Soil Bacterial and Archaeal Communities of the Periodic Flooding Zone of Three Main Reservoirs in the South Ural Region (Russia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sample Procedure and Soil Analysis
2.3. Water-Level Data and Meteorological Searches
2.4. DNA Analysis
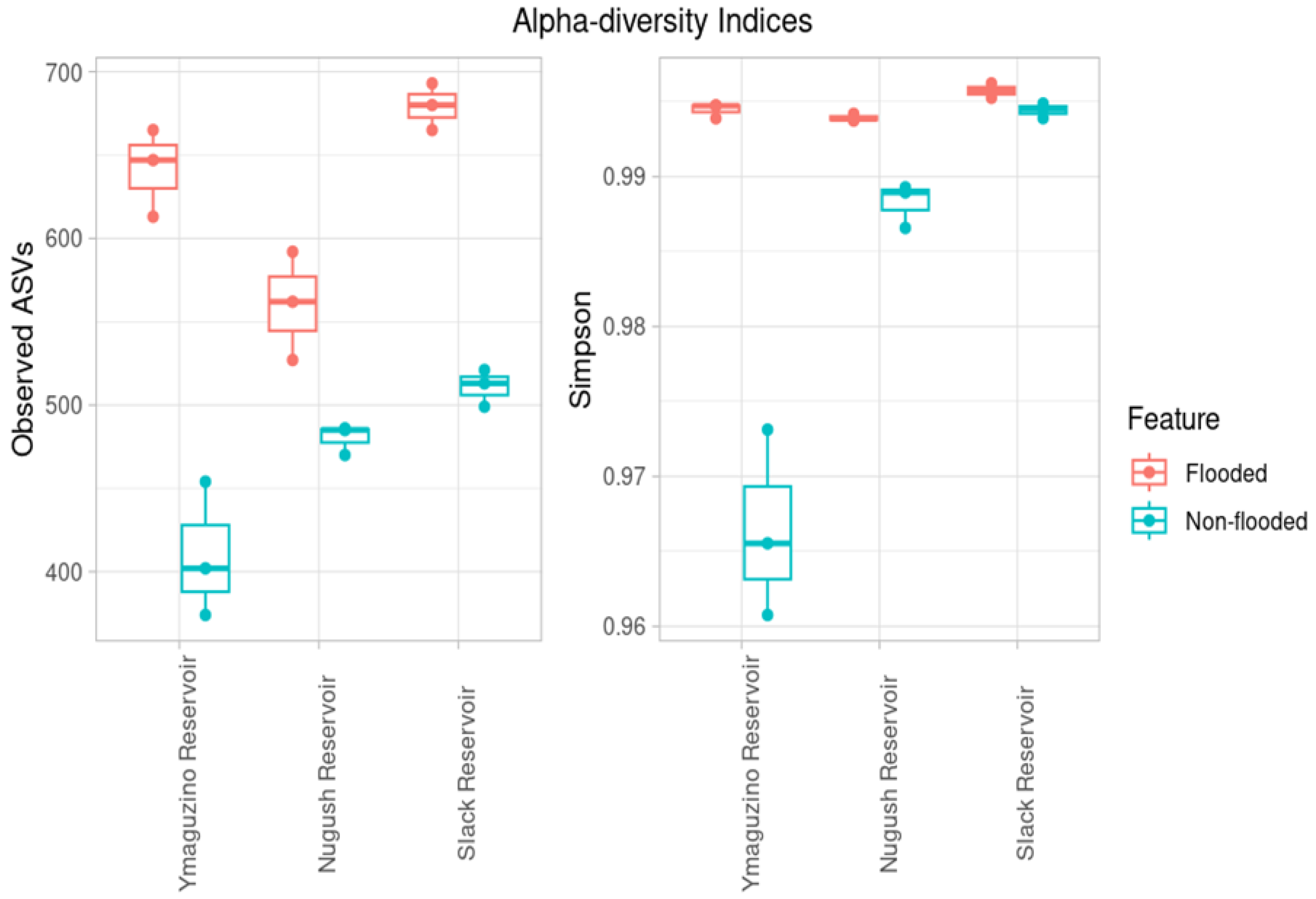
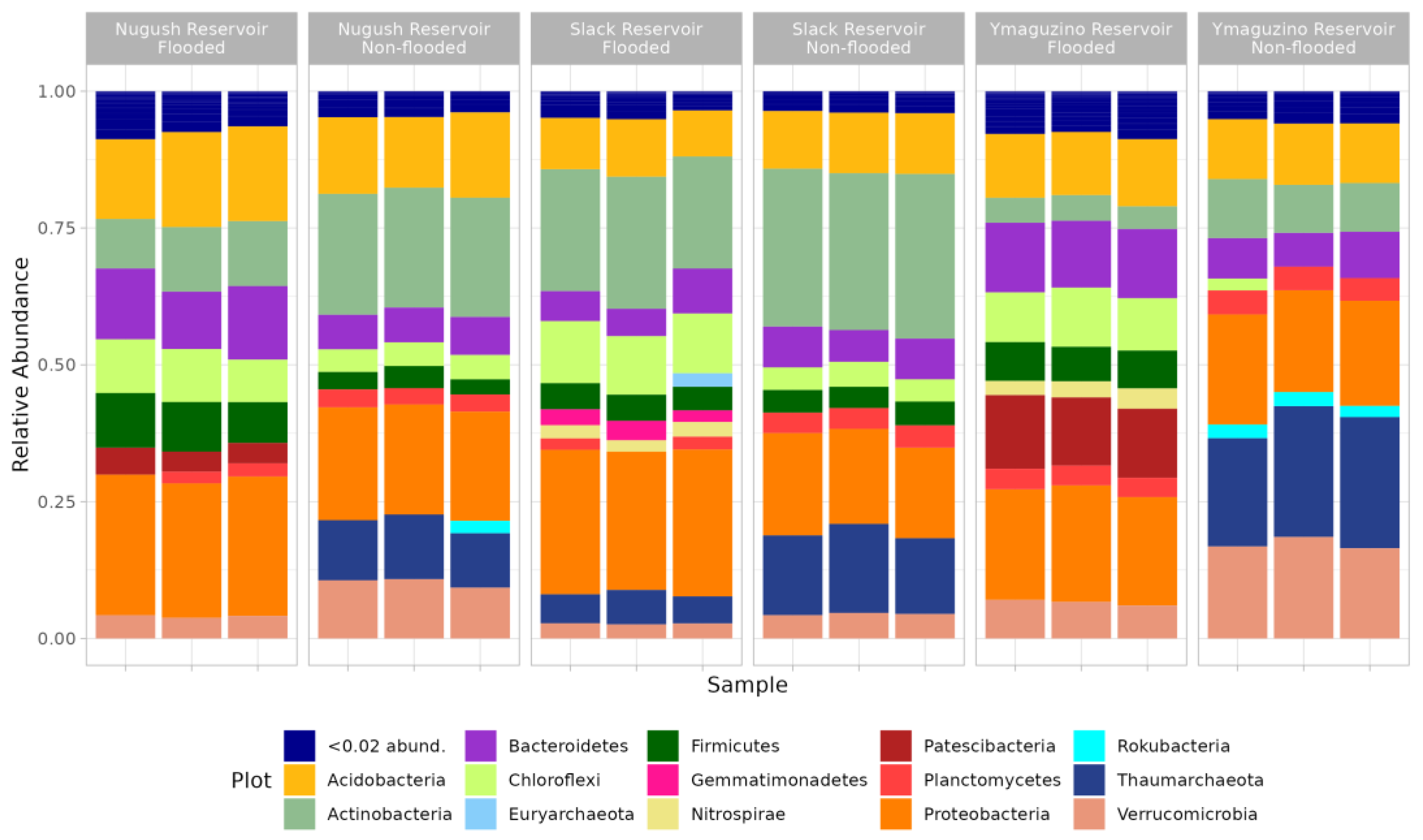
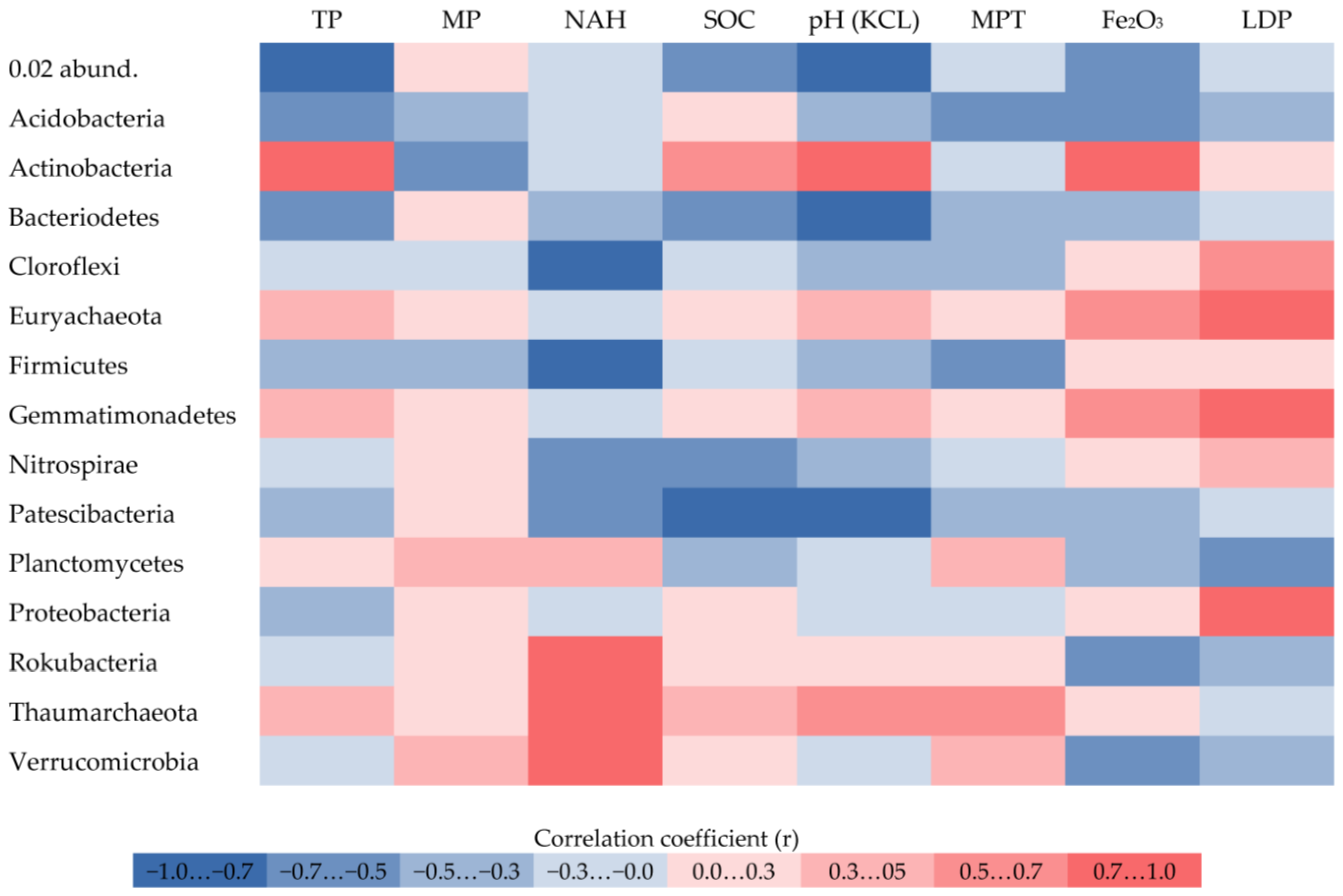
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Term “Reservoir”. Meridian Dictionary. Available online: https://www.merriam-webster.com/dictionary/reservoir (accessed on 7 April 2024).
- Felix-Faure, J.; Walter, C.; Balesdent, J.; Chanudet, V.; Avrillier, J.-N.; Hossann, C.; Baudoin, J.-M.; Dambrine, E. Soils drowned in water impoundments: A new frontier. Front. Environ. Sci. Sec. Soil Process. 2019, 7, 53. [Google Scholar] [CrossRef]
- Felix-Faure, J.; Caillard, J.; Descloux, S.; Chanudet, V.; Poirel, A.; Baudoin, J.-M.; Avrillier, J.-N.; Millery, A.; Dambrine, E. Contribution of flooded soils to sediment and nutrient fluxes in a hydropower reservoir (Sarrans, Central France). Ecosystems 2019, 22, 312–330. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The Chemistry of Submerged Soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar] [CrossRef]
- Houel, S.; Louchouarn, P.; Lucotte, M.; Canuel, R.; Ghaleb, B. Translocation of soil organic matter following reservoir impoundment in boreal systems: Implications for in situ producti. Limnol. Oceanogr. 2006, 51, 1497–1513. [Google Scholar] [CrossRef]
- Fonsega, R.M.F.; Barriga, F.J.A.S.; Fyfe, W.S. Dam Reservoir Sediments as Fertilizers and Artificial Soils. Case Studies from Portugal and Brazil. In Reservoir and River Basin Management. Exchange of Experiences from Brazil, Portugal and Germany; Gunkel, G., Sobral, M.C., Eds.; Universitätsverlag der TU Berlin: Berlin, Germany, 2007. [Google Scholar]
- Golosov, V.N.; Kumani, M.V.; Ivanova, N.N.; Belyaev, V.R.; Shamshurina, E.N. Siltation of a small reservoir under climatic changes and urbanization of its water catchment basin (the Popovsky pond, Kursk). Vestn. Mosk. Univ. Seriya 5 Geogr. 2020, 6, 51–62. (In Russian) [Google Scholar]
- Vinogradov, V.G. Classification of shallow water sections of reservoirs of the sod-podzolic zone and prospects for their use on the example of the Uchinsky reservoir. In Rational Use of Natural Resources and Environmental Protection; Science: Leningrad, Russia, 1978; Issue 2; pp. 46–51. (In Russian) [Google Scholar]
- Nurulhuda, K.; Gaydon, D.S.; Jing, Q.; Zakaria, M.P.; Struik, P.C.; Keesman, K.J. Nitrogen dynamics in flooded soil systems: An overview on concepts and performance of models. J. Sci. Food Agric. 2018, 98, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, Z.; Maavara, T.; Slowinski, S.; Van Cappellen, P. Effects of damming on river nitrogen fluxes: A global analysis. Global Biogeochem. Cycles 2019, 33, 1339–1357. [Google Scholar] [CrossRef]
- De Mello, J.W.V.; Barrón, V.; Torrent, J. Phosphorus and iron mobilization in flooded soils from Brazil. Soil Sci. 1998, 163, 122–132. [Google Scholar] [CrossRef]
- Rapin, A.; Rabiet, M.; Mourier, B.; Grybos, M.; Deluchat, V. Sedimentary phosphorus accumulation and distribution in the continuum of three cascade dams (Creuse River, France). Environ. Sci. Pollut. Res. 2020, 27, 6526–6539. [Google Scholar] [CrossRef]
- Qin, L.; Lei, P.; Lei, Q.; Liu, H. Evaluating the effect of dam construction on the phosphorus fractions in sediments in a reservoir of drinking water source, China. Environ. Monit. Assess. 2020, 192, 99. [Google Scholar] [CrossRef]
- Liesack, W.; Schnell, S.; Revbesch, N.P. Microbiology of flooded rice paddies. FEMS Microbiol. Rev. 2000, 24, 625–645. [Google Scholar] [CrossRef]
- King, G.M.; Henry, K. Impacts of experimental flooding on microbial communities and methane fluxes in an urban meadow, Baton Rouge, Louisiana. Front. Ecol. Evol. 2019, 7, 288. [Google Scholar] [CrossRef]
- Carnevali, P.B.M.; Lavy, A.; Thomas, A.D.; Crits-Christoph, A.; Diamond, S.; Méheust, R.; Olm, M.R.; Sharrar, A.; Lei, S.; Dong, W.; et al. Meanders as a scaling motif for understanding of floodplain soil microbiome and biogeochemical potential at the watershed scale. Microbiome 2021, 9, 121. [Google Scholar] [CrossRef]
- Furtak, K.; Grzadziel, J.; Galazka, A. Can Model Experiments Give Insight into the Response of the Soil Environment to Flooding? A Comparison of Microcosm and Natural Event. Biology 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Abdrakhmanov, R.F.; Tyur, V.A.; Poleva, A.O.; Yurov, V.M. Special features of hydrological and hydrochemical conditions in the big Southern Urals reservoirs. Proceedings of Voronezh State University. Series: Geography. Geoecology 2009, 1, 23–30. (In Russian) [Google Scholar]
- Orlov, D.S. Soil chemistry: A Textbook; Moscow State University: Moscow, Russia, 1985; 487p. (In Russian) [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006; 993p. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; 1264p. [Google Scholar] [CrossRef]
- Polyakov, V.; Orlova, K.; Abakumov, E. Evaluation of carbon stocks in the soils of Lena River Delta on the basis of application of “dry combustion” and Tyurin’s methods of carbon determination. Biol. Commun. 2017, 62, 67–72. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Ashwani K, T. A review on measurement of Alpha diversity in biology. Agric. Res. J. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Vodyanickij, Y.N. Gluing, olivization and hydrometamorphic process. Dokuchaev Soil Bull. 2008, 61, 12–20. (In Russian) [Google Scholar]
- Norgbey, E.; Li, Y.; Zhu, Y.; Nwankwegu, A.; Bofah-Buah, R.; Nuamah, L. Seasonal dynamics of iron and phosphorus in reservoir sediments in Eucalyptus plantation region. Ecol. Process. 2021, 10, 10. [Google Scholar] [CrossRef]
- Liu, X.; Sun, D.; Qin, J.; Zhang, J.; Yang, Y.; Yang, J.; Wang, Z.; Zhou, D.; Li, Y.; Wang, X.; et al. Spatial distribution of soil iron across different plant communities along a hydrological gradient in the Yellow River Estuary wetland. Front. Ecol. Evol. 2022, 10, 979194. [Google Scholar] [CrossRef]
- Liu, W.; Qin, T.; Wu, M.; Chen, Z.; Zhang, Y.; Abakumov, E.; Chebykina, E.; Wang, W.; Wu, D.; Han, C.; et al. Analyzing the phosphorus flow characteristics in the largest freshwater lake (Poyang Lake) watershed of China from 1950 to 2020 through a bottom-up approach of watershed-scale phosphorus substance flow model. Water Res. 2023, 245, 120546. [Google Scholar] [CrossRef] [PubMed]
- Khaziev, F.H.; Mukatanov, A.H.; Khabarov, I.K.; Koltsova, G.A.; Gabbasova, I.M.; Ramazanov, R.Y. Soils of Bashkortostan. T.1. Ecological-Genetic and Agricultural Production Characteristics; Gilem: Ufa, Russia, 1995; 384p. (In Russian) [Google Scholar]
- Hafeez, F.; Zafar, N.; Nazir, R.; Javeed, H.M.R.; Rizwan, M.; Faridullah; Asad, S.A.; Iqbal, A. Assessment of flood-induced changes in soil heavy metal and nutrient status in Rajanpur, Pakistan. Environ. Monit. Assess. 2019, 191, 234. [Google Scholar] [CrossRef]
- Gladkov, E.; Tereshonok, D.; Stepanova, A.; Gladkova, O. Plant–Microbe Interactions under the Action of Heavy Metals and under the Conditions of Flooding. Diversity 2023, 15, 175. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant-microbe interactions during flooding stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef]
- Wagner, D.; Eisenhauer, N.; Cesarz, S. Plant species richness does not attenuate responses of soil microbial and nematode communities to a flood event. Soil Biol. Biochem. 2015, 89, 135–149. [Google Scholar] [CrossRef]
- Mace, O.G.; Steinauer, K.; Jousset, A.; Eisenhauer, N.; Scheu, S. Flood-Induced Changes in Soil Microbial Functions as Modified by Plant Diversity. PLoS ONE 2016, 11, e0166349. [Google Scholar] [CrossRef]
- Zemskaya, T.I.; Bukin, S.V.; Bukin, Y.S.; Chernitsina, S.M.; Pogodaeva, T.V.; Rusanov, I.I.; Shubenkova, O.V.; Zakharenko, A.S.; Pimenov, N.V. Taxonomic diversity and metabolic activity of microbial communities in rivers and estuarine waters of Southern Baikal in summer. J. Great Lakes Res. 2022, 48, 125–142. [Google Scholar] [CrossRef]
- Kuznetsova, E.V.; Kosolapov, D.B.; Belkova, N.L. Diversity of Planktonic Bacteria in Durgun and Taishir Reservoirs (Western Mongolia). Microbiology 2020, 89, 595–602. [Google Scholar] [CrossRef]
- Lipko, I.A.; Krasnopeev, A.Y.; Tikhonova, I.V.; Timoshkin, O.A.; Kabilov, M.R.; Belykh, O.I. Characterization of microbial communities from the water and endemic sponges Lubomirskia baikalensis of Lake Baikal during environmental changes. AIP Conf. Proc. 2022, 2467, 070042. [Google Scholar] [CrossRef]
- Kurilkina, M.I.; Zakharova, Y.R.; Galachyants, Y.P.; Petrova, D.P.; Bukin, Y.S.; Domysheva, V.M.; Blinov, V.V.; Likhoshway, Y.V. Bacterial community composition in the water column of the deepest freshwater Lake Baikal as determined by next-generation sequencing. FEMS Microbiol. Ecol. 2016, 92, fiw094. [Google Scholar] [CrossRef] [PubMed]
- Matyugina, E.; Belkova, N.; Borzenko, S.; Lukyanov, P.; Kabilov, M.; Baturina, O.; Martynova-Van Kley, A.; Nalian, A.; Ptitsyn, A. Structure and diversity dynamics of microbial communities at day and night: Investigation of meromictic Lake Doroninskoe, Transbaikalia. Rus. J. Ocean. Limnol. 2018, 36, 1978–1992. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Angel, R.; Claus, P.; Conrad, R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012, 6, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.A.; Oshkin, I.Y.; Danilova, O.V.; Philippov, D.A.; Ravin, N.V.; Dedysh, S.N. Rokubacteria in Northern Peatlands: Habitat Preferences and Diversity Patterns. Microorganisms 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.R.; Besmer, M.D.; Langenegger, T.; Beck, K.; Walser, J.-C.; Ackermann, M.; Bürgmann, H.; Hammes, F. Phylogenetic clustering of small low nucleic acid-content bacteria across diverse freshwater ecosystems. ISME J. 2018, 12, 1344–1359. [Google Scholar] [CrossRef]
- Tian, R.; Ning, D.; He, Z.; Zhang, P.; Spencer, S.J.; Gao, S.; Shi, W.; Wu, L.; Zhang, Y.; Yang, Y.; et al. Small and mighty: Adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef]
- Kimble, J.C.; Winter, A.S.; Spilde, M.N.; Sinsabaugh, R.L.; Northup, D.E. A potential central role of Thaumarchaeota in N-Cycling in a semi-arid environment, Fort Stanton Cave, Snowy River passage, New Mexico, USA. FEMS Microbiol. Ecol. 2018, 94, fiy173. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-K.; Cho, J.-C. Environmental Variables Shaping the Ecological Niche of Thaumarchaeota in Soil: Direct and Indirect Causal Effects. PLoS ONE 2015, 10, e0133763. [Google Scholar] [CrossRef] [PubMed]
- Filippidou, S.; Wunderlin, T.; Junier, T.; Jeanneret, N.; Dorador, C.; Molina, V.; Johnson, D.R.; Junier, P. A Combination of Extreme Environmental Conditions Favor the Prevalence of Endospore-Forming Firmicutes. Front. Microbiol. 2016, 7, 1707. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-L.; Duan, G.-L.; Zhang, H.; Cheng, W.; Zhu, Y.-G. Microbiota in non-flooded and flooded rice culms. FEMS Microbiol. Ecol. 2019, 95, fiz036. [Google Scholar] [CrossRef] [PubMed]
- Furtak, K.; Grządziel, J.; Gałązka, A.; Niedźwiecki, J. Prevalence of unclassified bacteria in the soil bacterial community from floodplain meadows (fluvisols) under simulated flood conditions revealed by a metataxonomic approaches. CATENA 2020, 188, 104448. [Google Scholar] [CrossRef]
- Humphries, N.H.; Thornton, S.F.; Chen, X.; Bray, A.W.; Stewart, D.I. Response of soil bacterial populations to application of bio-solids under short-term flooding. Environ. Sci. Pollut. Res. 2023, 30, 72978–72992. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shah, S.; Shah, V. Impact of flooding on the soil microbiota. Environ. Chall. 2021, 4, 100134. [Google Scholar] [CrossRef]
- Abbas, A.; Duan, J.; Abdoulaye, A.H.; Fu, Y.; Lin, Y.; Xie, J.; Cheng, J.; Jiang, D. Deciphering Bacterial Community of the Fallow and Paddy Soil Focusing on Possible Biocontrol Agents. Agronomy 2022, 12, 431. [Google Scholar] [CrossRef]


| Indicator | Sites | |||||
|---|---|---|---|---|---|---|
| Yumaguzino Reservoir | Nugush Reservoir | Slak Reservoir | ||||
| Non-Flooded | Flooded | Non-Flooded | Flooded | Non-Flooded | Flooded | |
| Total phosphorous, mg/kg | 135 ± 7.0 | 152 ± 9.0 | 123 ± 7.0 | 135 ± 6.0 | 175 ± 8.0 | 210 ± 9.0 |
| Mobile phosphorous, mg/100 g of soils | 1.35 ± 0.04 | 2.23 ± 0.04 | 1.33 ± 0.03 | 0.43 ± 0.02 | 1.58 ± 0.04 | 0.97 ± 0.03 |
| Nitrogen alkaline hydrolysable, mg/kg | 210 ± 18.0 | 392 ± 27.0 | 266 ± 21.0 | 280 ± 24.0 | 262 ± 23.0 | 270 ± 21.0 |
| Soil organic carbon, % | 2.66 ± 0.4 | 5.59 ± 0.5 | 5.55 ± 0.6 | 5.05 ± 0.4 | 5.58 ± 0.6 | 5.34 ± 0.4 |
| pH (KCl) | 4.5 ± 0.2 | 5.6 ± 0.2 | 5.3 ± 0.1 | 5.6 ± 0.2 | 6.1 ± 0.3 | 6.3 ± 0.2 |
| pH (H2O) | 5.9 ± 0.2 | 6.4 ± 0.3 | 6.1 ± 0.3 | 6.4 ± 0.3 | 6.8 ± 0.3 | 6.9 ± 0.3 |
| Mobile potassium, mg/kg | 116 ± 6.1 | 195 ± 6.9 | 106 ± 5.2 | 92 ± 5.3 | 154 ± 5.8 | 138 ± 4.9 |
| Fe2O3, % | 0.72 ± 0.08 | 0.68 ± 0.09 | 1.15 ± 0.07 | 0.91 ± 0.09 | 3.11 ± 0.08 | 2.85 ± 0.08 |
| Sites | Sum of Effective Air Temperatures, °C | Length of the Drying Period, Days | ||||
|---|---|---|---|---|---|---|
| Min | Mean | Max | Min | Mean | Max | |
| Yumaguzino Reservoir | 0 (100%) | - | - | - | 0 (100%) | - |
| Nugush Reservoir | 0 (37.5%) | 54.0 | 309.5 | 0 (37.5%) | 6.4 | 33 |
| Slak Reservoir | 67.8 | 623.6 | 1598.6 | 6.0 | 51.8 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnegaliev, A.; Abakumov, E.; Suleymanov, R.; Zaitsev, G.; Davydychev, A.; Dorogaya, E.; Zverev, A.; Andronov, E.; Asylbaev, I. Soil Bacterial and Archaeal Communities of the Periodic Flooding Zone of Three Main Reservoirs in the South Ural Region (Russia). Ecologies 2024, 5, 233-247. https://doi.org/10.3390/ecologies5020015
Minnegaliev A, Abakumov E, Suleymanov R, Zaitsev G, Davydychev A, Dorogaya E, Zverev A, Andronov E, Asylbaev I. Soil Bacterial and Archaeal Communities of the Periodic Flooding Zone of Three Main Reservoirs in the South Ural Region (Russia). Ecologies. 2024; 5(2):233-247. https://doi.org/10.3390/ecologies5020015
Chicago/Turabian StyleMinnegaliev, Aleksandr, Evgeny Abakumov, Ruslan Suleymanov, Gleb Zaitsev, Alexandr Davydychev, Ekaterina Dorogaya, Aleksei Zverev, Evgeny Andronov, and Ilgiz Asylbaev. 2024. "Soil Bacterial and Archaeal Communities of the Periodic Flooding Zone of Three Main Reservoirs in the South Ural Region (Russia)" Ecologies 5, no. 2: 233-247. https://doi.org/10.3390/ecologies5020015
APA StyleMinnegaliev, A., Abakumov, E., Suleymanov, R., Zaitsev, G., Davydychev, A., Dorogaya, E., Zverev, A., Andronov, E., & Asylbaev, I. (2024). Soil Bacterial and Archaeal Communities of the Periodic Flooding Zone of Three Main Reservoirs in the South Ural Region (Russia). Ecologies, 5(2), 233-247. https://doi.org/10.3390/ecologies5020015









