (Rapid Res. Lett.) 2008, 2, 77–79. [Google Scholar] [CrossRef]
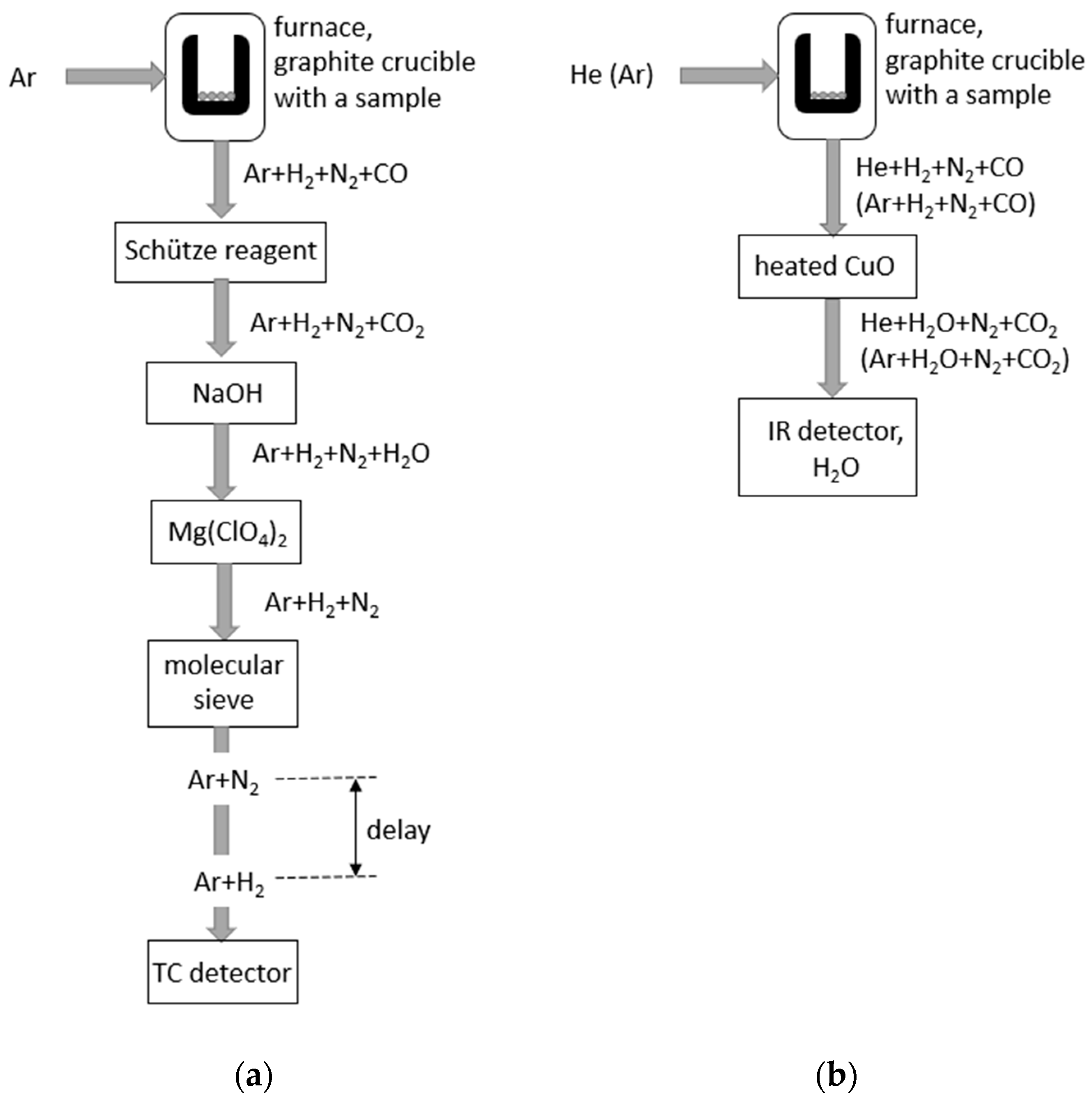
| Process | Reagent | Trade Name | |
|---|---|---|---|
| Conversion of CO to CO2 | CO → CO2 | Heated CuO | |
| I2O5 + H2SO4 | Schütze reagent 1 | ||
| Removal of CO2 | CO2 + 2NaOH → Na2CO3 + H2O | NaOH | |
| Conversion of H2 to H2O | H2 → H2O | Heated CuO | |
| Removal of H2O | H2O → Mg(ClO4)2·xH2O | Mg(ClO4)2 | Anhydrone™ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, Z. Analysis of Hydrogen in Inorganic Materials and Coatings: A Critical Review. Hydrogen 2021, 2, 225-245. https://doi.org/10.3390/hydrogen2020012
Weiss Z. Analysis of Hydrogen in Inorganic Materials and Coatings: A Critical Review. Hydrogen. 2021; 2(2):225-245. https://doi.org/10.3390/hydrogen2020012
Chicago/Turabian StyleWeiss, Zdeněk. 2021. "Analysis of Hydrogen in Inorganic Materials and Coatings: A Critical Review" Hydrogen 2, no. 2: 225-245. https://doi.org/10.3390/hydrogen2020012
APA StyleWeiss, Z. (2021). Analysis of Hydrogen in Inorganic Materials and Coatings: A Critical Review. Hydrogen, 2(2), 225-245. https://doi.org/10.3390/hydrogen2020012






