1. Introduction
Hydrogen storage is a key enabling technology for bringing forward hydrogen and fuel cell technologies in applications including transportation, stationary and portable power. For these applications, the volumetric and gravimetric hydrogen storage capacities are essential. Hydrogen can be physically stored as a gas under high pressure (35–70 MPa in high-pressure tanks) or as a liquid at cryogenic temperatures, since hydrogen boils at 20 K at ambient pressure. Besides, hydrogen can be stored in materials by adsorption (in the high surface solids, e.g., carbon materials or metal organic frameworks (MOFs) at temperatures < 77 K) or absorption (within solids, e.g., metal hydrides, complex metal hydrides, and alloys). In comparison to other methods, storage of absorbed hydrogen within metal hydrides or complex metal hydrides can offer high volumetric storage capacity up to 150 kg m
−3 [
1,
2].
Sodium aluminum hexahydride-Na
3AlH
6 with Ti catalysts was tested in our prior research as a storage material for the use in a lightweight aluminum alloy storage tank [
3]. It was shown that the storage material could practically absorb/release up to 2.0 wt % of hydrogen during the hydrogenation/dehydrogenation processes. Although the material exhibits stable behavior of the kinetics performance, the capacity and the cyclic stability of the material still needs further optimization for commercial applications. Hydrogen which can be used for recharging metal hydrides is a constituent of synthesis gas that is usually generated by the endothermic steam reforming of hydrocarbons, partial oxidation of methane, or gasification of coal and biomass [
4,
5]. The majority of hydrogen is produced by steam reforming of natural gas that is reacting with steam in the presence of Ni catalyst [
6] as follows (Equation (1)):
Partial oxidation of methane occurs when a sub-stoichiometric fuel–air mixture combusted forming a hydrogen-rich synthesis gas in an exothermic process according to Equation (2) [
7]:
Autothermal reforming combines the steam reforming process and fuel oxidation in one unit. The energy for the endothermic steam reforming is provided by the exothermic oxidation resulting in an overall reaction enthalpy close to zero (Equation (3)) [
8]:
During the above-mentioned synthesis gas processes, various gas compositions can be expected at the outlet of the appropriate reactor [
9]. The resulting gas contents are shown in
Table 1. The highest hydrogen content can be achieved during steam reforming and the lowest hydrogen content in the partial oxidation process.
The synthesis gas components such as CO, CO
2, CH
4, N
2, and H
2O are suspected to be harmful for complex metal hydrides. Limited information could be found on the influence of these gas constituencies on complex metal hydrides. In 2007, Sandia National Laboratories published the results of reactivity of sodium alanate with O
2, H
2O, and CO
2. It was shown that sodium alanate reacts exothermically with H
2O and O
2 producing various Na–O compounds and H
2 [
10]. The gas–solid reaction of CO
2 with alanates was investigated by Hugelshofer et al. where the authors observed the formation of intermediates like aluminum formate and methoxy compounds which then got converted into methane and metal oxides as final products [
11]. The influences of gas components on other metal hydrides are well investigated [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. In this work, the influence of different ratios of CO, CO
2, N
2 on the hydrogen storage performance of the complex metal hydride Na
3AlH
6 with 4 mol% TiCl
3, 8 mol% aluminum and 8 mol% activated carbon is explored.
2. Experimental Section
The detailed description of the synthesis of TiCl
3- doped Na
3AlH
6 was reported in our previous publication [
25]. NaH (60% dispersion in mineral oil, Sigma-Aldrich, St. Louis, MO, USA) and NaAlH
4 (95% technical grade, Sigma-Aldrich) both after purification, aluminum powder (99%, Sigma-Aldrich), activated carbon, and TiCl
3 (Sigma-Aldrich) were used. The composite was prepared by mechano-chemical synthesis of 2:1 molar ratio of NaH and NaAlH
4, 4 mol% of TiCl
3, 8 mol% of aluminum, and 8 mol% activated carbon under Argon inert atmosphere using hardened steel grinding bowls and balls in a Planetary Mill PULVERISETTE 5 (Fritsch GmbH, Idar-Oberstein, Germany).
Cycling tests of the composite were carried out in a fully automated Sieverts instrument PCTPro 2000 (SETARAM Inc., Caluire, France). Then, 2.0 g of material was used for each test. Hydrogenations were done at 2.5 MPa hydrogen pressure at 170 °C and dehydrogenations at 0.1 MPa at 170 °C. The XRD measurement was performed on the STOE STADI P (Cu-Kα radiation, 1.541 Å) in a 0.5 mm diameter capillary filled with purified argon (both H2O and O2 < 1 ppm). The IR-Spectrum measurements of the gaseous products were performed with a NICOLET MAGNA 560 FTIR spectrometer using Smart OMNI-Transmission cell with KBr windows. The gas sample was taken directly from the autoclave to the gas bag, being subsequently transferred into the Smart OMNI-Transmission cell. The gas phase spectra were taken at room temperature. Handling of the material was done in the MBraun glove box, in Argon atmosphere where oxygen and water levels always kept below 1 ppm.
The solid-state 13C NMR spectra were recorded on a Bruker Avance III HD 500WB spectrometer using a double-bearing magic angle spinning (MAS) probe (DVT BL4) at a resonance frequency of 125.8 MHz. The experimental conditions for 13C CP MAS NMR were as follows: 10–12 kHz spinning rate, 3 s recycle delay, 20,000–52,000 scans, 2 ms contact time, 3.3 µs 1H π/2 pulse, and high-power proton decoupling (spinal64). The 13C chemical shift was referenced with respect to neat TMS in a separate rotor.
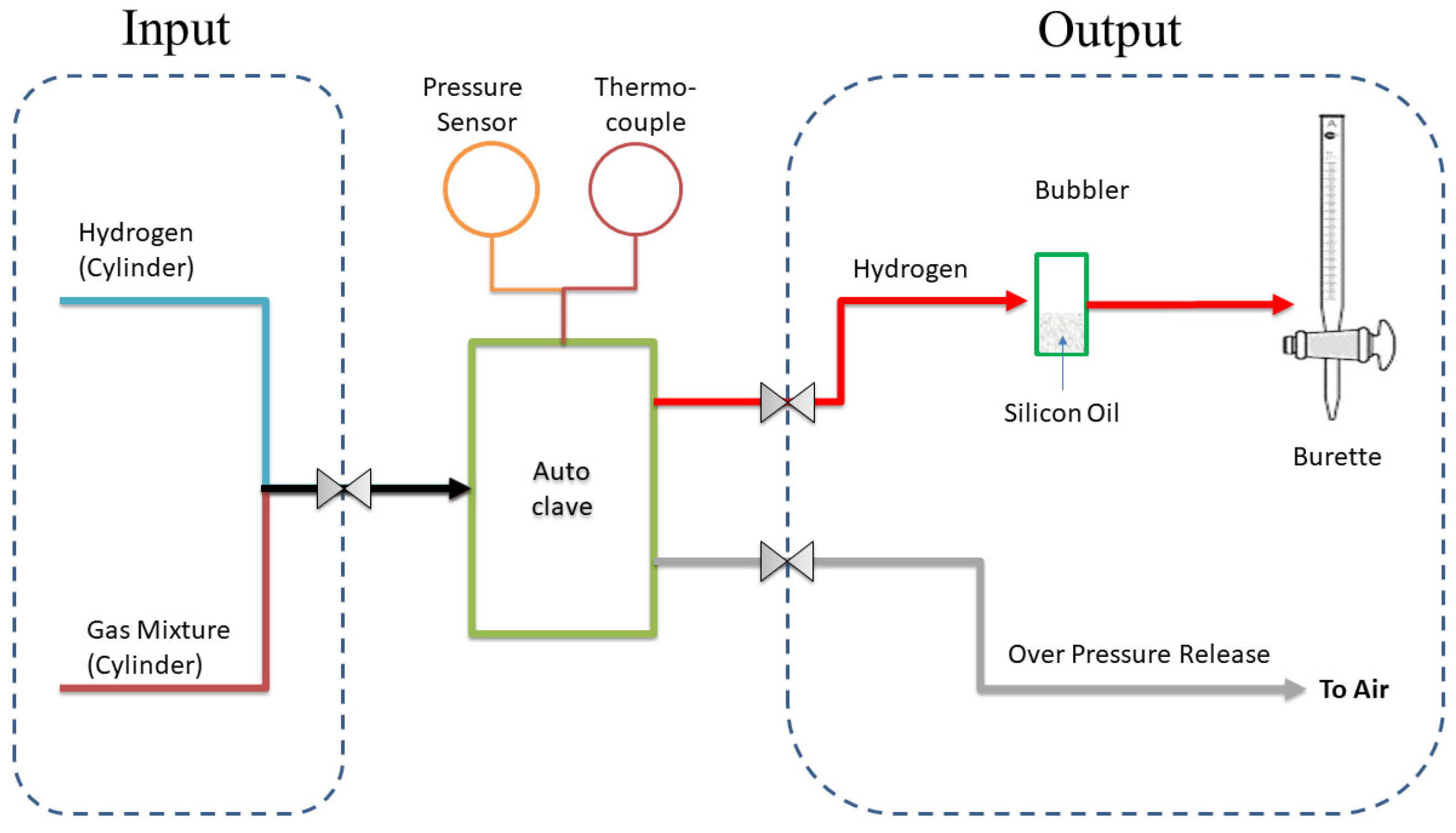
The hydrogenation and dehydrogenation cycles with gas mixtures were performed on a test rig shown in
Figure 1. The autoclave was charged with a total pressure of 3.0 MPa of pure hydrogen or gas mixtures (
Table 2) except of 11.0 MPa for the gas mixture in 7 (
Table 2). Gas mixture 7 was used as an example for autothermal syngas production from propane/butane mixtures of a commercial system. All gas mixtures were prepared in house. The autoclave was heated using a JUMO controller. The pressure inside the autoclave was monitored by JUMO pressure sensor and the temperature by a type K thermocouple. After the hydrogenation test, the hydrogen or gas mixture overpressure was released through the overpressure release valve. Immediately after releasing the gas pressure, desorbed hydrogen was collected and measured using a calibrated gas burette.
3. Results
3.1. Hydrogen Storage Performance of the Na3AlH6 Composite Material in Pure Hydrogen
Activated carbon (8 mol%) and aluminum (8 mol%) were added to Na
3AlH
6 and TiCl
3 (4 mol%) to enhance heat transfer of the composite material, which could improve the hydrogenation and dehydrogenation kinetic as well as dispersion, which can prevent the material from sintering during long time cycling. The other reason for addition of aluminum is that the forming of Ti-Al alloy during doping processes would consume part of Al from Na
3AlH
6. To replace this consumed Al metal and as the result to maintain the reversibility of the system, the amount of 8 mol% Al is added [
25].
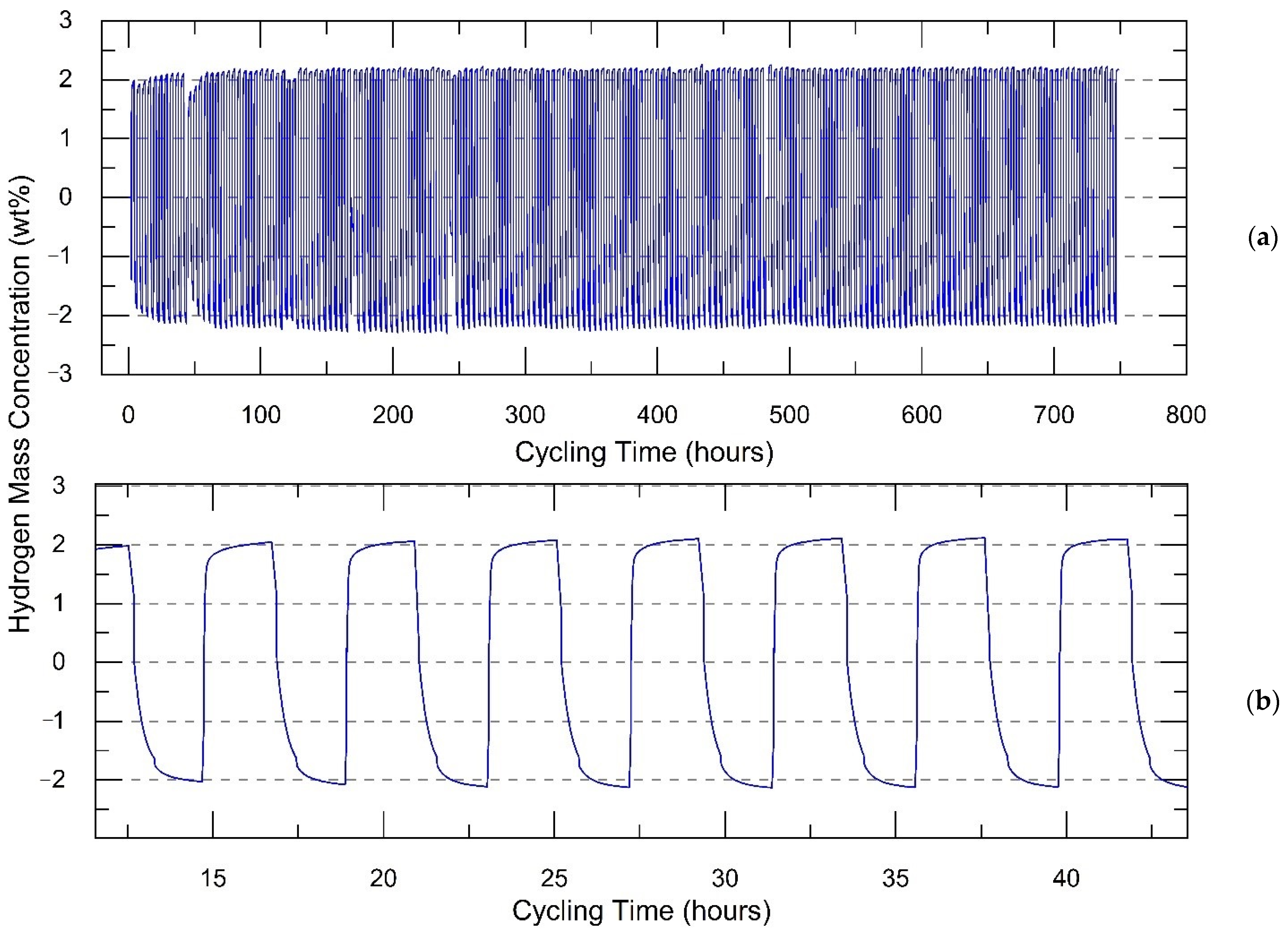
Here, we define that one “cycle” includes one hydrogenation and one dehydrogenation process. A complete cycle takes 4 h, including 2 h of hydrogenation and 2 h of dehydrogenation. As shown in
Figure 2, 187 cycles were achieved without notable degradation. Every hydrogenation cycle was performed at 2.5 MPa hydrogen pressure and 170 °C, while every dehydrogenation cycle was performed at 0.1 MPa pressure and 170 °C. Then, 2.15 ± 0.02 wt % of hydrogen was evolved and absorbed during hydrogenation or dehydrogenation. This value will be used as hydrogen normalized capacity in cycling experiments. In our prior research, the hydrogen capacity of the composite without additives of Al and activated carbon was less than 1.7 wt % [
26]. Addition of activated carbon and Al has improved stability and hydrogen storage capacity of the composite by more than 30% and therefore confirmed the benefits of these additives. In general, the addition of carbon materials to metal hydride compounds results in improved kinetics of dehydrogenation and hydrogenation and the ability to transport and to release the produced heat during the reaction with hydrogen [
27].
Table 3 shows the dehydrogenation H
2 capacity (0.1 MPa dehydrogenation pressure) of samples hydrogenated for 0.5, 1, and 2 h. The dehydrogenation time was varied between 1, 2, and 3 h. The hydrogenation and dehydrogenation temperatures were changed between 180 and 150 °C. It can be observed, that when temperature is higher than or equal to 160 °C, only 1 h is needed to fully hydrogenate the material at 3.0 MPa or dehydrogenate the material at 0.1 MPa. Increasing hydrogenation or dehydrogenation time to 2 or 3 h does not improve or only slightly improves the hydrogen capacity. At 150 °C, extending of hydrogenation and dehydrogenation time is necessary to obtain higher H
2 capacity. As result of these investigations, it was concluded to perform the cycling experiments with synthesis gas components at 170 °C and use 1 h for hydrogenation and 1 h for dehydrogenation processes.
3.2. The Influence of CO Gas on the Cycling Behavior of the Ti-Doped Na3AlH6
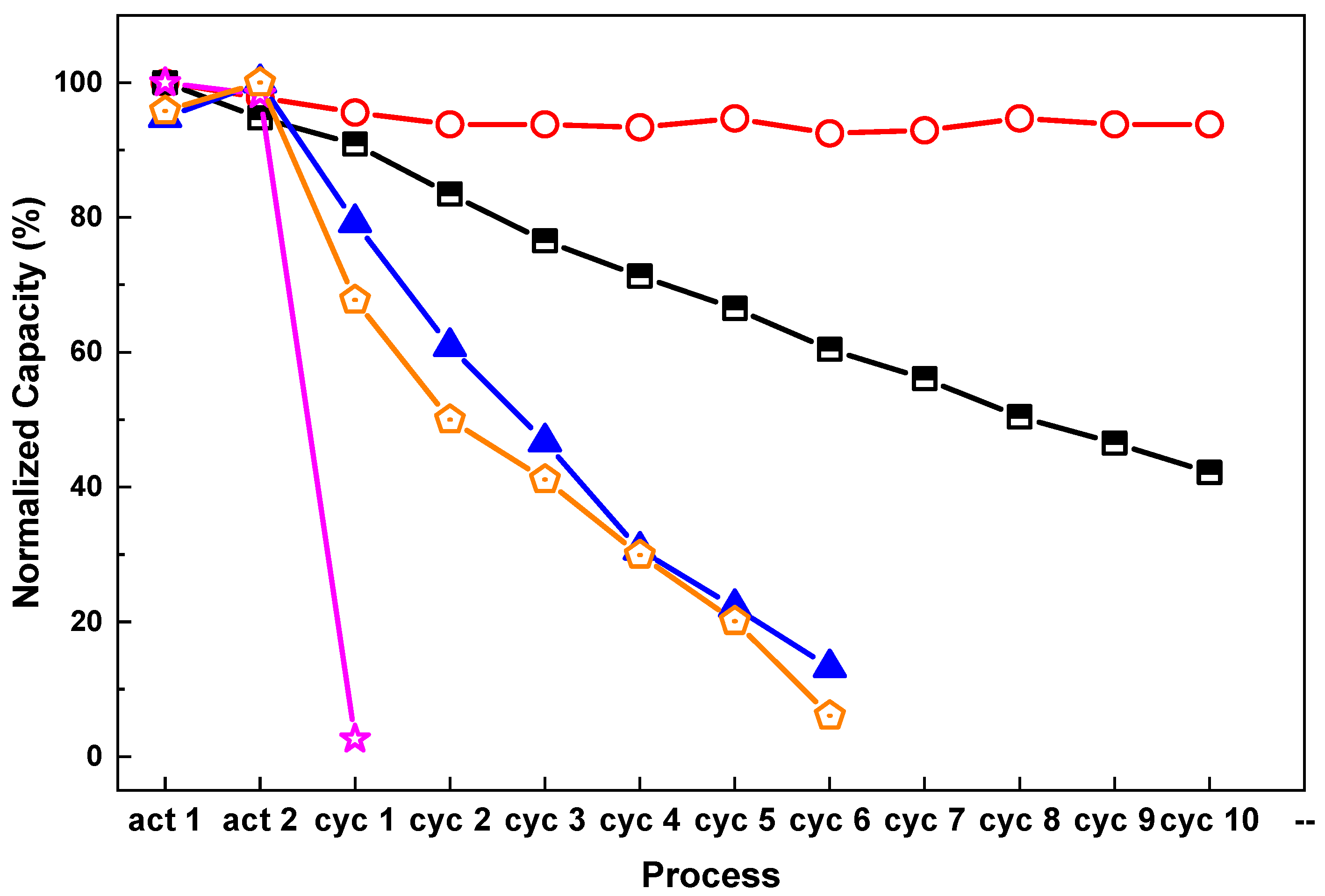
Figure 3 shows the hydrogen storage performance of the composite material in different ratios of CO and H
2 gas mixture. After 10 cycles with 0.1 vol % CO + 99.9 vol %H
2 and 1 vol % CO + 99 vol %H
2, the dehydrogenation storage capacity of the composite material decreased by 17.2% and 57.3%, respectively. The sample cycled with 10 vol % CO + 90 vol % H
2 gas mixture shows the most severe degradation. There was almost no hydrogen released after only 2 cycles after hydrogenation with 10 vol % CO + 90 vol % H
2 gas mixture. Attempts to recharge the sample with 5 MPa of pure hydrogen failed, which means that the changes during the cycling with the gas contained 10 vol % CO seem to be irreversible.
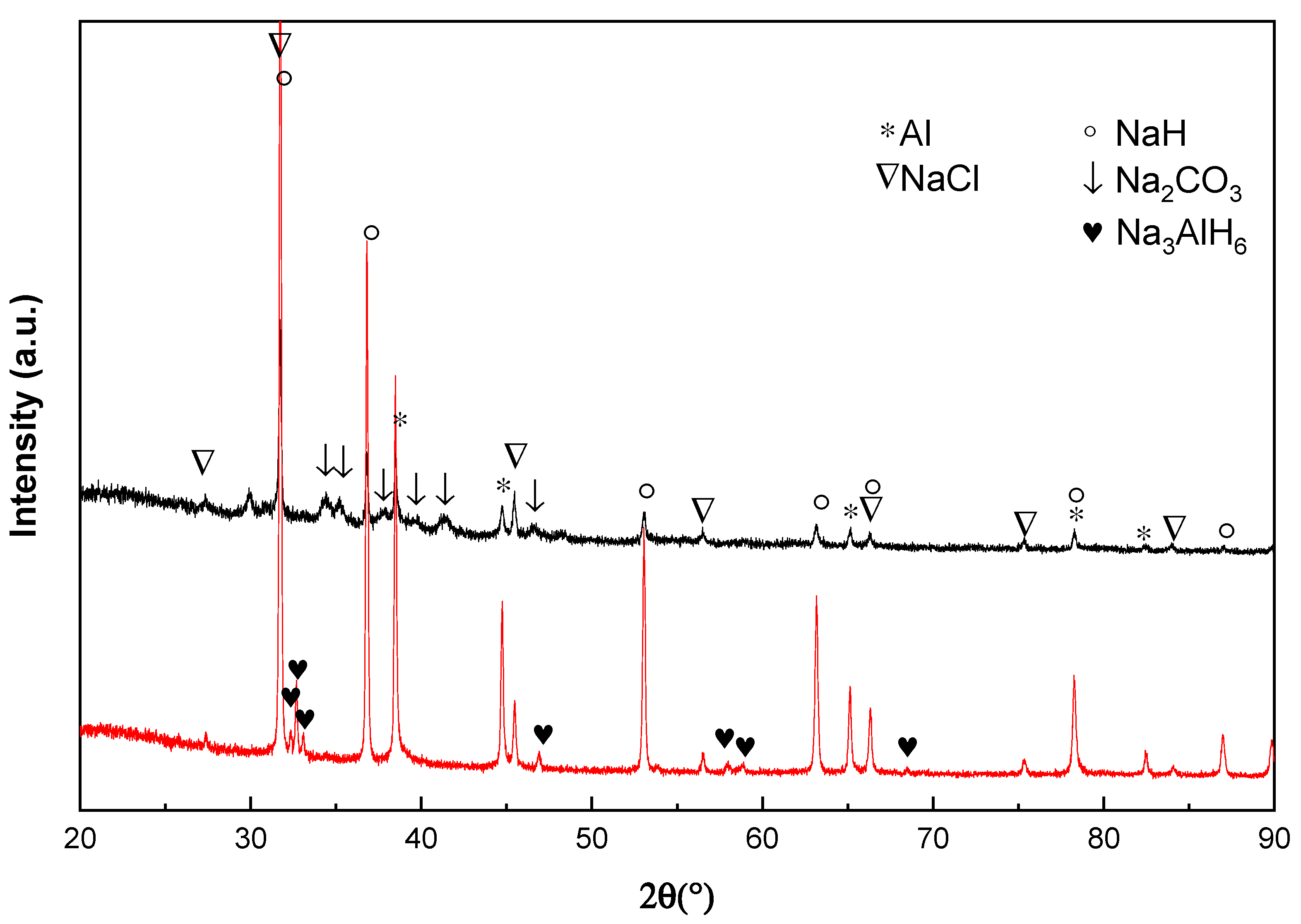
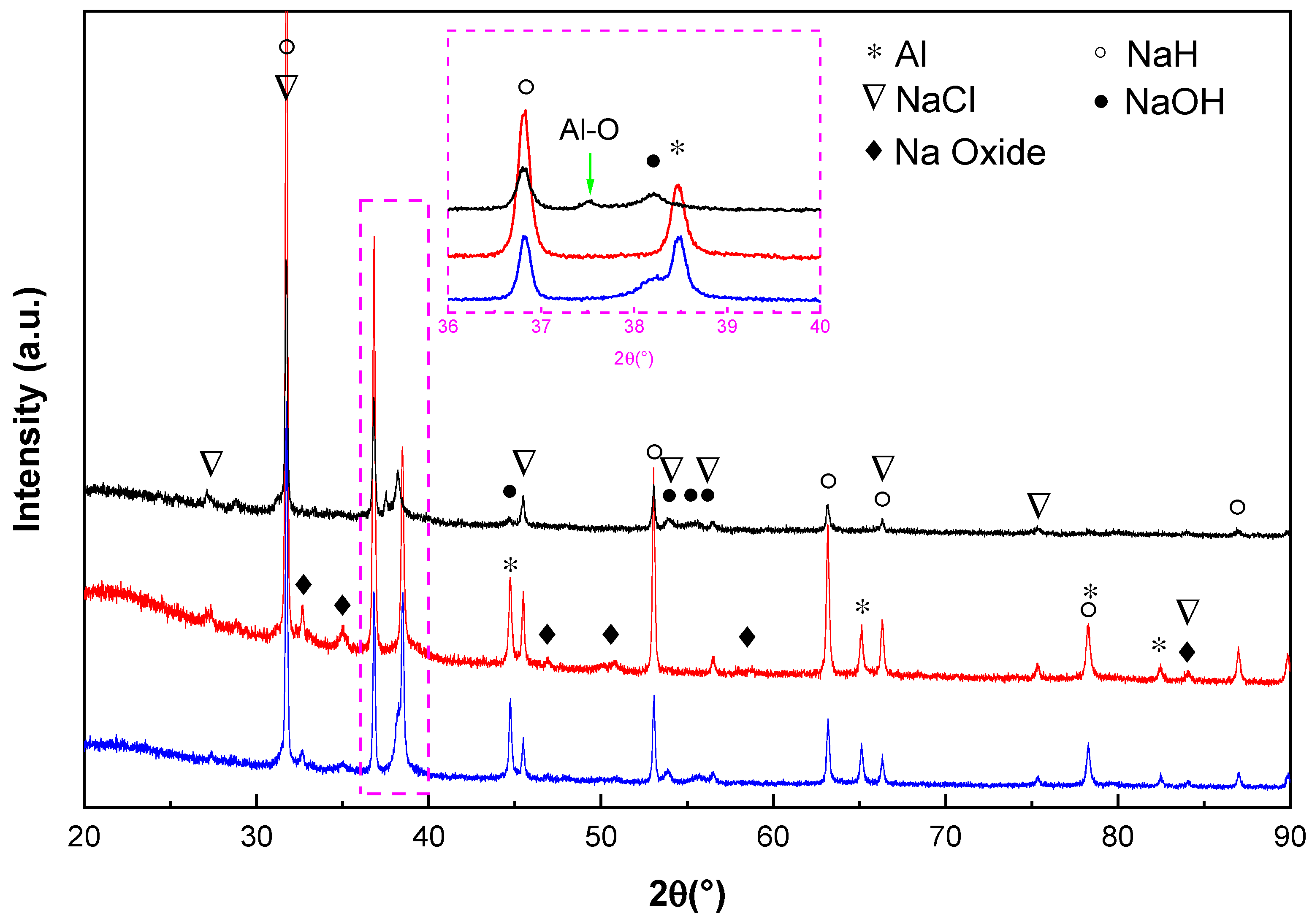
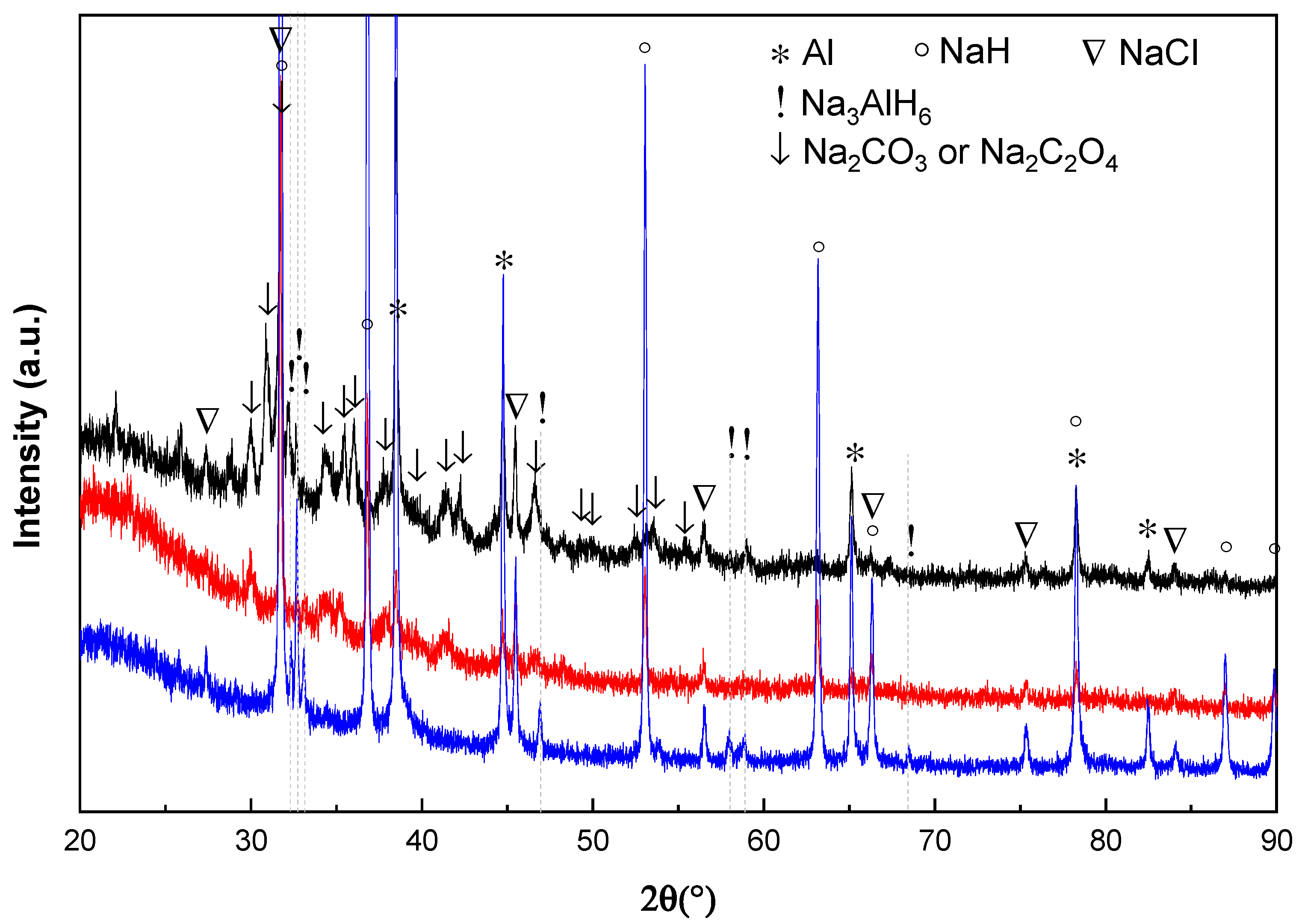
Figure 4 shows the X-ray diffraction pattern of the doped Na
3AH
6 material after treatment with different amounts of CO in H
2. It can be seen that Na oxides were formed after 10 cycles with 0.1 vol % CO + 99.9 vol % H
2 and 1 vol % CO + 99 vol % H
2. In case of 10 vol % CO + 90 vol % H
2 mixture, the formation of NaOH can be observed just after 2 cycles. The peak intensity ratio of Al:NaCl at around 45°(2θ) becomes smaller when the concentration of CO increases in the gas mixture. The Al peak with 10 vol % CO + 90 vol % H
2 sample almost disappears after 2 cycles. As shown in the embedded enlarged
Figure 4, a new weak reflection at round 37.4° (2θ) appeared. This peak may belong to some kind of Al oxygenates.
In order to get more information about the weak reflection at round 37.4° (2θ) observed in the X-ray diffraction pattern of
Figure 4, we measured a carbon solid state NMR-spectrum.
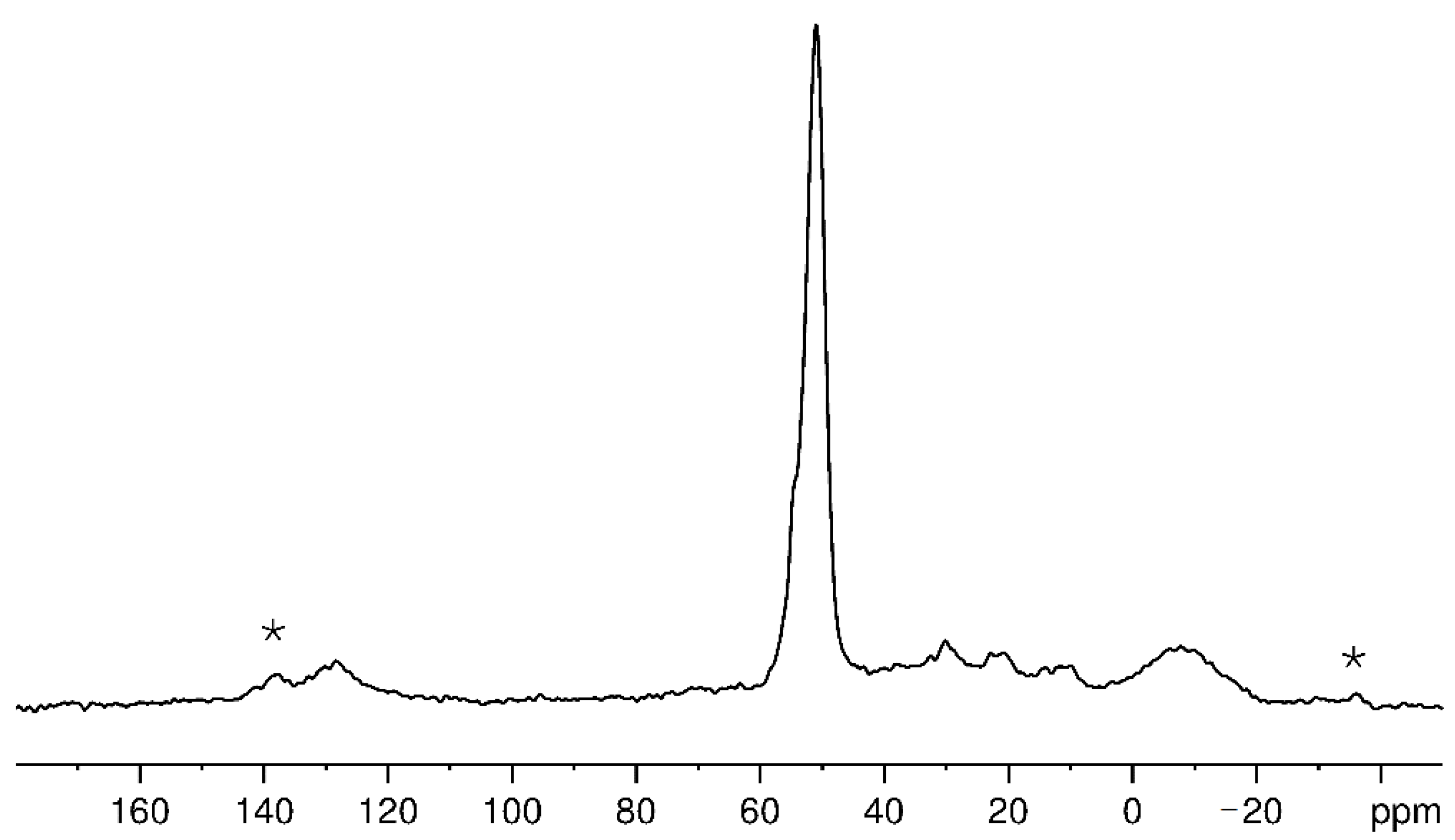
Figure 5 shows the
13C CP/MAS NMR spectrum of Ti-doped Na
3AlH
6 after the second hydrogenation with 10 vol % CO + 90 vol % H
2 gas mixture. The most intense and asymmetric line at about 51 ppm in the
13C NMR spectrum in
Figure 5 can be assigned to methoxy groups (O–CH
3) [
28]. These methoxy groups can be connected to Al- and/or Na-atoms. The observation of O–CH
3-groups also confirms the detection of Al–O-compounds in the X-ray diffraction pattern in
Figure 4. The high-field line at about −8 ppm stems most likely from methyl groups directly bound to aluminum atoms [
29]. The spectral intensity between 10 and 40 ppm is partly due to a small background signal of the probe.
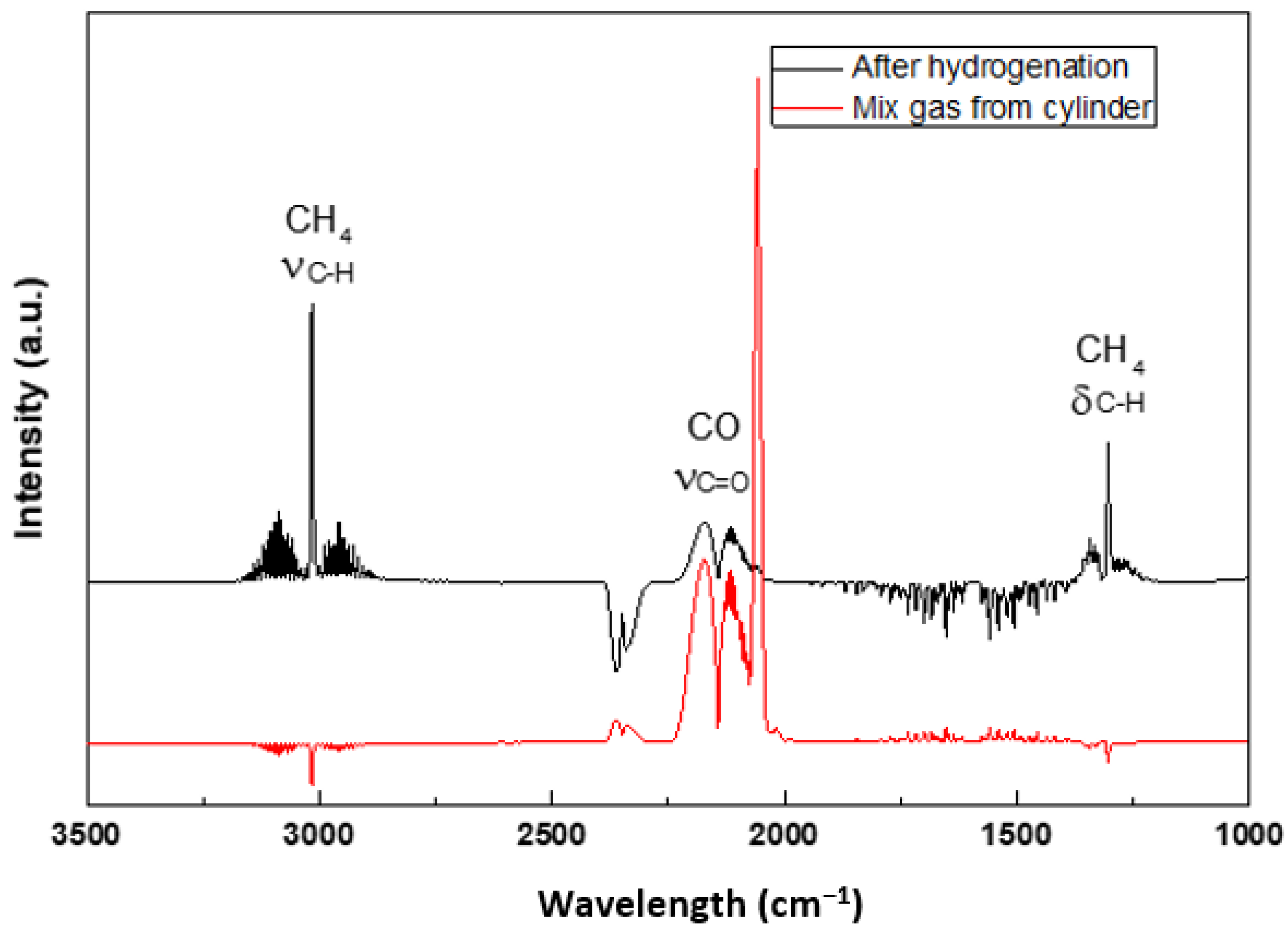
To gain a better understanding of the influences of CO on the molecular processes during the hydrogenation, the gas-phase FTIR spectra was measured and shown in
Figure 6. During the hydrogenation, part of the CO (ν
C=O = 2350 cm
−1) reacts with the complex hydride and produces CH
4 (ν
C–H = 3016 cm
−1, δ
C–H = 1305 cm
−1). No other gases can be detected in the IR-spectra.
3.3. The Influence of CO2 Gas on Cycling Behavior of the Complex Hydride Ti-Doped Na3AlH6
The hydrogen storage performance with different CO
2 + H
2 gas ratios were measured and are shown in
Figure 7. The influence of 1 vol % CO
2 on the storage capacity is comparable to a mixture with 1 vol % CO. After 10 cycles, the capacity degradation was 53.5% in the 1 vol % CO
2 + 99 vol % H
2 gas mixture and 57.3% in 1 vol % CO + 99 vol % H
2. After increasing the concentration of CO
2 up to 10 vol %, the capacity degradation becomes larger and reaches 86.8% after 6 cycles. The performance of hydride with 10 vol % CO
2 + 90 vol % H
2 is better than the one with 10 vol % CO + 90 vol % H
2. Several new compounds can be identified by X-ray powder diffractometry after cycling with different CO
2/H
2 gas mixtures as shown in
Figure 8. The diffraction pattern of dehydrogenated hydride (Al, NaH, NaCl) which was cycled in pure hydrogen, was used as a reference. The Al, NaH, NaCl peaks can be found in all these samples. The small amounts of Na
3AlH
6 in the composite in 10 vol % CO
2 + 90 vol % H
2 is due to the sluggish kinetics that is getting worse during cycling. The NaH phase is partly consumed during cycling to produce Na carbon oxides such as Na
2CO
3 and Na
2C
2O
4. The ratio of Al:NaH:NaCl becomes smaller that implies that the consumption of Al and NaH during cycling with 10 vol % CO
2 + 90 vol % H
2 gas mixture took place. The loss of Al and NaH leads to a reduction of the storage capacity of the material.
3.4. Collective Influence of CO, CO2, and N2 Gas Components on the Cycling Behavior of Ti-Doped Complex Hydride Na3AlH6
As mentioned in the introduction, the auto-thermal production of syngas with air contains in addition to H
2, CO, CO
2, H
2O also high amounts of N
2 gas. The high amount of neutral N
2 reduces the partial pressure of H
2 in the gas mixtures and changes the hydrogenation behavior of the complex hydride. Our experiments with 62 vol % N
2 + 38 vol % H
2 gas mixture shows that 3.0 MPa total pressure which corresponds to only 1.1 MPa of hydrogen partial pressure is not high enough to recharge the sample at 170 °C, see
Figure 9. This pressure of 1.1 MPa is below the equilibrium pressure at 170 °C, which is equal to 1.5 MPa. In order to reach partial H
2 pressure of 3 MPa in a gas mixture of 1.5 vol % CO + 10 vol % CO
2 + 27 vol % H
2 + 61.5 vol % N
2, the overall gas pressure must be increased to 11 MPa. As shown in
Figure 8, the degradation reaches 94% after 6 cycles with this gas mixture. The fast degradation process seems to be due to combined influence of the different gas impurities during cycling. Each kind of gas impurity affects the complex hydride in a different way.
Figure 10 shows the X-ray diffraction pattern of the complex hydride after 6 cycles with the 1.5 vol % CO + 10 vol % CO
2 + 27 vol % H
2 + 61.5 vol % N
2 gas mixture, and for comparison, the pattern of the decomposed complex hydride after cycling in pure H
2 as a reference. Only very small diffraction peaks of Na
3AlH
6 are observed after the cycling and decomposition. The main reflections belong to Al-metal, NaH, and NaCl from the doping process. No or very small reflections of Al-metal and NaH or Na
3AlH
6 can be observed in the diffraction pattern after only 6 cycles. Na
2CO
3 can be detected as newly formed compound, which can easily be explained by a reaction of NaO(H) with CO
2. No additional Al–O-compounds are detected.
4. Discussions
In this paper, we reported about the cycling performance of Ti-doped Na3AlH6 + Al + activated carbon composite in pure hydrogen and studied the influence of different ratios of CO, CO2, N2 gas components on the hydrogen storage properties of this complex hydride. By addition of activated carbon and aluminum to TiCl3-activated Na3AlH6, the cycling stability and hydrogen storage capacity could be successfully enhanced in comparison to undoped material in pure hydrogen. Both CO, CO2 gas components react with the hydride mixture and cause a serious decline in hydrogen storage capacity to form Al–O- and/or carboxyl compounds and Na–O- and/or carbonates, and CH4.
Several reaction pathways exist, which can explain the conversion of CO/CO2 to methane and water.
- -
reduction of CO/CO2 with Na3AlH6.
- -
reduction of CO/CO2 with NaH.
- -
reduction of CO/CO2 with H2 under the influence of TiAlx as catalyst.
Hugelshofer et al. [
11] described the reaction of LiAlH
4 with CO
2 and postulated the formation of various amorphous metal oxides, which cannot be identified by XRD. The similar reactivity of the complex aluminum hydrides LiAlH
4 and Na
3AlH
6 could explain the formation of some amorphous metal oxides compounds with the general formula Na
xAl
YO
z. The structure and the amount of formed oxide-compounds strongly depends on the amount of CO or CO
2 in the gas mixture, while CH
4 and H
2O are produced. Water seems to be the main reason for the production of metal oxide compounds and subsequently, reduced reversibility of the storage material.
The reduction of CO
2 to methane in the presence of mechanochemically activated alkali metal hydrides were discovered by Zhao et al. [
23]. They observed elemental carbon, hydrogen, alkali metal oxides, and carbonates as final products from the reaction of the oxides with CO
2. Similar reaction pathways can be expected in our case with the exception that in the presence of TiAl
x-catalyst hydrogenated gaseous compounds (CH
4) can be observed.
The in situ formed Al-Ti active species [
30], which are acting as a catalyst for the hydrogenation of NaH
/Al to Na
3AlH
6, can also act as catalyst in a hydrogen atmosphere for the reduction of CO to produce CH
4 [
11].
In comparison to these three examples, our system is more complicated, since all three different reaction pathways can take place at the same time. During these reduction processes, stoichiometric amounts of H2O are produced which consequently react with hydride species to produce Na- and/or Al-oxide compounds. In a following process, these oxide compounds can react with CO2 producing carbonates and in case of CO producing formic acid compounds. A loss of catalytic activity of Al-Ti species and the oxidation of Na- and Al-hydride species lower the reversibility of the composite system and hence degrade the hydrogen storage capacity.
5. Conclusions
Ti-doped Na3AlH6 is a medium temperature hydrogen storage material with a theoretical hydrogen storage capacity of 3 wt % H2 and an impressive stable capacity over several hundreds of de- and re-hydrogenation cycles in pure hydrogen. Nowadays, most of the worldwide used hydrogen is produced from natural gas. This means that significant amounts of CO and CO2 could be present in the produced hydrogen gas. Therefore, we have tested the reactivity of CO and CO2 against Ti-doped N3AlH6. Both gases, CO and CO2, show a high affinity to the complex hydrides and just after few hydrogenation/dehydrogenation cycles, cause irreversible damage. The complex hydride could not be regenerated with pure hydrogen under current experimental conditions. The N2 gas impurity does not react with the hydride, but it greatly reduces the partial pressure of H2 and therefore cause difficulties for the hydrogenation reaction. The experimental data show that syngas cannot be used for the recharging of these complex hydrides, therefore prior to the hydrogenation process, a thorough purification of hydrogen from CO, CO2, and of N2 is necessary. This purification process can be realized using well-known separation methods such as PSA (pressure swing adsorption), membrane techniques (Pd-based membranes, ceramic membranes, electrochemical membranes), and cryogenic separation. On the other hand, due to the high reactivity of Ti-doped Na3AlH6 towards CO and CO2, the material can be used for the absorption of small amounts of these gases and the production of high purity hydrogen for PEM-fuel cell applications.
Author Contributions
Conceptualization, R.U., K.P. and M.F.; formal analysis, T.S., R.U. and K.P.; investigation, T.S. and K.P.; writing—original draft preparation, T.S.; writing—review and editing, K.P., R.U. and M.F.; visualization, T.S., R.U. and K.P.; supervision, M.F.; funding acquisition, T.S. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by MOST Scientific Project (2018YFE0100700), Guangdong Scientific Project (No. 2017B030314081) and the GDAS Project of Science and Technology Development (No. 2017GDASCX-0506, 2019GDASYL-0503005) in China as well as EFRE NRW project funding Nr. EFRE-0800055. The basic financial support of the Max-Planck-Institut für Kohlenforschung is greatly appreciated.
Acknowledgments
The authors acknowledged the support of B. Zibrowius for the measurement of solid state NMR-spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A 2007, 365, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, R.; Peinecke, K.; Meggouh, M.; Minne, P.; Peil, S.; Bathen, D.; Felderhoff, M. Design and operation of an aluminium alloy tank using doped Na 3 AlH 6 in kg scale for hydrogen storage. J. Power Sources 2016, 324, 589–597. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Marcantonio, V.; Ferrario, A.M.; Di Carlo, A.; Del Zotto, L.; Monarca, D.; Bocci, E. Biomass Steam Gasification: A Comparison of Syngas Composition between a 1-D MATLAB Kinetic Model and a 0-D Aspen Plus Quasi-Equilibrium Model. Computation 2020, 8, 86. [Google Scholar] [CrossRef]
- Klein, H. Hydrogen and Carbon Monoxide: Synthesis Gases. In Industrial Gases Processing; Häring, H.W., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 145–149. [Google Scholar]
- Rostrup-Nielsen, J. Reforming and Gasification-Fossil Energy Carriers. In Hydrogen and Fuel Cells; Stolten, D., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 291–305. [Google Scholar]
- Ahmed, S. Hydrogen from hydrocarbon fuels for fuel cells. Int. J. Hydrog. Energy 2001, 26, 291–301. [Google Scholar] [CrossRef]
- Rampe, T. Entwicklung eines Bioethanol-Dampfreformers zur Erzeugung von Wasserstoff für den Einsatz in einem PEM-Brennstoffzellen-BHKW. Ph.D. Thesis, Universität Duisburg-Essen, Duisburg, Germany, 2004. [Google Scholar]
- Dedrick, D.E.; Behrens, R., Jr.; Bradshaw, R.W. The Reactivity of Sodium Alanates with O2, H2O, and CO2, An Investigation of Complex Metal Hydride Contamination in the Context of Automotive Systems; Sandia Report, SAND2007-4960; Sandia National Laboratories: Washington, DC, USA, 2007. [Google Scholar]
- Hugelshofer, C.L.; Borgschulte, A.; Callini, E.; Matam, S.K.; Gehrig, J.C.; Hog, D.T.; Züttel, A. Gas–Solid Reaction of Carbon Dioxide with Alanates. J. Phys. Chem. C 2014, 118, 15940–15945. [Google Scholar] [CrossRef]
- Sandrock, G.D.; Goodell, P.D. Surface poisoning of LaNi5, FeTi and (Fe,Mn)Ti by O2, Co and H2O. J. Less Common Met. 1980, 73, 161–168. [Google Scholar] [CrossRef]
- Han, J.I.; Lee, J.-Y. The effect of CO impurity on the hydrogenation properties of LaNi5, LaNi4.7Al0.3 and MmNi4.5Al0.5 during hydriding-dehydriding cycling. J. Less Common Met. 1989, 152, 319–327. [Google Scholar] [CrossRef]
- Sandrock, G.; Goodell, P. Cyclic life of metal hydrides with impure hydrogen: Overview and engineering considerations. J. Less Common Met. 1984, 104, 159–173. [Google Scholar] [CrossRef]
- Ono, S.; Ishido, Y.; Akiba, E.; Jindo, K.; Sawada, Y.; Kitagawa, I.; Kakutani, T. The effect of CO2, CH4, H2O and N2 on Mg-Ni alloys as hydrogen transporting media. Int. J. Hydrog. Energy 1986, 11, 381–387. [Google Scholar] [CrossRef]
- Varin, R.; Zbroniec, L. Decomposition behavior of unmilled and ball milled lithium alanate (LiAlH4) including long-term storage and moisture effects. J. Alloy Compd. 2010, 504, 89–101. [Google Scholar] [CrossRef]
- Dunikov, D.; Borzenko, V.; Malyshenko, S. Influence of impurities on hydrogen absorption in a metal hydride reactor. Int. J. Hydrog. Energy 2012, 37, 13843–13848. [Google Scholar] [CrossRef]
- Tai, Y.-L.; Wang, L.; Chen, H.-Q.; Dong, B.-X.; Kan, X.-T.; Teng, Y.-L. Improved mechanochemical methanation performance of the metal carbonate-hydride system. Solid State Sci. 2020, 109, 106398. [Google Scholar] [CrossRef]
- Gamba, N.S.; Puszkiel, J.; Larochette, P.A.; Gennari, F.C. Dual application of Ti-catalyzed Li-RHC composite for H2 purification and CO methanation. Int. J. Hydrog. Energy 2020, 45, 19493–19504. [Google Scholar] [CrossRef]
- Grasso, M.L.; Puszkiel, J.; Gennari, F.C.; Santoru, A.; Dornheim, M.; Pistidda, C. CO2 reactivity with Mg2NiH4 synthesized by in situ monitoring of mechanical milling. Phys. Chem. Chem. Phys. 2020, 22, 1944–1952. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, Y.-F.; Cai, Y.-L.; Wang, L.-Z.; Xie, J.; Teng, Y.-L.; Zhu, W.; Shen, M.; Dong, B.-X. Highly Selective and Efficient Reduction of CO2 to Methane by Activated Alkaline Earth Metal Hydrides without a Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 4831–4841. [Google Scholar] [CrossRef]
- Grasso, M.L.; Puszkiel, J.; Albanesi, L.F.; Dornheim, M.; Pistidda, C.; Gennari, F.C. CO2 reutilization for methane production via a catalytic process promoted by hydrides. Phys. Chem. Chem. Phys. 2019, 21, 19825–19834. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, B.-X.; Teng, Y.-L.; Wang, L.; Ping, C.; Li, Z.-W. Dehydrogenation reactions of mechanically activated alkali metal hydrides with CO2 at room temperature. Int. J. Hydrog. Energy 2018, 43, 5068–5076. [Google Scholar] [CrossRef]
- Dong, B.-X.; Zhao, J.; Wang, L.-Z.; Teng, Y.-L.; Liu, W.-L.; Wang, L. Mechanochemical synthesis of COx-free hydrogen and methane fuel mixtures at room temperature from light metal hydrides and carbon dioxide. Appl. Energy 2017, 204, 741–748. [Google Scholar] [CrossRef]
- Peinecke, K.; Meggouh, M.; Felderhoff, M. Mechanochemical synthesis and effect of various additives on the hydrogen absorption–desorption behavior of Na3AlH6. J. Mater. Sci. 2018, 53, 13742–13750. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peinecke, K.; Felderhoff, M.; Hauschild, K.; Kersten, W.; Peil, S.; Bathen, D. Aluminium alloy based hydrogen storage tank operated with sodium aluminium hexahydride Na3AlH6. Int. J. Hydrog. Energy 2014, 39, 17118–17128. [Google Scholar] [CrossRef]
- Adelhelm, P.; De Jongh, P.E. The impact of carbon materials on the hydrogen storage properties of light metal hydrides. J. Mater. Chem. 2010, 21, 2417–2427. [Google Scholar] [CrossRef]
- Wang, W.; Buchholz, A.; Arnold, A.; Xu, M.; Hunger, M. Effect of surface methoxy groups on the 27Al quadrupole parameters of framework aluminum atoms in calcined zeolite H–Y. Chem. Phys. Lett. 2003, 370, 88–93. [Google Scholar] [CrossRef]
- Slaughter, J.; Peel, A.J.; Wheatley, A.E.H. Reactions of Trimethylaluminium: Modelling the Chemical Degradation of Syn-thetic Lubricants. Chem. Eur. J. 2017, 23, 167–175. [Google Scholar] [CrossRef]
- Singh, S.; Eijt, S.; Huot, J.; Kockelmann, W.; Wagemaker, M.; Mulder, F. The TiCl3 catalyst in NaAlH4 for hydrogen storage induces grain refinement and impacts on hydrogen vacancy formation. Acta Mater. 2007, 55, 5549–5557. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 Pure H2,
Pure H2,  0.1 vol % CO + 99.9 vol % H2,
0.1 vol % CO + 99.9 vol % H2,  1 vol % CO + 99 vol % H2,
1 vol % CO + 99 vol % H2,  10 vol % CO + 90 vol % H2.
10 vol % CO + 90 vol % H2.
 Pure H2,
Pure H2,  0.1 vol % CO + 99.9 vol % H2,
0.1 vol % CO + 99.9 vol % H2,  1 vol % CO + 99 vol % H2,
1 vol % CO + 99 vol % H2,  10 vol % CO + 90 vol % H2.
10 vol % CO + 90 vol % H2.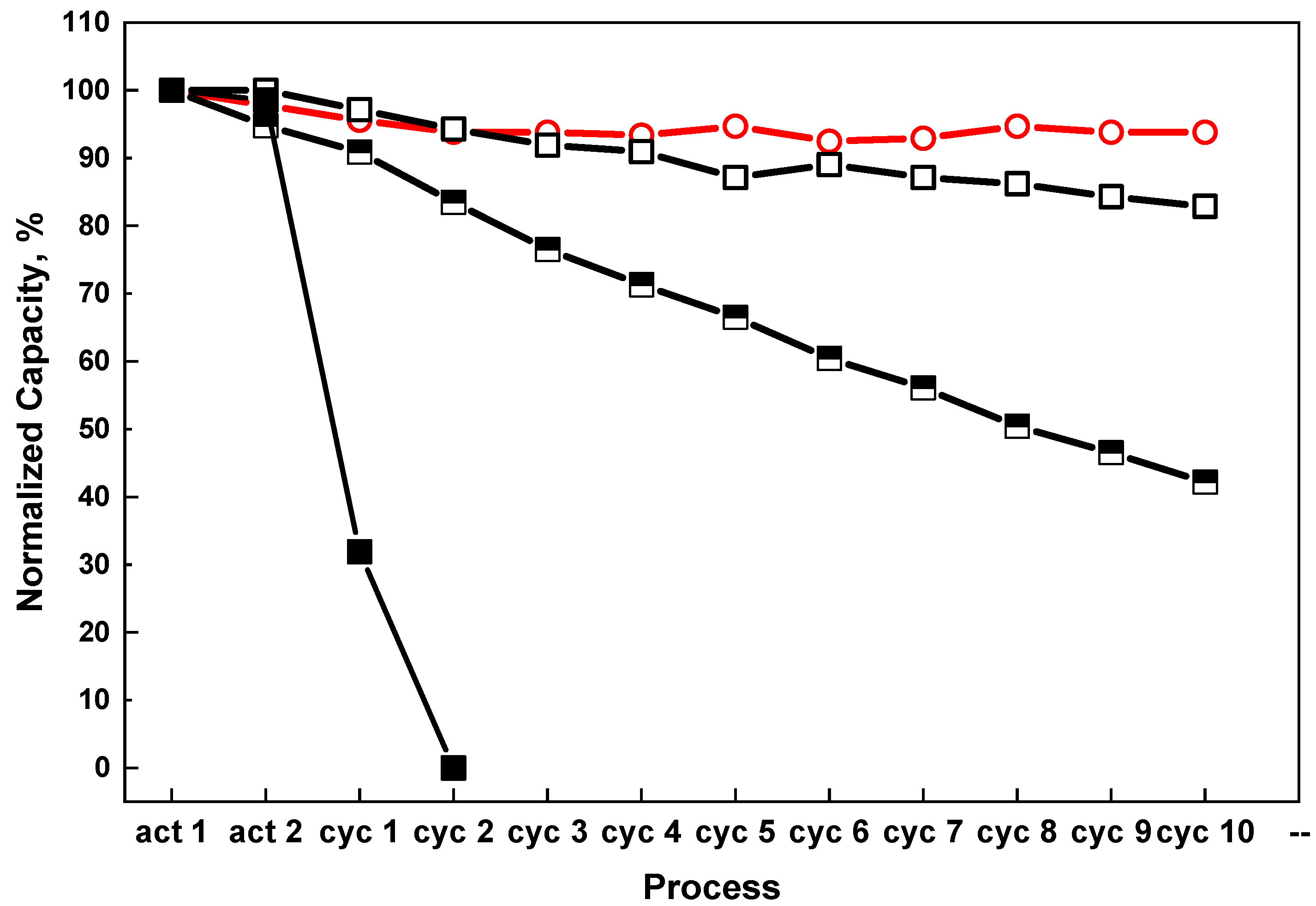
 Pure H2,
Pure H2,  1 vol % CO2 + 99 vol % H2,
1 vol % CO2 + 99 vol % H2,  10 vol % CO2 + 90 vol % H2.
10 vol % CO2 + 90 vol % H2.
 Pure H2,
Pure H2,  1 vol % CO2 + 99 vol % H2,
1 vol % CO2 + 99 vol % H2,  10 vol % CO2 + 90 vol % H2.
10 vol % CO2 + 90 vol % H2.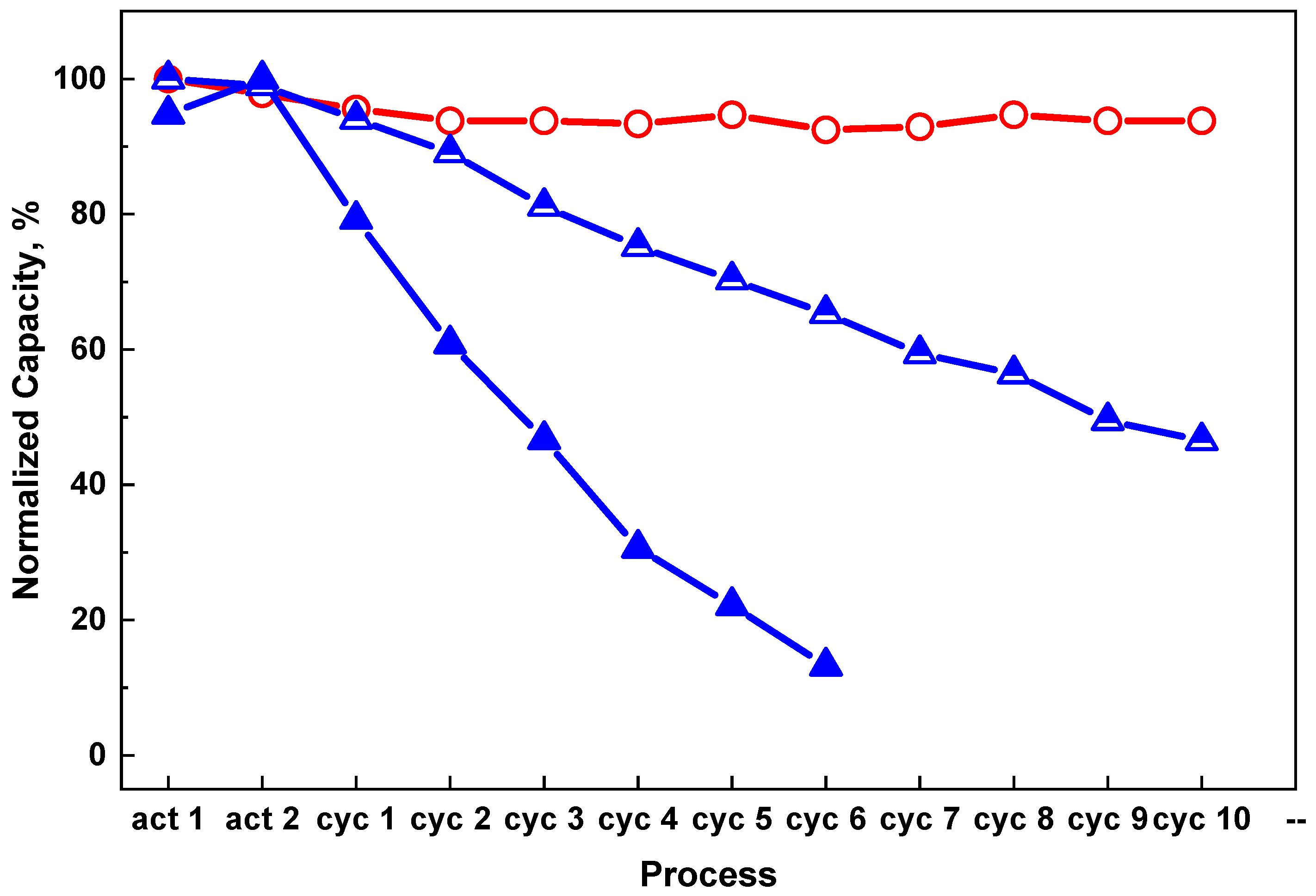
 gas mixture in comparison to cycling with pure H2
gas mixture in comparison to cycling with pure H2  , with 1 vol % CO + 99 vol % H2
, with 1 vol % CO + 99 vol % H2  , with 10 vol % CO2 + 90 vol % H2
, with 10 vol % CO2 + 90 vol % H2  and with 62 vol % N2 + 38 vol % H2
and with 62 vol % N2 + 38 vol % H2  at 3 MPa total pressure hydrogenation.
at 3 MPa total pressure hydrogenation.
 gas mixture in comparison to cycling with pure H2
gas mixture in comparison to cycling with pure H2  , with 1 vol % CO + 99 vol % H2
, with 1 vol % CO + 99 vol % H2  , with 10 vol % CO2 + 90 vol % H2
, with 10 vol % CO2 + 90 vol % H2  and with 62 vol % N2 + 38 vol % H2
and with 62 vol % N2 + 38 vol % H2  at 3 MPa total pressure hydrogenation.
at 3 MPa total pressure hydrogenation.