Abstract
This study examines the spatio-temporal dynamics of Acacia auriculiformis in Lubumbashi city, southeastern Democratic Republic of Congo, in the context of rapid urbanization following the liberalization of the mining sector. The city has experienced significant demographic growth and unplanned spatial expansion, resulting in a decrease in vegetation cover. The introduction and proliferation of A. auriculiformis, an exotic tree species, have occurred without strategic planning or monitoring. Utilizing digitized remote sensing imagery from 2006, 2014, and 2021, we quantified the expansion of A. auriculiformis along the urban–rural gradient. Additionally, a floristic inventory conducted in 2021 provided insights into tree diversity within A. auriculiformis plantations. Our findings indicate a substantial increase in the number and area of A. auriculiformis patches, predominantly in urban zones. However, the patch values, highest in 2006, were shown to decline by 2021, especially in urban areas. The floristic inventory identified 39 tree species within A. auriculiformis plantations, including predominant species such as Albizia lebbeck, Albizia alba, and Leucaena leucocephala. Notably, 20 of these species are exotic, with half being invasive. In contrast, the 19 indigenous species were primarily found in peri-urban areas. While a greater number of tree species were observed in urban zones, larger average diameters were recorded in peri-urban zones. The persistence and expansion of A. auriculiformis in a landscape characterized by declining tree cover suggest its potential sustainability in this setting. However, A. auriculiformis plantations have facilitated the establishment of predominantly exotic and potentially invasive species. These findings highlight the need for the strategic management of A. auriculiformis and associated exotic flora to mitigate their spread and to consider their role in the restoration of degraded lands.
1. Introduction
More than half of the world’s population now lives in cities, and by 2050 this is expected to increase by about 3 billion people [1]. Accordingly, 90% of this growth will be concentrated in Africa and Asia, where urbanization rates are expected to be around 56% and 64%, respectively. Landscape transformations due to urban development, which occurred in the northern hemisphere in the 20th century [2], currently dominate the landscape dynamics of southern countries [3,4,5], particularly due to rural–urban migration and the intrinsic demographic changes of the urban population itself [3]. This situation is particularly crucial on the African continent [6], where several generations of urban development master plans have proved to be completely inadequate for the extent of demographic growth and the economic fragility of the population [7]. In particular, the situation is worrying in sub-Saharan Africa, where the urban population is expected to grow fivefold from 200 million in 2000 to one billion in 2050, while the urbanized area is expected to increase twelvefold from ±26,500 km2 in 2000 to ±325,500 km2 in 2050 [3]. Increasing urban land cover is expected to have profound effects on local and global species’ diversity patterns [4]. Consequently, the future of vegetation is thus largely dependent on the management of the spatial pattern of cities [8], which are constantly expanding in peripheral rural areas [9]. In response, decision-makers, planners, and scientists are focusing their efforts in and around cities to restore the remnants of natural diversity. Indeed, as the restoration of the natural landscape in urban areas receives considerable attention, urbanization is promoting the creation of novel ecosystems where alien plant species are being introduced [10] to provide, augment, or restore specific ecosystem services [11]. Yet, cities are the sources of non-native species’ invasions into the natural habitats of the surrounding suburban and rural areas [12]. For example, in Singapore, nine invasive alien species (Acacia auriculiformis, Cecropia pachystachya, Falcataria moluccana, Leucaena leucocephala, Manihot carthaginensis subp. glaziovii, Muntingia calabura, Piper aduncum, Pipturus argenteus, and Spathodea campanulata) are dominant in all stages of plant succession on most of the abandoned land, where they have developed a dense canopy forest dominated by invasive species [13]. The presence of alien species may negatively alter ecosystem functions, reduce the flows of ecosystem services, and reduce native biodiversity, including in surrounding natural areas [14]. Ultimately, this hurts local economies and human well-being [15].
Invasive species are under-studied in sub-Saharan Africa compared to other parts of the world [10,16], with the spatial expansion of invasive alien plants and its impact on the phytodiversity receiving little recent attention in the literature. The south-eastern part of the DRC, which has some of the largest copper and cobalt deposits in the world [17,18], is no exception to this trend [19]. The exploitation of these deposits is prone to damaging the land cover, as the greater fragmentation and less diverse vegetation observed in the vegetation are generally found on soils polluted by atmospheric deposits resulting from mining activities. However, the natural ecosystems within and around Lubumbashi city are also modified to fit the needs of the human population, like housing, energy, agriculture [20,21]. In this area, Lubumbashi city, which was created ex nihilo in a formerly rural area within a miombo woodland, represents an interesting case study for the anthropization of natural landscapes [22,23]. The city has been strongly modified by mining activities and through strong industrialization of the landscape [24], attracting people from the surrounding rural areas in search of gainful employment [25]. Rapid population growth has led to various anthropogenic effects, including deforestation [24], and rapid and poorly planned spatial urban growth after the country’s independence in 1960 [19,24]. Within 25 years (1984–2009), Lubumbashi City experienced a drastic rise in its spatial expansion rate from 100 ha to 500 ha [24].
Although the deforestation around the city was accelerated as of 1960, after the country’s independence, it began as soon as the city was created in 1910 and has continued ever since, following the demographic growth commonly associated with an increased consumption of charcoal and agricultural products [22]. As a result, a 70% loss of forest was noted within a 25 km radius of Lubumbashi city between 1956 and 2009 [24]. Within the city, the set of admirable green spaces that were once carefully maintained have been neglected and replaced by anthropogenic land cover/use [22], with numerous harmful effects on the environment. Indeed, urbanization and industrialization are therefore accompanied by a strong presence of bare soil in the urban and peri-urban areas of Lubumbashi city. However, green spaces, which correspond to surfaces covered with vegetation [26], contribute to the purification of air and water, the treatment of waste, the regulation of the microclimate, etc. [27]. In addition, their presence also provides people with aesthetic pleasures, recreational opportunities, and physical and psychological well-being [28].
In the context of Lubumbashi city, the spread of invasive species is directly encouraged by human activity, through the deliberate and accidental movement of plants. Particularly, invasive species are spread through the creation of green spaces planted with exotic and fast-growing trees, like A. auriculiformis. A. auriculiformis is an alien invasive species of the Fabaceae family with creamy yellow fragrant flowers that tolerates many types of soil, including degraded lands. A. auriculiformis has a high N2-fixing potential, given its excellent nodulation, observed in many soils [29]. The species is known for its high litter content, which can be used for soil fertility enhancement, and its prunings have also been used in alley cropping as a biofertilizer [30].
Introduced into Lubumbashi city in early 2000, it is considered a beneficial nurse crop used as an ornamental plant, shade tree, and as firewood [31]. Although the urban and peri-urban woody green spaces of Lubumbashi city are dominated by miombo woodland species, A. auriculiformis has remained the most common species [32]. Within the landscape of Lubumbashi city, A. auriculiformis is often used to compensate for the loss of native forests, to restore disturbed minelands or other types of wasteland [33]. However, Richardson et al.’s [34] findings revealed the reduction in the species richness of indigenous plants as one of the major problems associated with the presence of dense stands of invasive alien trees and shrubs in the Fynbos Biome (South Africa), but this aspect is not understood, as far as A. auriculiformis stands, in Lubumbashi city. Yet, there are no studies that have investigated the change in landscape pattern due to its expansion, and its impact on plant diversity since its introduction in Lubumbashi. Likewise, a greater understanding of the spatial expansion and the ecological role of alien species might help to reduce controversy surrounding their purposeful use in restoration [35,36], as recently documented by Watanabe et al. [37], who advocate for the use of native species (Brachystegia spiciformis, Combretum collinum, and Pterocarpus tinctorius) in the restoration of land degraded by human activities, instead of Acacia or Eucalyptus.
With the recent development of remote sensing images coupled to GIS and landscape ecology, the spatial expansion of plant communities can be mapped and quantified [37]. The expansion of plant communities that modify the ecological functioning of landscapes can be highlighted by an assessment of the properties of landscapes and the ecosystem services they provide [38,39], particularly through biodiversity analysis [40]. The present study characterizes the spatial evolution and the phytodiversity in A. auriculiformis plantations along the urban–rural gradient. We hypothesized that the positive anthropization of the landscape through the creation of A. auriculiformis plantations could be accompanied by an increase in the patch number as well as area, either in urban or peri-urban zones, promoting the establishment of mostly exotic plant species.
2. Materials and Methods
2.1. Study Area: The Urban Zone and Peri-Urban Zone of Lubumbashi City
Lubumbashi city (Figure 1) covers an area of nearly 747 km2 (11°27′–11°47′ S and 27°19′–27°40′ E) at an altitude varying between 1200 and 1300 m. The climate is Cw type of the Köppen classification system [41], with a dry season (May to September), a rainy season (November to March), and two transition months (April and October).
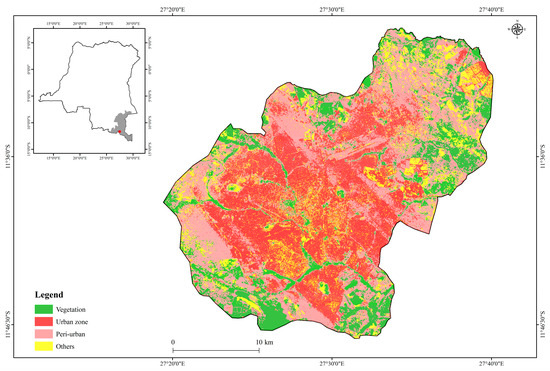
Figure 1.
Geographical location of the study area, the city of Lubumbashi (The red dot on the map above) in the Haut-Katanga province, the Democratic Republic of the Congo. The land cover map of the city of Lubumbashi was created from a Landsat image taken in July 2021. The urban zone occupies the central part of the town and is surrounded by an aureole formed by the peri-urban zone. The vegetation in these two zones is very sparse.
In the second half of the last century, the average annual temperature was around 20.1 °C [41], though recent reports indicate ongoing warming [42]. In the dry season, winds from the Indian Ocean generally prevail, blowing in the east-southeast and east-northeast directions, which accelerates the spread of fumes from mining companies, and contributes to the pollution of the city [43]. The soils of the city are very ferralitic [41]. Natural wooded vegetation, currently in a fragmented state, is located several kilometers away from the city [24]. The population is around 2.5 million and is mainly engaged in agriculture, residential livestock, services, mining, and trade [44].
2.2. Exploratory Visits and Spatial Analysis
Exploratory field visits were carried out from 1 to 19 June 2021 to locate A. auriculiformis plantations within the administrative boundaries of the city of Lubumbashi, considered to be areas with a collection of A. auriculiformis trees planted either by the public authority or by private individuals. In each municipality of Lubumbashi city, we communicated with local authorities to present the objectives of the study to the local authorities and seek their permission to undertake the study. The person in charge of administration or an influential member of the community was then instructed by the leaders to establish and deliver a list and the addresses of all A. auriculiformis within their jurisdictions. The listed plantations have been visited based on local knowledge and located using a GPS Garmin 64st (±3 m precision). In addition, for each plantation visited, its position in the urban–rural gradient was given using the decision tree of [9], which is based on the morphological characteristics of urbanization (proportion, density, and continuity of the built-up). Accordingly, the urban zone is characterized by densified and continuous built-up areas while the discontinuity of the otherwise less dense built-up areas characterizes the peri-urban zone.
This study utilized high-resolution imagery from Google Earth, captured in July of 2006, 2014, and 2021. These intervals represent around 5, 13, and 20 years post the introduction of A. auriculiformis in the study area, respectively. Google Earth provides a comprehensive combination of satellite and aerial imagery, predominantly from the past decade, enabling detailed terrestrial visualization [45]. Lubumbashi city, as a focal economic hub, benefits from comprehensive coverage in Google Earth’s database. This facilitates accurate land cover and land use analysis, essential for monitoring urban and environmental changes. The ESRI ArcGIS software (version 10.5) suite was employed for cartographic representation. The spatial resolution of Google Earth imagery was meticulously considered, taking into account both the geographical coordinates and the specific year of image capture [46]. For this study, individual A. auriculiformis trees situated within residential areas and rural settings—areas minimally affected by urbanization—as well as plantations smaller than 250 square meters, were excluded from the analysis. In contrast, larger plantations of A. auriculiformis were digitized from the Google Earth imagery and subsequently converted into vector formats using the ESRI ArcGIS software (version 10.5). To quantitatively assess the distribution pattern of A. auriculiformis across the urban–rural gradient, three key landscape metrics were computed: the total class area (CA), rate of change (RC), and patch number (PN). These indices are critical in evaluating the anthropogenic influences on landscape patterns [47].
2.3. Floristic Data Collection and Analysis
To determine species richness, plots of 100 m2 were established on each A. auriculiformis plantation and the number of plots was adapted to the area of each plantation. A total of 27 plots, including 13 plots on a single peri-urban plantation and 14 plots in urban plantations (2–4 per plantation), were studied from July to August 2021. It has been noted that for species richness determination, Liang et al. [48] suggest plots of 1 m2, 4 m2, and 100 m2 for herbaceous species, shrubs, and trees, respectively. This period of floristic inventory corresponds to the dry season in the Lubumbashi plain, which is why the herbaceous plants which develop favorably during the rainy season have not been studied. Indeed, many herbaceous plants in the Lubumbashi plain spend the unfavorable period, the dry season, in a state of organ regeneration (seeds, tubers, stumps, rhizomes). Herbaceous flora has not been studied as it is more sensitive to disturbance (i.e., droughts) and even edaphic variation than woody flora [49]. However, the tree age was estimated based on its stage of development (seedling and adult). Then, the average height and circumference at 1.30 m above the ground of adult A. auriculiformis trees were estimated and measured, respectively. The data collected made it possible to define the species richness with a floristic list of plant species present in a site.
In terms of the identification of plant species, some species were identified using our knowledge of plant systematics and others using available flora [50]; specialized literature [10,51] was also utilized. The origin status of species was determined. Thus, exotic species were considered species that were not indigenous to Africa; while Afro-Asian species were considered indigenous [10]. Among these exotic species, the biological invasion status of each species was assembled from online databases and specialized literature [52,53,54,55,56]. According to Meerts et al. [57], an alien invasive species is a non-native, naturalized species, showing a rapid expansion dynamic in its territory of introduction. The status of native miombo species was specifically determined based on the checklists established by [58]. For each identified species, a specific abundance and specific frequency were calculated. The specific abundance was defined as the number of individual trees for each species, while the specific frequency was considered to be the number of sites where the species was present [59]. Indeed, the relative frequency was calculated as the ratio between the frequency of the species and the total number of sites surveyed [60]. It should be noted that abundance and frequency were used to calculate diversity indices such as Simpson’s index and Shannon’s index using Paleontological Statistics software (PAST version 4.03). Shannon’s index is believed to emphasize the richness component of diversity, and Simpson’s index to emphasize the evenness component [61].
3. Results
3.1. The Mapping and Spatial Pattern of A. auriculiformis Plantations
It was revealed from the spatial pattern dynamics analysis that there was an increase of about 30% in A. auriculiformis plantations from 2006 (12 plantations) to 2021 (17 plantations). In terms of surface area, two tendencies were noted. A twofold area increase (15.28 ha to 29.38 ha) of A. auriculiformis plantations was observed between 2006 and 2014, followed by a slight decrease of about 5% (29.38 ha to 27.89 ha) in the period from 2014 to 2021 (Table 1).

Table 1.
Types of green space covered by Acacia plantations in the urban and peri-urban zones of the city of Lubumbashi from the digitization of Google Earth images from 2006, 2014, and 2021. PN: patch number; CA: class area (ha); PZ: peri-urban zone; UZ: urban zone; and RC: rate of change (%). These area values correspond to the total area covered by A. auriculiformis plantations.
Analysis along the urban–rural gradient reveals an increase in the number of A. auriculiformis plantation patches from 11 (2006) to 14 (2014) and from 14 (2014) to 15 (2021) in urban areas. Meanwhile, the area occupied by A. auriculiformis plantations almost doubled from 13.68 ha in 2006 to 21.97 ha in 2021. In the peri-urban zone, the period of 2006–2014 was characterized by an increase in the patch number, which tripled, while the class area increased fivefold. In the following period (2014–2021), the patch number of A. auriculiformis plantations decreased from 3 to 2. Over the same period, the class area was reduced by about 31% (Table 1 and Figure 2). In addition, it is important to note that the rate of land use change by A. auriculiformis plantations in the urban area has continued to increase significantly over time. Between 2006 and 2014, this rate was 52.33 and, between 2014 and 2021, it rose to 113. These figures demonstrate the continued expansion of A. auriculiformis plantations in the urban area. As for the peri-urban area, the rate of change in land use was positive, at a high rate of 433.75 between 2006 and 2014, also indicating a significant increase in A. auriculiformis plantations. However, between 2014 and 2022, this rate became negative, at −30.68, suggesting a reduction or decline in the presence of A. auriculiformis plantations in the peri-urban area. These results highlight an overall expansion of A. auriculiformis plantations in the landscape studied, particularly in the urban zone.
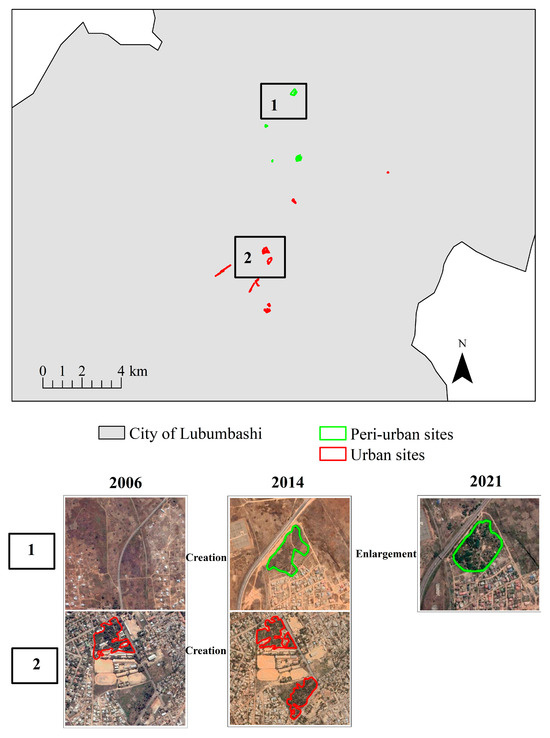
Figure 2.
Maps of the evolutionary trends in the area and the number of patches of A. auriculiformis plantations in urban and peri-urban zones of the city of Lubumbashi from the digitization of Google Earth images from 2006, 2014, and 2021, according to Bogaert et al. [47].
These results also show that the A. auriculiformis plantations belong to three green space types: parks, street trees, and attached green spaces. Parks and street trees showed a simultaneous increase in their patch number and in their class area. By contrast, in the case of parks, the number of patches doubled from 1 in 2006 to 2 in 2021, while the area increased 39-fold from 0.14 ha to 5.41 ha in the same period. As for street trees, the number of patches and the class area have, respectively, doubled and tripled. Indeed, the number of patches almost doubled from 3 in 2006 to 5 in 2021, while the class area increased by about threefold from 1.21 ha to 3.62 ha in the same period. Conversely, although the patch number of attached Acacia and its class area increased by 20% and 40% from 2006 to 2014, respectively, a slight decrease (18%) in its class area was recorded from 2014 to 2021 (Table 1).
3.2. Flora Richness and Diversity in the Plantations of A. auriculiformis along the Urban–Rural Gradient of Lubumbashi City
The data on floristic spectra revealed a total of 39 species in the plantations of A. auriculiformis, with 29 species in the urban zone and 10 in the peri-urban zone. Slightly higher diversity index values were recorded in the urban zone compared to the peri-urban zone (Table 2). Moreover, Table 3 shows that the proportions of adult trees are higher than those of seedlings in the A. auriculiformis plantations in both urban (94.2% against 5.8%) and peri-urban plantations (89.3% against 10.7%). However, no significant variation (p > 0.05) was found regarding height and tree diameter in the studied zones of the urban–rural gradient (Table 4).

Table 2.
Diversity of trees and shrubs in A. auriculiformis plantations in the urban and peri-urban zones of Lubumbashi city. PZ: peri-urban zone; UZ: urban zone.

Table 3.
Proportions of age (adult and seedling) of A. auriculiformis trees in plantations in urban and peri-urban areas. PZ: peri-urban zone; UZ: urban zone.

Table 4.
Average height and average diameter of A. auriculiformis trees in urban and peri-urban plantations in Lubumbashi. PZ: peri-urban zone; UZ: urban zone. T= student’s t-test, ns = p > 0.05.
Table 5 shows that 39 species were inventoried across all A. auriculiformis plantations in Lubumbashi. A. auriculiformis plantations in the urban zone revealed 29 species, while the plantations in the peri-urban zone revealed only 20 species. Naturally, A. auriculiformis was the most abundant species in the plantations of the urban and peri-urban zones, with a proportion of 46.6% and 45.5%, respectively, while the other species had abundance proportions of less than 5%, except L. leucocephala, which had an abundance of 30.6% in the peri-urban zone. Moreover, the relative frequency was 100% for A. auriculiformis in all plantations. This is followed by A. lebbeck, A. alba, and L. leucocephala, with 50% frequencies in the plantations of the urban zone, while the remaining species represented 25% of flora. In the A. auriculiformis plantations of the peri-urban zone, species showed a relative frequency of 50%. The results also showed that 20 species out of the 39 species found were exotic (51.3%) and only 19 species were indigenous (48.7%). It should be noted that 18 of the 19 indigenous species inventoried are characteristic of the miombo woodland (94.7%), all except E. guineensis. Furthermore, our results show that 10 of the 20 exotic species recorded have been reported in the literature as invasive species, namely Acacia melanoxylon, Acacia heterophylla, Acacia auriculiformis, Acacia mangium, Melia azedarach, Leucaena leucocephala, Callistemon viminalis, Eucalyptus camaldulensis, and Eucalyptus globulus.

Table 5.
Relative abundance (RA), relative frequency (RF), species status, and origin of species in A. auriculiformis plantations in the urban zone (UZ) and peri-urban zone (PZ) of Lubumbashi city. Ex: exotic species; In: indigenous species. Species preceded by * are characteristic of the miombo woodland. RA is calculated as the ratio of the total number of individuals of a given species to the total number of individuals of all species in the plantations. RF is the ratio of the total number of observations of the species to the total number of investigated plantations. + Invasive species according to [34,52,53,54,55].
These species are principally located in the peri-urban zone. All species inventoried belong to 15 families, the Anacardiaceae, Apocynaceae, Arecaceae, Ebenaceae, Fabaceae, Kirkiaceae, Lamiaceae, Lauraceae, Meliaceae, Mimosaceae, Moraceae, Myrtaceae, Phyllanthaceae, Pinaceae, and Rhamnaceae. In the A. auriculiformis plantations of the urban zone and peri-urban zone, the Fabaceae family predominated with, respectively, 37.9% and 50% of the species abundance, followed by the Mimosaceae family.
4. Discussion
4.1. Spatial Pattern Dynamics of A. auriculiformis and Its Impact on Phytodiversity
In Lubumbashi city, a thorough diagnosis of governance management has revealed unclear role attributions among different services in this city (municipality, province, state/division of housing, urban planning, land registry, roads/technical concessionaires), resulting in competence conflicts [62]. Moreover, a systematic lack of public budgeting has been noted, particularly for equipment and maintenance costs, especially regarding public green spaces. On the overall scale of the city, unplanned urbanization is accompanied by a dramatic regression of green spaces, through the densification of buildings and peri-urbanization, in which new housing estates are created [19]. Useni et al. [22] reported that the built-up area tripled from 94.14 km2 to 291.31 km2 between 1989 and 2014 in the city of Lubumbashi, while green areas diminished from 575.27 km2 to 484.64 km2 during the same period, with the most concerning loss being the cover of public green spaces. Further, GROUPE HUIT [62] concluded that there is virtually no provision for green spaces in most neighborhoods, with green spaces systematically destroyed to make way for constructions, notably fuel stations, mostly for those located at crossroads. This situation is exacerbated by the policy of land speculation, which means that even vacant, untended green spaces have a high monetary value when they are divided up and sold as plots. The populations in search of a space to erect buildings care little about the sustainability of green spaces [22], probably due to their lack of knowledge of the various functions performed by them. To get around this bleak situation, in certain sectors of life, the population has begun to organize what the ‘bankrupt’ state cannot do, through solidarity networks and neighborhood, professional, and even religious associations. It is in this context that A. auriculiformis plantations have been created to compensate for the loss of green spaces. As these plantations are often located in private areas, they enjoy the security of tenure and are thus protected from urbanization pressure. Jim [63] reported that formal green spaces are usually well protected. On the other hand, public green spaces, especially parks, mostly belonging to the state, are easily converted into other land cover/uses because they are not legally secure.
A visual analysis of change maps in the study areas showed an expansion of A. auriculiformis plantations, most noticeable in the urban area between 2006 and 2021, probably due to human preferences and for ornamental purposes. Indeed, the urban flora constitutes a subset of the species pool after passing through several filters, including the strong influences of private and public landowners’ preferences [64]. Due to its rapid growth, A. auriculiformis is used to establish plantations; as reported by Williams et al. [65], in the absence of human disturbance, it takes nearly 40 years for native miombo species to reach the adult stage. Nevertheless, compared to the peri-urban zone, the number of A. auriculiformis patches in the urban zone is reported to be higher. Due to limited budgets, the attention of public services is mostly drawn in the urban zone to increase its visibility [66], justifying the increased number of parks and street trees planted with A. auriculiformis.
On the other hand, most of the public service buildings around which A. auriculiformis plantations are located occur in urban zones. The almost complete absence of public infrastructure in peri-urban areas has already been reported in Central Africa [66]. The increase in the patch number of A. auriculiformis plantations is accompanied by an increase in their area. This is not only due to the growth of the tree crown over time, but also to the densification of the plantation as the consequence of the development of new plants from seeds. Janzen [67] and Connell [68] hypothesized that specific seed and seedling viabilities depend on their distance to the parent plant or the density of young individuals. Mortality, due to pathogens and predators, modifies the initial distribution of seeds and leads to maximum recruitment at an intermediate distance from the parent plant [69]. It should be noted, however, that within the concessions of private or public landowners, the security of tenure also contributes to the easy expansion of A. auriculiformis trees. In this context, land insecurity could be a threat to the ecological restoration project within the city, particularly in the peri-urban zone, which is a dynamic zone that is characterized by rapid change because of an extension of the city and its associated infrastructures [70].
Although the regression of green space coverage has already been revealed in Lubumbashi city [22], our results showed an increase in the acreage of introduced patches of green space planted with A. auriculiformis, which follows the theory of patch origin [71]. Although progress is being made, it still appears that this landscape restoration with A. auriculiformis will not succeed in compensating for the loss of natural patches. This is in line with the findings of Toyi et al. [72], since exotic and potentially invasive species are used for this purpose, resulting in the ecosystem’s damage, particularly when juxtaposed with native forests. Moreover, there is no longer sufficient space to implement large-scale reforestation programs in the urban zone and peri-urban zone of Lubumbashi city [62]. More plant species are accompanying A. auriculiformis in the urban zone than in the peri-urban zone, particularly exotic species, leading to high plant diversity. The process of urbanization has greatly transformed the landscape of Lubumbashi and has created heterogeneous urban vegetated lands with new environmental conditions, leading to an installation of exotic species [19]. However, the older plantations in the urban zone would explain this, as sites planted with A. auriculiformis have been recently developed in the peri-urban zone. This corroborates the results of Elliott et al. [73], which revealed a much higher species richness for ground flora in a 28-year-old forest compared to a 1-year-old field in the southern Appalachian watershed. In addition, the environmental conditions specific to the urban area (high temperatures, low water availability) constitute a filter that would have eliminated species not adapted to these conditions [74]. Indeed, urbanization mostly excludes species (native and non-native) with limited dispersal capacity and selects species capable of long-distance dispersal [64]. Since most of the native tree felling left the stumps, it appears that the presence of some miombo woodland species under A. auriculiformis plantations could be explained by stump regeneration, especially in the peri-urban zone. Indeed, a few years later they would have regenerated from the stumps. Several authors have shown that the stumps of miombo woodland species regenerate when there is no disturbance [75,76].
The nature of the region’s economy, with a majority of poor populations, has a bearing on the popularity of Acacia or its receptiveness to the detriment of native species. Acacia does not require a great deal of labor to maintain its trees. Due to its ability to fix atmospheric N, over 350 ha of A. auriculiformis agroforestry plantations have been established on the outskirts of Lubumbashi, with 133 agroforestry farming families growing maize in association with it. Properly maintained, a half-hectare plot of A. auriculiformis can provide around 12 tons of charcoal after ten years [77]. On the other hand, it is known to be invasive [78], leaving the possibility for other species, more often invasive, to establish themselves. This was confirmed by our results, which indicate that half of the identified alien species are invasive and echo the hypothesis of ecological facilitation [79]. The results of the present study show an expansion of A. auriculiformis plantations in Lubumbashi. This is good news on the one hand, given the capacity of this species to improve edaphic and carbon storage factors. The ability of A. auriculiformis fallows to improve soil fertility has been demonstrated in the Bateke Plateau (D.R. Congo), notably through significant increases in organic carbon content, total nitrogen content, cation exchange capacity and the sum of base cations, OM content, and the soil pH in A. auriculiformis plantations [80]. Similarly, Kasongo et al. [81] demonstrated higher phosphorus levels under A. auriculiformis than in natural forests; Nsombo et al. [82] demonstrated a high carbon stock in A. auriculiformis plantations in forests in southern Benin. All these are important indicators in the current context of the pronounced regression of the miombo woodland in the Lubumbashi lowlands [19].
However, the expansion of A. auriculiformis plantations could determine a progressive loss of floristic identity if the management of these plantations is not assured, since this species is classified as potentially invasive [83]. Already, Gnahoua and Louppe [84] have reported a dominance of more than 85% of the soil cover by A. auriculiformis at the expense of other species in the classified forests of Ouèdo in Benin. The results of the floristic inventories carried out in this study show that native species are established in A. auriculiformis plantations. However, the ecological impacts of A. auriculiformis plantations are still unclear in the Lubumbashi region and in DR Congo. A report on the potential risks of A. auriculiformis plantations in the Bateke plateau was prepared by Hardarson et al. [78]. Based on their preliminary findings, the most important potential risks associated with non-native Acacia plantations were invasion of the species, the depletion of groundwater reserves, and reduced soil productivity. Indeed, with a trend towards increasing the area planted with A. auriculiformis, there could also be a risk to groundwater resources. Some exotic species can release chemical substances or modify the soil composition, thus altering groundwater quality. In addition, they can consume large quantities of water, reducing groundwater recharge. Also, the roots of exotic plants can modify the soil structure, affecting the soil’s capacity to retain water [85].
The difference in plant diversity under A. auriculiformis plantations also results from the fact that urban landscapes have a different species composition of introduced species, following the landscape-divergence hypothesis. Fragments within the same landscape tend to converge in species composition, whereas those in different landscapes diverge in composition [86]. Accordingly, the highest plant diversity noted in the urban zone could be due to local-level landscaping aesthetics and socioeconomic characteristics, acting as dominant bottom-up anthropogenic forces [87]. The propagation of similar plants or landscape elements in neighborhoods is indeed due to the actions of residents that copy, adapt, exchange plants, and suggest ideas, a phenomenon referred to as the “neighbor mimicry effect” [88].
A. lebeck is one of the most abundant species accompanying A. auriculiformis. The choice of this species in the vegetation of disturbed areas is due to its adaptability and rapid growth as well as its high capacity to produce seeds to generate other trees [89]. However, these characteristics can increase the invasiveness of the species. Furthermore, some introduced species in cities have high invasiveness [14] due to their prolific seed production, widely dispersed seeds, and nitrogen-rich and warm habitat preference, making them able to establish and thrive along edges [90]. However, A. auriculiformis trees are larger in size and stem diameter in the peri-urban zone due to the lower plant diversity, which limits interspecific competition [91].
4.2. Implications for the (Peri-)Urban Landscape’s Ecological Restoration
A. auriculiformis appears to be highly sustainable as a species in this landscape, in that it has persisted and spread in a landscape with degrading tree cover resources. In addition, A. auriculiformis plantations promote the establishment of species that are, however, mostly exotic and potentially invasive, with the risk of further degrading surrounding ecosystems. However, the peri-urban area of Lubumbashi where A. auriculiformis plantations are present constitutes an edge between urban and rural areas [92]. It is therefore possible that the species escapes from the peri-urban area and colonizes the adjacent rural area, where the vegetation still retains a certain level of naturalness [23]. This is not without socio-environmental problems. In rural areas, invasive species also harm livelihoods and increase human vulnerability through encroaching on land and reducing mobility or access. They can also decrease the supply of natural resources used by households and reduce agricultural production (livestock and/or crops), thus resulting in losses of income and increased vulnerability. Furthermore, some invasive species were seen to have negative implications for human health and safety and reduce the cultural value of landscapes [93]. Despite its various advantages (the creation of green space plots, ornamentation and shade, production of firewood, etc.), A. auriculiformis increases the level of soil acidity, as demonstrated in the Batéké plateau in Kinshasa [94]. Consequently, the process of restoring degraded land should favor native species like Brachystegia spiciformis, Combretum collinum, and Pterocarpus tinctorius, which are the most productive potential candidates for restoration, based on [95]. For this reason, managing the expansion of A. auriculiformis plantations and their associated risks requires an assessment of the potential impact on the local ecosystem, the regular monitoring of affected areas based on accurate mapping, and the selective elimination of other invasive species from the accompanying flora, using appropriate methods (e.g., selective cutting or uprooting). In addition, private landowners should be introduced to innovative management practices for exotic species, such as using their biomass for soil fertilization or cutting their wood for carbonization [96]. Finally, cooperative efforts between the government, non-governmental organizations, and local communities are needed to effectively combat the invasion of A. auriculiformis [97].
Although our study quantified the impact of the presence of A. auriculiformis plantations on plant diversity, it did not assess the influence of the edge effect or the seed bank on this plant diversity. Nevertheless, this study provides the first information on the importance of A. auriculiformis plantations as habitats and corridors for local and exotic flora. By monitoring the plant community over time, it becomes possible to assess the effectiveness of restoration efforts. Indeed, changes in floristic composition can reflect the success or challenges encountered in the restoration process. Also, a thorough knowledge of the plant community helps to avoid the excessive dominance of a single species, which could lead to ecological imbalances. Finally, some plants can have beneficial effects on others, promoting growth and regeneration. Others may play a role in nitrogen fixation, protection against pests, or the creation of a favorable microclimate.
5. Conclusions
The current study provides a detailed analysis of the spatio-temporal dynamics of A. auriculiformis plantations and their influence on plant diversity in degraded lands across the urban–rural gradient in Lubumbashi. Our findings demonstrate a significant increase in the extent of these plantations over a 15-year period, growing from 13.68 hectares in 2006 to 21.97 hectares in 2021. This expansion is attributed to the restoration efforts made in landscapes degraded by urbanization.
In terms of composition, A. auriculiformis plantations predominantly feature members of the Fabaceae family and a higher proportion of exotic species, especially in urban areas compared to peri-urban zones. Furthermore, 19 indigenous species were identified within these plantations, including 18 tree species typical of the miombo woodlands. Notably, a greater diversity of plant species was observed in urban plantations compared to those in peri-urban areas.
The increase in plantation area indicates a trend towards the re-vegetation of degraded lands, with A. auriculiformis plantations supporting both exotic and native miombo species. However, the long-term sustainability of these plantations is questionable due to the presence of numerous invasive alien tree species, which could potentially threaten local biodiversity. This study’s methodology and findings offer a foundation for developing policies and technical strategies for ecological restoration in Lubumbashi.
Author Contributions
Y.U.S.: conceptualization, methodology, and writing—original draft preparation; H.K.M. and J.Y.M.: data curation and writing—original draft preparation; M.M.M. and F.M.: writing—review and editing; J.B.: supervision, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ARES-CCD (Belgium), through the research for development project ‘Renforcement des capacités de gestion durable de la forêt claire de miombo par l’évaluation de l’impact environnemental de la production de charbon de bois et l’amélioration des pratiques vis-à-vis des ressources forestières (CHARLU)’.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this published paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations, Department of Economic and Social Affairs, Population Division. The World’s Cities in 2018—Data Booklet (ST/ESA/ SER.A/417), 2018. Available online: https://digitallibrary.un.org/record/3799524 (accessed on 2 January 2023).
- Antrop, M. The language of landscape ecologists and planners: A comparative content analysis of concepts used in landscape ecology. Landsc. Urban. Plan. 2001, 55, 163–173. [Google Scholar] [CrossRef]
- Angel, S.; Parent, J.; Civco, D.L.; Blei, M.A. Making Room for a Planet of Cities. In Policy Focus Report; Lincoln Institute of Land Policy: Cambridge, MA, USA, 2011. [Google Scholar]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Saley, A. Utilisation des Nouvelles Techniques de Cartographie Pour L’étude de la Dynamique de L’occupation du Sol Dans la Commune Rurale de Namaro; CRESA: Niamey, Niger, 2006; 82p. [Google Scholar]
- Cilliers, S.S.; Cilliers, J.; Lubbe, R.; Siebert, S. Ecosystem services of urban green spaces in African countries—Perspectives and challenges. Urban. Ecosyst. 2013, 16, 681–702. [Google Scholar] [CrossRef]
- Watson, V. ‘The planned city sweeps the poor away…’: Urban planning and 21st century urbanisation. Prog. Plan. 2009, 72, 151–193. [Google Scholar] [CrossRef]
- Alberti, M. The effects of urban patterns on ecosystem functions. Int. Reg. Sci. Rev. 2005, 28, 168–192. [Google Scholar] [CrossRef]
- André, M.; Mahy, G.; Lejeune, P.; Bogaert, J. Vers une synthèse de la conception et d’une définition des zones dans le gradient urbain-rural. Biotechnol. Agron. Société Et Environ. 2014, 18, 61–74. [Google Scholar]
- Bigirimana, J.; Bogaert, J.; De Cannière, C.; Lejoly, J.; Parmentier, I. Alien plant species dominate the vegetation in a city of sub-Saharan Africa. Landsc. Urban. Plan. 2011, 100, 251–267. [Google Scholar] [CrossRef]
- Bernholt, H.; Kehlenbeck, K.; Gebauer, J.; Buerkert, A. Plant species richness and diversity in urban and peri-urban gardens of Niamey, Niger. Agrofor. Syst. 2009, 77, 159–179. [Google Scholar] [CrossRef]
- Pyšek, P. Alien and native species in Central European urban floras: A quantitative comparison. J. Biogeogr. 1998, 25, 155–163. [Google Scholar] [CrossRef]
- Nghiem, L.T.; Tan, H.T.; Corlett, R.T. Invasive trees in Singapore: Are they a threat to native forests? Trop. Conserv. Sci. 2015, 8, 201–214. [Google Scholar] [CrossRef]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 52–66. [Google Scholar] [CrossRef]
- Reid, W.V.; Mooney, H.A.; Cropper, A.; Capistrano, D.; Carpenter, S.R.; Chopra, K.; Zurek, M.B. Ecosystems, and Human Well-Being-Synthesis: A Report of the Millennium Ecosystem Assessment; Island Press: Oxford, UK, 2005. [Google Scholar]
- Bordbar, F.; Meerts, P.J. Alien flora of DR Congo: Improving the checklist with digitised herbarium collections. Biol. Invasions 2022, 24, 939–954. [Google Scholar] [CrossRef]
- El Desouky, H.A.; Muchez, P.; Cailteux, J. Two Cu–Co sulfide phases and contrasting fluid systems in the Katanga Copperbelt, Democratic Republic of Congo. Ore Geol. Rev. 2009, 36, 315–332. [Google Scholar] [CrossRef]
- Decrée, S.; Deloule, É.; De Putter, T.; Dewaele, S.; Mees, F.; Yans, J.; Marignac, C. SIMS U–Pb dating of uranium mineralization in the Katanga Copperbelt: Constraints for the geodynamic context. Ore Geol. Rev. 2011, 40, 81–89. [Google Scholar] [CrossRef]
- Useni, S.Y.; Cabala, K.; Halleux, J.M.; Bogaert, J.; Munyemba, K. Caractérisation de la croissance spatiale urbaine de la ville de Lubumbashi (Haut-Katanga, RD Congo) entre 1989 et 2014. Tropicultura 2018, 38, 98–108. [Google Scholar]
- Mwitwa, J.; German, L.; Muimba-Kankolongo, A.; Puntodewo, A. Governance and sustainability challenges in landscapes shaped by mining: Mining-forestry linkages and impacts in the Copper Belt of Zambia and the DR Congo. For. Policy Econ. 2012, 25, 19–30. [Google Scholar] [CrossRef]
- Cabala, K.S.; Useni, S.Y.; Sambieni, K.R.; Bogaert, J.; Munyemba, K.F. Dynamique des écosystèmes forestiers de l’Arc Cuprifère Katangais en République Démocratique du Congo. Causes, Transformations spatiales et ampleur. Tropicultura 2017, 35, 192–202. [Google Scholar]
- Useni, S.Y.; Cabala, K.S.; Nkuku, K.C.; Amisi, M.Y.; Malaisse, F.; Bogaert, J.; Munyemba, K.F. Vingt-cinq ans de monitoring de la dynamique spatiale des espaces verts en réponse à l’urbanisation dans les communes de la ville de Lubumbashi (Haut-Katanga, RD Congo). Tropicultura 2017, 35, 300–311. [Google Scholar]
- André, M.; Vranken, I.; Boisson, S.; Mahy, G.; Rüdisser, J.; Visser, M.; Lejeune, P.; Bogaert, J. Quantification of anthropogenic effects in the landscape of Lubumbashi. In Anthropisation des Paysages Katangais; Bogaert, J., Colinet, G., Mahy, G., Eds.; Les Presses Universitaires de Liège: Liège, Belgium, 2018; pp. 231–251. [Google Scholar]
- Cabala, K.S.; Useni, S.Y.; Munyemba, K.F.; Bogaert, J. Activités anthropiques et dynamique spatiotemporelle de la forêt claire dans la Plaine de Lubumbashi. In Anthropisation des Paysages Katangais; Bogaert, J., Colinet, G., Mahy, G., Eds.; Les presses universitaires de Liège: Liège, Belgique, 2018; pp. 253–266. [Google Scholar]
- Useni, S.Y.; Mpibwe, K.A.; Yona, M.J.; N’Tambwe, N.D.; Malaisse, F.; Bogaert, J. Assessment of Street Tree Diversity, Structure and Protection in Planned and Unplanned Neighborhoods of Lubumbashi City (DR Congo). Sustainability 2022, 14, 3830. [Google Scholar] [CrossRef]
- Klompmaker, J.O.; Hoek, G.; Bloemsma, L.D.; Gehring, U.; Strak, M.; Wijga, A.H.; den Brink, C.; Brunekreef, B.; Lebret, L.; Janssen, N.A. Green space definition affects associations of green space with overweight and physical activity. Environ. Res. 2018, 160, 531–540. [Google Scholar] [CrossRef]
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Tzoulas, K.; Korpela, K.; Venn, S.; Yli-Pelkonen, V.; Kaźmierczak, A.; Niemela, J.; James, P. Promoting ecosystem and human health in urban areas using Green Infrastructure: A literature review. Landsc. Urban. Plan. 2007, 81, 167–178. [Google Scholar] [CrossRef]
- Akondé, T.P. Potential of Alley Cropping with “Leucaena leucocephala”(Lam.) de Wit and ”Cajanus cajan (L.) Millsp. for Maize (Zea mays L.) and Cassava, (Manihot Esculenta Crantz) Production on Acrisol in Benin Republic (West Africa). Doctoral Dissertation, Universität Hohenheim, Stuttgart, Germany, 1995. [Google Scholar]
- Wuenschel, A. Impacts Ecologiques Potentiels à Long-Terme des Plantations d’Acacias Non-Natifs Dans la Région de Kinshasa, en RDC; 2019; 37p. Available online: https://usfscentralafrica.org/wp-content/uploads/2020/01/DRC_AcaciaNon-Native_EcoImpacts_Fr_120.pdf (accessed on 9 January 2024).
- Yang, L.; Liu, N.; Ren, H.; Wang, J. Facilitation by two exotic Acacia: Acacia auriculiformis and Acacia mangium as nurse plants in South China. For. Ecol. Manag. 2009, 257, 1786–1793. [Google Scholar] [CrossRef]
- Useni, S.Y.; Malaisse, F.; Cabala, K.S.; Kalumba, M.A.; Mwana, Y.A.; Nkuku, K.C.; Bogaert, J.; Munyemba, K.F. Tree diversity and structure on green space of urban and peri-urban zones: The case of Lubumbashi City in the Democratic Republic of Congo. Urban. For. Urban. Green. 2019, 41, 67–74. [Google Scholar] [CrossRef]
- Mwanasomwe, J.K.; Langunu, S.; Shutcha, M.N.; Colinet, G. Effects of 15-Year-Old Plantation on Soil Conditions, Spontaneous Vegetation, and the Trace Metal Content in Wood Products at Kipushi Tailings Dam. Front. Soil. Sci. 2022, 2, 934491. [Google Scholar] [CrossRef]
- Richardson, D.M.; Macdonald, I.A.W.; Forsyth, G.G. Reductions in plant species richness under stands of alien trees and shrubs in the fynbos biome. S. Afr. For. J. 1989, 149, 1–8. [Google Scholar] [CrossRef]
- D’antonio, C.A.R.L.A.; Meyerson, L.A. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Restor. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- Goodenough, A. Are the ecological impacts of alien species misrepresented? A review of the “native good, alien bad” philosophy. Community Ecol. 2010, 11, 13–21. [Google Scholar] [CrossRef]
- Watanabe, S.; Sumi, K.; Ise, T. Identifying the vegetation type in Google Earth images using a convolutional neural network: A case study for Japanese bamboo forests. BMC Ecol. 2020, 20, 65. [Google Scholar] [CrossRef]
- Koua, K.A.N.; Bamba, I.; Barima, Y.S.S.; Kouakou, A.T.M.; Kouakou, K.A.; Sangne, Y.C. Echelle spatiale et dynamique de la forêt classée du Haut-Sassandra (Centre-Ouest de la Côte d’Ivoire) en période de conflits. Rev. Environ. Biodiversité-PASRES 2017, 2, 54–68. [Google Scholar]
- Gong, C.; Chen, J.; Yu, S. Biotic homogenization and differentiation of the flora in artificial and near-natural habitats across urban green spaces. Landsc. Urban. Plan. 2013, 120, 158–169. [Google Scholar] [CrossRef]
- Kohli, R.K.; Dogra, K.S.; Batish, D.R.; Singh, H.P. Impact of invasive plants on the structure and composition of natural vegetation of northwestern Indian Himalayas. Weed Technol. 2004, 18, 1296–1300. Available online: http://www.jstor.org/stable/3989638 (accessed on 9 January 2024).
- Cabala, K.S.; Useni, S.Y.; Amisi, M.Y.; Munyemba, K.F.; Bogaert, J. Activités anthropiques et dynamique des écosystèmes forestiers dans les zones territoriales de l’Arc Cuprifère Katangais (RD Congo). Tropicultura 2022, 40, 27. Available online: https://popups.uliege.be/2295-8010/index.php?id=2100 (accessed on 9 January 2024). [CrossRef]
- Kalombo, K.D. Evaluation des Eléments du Climat en R.D.C.; Editions Universitaires Européennes: Saarbrücken, Allemagne, 2016; 220p. [Google Scholar]
- Vranken, I.; Amisi, Y.M.; Munyemba, K.F.; Bamba, I.; Veroustraete, F.; Visser, M.; Bogaert, J. The Spatial Footprint of the Non-ferrous Mining Industry in Lubumbashi. Tropicultura 2013, 31, 22–29. [Google Scholar]
- Peroches, A.; Nge Okwe, A.; Gazull, L.; Dubiez, E. Rapport D’étude de la Filière bois-Energie de la ville de Lubumbashi. 2021. Available online: https://publications.cirad.fr/une_notice.php?dk=600201 (accessed on 12 October 2023).
- Ozer, P. Catastrophes naturelles et aménagement du territoire: De l’intérêt des images Google Earth dans les pays en développement. Geo-Eco-Trop 2014, 38, 209–220. [Google Scholar]
- Vranken, I.; Marielle, A.; Mujinya, B.B.; Munyemba, K.F.; Baert, G.; Van Ranst, E.; Visser, M.; Bogaert, J. Termite mound identification through aerial photographic interpretation in Lubumbashi, Democratic Republic of the Congo: Methodology evaluation. Trop. Conserv. Sci. 2014, 7, 733–746. [Google Scholar]
- Bogaert, J.; Ceulemans, R.; Salvador-Van Eysenrode, D. Decision tree algorithm for detection of spatial processes in landscape transformation. Environ. Manag. 2004, 33, 62–73. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Li, J.-W.; Li, J.; Valimaki, S.K. Impact of urbanization on plant diversity: A case study in built-up area of Beijing. For. Stud. China 2008, 10, 179–188. [Google Scholar] [CrossRef]
- Schippers, P.; Van Groenendael, J.M.; Vleeshouwers, L.M.; Hunt, R. Herbaceous plant strategies in disturbed habitats. Oikos 2001, 95, 198–210. [Google Scholar] [CrossRef]
- Lebrun, J.P.; Stork, A.L. Enumération des plantes à fleurs d’Afrique tropicale et Tropical African Flowering Plants: Ecology and Distribution. Conserv. Et Jard. Bot. De La Ville De Genève 1991, 2015, 1–7. [Google Scholar]
- Rija, A.A.; Said, A.; Mwamende, K.A.; Hassan, S.H.; Madoffe, S.S. Urban sprawl and species movement may decimate natural plant diversity in an Afro-tropical city. Biodivers. Conserv. 2014, 23, 963–978. [Google Scholar] [CrossRef]
- Kull, C.A.; Tassin, J.; Rambeloarisoa, G.; Sarrailh, J.M. Invasive Australian acacias on western Indian Ocean islands: A historical and ecological perspective. Afr. J. Ecol. 2008, 46, 684–689. [Google Scholar] [CrossRef]
- Shinde, P.R.; Patil, P.S.; Bairagi, V.A. Pharmacognostic, Phytochemical properties and anti-bacterial activity of Callistemon citrinus viminalis leaves and stems. Int. J. Pharm. Pharm. Sci. 2012, 4, 406–408. [Google Scholar]
- Arán, D.; García-Duro, J.; Reyes, O.; Casal, M. Fire and invasive species: Modifications in the germination potential of Acacia melanoxylon, Conyza canadensis and Eucalyptus globulus. For. Ecol. Manag. 2013, 302, 7–13. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Rubido-Bará, M. Invasive potential of Eucalyptus globulus: Seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For. Ecol. Manag. 2013, 305, 129–137. [Google Scholar] [CrossRef]
- Dzikiti, S.; Gush, M.B.; Le Maitre, D.C.; Maherry, A.; Jovanovic, N.Z.; Ramoelo, A.; Cho, M.A. Quantifying potential water savings from clearing invasive alien Eucalyptus camaldulensis using in situ and high-resolution remote sensing data in the Berg River Catchment, Western Cape, South Africa. For. Ecol. Manag. 2016, 361, 69–80. [Google Scholar] [CrossRef]
- Meerts, P.; Dassonville, N.; Vanderhoeven, S.; Chapuis-Lardy, L.; Koutika, L.S.; Jacquemart, A.L. Les plantes exotiques envahissantes et leurs impacts. In Biodiversité. Etat, Enjeux et Perspectives; De Boeck: Louvain-la-Neuve, Belgium, 2006; pp. 109–120. [Google Scholar]
- Meerts, P. An annotated checklist to the trees and shrubs of the Upper Katanga (D.R. Congo). Phytotaxa 2016, 258, 201–250. [Google Scholar] [CrossRef][Green Version]
- Sillett, T.S.; Chandler, R.B.; Royle, J.A.; Kery, M.; Morrison, S.A. Hierarchical distance-sampling models to estimate population size and habitat-specific abundance of an island endemic. Ecol. Appl. 2012, 22, 1997–2006. [Google Scholar] [CrossRef]
- Useni, S.Y.; Malaisse, F.; Yona, M.Y.; Mwamba, M.T.; Bogaert, J. Diversity, use and management of household-located fruit trees in two rapidly developing towns in Southeastern DR Congo. Urban. For. Urban. Green. 2021, 63, 127220. [Google Scholar] [CrossRef]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- GROUPE HUIT. Elaboration du Plan Urbain de Référence de Lubumbashi; BEAU: Kinshasa, Democratic Republic of the Congo, 2009; 62p. [Google Scholar]
- Jim, C.Y. Green-space preservation and allocation for sustainable greening of compact cities. Cities 2004, 21, 311–320. [Google Scholar] [CrossRef]
- Bruneau, J.C.; Pain, M. Atlas de Lubumbashi; Centre d’Etude Géographique sur l’Afrique Noire, Université Paris X: Nanterre, France, 1990; 201p. [Google Scholar]
- Williams, N.S.G.; Schwartz, M.W.; Vesk, P.A.; McCarthy, M.A.; Hahs, A.K.; Clemants, S.E.; Corlett, R.T.; Duncan, R.P.; Norton, B.A.; Thompson, K.; et al. A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 2009, 97, 4–9. [Google Scholar] [CrossRef]
- Trefon, T.; Kabuyaya, N. Les espaces périurbains en Afrique centrale. In Territoires Périurbains: Développement, Enjeux et Perspectives Dans les Pays du Sud; Bogaert, J., Halleux, J.M., Eds.; Les Presses Agronomiques de Gembloux: Gembloux, Belgique, 2015; pp. 33–42. [Google Scholar]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In Dynamics of Populations; Den Boer, P.J., Gradwell, G., Eds.; PUDOC: Wageningen, The Netherlands, 1971; pp. 298–312. [Google Scholar]
- Azihou, A.F.; Kakaï, R.G.; Bellefontaine, R.; Sinsin, B. Distribution of tree species along a gallery forest–savanna gradient: Patterns, overlaps and ecological thresholds. J. Trop. Ecol. 2013, 29, 25–37. [Google Scholar] [CrossRef]
- Shackleton, S.; Chinyimba, A.; Hebinck, P.; Shackleton, C.M.; Kaoma, H. Multiple benefits and values of trees in urban landscapes in two towns in northern South Africa. Landsc. Urban. Plan. 2015, 136, 76–86. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Godron, M. Landscape Ecology; John Wiley & Sons: New York, NY, USA, 1986; 640p. [Google Scholar]
- Toyi, M.S.; Barima, Y.S.S.; Mama, A.; André, M.; Bastin, J.-F.; De Cannière, C.; Sinsin, B.; Bogaert, J. Tree plantation will not compensate natural woody vegetation cover loss in the Atlantic department of southern Benin. Tropicultura 2013, 31, 62–70. Available online: https://hdl.handle.net/2268/160471 (accessed on 9 January 2024).
- Elliott, K.J.; Boring, L.R.; Swank, W.T. Changes in vegetation structure and diversity after grass-to-forest succession in a southern Appalachian watershed. Am. Midl. Nat. 1998, 140, 219–232. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Sciences 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Chidumayo, E.N. Forest degradation and recovery in a miombo woodland landscape in Zambia: 22 years of observations on permanent sample plots. For. Ecol. Manag. 2013, 291, 154–161. [Google Scholar] [CrossRef]
- Syampungani, S.; Tigabu, M.; Matakala, N.; Handavu, F.; Oden, P.C. Coppicing ability of dry miombo woodland species harvested for traditional charcoal production in Zambia: A win–win strategy for sustaining rural livelihoods and recovering a woodland ecosystem. J. For. Res. 2017, 28, 549–556. [Google Scholar] [CrossRef]
- Peltier, R.; Bisiaux, F.; Dubiez, E.; Marien, J.N.; Muliele, J.C.; Proces, P.; Vermeulen, C. De la Culture Itinérante sur Brulis aux Jacheres Enrichies Productrices de Charbon de Bois, en RD du Congo; Cirad-Inra-SupAgro: Paris, France, 2010; 16p. [Google Scholar]
- Hardarson, G.; Danso, S.K.A. Methods for measuring biological nitrogen fixation in grain legumes. In Enhancement of Biological Nitrogen Fixation of Common Bean in Latin America; Bliss, F.A., Hardarson, G., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 152, pp. 19–23. [Google Scholar] [CrossRef]
- Dzikiti, S.; Schachtschneider, K.; Naiken, V.; Gush, M.; Moses, G.; Le Maitre, D.C. Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape, South Africa. J. Arid. Environ. 2013, 90, 103–113. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Callaway, R.M. Ecological facilitation may drive major evolutionary transitions. BioScience 2009, 59, 399–404. [Google Scholar] [CrossRef][Green Version]
- Kasongo, R.K.; Van Ranst, E.; Verdoodt, A.; Kanyankogote, P.; Baert, G. Impact of Acacia auriculiformis on the chemical fertility of sandy soils on the Batéké plateau, D.R Congo. Soil. Use Manag. 2009, 25, 21–27. [Google Scholar] [CrossRef]
- Nsombo, B.M.; Lumbuenamo, R.S.; Lejoly, J.; Aloni, J.K.; Mafuka, P.M.M. Caractéristiques des sols sous savane et sous forêt naturelle sur le plateau des Batéké en République démocratique du Congo. Tropicultura 2016, 34, 87–97. [Google Scholar]
- Kooke, G.X.; Ali, R.K.F.M.; Djossou, J.M. Estimation du stock de carbone organique dans les plantations de Acacia auriculiformis A. Cunn. ex Benth. des forêts classées de Pahou. Int. J. Bio Chem. Sci. 2019, 13, 277–293. [Google Scholar] [CrossRef]
- Gnahoua, G.M.; Louppe, D. Acacia auriculiformis; CIRAD: Paris, France, 2003. [Google Scholar]
- Kolawolé, R.F.M.A. Phytodiversité dans les plantations de Acacia auriculiformis de la forêt classée de Ouèdo au Sud du Bénin. Asian J. Sci. Technol. 2019, 10, 10056–10066. [Google Scholar]
- Laurance, W.F.; Nascimento, H.E.; Laurance, S.G.; Andrade, A.; Ewers, R.M.; Harms, K.E.; Luizão, R.C.C.; Ribeiro, J.E. Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2007, 2, e1017. [Google Scholar] [CrossRef]
- Lubbe, C.S.; Siebert, S.J.; Cilliers, S.S. Political legacy of South Africa affects the plant diversity patterns of urban domestic gardens along a socio-economic gradient. Sci. Res. Essays 2010, 5, 2900–2910. [Google Scholar]
- Zmyslony, J.; Gagnon, D. Residential management of urban front-yard landscape: A random process? Landsc. Urban. Plan. 1998, 40, 295–307. [Google Scholar] [CrossRef]
- Lowry, J.B.; Prinsen, J.H.; Burrows, D.M. Albizia lebbeck-a promising forage tree for semiarid regions. In Forage Tree Legumes in Tropical Agriculture; Oxford University Press: Oxford, UK, 1994; pp. 75–83. [Google Scholar]
- Godefroid, S. Temporel analysis of the Brussels flora as indicator for changing environmental quality. Landsc. Urban. Plan. 2001, 52, 203–224. [Google Scholar] [CrossRef]
- Villalobos, F.J.; Sadras, V.O.; Fereres, E. Plant density and competition. In Principles of Agronomy for Sustainable Agriculture; Springer: Cham, Switzerland, 2016; pp. 159–168. [Google Scholar] [CrossRef]
- Useni, S.Y.; Sambiéni, K.R.; Maréchal, J.; Ilunga, W.I.E.; Malaisse, F.; Bogaert, J.; Munyemba, K.F. Changes in the Spatial Pattern and Ecological Functionalities of Green Spaces in Lubumbashi (the Democratic Republic of Congo) in Relation with the Degree of Urbanization. Trop. Conserv. Sci. 2018, 11, 1940082918771325. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Shackleton, C.M.; Kull, C.A. The role of invasive alien species in shaping local livelihoods and human well-being: A review. J. Environ. Manag. 2019, 229, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Amandine, S. Impact de l’Acacia auriculiformis sur les Propriétés des sols Sableux du Plateau Batéké, République Démocratique du Congo. Master’s Thesis, Université Catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium, 2011; 98p. [Google Scholar]
- Kaumbu, J.M.K.; Mpundu, M.M.M.; Kasongo, E.L.M.; Ngoy Shutcha, M.; Kalambulwa, A.N.; Khasa, D. Early Selection of Tree Species for Regeneration in Degraded Woodland of Southeastern Congo Basin. Forests 2021, 12, 117. [Google Scholar] [CrossRef]
- Chirwa, P.W. Pratiques de Restauration dans les Zones Dégradées d’Afrique de l’Est. 2014. Available online: https://afforum.org/oldaff/sites/default/files/French/French_63.pdf (accessed on 2 January 2023).
- Otts, S.S.; Janasie, C.; Cotter, P. Working together to combat invasive species threats: Strategies for facilitating cooperation between the national park service and states. Nat. Resour. J. 2016, 56, 117–143. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).