Abstract
In this study, we examine the cooperative effect between vitamins C and E that mitigates oxidative stress by using experimental and computational methods. We performed superoxide scavenging experiments on each vitamin individually and their combination using rotating ring–disk electrode voltammetry. The results indicate that vitamins E and C together produce more effective scavenging of superoxide as evaluated by a steeper slope in the efficiency graph, −7.2 × 104, compared to that of vitamin E alone, −1.8 × 103, or vitamin C alone, −1.3 × 104. Density Functional Theory calculations agree with our experimental results, and we describe a mechanism for the antioxidant action of individual vitamins E and C, plus the synergistic action when both vitamins interact. This process involves the restoration of vitamin E by vitamin C and includes π-π interactions between superoxide and scavengers. The overall result produces an increase in scavenging superoxide radicals when both vitamins act together.
1. Introduction
The in vivo and in vitro relationship between vitamin C and vitamin E has been known and studied for some time [1,2,3,4]. The in vivo results of a pioneering study on healthy young adults by Jeng et al. [5] showed that combined supplementation with vitamins C and E resulted in an enhanced immune response over supplementation by either vitamin alone. Similarly, beneficial results of in vivo interaction between vitamins C and E were reported after sequential antioxidant vitamin C and E supplementation to the diet of human subjects [6]. More recently, elderly adult immune function was improved with supplementation of vitamins C and E [7]. Also, the fertility of male rats under conditions of high oxidative stress was restored by a diet containing tocopherols and vitamin C [8].
Vitamin E’s cooperative role with vitamin C as an antioxidant species in diseases associated with oxidative stress is much studied [9]. These essential nutrients play a major role in reducing and controlling oxidative stress by protecting the immune system and enhancing resistance against infectious microbes such as bacteria, viruses, and parasites. The beneficial effects of these two antioxidant vitamins on the immune system are described in several reviews [10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Relevant to this work, supplementation of vitamins C and E decreased O2•− concentration in the bloodstream and improved antioxidant activity by enhancing NADPH oxidase and superoxide dismutase activity [4].
Many literature studies on the individual action of these two antioxidant vitamins exist. Vitamin C (ascorbic acid) supplementation, for example, has been shown to reduce the duration and severity of upper respiratory infections, including colds [24] and other respiratory infections, including COVID-19 [25]. Water-soluble vitamin C is an important non-enzymatic antioxidant found in human tissue that is involved in many biological functions by acting as a reducing agent through the donation of two electrons. This redox ability is crucial to its role (1) as a cofactor in either monooxygenase or dioxygenase enzymatic reactions, including collagen synthesis, and (2) as an antioxidant against cellular oxidative stress [26]. Vitamin C also restores vitamin E through redox recycling and facilitates the absorption of iron by enhancing its intestinal absorption [27]. One of its biological roles is as a normal skin constituent, both dermis and epidermis, and it plays an important role in tissue repair and wound and burn healing. Scurvy was one of the first examples of a serious disease caused by vitamin C deficiency [28]. Current reviews describe the helpful effects of vitamin C on several other infections [13,29]. However, the relationship between insufficient vitamin C and altered inflammatory and immune responses is not yet clear. As such, the biochemical mechanisms by which vitamin C influences NF-κβ activation to produce pro-inflammatory cytokines are not obvious, and this topic is the subject of many research studies [11,30].
Vitamin E is chemically more complex and consists of a family of eight different compounds: α-, β-, γ-, and δ-tocopherols and four α-, β-, γ-, and δ-tocotrienols (Figure 1). α-Tocopherol is the predominant form in the body and has been given the greatest attention. It is a fat-soluble antioxidant, and its role in human health, particularly as it interacts with peroxyl radicals to prevent lipid peroxidation and subsequent membrane damage, is much investigated and not yet completely explained [31,32]. All naturally occurring vitamin E forms are strong antioxidants with anti-inflammatory activities [33]. A recent review describes the capability of vitamin E to behave as an antioxidant and its effects on diseases caused by oxidative stress [34]. However, despite many studies that have reported on vitamin E’s relationship with the immune system, the mechanistic details are lacking [35,36].
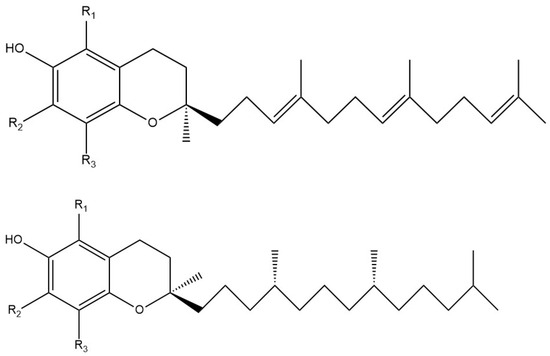
Figure 1.
Chemical structures of vitamin E components. (Top) Tocotrienols: α-Tocotrienol: R1, R2, R3 = Me; β-Tocotrienol: R1 = Me, R2 = H, R3 = Me; γ-Tocotrienol: R1 = H, R2 = Me, R3 = Me; δ-Tocotrienol: R1 = H, R2 = H, R3 = Me. (Bottom) Tocopherols have the C12 long chain saturated: α-tocopherol, R1, R2, R3 = Me; β-tocopherol, R1 = Me, R2 = H, R3 = Me; γ-tocopherol, R1 = H, R2 = Me, R3 = Me; δ-tocopherol, R1 = H, R2 = H, R3 = Me.
The antioxidant role of vitamins C and E is a subject of continual investigation, and studying their interactions with reactive oxygen species (ROS), especially the superoxide radical, is of importance. The latter is biologically obtained through two main processes: (1) leakage from the electron transport chain during oxidative phosphorylation [37] and (2) the generation of the superoxide radical by NADPH oxidases in the immune system to destroy ingested microbes by neutrophils and other leukocytes [38]. However, excess superoxide is damaging and needs to be regulated. This is performed enzymatically by superoxide dismutases (SODs) and assisted through human diets that provide natural antioxidant compounds from fruits and vegetables, including vitamins C and E. Therefore, we focus our attention on trying to understand the mechanism by which vitamin C and α-tocopherol exhibit a cooperative effect to ameliorate oxidative stress, specifically due to the superoxide radical. Among these antioxidant mechanisms, π-π interactions between superoxide and its scavengers permit superoxide to act as a reducing agent, mimicking SOD action, as recently shown for isoflavones [39].
A recent review on superoxide chemistry [40] describes the role played by the electrodes in the electrochemical generation of O2•–. This is relevant to the rotating ring–disk electrode (RRDE) voltammetry technique that we developed and used in this study. In fact, we perform voltaic experiments to measure individual superoxide scavenging by each vitamin as well as examine their combined effects. The advantage of our method is that the concentration of superoxide present in the solution can be calculated directly. Density Functional Theory (DFT) calculations supporting a synergistic effect for both vitamins are evaluated in a similar environment to the natural location of vitamin E in the cell membrane. This chemical mechanism agrees with our experimental results.
2. Materials and Methods
2.1. Hydrodynamic Voltammetry (RRDE)
Hydrodynamic Voltammetry was performed at a rotating ring–disk electrode (RRDE) using the WaveDriver 20 bipotentiostat (Pine Research, Durham, NC, USA) with the MSR Electrode Rotator, also from Pine Research, Durham, NC, USA. In RRDE, the bipotentiostat measures, at the same time, both the currents at the disk and ring electrodes (that correspond to charge movements among the ring, the disk, and the counter electrode) and the potentials of the disk and ring electrodes relating to the single reference electrode. The RRDE cell contains four electrodes: the two gold working electrodes, both the rotating ring and disk (Pine Research, Durham, NC, USA), one coiled platinum wire counter electrode, and one reference electrode consisting of a platinum wire immersed in 0.1 M dried tetrabutylammonium bromide, TBAB (Sigma-Aldrich, St. Louis, MO, USA), dissolved in 50 mL dimethyl sulfoxide, DMSO, anhydrous, ≥99.9% (Sigma-Aldrich, St. Louis, MO, USA), in a fritted glass tube. The electrodes were placed in a 5-neck electrochemical cell together with means for either bubbling or blanketing the solution with gas. The solution was subsequently bubbled with dry O2/N2 (35%/65%) for five minutes to establish the required dissolved molecular oxygen level. Careful initial cleaning of the electrodes was performed to clear potential film formation.
Aftermath software release 1.6.10523 (Pine Research, Durham, NC, USA) was used to set up the parameters needed for the experiment: the potential sweep was applied to the disk from 0.2 V to −1.2 V and then reversed to 0.2 V, while the potential of the ring electrode was held constant at 0.0 V. The disk voltage sweep rate was set at 25 mV/s. The rotation setting used for the rotation of the Au/Au disk electrode was chosen to be 1000 rpm at the disk electrode. The superoxide radical is generated through molecular oxygen reduction, and the peak was detected around −0.6 V. Meanwhile, the reverse oxidation reaction of the remaining unreacted superoxide radicals was detected at the ring electrode. An initial blank solution consisting of bubbled N2/O2, the electrolyte TBMB, and DMSO was run. The ratio of the ring/disk current was defined as “efficiency”. Next, an antioxidant aliquot of vitamin E (DL-α-tocopherol) or vitamin C (0.03 M) was introduced. The solution containing antioxidant in the voltaic cell was bubbled with the gas mixture for 5 min, a revised voltammogram was documented, and the corresponding efficiency was calculated. In this way, the rate at which the increasing concentration of the antioxidant scavenges the generated superoxide radicals during the electrochemical reaction is determined upon the addition of each antioxidant aliquot. Aftermath software was used to record the results from each run, represented as voltammograms showing the current vs. potential graphs. These were later evaluated using Microsoft Excel. The volume amount used in each of the aliquots is indicated in the related RRDE graph. Finally, the decreasing slope of the curve, describing the overall decrease in efficiency with the incremental addition of the antioxidant, serves as a quantitative measure of the antioxidant activity of the vitamins. Any decrease in the collection efficiency is anticipated to be due to the amount of superoxide consumed by the antioxidant. This method was developed in our laboratory [41].
2.2. Computational Study
Calculations were run using BIOVIA Materials Studio DMoL3, implemented in Materials Studio 7.0. DMoL3 is a modeling program (Dassault Systèmes, San Diego, CA, USA) that utilizes Density Functional Theory (DFT) to calculate properties of molecules such as energy, geometry, and transition state optimizations [42]. The results allow the relationships between the molecular structure with its antioxidant properties and scavenging behavior to become evident. We employed the double numerical polarized (DNP) basis set, including all the occupied atomic orbitals plus a second set of valence atomic orbitals, as well as polarized d-valence orbitals [43]. Correlation generalized gradient approximation (GGA) was used, including BLYP correlation plus BLYP-D and Becke exchange [44]. We also included Grimme’s correction when van der Waals interactions were involved [45]. The continuous model of Dmol3 was applied for solvent effects and calculation, including polar DMSO, non-polar n-hexane, or non-solvent (gas phase), showing no important variation in dimensions (bond distances or separations between isolated species) [46]. All electrons were treated explicitly, and the real space cutoff of 5 Å was set for the numerical integration of the Hamiltonian matrix elements. The self-consistent field convergence criterion was established for the root mean square variation in the electronic density to be less than 10−6 electron/Å3. The convergence criteria applied during geometry optimization were 2.72 × 10−4 eV for energy and 0.054 eV/Å for force.
An attempt to use a functional of higher quality, B3LYP, which is better at estimating potential barrier heights, was made, but calculations became extremely lengthy. Ultimately, these B3LYP calculations confirmed what we saw using BLYP, e.g., some calculated potential energy minimums for our molecules are extremely shallow, which makes for extremely prolonged calculations to reach the needed energy minimum. (After almost a week for completion of the first calculation where we get convergence, we needed to refine the entire system to look for the true minimum through other very lengthy iterative refinements). Therefore, the dimensions of our molecules pose a problem, as using the strong functional (B3LYP) instead of BLYP is markedly time-consuming. From our computational studies, we are looking mainly for trends in the barriers to identify fast and slow steps for correlation with our experimental studies, which can be achieved using the simpler and faster BLYP functional. These calculations try to correlate experimental RRDE features.
3. Results and Discussion
3.1. DFT of Vitamin E-Model and Vitamin C
3.1.1. Vitamin E-Model Scavenges Superoxide
DFT methods were applied to study the antioxidant properties of vitamins E and C. To decrease the calculation time, we used a model of vitamin E α-tocopherol, which we call vitamin E-model (Figure 1), which consists of the 6-chromanol nucleus contained in the α-tocopherol isomer but with the long, hydrophobic, saturated side chain replaced by a methyl group. The model then has a total of five methyl groups on the 6-chromanol nucleus (methyl groups in position R1, R2, and R3 of the aromatic ring plus the two methyl groups at the C2 position of the 6-chromanol). This model is a suitable representative of all forms of vitamin E, and a published report concluded that the antioxidant capacity of vitamin E and a model without the side chain were quite similar [47].
The superoxide radical anion, considered to be of major importance in cell biology, is primarily obtained when an oxygen molecule acquires an extra electron leaked from the mitochondria during aerobic cellular respiration that ultimately produces ATP [42,48,49]. The reactivity of the superoxide radical is controlled by endogenous enzymes, superoxide dismutases, catalase, and glutathione peroxidase, which help to cope with oxidative stress by effectively quenching free radicals [50,51]. Substantial evidence links increases in oxidative stress [52] with the decline of enzyme regulation of the superoxide anion during aging. Leakage of this radical anion during oxidative stress creates conditions for attack on DNA, proteins, and other important biological molecules, leading to disease states [53].
Due to the lipidic character of vitamin E with its long saturated hydrophobic side chain, α-tocopherol is recognized as the major antioxidant in membrane-rich fractions such as mitochondria and microsomes [54,55]. This strategic position makes vitamin E an important means of scavenging the superoxide radical inside the cell membrane [56]. It is, therefore, important to describe the initial interaction between the 6-hydroxyl in vitamin E-model and superoxide. Using computational methods, the reaction, with the minimized structures of reagent, transition state (TS), and product, shown in Figure 2, has ΔG of −0.4 Kcal/mol and an easily attainable E(barrier) of 0.8 Kcal/mol. Figure 3 shows details of the transition state search. These results demonstrate that vitamin E-model can intercept and neutralize superoxide. A potential ensuing step on the product shown in Figure 2 is described in Figure 4, where a proton is placed at van der Waals separation from the added superoxide, 2.60 Å. The minimization of Figure 4 arrangement (Figure 5) shows H2O2 formation and separated 1.654 Å from the O(polyphenol) in position 6, which is longer than the initial input conditions, 1.498 Å. Therefore, hydrogen peroxide was eliminated, and the residual semiquinone vitamin E-model was minimized. The next step in this study involves the π-π interaction with an additional molecule of superoxide. This process was shown to mimic superoxide dismutase action by some natural organic molecules and is described in previous studies and a recent review [57].
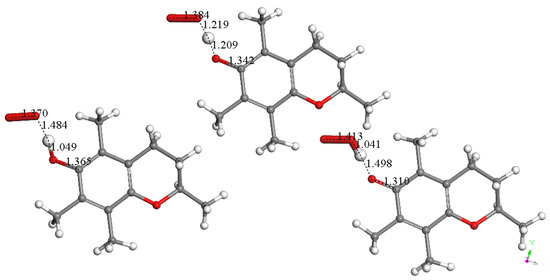
Figure 2.
Superoxide scavenges H(hydroxyl) in position 6 of vitamin E-model. Minimized structures of reagent (left), transition state (center), and product (bottom right) are shown. This reaction has ΔG of −0.4 Kcal/mol and E(barrier) of 0.8 Kcal/mol. The transferred H atom is equidistant from both moieties in this TS: O(superoxide)—H = 1.219 Å, O6—H = 1.209 Å. O atoms, red; C atoms, black; H atoms, light grey.
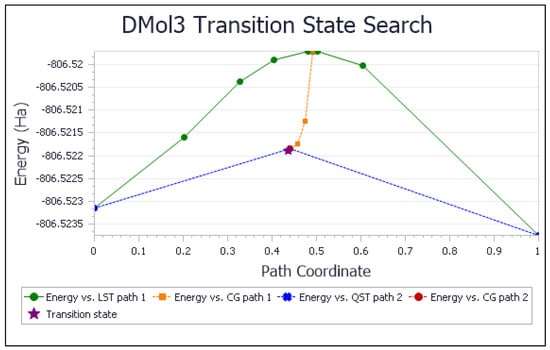
Figure 3.
TS search of scavenging superoxide by vitamin E-model. ΔG = −0.4 kcal/mol; E(barrier) = 0.8 kcal/mol.
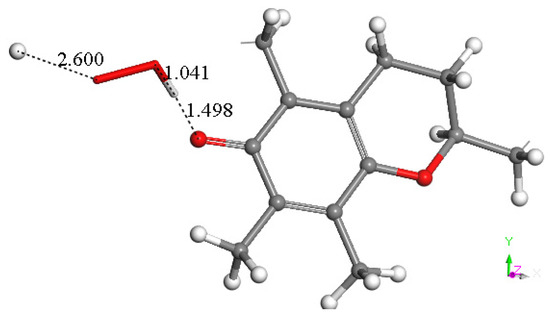
Figure 4.
A proton is placed near the HO2 moiety of Figure 2 product, having van der Waals separation of 2.60 Å.
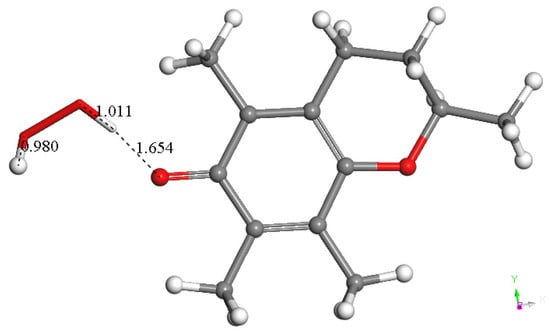
Therefore, the semiquinone vitamin E-model was minimized, and a superoxide radical was π-π placed above its aromatic ring (van der Waals distance, 3.50 Å). Upon geometry minimization, there is a shortening of the distance between both centroids, 2.899 Å, indicating bond formation. In the meantime, the initial superoxide bond distance of 1.373 Å shortens to 1.302 Å, which implies that some electron density becomes directed toward the ring (Figure 6). This overall arrangement is non-radical due to its even number of electrons, arising from two superoxides (each one contributing with an odd number of electrons), and it is negatively charged (−1), resulting from two negative charges of the two superoxides (from Figure 2 and Figure 6) and one proton (from Figure 4).
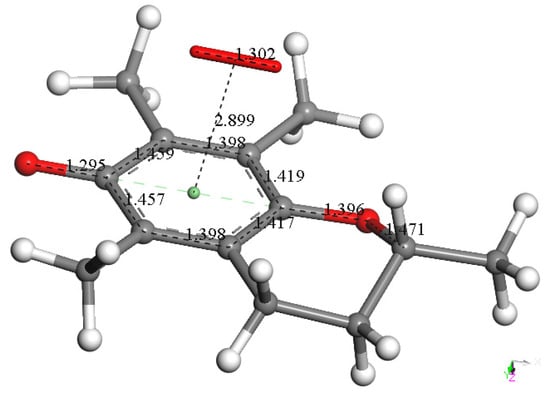
Figure 6.
After elimination of H2O2 from the previous arrangement (Figure 5), the remaining species, semiquinone vitamin E-model, was minimized, and a superoxide radical was π-π placed above the aromatic ring (van der Waals distance, 3.50 Å). Its geometry minimization shows shortening between both centroids, 2.899 Å, indicating bond formation. Meanwhile, the initial superoxide bond distance of 1.373 Å shortens to 1.302 Å, which indicates some electron density was directed toward the ring.
It can be expected that a cation will react with this arrangement, and so a proton was van der Waals placed near O6 of the anion arrangement, with an O6-proton distance of 2.60 Å. Its DFT optimization shows O6-H6 bond formation, 0.976 Å (Figure 7). This reaction has a small energy barrier, as observed after a TS search, and the resulting structure is shown in Figure S1. The C6-O6 bond length, 1.378 Å, is characteristic of a single bond (Figure 7) and is longer than the partial double bond in the initial species, 1.295 Å (Figure 6). The effects of proton addition to O6 are also evident in the aromatic ring, with four bond lengths becoming more similar, range 1.42–1.43 Å, than those in the reacting species, range 1.42–1.46 Å, while the remaining two bond lengths in the ring continue to be shorter and unaltered (1.398–1.399 Å). These effects are related to the shorter distance between superoxide and ring centroids, 2.664 Å, compared with the initial species, 2.899 Å. Thus, the approach of a positive charge, a proton, to the reacting species makes the π-π bound superoxide shift more electron density toward the ring. This forms a neutral and non-radical species, η-O2-vitamin E-model, and H2O2, resulting from the interaction of vitamin E-model with two superoxide molecules (one π and one σ added to the polyphenol), plus two protons, shown in Reaction (1).
2O2•− + vitE-model + 2H+ → H2O2 + η-O2-vitE-model
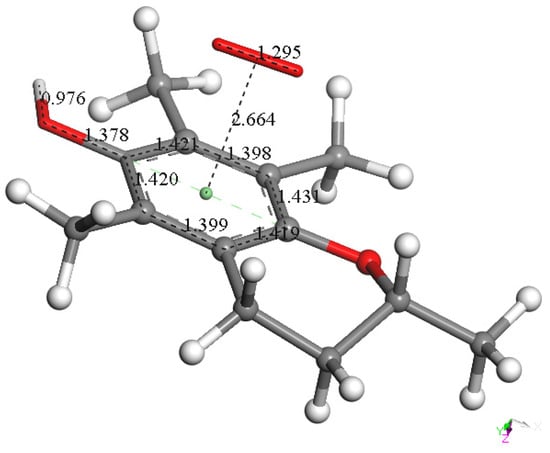
Figure 7.
DFT optimization result after a proton was placed near O6 of the previous arrangement, Figure 6, at O6-proton distance 2.60 Å. It shows O6-H6 bond formation, 0.976 Å; the C6-O6 bond length, 1.378 Å, is a typical single bond and is longer than the partial double bond in the reacting species shown above, 1.295 Å (Figure 6). This reaction has a small barrier, observed after a TS search, whose resulting structure is shown in Figure S1.
Earlier studies using UV–Vis and ESR techniques involving the scavenging of DPPH and galvinoxyl radicals by a vitamin E derivative, IRFI005, describe a “two electrons and/or 2 H-atoms” donation mechanism for each molecule of the scavenger [58].
Our mechanism is shown in Scheme 1. The scavenging mechanism here described for superoxide, a biologically relevant radical, includes partial π-π donation of an electron to the aromatic ring of vitamin E and capture of a H atom by another superoxide. In a related excellent theoretical study about vitamin E, π-π interactions have not been considered for scavenging superoxide [59].
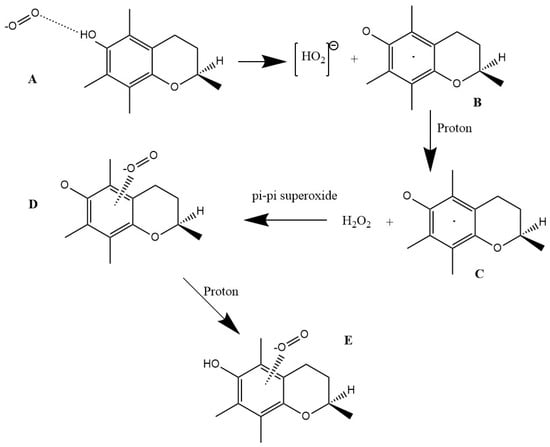
Scheme 1.
Vitamin E scavenges superoxide. (A) Superoxide approaches vitamin E hydroxyl in position 6; (B) H is captured by superoxide, forming [HO2]− plus vitamin E semiquinone; (C) a proton is captured by [HO2]−, forming H2O2; (D) an additional superoxide interacts π-π with the aromatic ring; (E) an additional proton interacts with the semiquinone, forming η-O2-vitamin E-model.
We compare the energy barriers for the higher-quality B3LYP functional and the BLYP used in Figure 2. A TS Optimization for the TS shown in Figure 2 shows equal energy for both systems BLYP and B3LYP, suggesting no underestimation of the energy barrier by BLYP: energy of transition state: −806.5218885 Ha (BLYP), −806.521889 Ha (B3LYP). In addition, Figure 2 TS for the transferred proton has O(superoxide)-H = 1.219 Å, O(VitaminE)-H = 1.209 Å, and the corresponding distances are very similar to those obtained using B3LYP calculation (1.211 Å and 1.204 Å), as shown in Figure S1.
3.1.2. Vitamin C Restores Vitamin E-Model
As shown in Figure 7, a reacting proton is needed to accomplish Scheme 1. Therefore, the interaction between the semiquinone vitamin E-model and vitamin C, with an initial separation of 2.60 Å (Figure 8), was studied with DFT, resulting in a proton transfer to the semiquinone (Figure 9). This reaction is possible as shown by ΔG = −2.7 Kcal/mol and E(barrier) = 0.7 Kcal/mol. Since the total charge of the system is zero, the result of this interaction is an ascorbate radical and vitamin E-model.
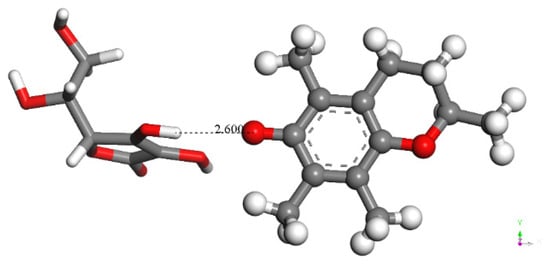
Figure 8.
Initial state for vitamin C (left) transferring a H atom to the vitamin E-model semiquinone (right), which previously gave up a proton to superoxide; the total charge of this radical is 0.
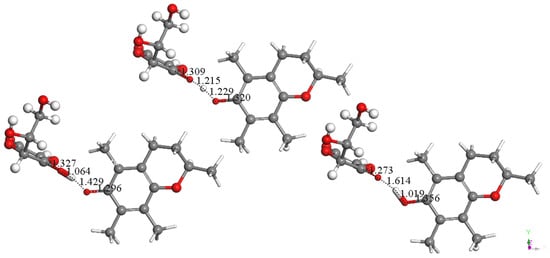
Figure 9.
Vitamin C transfers a hydrogen atom to vitamin E-model semiquinone. Left: Minimum of energy after approaching H(vitamin C) to vitamin E-model semiquinone (obtained after DFT minimization of Figure 8). Right: Product of reaction. Center: Transition state showing H located midway between both O atoms, 1.215 Å from vitamin C and 1.229 Å from vitamin E. This reaction is characterized by ΔG = −2.7 Kcal/mol and E(barrier) = 0.7 Kcal/mol.
3.1.3. Antioxidant Vitamin C Scavenges Superoxide
Our DFT calculations support that vitamin C is an antioxidant, as seen in Figure 10, Figure 11, Figure 12, Figure 13 and Figure S2. Figure 10 shows the initial σ approach of superoxide to the acidic proton of vitamin C. Figure 11 shows the result of DFT minimization using an n-hexane solvent effect, whose low dielectric constant is closely related to the lipidic environment in the membrane cell, the natural location of vitamin E. Figure S2 applies a DFT DMSO solvent effect, which is related to the experimental aprotic solvent used in RRDE, vide infra, and the structural results of Figure 11 and Figure S2 are similar. The expected subsequent step for Figure 11 is a proton addition to obtain H2O2, which is then a substrate in the catalase enzymatic reaction to yield H2O plus ½ O2. H2O2 is obtained after a second ascorbic acid approaches the most exposed O atom of superoxide in Figure 11. The separation between H2O2 and both ascorbates is 1.680 Å and 1.698 Å (Figure 12).
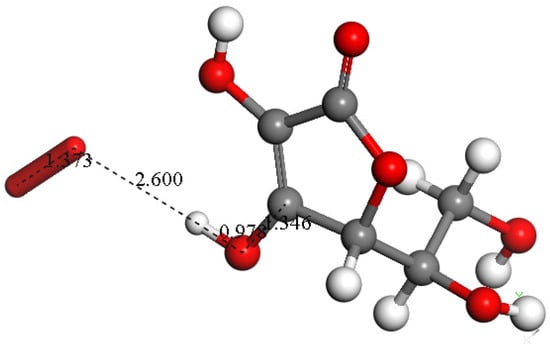
Figure 10.
Vitamin C scavenging superoxide. Initial state, superoxide is placed at van der Waals separation, 2.60 Å, from the acidic proton of vitamin C.
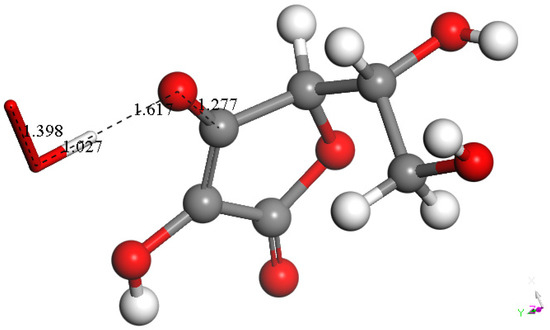
Figure 11.
Superoxide forms a HO2 moiety, well separated from the remaining species. DFT calculation performed in n-hexane, an organic environment that can mimic the membrane cell. Bond distances within HO2, O-H = 1.027 Å, O-O = 1.398 Å; separation between both units = 1.617 Å; within the vitamin C derivative C-O = 1.277 Å.
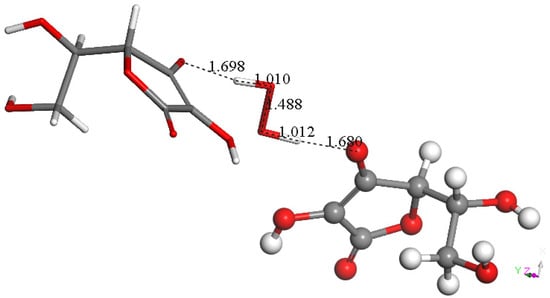
Figure 12.
A 2nd vitamin C molecule, stick style, was placed near the most exposed O(superoxide) of the product arrangement shown in Figure 11. DFT showed formation of H2O2 well separated from two vitamin C species, 1.680 Å and 1.698 Å. Therefore, H2O2 is a product of vitamin C scavenging superoxide.
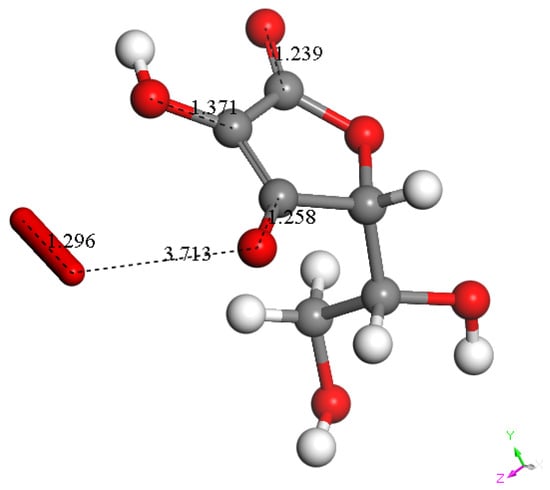
Figure 13.
Interaction between superoxide and the vitamin C radical shown in Figure 12 (right). Superoxide gives its electron to the scavenger, which becomes a monoanionic quinone-like species. Meanwhile, the short O-O bond distance of the incoming radical, 1.296 Å, may be associated with a leaving O2 molecule, as shown by the separation between both units, 3.713 Å, longer than the van der Waals separation, 2.80 Å.
Since one of the two remaining vitamin C derivatives is a radical (Figure 12), this can easily react with an additional superoxide and yield a quinone-like vitamin C derivative (see Figure 13). This can be considered part of the quenching action of stopping lipid peroxidation. The alternative reaction involving a π-π interaction, instead of the σ superoxide attack on the quinone vitamin C derivative of Figure 10, was shown not to be feasible in performing DFT calculations. Therefore, a vitamin C quinone formation is suggested, along with H2O2, for superoxide scavenging of vitamin C. A detailed theoretical mechanism for vitamin C oxidation by the superoxide anion radical reveals the presence of radical vitamin C quinone derivatives. These compounds have also been modeled with water molecules [60]. However, in the RRDE method used here, water molecules do not play a role as they react with superoxide when they arrive at the disk electrode. We use anhydrous DMSO solvent to have minimal water solvation for vitamins C and E, using the disk electrode where superoxide is generated.
3.2. Hydrodynamic Voltammetry (RRDE)
The superoxide radical must be generated experimentally to measure its scavenging. There are several methods to do this: (1) an enzymatic reaction with xanthine dehydrogenase [61] and (2) a non-enzymatic option using phenazine methosulphate, NADH, and molecular oxygen [62]. A third non-enzymatic option to generate superoxide is by dissolving potassium superoxide, KO2, in a given solvent [63,64]. However, this method suffers from complex starting conditions since superoxide reacts readily with protons. Thus, for KO2 use, it is necessary to operate either in an anhydrous solution or a strongly basic water environment [65]. Once generated, the superoxide concentration is followed using spectrophotometric, colorimetric, chemiluminescence, and fluorescence methods. Also, the superoxide can be trapped with 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), and the resultant DMPO-OH adduct is detectable by ESR. These methods all indirectly measure superoxide concentration as they consist of measuring a product generated through superoxide consumption, whose concentration is decreased with the addition of an antioxidant.
A simpler method to generate the superoxide radical is provided by classical cyclic voltammetry in a voltaic cell. Increasing the concentration of antioxidants in the voltaic cell detects the decrease in the current intensity of the superoxide signal [66]. However, the differences among several voltammograms, obtained after successive additions of scavenger, are not well distinguished, and a quantitative measure of superoxide is difficult [41]. We improved this system by splitting reduction and oxidation using a rotating ring–disk electrode (RRDE). At the disk, reduction will produce superoxide following Reaction (2) (lower part of Figure 14), while at the ring, the opposite oxidation reaction will be performed (upper part of Figure 14). This RRDE method measures superoxide scavenging directly, e.g., a real superoxide concentration is detected. A recent review describes several natural polyphenols analyzed with the RRDE technique [57].
O2 + e− → O2−
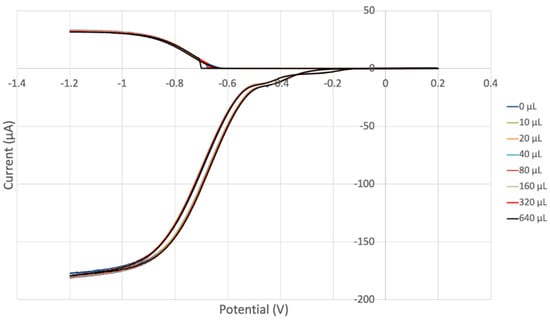
Figure 14.
RRDE voltammograms of vitamin E. Each run is associated with a specific color (e.g., red) and corresponds to the red oxidation curve (top, positive current) detected at the ring electrode and the red reduction curve detected at the disk electrode (bottom, negative current). These individual voltammograms show almost no variation; that is, the superoxide destroyed at the ring electrode has a similar value for all runs, suggesting that vitamin E is not a strong antioxidant.
Our first experiment (Figure 14) shows voltammograms of vitamin E in which the different runs are not easily distinguished; that is, the measurements in the variation in superoxide concentration destroyed at the ring electrode, upper part, are almost all similar, which suggests that the vitamin E is not a strong antioxidant in this experiment. This is more clearly and quantitatively indicated in Figure 15, showing the efficiency collection. The estimated line equation, y = −0.0018x + 18.391, R2 = 0.8981, has a slope of −1.8 × 103, which is associated with vitamin E antioxidant capability. Even so, the vitamin E slope is slightly better than that of the previously measured commercial antioxidant butylated hydroxytoluene, BHT (−1.6 × 103) [57]. Vitamin E is a component of olive oil, previously analyzed by us [67], and both show closely related scavenging of superoxide.
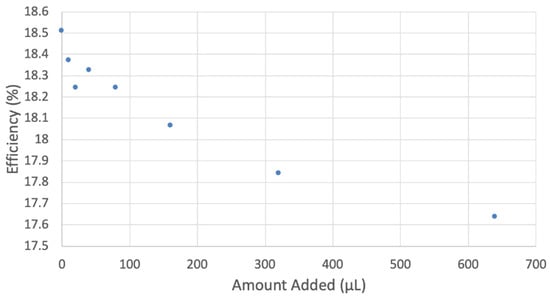
Figure 15.
Collection efficiency of vitamin E cyclovoltammetry, y = −0.0012x + 18.352, R2 = 0.8969 considering all points; y = −0.0018x + 18.391, R2 = 0.8981, excluding the 640 µL data. In experiments at high concentrations of some antioxidants, for instance, quercetin [41], a related decreasing pattern in efficiency is seen, and the last data point is not included.
RRDE vitamin C results (Figure 16 and Figure S3), with slope −2.6 × 104, indicate a stronger scavenging activity than that of vitamin E alone. The voltammograms (Figure 16) are much more separated from each other than those shown in Figure 14 for vitamin E. The vitamin C slope of the efficiency graph, −2.6 × 104, is like eriodictyol (−2.2 × 104), weaker than butein (−11.2 × 104) and stronger than the commercial antioxidant BHT (−0.16 × 104) [57].
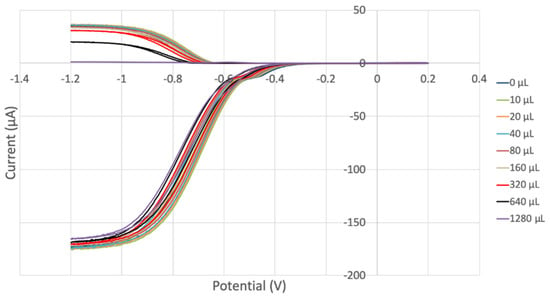
Figure 16.
Voltammograms for 0.03 M vitamin C. They are much more separated from each other than in Figure 14, indicating a stronger antioxidant activity with increasing concentration of vitamin C than vitamin E.
We were interested in analyzing a potential interaction between both vitamins regarding superoxide scavenging. Thus, to a fixed concentration of vitamin E, 640 μL, aliquots of vitamin C were added, and measurements were taken (Figure 17, Figure 18, Figures S3 and S4). Both vitamins are more effective together than individually. Therefore, combining vitamins E and C produces a steeper slope, −7.2 × 104 (Figure 18), than that of vitamin E alone, −1.8 × 103 (Figure 15), or vitamin C alone, −1.3 × 104 (Figure 18). This supports earlier studies describing a cooperative association between the two antioxidant vitamins [1,14,68].
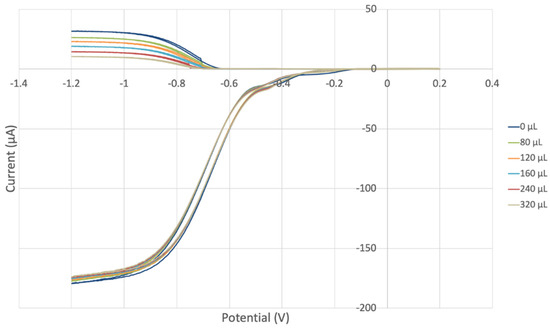
Figure 17.
Voltammograms for vitamin C aliquots in fixed amount of vitamin E, 640 µL.
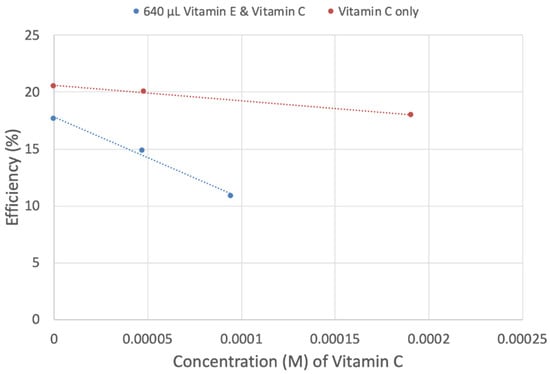
Figure 18.
Different slopes of vitamin C and vitamins C plus E. Vitamin C (red line): y = −13,359x + 20.573 R2 = 0.9947; vitamin E + vitamin C (blue line): y = −71,724x + 17.833 R2 = 0.99.
4. Conclusions
The antioxidant vitamins C and E are essential nutrients that participate in crucial immune system reactions, and they mitigate the results of many diseases related to oxidative stress [6].
In this work, we use the RRDE method to measure the presence of superoxide radicals in a voltaic cell directly and have shown that when administered together, vitamin C and vitamin E antioxidant activity is enhanced. The described superoxide scavenging mechanisms for vitamin C alone and together with vitamin E show marked differences. Vitamin C reacts through its acidic proton and primarily produces H2O2, while vitamin C is transformed into a quinone derivative (Figure 13). In contrast, vitamin E possesses an aromatic ring that is also susceptible to π-π interaction (not feasible for vitamin C) with a second superoxide radical, and, in fact, the second superoxide remains trapped by vitamin E to form a molecular complex, η-O2-vitamin E-model (Figure 7). Moreover, in Scheme 1, the antioxidant action of vitamin E includes the interaction of vitamin E semiquinone with an acidic reagent. This process has been studied in depth using DFT when the acidic compound is vitamin C (Figure 8) and involves the transfer of an H atom to vitamin E semiquinone (Figure 9) to produce an ascorbate radical plus restored vitamin E. This concurs with the results of earlier studies that ascorbic acid can donate a hydrogen atom to a tocopheroxyl radical at the rate of 2 × 105 Mol/s because of the difference of 1-electron reduction potential between ascorbic acid (282 mV) and a tocopheroxyl radical (480 mV) [69].
Also, an ESR study in plasma of vitamin C and vitamin E reacting with superoxide [70], in which ascorbate and tocopheroxyl free radicals were subjected to oxidative stress, showed an immediate increase in the concentration of ascorbate radicals, which then steadily declined. Only after the virtual disappearance of the ascorbate radical was the tocopheroxyl radical detected. This seems consistent with our study, where the role of vitamin C is to reform vitamin E, and provides an explanation as to why the ESR signal [70] for the vitamin E radical shows up only when vitamin C is exhausted. In conclusion, these results are important to enhancing our understanding of the synergistic features of these two antioxidant vitamins in different disease states and aging effects in humans.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biophysica4020022/s1, Figure S1. TS (B3LYP calculation): O(superoxide)-H = 1.211 Å, O(VitE)-H = 1.204 Å; Figure S2. The TS structural model after approaching a proton near O6 (Figure 7), which determines O6-H6 formation. E(barrier) = 1.1 Kcal/mol ΔG = −82.2 Kcal/mol. We name the related product η-O2-vitamin E-model; Figure S3. Same calculation of Figure 11 but including the DMSO solvent effect, used in the RRDE experimental section. Distances have similar relevant distances seen in Figure 11. They are 1.025 Å, 1.417 Å, 1.611 Å, and 1.278 Å, respectively; Figure S4. Collection efficiency for Vit C, y = −26,555x + 21.433, R2 = 0.986, all data included; Figure S5. Collection efficiency of vitamins C and E, referred to Figure 17; all data included (y = −63,236x + 17.502, R2 = 0.9913).
Author Contributions
Conceptualization, F.C. and M.R.; Methodology, S.B.; Data curation, R.S., J.Z.P. and S.I.; Writing manuscript, M.R. and F.C.; Visualization, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Packer, J.; Slater, T.; Willson, R. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979, 278, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.J.; Mulholland, C.W. Vitamin C and Vitamin E—Synergistic Interactions In Vivo? In Free Radicals and Aging; EXS; Emerit, I., Chance, B., Eds.; Birkhäuser: Basel, Switzerland, 1992; Volume 62. [Google Scholar] [CrossRef]
- Fleming, E.; Luo, Y. Co-delivery of synergistic antioxidants from food sources for the prevention of oxidative stress. J. Agric. Food Res. 2021, 3, 100107. [Google Scholar] [CrossRef]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertens. J. Am. Heart Assoc. 2001, 38, 606–611. Available online: https://api.semanticscholar.org/CorpusID:2072452 (accessed on 1 January 2023). [CrossRef] [PubMed]
- Jeng, K.C.; Yang, C.S.; Siu, W.Y.; Tsai, Y.S.; Liao, W.J.; Kuo, J.S. Supplementation with vitamins C and E enhances cytokine production by peripheral blood mononuclear cells in healthy adults. Am. J. Clin. Nutr. 1996, 64, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, I.M.; Gilmore, W.S.; Benzie, I.F.; Mulholland, C.W.; Strain, J.J. Interactions between vitamins C and E in human subjects. Br. J. Nutr. 2000, 84, 261–267. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Sánchez, C.; Vallejo, C.; Díaz-Del Cerro, E.; Arnalich, F.; Hernanz, Á. Vitamin C and vitamin C plus E improve the immune function in the elderly. Exp. Gerontol. 2020, 142, 111118. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, E.; Fernandez, M.C.; O’Flaherty, C. A novel combination of γ-tocopherol-rich mixture of tocopherols and ascorbic acid restores fertility in cases of tyrosine nitration-associated male infertility in mice. Antioxidants 2020, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Khadim, R.M.; Al-Fartusie, F.S. Antioxidant vitamins and their effect on immune system. J. Phys. Conf. Ser. 2021, 1853, 012065. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Nazarian, B.; Sarreshtedari, M.; Mozaffari-Khosravi, H.; Maleki, V.; Alizadeh, M.; Shokri, A.; Sadeghi, O. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 17234. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Military Nutrition Research. Military Strategies for Sustainment of Nutrition and Immune Function in the Field. In Volume 13, Vitamin E, Vitamin C, and Immune Response: Recent Advances; National Academies Press: Washington, DC, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK230984/ (accessed on 1 January 2024).
- Victor, V.M. Ascorbic acid as modulator of immune function in sepsis. Lett. Drug Des. Discov. 2005, 2, 239–244. [Google Scholar] [CrossRef]
- Gumpricht, E.; Rockway, S. Can ω-3 fatty acids and tocotrienol-rich vitamin E reduce symptoms of neurodevelopmental disorders? Nutrition 2014, 30, 733–738. [Google Scholar] [CrossRef]
- Girndt, M.; Kaul, H.; Lengler, S.; Sester, U.; Sester, M.; Kohler, H. Immunological biocompatibility characterization of a vitamin E-bonded membrane. Contribut. Nephrol. 1999, 127, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, M.A.; Puertollano, E.; Alvarez de Cienfuegos, G.; de Pablo, M.A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Maneesh, M.; Jayalekshmi, H. Effect of ascorbic acid, α-tocopherol, lecithin and L-ornithine-L-aspartate on Ethanol induced hypoproteinemia and hyperlipidemia in rats. Ind. J. Physiol. Pharmacol. 2005, 49, 422–426. [Google Scholar]
- Anderson, R. Mechanisms of vitamin-mediated anti-inflammatory and immunomodulatory activity. Biblioth. Nutr. Dieta 2001, 55, 135–147. [Google Scholar] [CrossRef]
- Evans, W.J. Vitamin E, vitamin C, and exercise. Am. J. Clin. Nutr. 2000, 72, 647S–652S. [Google Scholar] [CrossRef]
- Bendich, A. Immunological role of antioxidant vitamins. In Antioxidants in Human Health and Disease; Basu, T.K., Temple, N.J., Garg, M.L., Eds.; CAB International: Wallingford, UK, 1999; Available online: https://epdf.pub/antioxidants-in-human-health-and-disease.html (accessed on 1 January 2024).
- Bucher, A.; White, N. Vitamin C in the prevention and treatment of the common cold. Am. J. Lifestyle Med. 2016, 10, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Khanna, D. The Role of Vitamin C in human immunity and its treatment potential against COVID-19: A review article. Cureus 2023, 15, e33740. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Sajedianfard, J. An overview of the characteristics and function of vitamin C in various tissues: Relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18, e4037. [Google Scholar] [CrossRef]
- Dresen, E.; Lee, Z.-Y.; Hill, A.; Notz, Q.; Patel, J.J.; Stoppe, C. History of scurvy and use of vitamin C in critical illness: A narrative review. Nutr. Clin. Pract. 2023, 38, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Kirby, T.O.; Sapp, P.A.; Gonzalez, A.M.; Marshall, T.M.; Esposito, R. Nutrient synergy: Definition, evidence, and future directions. Front. Nutr. 2023, 10, 1279925. [Google Scholar] [CrossRef] [PubMed]
- Alboaklah, H.K.M.; Leake, D.S. Effect of vitamin E on low density lipoprotein oxidation at lysosomal pH. Free Radic. Res. 2020, 54, 574–584. [Google Scholar] [CrossRef]
- Catalgol, B.; Ozer, N.K. Protective effects of vitamin E against hypercholesterolemia-induced age-related diseases. Genes Nutr. 2012, 7, 91–98. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Ehizuelen Ebhohimen, I.; Stephen Okanlawon, T.; Ododo Osagie, A.; Norma Izevbigie, O. Vitamin E in Human Health and Oxidative Stress Related Diseases; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Caruso, F.; Belli, S.; Rossi, M. Scavenging of superoxide in aprotic solvents of four isoflavones that mimic superoxide dismutase. Int. J. Mol. Sci. 2023, 24, 3815. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.; Rossi, M.; Molasky, N.; Middleton, L.; Caldwell, C.; Bartow-McKenney, C.; Duong, M.; Chiu, J.; Gibbs, E.; Caldwell, A.; et al. Effective and novel application of superoxide radical scavenging by natural phenolic antioxidants. Antioxidants 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Delley, B.J. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Kawakami, A.; Saito, M.; Yamamoto, Y.; Tsuchiya, J.; Kamiya, Y. Effect of phytyl side chain of vitamin E on its antioxidant activity. J. Biol. Chem. 1985, 260, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Dighe, P.A.; Mezera, V.; Monternier, P.A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yan, T.; Lim, R.; Oberley, L.W. Expression of superoxide dismutases, catalase, and glutathione peroxidase in glioma cells. Free Radic. Biol. Med. 1999, 27, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, D.; Smits, K.; Osório, N.; Caseiro, A. Oxidative stress in relation to aging and exercise. Encyclopedia 2022, 2, 1545–1558. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Wang, X.; Quinn, P.J. The location and function of vitamin E in membranes (Review). Mol. Membr. Biol. 2000, 3, 143–156. [Google Scholar] [CrossRef]
- Atkinson, J.; Harroun, T.; Wassall, S.R.; Stillwell, W.; Katsaras, J. The location and behavior of alpha-tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of plasma membrane repair by vitamin E. Nat. Commun. 2011, 2, 597. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic polyphenol π-π interactions with superoxide radicals contribute to radical scavenging and can make polyphenols mimic superoxide dismutase activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Pedersen, J.Z.; Incerpi, S.; Moosavi-Movahedi, A.A.; Saso, L. The mechanism of antioxidant activity of IRFI005 as a synthetic hydrophilic analogue of vitamin E. Biochimie 2011, 93, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Honda, R.; Kuwata, K.; Usui, S.; Uno, B. Electrochemical and mechanistic study of reactivities of α-, β-, γ-, and δ-tocopherol toward electrogenerated superoxide in N,N-dimethylformamide through proton-coupled electron transfer. Antioxidants 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO˙/O2˙− radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, M.; Kaspari, K.; Stagge, S.; Rethmeier, R.; Mendel, R.R.; Bittner, F. Xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant Mol. Biol. 2010, 72, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, C.M.; Debiton, E.; Barbakadze, V.V.; Kemertelidze, E.P. Evaluation of the dietetic and therapeutic potential of a high molecular weight hydroxycinnamate-derived polymer from Symphytum asperum Lepech. Regarding its antioxidant, antilipoperoxidant, antiinflammatory, and cytotoxic properties. J. Agric. Food Chem. 2001, 49, 3942–3946. [Google Scholar] [CrossRef]
- Grivennikova, V.G.; Vinogradov, A.D. Generation of superoxide by the mitochondrial complex I. Biochim. Biophys. Acta Bioenergy 2006, 1757, 553–561. [Google Scholar] [CrossRef]
- Kladna, A.; Berczynski, P.; Kruk, I.; Michalska, T.; Aboul-Enein, H.Y. Superoxide anion radical scavenging property of catecholamines. Luminescence 2013, 28, 450–455. [Google Scholar] [CrossRef]
- Severino, J.F.; Goodman, B.A.; Kay, C.W.M.; Stolze, K.; Tunega, D.; Reichenauer, T.G.; Pirker, K.F. Free radicals generated during oxidation of green tea polyphenols: Electron paramagnetic resonance spectroscopy combined with density functional theory calculations. Free Radic. Biol. Med. 2009, 46, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Hauchard, D.; Darchen, A.; Burgot, J.L.; Abasq, M.L. Validation of a new method using the reactivity of electrogenerated superoxide radical in the antioxidant capacity determination of flavonoids. Talanta 2008, 75, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Caruso, F.; Kwok, L.; Lee, G.; Caruso, A.; Gionfra, F.; Candelotti, E.; Belli, S.L.; Molasky, N.; Raley-Susman, K.M.; et al. Protection by extra virgin olive oil against oxidative stress in vitro and in vivo. Chemical and biological studies on the health benefits due to a major component of the Mediterranean diet. PLoS ONE 2017, 12, e0189341. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compreh. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Sharma, M.K.; Buettner, G.R. Interaction of vitamin C and vitamin E during free radical stress in plasma: An ESR study. Free Radic. Biol. Med. 1993, 14, 649–653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
