Comparisons of Twelve Freshwater Mussel Bed Assemblages Quantitatively Sampled at a 15-year Interval in the Buffalo National River, Arkansas, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
Study Area
2.2. Monitoring and Sampling Techniques
2.2.1. Qualitative Reconnaissance
2.2.2. Mussel Bed Delineation
2.2.3. Mesohabitat Determinations and Stratification
2.2.4. Stratified Random Sampling
2.2.5. Sample Processing
2.2.6. In-Field Sampling Confidence Level Determination and Additional Sampling
2.3. Data Analysis
3. Results
3.1. An Overview of 2006 and 2020–21 Sampling Events
3.2. Mussel Assemblage Sampling Site Variables’ Descriptions
3.3. Sampling Confidence Levels of 2006 and 2020–21
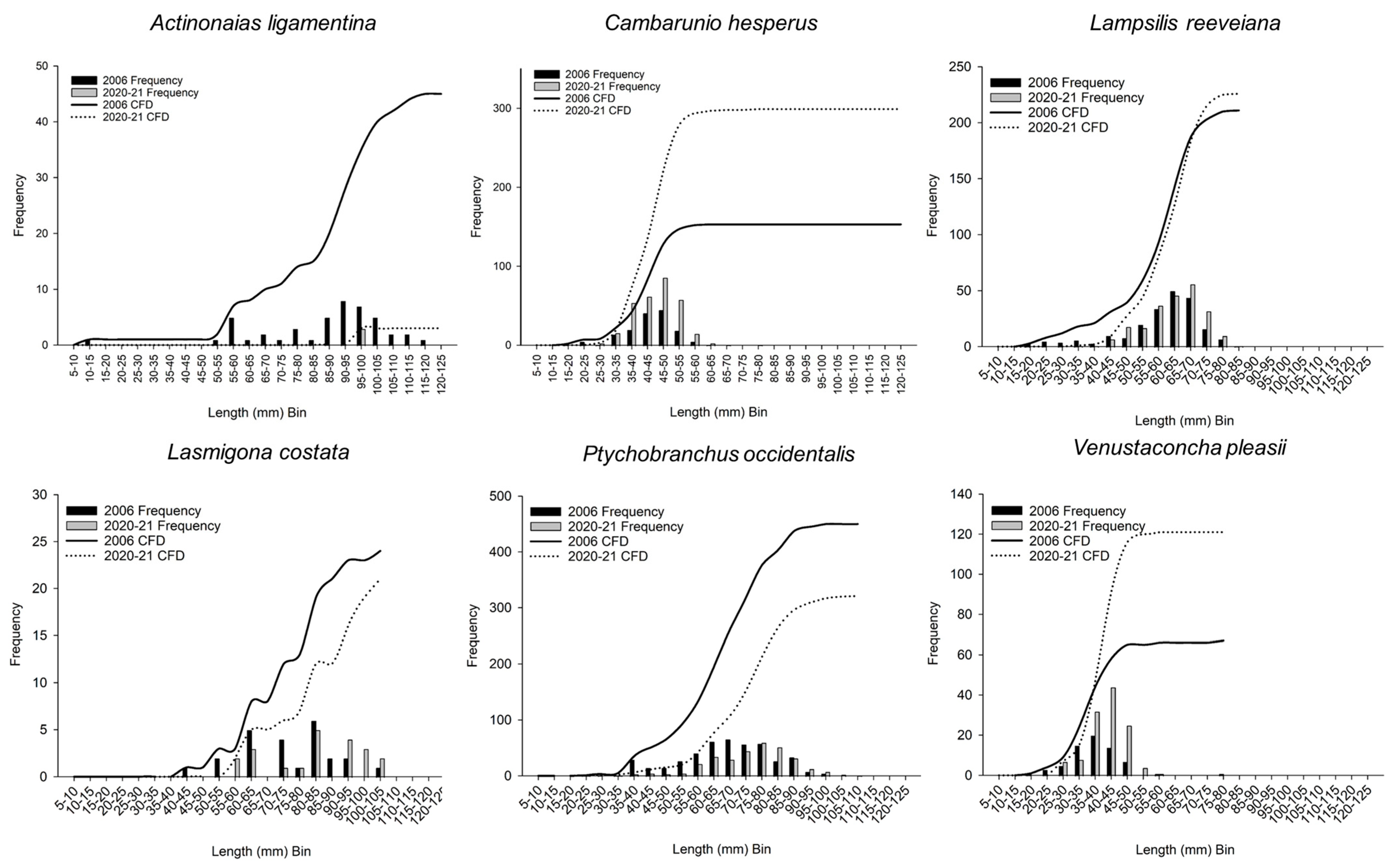
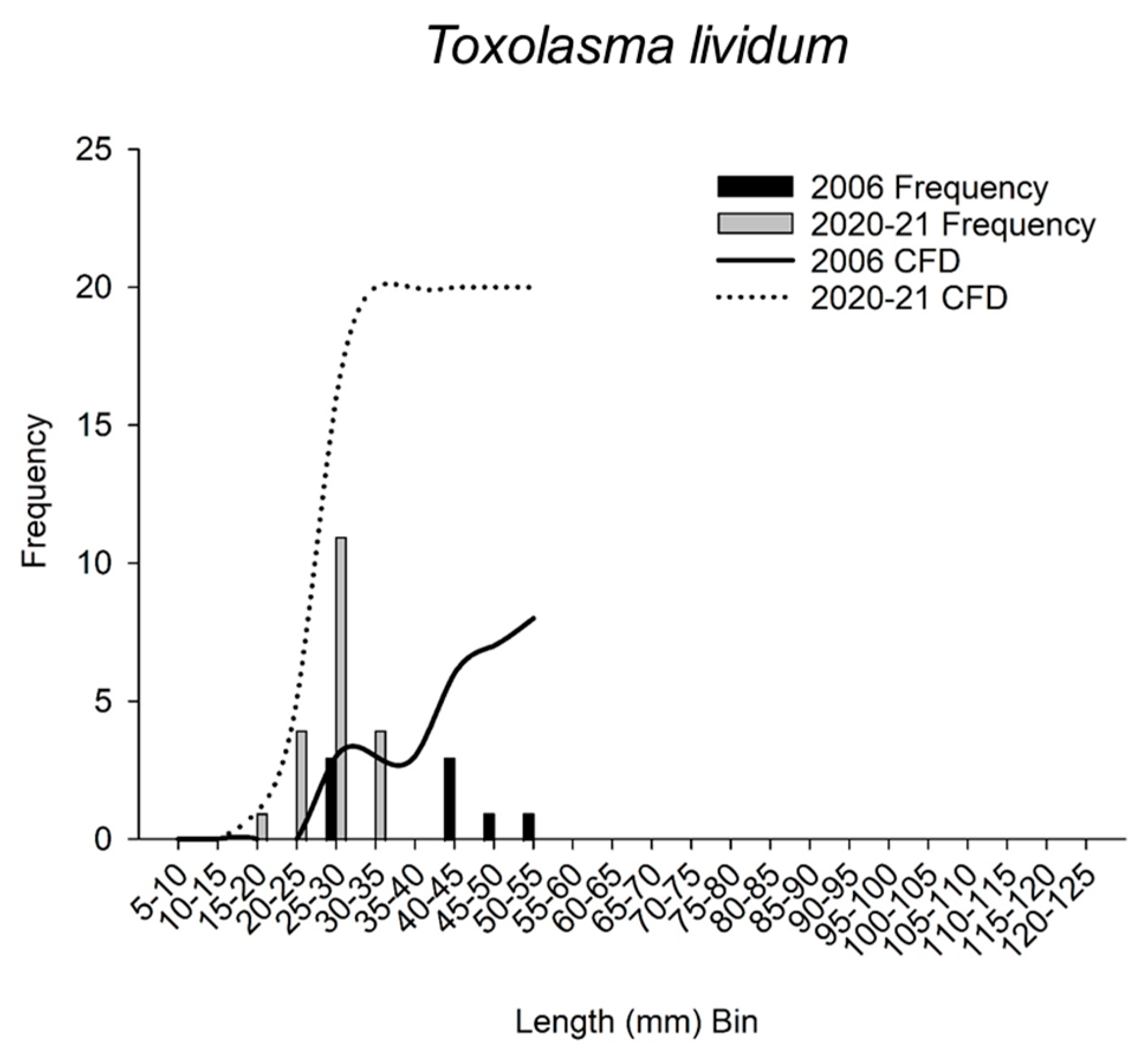
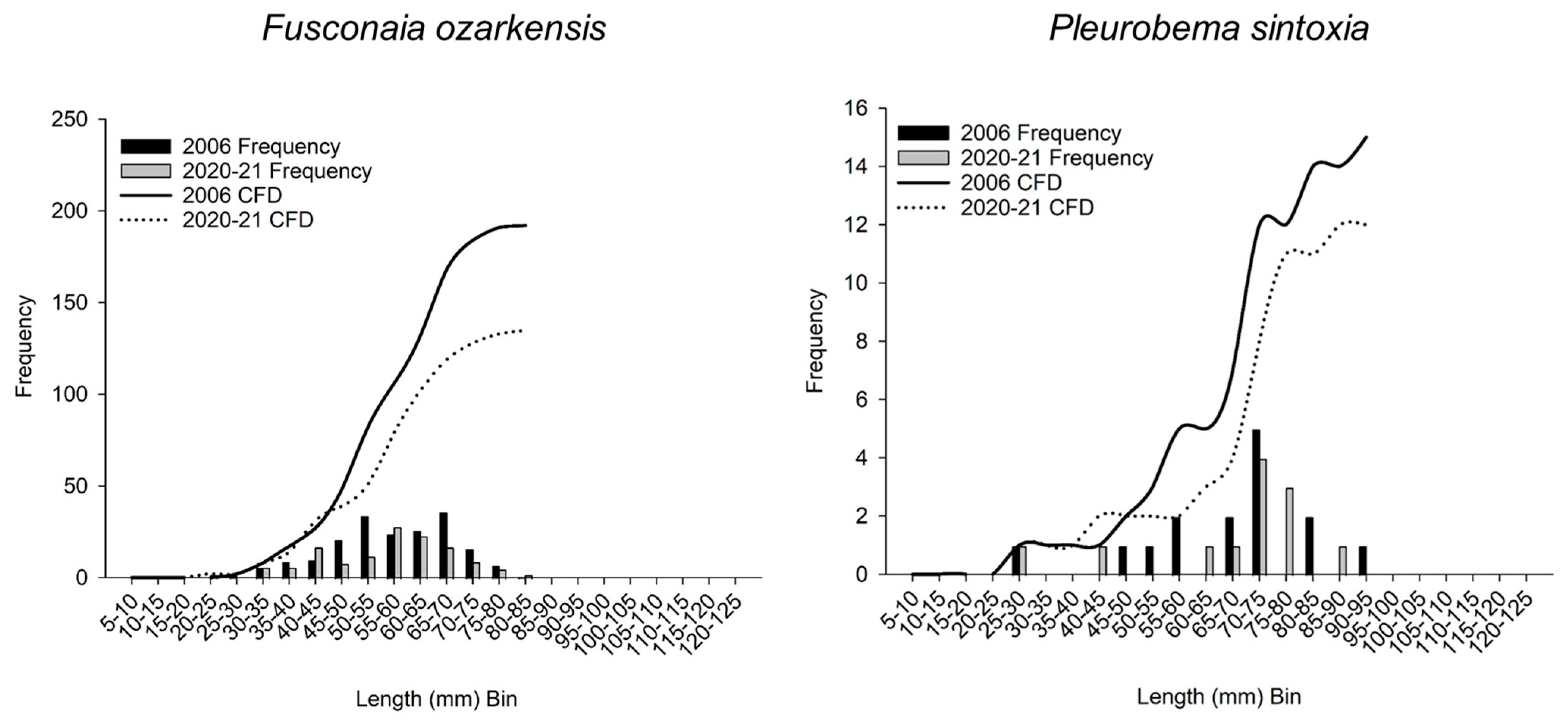
3.4. Size and Cumulative Frequency Distributions
3.5. The Paired t-Test of Common 2006 and 2020–21 Sites
3.6. The Pairwise Density and Richness Comparisons of 2006 and 2020–21
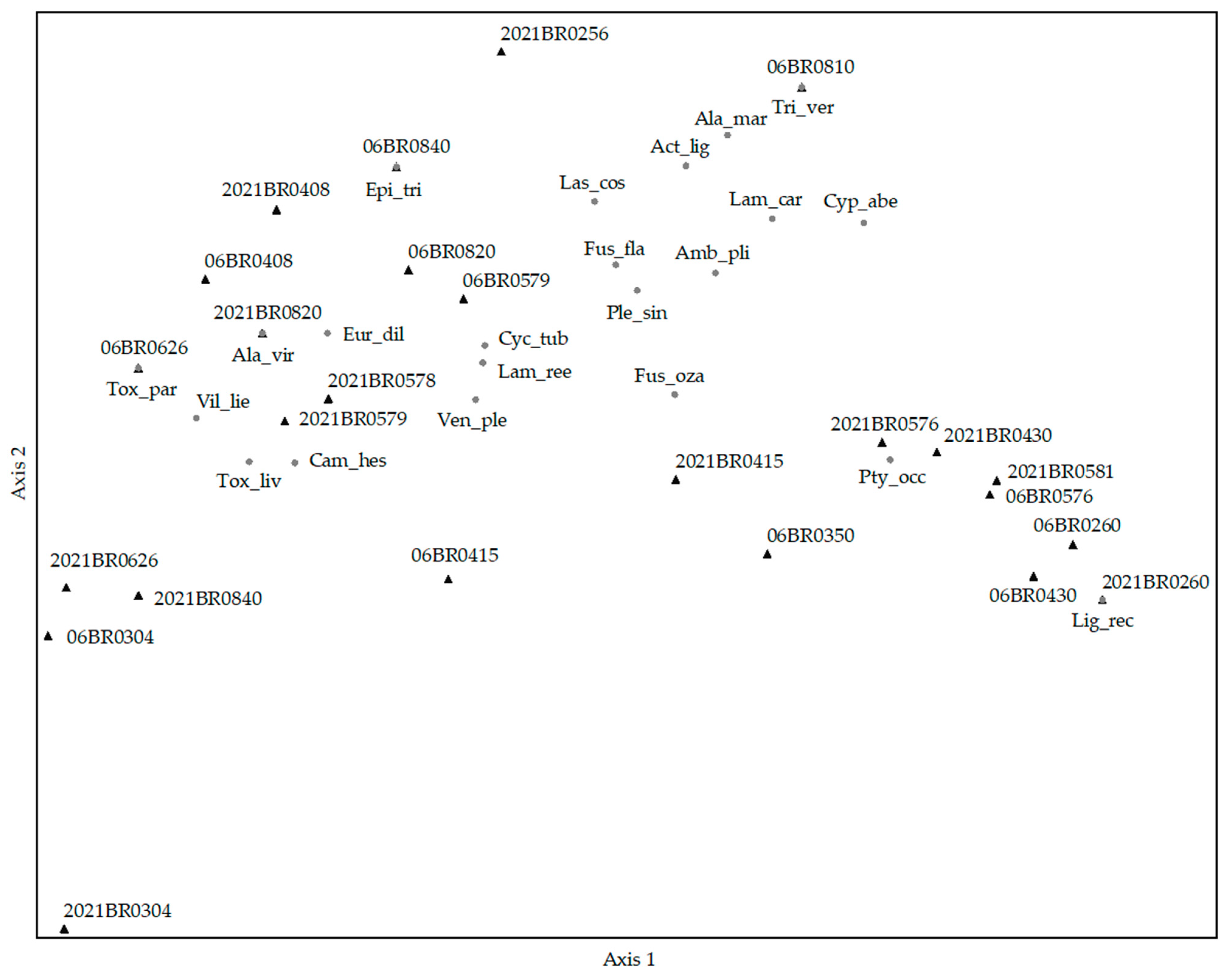
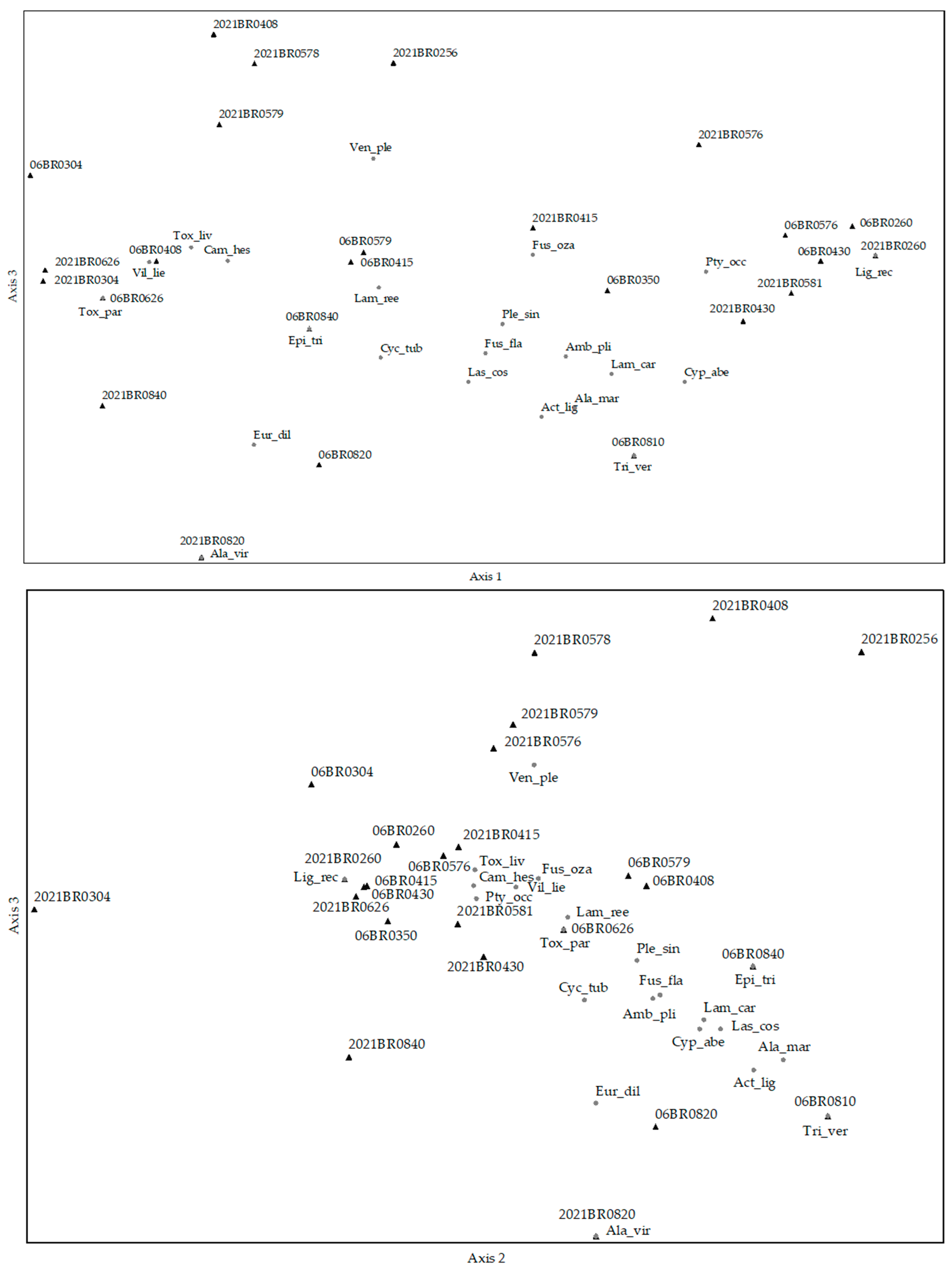
3.7. The Assemblage Comparisons of 2006 and 2020–21
4. Discussion
4.1. Overview
4.2. Sampling Effort
4.3. Species and Assemblage Composition and Structure Changes
4.4. Mussel Bed Persistence and Outliers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haag, W.R.; Williams, J.D. Biodiversity on the Brink: An Assessment of Conservation Strategies for North American Freshwater Mussels. Hydrobiologia 2014, 735, 45–60. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of Freshwater Bivalves at the Global Scale: Diversity, Threats and Research Needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- Strayer, D.L.; Downing, J.A.; Haag, W.R.; King, T.L.; Layzer, J.B.; Newton, T.J.; Nichols, S.J. Changing Perspectives on Pearly Mussels, North America’s Most Imperiled Animals. Bioscience 2004, 54, 429–439. [Google Scholar] [CrossRef]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The Global Decline of Nonmarine Mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Régnier, C.; Fontaine, B.; Bouchet, P. Not Knowing, Not Recording, Not Listing: Numerous Unnoticed Mollusk Extinctions. Conserv. Biol. 2009, 23, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Bogan, A.E. Freshwater Bivalve Extinctions (Mollusca: Unionidae): A Search for Causes. Am. Zool. 1993, 33, 599–609. [Google Scholar] [CrossRef]
- Williams, J.D.; Warren, M.L.; Cummings, K.S.; Harris, J.L.; Neves, R.J. Conservation Status of Freshwater Mussels of the United States and Canada. Fisheries 1993, 18, 6–22. [Google Scholar] [CrossRef]
- Freshwater Mollusk Conservation Society. A National Strategy for the Conservation of Native Freshwater Mollusks. Freshw. Mollusk Biol. Conserv. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Neves, R.J.; Bogan, A.E.; Williams, J.D.; Ahlstedt, S.A.; Hartfield, P.W. Status of Aquatic Mollusks in the Southeastern United States: A Downward Spiral of Diversity. In Aquatic Fauna in Peril: The Southeastern Perspective; Benz, G.W., Collins, D.E., Eds.; Special Publication 1; Southeast Aquatic Research Institute, Lenz Design & Communications: Decatur, GA, USA, 1997; pp. 43–86. [Google Scholar]
- Campbell, D.C.; Serb, J.M.; Buhay, J.E.; Roe, K.J.; Minton, R.L.; Lydeard, C. Phylogeny of North American Amblemines (Bivalvia, Unionoida): Prodigious Polyphyly Proves Pervasive across Genera. Invertebr. Biol. 2005, 124, 131–164. [Google Scholar] [CrossRef]
- Downing, J.A.; Van Meter, P.; Woolnough, D.A. Suspects and Evidence: A Review of the Causes of Extirpation and Decline in Freshwater Mussels. Anim. Biodivers. Conserv. 2010, 33, 151–185. [Google Scholar] [CrossRef]
- Strayer, D.L. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2008; Volume 1. [Google Scholar]
- Vaughn, C.C.; Taylor, C.M. Impoundments and the Decline of Freshwater Mussels: A Case Study of an Extinction Gradient. Conserv. Biol. 1999, 13, 912–920. [Google Scholar] [CrossRef]
- Nobles, T.; Zhang, Y. Biodiversity Loss in Freshwater Mussels: Importance, Threats, and Solutions. Biodivers. Loss A Chang. Planet 2011, 318, 17–162. [Google Scholar]
- Vaughn, C.C. Life History Traits and Abundance Can Predict Local Colonisation and Extinction Rates of Freshwater Mussels. Freshw. Biol. 2012, 57, 982–992. [Google Scholar] [CrossRef]
- Galbraith, H.S.; Spooner, D.E.; Vaughn, C.C. Synergistic Effects of Regional Climate Patterns and Local Water Management on Freshwater Mussel Communities. Biol. Conserv. 2010, 143, 1175–1183. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as Ecosystem Engineers: The Role of Shell Production in Aquatic Habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hakenkamp, C.C. The Functional Role of Burrowing Bivalves in Freshwater Ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity Losses and Ecosystem Function in Freshwaters: Emerging Conclusions and Research Directions. J. Biosci. 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Reis, J.; Alvarez, M.G.; Anastácio, P.M.; Banha, F.; Beja, P.; Castro, P.; Gama, M.; Gil, M.G.; Gomes-dos-Santos, A.; et al. The Silent Extinction of Freshwater Mussels in Portugal. Biol. Conserv. 2023, 285, 110244. [Google Scholar] [CrossRef]
- Arkansas Pollution Control and Ecology. Regulation No. 2: Regulation Establishing Water Quality Standards for Surface Waters of the State of Arkansas; Arkansas Pollution Control and Ecology Commission: Little Rock, AR, USA, 2015. [Google Scholar]
- National Park Service, Department of the Interior. Learn about the Park. Available online: https://www.nps.gov/buff/learn/index.htm#:~:text=Buffalo%20National%20River%20was%20established%20by%20Congress%20in,the%20overall%20watershed%20is%20under%20direct%20NPS%20management (accessed on 19 June 2019).
- American Rivers. America’s Most Endangered Rivers (R) 2019; American Rivers: Washington, DC, USA, 2019; p. 26. Available online: https://s3.amazonaws.com/american-rivers-website/wp-content/uploads/2019/04/12112920/MER-Report-2019_Full-Layout_FNL1.pdf (accessed on 18 June 2019).
- Arkansas Department of Environmental Quality. Arkansas’s 2018 List of Impaired Waterbodies Executive Summary Section; Aransas Department of Environmental Quality: Little Rock, AR, USA, 2018; p. 6. [Google Scholar]
- FTN Associates, Ltd. Buffalo River Watershed-Based Management Plan; Aransas Department of Environmental Quality: Little Rock, AR, USA, 2018; Available online: https://www.adeq.state.ar.us/water/planning/integrated/303d/pdfs/2018/2018-05-22-final-buffalo-river-wmp.pdf (accessed on 24 September 2019).
- Meek, S.E.; Clark, H.W. The Mussels of the Big Buffalo Fork of White River, Arkansas; Bureau of Fisheries Document No 759; Department of Commerce and Labor Bureau of Fisheries, Government Printing Office: Washington, DC, USA, 1912. [Google Scholar]
- Watershed Conservation Resource Center. Surface-Water Quality in the Buffalo National River; Watershed Conservation Resource Center: Fayetteville, AR, USA, 2017; p. 71. Available online: https://buffaloriveralliance.org/resources/Pictures/Buffalo%20National%20River%20Water%20Quality%20Report%201985%20-%202011%20Final.pdf (accessed on 1 July 2020).
- Cullinane, T.; Flyr, M.; Koontz, L. 2021 National Park Visitor Spending Effects: Economic Contributions to Local Communities, States, and the Nation; Natural Resource Report NPS/NRSS/EDQ/NRR-2022/2395; National Park Service: Fort Collins, CO, USA, 2021. [Google Scholar] [CrossRef]
- Harris, J.L. The Freshwater Mussel Resources of the Buffalo National River, Arkansas Phase I Qualitative Survey: Location, Species Composition, and Status of Mussel Beds; Report; Buffalo National River, John L. Harris: Little Rock, AR, USA, 1996. [Google Scholar]
- Matthews, M.W. Freshwater Bivalve (Mollusca: Unionidae, Corbiculidae) Assemblages in an Ozark River: Structure and Role in Nutrient Recycling. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 2007. [Google Scholar]
- Matthews, M.W.; Usrey, F.; Hodges, S.; Harris, J.L.; Christian, A.D. Species Richness, Distribution, and Relative Abundance of Freshwater Mussels (Bivalvia: Unionidae) of the Buffalo National River, Arkansas. J. Ark. Acad. Sci. 2009, 63, 113–130. [Google Scholar]
- Master, L.L.; Faber-Langendoen, D.; Bittman, R.; Hammerson, G.; Heidel, B.; Ramsay, L.; Snow, K.; Teucher, A.; Tomaino, A. NatureServe Conservation Status Assessments: Factors for Evaluating Species and Ecosystem Risk; NatureServe: Arlington, VA, USA, 2012. [Google Scholar]
- Pieri, A.M.; Harris, J.L.; Bouldin, J.L.; Schaeffer, T.W.; Steevens, J.A.; Hodges, S.W.; Rodman, A.R. A Quantitative Survey of Freshwater Mussels of the Buffalo National River, Arkansas from 2019 to 2021A Quantitative Survey of Freshwater Mussels of the Buffalo National River, Arkansas from 2019 to 2021; Final Report. Buffalo National River: Harrison, AR, USA, 2022. [Google Scholar] [CrossRef]
- United States Geological Survey. StreamStats; StreamStats Application Version 4.10.1; United States Geological Survey: Reston, VA, USA, 2021. [Google Scholar]
- Smith, D.R.; Strayer, D.L. A Guide to Sampling Freshwater Mussel Populations; American Fisheries Society Monograph 8; American Fisheries Society: Bethesda, MD, USA, 2003. [Google Scholar]
- Williams, J.D.; Bogan, A.E.; Butler, R.S.; Cummings, K.S.; Garner, J.T.; Harris, J.L.; Johnson, N.A.; Watters, G.T. A Revised List of the Freshwater Mussels (Mollusca: Bivalvia: Unionida) of the United States and Canada. Freshw. Mollusk Biol. Conserv. 2017, 20, 33–58. [Google Scholar] [CrossRef]
- Freshwater Mollusk Conservation Society. Scientific and Common Names of Freshwater Bivalves of the US and Canada. Available online: https://molluskconservation.org/MServices_Names-Bivalves.html (accessed on 1 November 2023).
- McCain, M.E.; Fuller, D.; Decker, L.; Overton, K. Stream Habitat Classification and Inventory Procedures For Northern California. Fish Habitat Relatsh. Tech. Bull. 1990, 1, 1–15. [Google Scholar]
- Clingenpeel, J.A.; Cochran, B.G. Using Physical, Chemical, and Biological Indicators to Assess Water Quality on the Ouachita National Forest Utilizing Basin Area Stream Survey Methods. Proc. Ark. Acad. Sci. 1992, 46, 33–35. [Google Scholar]
- Wolman, M.G. A Method of Sampling Coarse River-bed Material. EOS Trans. Am. Geophys. Union 1954, 35, 951–956. [Google Scholar]
- Christian, A.D.; Harris, J.L. Development and Assessment of a Sampling Design for Mussel Assemblages in Large Streams. Am. Midl. Nat. 2005, 153, 284–292. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Ecological Methods; Chapman and Hall: London, UK, 1978. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; Version 7.08 MjM Software Design: Gleneden Beach, OR, USA, 2018. [Google Scholar]
- Huebner, J.D.; Malley, D.F.; Donkersloot, K. Population Ecology of the Freshwater Mussel Anodonta Grandis Grandis in a Precambrian Shield Lake. Can. J. Zool. 1990, 68, 1931–1941. [Google Scholar] [CrossRef]
- Systat Software, Inc. SigmaStat for Windows; Systat Software, Inc.: London, UK, 2016. [Google Scholar]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. 2023. Available online: https://www.xlstat.com/en (accessed on 15 February 2023).
- Rust, P.J. Analysis of the Commercial Mussel Beds in the Black, Spring, Strawberry and Current Rivers in Arkansas. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 1993. [Google Scholar]
- Christian, A.D. Analysis of the Commercial Mussel Beds in the Cache and White Rivers in Arkansas. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 1995. [Google Scholar]
- Posey, W.R., II. Location, Species Composition and Community Estimates for Mussel Beds in the St. Francis and Ouachita Rivers in Arkansas. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 1997. [Google Scholar]
- Christian, A.D.; Harris, J.L.; Posey, W.R.; Hockmuth, J.F.; Harp, G.L. Freshwater Mussel (Bivalvia: Unionidae) Assemblages of the Lower Cache River, Arkansas. Southeast. Nat. 2005, 4, 487–512. [Google Scholar] [CrossRef]
- Christian, A.D.; Atwood, A.; Bethel, D.; Dimino, T.; Garner, N.; Garrison, J.R.; Gulich, L.; McCanty, S. Baseline Qualitative and Quantitative Mussel Surveys of the Mill River System, Massachusetts, Prior to Final Dam Removal. Freshw. Mollusk Biol. Conserv. 2019, 22, 1–11. [Google Scholar] [CrossRef]
- Gordon, M.E.; Kraemer, L.R.; Brown, A.V. Unionaceae of Arkansas: Historical Review, Checklist, and Observations on Distributional Patterns. Bull. Am. Malacol. Union Inc. 1980, 31–37. [Google Scholar]
- Harris, J.L.; Posey, W.R., II; Davidson, C.L.; Farris, J.L.; Oetker, S.R.; Stoeckel, J.N.; Crump, B.G.; Barnett, M.S.; Martin, H.C.; Seagraves, J.H.; et al. Unionoida (Mollusca: Margaritiferidae, Unionidae) in Arkansas, Third Status Review. J. Ark. Acad. Sci. 2009, 63, 50–84. [Google Scholar]
- Haag, W.R. North American Freshwater Mussels: Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Spooner, D.E.; Vaughn, C.C. A Trait-Based Approach to Species’ Roles in Stream Ecosystems: Climate Change, Community Structure, and Material Cycling. Oecologia 2008, 158, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.W.; DuBose, T.P.; Franzen, A.J.; Atkinson, C.L.; Vaughn, C.C. Long-term Monitoring Shows That Drought Sensitivity and Riparian Land Use Change Coincide with Freshwater Mussel Declines. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1571–1583. [Google Scholar] [CrossRef]
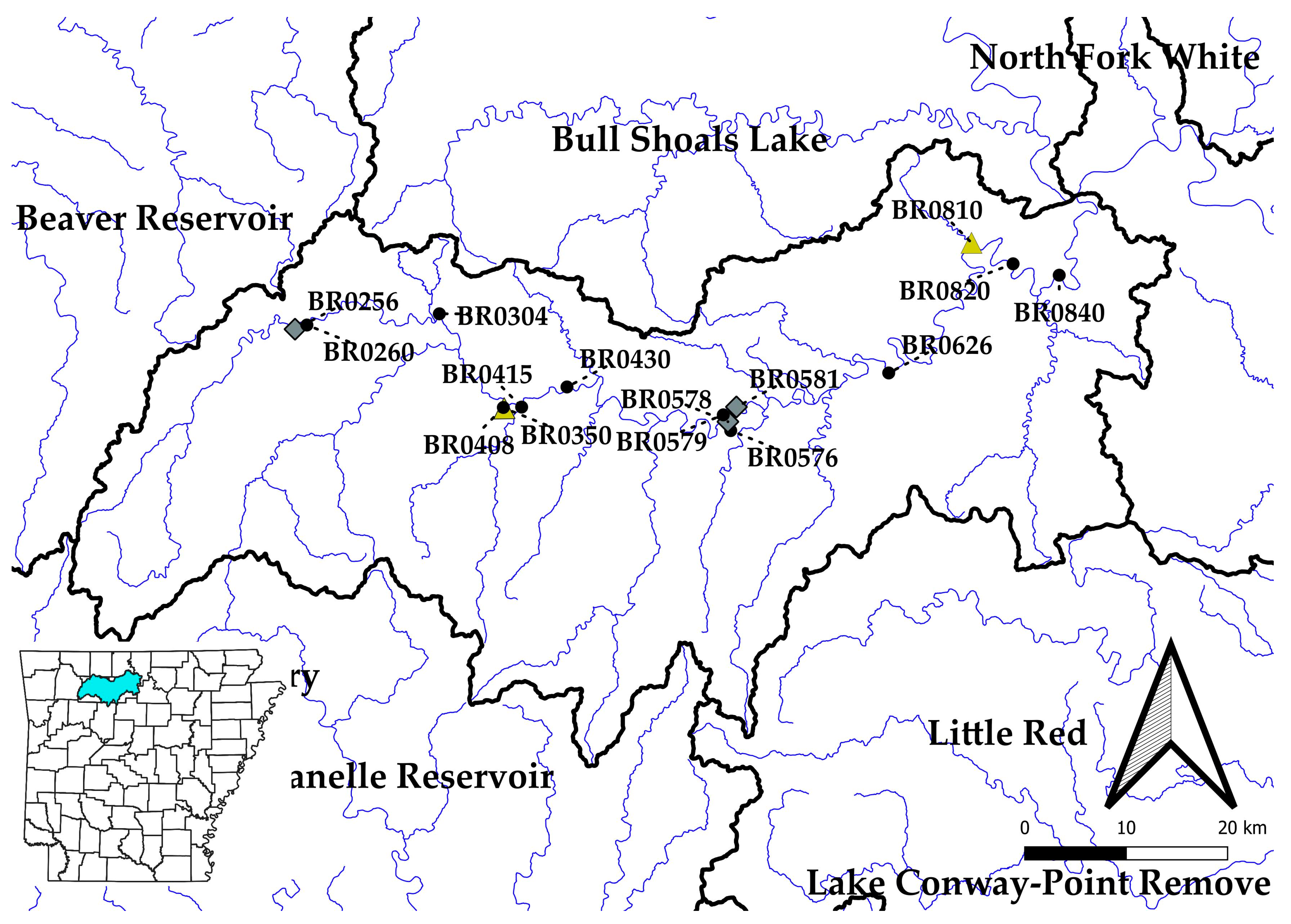

| Site | River Km | Stream Order | Drainage Area (km2) | Slope (m/km) | % Dev. | % Imp. | % Pas. | MAP (cm) | Matthews 2006 Sampling Sites | 2020–2021 Sampling Sites | Pairwise Comparison Sites |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BR0256 | 188.6 | 4 | 365 | 5.097 | 2.38 | 0.14 | 6.68 | 126.5 | X | ||
| BR0260 | 186.6 | 4 | 373 | 4.997 | 2.39 | 0.14 | 6.86 | 126.5 | X | X | X |
| BR0304 | 164.4 | 5 | 552 | 3.815 | 2.70 | 0.18 | 7.82 | 124.5 | X | X | X |
| BR0350 | 157.1 | 6 | 976 | 3.549 | 3.15 | 0.24 | 8.45 | 125.2 | X | Extirpated | |
| BR0408 | 149.4 | 6 | 1020 | 3.248 | 3.20 | 0.24 | 8.50 | 125.0 | X | X | X |
| BR0415 | 147.4 | 6 | 1259 | 3.207 | 3.22 | 0.25 | 9.54 | 125.2 | X | X | X |
| BR0430 | 141.0 | 6 | 1290 | 3.014 | 3.23 | 0.25 | 9.49 | 125.0 | X | X | X |
| BR0576 | 107.9 | 6 | 1950 | 2.238 | 3.14 | 0.22 | 9.62 | 124.2 | X | X | X |
| BR0578 | 106.3 | 6 | 1953 | 2.197 | 3.14 | 0.22 | 9.62 | 124.2 | X | ||
| BR0579 | 105.3 | 6 | 1966 | 2.177 | 3.15 | 0.22 | 9.86 | 124.2 | X | X | X |
| BR0581 | 103.5 | 6 | 1968 | 2.142 | 3.15 | 0.22 | 9.88 | 124.2 | X | ||
| BR0626 | 73.8 | 6 | 2616 | 1.791 | 3.38 | 0.28 | 14.40 | 122.4 | X | X | x |
| BR0810 | 36.1 | 6 | 2953 | 1.392 | 3.37 | 0.27 | 14.10 | 121.9 | X | Extirpated | |
| BR0820 | 29.9 | 6 | 2979 | 1.345 | 3.36 | 0.27 | 14.00 | 121.7 | X | X | X |
| BR0840 | 18.7 | 6 | 3367 | 1.257 | 3.38 | 0.27 | 14.80 | 121.4 | X | X | X |
| Entire Basin | NA | 6 | 3471 | 1.183 | 3.38 | 0.27 | 14.30 | 121.2 |
| Species | G-Rank | S-Rank | 2006 Abundance | 2006 Relative Abundance | 2020–21 Abundance | 2020–21 Relative Abundance |
|---|---|---|---|---|---|---|
| Actinonaias ligamentina | G5 | S4 | 174 | 9.3 | 7 | 0.3 |
| Alasmidonta marginata | G4 | S3 | 6 | 0.3 | 1 | <0.1 |
| Alasmidonta viridis | G4/G5 | S1 | 0 | 0.0 | 1 | <0.1 |
| Amblema plicata | G5 | S5 | 13 | 0.7 | 8 | 0.3 |
| Cambarunio hesperus | G5 | S3 | 165 | 8.8 | 426 | 18.3 |
| Cyclonaias tuberculata | G5 | S4 | 109 | 5.8 | 215 | 9.2 |
| Cyprogenia aberti | G2/G3 | S4 | 3 | 0.2 | 0 | 0.0 |
| Epioblasma triquetra | G3 | S1 | 1 | 0.1 | 0 | 0.0 |
| Eurynia dilatata | G5 | S5 | 28 | 1.5 | 106 | 4.5 |
| Fusconaia flava | G5 | S5 | 11 | 0.6 | 0 | 0.0 |
| Fusconaia ozarkensis | G3/G4 | S3 | 257 | 13.7 | 270 | 11.6 |
| Lampsilis cardium | G5 | S5 | 17 | 0.9 | 6 | 0.3 |
| Lampsilis reeveiana | G4 | S4 | 284 | 15.1 | 351 | 15.1 |
| Lasmigona costata | G5 | S4 | 89 | 4.7 | 27 | 1.2 |
| Leaunio lienosus | G5 | S4 | 7 | 0.4 | 10 | 0.4 |
| Ligumia recta | G4/G5 | S4 | 0 | 0.0 | 1 | <0.1 |
| Pleurobema sintoxia | G4/G5 | S3 | 41 | 2.2 | 36 | 1.5 |
| Ptychobranchus occidentalis | G3/G4 | S3 | 585 | 31.2 | 524 | 22.5 |
| Toxolasma lividum | G3 | S3 | 8 | 0.4 | 28 | 1.2 |
| Toxolasma parvum | G5 | S3 | 5 | 0.3 | 0 | 0.0 |
| Tritogonia verrucosa | G4/G5 | S5 | 1 | 0.1 | 0 | 0.0 |
| Venustaconcha pleasii | G3/G4 | S3 | 74 | 3.9 | 315 | 13.5 |
| Total Abundance | 1878 | 100.0 | 2331 | 100.0 | ||
| Total Richness | 20 | 17 |
| Year | Site | Area (m2) | Samples (n) | Proportion of Bed Sampled (%) | Mean Density (#/m2) | Average Density SD | CNSC | CNSC 95% CI (±) | Total Richness | S1–S3 Richness | Average Richness (Richness/m2) | E | H′ | D | # Species with 1 Occurrence | # Species with 2 Occurrences | S1–S3 Relative Abundance | Average Richness SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | BR0256 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 2020–21 | BR0256 | 210 | 25 | 12 | 2.6 | 2.4 | 546 | 167 | 6 | 3 | 1.6 | 0.757 | 1.356 | 0.6907 | 2 | 0 | 50.8 | 1.3 |
| 2006 | BR0260 | 240 | 24 | 10 | 17.0 | 16.2 | 4090 | 1321 | 4 | 3 | 2.4 | 0.645 | 0.894 | 0.4930 | 0 | 0 | 93.4 | 1.2 |
| 2020–21 | BR0260 | 240 | 25 | 10 | 7.4 | 8.2 | 1776 | 679 | 5 | 3 | 1.6 | 0.474 | 0.763 | 0.4099 | 1 | 0 | 95.7 | 1.3 |
| 2006 | BR0304 | 160 | 15 | 9 | 1.3 | 1.8 | 213 | 124 | 5 | 3 | 0.8 | 0.859 | 1.383 | 0.7050 | 1 | 1 | 75.0 | 0.9 |
| 2020–21 | BR0304 | 160 | 25 | 16 | 2.4 | 3.8 | 378 | 198 | 5 | 4 | 0.9 | 0.363 | 0.651 | 0.2758 | 3 | 0 | 93.2 | 1.1 |
| 2006 | BR0350 | 61 | 13 | 21 | 3.7 | 4.7 | 256 | 131 | 6 | 4 | 1.8 | 0.839 | 1.504 | 0.7292 | 1 | 0 | 77.1 | 1.7 |
| 2020–21 | BR0350 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 2006 | BR0408 | 91 | 15 | 17 | 2.6 | 1.7 | 237 | 65 | 11 | 5 | 2.1 | 0.894 | 2.144 | 0.8547 | 2 | 3 | 51.3 | 1.4 |
| 2020–21 | BR0408 | 91 | 18 | 20 | 3.3 | 3.7 | 298 | 127 | 10 | 6 | 2.1 | 0.875 | 2.015 | 0.8423 | 2 | 1 | 61.0 | 2.1 |
| 2006 | BR0415 | 195 | 17 | 9 | 4.6 | 3.7 | 811 | 213 | 8 | 5 | 2.4 | 0.751 | 1.561 | 0.7498 | 2 | 2 | 70.5 | 1.5 |
| 2020–21 | BR0415 | 704 | 25 | 4 | 9.2 | 8.8 | 6477 | 2127 | 9 | 5 | 3.5 | 0.781 | 1.716 | 0.7835 | 2 | 0 | 75.2 | 1.8 |
| 2006 | BR0430 | 48 | 6 | 13 | 14.3 | 4.9 | 687 | 138 | 10 | 4 | 4.7 | 0.560 | 1.289 | 0.5411 | 4 | 0 | 79.1 | 2.0 |
| 2020–21 | BR0430 | 48 | 10 | 21 | 10.0 | 4.3 | 467 | 106 | 12 | 6 | 4.6 | 0.696 | 1.729 | 0.7312 | 4 | 2 | 80.0 | 1.8 |
| 2006 | BR0576 | 196 | 19 | 10 | 4.5 | 3.3 | 781 | 233 | 10 | 5 | 2.3 | 0.597 | 1.374 | 0.6131 | 5 | 1 | 78.8 | 1.8 |
| 2020–21 | BR0576 | 196 | 15 | 8 | 3.5 | 2.4 | 657 | 194 | 6 | 5 | 2.0 | 0.721 | 1.403 | 0.6890 | 3 | 0 | 76.5 | 0.9 |
| 2006 | BR0578 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 2020–21 | BR0578 | 3625 | 25 | 1 | 23.5 | 12.0 | 83,558 | 15,369 | 11 | 6 | 5.5 | 0.798 | 1.913 | 0.8237 | 1 | 1 | 74.7 | 1.9 |
| 2006 | BR0579 | 375 | 25 | 7 | 6.1 | 6.0 | 2280 | 764 | 12 | 5 | 3.4 | 0.819 | 2.036 | 0.8510 | 3 | 1 | 65.1 | 2.5 |
| 2020–21 | BR0579 | 375 | 15 | 4 | 10.3 | 6.4 | 3825 | 1105 | 11 | 7 | 4.4 | 0.756 | 1.814 | 0.8083 | 5 | 0 | 75.2 | 1.2 |
| 2006 | BR0581 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 2020–21 | BR0581 | 1140 | 25 | 2 | 11.9 | 11.3 | 13,543 | 4465 | 12 | 5 | 3.7 | 0.655 | 1.627 | 0.6861 | 1 | 1 | 75.8 | 2.8 |
| 2006 | BR0626 | 216 | 21 | 10 | 8.4 | 6.0 | 1810 | 477 | 13 | 5 | 4.0 | 0.754 | 1.935 | 0.8047 | 3 | 0 | 48.6 | 2.4 |
| 2020–21 | BR0626 | 216 | 25 | 12 | 4.4 | 6.3 | 907 | 482 | 7 | 4 | 1.6 | 0.620 | 1.206 | 0.6213 | 2 | 1 | 64.8 | 1.2 |
| 2006 | BR0810 | 655 | 24 | 4 | 22.6 | 9.9 | 14,801 | 2227 | 16 | 6 | 6.9 | 0.762 | 2.113 | 0.8492 | 3 | 0 | 39.5 | 1.2 |
| 2020–21 | BR0810 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 2006 | BR0820 | 354 | 25 | 7 | 6.3 | 4.8 | 2267 | 566 | 15 | 6 | 4.1 | 0.834 | 2.260 | 0.8741 | 3 | 2 | 40.5 | 2.7 |
| 2020–21 | BR0820 | 354 | 25 | 7 | 15.0 | 6.3 | 5346 | 759 | 12 | 7 | 5.3 | 0.754 | 1.874 | 0.8179 | 1 | 2 | 33.8 | 1.5 |
| 2006 | BR0840 | 520 | 25 | 5 | 4.9 | 10.5 | 1789 | 1876 | 13 | 6 | 2.5 | 0.831 | 2.130 | 0.8426 | 4 | 0 | 41.9 | 2.6 |
| 2020–21 | BR0840 | 520 | 25 | 5 | 3.1 | 2.7 | 1560 | 470 | 8 | 4 | 2.2 | 0.825 | 1.715 | 0.7765 | 1 | 0 | 56.0 | 1.4 |
| Density | Richness | ||||||
|---|---|---|---|---|---|---|---|
| Year | Site | Calculated CL | n Required for 80% CL | n Required for 90% CL | Calculated CL | n Required for 80% CL | n Required for 90% CL |
| 2006 | BR0256 | NS | NS | NS | NS | NS | NS |
| 2020–21 | BR0256 | 81.4 | 21.6 | 86.3 | 83.9 | 16.3 | 65.1 |
| 2006 | BR0260 | 80.7 | 22.4 | 89.8 | 89.3 | 6.9 | 27.5 |
| 2020–21 | BR0260 | 78.3 | 30.5 | 122.1 | 84.2 | 16.2 | 64.9 |
| 2006 | BR0304 | 65.9 | 43.5 | 174.1 | 72.2 | 29.0 | 116.1 |
| 2020–21 | BR0304 | 67.5 | 66.2 | 264.7 | 76.1 | 35.8 | 143.3 |
| 2006 | BR0350 | 65.0 | 39.9 | 159.4 | 73.5 | 22.8 | 91.3 |
| 2020–21 | BR0350 | NS | NS | NS | NS | NS | NS |
| 2006 | BR0408 | 83.3 | 10.5 | 41.8 | 82.0 | 12.1 | 48.4 |
| 2020–21 | BR0408 | 73.3 | 32.0 | 127.9 | 75.6 | 26.8 | 107.1 |
| 2006 | BR0415 | 80.6 | 16.0 | 64.2 | 85.9 | 9.7 | 38.8 |
| 2020–21 | BR0415 | 80.6 | 22.6 | 90.5 | 89.4 | 6.7 | 26.9 |
| 2006 | BR0430 | 86.1 | 2.9 | 11.6 | 82.8 | 4.4 | 17.8 |
| 2020–21 | BR0430 | 86.3 | 4.7 | 18.9 | 87.8 | 3.7 | 14.9 |
| 2006 | BR0576 | 83.2 | 13.4 | 53.5 | 82.2 | 15.0 | 59.9 |
| 2020–21 | BR0576 | 82.8 | 11.8 | 47.4 | 88.0 | 5.4 | 21.4 |
| 2006 | BR0578 | NS | NS | NS | NS | NS | NS |
| 2020–21 | BR0578 | 89.6 | 6.5 | 26.0 | 93.2 | 2.8 | 11.1 |
| 2006 | BR0579 | 80.3 | 24.3 | 97.4 | 85.4 | 13.2 | 53.0 |
| 2020–21 | BR0579 | 83.9 | 9.7 | 38.8 | 92.7 | 2.0 | 8.0 |
| 2006 | BR0581 | NS | NS | NS | NS | NS | NS |
| 2020–21 | BR0581 | 81.0 | 22.5 | 89.9 | 84.8 | 14.5 | 58.1 |
| 2006 | BR0626 | 84.3 | 12.9 | 51.8 | 86.6 | 9.4 | 37.7 |
| 2020–21 | BR0626 | 71.6 | 50.5 | 201.9 | 84.7 | 14.6 | 58.5 |
| 2006 | BR0810 | 91.1 | 4.8 | 19.2 | 96.4 | 0.8 | 3.1 |
| 2020–21 | BR0810 | NS | NS | NS | NS | NS | NS |
| 2006 | BR0820 | 84.8 | 14.4 | 57.7 | 86.9 | 10.7 | 42.6 |
| 2020–21 | BR0820 | 91.6 | 4.5 | 17.8 | 94.3 | 2.1 | 8.2 |
| 2006 | BR0840 | 57.2 | 114.3 | 457.5 | 78.9 | 27.9 | 111.0 |
| 2020–21 | BR0840 | 82.1 | 19.2 | 76.9 | 86.8 | 10.5 | 42.0 |
| Mann–Whitney U Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Event | N | Median | 25% | 75% | M-W U Statistic | p-Value | |
| Actinonaias ligamentina | 2006 | 45 | 91.1 | 75.7 | 98.0 | |||
| 2020–21 | 3 | 98.1 | 96.9 | 99.8 | 34.0 | 0.169 | ||
| Cambarunio hesperus | 2006 | 153 | 44.3 | 38.7 | 48.1 | |||
| 2020–21 | 299 | 45.9 | 40.0 | 50.2 | 19,473.0 | 0.01 | ||
| Cyclonaias tuberculata | 2006 | 65 | 57.3 | 43.8 | 70.4 | |||
| 2020–21 | 134 | 46.8 | 34.2 | 59.9 | 3185.5 | 0.002 | ||
| Eurynia dilatata | 2006 | 26 | 53.4 | 43.0 | 62.1 | |||
| 2020–21 | 83 | 56.8 | 48.2 | 61.7 | 930.5 | 0.293 | ||
| Fusconaia ozarkensis | 2006 | 192 | 57.8 | 49.9 | 66.3 | |||
| 2020–21 | 135 | 57.7 | 48.1 | 64.8 | 21,603.0 | 0.524 | ||
| Lampsilis reeveiana | 2006 | 211 | 61.0 | 53.9 | 66.8 | |||
| 2020–21 | 226 | 63.6 | 56.8 | 68.2 | 20,421.0 | 0.010 | ||
| Lasmigona costata | 2006 | 24 | 74.9 | 62.8 | 82.5 | |||
| 2020–21 | 21 | 84.1 | 68.2 | 95.0 | 164.0 | 0.047 | ||
| Pleurobema sintoxia | 2006 | 15 | 70.3 | 56.8 | 74.9 | |||
| 2020–21 | 12 | 71.3 | 64.7 | 75.6 | 82.0 | 0.714 | ||
| Ptychobranchus occidentalis | 2006 | 450 | 66.9 | 57.4 | 76.5 | |||
| 2020–21 | 321 | 75.8 | 65.2 | 82.9 | 49,580.5 | <0.001 | ||
| Toxolasma lividum | 2006 | 8 | 41.5 | 26.1 | 44.9 | |||
| 2020–21 | 20 | 27.0 | 22.8 | 29.9 | 36.0 | 0.027 | ||
| Venustaconcha pleasii | 2006 | 67 | 37.6 | 32.6 | 41.1 | |||
| 2020–21 | 121 | 41.0 | 38.0 | 45.0 | 2622.0 | <0.001 | ||
| Two-sample Kolmogorov–Smirnov Test | ||||||||
| Species | Event | N | Mean | SD | Min | Max | K–S D Statistic | p-value |
| Actinonaias ligamentina | 2006 | 45 | 85.8 | 20.7 | 10.1 | 118.2 | ||
| 2020–21 | 3 | 98.3 | 1.5 | 96.9 | 99.8 | 0.711 | <0.0001 | |
| Cambarunio hesperus | 2006 | 153 | 43.1 | 7.9 | 18.2 | 60.3 | ||
| 2020–21 | 299 | 45.5 | 7.0 | 25.8 | 77.2 | 0.139 | 0.0050 | |
| Cyclonaias tuberculata | 2006 | 65 | 56.1 | 19.7 | 12.3 | 107.1 | ||
| 2020–21 | 134 | 48.1 | 15.8 | 22.5 | 96.5 | 0.243 | 0.0010 | |
| Eurynia dilatata | 2006 | 26 | 50.2 | 15.3 | 15.7 | 71.9 | ||
| 2020–21 | 83 | 54.7 | 12.2 | 19.5 | 79.9 | 0.228 | 0.0230 | |
| Fusconaia ozarkensis | 2006 | 192 | 57.4 | 11.3 | 25.1 | 81.2 | ||
| 2020–21 | 135 | 56.4 | 12.4 | 21.0 | 82.8 | 0.098 | 0.2910 | |
| Lampsilis reeveiana | 2006 | 211 | 58.2 | 13.2 | 15.0 | 82.1 | ||
| 2020–21 | 226 | 62.0 | 8.8 | 30.4 | 82.0 | 0.141 | 0.0190 | |
| Lasmigona costata | 2006 | 24 | 73.7 | 14.1 | 43.7 | 101.0 | ||
| 2020–21 | 21 | 82.8 | 14.8 | 58.7 | 103.1 | 0.411 | 0.0300 | |
| Pleurobema sintoxia | 2006 | 15 | 66.6 | 16.1 | 28.7 | 92.0 | ||
| 2020–21 | 12 | 67.0 | 16.5 | 27.4 | 87.0 | 0.183 | 0.9380 | |
| Ptychobranchus occidentalis | 2006 | 450 | 66.1 | 14.7 | 23.0 | 98.5 | ||
| 2020–21 | 321 | 73.9 | 13.0 | 21.2 | 107.0 | 0.271 | <0.0001 | |
| Toxolasma lividum | 2006 | 8 | 38.0 | 10.6 | 25.4 | 53.4 | ||
| 2020–21 | 20 | 27.0 | 4.4 | 19.9 | 34.8 | 0.625 | 0.0000 | |
| Venustaconcha pleasii | 2006 | 67 | 37.4 | 8.2 | 19.7 | 75.8 | ||
| 2020–21 | 121 | 40.8 | 5.9 | 25.2 | 56.6 | 0.318 | <0.0001 | |
| Variable | Treatment Name | N | Mean | SD | SEM | t | df | Two-Tailed (p-Value) | Shapiro–Wilk Normality (p-Value) | Power |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | ||||||||||
| 2006 | 10 | 19.2000 | 6.1610 | 1.9480 | ||||||
| 2020–21 | 10 | 20.8000 | 5.7500 | 1.8180 | ||||||
| Difference | 10 | −1.6000 | 5.7390 | 1.8150 | −0.8820 | 9 | 0.401 | 0.7630 | 0.204 | |
| % Bed Sampled | ||||||||||
| 2006 | 10 | 9.7000 | 3.3680 | 1.0650 | ||||||
| 2020–21 | 10 | 10.5300 | 6.3520 | 2.0090 | ||||||
| Difference | 10 | −0.8300 | 4.1230 | 1.3040 | −0.6370 | 9 | 0.540 | 0.7820 | 0.146 | |
| Density (#/m2) | ||||||||||
| 2006 | 10 | 7.0030 | 5.0080 | 1.5840 | ||||||
| 2020–21 | 10 | 6.8600 | 4.1870 | 1.3240 | ||||||
| Difference | 10 | 0.1430 | 5.2460 | 1.6590 | 0.0862 | 9 | 0.933 | 0.9910 | 0.059 | |
| Total Richness | ||||||||||
| 2006 | 10 | 10.1000 | 3.5420 | 1.1200 | ||||||
| 2020–21 | 10 | 8.5000 | 2.7180 | 0.8600 | ||||||
| Difference | 10 | 1.6000 | 2.7570 | 0.8720 | 1.8350 | 9 | 0.100 | 0.5150 | 0.375 | |
| S1–S3 Richness | ||||||||||
| 2006 | 10 | 4.6000 | 1.0750 | 0.3400 | ||||||
| 2020–21 | 10 | 5.1000 | 1.3700 | 0.4330 | ||||||
| Difference | 10 | −0.5000 | 1.2690 | 0.4010 | −1.2460 | 9 | 0.244 | 0.2380 | 0.311 | |
| S1–S3 Relative Abundance | ||||||||||
| 2006 | 10 | 64.4160 | 18.0000 | 5.6920 | ||||||
| 2020–21 | 10 | 71.1300 | 18.2500 | 5.7710 | ||||||
| Difference | 10 | −6.7150 | 8.2840 | 2.6200 | −2.5630 | 9 | 0.305 | 0.8300 | 0.627 | |
| Evenness (E) | ||||||||||
| 2006 | 10 | 0.7540 | 0.1160 | 0.0367 | ||||||
| 2020–21 | 10 | 0.6870 | 0.1590 | 0.0504 | ||||||
| Difference | 10 | 0.0679 | 0.1800 | 0.0570 | 1.1910 | 9 | 0.264 | 0.1100 | 0.294 | |
| Shannon’s diversity (H′) | ||||||||||
| 2006 | 10 | 1.7010 | 0.4600 | 0.1460 | ||||||
| 2020–21 | 10 | 1.4890 | 0.4720 | 0.1490 | ||||||
| Difference | 10 | 0.2120 | 0.3710 | 0.1170 | 1.8060 | 9 | 0.104 | 0.8300 | 0.509 | |
| Simpson’s diversity (D) | ||||||||||
| 2006 | 10 | 0.7330 | 0.1400 | 0.0442 | ||||||
| 2020–21 | 10 | 0.6760 | 0.1900 | 0.0600 | ||||||
| Difference | 10 | 0.0573 | 0.1650 | 0.0521 | 1.1000 | 9 | 0.300 | 0.3130 | 0.265 | |
| # Singlets or Doublets | ||||||||||
| 2006 | 10 | 3.7000 | 1.7030 | 0.5390 | ||||||
| 2020–21 | 10 | 3.0000 | 1.5630 | 0.4940 | ||||||
| Difference | 10 | 0.7000 | 1.8890 | 0.5970 | 1.1720 | 9 | 0.271 | 0.0930 | 0.288 |
| Site | Variable | Event | N | Median | 25% | 75% | M-W U Statistic | p-Value | 2006 vs. 2020–2021 |
|---|---|---|---|---|---|---|---|---|---|
| BR0260 | Density | 2006 | 24 | 17.5 | 1.3 | 31.8 | |||
| 2020–21 | 26 | 5.0 | 1.0 | 12.3 | 230.0 | 0.111 | No significant difference | ||
| Richness | 2006 | 24 | 3.0 | 1.0 | 3.0 | ||||
| 2020–21 | 26 | 2.0 | 0.0 | 2.3 | 201.5 | 0.029 | 20–21 significantly lower | ||
| BR0304 | Density | 2006 | 15 | 1.0 | 0.0 | 2.0 | |||
| 2020–21 | 25 | 1.0 | 0.0 | 3.0 | 180.5 | 0.847 | No significant difference | ||
| Richness | 2006 | 15 | 1.0 | 0.0 | 2.0 | ||||
| 2020–21 | 25 | 1.0 | 0.0 | 1.5 | 186.0 | 0.976 | No significant difference | ||
| BR0408 | Density | 2006 | 16 | 3.0 | 2.0 | 4.0 | |||
| 2020–21 | 18 | 2.0 | 0.0 | 5.0 | 125.5 | 0.526 | No significant difference | ||
| Richness | 2006 | 16 | 2.0 | 1.0 | 3.8 | ||||
| 2020–21 | 18 | 2.0 | 0.0 | 3.3 | 123.0 | 0.471 | No significant difference | ||
| BR0415 | Density | 2006 | 17 | 4.0 | 1.5 | 6.5 | |||
| 2020–21 | 25 | 7.0 | 3.0 | 12.5 | 137.0 | 0.054 | No significant difference | ||
| Richness | 2006 | 17 | 2.0 | 1.0 | 4.0 | ||||
| 2020–21 | 25 | 3.0 | 2.0 | 5.0 | 142.5 | 0.071 | No significant difference | ||
| BR0430 | Density | 2006 | 6 | 15.0 | 10.0 | 18.0 | |||
| 2020–21 | 10 | 10.0 | 6.8 | 14.3 | 14.5 | 0.103 | No significant difference | ||
| Richness | 2006 | 6 | 4.5 | 3.5 | 7.8 | ||||
| 2020–21 | 10 | 5.0 | 3.0 | 3.0 | 27.0 | 0.780 | No significant difference | ||
| BR0576 | Density | 2006 | 19 | 4.0 | 2.0 | 6.0 | |||
| 2020–21 | 15 | 2.0 | 2.0 | 5.0 | 119.0 | 0.421 | No significant difference | ||
| Richness | 2006 | 19 | 2.0 | 1.0 | 4.0 | ||||
| 2020–21 | 15 | 2.0 | 1.0 | 3.0 | 133.5 | 0.762 | No significant difference | ||
| BR0579 | Density | 2006 | 25 | 3.0 | 2.0 | 10.5 | |||
| 2020–21 | 15 | 9.0 | 6.0 | 16.0 | 107.5 | 0.026 | 20–21 significantly higher | ||
| Richness | 2006 | 25 | 3.0 | 2.0 | 5.5 | ||||
| 2020–21 | 15 | 5.0 | 4.0 | 5.0 | 136.5 | 0.153 | No significant difference | ||
| BR0626 | Density | 2006 | 21 | 7.0 | 5.0 | 11.5 | |||
| 2020–21 | 24 | 2.0 | 1.0 | 4.5 | 128.5 | 0.003 | 20–21 significantly lower | ||
| Richness | 2006 | 24 | 3.0 | 1.0 | 1.0 | ||||
| 2020–21 | 26 | 2.0 | 0.0 | 0.0 | 201.5 | 0.029 | 20–21 significantly lower | ||
| BR0820 | Density | 2006 | 25 | 6.0 | 2.0 | 9.0 | |||
| 2020–21 | 24 | 2.5 | 1.0 | 4.0 | 172.5 | 0.011 | 20–21 significantly lower | ||
| Richness | 2006 | 25 | 5.0 | 2.0 | 6.0 | ||||
| 2020–21 | 24 | 2.5 | 1.0 | 3.0 | 160.5 | 0.005 | 20–21 significantly lower | ||
| BR0840 | Density | 2006 | 25 | 2.0 | 0.0 | 5.0 | |||
| 2020–21 | 24 | 2.5 | 1.0 | 4.0 | 283.0 | 0.739 | No significant difference | ||
| Richness | 2006 | 25 | 2.0 | 0.0 | 4.0 | ||||
| 2020–21 | 24 | 2.5 | 1.0 | 3.0 | 293.5 | 0.903 | No significant difference |
| Axis 1 | Axis 2 | Axis 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | r | r2 | tau | r | r2 | tau | r | r2 | tau |
| Actinonaias ligamentina | 0.126 | 0.016 | 0.086 | 0.402 | 0.161 | 0.377 | −0.364 | 0.132 | −0.479 |
| Alasmidonta marginata | 0.132 | 0.017 | 0.025 | 0.369 | 0.137 | 0.227 | −0.284 | 0.080 | −0.025 |
| Alasmidonta viridis | −0.156 | 0.024 | −0.141 | 0.107 | 0.011 | 0.118 | −0.494 | 0.244 | −0.283 |
| Amblema plicata | 0.214 | 0.046 | −0.026 | 0.343 | 0.118 | 0.384 | −0.315 | 0.100 | −0.231 |
| Cambarunio hesperus | −0.542 | 0.294 | −0.554 | −0.125 | 0.016 | −0.044 | 0.007 | 0.000 | −0.180 |
| Cyclonaias tuberculata | −0.084 | 0.007 | 0.025 | 0.294 | 0.086 | 0.269 | −0.501 | 0.251 | −0.398 |
| Cyprogenia aberti | 0.263 | 0.069 | 0.211 | 0.306 | 0.094 | 0.143 | −0.274 | 0.075 | −0.109 |
| Epioblasma triquetra | −0.078 | 0.006 | −0.047 | 0.284 | 0.081 | 0.236 | −0.112 | 0.013 | −0.189 |
| Eurynia dilatata | −0.190 | 0.036 | 0.024 | 0.172 | 0.030 | 0.367 | −0.490 | 0.240 | −0.327 |
| Fusconaia flava | 0.131 | 0.017 | 0.146 | 0.482 | 0.232 | 0.361 | −0.410 | 0.168 | −0.234 |
| Fusconaia ozarkensis | 0.341 | 0.116 | 0.332 | 0.172 | 0.030 | 0.251 | 0.049 | 0.002 | −0.034 |
| Lampsilis cardium | 0.246 | 0.061 | 0.307 | 0.404 | 0.163 | 0.316 | −0.330 | 0.109 | −0.179 |
| Lampsilis reeveiana | −0.171 | 0.029 | −0.054 | 0.457 | 0.209 | 0.409 | −0.264 | 0.070 | −0.094 |
| Lasmigona costata | 0.066 | 0.004 | −0.284 | 0.448 | 0.201 | 0.480 | −0.363 | 0.131 | −0.298 |
| Leaunio lienosus | −0.577 | 0.333 | −0.528 | 0.049 | 0.002 | 0.093 | 0.001 | 0.000 | −0.147 |
| Ligumia recta | 0.331 | 0.109 | 0.283 | −0.178 | 0.032 | −0.236 | 0.011 | 0.000 | 0.047 |
| Pleurobema sintoxia | 0.161 | 0.026 | 0.082 | 0.403 | 0.162 | 0.377 | −0.272 | 0.074 | −0.105 |
| Ptychobranchus occidentalis | 0.715 | 0.511 | 0.760 | −0.098 | 0.010 | −0.077 | −0.057 | 0.003 | −0.010 |
| Toxolasma lividum | −0.482 | 0.232 | −0.529 | −0.089 | 0.008 | 0.036 | 0.071 | 0.005 | −0.004 |
| Toxolasma parvum | −0.228 | 0.052 | −0.189 | 0.070 | 0.005 | 0.094 | −0.060 | 0.004 | −0.141 |
| Tritigonia verrucosa | 0.156 | 0.024 | 0.118 | 0.370 | 0.137 | 0.259 | −0.324 | 0.105 | −0.236 |
| Venustaconcha pleasii | −0.080 | 0.006 | 0.217 | 0.091 | 0.008 | 0.300 | 0.430 | 0.185 | 0.341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieri, A.M.; Harris, J.L.; Matthews, M.W.; Hodges, S.W.; Rodman, A.R.; Bouldin, J.L.; Christian, A.D. Comparisons of Twelve Freshwater Mussel Bed Assemblages Quantitatively Sampled at a 15-year Interval in the Buffalo National River, Arkansas, USA. Ecologies 2024, 5, 1-24. https://doi.org/10.3390/ecologies5010001
Pieri AM, Harris JL, Matthews MW, Hodges SW, Rodman AR, Bouldin JL, Christian AD. Comparisons of Twelve Freshwater Mussel Bed Assemblages Quantitatively Sampled at a 15-year Interval in the Buffalo National River, Arkansas, USA. Ecologies. 2024; 5(1):1-24. https://doi.org/10.3390/ecologies5010001
Chicago/Turabian StylePieri, Anna M., John L. Harris, Mickey W. Matthews, Shawn W. Hodges, Ashley R. Rodman, Jennifer L. Bouldin, and Alan D. Christian. 2024. "Comparisons of Twelve Freshwater Mussel Bed Assemblages Quantitatively Sampled at a 15-year Interval in the Buffalo National River, Arkansas, USA" Ecologies 5, no. 1: 1-24. https://doi.org/10.3390/ecologies5010001
APA StylePieri, A. M., Harris, J. L., Matthews, M. W., Hodges, S. W., Rodman, A. R., Bouldin, J. L., & Christian, A. D. (2024). Comparisons of Twelve Freshwater Mussel Bed Assemblages Quantitatively Sampled at a 15-year Interval in the Buffalo National River, Arkansas, USA. Ecologies, 5(1), 1-24. https://doi.org/10.3390/ecologies5010001







