Assessing the Impacts of Climate Change on the At-Risk Species Anaxyrus microscaphus (The Arizona Toad): A Local and Range-Wide Habitat Suitability Analysis
Abstract
:1. Introduction
2. Materials and Methods
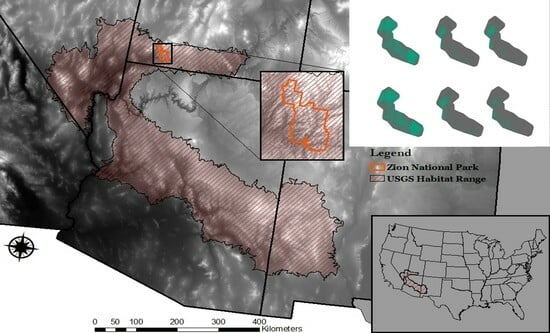
2.1. Study Area
2.2. Data Acquisition
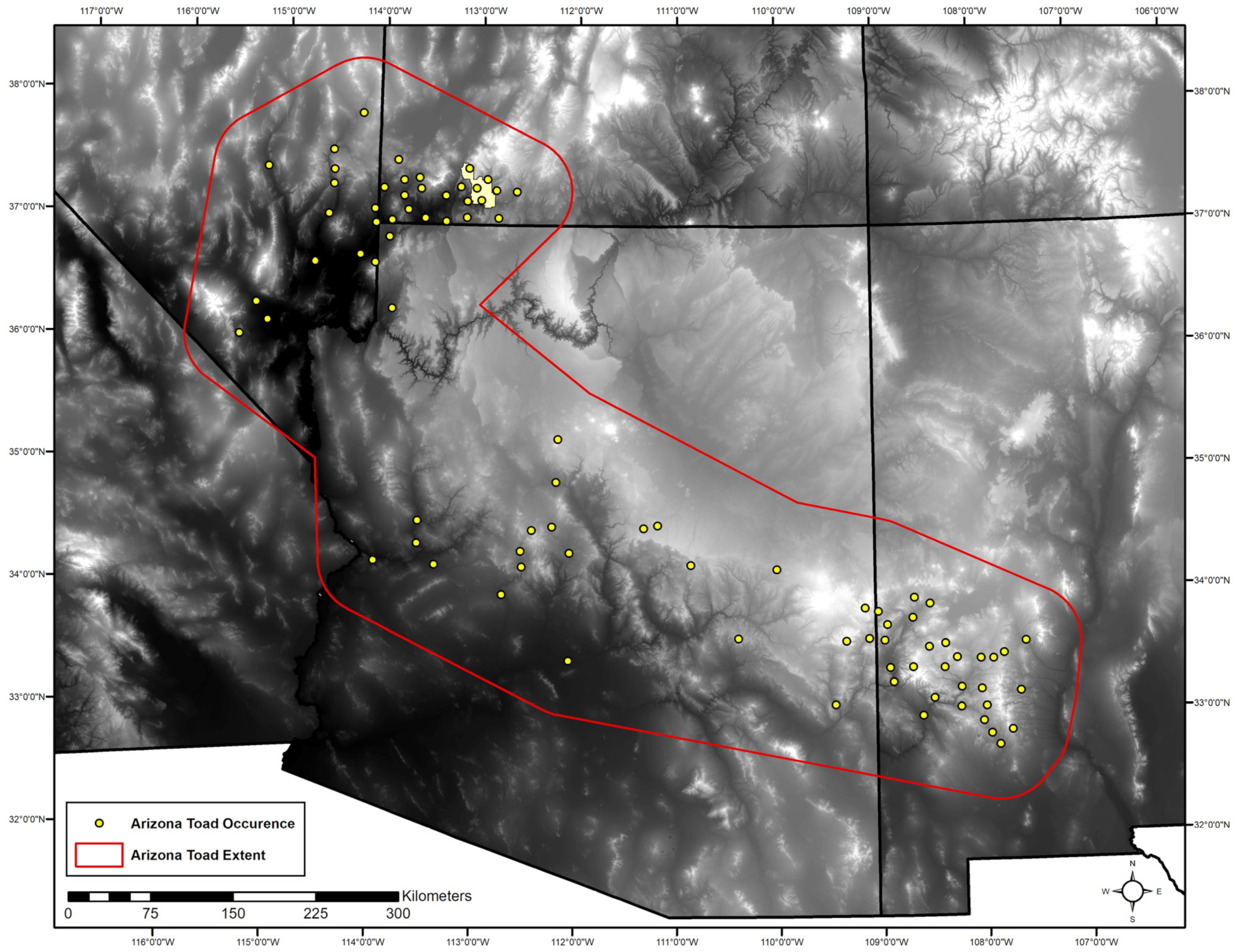
2.2.1. Occurrence Data
2.2.2. Spatial Scale and Resolution
2.2.3. Elevation and Remote Sensing Data
2.2.4. Climatic Data
2.2.5. Environmental Variables
2.3. Modeling Procedure
2.4. Environmental Variable Justification
2.4.1. Digital Elevation Model
2.4.2. Slope
2.4.3. Aspect
2.4.4. Terrain Ruggedness Index
2.4.5. Topographic Position Index
2.4.6. Normalized Difference Vegetation Index
2.4.7. Bare Soil Index
2.5. Model Evaluation
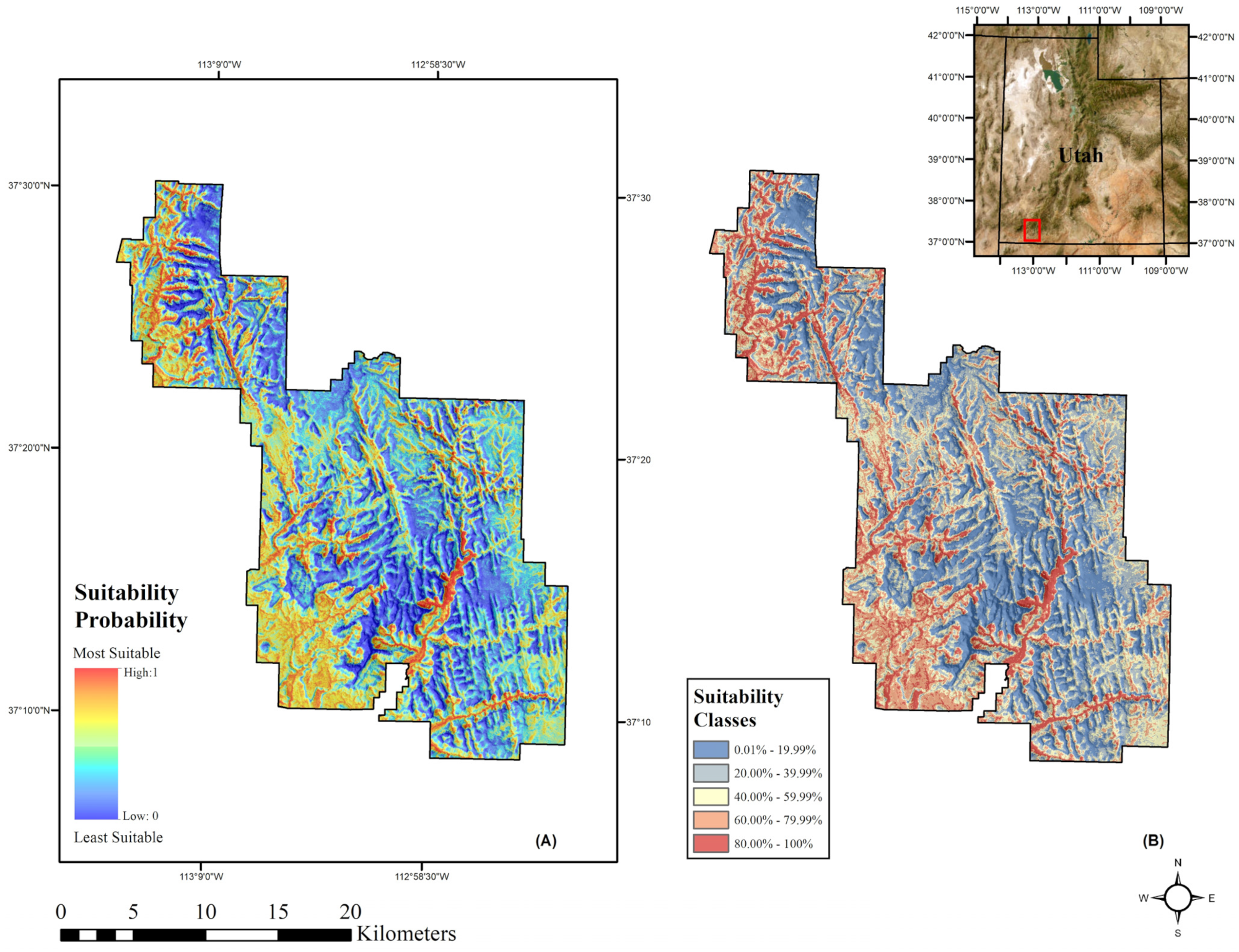
2.5.1. Forecasting
2.5.2. Model Assessment and Validation
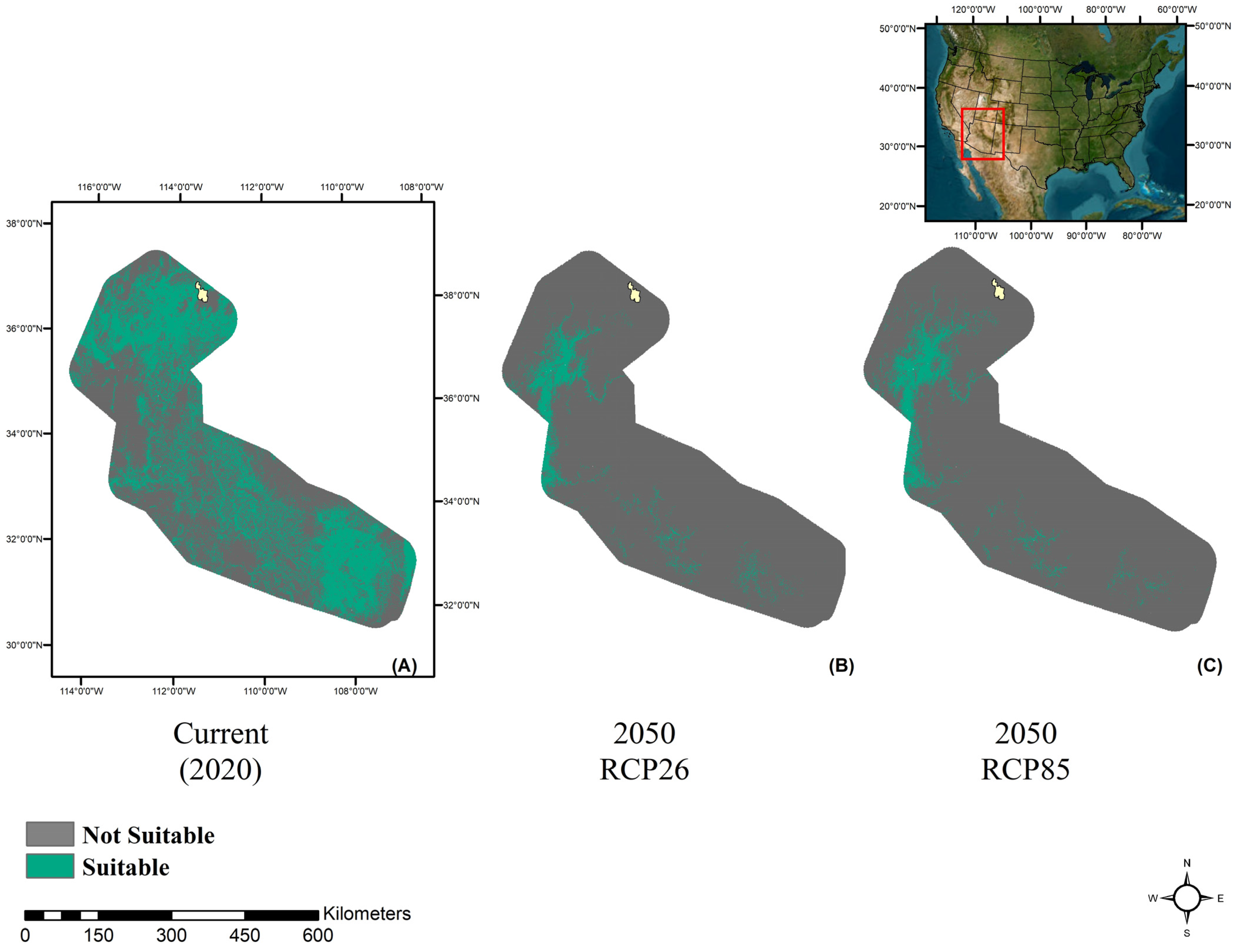
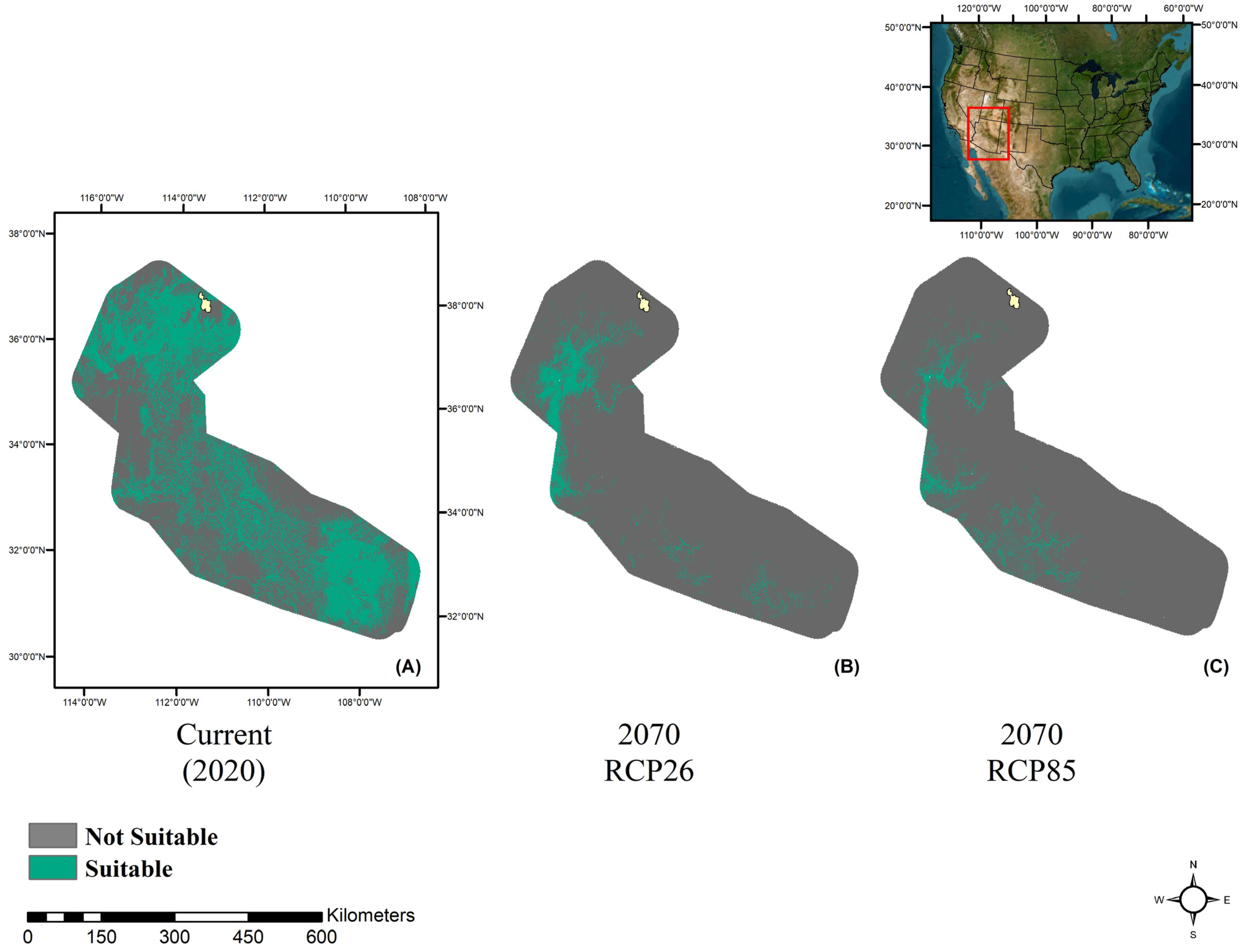
3. Results
3.1. Model Performance
3.2. Future Climate Trends
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raes, N. Partial versus Full Species Distribution Models. Nat. Conserv. 2012, 10, 127–138. [Google Scholar] [CrossRef]
- Addison, P.F.E.; Rumpff, L.; Bau, S.S.; Carey, J.M.; Chee, Y.E.; Jarrad, F.C.; McBride, M.F.; Burgman, M.A. Practical Solutions for Making Models Indespensible in Conservation Decision Making. Divers. Distrib. 2013, 19, 490–502. [Google Scholar] [CrossRef]
- Silva Angellieri, C.C.; Adams-Hosking, C.; de Barros Ferraz, K.M.P.M.; de Souza, M.P.; McAlpine, C.A. Using Species Distribution Models to Predict Potential Landscape Restoration Effects on Puma Conservation. PLoS ONE 2016, 11, e0145232. [Google Scholar] [CrossRef]
- Villero, D.; Pla, M.; Camps, D.; Ruiz-Olmo, J.; Brotons, L. Integrating species distribution modelling into decision-making to inform conservation actions. Biodivers. Conserv. 2016, 26, 251–271. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Pourhallaji, M.; Dargahi, M.D.; Kami, H.G.; Hakimiabed, M. Species distribution modeling and environmental suitability of the Southern crested newt, Triturus karelinii (Strauch, 1870) (Amphibia: Caudata) in Iran. J. Wildl. Biodivers. 2021, 5, 44–52. [Google Scholar]
- Fong, G.A.; Dávila, N.V.; López-Iborra, G.M. Amphibian Hotspots and Conservation Priorities in Eastern Cuba Identified by Species Distribution Modeling. Biotropica 2015, 47, 119–127. [Google Scholar] [CrossRef]
- Pineda, E.; Lobo, J.M. Assessing the accuracy of species distribution models to predict amphibian species richness patterns. J. Anim. Ecol. 2009, 78, 182–190. [Google Scholar] [CrossRef]
- Spiers, J.A.; Oatham, M.P.; Rostant, L.V.; Farrell, A.D. Applying species distribution modelling to improving conservation based decisions: A gap analysis of Trinidad and Tobago’s endemic vascular plants. Biodivers. Conserv. 2018, 27, 2931–2949. [Google Scholar] [CrossRef]
- Gerick, A.A.; Munshaw, R.G.; Palen, W.J.; Combes, S.A.; O’Regan, S.M. Thermal physiology and species distribution models reveal climate vulnerability of temperate amphibians. J. Biogeogr. 2014, 41, 713–723. [Google Scholar] [CrossRef]
- Rawien, J.; Jairam-Doerga, S. Predicted Batrachochytrium dendrobatidis infection sites in Guyana, Suriname, and French Guiana using the species distribution model maxent. PLoS ONE 2022, 17, e0270134. [Google Scholar] [CrossRef] [PubMed]
- Trainor, A.M.; Schmitz, O.J.; Ivan, J.S.; Shenk, T.M. Enhancing species distribution modeling by characterizing predator–prey interactions. Ecol. Appl. 2014, 24, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Battini, N.; Farías, N.; Giachetti, C.; Schwindt, E.; Bortolus, A. Staying ahead of invaders: Using species distribution modeling to predict alien species’ potential niche shifts. Mar. Ecol. Prog. Ser. 2019, 612, 127–140. [Google Scholar] [CrossRef]
- Peñalver-Alcázar, M.; Jiménez-Valverde, A.; Aragón, P. Niche differentiation between deeply divergent phylogenetic lineages of an endemic newt: Implications for Species Distribution Models. Zoology 2021, 144, 125852. [Google Scholar] [CrossRef] [PubMed]
- Rathore, M.K.; Sharma, L.K. Efficacy of species distribution models (SDMs) for ecological realms to ascertain biological conservation and practices. Biodivers. Conserv. 2023, 32, 3053–3087. [Google Scholar] [CrossRef]
- Laxton, M.R.; De Rivera, O.R.; Soriano-Redondo, A.; Illian, J.B. Balancing structural complexity with ecological insight in Spatio-temporal species distribution models. Methods Ecol. Evol. 2023, 14, 162–172. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Ricketts, T.H. The Matrix Matters: Effective Isolation in Fragmented Landscapes. Am. Nat. 2001, 158, 87–99. [Google Scholar] [CrossRef]
- Vrba, E.S. Ecology in relation to speciation rates: Some case histories of Miocene-Recent mammal clades. Evol. Ecol. 1987, 1, 283–300. [Google Scholar] [CrossRef]
- Dodd, C.K. Frogs of the United States and Canada; John Hopkins University Press: Baltimore, MD, USA, 2013; pp. 127–132. [Google Scholar]
- Blais, B.; Ryan, M.J.; Gustafson, G.; Giermakowski, J.T. Natural History Notes. Herpetol. Rev. 2016, 47, 436–438. [Google Scholar]
- Sullivan, B.K.; Lamb, T. Hybridization Between the Toads Bufo microscaphus and Bufo woodhousii in Arizona: Variation in Release Calls and Allozymes. Herpetologica 1988, 44, 325–333. [Google Scholar]
- Ryan, M.J.; Latella, I.M.; Giermakowski, J.T.; Snell, H.L. Final Report: Status of the Arizona Toad (Anaxyrus microscaphus) in New Mexico; New Mexico Department of Game and Fish: Santa Fe, NM, USA, 2015; 35p.
- Sweet, S.S. Initial Report on the Ecology and Status of the Arroyo Toad (Anaxyrus Microscaphus Californicus) on the Los Padres National Forest of Southern California, with Management Recommendations; Contract Report to USDA; Forest Service, Los Padres National Forest: Goleta, CA, USA, 1992; p. 198.
- Schwaner, T.D.; Sullivan, B.K. Fifty Years of Hybridization: Introgression Between the Arizona Toad (Bufo microscaphus) and Woodhouse’s Toad (B. woodhousii) Along Beaver Dam Wash in Utah. Herpetol. Conserv. Biol. 2009, 4, 198–206. [Google Scholar]
- Hammerson, G.; Schwaner, T.D. Anaxyrus microscaphus. In IUCN 2011 IUCN Red List of Threatened Species; Version 2011.2; IUCN: Gland, Switzerland, 2004. [Google Scholar]
- McNab, W.H.; Avers, P.E. Ecological Subregions of the United States; Section Descriptions; USDA Forest Service, Ecosystem Management: Washington, DC, USA, 1994; Volume 5.
- Sleeter, B.M.; Sohl, T.L.; Bouchard, M.A.; Reker, R.R.; Soulard, C.E.; Acevedo, W.; Griffith, G.E.; Sleeter, R.R.; Auch, R.F.; Sayler, K.L.; et al. Scenarios of land use and land cover change in the conterminous United States: Utilizing the special report on emission scenarios at ecoregional scales. Glob. Environ. Change 2012, 22, 896–914. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A Python-based GIS Toolkit for Landscape Genetic, Biogeographic, and Species Distribution Model Analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2006, 34, 102–117. [Google Scholar] [CrossRef]
- Anderson, R.P.; Raza, A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: Preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J. Biogeogr. 2010, 37, 1378–1393. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using Species Distribution Models to Predict New Occurrences for Rare Plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Hutchinson, M.F.; Xu, T. ANUSPLIN Version 4.4 User Guide; Fenner School of Environment and Society, Australian National University: Canberra, ACT, Australia, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 August 2020).
- Morgan, B.; Guénard, B. New 30 m resolution Hong Kong climate, vegetation, and topography rasters indicate greater spatial variation than global grids within an urban mosaic. Earth Syst. Sci. Data 2019, 11, 1083–1098. [Google Scholar] [CrossRef]
- Meineri, E.; Hylander, K. Fine-grain, large-domain climate models based on climate station and comprehensive topographic information improve microrefugia detection. Ecography 2017, 40, 1003–1013. [Google Scholar] [CrossRef]
- Sappington, J.M.; Longshore, K.M.; Thompson, D.B. Quantifying Landscape Ruggedness for Animal Habitat Analysis: A Case Study Using Bighorn Sheep in the Mojave Desert. J. Wildl. Manag. 2007, 71, 1419–1426. [Google Scholar] [CrossRef]
- Jenness, J. Topographic Position Index Extension for ArcView 3.x. Jenness Enterprises. 2006. Available online: http://www.jennessent.com/arcview/tpi.htm (accessed on 15 August 2020).
- Zhao, H.M.; Chen, X. Use of Normalized Difference Bareness Index in Quickly Mapping Bare Areas from TM/ETM+. Geosci. Remote Sens. Symp. 2003, 3, 1666–1668. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions. Version 3.4.1. 2017. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 15 August 2020).
- Esquivel, D.A.; Penagos, A.P.; García, R.S.; Bennett, D. New records of Pygmy Round-eared Bat, Lophostoma brasiliense Peters, 1867 (Chiroptera, Phyllostomidae), and updated distribution in Colombia. Check List 2020, 16, 277–285. [Google Scholar] [CrossRef]
- Stryszowska, K.M.; Johnson, G.; Mendoza, L.R.; Langen, T.A. Species Distribution Modeling of the Threatened Blanding’s Turtle’s (Emydoidea blandingii) Range Edge as a Tool for Conservation Planning. J. Herpetol. 2016, 50, 366–373. [Google Scholar] [CrossRef]
- Montgomery, B. Occupy ToadStreet: Occupancy and Habitat Use of the Arizona Toad in Streams of Arizona. Master’s Thesis, Arizona State University, Phoeniz, AZ, USA, 2023; 61p. [Google Scholar]
- De Albuquerque, F.S.; Bateman, H.L.; Ryan, M.J.; Montgomery, B. Model transferability and predicted response of a dryland anuran to climate change in the Southwest United States. J. Biogeogr. 2023, in press. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Grant, E.H.C.; Veran, S. Presence-only Modelling Using MAXENT: When Can We Trust the Inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1998, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Ayoro, J.H.; Nicolas, V.; Segniagbeto, G.H.; Hema, E.M.; Ohler, A.; Kabré, G.B. Potential impact of climate change on spatial distribution of two savannah amphibian species in West Africa. Afr. J. Ecol. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, Z.; Mammola, S.; Liang, Z.; Capinha, C.; Wei, Q.; Wu, Y.; Zhou, J.; Wang, C. Future climate change will severely reduce habitat suitability of the Critically Endangered Chinese giant salamander. Freshw. Biol. 2020, 65, 971–980. [Google Scholar] [CrossRef]
- Ryan, M.J.; Giermakowski, J.T.; Latella, I.M.; Snell, H.L. No evidence of hybridization between the Arizona toad (Anaxyrus microscaphus) and Woodhouse’s toad (A. woodhousii) in New Mexico, USA. Herpetol. Conserv. Biol. 2017, 12, 565–575. [Google Scholar]
- Thurtell, L.; Rajanathan, R.; Thomas, P.; Ballard, G.; Bayne, P.; Vernes, K. Predictively modeling the distribution of the threatened brush-tailed rock-wallaby (Petrogale penicillate) in Oxley Wild Rivers National Park, north-eastern New South Wales, Australia. Wildl. Res. 2022, 49, 169–182. [Google Scholar] [CrossRef]
- Liang, C.T.; Stohlgren, T.J. Habitat suitability of patch types: A case study of the Yosemite toad. Front. Earth Sci. 2011, 5, 217–228. [Google Scholar] [CrossRef]
- Giovannini, A.; Seglie, D.; Giacoma, C. Identifying priority areas for conservation of spadefoot toad, Pelobates fuscus insubricus using a maximum entropy approach. Biodivers. Conserv. 2014, 23, 1427–1439. [Google Scholar] [CrossRef]
- Ganesh, S.R.; Brihadeesh, S.; Narayana, B.L.; Hussain, S.; Kumar, G.C. A contribution on morphology and distribution of the Rock Toad, Duttaphrynus hololius (Günther, 1876) with first report on deformity, calling and breeding behaviours (Amphibia: Anura: Bufonidae). Asian J. Conserv. Biol. 2020, 9, 71–78. [Google Scholar]
- Guisan, A.; Brönnimann, O.; Engler, R.; Vust, M.; Yoccoz, N.G.; Lehmann, A.; Zimmermann, N.E. Using Niche-Based Models to Improve the Sampling of Rare Species. Conserv. Biol. 2006, 20, 501–511. [Google Scholar] [CrossRef]

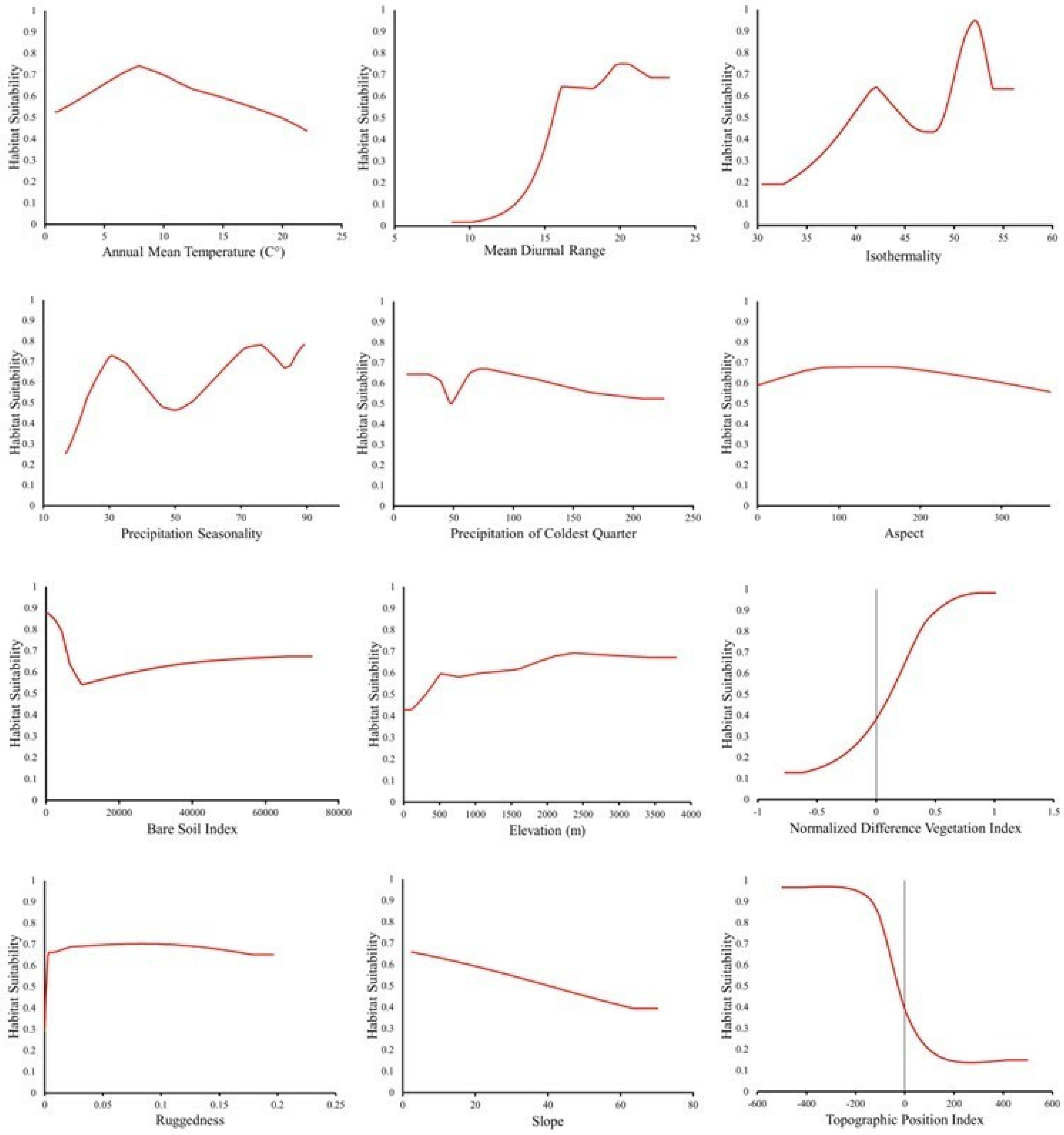
| Variable | Permutation Importance (%) |
|---|---|
| Annual Mean Temperature | 22.9 |
| Elevation | 22.7 |
| Isothermality | 11.5 |
| Normalized Difference Vegetation Index | 11.1 |
| Mean Diurnal Range | 11.0 |
| Precipitation Seasonality | 5.7 |
| Topographic Position Index | 3.5 |
| Slope | 3.2 |
| Precipitation of Coldest Quarter | 2.7 |
| Ruggedness | 2.4 |
| Aspect | 2.1 |
| Bare Soil Index | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driver, S.M.; Eversole, C.B.; Unger, D.R.; Kulhavy, D.L.; Schalk, C.M.; Hung, I.-K. Assessing the Impacts of Climate Change on the At-Risk Species Anaxyrus microscaphus (The Arizona Toad): A Local and Range-Wide Habitat Suitability Analysis. Ecologies 2023, 4, 762-778. https://doi.org/10.3390/ecologies4040050
Driver SM, Eversole CB, Unger DR, Kulhavy DL, Schalk CM, Hung I-K. Assessing the Impacts of Climate Change on the At-Risk Species Anaxyrus microscaphus (The Arizona Toad): A Local and Range-Wide Habitat Suitability Analysis. Ecologies. 2023; 4(4):762-778. https://doi.org/10.3390/ecologies4040050
Chicago/Turabian StyleDriver, Sam M., Cord B. Eversole, Daniel R. Unger, David L. Kulhavy, Christopher M. Schalk, and I-Kuai Hung. 2023. "Assessing the Impacts of Climate Change on the At-Risk Species Anaxyrus microscaphus (The Arizona Toad): A Local and Range-Wide Habitat Suitability Analysis" Ecologies 4, no. 4: 762-778. https://doi.org/10.3390/ecologies4040050
APA StyleDriver, S. M., Eversole, C. B., Unger, D. R., Kulhavy, D. L., Schalk, C. M., & Hung, I.-K. (2023). Assessing the Impacts of Climate Change on the At-Risk Species Anaxyrus microscaphus (The Arizona Toad): A Local and Range-Wide Habitat Suitability Analysis. Ecologies, 4(4), 762-778. https://doi.org/10.3390/ecologies4040050