Vegetative Growth Analysis of Schoenoplectus californicus (Totora): Dynamics and Physiological Mechanisms in High-Altitude Andean Lakes
Abstract
1. Introduction
2. Materials and Methods
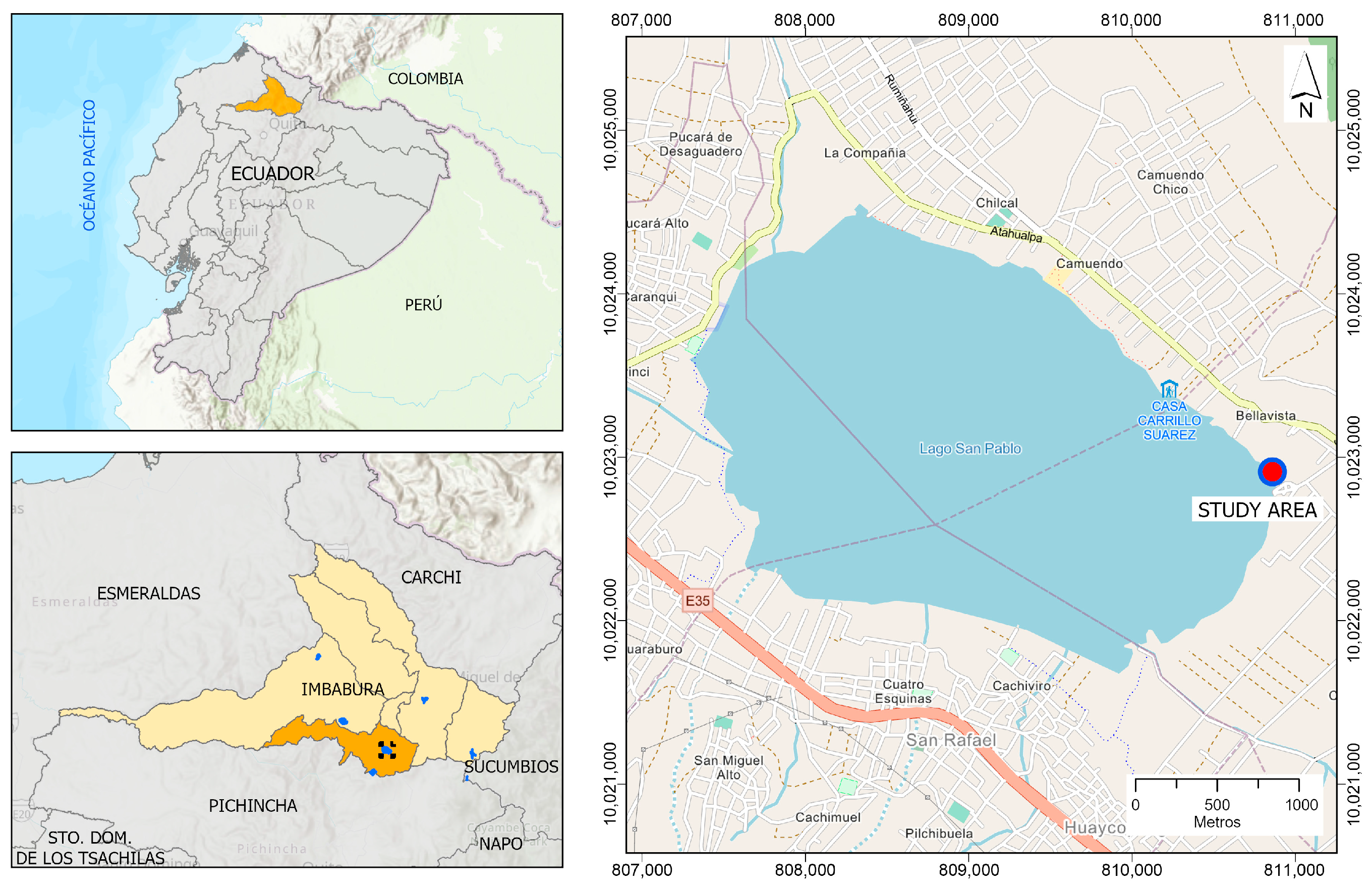
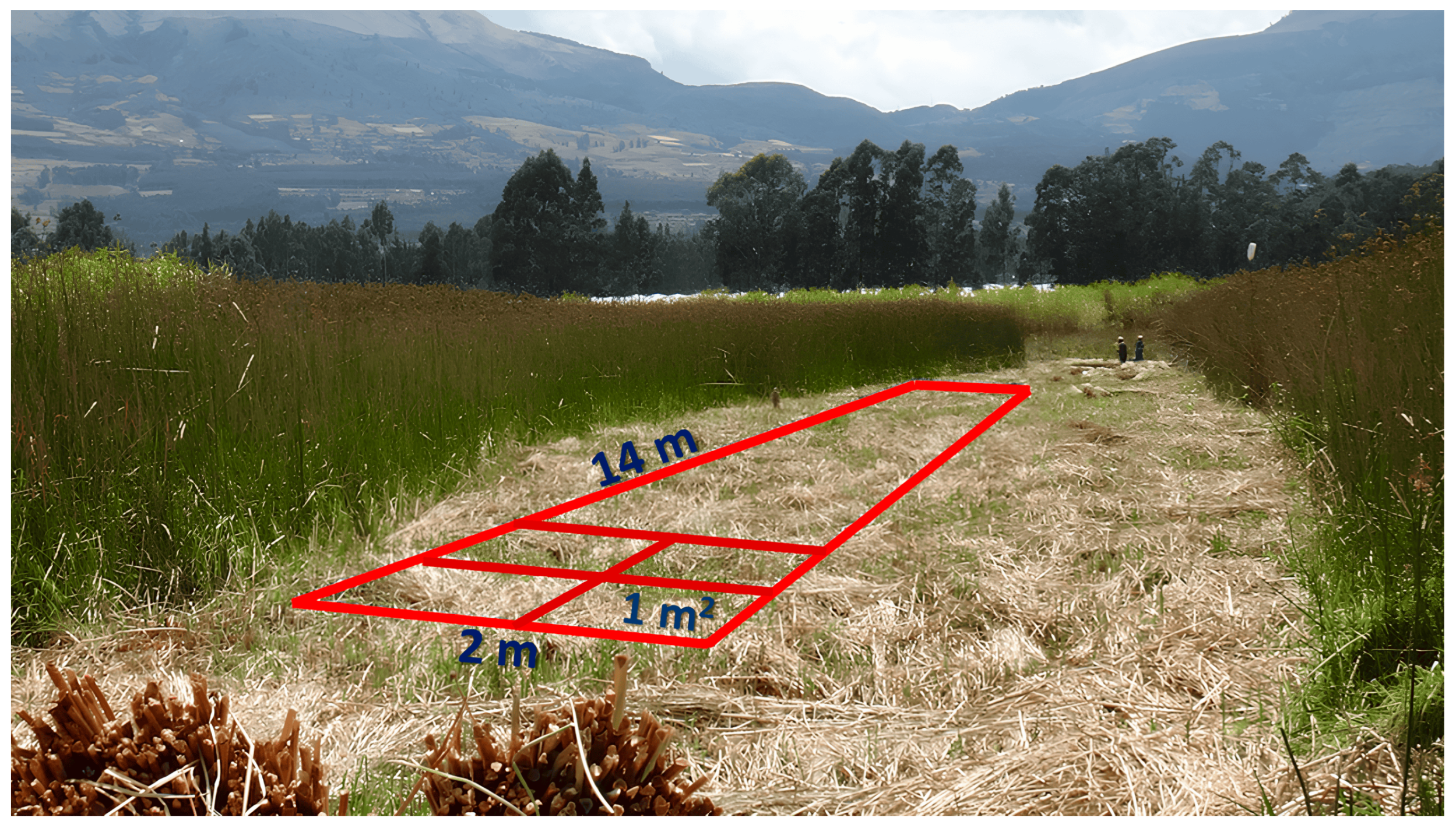
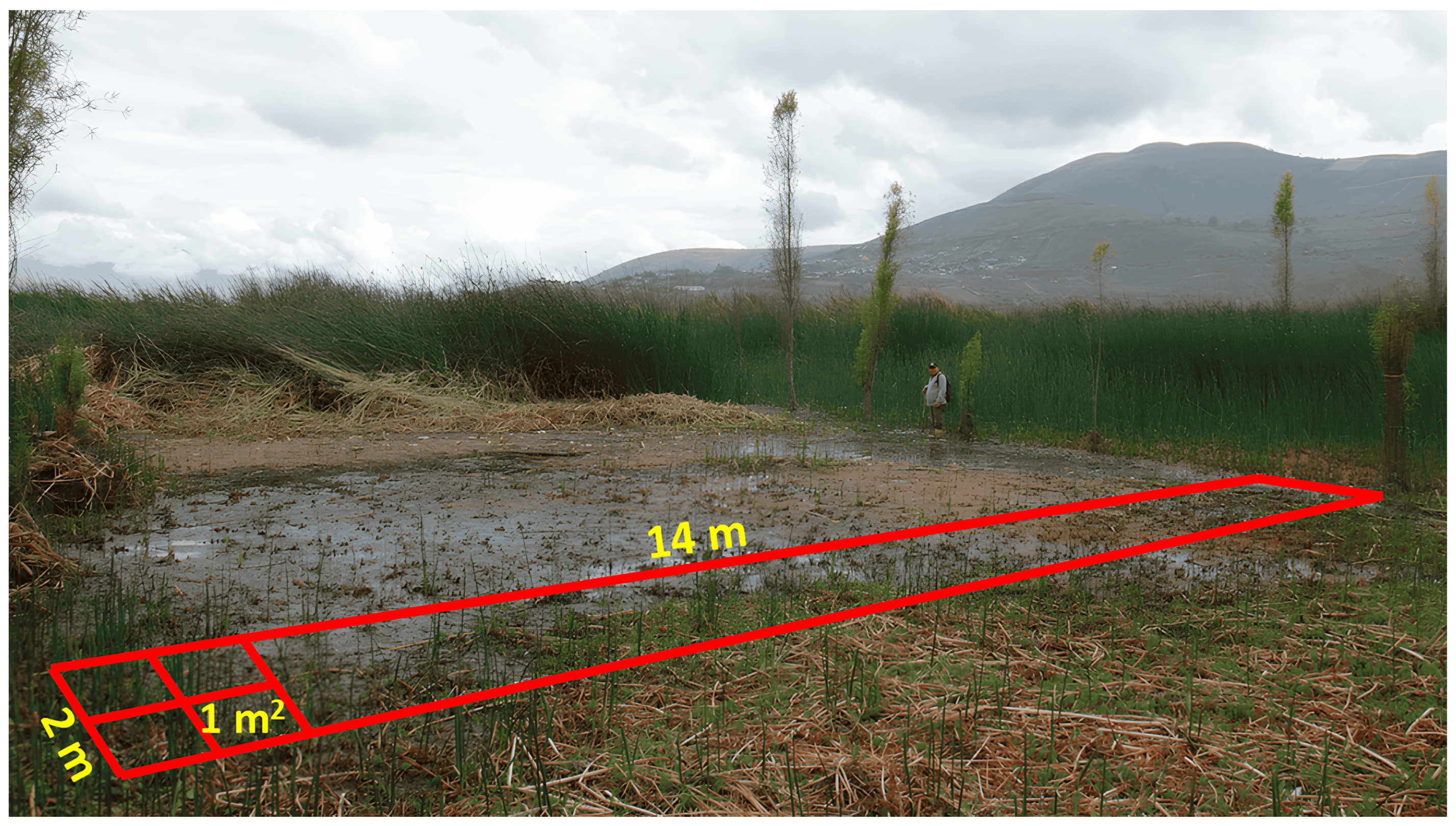
Experimental Area and Sampling Strategies
- (a)
- Photosynthetic stem area (defined as the total photosynthetic surface area of aerial stems in one square meter of the plant growth zone);
- (b)
- Dry mass of the stems;
- (c)
- Dry mass of the roots.
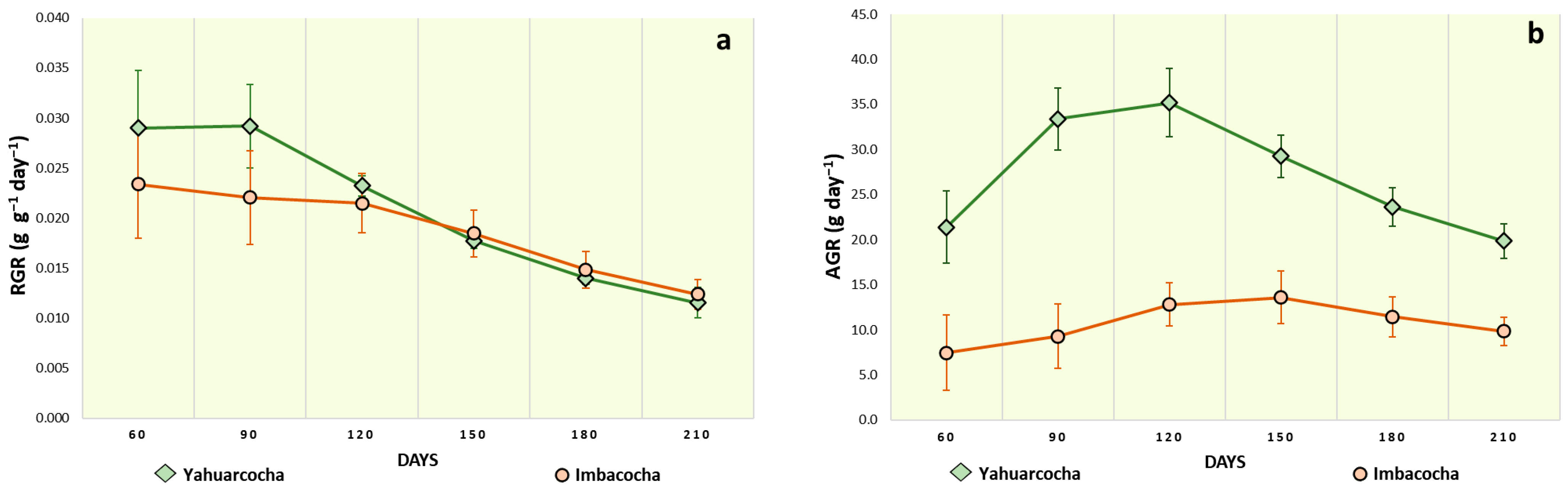
- Relative Growth Rate (RGR): Increase in plant material per unit of existing plant material per unit of time [57].
- Absolute Growth Rate (AGR): Increase in dry mass of plant material per unit of time [36].
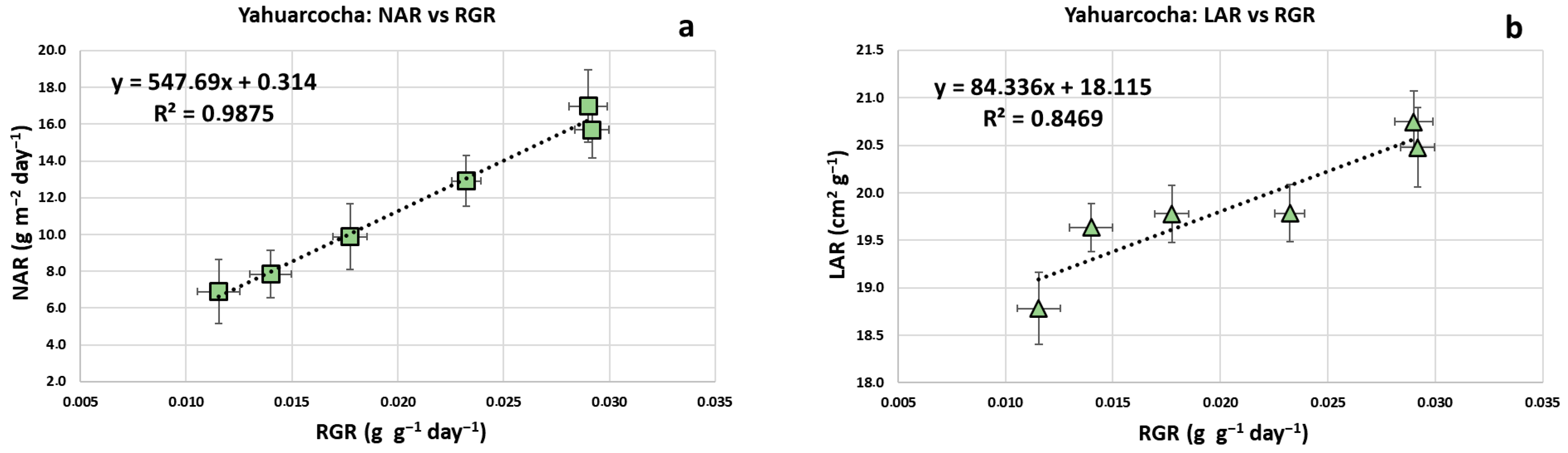
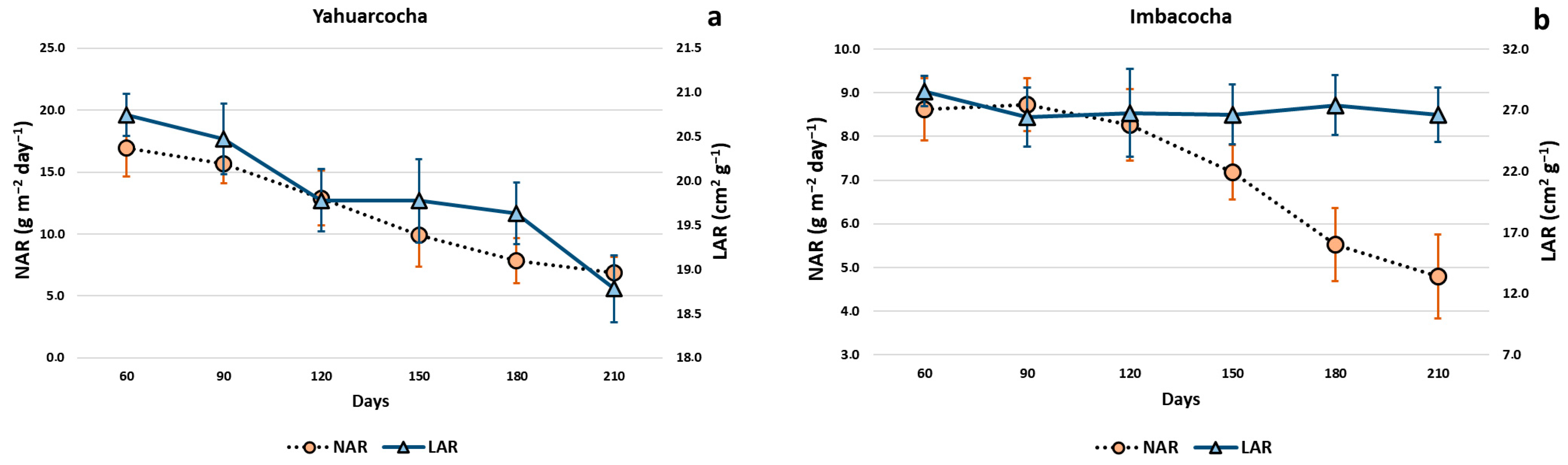
- Net Assimilation Rate (NAR): Estimates the plant’s photosynthetic capacity; represents the rate of increase in plant mass per unit of leaf area [58].
- Leaf Area Ratio (LAR): Ratio of leaf area to total plant mass [56].
- Leaf Area Index (LAI): Instantaneous measurement that relates the assimilable leaf area (A) per unit of soil Surface (S) [36].
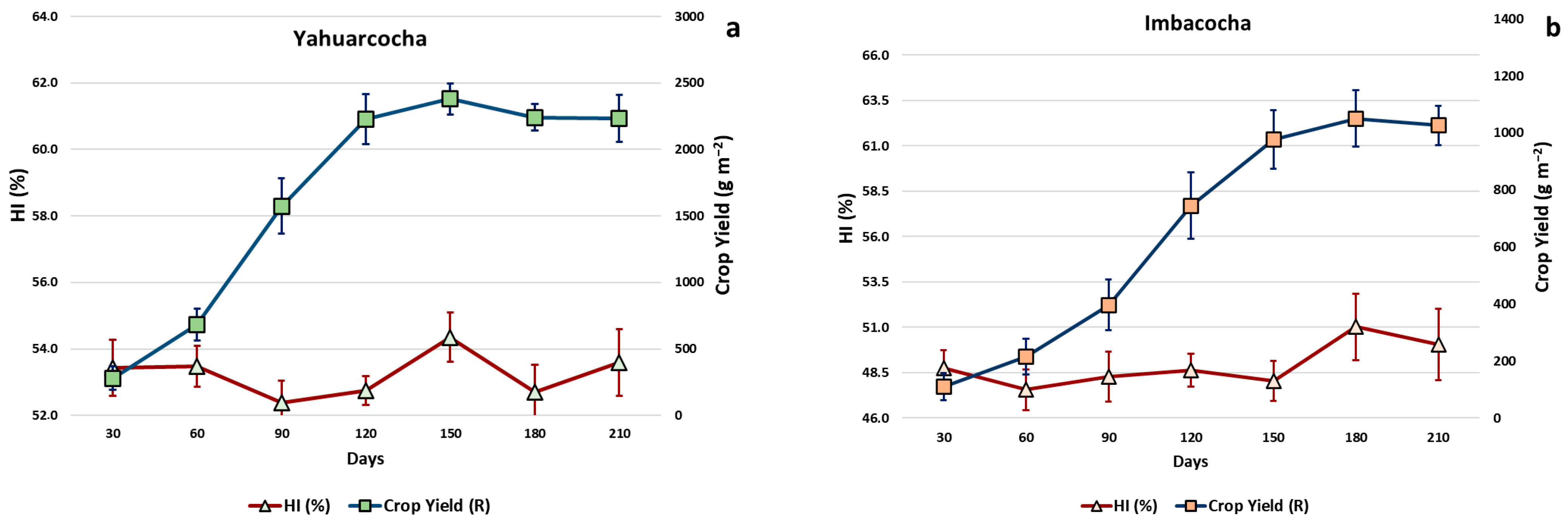
- Harvest Index (HI): Ratio between the yield of the harvestable organ and the plant biomass [36].
- Crop Yield (R): Product of biomass and the harvest index (HI) [36].
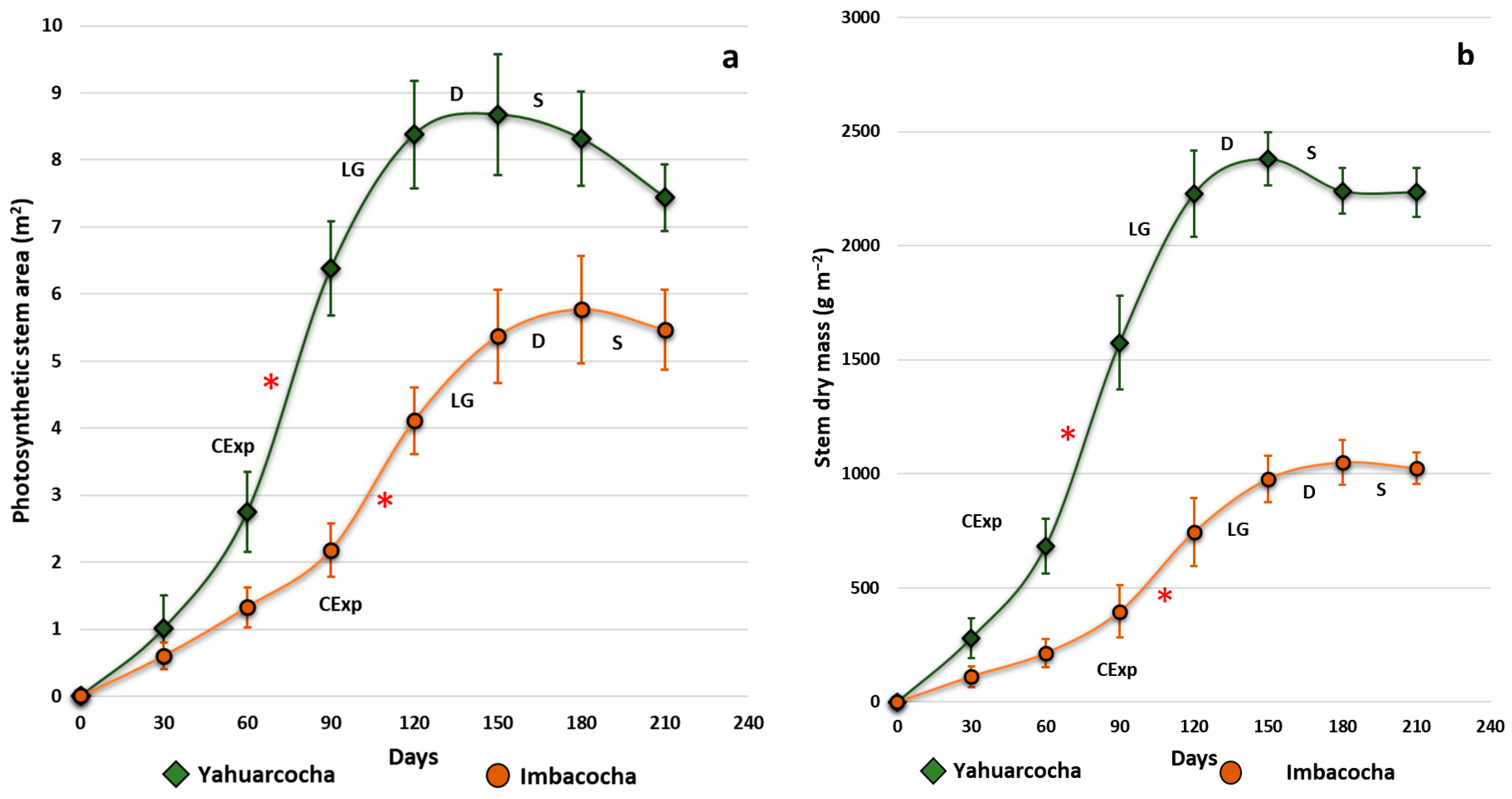
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simbaña, A. La totora y el desarrollo sustentable del Imbakucha, Lago San Pablo, se fortalece [Totora and the Sustainable Development of Imbakucha, Lake San Pablo, is Strengthened] AXIOMA 2015, 1, 6. Available online: https://axioma.pucesi.edu.ec/index.php/axioma/article/view/17 (accessed on 5 August 2025). (In Spanish).
- Paredes, R.; Hopkins, A.L. Dynamism in Traditional Ecological Knowledge: Persistence and Change in the Use of Totora (Schoenoplectus californicus) for Subsistence in Huanchaco, Peru. Ethnobiol. Lett. 2018, 9, 169–179. [Google Scholar] [CrossRef]
- Blanco, J.; Alvarado, R.; Flores, J.; Zurita, F. Suitability of Totora (Schoenoplectus californicus (C.A. Mey.) Soják) for its use in constructed wetlands in areas polluted with heavy metals. Sustainability 2019, 11, 19. [Google Scholar] [CrossRef]
- Hidalgo-Cordero, J.F.; Němec, M.; Castro, P.H.; Hájková, K.; Castro, A.O.; Hýsek, Š. Macromolecular composition of Totora (Schoenoplectus californicus C.A. Mey, Soják) stem and its correlation with stem mechanical properties. J. Nat. Fibers 2023, 20, 2282049. [Google Scholar] [CrossRef]
- Macía, M.J.; Balslev, H. Use and management of Totora (Schoenoplectus californicus, Cyperaceae) in Ecuador. Econ. Bot. 2000, 54, 82–89. [Google Scholar] [CrossRef]
- Banack, S.A.; Rondón, X.J.; Diaz-Huamanchumo, W. COVER ARTICLE: Indigenous Cultivation and Conservation of Totora (Schoenoplectus californicus, Cyperaceae) in Peru. Econ. Bot. 2004, 58, 11–20. [Google Scholar] [CrossRef]
- Galindo Acuña, L.A.; Córdoba Sánchez, M.P. Bioindication and phytostabilization of potentially toxic elements by Schoenoplectus californicus in a Ramsar urban wetland, Colombia. Int. J. Phytoremediat. 2025, 27, 1765–1773. [Google Scholar] [CrossRef]
- Murray-Gulde, C.L.; Huddleston, G.M.; Garber, K.V.; Rodgers, J.H. Contributions of Schoenoplectus californicus in a constructed wetland system receiving copper-contaminated wastewater. Water Air Soil Pollut. 2005, 163, 355–378. [Google Scholar] [CrossRef]
- De Rito, M.; Borrelli, N.; Natal, M.; Fernández Honaine, M. Schoenoplectus californicus (Cyperaceae) amorphous silica contribution to the silicon cycle in Pampean shallow lakes: An analysis of spatio-temporal variation and silicon–lignin relations. Aust. J. Bot. 2024, 72, BT23084. [Google Scholar] [CrossRef]
- Sabaj, V.; Conde, D.; Rodríguez-Gallego, L.; Kandus, P. Postharvest growth dynamic of Schoenoplectus californicus along fluvio-estuarine and flooding gradients. Wetl. Ecol. Manag. 2018, 26, 125–138. [Google Scholar] [CrossRef]
- Romero, M.; Flores, M.; Bravo-Thais, S.; Guzman, M. Phytoremediation potential of Schoenoplectus californicus in mine drainage waters. Environ. Sci. Pollut. Res. 2023, 30, 9876–9885. [Google Scholar] [CrossRef]
- Aza-Medina, L.C.; Palumbo, M.; Lacasta, A.M.; Gonzalez-Lezcano, R.A. Characterization of the Thermal Behavior, Mechanical Resistance, and Reaction to Fire of Totora (Schoenoplectus californicus (C.A. Mey.) Sojak) Panels and Their Potential Use as a Sustainable Construction Material. J. Build. Eng. 2023, 69, 105984. [Google Scholar] [CrossRef]
- Departamento de Botánica, Instituto de Biología (IBUNAM). Schoenoplectus californicus (C.A.Mey.) Soják. Ejemplar de: Herbario Nacional de México (MEXU), Frutos y Semillas [Internet]; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2018; Available online: http://datosabiertos.unam.mx/IBUNAM:MEXU:FS9187 (accessed on 19 September 2025).
- Maldonado, G.; Voeks, R. The paradox of culturally useful invasive species: Southern cattail (Typha domingensis) crafts of Lake Pátzcuaro, Mexico. J. Lat. Am. Geogr. 2021, 20, 148–174. [Google Scholar] [CrossRef]
- Sloey, T.M.; Howard, R.J.; Hester, M.W. Response of Schoenoplectus acutus and Schoenoplectus californicus at Different Life-History Stages to Hydrologic Regime. Wetlands 2016, 36, 37–46. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Zedler, J.B. Progress in wetland restoration ecology. Trends Ecol. Evol. 2000, 15, 402–407. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; Available online: https://sswm.info/sites/default/files/reference_attachments/KADLEC%20WALLACE%202009%20Treatment%20Wetlands%202nd%20Edition_0.pdf (accessed on 5 August 2025).
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Available online: https://download.e-bookshelf.de/download/0000/5929/20/L-G-0000592920-0002363128.pdf (accessed on 5 August 2025).
- USDA NRCS. Schoenoplectus californicus (California bulrush). Plant Guide. 2000. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_scca11.pdf (accessed on 5 August 2025).
- Noriega-Rico, E.; Rico, Y.; Lobato-de Magalhães, T. 2023 SWS Student Research Grant: Research in Progress—Functional Connectivity of the California Bulrush (Schoenoplectus californicus) in Central-Western Wetlands in Mexico. Wetl. Sci. Pract. 2023, 40, 106–111. Available online: https://growthzonesitesprod.azureedge.net/wp-content/uploads/sites/1889/2024/02/WSP_January2024_ARTICLE14.pdf (accessed on 5 August 2025).
- Rigotti, J.A.; Paqualini, J.P.; Rodrigues, L.R. Root Growth and Nutrient Removal of Typha domingensis and Schoenoplectus californicus over the Period of Plant Establishment in a Constructed Floating Wetland. Environ. Sci. Pollut. Res. 2021, 28, 8927–8935. [Google Scholar] [CrossRef]
- Heiser, C. The Totora (Scirpus californicus) in Ecuador and Peru. Econ. Bot. 1978, 32, 222–236. [Google Scholar] [CrossRef]
- Terneus Jácome, E. Vegetación acuática y estado trófico de las lagunas andinas de San Pablo y Yahuarcocha, provincia de Imbabura, Ecuador [Aquatic Vegetation and Trophic Status of the Andean Lagoons of San Pablo and Yahuarcocha, Imbabura Province, Ecuador]. Rev. Ecuat. Med. Cienc. Biol. 2017, 35, 121–123. (In Spanish) [Google Scholar]
- Hidalgo-Cordero, J.F.; García-Navarro, J. Totora (Schoenoplectus californicus (C.A. Mey.) Soják) and its potential as a construction material. Ind. Crops Prod. 2018, 112, 467–480. [Google Scholar] [CrossRef]
- Hester, M.W.; Willis, J.M.; Sloey, T.M. Field assessment of environmental factors constraining the development and expansion of Schoenoplectus californicus marsh at a California tidal freshwater restoration site. Wetl. Ecol. Manag. 2016, 24, 33–44. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Liu, Y.; He, L.; Yang, S.; Gong, H.; Xu, R.; Yao, X.; Ge, G. Interactive Effects of Flooding Duration and Sediment Texture on the Growth and Adaptation of Three Plant Species in the Poyang Lake Wetland. Biology 2023, 12, 944. [Google Scholar] [CrossRef]
- Hidalgo Cordero, J.F.; García Navarro, J. Review on the traditional uses and potential of Totora (Schoenoplectus californicus) as construction material. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 022068. [Google Scholar] [CrossRef]
- Arévalo, A.; Cadena, M.; Ontaneda, D. Diversidad fitoplanctónica y estado trófico actual de un lago de alta montaña en la provincia de Imbabura, Ecuador. ACI Av. Cienc. Ing. 2025, 17, e3397. [Google Scholar] [CrossRef]
- Arce, W.A.; Achá, D. Allometric determinations in the early development of Schoenoplectus californicus to monitor nutrient uptake in constructed wetlands. Ecohydrol. Hydrobiol. 2025, 25, 34–41. [Google Scholar] [CrossRef]
- Keddy, P.A. Wetland Ecology: Principles and Conservation, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; 497p. [Google Scholar] [CrossRef]
- Cronk, J.K.; Fennessy, M.S. Wetland plants: Biology and ecology; CRC Press: Boca Raton, FL, USA, 2001; Available online: https://www.crcpress.com/Wetland-Plants-Biology-and-Ecology/Cronk-Fennessy/p/book/9781566703727 (accessed on 5 August 2025).
- Dykyjová, D.; Květ, J. (Eds.) Pond Littoral Ecosystems: Structure and Functioning: Methods and Results of Quantitative Ecosystem Research in the Czechoslovakian IBP Wetland Project. In Ecological Studies; Springer-Verlag: Berlin/Heidelberg, Germany, 1978; Volume 25, 466p. [Google Scholar] [CrossRef]
- Poorter, H. Plant growth analysis: Towards a synthesis of the classical and the functional approach. Physiol. Plant. 1989, 75, 237–244. [Google Scholar] [CrossRef]
- Gardner, F.P.; Pearce, R.B.; Mitchell, R.L. Physiology of Crop Plants, 2nd ed.; Scientific Publishers: Jodhpur, India, 2017; 327p. [Google Scholar]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Tessmer, O.L.; Jiao, Y.; A Cruz, J.; Kramer, D.M.; Chen, J. Functional approach to high throughput plant growth analysis. BMC Syst. Biol. 2013, 7 (Suppl. S6), S17. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Tognetti, J. Técnicas de análisis de crecimiento de plantas: Su aplicación a cultivos intensivos. RIA. Rev. Investig. Agropecu. 2016, 42, 258–282. Available online: https://www.redalyc.org/articulo.oa?id=86449712008 (accessed on 7 August 2025).
- Neubauer, M.E.; Plaza de los Reyes, C.; Pozo, G.; Villamar, C.A.; Vidal, G. Growth and nutrient uptake by Schoenoplectus californicus in a constructed wetland fed with swine slurry. J. Soil Sci. Plant Nutr. 2012, 12, 421–430. [Google Scholar] [CrossRef]
- Hýsková, P.; Gaff, M.; Hidalgo-Cordero, J.F.; Hýsek, Š. Composite materials from totora (Schoenoplectus californicus C.A. Mey, Soják): Is it worth it? Compos. Struct. 2020, 232, 111572. [Google Scholar] [CrossRef]
- Pugnaire, F.; Valladares, F. (Eds.) Functional Plant Ecology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Villar, R.; Ruiz-Robleto, J.; Quero, J.; Poorter, H.; Valladares, F.; Marañón, T. Tasas de crecimiento en especies leñosas: Aspectos funcionales e implicaciones ecológicas [Growth Rates in Woody Species: Functional Aspects and Ecological Implications]. In Ecología del Bosque Mediterráneo en un Mundo Cambiante, 2nd ed.; Valladares, F., Ed.; Ministerio de Medio Ambiente: Madrid, Spain, 2008; pp. 193–230. (In Spanish) [Google Scholar]
- Evans, G.C. The Quantitative Analysis of Plant Growth; Blackwell Scientific Publications: Oxford, UK, 1972; p. 26. 734p. [Google Scholar]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Poorter, H.; Garnier, E. Plant growth analysis: An evaluation of experimental design and computational methods. J. Exp. Bot. 1996, 47, 1343–1351. [Google Scholar] [CrossRef]
- Grime, J.P.; Hunt, R. Relative Growth-Rate: Its Range and Adaptive Significance in a Local Flora. J. Ecol. 1975, 63, 393–422. [Google Scholar] [CrossRef]
- Poorter, H.; Remkes, C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 1990, 83, 553–559. [Google Scholar] [CrossRef]
- Puntieri, J.G.; Gomez, I.A. Análisis del crecimiento vegetativo del amancay (Alstroemeria aurantiaca D. Don) en dos poblaciones naturales [Vegetative Growth Analysis of Amancay (Alstroemeria aurantiaca D. Don) in Two Natural Populations] Rev. Chil. Hist. Nat. 1988, 61, 177–185. (In Spanish) [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Poorter, H. Avoiding Bias in Calculations of Relative Growth Rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef]
- Poorter, H.; De Jong, R. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol. 1999, 143, 163–176. [Google Scholar] [CrossRef]
- Santos Castellanos, M.; Segura Abril, M.; Ñústez López, C.E. Análisis de crecimiento y relación fuente-demanda de cuatro variedades de papa (Solanum tuberosum L.) en el municipio de Zipaquirá (Cundinamarca, Colombia). Rev. Fac. Nac. Agron. Medellín 2010, 63, 5253–5266. [Google Scholar]
- Shipley, B. Net Assimilation Rate, Specific Leaf Area and Leaf Mass Ratio: Which Is Most Closely Correlated with Relative Growth Rate? A Meta-Analysis. Funct. Ecol. 2006, 20, 565–574. Available online: http://www.jstor.org/stable/3806604 (accessed on 7 August 2025). [CrossRef]
- Pabón, G.; Rodés, R.; Pérez, L.; Vásquez, L.; Ortega, E. Relaciones morfológicas en Schoenoplectus californicus (Cyperaceae) en lagos alto-andinos de Ecuador [Morphological Relationships in Schoenoplectus californicus (Cyperaceae) in High-Andean Lakes of Ecuador] Rev. Jardín Botánico Nac. 2019, 40, 109–119. Available online: https://www.jstor.org/stable/26937052 (accessed on 7 August 2025). (In Spanish).
- Hunt, R. Basic Growth Analysis. In Plant Growth Analysis for Beginners; Unwin Hyman: London, UK, 1990. [Google Scholar] [CrossRef]
- Shipley, B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: Relationship with daily irradiance. Funct. Ecol. 2002, 16, 682–689. [Google Scholar] [CrossRef]
- Rees, M.; Osborne, C.P.; Woodward, F.I.; Hulme, S.P.; Turnbull, L.A.; Taylor, S.H. Partitioning the components of relative growth rate: How important is plant size variation? Am. Nat. 2010, 176, E152–E161. [Google Scholar] [CrossRef]
- Li, X.; Schmid, B.; Wang, F.; Paine, C.E.T. Net assimilation rate determines the growth rates of 14 species of subtropical forest trees. PLoS ONE 2016, 11, e0150644. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Cao, T.; Ni, L.; Li, W.; Zhu, G. Relationships between relative growth rate and its components across 11 submersed macrophytes. J. Freshw. Ecol. 2012, 27, 471–480. [Google Scholar] [CrossRef]
- Medek, D.E.; Ball, M.C.; Schortemeyer, M. Relative contributions of leaf area ratio and net assimilation rate to change in growth rate depend on growth temperature: Comparative analysis of subantarctic and alpine grasses. New Phytol. 2007, 175, 612–623. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Cross-species relationships between seedling relative growth rate, nitrogen productivity and root vs leaf function in 28 Australian woody species. Funct. Ecol. 2000, 14, 97–107. [Google Scholar] [CrossRef]
- Rejmánková, E.; Sirová, D.; Castle, S.T.; Bárta, J.; Carpenter, H. La fijación heterotrófica de N2 contribuye a la economía de nitrógeno de un junco común de humedales, Schoenoplectus californicus. PLoS ONE 2018, 13, e0195570. [Google Scholar] [CrossRef]
- Pabon Garces, G.J.; Rodés, R.; Pérez, L.; Vásquez Hernández, L.D.R.; Ortega, E. Estudio comparativo de germinación de semillas de totora provenientes de tres lagos norte-andinos de Ecuador (Comparative Study of Germination of Totora Seeds from Three North-Andean Lakes of Ecuador). Rev. Cubana Cienc. Biol. 2018, 6, 1–12. Available online: https://scispace.com/pdf/estudio-comparativo-de-germinacion-de-semillas-de-totora-31tf55vypf.pdf (accessed on 7 August 2025).
- Liang, Z.; Soranno, P.A.; Wagner, T. El papel del fósforo y el nitrógeno en la clorofila a: Evidencia de cientos de lagos. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef]
- López, D.; Sepúlveda, M.; Vidal, G. Phragmites australis and Schoenoplectus californicus in constructed wetlands: Development and nutrient uptake. J. Soil Sci. Plant Nutr. 2016, 16, 763–777. Available online: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0718-95162016000300015 (accessed on 7 August 2025). [CrossRef]
- Sabaj, V. Extracción de “juncos” de Schoenoplectus californicus en el Área Protegida Humedales de Santa Lucía (Uruguay): Contexto Ecológico, Socio–Espacial y Perspectivas de Manejo Sustentable [Harvesting of Schoenoplectus californicus Reeds in the Santa Lucía Wetlands Protected Area (Uruguay): Ecological, Socio-Spatial Context and Sustainable Management Perspectives]. Master’s Thesis, Universidad de la República, Montevideo, Uruguay, 2011. Available online: https://www.colibri.udelar.edu.uy/jspui/bitstream/20.500.12008/3910/1/uy24-15287.pdf (accessed on 4 August 2025). (In Spanish).
- Yang, Z.; Wang, Q.; Zhang, J.; Xie, H.; Feng, S. Effect of Plant Harvesting on the Performance of Constructed Wetlands during Summer. Water 2016, 8, 24. [Google Scholar] [CrossRef]
- Silveira, T.C.L.; Rodrigues, G.G.; de Souza, G.P.C.; Würdig, N.L. Effects of cutting disturbance in Schoenoplectus californicus (C.A. Mey.) Soják on the benthic macroinvertebrates. Acta Sci. Biol. Sci. 2011, 33, 31–39. [Google Scholar] [CrossRef]
- Rejmankova, E. The role of macrophytes in wetland ecosystems. J. Ecol. Environ. 2011, 34, 333–345. [Google Scholar] [CrossRef]
- Chotikarn, P.; Pramneechote, P.; Sinutok, S. Photosynthetic Responses of Freshwater Macrophytes to the Daily Light Cycle in Songkhla Lagoon. Plants 2022, 11, 2806. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Reitsema, R.E.; Wolters, J.-W.; Preiner, S.; Meire, P.; Hein, T.; De Boeck, G.; Blust, R.; Schoelynck, J. Response of Submerged Macrophyte Growth, Morphology, Chlorophyll Content and Nutrient Stoichiometry to Increased Flow Velocity and Elevated CO2 and Dissolved Organic Carbon Concentrations. Front. Environ. Sci. 2020, 8, 527801. [Google Scholar] [CrossRef]
- Costa, M.L.R.; Henry, R. Phosphorus, nitrogen, and carbon contents of macrophytes in lakes lateral to a tropical river (Paranapanema River, São Paulo, Brazil). Acta Limnol. Bras. 2010, 22, 122–132. [Google Scholar] [CrossRef]
- Verhofstad, M.J.J.M.; Poelen, M.D.M.; van Kempen, M.M.L.; Bakker, E.S.; Smolders, A.J.P. Finding the harvesting frequency to maximize nutrient removal in a constructed wetland dominated by submerged aquatic plants. Ecol. Eng. 2017, 106, 423–430. [Google Scholar] [CrossRef]
- Hong, Z.; Ding, S.; Zhao, Q.; Qiu, P.; Chang, J.; Peng, L.; Wang, S.; Hong, Y.; Liu, G.-J. Plant trait–environment trends and their conservation implications for riparian wetlands in the Yellow River. Sci. Total Environ. 2021, 767, 144867. [Google Scholar] [CrossRef]
- Maksimov, A.; Apaseev, V.; Maksimov, E.; Alekseev, N.; Pushkarenko, N.; Maksimov, N. Towards a mathematical model of plant growth. IOP Conf. Ser. Earth Environ. Sci. 2021, 935, 012031. [Google Scholar] [CrossRef]
- Singh, P.; Singh, G.; Singh, A.; Mishra, V.K.; Shukla, R. Macrophytes for Utilization in Constructed Wetland as Efficient Species for Phytoremediation of Emerging Contaminants from Wastewater. Wetlands 2024, 44, 22. [Google Scholar] [CrossRef]
- Mahmud, K.; Hossain, T.; Haque Mou, T.; Ali, A.; Islam, M. Effect of Nitrogen On Growth and Yield of Chili (Capsicum annuum L.) in Roof Top Garden. Turk. J. Agric. Food Sci. Technol. 2020, 8, 246–251. [Google Scholar] [CrossRef]
- Buelna-Tarín, S.; Romero-Félix, C.S.; Bojórquez-Ramos, C.; Lugo-García, G.A.; Sánchez-Soto, B.H. Bioestimulantes y solución Steiner en crecimiento y producción de Capsicum annuum L. [Biostimulants and Steiner solution in growth and production of Capsicum annuum L.] Rev. Mex. Cienc. Agríc. 2024, 15, e3255. (In Spanish) [Google Scholar] [CrossRef]
- Kalaitzidis, A.; Kadoglidou, K.; Mylonas, I.; Ghoghoberidze, S.; Ninou, E.; Katsantonis, D. Investigating the Impact of Tillering on Yield and Yield-Related Traits in European Rice Cultivars. Agriculture 2025, 15, 616. [Google Scholar] [CrossRef]
- Degiovanni Beltramo, V.M.; Martínez Racines, C.P.; Motta, O.F. Producción Eco-Eficiente del Arroz en América Latina; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 2010; 487p, Available online: https://hdl.handle.net/10568/54233 (accessed on 23 September 2025).
- Ntanos, D.A.; Koutroubas, S.D. Dry matter and N accumulation and translocation for Indica and Japonica rice under Mediterranean conditions. Field Crops Res. 2002, 74, 93–101. [Google Scholar] [CrossRef]
- Yamada, T.; Okuda, T.; Abdullah, M.; Awang, M.; Furukawa, A. The leaf development process and its significance for reducing self-shading of a tropical pioneer tree species. Oecologia 2000, 125, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, H.; Nakabayashi, M.; Yamada, T. Newly found leaf arrangement to reduce self-shading within a crown in Japanese monoaxial tree species. J. Plant Res. 2024, 137, 203–213. [Google Scholar] [CrossRef]
- Pratolongo, P.; Kandus, P.; Brinson, M.M. Net aboveground primary production and biomass dynamics of Schoenoplectus californicus (Cyperaceae) marshes growing under different hydrological conditions. Darwiniana 2008, 46, 258–269. [Google Scholar]
- Hidalgo-Cordero, J.F.; Aza-Medina, L.C. Analysis of the thermal performance of elements made with totora using different production processes. J. Build. Eng. 2023, 65, 105777. [Google Scholar] [CrossRef]
- Zhang, P.; Hefting, M.M.; Soons, M.B.; Kowalchuk, G.A.; Rees, M.; Hector, A.; Turnbull, L.A.; Zhou, X.; Guo, Z.; Chu, C.; et al. Fast and furious: Early differences in growth rate drive short term plant dominance and exclusion under eutrophication. Ecol. Evol. 2020, 10, 10116–10129. [Google Scholar] [CrossRef]
- Coley, P.D. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 1988, 74, 531–536. [Google Scholar] [CrossRef]
- Kitajima, K.; Fenner, M. Ecology of seedling regeneration. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 331–359. [Google Scholar] [CrossRef]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.F.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Almeida-Cortez, J.S.; Shipley, B.; Arnason, J.T. Do plant species with high relative growth rates have poorer chemical defences? Funct. Ecol. 1999, 13, 819–827. [Google Scholar] [CrossRef]
- Chen, Q.; He, A.; Wang, W.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Comparisons of regeneration rate and yields performance between inbred and hybrid rice cultivars in a direct seeding rice-ratoon rice system in central China. Field Crops Res. 2018, 223, 164–170. [Google Scholar] [CrossRef]
- Sloey, T.M.; Hester, M.W. Impact of nitrogen and importance of silicon on mechanical stem strength in Schoenoplectus acutus and S. californicus: Applications for restoration. Wetl. Ecol. Manag. 2018, 26, 459–474. [Google Scholar] [CrossRef]
- Gaudet, C.L.; Keddy, P.A. Competitive performance and species distribution in shoreline plant communities: A comparative approach. Ecology 1995, 76, 280–291. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; Wiley: Chichester, UK, 2006. [Google Scholar]
- Ruiz-Robleto, J.; Villar, R. Relative growth rate and biomass allocation in ten woody species with different leaf longevity using phylogenetic independent contrasts (PICs). Plant Biol. 2005, 7, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; van der Werf, A. Is inherent variation in RGR determined by LAR at low irradiance and by NAR at high irradiance? A review of herbaceous species. In Inherent Variation in Plant Growth. Physiological Mechanisms and Ecological Consequences; Lambers, H., Poorter, H., van Vuuren, M.M.I., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 309–336. [Google Scholar]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Skovsholt, L.J.; Matheson, F.; Riis, T.; Hawes, I. Trait-specific groups of aquatic macrophytes respond differently to eutrophication of unshaded lowland streams. Sci. Total Environ. 2024, 954, 176724. [Google Scholar] [CrossRef]
- Osone, Y.; Ishida, A.; Tateno, M. The correlation between relative growth rate and specific leaf area requires associations of specific leaf area with root nitrogen absorption rate. New Phytol. 2008, 179, 417–427. [Google Scholar] [CrossRef]
- Ruiz-Checa, R.; Pérez-Jordán, H.; García-Gómez, H.; Prieto-Benítez, S.; Gónzalez-Fernández, I.; Alonso, R. Foliar nitrogen uptake in broadleaf evergreen Mediterranean forests: Fertilisation experiment with labelled nitrogen. Sci. Total Environ. 2024, 926, 171865. [Google Scholar] [CrossRef]
- Wang, X.; Wen, H.; Suprun, A.; Zhu, H. Ethylene Signaling in Regulating Plant Growth, Development, and Stress Responses. Plants 2025, 14, 309. [Google Scholar] [CrossRef]
- Visser, E.J.; Voesenek, L.A. Aclimatacióln a la inundación del suelo: Detección y transducción de señales. In Root Physiology: From Gene to Function; Lambers, H., Colmer, T.D., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 375–392. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, J.; Fu, Q. Quantitative Relationship of Plant Height and Leaf Area Index of Spring Maize under Different Water and Nitrogen Treatments Based on Effective Accumulated Temperature. Agronomy 2024, 14, 1018. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Sylvester-Bradley, R.; Weightman, R.; Snape, J.W. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Res. 2007, 103, 11–24. [Google Scholar] [CrossRef]
- Gifford, R.M.; Evans, L.T. Fotosíntesis, reparto del carbono y rendimiento. Annu. Rev. Plant Physiol. 1981, 32, 485–509. [Google Scholar] [CrossRef]
- Diez, J.; Orellana, F.; Searles, P.; Acreche, M.M. Supplemental irrigation during the critical period for yield ensures higher radiation capture and use efficiency, water use efficiency, and grain yield in chia. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef]
- Golubkov, M.; Golubkov, S. The role of total phosphorus in eutrophication of freshwater and brackish-water parts of the Neva River estuary (Baltic Sea). Mar. Environ. Res. 2025, 209, 107232. [Google Scholar] [CrossRef] [PubMed]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Stafford Smith, D.M.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global Desertification: Building a Science for Dryland Development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consecuencias del cambio de la biodiversidad. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Toet, S.; Bouwman, M.; Cevaal, A.; Verhoeven, J.T.A. Nutrient Removal through Autumn Harvest of Phragmites australis and Typha latifolia Shoots in Relation to Nutrient Loading in a Wetland System Used for Polishing Sewage Treatment Plant Effluent. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2005, 40, 1133–1156. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.C.; Ge, Y.; Dzakpasu, M.; Zhao, Y.; Xiong, J. Effects of Annual Harvesting on Plant Growth and Nutrient Removal in Surface-Flow Constructed Wetlands in Northwestern China. Ecol. Eng. 2015, 83, 268–275. [Google Scholar] [CrossRef]
- Carrillo, V.; Collins, C.; Brisson, J.; Vidal, G. Evaluation of long-term phosphorus uptake by Schoenoplectus californicus and Phragmites australis plants in pilot-scale constructed wetlands. Int. J. Phytoremediat. 2021, 24, 610–621. [Google Scholar] [CrossRef]
- Choudhury, M.I.; Nilsson, J.E.; Hylander, S.; Hauber, M.; Ehde, P.M.; Weisner, S.E.B.; Liess, A. Enhancing Nitrogen Removal through Macrophyte Harvest and Installation of Woodchips-Based Floating Beds in Surface-Flow Constructed Wetlands. Chemosphere 2024, 359, 142284. [Google Scholar] [CrossRef]
- Camacho, A.; Picazo, A.; Rochera, C.; Peña, M.; Morante, D.; Miralles-Lorenzo, J.; Santamans, A.C.; Estruch, H.; Montoya, T.; Fayos, G.; et al. Uso en Serie de Helosciadum nodiflorum y Typha latifolia en Humedales Artificiales Mediterráneos para Naturalizar los Efluentes de Plantas de Tratamiento de Aguas Residuales. Water 2018, 10, 717. [Google Scholar] [CrossRef]




| Growth Index | Symbol | Average Value over a Time Interval (t2–t1) | Units |
|---|---|---|---|
| Relative Growth Rate | RGR | g g−1 day−1 | |
| Absolute Growth Rate | AGR | g day−1 | |
| Net Assimilation Rate | NAR | g m−2 day−1 | |
| Leaf Weight Ratio | LWR | g g−1 | |
| Leaf Area Index | LAI | Dimensionless | |
| Harvest Index | HI | Dimensionless 100 = % | |
| Crop Yield | R | g m−2 |
| Lake | Chemical Oxygen Demand (COD) (mg L−1) | Biological Oxygen Demand (BOD5) (mg L−1) | Total Phosphorus (TP) (µg L−1) | Total Nitrogen (TN) (µg L−1) | Chlorophyll a (µg L−1) |
|---|---|---|---|---|---|
| Yahuarcocha | 103 | 16 | 272 | 4125 | 71.1 |
| Imbacocha | 24 | 7 | 149 | 1558 | 23.4 |
| Schoenoplectus californicus in Lakes Yahuarcocha and Imbacocha | ||||||
|---|---|---|---|---|---|---|
| Days | Photosynthetic Area PA (m2) | Stem Dry Mass (g m−2) | Total Plant Dry Mass (g m−2) | |||
| Yah 1 | Imb 1 | Yah 1 | Imb 1 | Yah 1 | Imb 1 | |
| 30 | 1.01 ± 0.51 | 0.60 ± 0.15 | 280 ± 88 | 110 ± 47 | 508 ± 19 | 223 ± 18 |
| 60 | 2.75 ± 0.59 | 1.33 ± 0.28 | 682 ± 121 | 214 ± 63 | 1283 ± 55 | 448 ± 29 |
| 90 | 6.37 ± 0.70 | 2.18 ± 0.42 | 1575 ± 206 | 397 ± 115 | 3005 ± 23 | 837 ± 59 |
| 120 | 8.38 ± 0.83 | 4.11 ± 0.45 | 2228 ± 188 | 745 ± 148 | 4222 ± 54 | 1537 ± 62 |
| 150 | 8.68 ± 0.85 | 5.37 ± 0.66 | 2380 ± 116 | 977 ± 103 | 4390 ± 253 | 2038 ± 123 |
| 180 | 8.32 ± 0.70 | 5.77 ± 0.77 | 2240 ± 100 | 1050 ± 99 | 4253 ± 81 | 2068 ± 177 |
| 210 | 7.44 ± 0.52 | 5.47 ± 0.62 | 2234 ± 108 | 1026 ± 69 | 4168 ± 86 | 2061 ± 165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabón-Garcés, G.; Vásquez-Hernández, L.; Yaguana-Jiménez, G.; Aguirre-Mejía, P. Vegetative Growth Analysis of Schoenoplectus californicus (Totora): Dynamics and Physiological Mechanisms in High-Altitude Andean Lakes. Ecologies 2025, 6, 71. https://doi.org/10.3390/ecologies6040071
Pabón-Garcés G, Vásquez-Hernández L, Yaguana-Jiménez G, Aguirre-Mejía P. Vegetative Growth Analysis of Schoenoplectus californicus (Totora): Dynamics and Physiological Mechanisms in High-Altitude Andean Lakes. Ecologies. 2025; 6(4):71. https://doi.org/10.3390/ecologies6040071
Chicago/Turabian StylePabón-Garcés, Galo, Lucía Vásquez-Hernández, Gladys Yaguana-Jiménez, and Patricia Aguirre-Mejía. 2025. "Vegetative Growth Analysis of Schoenoplectus californicus (Totora): Dynamics and Physiological Mechanisms in High-Altitude Andean Lakes" Ecologies 6, no. 4: 71. https://doi.org/10.3390/ecologies6040071
APA StylePabón-Garcés, G., Vásquez-Hernández, L., Yaguana-Jiménez, G., & Aguirre-Mejía, P. (2025). Vegetative Growth Analysis of Schoenoplectus californicus (Totora): Dynamics and Physiological Mechanisms in High-Altitude Andean Lakes. Ecologies, 6(4), 71. https://doi.org/10.3390/ecologies6040071






