The Effect of UV-Vis Radiation on DNA Systems Containing the Photosensitizers Methylene Blue and Acridine Orange
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Irradiation Studies
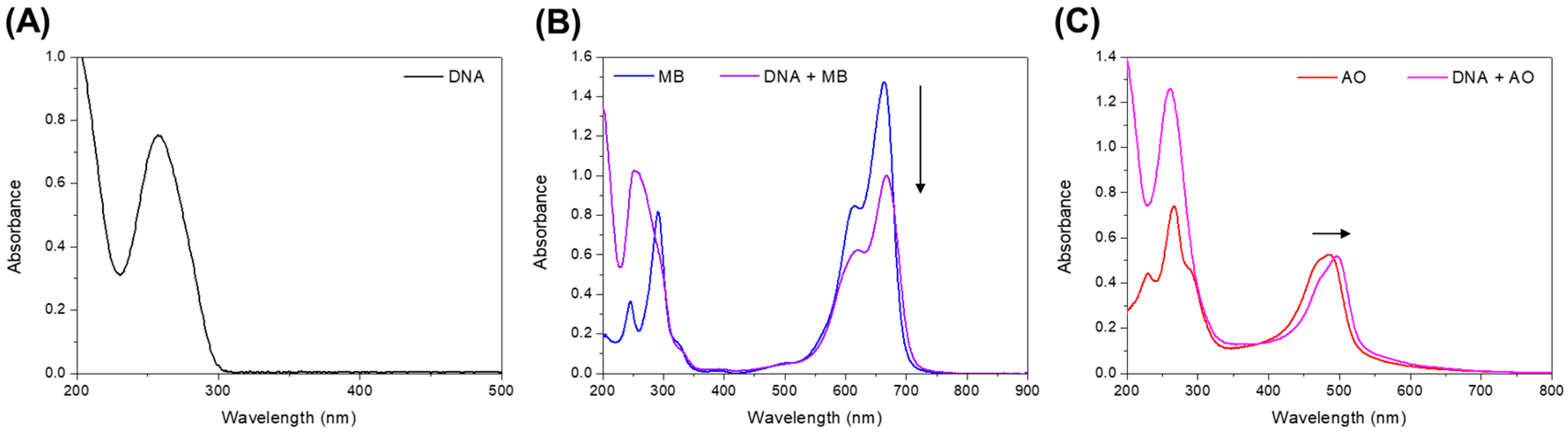
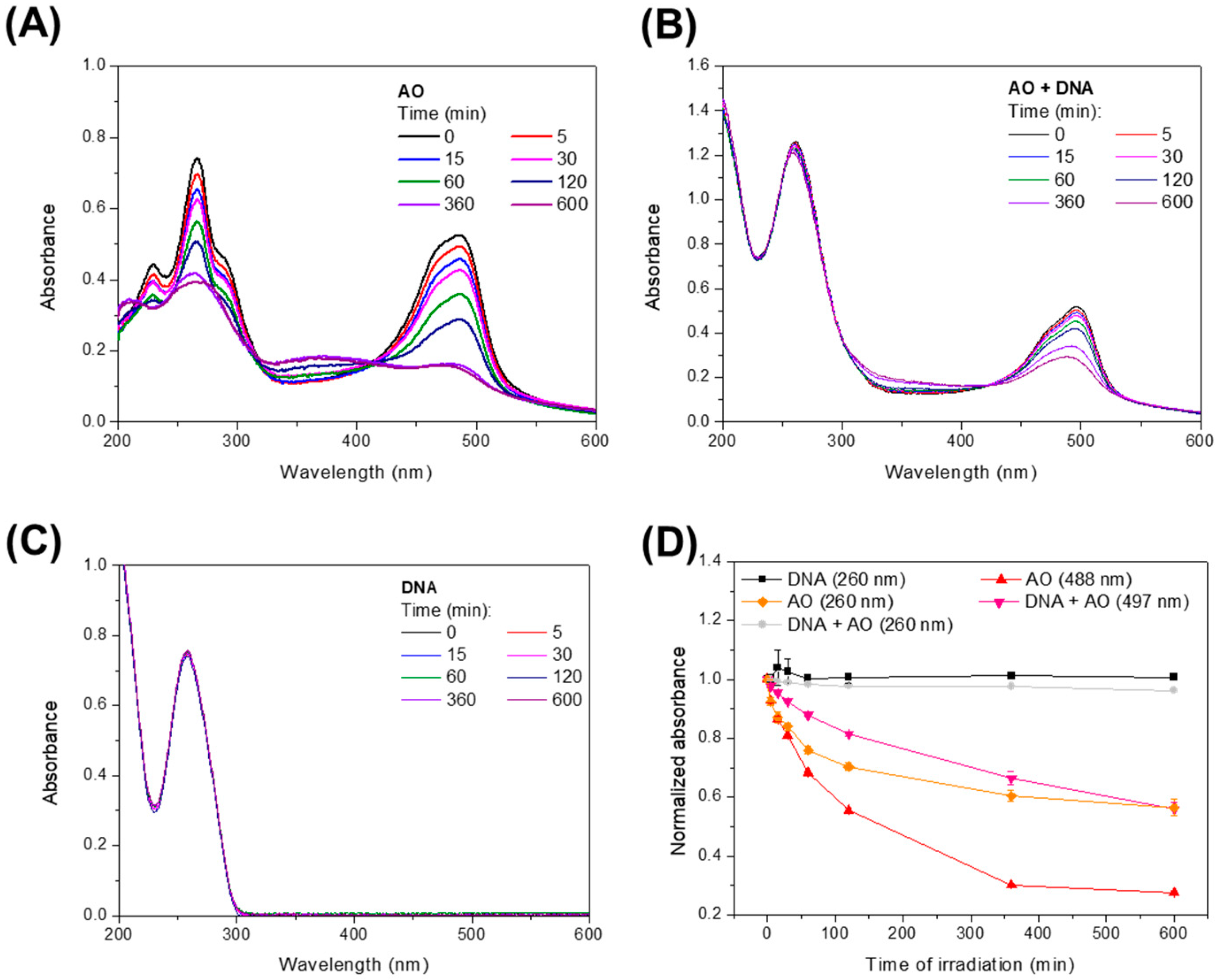
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sayed, M.; Krishnamurthy, B.; Pal, H. Unraveling Multiple Binding Modes of Acridine Orange to DNA Using a Multispectroscopic Approach. Phys. Chem. Chem. Phys. 2016, 18, 24642–24653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sheng, H.; Wang, S.; Ma, Y.; Cai, C. Screening DNA-Targeted Anticancer Drug in Vitro Based on Cancer Cells DNA-Templated Silver Nanoclusters. Sci. Rep. 2019, 9, 8911. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.R.; Harun, S.N.; Halder, S.; Boghozian, R.A.; Ramadan, K.; Ahmad, H.; Vallis, K.A. A Ruthenium Polypyridyl Intercalator Stalls DNA Replication Forks, Radiosensitizes Human Cancer Cells and Is Enhanced by Chk1 Inhibition. Sci. Rep. 2016, 6, 31973. [Google Scholar] [CrossRef] [PubMed]
- Palchaudhuri, R.; Hergenrother, P.J. DNA as a Target for Anticancer Compounds: Methods to Determine the Mode of Binding and the Mechanism of Action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C.; Drummond, C.J.; Chen, Y.Y.; Finlay, G.J. DNA-Binding Anticancer Drugs: One Target, Two Actions. Molecules 2021, 26, 552. [Google Scholar] [CrossRef]
- Kitamura, N.; Kohtani, S.; Nakagaki, R. Molecular Aspects of Furocoumarin Reactions: Photophysics, Photochemistry, Photobiology, and Structural Analysis. J. Photochem. Photobiol. C 2005, 6, 168–185. [Google Scholar] [CrossRef]
- Vangipuram, R.; Feldman, S.R. Ultraviolet Phototherapy for Cutaneous Diseases: A Concise Review. Oral Dis. 2016, 22, 253–259. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Panikar, S.S.; Ramírez-García, G.; Banu, N.; Vallejo-Cardona, A.A.; Lugo-Fabres, P.; Camacho-Villegas, T.A.; Salas, P.; De la Rosa, E. Ligand-Targeted Theranostic Liposomes Combining Methylene Blue Attached Upconversion Nanoparticles for NIR Activated Bioimaging and Photodynamic Therapy against HER-2 Positive Breast Cancer. J. Lumin. 2021, 237, 118143. [Google Scholar] [CrossRef]
- Wu, P.-T.; Lin, C.-L.; Lin, C.-W.; Chang, N.-C.; Tsai, W.-B.; Yu, J. Methylene-Blue-Encapsulated Liposomes as Photodynamic Therapy Nano Agents for Breast Cancer Cells. Nanomaterials 2018, 9, 14. [Google Scholar] [CrossRef]
- Santos, G.M.P.; Oliveira, S.C.P.S.; Monteiro, J.C.S.; Fagnani, S.R.; Sampaio, F.P.; Correia, N.A.; Crugeira, P.J.L.; Pinheiro, A.L.B. ROS-Induced Autophagy Reduces B16F10 Melanoma Cell Proliferative Activity. Lasers Med. Sci. 2018, 33, 1335–1340. [Google Scholar] [CrossRef]
- Yu, J.; Hsu, C.-H.; Huang, C.-C.; Chang, P.-Y. Development of Therapeutic Au–Methylene Blue Nanoparticles for Targeted Photodynamic Therapy of Cervical Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Saboury, A.A.; Keramat, N.; Neault, J.-F.; Tajmir-Riahi, H.-A. Stability and Structural Features of DNA Intercalation with Ethidium Bromide, Acridine Orange and Methylene Blue. J. Mol. Struct. 2007, 827, 35–43. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Tang, G.-Q. The Binding Properties of Photosensitizer Methylene Blue to Herring Sperm DNA: A Spectroscopic Study. J. Photochem. Photobiol. B 2004, 74, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Vardevanyan, P.O.; Antonyan, A.P.; Parsadanyan, M.A.; Shahinyan, M.A.; Petrosyan, N.H. Study of Interaction of Methylene Blue with DNA and Albumin. J. Biomol. Struct. Dyn. 2021, 40, 7779–7785. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.; Coelho, M.; Ribeiro, P.A.; Raposo, M. Methylene Blue: A Trendy Photosensitizer in Medicine and in Solar-Energy Conversion Systems. In Proceedings of the 2016 4th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS), Rome, Italy, 27–29 February 2016; pp. 1–5. [Google Scholar]
- Pitchaimani, A.; Renganathan, A.; Cinthaikinian, S.; Premkumar, K. Photochemotherapeutic Effects of UV-C on Acridine Orange in Human Breast Cancer Cells: Potential Application in Anticancer Therapy. RSC Adv. 2014, 4, 22123–22128. [Google Scholar] [CrossRef]
- Pierzyńska-Mach, A.; Janowski, P.A.; Dobrucki, J.W. Evaluation of Acridine Orange, LysoTracker Red, and Quinacrine as Fluorescent Probes for Long-Term Tracking of Acidic Vesicles. Cytom. Part A 2014, 85, 729–737. [Google Scholar] [CrossRef]
- Damas-Souza, D.M.; Nunes, R.; Carvalho, H.F. An Improved Acridine Orange Staining of DNA/RNA. Acta Histochem. 2019, 121, 450–454. [Google Scholar] [CrossRef]
- Thomé, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric Analysis of Acridine Orange Staining in the Study of Acidic Organelles and Autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, J.-F.; Tsai, T.-F.; Chen, H.-E.; Chou, K.-Y.; Yang, S.-C.; Tang, Y.-M.; Hwang, T.I.S. Acridine Orange Exhibits Photodamage in Human Bladder Cancer Cells under Blue Light Exposure. Sci. Rep. 2017, 7, 14103. [Google Scholar] [CrossRef]
- Osman, H.; Elsahy, D.; Saadatzadeh, M.R.; Pollok, K.E.; Yocom, S.; Hattab, E.M.; Georges, J.; Cohen-Gadol, A.A. Acridine Orange as a Novel Photosensitizer for Photodynamic Therapy in Glioblastoma. World Neurosurg. 2018, 114, e1310–e1315. [Google Scholar] [CrossRef] [PubMed]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; Satonaka, H.; Wakabayashi, T.; Matsumine, A.; Uchida, A. Acridine Orange Could Be an Innovative Anticancer Agent under Photon Energy. In Vivo 2007, 21, 205–214. [Google Scholar] [PubMed]
- Amado, A.M.; Pazin, W.M.; Ito, A.S.; Kuzmin, V.A.; Borissevitch, I.E. Acridine Orange Interaction with DNA: Effect of Ionic Strength. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Ferreira, Q.; Vieira, T.; Silva, J.C.; Simões, S.; Ribeiro, P.A.; Raposo, M. Liposomes Encapsulating Methylene Blue and Acridine Orange: An Approach for Phototherapy of Skin Cancer. Colloids Surf. B Biointerfaces 2022, 220, 112901. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Vieira, T.; Silva, J.C.; Ribeiro, P.A.; Raposo, M. Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening. Int. J. Mol. Sci. 2023, 24, 5602. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Jochelavicius, K.; Wrobel, E.C.; Balogh, D.T.; Oliveira, O.N.; Ribeiro, P.A.; Raposo, M. Incorporation of Acridine Orange and Methylene Blue in Langmuir Monolayers Mimicking Releasing Nanostructures. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2023, 1865, 184156. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.; Geraldo, V.P.N.; Antunes, A.; Marletta, A.; Oliveira, O.N., Jr.; Raposo, M. On the Role of Epigallocatechin-3-Gallate in Protecting Phospholipid Molecules against UV Irradiation. Colloids Surf. B Biointerfaces 2019, 173, 312–319. [Google Scholar] [CrossRef]
- Noda, I. Advances in Two-Dimensional Correlation Spectroscopy. Vib. Spectrosc. 2004, 36, 143–165. [Google Scholar] [CrossRef]
- Raposo, M.; Coelho, M.; Gomes, P.J.; Vieira, P.; Ribeiro, P.A.; Mason, N.J.; Hunniford, C.A.; McCullough, R.W. DNA Damage Induced by Carbon Ions (C3+) Beam Accessed by Independent Component Analysis of Infrared Spectra. Int. J. Radiat. Biol. 2014, 90, 344–350. [Google Scholar] [CrossRef]
- Isaacson, M. Interaction of 25 KeV Electrons with the Nucleic Acid Bases, Adenine, Thymine, and Uracil. I. Outer Shell Excitation. J. Chem. Phys. 1972, 56, 1803–1812. [Google Scholar] [CrossRef]
- Gomes, P.J.; Ribeiro, P.A.; Shaw, D.; Mason, N.J.; Raposo, M. UV Degradation of Deoxyribonucleic Acid. Polym. Degrad. Stab. 2009, 94, 2134–2141. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Dang, A.; Urban, J.; Tureček, F. Charge-Tagged DNA Radicals in the Gas Phase Characterized by UV/Vis Photodissociation Action Spectroscopy. Angew. Chem. Int. Ed. 2020, 59, 7772–7777. [Google Scholar] [CrossRef]
- Párkányi, C.; Boniface, C.; Aaron, J.J.; Maafi, M. A Quantitative Study of the Effect of Solvent on the Electronic Absorption and Fluorescence Spectra of Substituted Phenothiazines: Evaluation of Their Ground and Excited Singlet-State Dipole Moments. Spectrochim. Acta A 1993, 49, 1715–1725. [Google Scholar] [CrossRef]
- Heger, D.; Jirkovský, J.; Klán, P. Aggregation of Methylene Blue in Frozen Aqueous Solutions Studied by Absorption Spectroscopy. J. Phys. Chem. A 2005, 109, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Le Bahers, T.; Di Tommaso, S.; Peltier, C.; Fayet, G.; Giacovazzi, R.; Tognetti, V.; Prestianni, A.; Labat, F. Acridine Orange in a Pumpkin-Shaped Macrocycle: Beyond Solvent Effects in the UV–Visible Spectra Simulation of Dyes. J. Mol. Struct. 2010, 954, 45–51. [Google Scholar] [CrossRef]
- Hajian, R.; Shams, N.; Mohagheghian, M. Study on the Interaction between Doxorubicin and Deoxyribonucleic Acid with the Use of Methylene Blue as a Probe. J. Braz. Chem. Soc. 2009, 20, 1399–1405. [Google Scholar] [CrossRef]
- Kapuscinski, J.; Darzynkiewicz, Z. Interactions of Acridine Orange with Double Stranded Nucleic Acids. Spectral and Affinity Studies. J. Biomol. Struct. Dyn. 1987, 5, 127–143. [Google Scholar] [CrossRef]
- Mondek, J.; Mravec, F.; Halasová, T.; Hnyluchová, Z.; Pekař, M. Formation and Dissociation of the Acridine Orange Dimer as a Tool for Studying Polyelectrolyte–Surfactant Interactions. Langmuir 2014, 30, 8726–8734. [Google Scholar] [CrossRef]
- Goto, N.; Bazar, G.; Kovacs, Z.; Kunisada, M.; Morita, H.; Kizaki, S.; Sugiyama, H.; Tsenkova, R.; Nishigori, C. Detection of UV-Induced Cyclobutane Pyrimidine Dimers by near-Infrared Spectroscopy and Aquaphotomics. Sci. Rep. 2015, 5, 11808. [Google Scholar] [CrossRef]
- dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.M.; Oliveira, T.C.; Gomes, V.d.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene Blue Photodynamic Therapy Induces Selective and Massive Cell Death in Human Breast Cancer Cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef]
- Saraswathi, S.K.; Karunakaran, V.; Maiti, K.K.; Joseph, J. DNA Condensation Triggered by the Synergistic Self-Assembly of Tetraphenylethylene-Viologen Aggregates and CT-DNA. Front. Chem. 2021, 9, 716771. [Google Scholar] [CrossRef]
- Kapuscinski, J.; Darzynkiewicz, Z. Condensation of Nucleic Acids by Intercalating Aromatic Cations. Proc. Natl. Acad. Sci. USA 1984, 81, 7368–7372. [Google Scholar] [CrossRef] [PubMed]
- Byvaltsev, V.A.; Bardonova, L.A.; Onaka, N.R.; Polkin, R.A.; Ochkal, S.V.; Shepelev, V.V.; Aliyev, M.A.; Potapov, A.A. Acridine Orange: A Review of Novel Applications for Surgical Cancer Imaging and Therapy. Front. Oncol. 2019, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.H.; Mercado-Uribe, H. Visible Light Neutralizes the Effect Produced by Ultraviolet Radiation in Proteins. J. Photochem. Photobiol. B 2017, 167, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Freifelder, D.; Davison, P.F.; Geiduschek, E.P. Damage by Visible Light to the Acridine Orange-DNA Complex. Biophys. J. 1961, 1, 389–400. [Google Scholar] [CrossRef]
- Hendershot, J.M.; O’Brien, P.J. Critical Role of DNA Intercalation in Enzyme-Catalyzed Nucleotide Flipping. Nucleic Acids Res. 2014, 42, 12681–12690. [Google Scholar] [CrossRef]
- Bohne, C.; Faulhaber, K.; Giese, B.; Häfner, A.; Hofmann, A.; Ihmels, H.; Köhler, A.-K.; Perä, S.; Schneider, F.; Sheepwash, M.A.L. Studies on the Mechanism of the Photo-Induced DNA Damage in the Presence of Acridizinium SaltsInvolvement of Singlet Oxygen and an Unusual Source for Hydroxyl Radicals. J. Am. Chem. Soc. 2005, 127, 76–85. [Google Scholar] [CrossRef]
- Gicquel, E.; Souchard, J.-P.; Magnusson, F.; Chemaly, J.; Calsou, P.; Vicendo, P. Role of Intercalation and Redox Potential in DNA Photosensitization by Ruthenium(Ii) Polypyridyl Complexes: Assessment Using DNA Repair Protein Tests. Photochem. Photobiol. Sci. 2013, 12, 1517. [Google Scholar] [CrossRef]
- Bernas, T.; Asem, E.K.; Robinson, J.P.; Cook, P.R.; Dobrucki, J.W. Confocal Fluorescence Imaging of Photosensitised DNA Denaturation in Cell Nuclei. Photochem. Photobiol. 2005, 81, 960–969. [Google Scholar] [CrossRef]



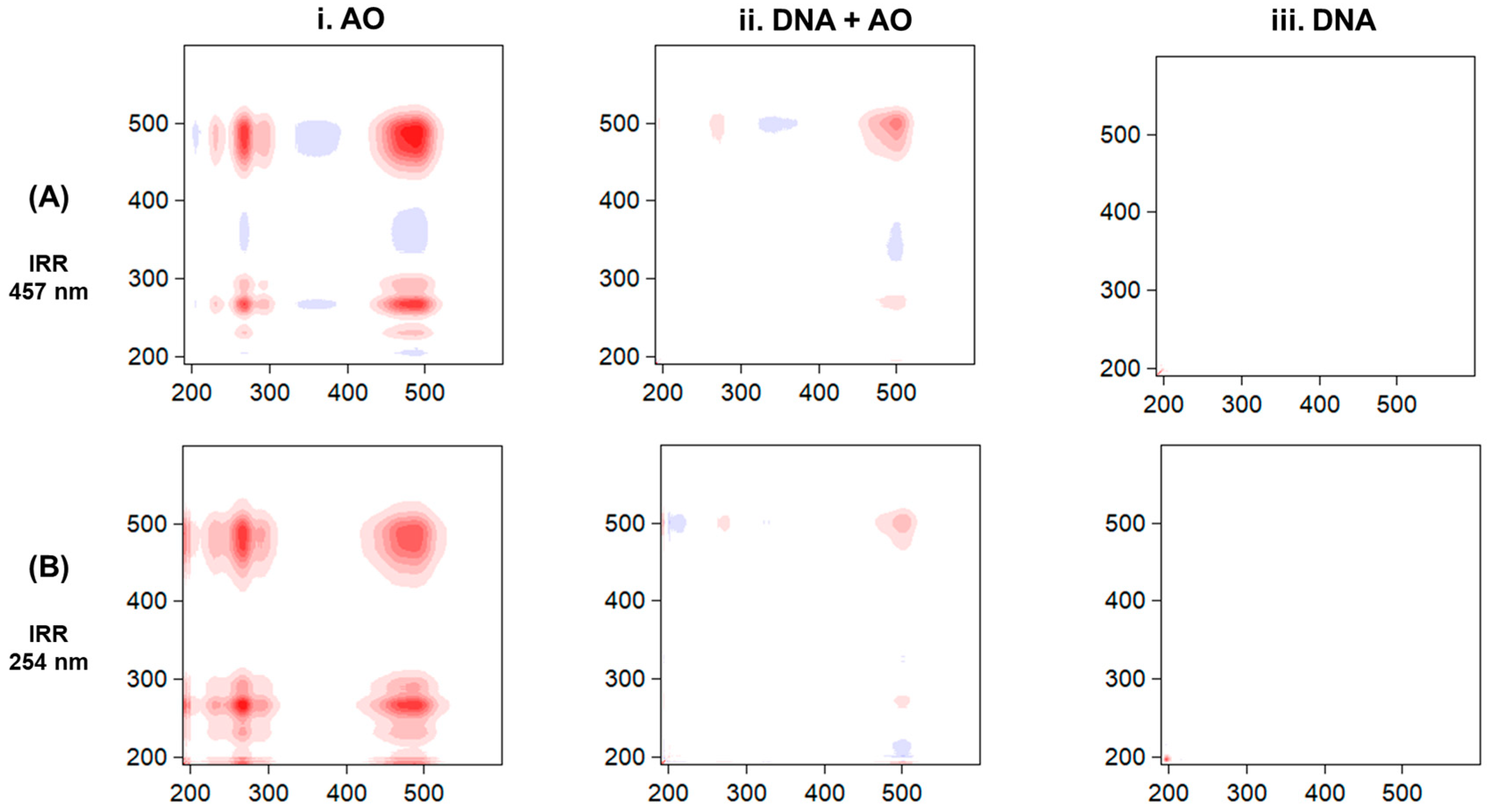
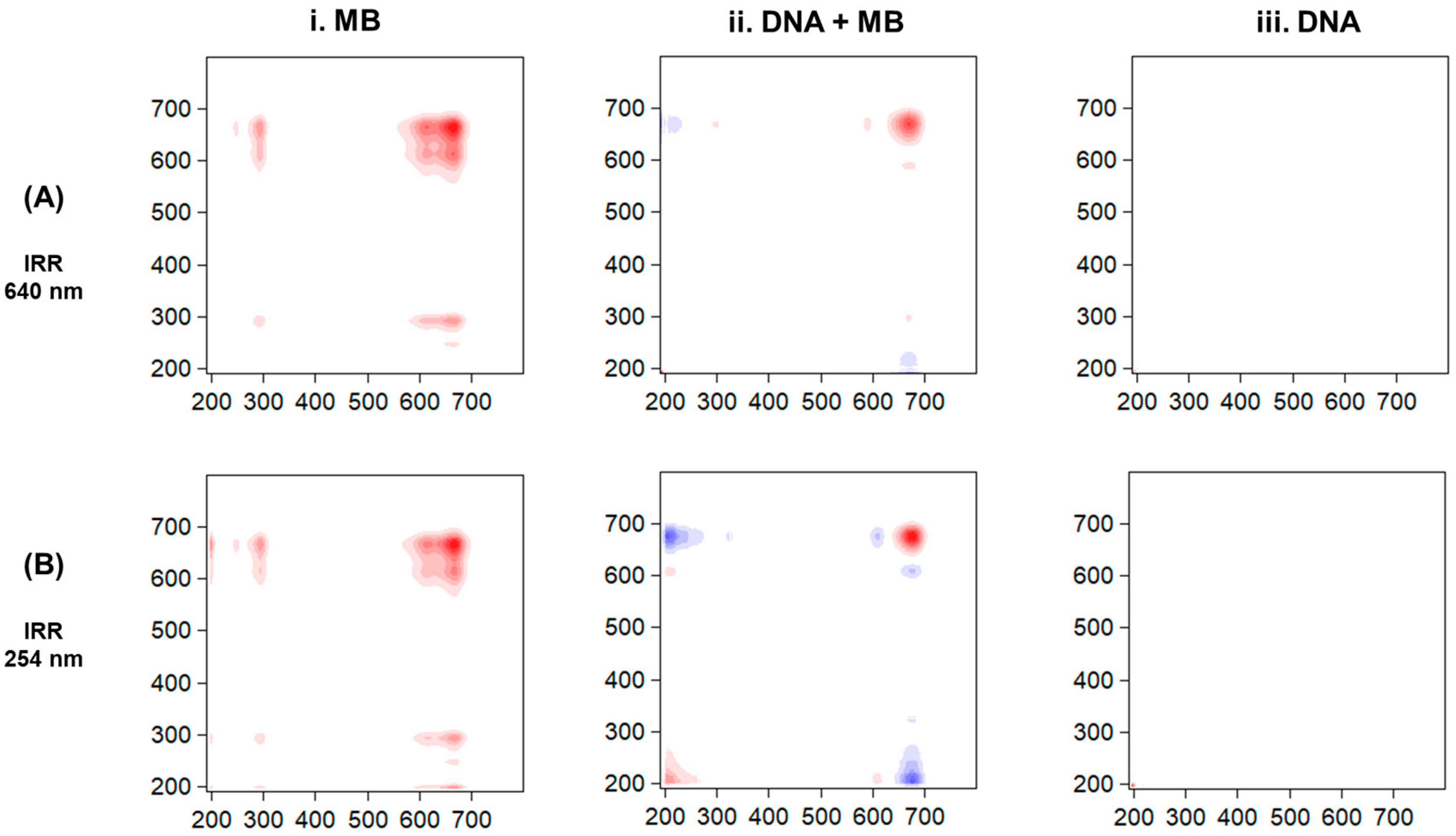
| Sample | Peak (nm) | τ (min) | ||
|---|---|---|---|---|
| Red Light (640 nm) | Blue Light (457 nm) | UVC (254 nm) | ||
| MB | 664 | 639.4 ± 191.3 | - | 181.1 ± 87.3 |
| AO | 488 | - | 129.4 ± 14.8 | 179.2 ± 60.2 |
| DNA + MB | 667 | 1167.3 ± 258.3 | - | - |
| DNA + AO | 497 | - | 279.5 ± 43.9 | 328.9 ± 71.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivetta, T.P.; Ribeiro, P.A.; Raposo, M. The Effect of UV-Vis Radiation on DNA Systems Containing the Photosensitizers Methylene Blue and Acridine Orange. Biophysica 2024, 4, 22-33. https://doi.org/10.3390/biophysica4010002
Pivetta TP, Ribeiro PA, Raposo M. The Effect of UV-Vis Radiation on DNA Systems Containing the Photosensitizers Methylene Blue and Acridine Orange. Biophysica. 2024; 4(1):22-33. https://doi.org/10.3390/biophysica4010002
Chicago/Turabian StylePivetta, Thais P., Paulo A. Ribeiro, and Maria Raposo. 2024. "The Effect of UV-Vis Radiation on DNA Systems Containing the Photosensitizers Methylene Blue and Acridine Orange" Biophysica 4, no. 1: 22-33. https://doi.org/10.3390/biophysica4010002
APA StylePivetta, T. P., Ribeiro, P. A., & Raposo, M. (2024). The Effect of UV-Vis Radiation on DNA Systems Containing the Photosensitizers Methylene Blue and Acridine Orange. Biophysica, 4(1), 22-33. https://doi.org/10.3390/biophysica4010002








