The Influence of Magnetic Fields, Including the Planetary Magnetic Field, on Complex Life Forms: How Do Biological Systems Function in This Field and in Electromagnetic Fields?
Abstract
:1. Introduction
1.1. Purpose
1.2. Background
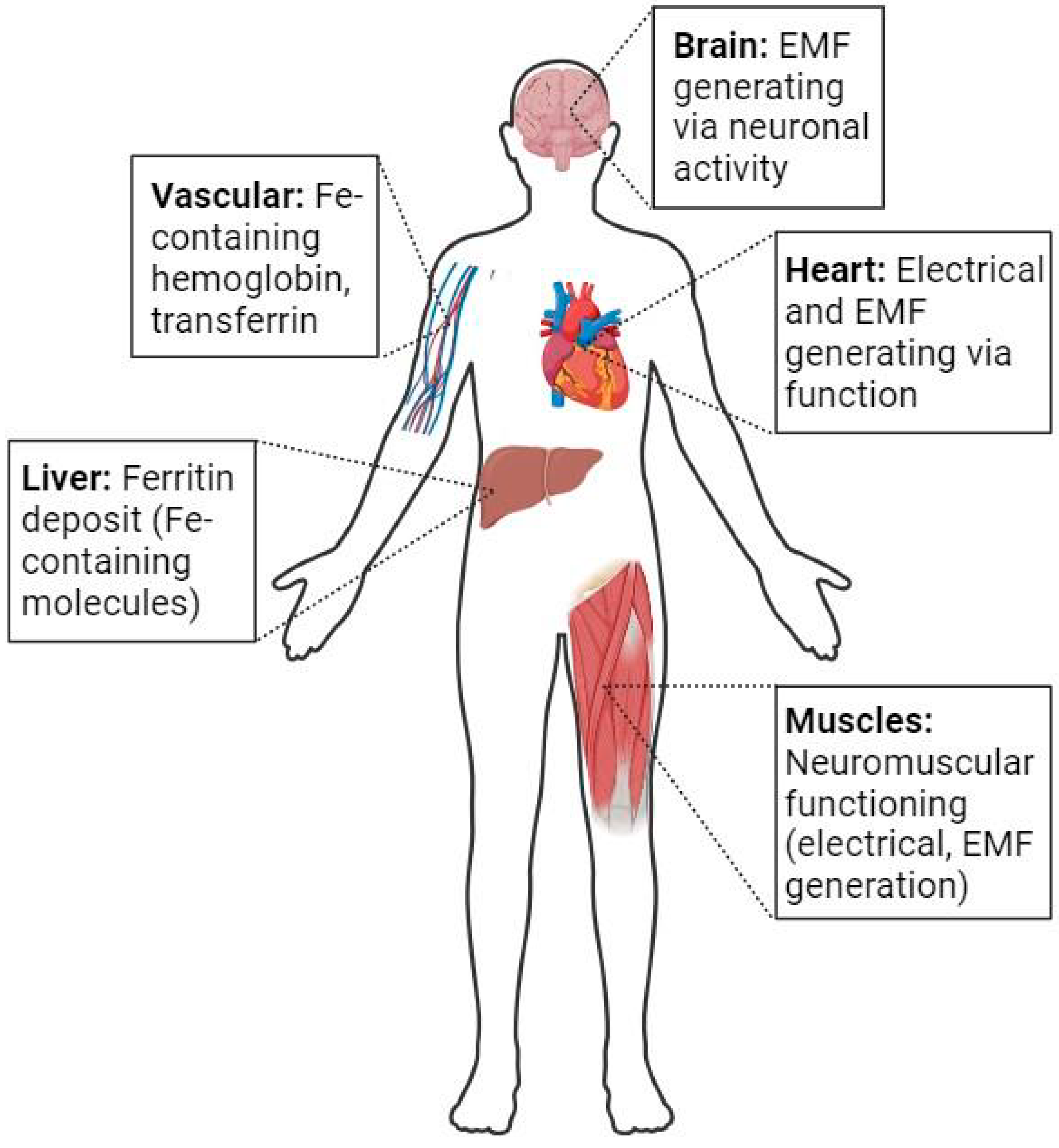
2. Iron-Containing Molecules, Magnetic Fields, and Evolution to Complex Lifeforms
2.1. Incorporation of Ferro-Magnetic Ions in Essential Systems and Molecules
2.2. Magnetic Fields and Navigation
2.3. Magnetic Fields and Cognition
3. Exogenous Electromagnetic Field Effects on Biological Systems
3.1. Power Lines and Cancer
3.2. Cell Phone Use
4. Uses of Magnetic Fields for Health Applications
4.1. The Musculoskeletal System (MSK)
4.2. The Brain and Neurological Integrity
4.2.1. Detection of Brain Injury or Diseases
4.2.2. Health Benefits of Magnetic Fields on the Brain
4.3. Magnetic Field Effects on Wound Healing
5. Summary
- Evolution of complex organisms leading to Homo sapiens occurred in a complex set of boundary conditions of Earth, including both the planetary magnetic field and magnetic fields associated with local concentrations of iron.
- Evolution led to the development and incorporation of biological systems that generate electromagnetic fields as a consequence of functioning (i.e., cardiovascular, neuromuscular, and neuronal networks) or use incorporated iron ions for function. Thus, against a background of magnetic fields, evolution led to the use of systems that generated electromagnetic fields, but such fields were not overtly impacted by the exogenous fields regarding function.
- Many species have evolved navigation systems that use magnetic fields for guidance, and thus, they recognize that the geomagnetic field exists and can be used for directional migration. Furthermore, species that use such navigation systems can apparently filter endogenous electromagnetic fields from the geomagnetic field orientations.
- The functioning of several biological systems can be acutely compromised or altered using exogenous magnetic fields, but such exposure does not lead to overt disruption of long-term regulation. Thus, exposure of the human body to high magnetic fields in an MRI machine (i.e., 3 Tesla), including the heart and brain, does not lead to disruption of the functioning of the brain processes or the functioning of the cardiac muscle.
- Chronic exposure to varying levels of electromagnetic fields, likely not anticipated by evolution, does not lead to altered functioning of Homo sapiens.
- Exposure to short-term drastically diminished geomagnetic fields via space flight to the moon did not overtly affect the functioning of astronauts.
6. Conclusions and Suggestions Going Forward
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binhi, V.N.; Sarimov, R.M. Zero magnetic field effect observed in human cognitive processes. Electromagn. Bio. Med. 2009, 28, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytic review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Homo sapiens-A species not designed for space flight: Health risks in low Earth orbit and beyond, including potential risks when traveling beyond the geomagnetic field of Earth. Life 2023, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A.; Zernicke, R.F. Optimal human functioning requires exercise across the lifespan: Mobility in a 1g environment is intrinsic to the integrity of multiple biological systems. Front. Physiol. 2020, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, W.; Kmita, H.; Kosicki, J.Z.; Karzmarek, L. How the geomagnetic field influences life on Earth- an integrated approach to geomagnetobiology. Orig. Life Evol. Biosph. 2021, 51, 231–257. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ali, Y.F.; Liu, C.; Hong, Z.; Luo, W.; Nie, J.; Li, B.; Jiao, Y.; Liu, N.A. Geomagnetic shielding enhances radiation resistance by promoting DNA repair process in human bronchial epithelial cells. Int. J. Mol. Sci. 2020, 21, 9304. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Martyniuk, C.J. The bioelectric code: An ancient computational medium for dynamic control of growth and form. BioSystems 2018, 164, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Rubin, A.B. Theoretical concepts in magnetobiology after 40 years of research. Cells 2022, 11, 274. [Google Scholar] [CrossRef]
- Gonciarz, R.L.; Rensio, A.R. Emerging role of ferrous iron in bacterial growth and host-pathogen interaction: New tools for chemical (micro)biology and antibacterial therapy. Curr. Opin. Chem. Biol. 2021, 61, 170–178. [Google Scholar] [CrossRef]
- Baatjies, L.; Loxton, A.G.; Williams, M.J. Host and bacterial iron homeostasis, an underexplored area in tuberculosis biomarker research. Front. Immunol. 2021, 12, 742059. [Google Scholar] [CrossRef]
- Seyoum, Y.; Baye, K.; Humblot, C. Iron homeostasis in host and gut bacteria-a complex relationship. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.Z. Cellular iron metabolism and regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.S. Kinetic mechanisms for O2 binding to myoglobins and hemoglobins. Mol. Aspects Med. 2022, 84, 101024. [Google Scholar] [CrossRef]
- Nagatomo, S.; Naga, M.; Kitagawa, T. Structural origin of cooperativity in human hemoglobin: A view from different roles of alpha and beta subunits in the alpha2beta2 tetramer. Biophys Rev. 2022, 14, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Wright, N. Acyl-carbon bond cleaving cytochrome P450 enzymes: CYP17A1, CYO19A1 and CYO51A1. Adv. Exp. Med. Biol. 2015, 851, 107–130. [Google Scholar] [CrossRef]
- Kumar, N.; Chugh, H.; Sood, D.; Singh, S.; Singh, A.; Awasthi, A.D.; Tomar, R.; Tomar, V.; Changdra, R. Biology of heme: Drug interactions and adverse drug reactions with CYO450. Curr. Top. Med. Chem. 2019, 18, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L.; Follmer, A.H. Updating the paradigm: Redox partner binding and conformational dynamics in cytochromes P450. Acc. Chem. Res. 2022, 55, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Luck, A.N.; Mason, A.B. Transferrin-mediated cellular iron delivery. Curr. Top. Membr. 2012, 69, 3–35. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Hong, J.; Tang, A.; Liu, Y.; Xie, N.; Nie, G.; Yan, X.; Liang, M. Biochemistry of mammalian ferritins in the regulation of cellular iron homeostasis and oxidative responses. Sci. China Life Sci. 2021, 64, 353–362. [Google Scholar] [CrossRef]
- Zhao, X.; Kruzel, M.; Aronowski, J. Lactoferrin and hematoma detoxification after intracerebral hemorrhage. Biochem. Cell Biol. 2021, 99, 97–101. [Google Scholar] [CrossRef]
- Meyer, O.; Gremer, L.; Ferner, R.; Dobbek, H.; Meyer-Klaucke, W.; Huber, R. The role of Se, Mo, Fe in the structure and function of carbon monoxide dehydrogenase. Biol. Chem. 2000, 38, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Saini, S.K.; Mankowski, R.T.; Kamenov, G.; Anton, S.D.; Manini, T.M.; Buford, T.W.; Wohlgemuth, S.F.; Xiao, R.; Calvani, R.; et al. Altered expression of mitoferrin and frataxin, larger labile iron pool and greater mitochondrial DNA damage in the skeletal muscle of older adults. Cells 2020, 9, 2579. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, D.C.; Moran, G.R. Mammalian dihydropyrimidine dehydrogenase. Ach. Biochem. Biophys. 2021, 714, 109066. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Tsukihara, T.; Yoshikawa, S. Recent progress in experimental studies on the catalytic mechanism of cytochrome c oxidase. Front. Chem. 2023, 11, 1108190. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kong, Q.; Luo, X.; Petersen, R.B.; Meyerson, H.; Singh, N. Prion protein (PrP) knockout mice show altered iron metabolism: A functional role for PrP in iron uptake and transport. PLoS ONE 2009, 4, e6115. [Google Scholar] [CrossRef] [PubMed]
- Alayash, A.I. Oxidation reactions of cellular and acellular hemoglobins: Implications for human health. Front. Med. Technol. 2022, 4, 1068972. [Google Scholar] [CrossRef]
- Pires, I.S.; Berthiaume, F.; Palmere, A.F. Engineering therapeutics to detoxify hemoglobin, heme and iron. Annu. Rev. Biomed. Eng. 2023, 25, 1–21. [Google Scholar] [CrossRef]
- Piperno, A.; Pelucchi, S.; Mariani, R. Hereditary hyperferritinemia. Int. J. Mol. Sci. 2023, 24, 2560. [Google Scholar] [CrossRef]
- Lommaert, E.; Verlinden, W.; Duysburgh, I.; Holvoet, T.; Schouten, J. Hyperferritinemia and non-HFE hemochromatosis: Differential diagnosis and workup. Acta Gastroenterol. Belg. 2023, 86, 356–359. [Google Scholar] [CrossRef]
- Bruno, F.; Albano, D.; Agostini, A.; Benenati, M.; Cannella, R.; Caruso, D.; Cellina, M.; Cozzi, D.; Danti, G.; De Muzio, F.; et al. Imaging of metabolic and overload disorders in tissues and organs. Jpn J. Radiol. 2023, 41, 571–595. [Google Scholar] [CrossRef]
- De Simone, G.; Varriccho, R.; Ruberto, T.F.; di Masi, A.; Ascenzi, P. Heme scavenging and delivery: The role of human serum albumin. Biomolecules 2023, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.O. Maternal, fetal and placental regulation of placental iron trafficking. Placenta 2022, 125, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Navas, F.J.; Cordova, A. Iron distribution in different tissues in rats following exercise. Biol. Trace Elem. Res. 2000, 73, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.M.; Smith, M.A.; LaManna, J.C.; Wilson, A.C.; Perry, G.; Atwood, C.S. Iron homeostasis is maintained in the brain, but not the liver following mild hypoxia. Redox Rep. 2007, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Djordjevich, D.M.; De Luca, S.R.; Milovanovich, I.D.; Jankovic, S.; Stefanovic, S.; Veskovic-Moracanin, S.; Cirkovic, S.; Ilic, A.Z.; Ristic-Djurovic, J.L.; Trbovich, A.M. Hemotological parameters’ changes in mice subchronically exposed to static magnetic fields of different orientations. Ecotoxicol. Environ. Saf. 2012, 81, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kopani, M.; Panik, J.; Filova, B.; Bijdos, M.; Misek, J.; Kohan, M.; Jakus, J.; Povinec, P. PIXE analysis of iron in rabbit cerebellum after exposure to radiofrequency electromagnetic fields. Bratisl. Lek. Listy. 2022, 123, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Schildroth, S.; Kordas, K.; Bauer, J.A.; Wright, R.O.; Henn, B.C. Environmental metal exposure, neurodevelopment, and the role of iron status: A review. Curr. Environ. Health Rep. 2022, 9, 758–787. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.K.; Kosman, D.J. Is brain iron trafficking part of the physiology of the amyloid precursor protein? J. Biol. Inorg. Chem. 2019, 24, 1171–1177. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, X.; Lang, M. Iron homeostasis disorder and Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Dirksen, R.T.; Wojtovich, A.P. Iron dysregulation in mitochondrial dysfunction and alzheimer’s disease. Antioxidants 2022, 11, 692. [Google Scholar] [CrossRef]
- Rao, S.S.; Adland, P.A. Untangling tau and iron: Exploring the interaction between iron and tau in neurodegeneration. Front. Mol. Neurosci. 2018, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Joppe, K.; Roser, A.E.; Maass, F.; Lingor, P. The contribution of iron to protein aggregation disorders in the central nervous system. Front. Neurosci. 2019, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Cahill, C.M. Iron-responsive-like elements and neurodegenerative ferroptosis. Learn. Mem. 2020, 27, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Panda, D. Contrasting effects of ferric and ferrous ions on oligomerization and droplet formation of tau: Implications in taupathies and neurodegeneration. ACS Chem. Neurosci. 2021, 12, 4393–4405. [Google Scholar] [CrossRef] [PubMed]
- Trojsi, F.; Sorrentino, P.; Sorrentino, G.; Tedeschi, G. Neurodegeneration of brain networks in the amyotrophic lateral sclerosis-frontotemporal lobar degeneration (ALS-FTLD) continuum: Evidence from MRI and MEG studies. CNS Spectr. 2018, 23, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Cope, T.E.; Weil, R.S.; Duzel, E.; Dickerson, B.C.; Rowe, J.B. Advances in neuroimaging to support translational medicine in dementia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pilozzi, A.; Huang, X. An overview of ICA/BSS-based application to Alzheimer’s brain signal processing. Biomedicines 2021, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Fred, A.L.; Kumar, S.N.; Haridhas, A.K.; Ghosh, S.; Bhuvana, H.P.; Sim, W.K.J.; Vimalan, V.; Givo, F.A.S.; Jousmaki, V.; Padmanabhan, P.; et al. A brief introduction to magnetoencephalography (MEG) and its clinical applications. Brain Sci. 2022, 12, 788. [Google Scholar] [CrossRef]
- Heyers, D.; Musielak, I.; Haase, K.; Herold, C.; Bolte, P.; Gunturkun, O.; Mouritsen, H. Morphology, biochemistry and connectivity of cluster N and the hippocampal formation in a migratory bird. Brain Struct. Funct. 2022, 227, 2731–2749. [Google Scholar] [CrossRef]
- Karwinkel, T.; Winklhofer, M.; Janner, L.E.; Brust, V.; Huppop, O.; Bairlein, F.; Schmaljohann, H. A magnetic pulse does not affect free-flight navigation behaviour of a medium-distance songbird migrant in spring. J. Exp. Biol. 2022, 225, jeb244473. [Google Scholar] [CrossRef]
- Tonelli, B.A.; Youngflesh, C.; Tingley, M.W. Geomagnetic disturbance associated with increased vagrancy in migratory landbirds. Sci. Rep. 2023, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Kremers, D.; Manulanda, J.L.; Hausberger, M.; Lemasson, A. Bahvoural evidence of magnetoreception in dolphins: Detection of experimental fields. Naturwissenschaften 2014, 101, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Formicki, K.; Korzelecka-Orkisz, A.; Tanski, A. Magnetoreception in fish. J. Fish. Biol. 2019, 95, 73–91. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.; Kehl, C.E.; Taylor, B.K.; Piacenza, J.; Piacenza, S.; Li, K.J.F. A computational framework for studying energetics and resource management in sea turtle migration and autonomous systems. J. Theor. Biol. 2021, 527, 110815. [Google Scholar] [CrossRef] [PubMed]
- Komolkin, A.V.; Kupriyanov, P.; Chudin, A.; Bojarinova, J.; Kavokin, K.; Chernetsov, N. Theoretically possible spatial accuracy of geomagnetic maps used by migrating animals. J. R. Soc. Interface 2017, 14, 20161002. [Google Scholar] [CrossRef]
- Chae, K.S.; Kim, S.C.; Kwon, H.J.; Kim, Y. Human magnetic sense is mediated by a light and magnetic field resonance-dependent mechanism. Sci. Rep. 2022, 12, 8997. [Google Scholar] [CrossRef]
- Chae, K.S.; Oh, I.T.; Lee, S.H.; Kim, S.C. Blue light-dependent human magnetoreception in geomagnetic food orientation. PLoS ONE 2019, 14, 1826. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhu, F.; Hong, Y. Identification of medaka magnetoreceptor and cryptochromes. Sci. China Life Sci. 2017, 60, 271–278. [Google Scholar] [CrossRef]
- Fitak, R.R.; Wheeler, B.R.; Ernst, D.A.; Lohman, K.J.; Johnsen, S. Candidate genes mediating magnetoreception in rainbow trout (Oncorhynchus mykiss). Biol. Lett. 2017, 13, 20170142. [Google Scholar] [CrossRef]
- Liedvogel, M.; Mouritsen, H. Cryptochromes—A potential magnetoreceptor: What do we know and what do we want to know? J. R. Soc. Interface 2010, 7 (Suppl. S2), S147–S162. [Google Scholar] [CrossRef]
- Landler, L.; Keays, D.A. Cryptochrome: The magnetoreceptor with a sinister side? PLoS ONE 2018, 16, e3000018. [Google Scholar] [CrossRef]
- van Horik, J.; Emery, N.J. Evolution of cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Heft, H. Evolution of human cognition. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 252–258. [Google Scholar]
- Vonk, J.; Aradhye, C. Evolution of Cognition. In Basics in Human Evolution; Muehlenbein, M.P., Ed.; Elesevier Inc.: Amsterdam, The Netherlands, 2015; Volume 3, pp. 479–491. [Google Scholar] [CrossRef]
- Roth, G.; Dicke, U. Origin and evolution of human cognition. Prog. Brain Res. 2019, 250, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, M.J. Origins, trends and perspectives of historical epistemological research on Piaget. Integr. Psychol. Behav. Sci. 2023, 27. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J. Biomagnetism: The first sixty years. Sensors 2023, 23, 4218. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S. Studies on magnetism and bioelectromagnetics for 45 years: From magnetic analog memory to human brain stimulation and imaging. Bioelectromagnetics 2012, 33, 3–33. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Chevalier, G.; Ojcius, D.M.; Young, J.D. Influence of electromagnetic fields on the circadian rhythm: Implications for human health and disease. Biomed. J. 2023, 46, 48–59. [Google Scholar] [CrossRef]
- Stock, J.B.; Zhang, S. The biochemistry of memory. Curr. Biol. 2013, 23, R741–R745. [Google Scholar] [CrossRef]
- Bickle, J.; Sarwich, S.S. Introduction to molecular and cellular cognition. In Mind, Cognition and Neuroscience. A Philosophical Introduction; Young, B., Jennings, C.D., Eds.; Routledge: Oxfordshire, UK, 2022; Chapter 3; pp. 32–50. [Google Scholar]
- McFadden, J. Consciousness: Matter or EMF? Front. Hum. Neurosci. 2023, 16, 1024934. [Google Scholar] [CrossRef]
- McFadden, J. The conscious electromagnetic information (cemi) field theory. The hard problem made easy? J. Conscious. Stud. 2002, 9, 45–60. [Google Scholar]
- McFadden, J. The cemi field theory. Gestalt information and the meaning of meaning. J. Conscious. Stud. 2013, 20, 152–182. [Google Scholar]
- McFadden, J. The cemi field theory. Closing the loop. J. Conscious. Stud. 2013, 20, 153–168. [Google Scholar]
- McFadden, J. Integrating information in the brain’s EM field: The cemi field theory of consciousness. Neurosci. Conscious. 2020, 2020, niaa016. [Google Scholar] [CrossRef] [PubMed]
- Banaclocha, M.A.M. Magnetic storage of information in the human cerebral cortex: A hypothesis for memory. Int. J. Neurosci. 2005, 115, 329–337. [Google Scholar] [CrossRef]
- Brignani, D.; Bortoletto, M.; Miniussi, C.; Maioli, C. The when and where of spatial storage in memory-guided saccades. Neuroimage 2010, 52, 1611–1620. [Google Scholar] [CrossRef]
- Edwards, J.C.W. EM fields and the meaning of meaning. Response to Johnjoe McFadden. J. Conscious. Stud. 2013, 20, 159–167. [Google Scholar]
- Crasson, M. 50-60 Hz electric and magnetic field effects on cognitive function in humans: A review. Radiat. Prot. Dosim. 2003, 106, 333–340. [Google Scholar] [CrossRef]
- Benke, G.; Abramson, M.J.; Zeleke, B.M.; Kaaufman, J.; Karipidis, K.; Kelsall, H.; McDonald, S.; Brzozek, C.; Feychting, M.; Brennan, S. The effect of long-term radiofrequency exposure on cognition in human observational studies: A protocol for a systematic review. Environ. Int. 2022, 159, 106972. [Google Scholar] [CrossRef]
- Heinrich, A.; Szostek, A.; Nees, F.; Meyer, P.; Semmler, W.; Flor, H. Effects of static magnetic fields on cognition, vital signs, and sensory perception: A meta-analysis. J. Magn. Reson. Imaging 2011, 34, 758–763. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Y.; Yang, J.; Shang, P.; Yuan, X. Biological effects of hypomagnetic field: Ground-based data for space exploration. Bioelectromagnetics 2021, 42, 516–531. [Google Scholar] [CrossRef]
- Stahn, A.C.; Kuhn, S. Brains in space: The importance of understanding the impact of long-duration spaceflight on spatial cognition and its neural circuitry. Cogn. Process. 2021, 22 (Suppl. S1), 105–114. [Google Scholar] [CrossRef] [PubMed]
- Arshad, I.; Ferre, E.R. Cognition in zero gravity: Effects of non-terrestrial gravity on human behavior. Q. J. Exp. Psychol. 2023, 76, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.P.; McGregor, H.R.; Hupfeld, K.E.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Riascos, R.F.; Reuter-Lorenz, P.A.; Bloomberg, J.J.; Mulavara, A.P.; et al. Changing in working memory brain activity and task-based connectivity after long-duration spaceflight. Cereb. Cortex. 2023, 33, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O. Human disease resulting from exposure to electromagnetic fields. Rev. Environ. Health 2013, 28, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C. Carcinogenesis from chronic exposure to radio-frequency radiation. Front. Public Health 2022, 10, 1042478. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Malavolti, M.; Wise, L.A.; Balboni, E.; Fabbi, S.; Teggi, S.; Palazzi, G.; Cellini, M.; Poli, M.; Zanichelli, P.; et al. Residential exposure to magnetic fields from high-voltage power lines and risk of childhood leukemias. Environ. Res. 2023, 232, 116320. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Bilodeau-Bertrand, M.; Marcoux, S.; Kosatsky, T. Residential exposure to electromagnetic fields during pregnancy and risk for child cancer: A longitudinal cohort study. Environ. Res. 2019, 176, 108524. [Google Scholar] [CrossRef] [PubMed]
- Repacholi, M. Concern that “EMF” magnetic fields from power lines cause cancer. Sci. Total Environ. 2012, 426, 454–458. [Google Scholar] [CrossRef]
- Amoon, A.T.; Swanson, J.; Magnani, C.; Johansen, C.; Kheifets, L. Pooled analysis of recent studies of magnetic fields and childhood leukemia. Environ. Res. 2022, 204, 111993. [Google Scholar] [CrossRef]
- Brabant, C.; Geerinck, A.; Beaudart, C.; Tirelli, E.; Geuzaine, C.; Bruyere, O. Exposure to magnetic fields and childhood leukemia: A systematic review and meta-analysis of case-control and cohort studies. Rev. Environ. Health 2022, 38, 229–253. [Google Scholar] [CrossRef]
- Philips, A. Risk of cancer and exposure to power lines. Still no answers. BMJ 1994, 308, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.M.; Swanson, J.; Vergara, X.P.; Kheifets, L. Childhood leukemia risk in the California Power Line study: Magnetic fields versus distance from power lines. Environ. Res. 2019, 171, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Carles, C.; Esquirol, Y.; Turuban, M.; Piel, C.; Migualt, L.; Pouchieu, C.; Bouvier, G.; Fabbro-Peray, P.; Lebailly, P.; Baldi, I. Residential proximity to power lines and risk of brain tumor in the general population. Environ. Res. 2020, 185, 109473. [Google Scholar] [CrossRef]
- Carpenter, D.O. Extremely low frequency electromagnetic fields and cancer: How source of funding affects results. Environ. Res. 2019, 178, 108688. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Taylor, H.S.; Baldwin, H.; Ben-Ishai, P.; Davis, D. RE: Cellular telephone use and the risk of brain tumors: Update of the UK million women study. J. Natl. Cancer Inst. 2022, 114, 1551–1552. [Google Scholar] [CrossRef]
- Bhargav, H.; Srinivasan, T.M.; Varambally, S.; Gangadhar, B.N.; Koka, P. Effect of mobile phone-induced electromagnetic field on brain hemodynamics and human stem cell functioning: Possible mechanistic link to cancer risk and early diagnostic value of electronphotonic imaging. J. Stem Cells 2015, 10, 287–294. [Google Scholar]
- Nelson, N. Recent studies show cell phone use is not associated with increased cancer risk. J. Natl. Cancer Inst. 2001, 93, 170–172. [Google Scholar] [CrossRef]
- Jagetia, G.C. Genotoxic effects of electromagnetic field radiation from mobile phones. Environ. Res. 2022, 212, 113321. [Google Scholar] [CrossRef]
- Farashi, S.; Bashirian, S.; Khazaei, S.; Khazaei, M. Mobile phone electromagnetic radiation and the risk of headache: A systematic review and meta-analysis. Int. Arch. Occup. Environ. Health 2022, 95, 1587–1601. [Google Scholar] [CrossRef]
- Herbert, M.R.; Sage, C. Autism and EMF? Plausibility of a pathophysiological link-part 1. Pathophysiology 2013, 20, 191–209. [Google Scholar] [CrossRef]
- Zabroda, N.N.; Artemenko, M.V. Hygienic characteristics of the Kursk magnetic anomaly area and morbidity in the aboriginal population. Gig. Sanit. 2008, 5, 35–38. (In Russian) [Google Scholar]
- Wei, Y.; Wang, X. Biological effects of rotating magnetic field: A review from 1969 to 2021. Prog. Biophs. Mol. Biol. 2023, 178, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A. Beneficial effects of electromagnetic fields. J. Cell Biochem. 1993, 51, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Trock, D.H. Electromagnetic fields and magnets. Investigational treatment for musculoskeletal disorders. Rheum. Dis. Clin. N. Am. 2000, 26, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Bandeira, J.P.; Chumbiauca, C.N.P.; Lahiri, D.K.; Morisaki, J.; Rizkalla, M. Multidimential insights into the repeated electromagnetic field stimulation and biosystem interaction in aging and age-related diseases. J. Biomed. Sci. 2022, 29, 39. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Maqsood, R.; Tawakalzada, A.S. Do magnetic fields have a place in treating vascular complications in diabetes? Cureus 2022, 14, e24883. [Google Scholar] [CrossRef] [PubMed]
- Valone, T.F. Bioelectromagnetic healing, its history and a rationale for its use. In Proceedings of the Whole Person Healing Conference; Lumiverse Inc.: Bethesda, MD, USA, 2003; pp. 1–17. [Google Scholar]
- Soltani, D.; Samini, S.; Vasheghani-Farahani, A.; Shariatpanahi, S.P.; Abodolmaleki, P.; Ansari, A.M. Electromagnetic field therapy in cardiovascular diseases: A review of patents, clinically effective devices, and mechanism of therapeutic effects. Trends Cardiovasc. Med. 2023, 33, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, F.; Trentin, M.; Zanolla, I.; Teingo, E.; Mantarro, C.; Paola, L.D.; Tremoli, E.; ambataro, M.; Sambado, L.; Picari, M.; et al. Playing with biophysics: How a symphony of different electromagnetic fields acts to reduce the inflammation in diabetic derived cells. Int. J. Mol. Sci. 2023, 24, 1754. [Google Scholar] [CrossRef]
- Littman, J.; Aaron, R.K. Stimulation of chondrogenesis in a developmental model of endochondral bone formation by pulsed electromagnetic fields. Int. J. Mol. Sci. 2023, 24, 3275. [Google Scholar] [CrossRef]
- Haddad, J.B.; Obolensky, A.G.; Shinnick, P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: New findings and a review of earlier work. J. Altern. Complement Med. 2007, 13, 485–490. [Google Scholar] [CrossRef]
- Frank, C.; Schachar, N.; Dittrich, D.; Shrive, N.; deHaas, W.; Edwards, G. Electromagnetic stimulation of ligament healing in rabbits. Clin. Orthop. Relat. Res. 1983, 175, 263–272. [Google Scholar]
- Lin, Y.; Nishimura, R.; Nozaki, K.; Sasaki, N.; Kadosawa, T.; Goto, N.; Date, M.; Takeuchi, A. Effects of pulsing electromagnetic fields on the ligament healing in rabbits. J. Vet. Med. Sci. 1992, 54, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, T.; Qu, J.; Hu, J.; Lu, H. Enhanced patella-patella tendon healing using combined magnetic fields in a rabbit model. Am. J. Sports Med. 2014, 42, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, T.; Xu, D.; Qu, J.; Qin, L.; Zhou, J.; Lu, H. Combined magnetic fields accelerate bone-tendon junction injury healing through osteogenesis. Scand. J. Med. Sci. Sports 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Feng, L.; Zhang, X.; Wang, H.; Zhang, N.; Viohl, I.; Li, G. Pulsed electromagnetic field enhances healing of a meniscal tear and mitigates posttraumatic osteoarthritis in a rat model. Am. J. Sports Med. 2022, 50, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Hulme, J.; Robinson, V.; DeBie, R.; Wells, G.; Judd, M.; Tugwell, P. Electromagnetic fields for the treatment of osteoarthritis. Cochrane Database Syst. Rev. 2002, 1, CD003523. [Google Scholar] [CrossRef]
- Li, S.; Yu, B.; Zhou, D.; He, C.; Zhuo, Q.; Hulme, J.M. Electromagnetic fields for treating osteoarthritis. Cochrane Database Syst. Rev. 2013, 12, CD003523. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed electromagnetic field stimulation of bone healing and joint preservation: Cellular mechanisms of skeletal response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e1900155. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, W.; Li, F.; Zhao, H.; Deng, X.; Wang, H.-L. Electric/magnetic intervention for bone regeneration: A systematic review and network meta-analysis. Tissue Eng. Part B Rev. 2023, 29, 217–231. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Flores, M.; Sadureira, S.; Zanotto, F.; Monteiro, F.; Laranjeira, M.S. Magnetic bone tissue engineering: Reviewing the effects of magnetic stimulation on bone regeneration and angiogenesis. Pharmaceutics 2023, 15, 1045. [Google Scholar] [CrossRef]
- Caliogna, L.; Bina, V.; Brancato, A.M.; Gastaldi, G.; Annumziata, S.; Mosconi, M.; Grassi, F.A.; Benazzo, F.; Pasta, G. The role of pemfs on bone healing: An in vitro study. Int. J. Mol. Sci. 2022, 23, 14298. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, M.A.; Darendeliler, A.; Sinclair, P.M. Effects of static magnetic and pulsed electromagnetic fields on bone healing. Int. J. Adult Orthodon. Orthognath. Surg. 1997, 12, 43–53. [Google Scholar] [PubMed]
- Pereira, A.; Diaz, J.J.H.; Saur, M.; Botero, S.S.; Facca, S.; Liverneaux, P. Carpal scaphoid non-union treatment: A retrospective trial comparing simple retrograde percutaneous screw fixation versus percutaneous screw fixation plus pulsed electromagnetic fields (Physiotim). Eur. J. Orthop. Surg. Traumatol. 2017, 27, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Costa, M.L.; Parsons, N.; Smith, N. Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults. Cochrane Database Syst. Rev. 2011, 13, CD008471. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, V.; Anazor, F.; Relwani, J.; Dhinsa, B.S. Outcomes of the treatment of fracture non-union using combined magnetic field bone growth stimulation: Experiences from a UK trauma unit. Cureus 2012, 14, e25100. [Google Scholar] [CrossRef] [PubMed]
- Assiotis, A.; Sachinis, N.P.; Chalidis, B.E. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J. Orthop. Surg. Res. 2012, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, P.F.W.; Mommers, E.H.H.; Schots, J.P.M.; Brink, P.R.G.; Poeze, M. The effects of low-intensity pulsed ultrasounds and pulsed electromagnetic fields bone growth stimulation in acute fractures: A systematic review and meta-analysis of randomized controlled trials. Arch. Orthop. Truam Surg. 2014, 134, 1093–1106. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Solarino, D.; Moretti, F.; Ottaviani, G.; Simone, F.; Zavattini, G.; Maccagnano, G.; Noia, G.; Moretti, B. The role of biophysical stimulation with pemfs in fracture healing from bench to bedside. J. Biol. Regl. Homeost. Agents 2020, 34, 131–135. [Google Scholar]
- Lv, H.; Wang, Y.; Zhen, C.; Liu, J.; Chen, X.; Zhang, G.; Yao, W.; Guo, H.; Wei, Y.; Wang, S.; et al. A static magnetic field improves bone quality and balances the function of bone cells with regulation on iron metabolism and redox status in type 1 diabetes. FASEB J. 2023, 37, e22985. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Hua, Z.; Nie, S.; Xu, S.; Zhou, C.; Chen, F.; Li, M.; Yu, Q.; Sun, Y.; et al. Rotating magnetic field mitigates ankylosing spondylitis targeting osteocytes and chondrocytes via ameliorating immune dysfunctions. Cells 2023, 12, 972. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Y.; Liu, J.; Zhen, C.; Zhang, X.; Liu, Y.; Lou, C.; Guo, H.; Wei, Y. Exposure to a static magnetic field attenuates hepatic damage and function abnormality in obese and diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166719. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Li, X.Y.; Tian, Y.H.; Chen, X.R.; Zhou, J.; Zhu, B.Y.; Xi, H.R.; Gao, Y.H.; Xian, C.J.; Chen, K.M. Pulsed electromagnetic fields prevented the decrease of bone formation in hindlimb-suspended rats by activating sAC/cAMP/PKA/CREB signaling pathway. Bioelectromagnetics 2018, 39, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, S.; Lv, H.; Wei, M.; Fang, Y.; Shang, P. Static magnetic field of 0.2-0.4 T promotes the recovery of hindlimb unloading-induced bone loss in mice. Int. J. Radiat. Biol. 2021, 97, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Salmelin, R. Magnetocephalography: From SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage 2012, 61, 386–396. [Google Scholar] [CrossRef]
- Lowery, C.L.; Govindan, R.B.; Preissl, H.; Murphy, P.; Eswaran, H. Fetal neurological assessment using noninvasive magnetocephalography. Clin. Perinatol. 2009, 36, 701–709. [Google Scholar] [CrossRef]
- Anninos, P.; Adamopoulos, A.; Kotini, A. MEG as a medical diagnostic tool in the Greek population. Acta Medica 2015, 58, 71–78. [Google Scholar] [CrossRef]
- Rizkalla, J.; Botros, D.; Alqahtani, N.; Patnala, M.; Salama, P.; Perez, F.P.; Rizkalla, M. Electromagnetic detection of mild brain injury: A novel imaging approach to post concussive syndrome. J. Biomed. Sci. Eng. 2021, 14, 347–360. [Google Scholar] [CrossRef]
- Burgess, R.C. Magnetoencephalography for localizing and characterizing the epileptic focus. Handb. Clin. Neurol. 2019, 160, 203–214. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, F.; Lei, W.; Ke, J.; Dai, Y.; Qi, R.; Lu, G.; Zhong, Y. Transcriptional signal and cell specificity of genes related to cortical structural differences of post-traumatic stress disorder. J. Psychiatr. Res. 2023, 160, 28–37. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.E.; Guarnaccia, L.; Capanella, R.; Locatelli, M.; Miozzo, M.; Perelli, P.; Morte, G.D.; Catamo, L.; Tondo, P.; et al. Space flight and central nervous system: Friends or enemies: Challenges and opportunities for neuroscience and neuro-oncology. J. Neurosci. Res. 2020, 100, 1649–1663. [Google Scholar] [CrossRef]
- Berles, F.; Williams, R.; Berger, L.; Pike, G.B.; Lebel, C.; Iaria, G. The unresolved methodological challenge of detecting neuroplastic changes in astronauts. Life 2023, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Pusil, S.; Zegarra-Valdivia, J.; Cuesta, P.; Laohathai, C.; Cebolta, A.M.; Haueisen, J.; Fiedler, P.; Funke, M.; Maestu, F.; Cheron, G. Effects of spaceflight on the EEG alpha power and functional connectivity. Sci. Rep. 2023, 13, 9489. [Google Scholar] [CrossRef] [PubMed]
- Barkaszi, I.; Ehmann, B.; Tolgyesi, B.; Balazs, L.; Altbacker, A. Are head-down tilt bedrest studies capturing the true nature of spaceflight-induced cognitive changes? A review. Front. Physiol. 2022, 13, 1008508. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Robertson, A.D.; Arbeille, P.; Shoemaker, K.; Rush, J.W.E.; Fraser, K.S.; Greaves, D.K. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H628–H638. [Google Scholar] [CrossRef] [PubMed]
- Genik, R.J., 2nd; Green, C.C.; Graydon, F.X.; Armstrong, R.E. Cognitive avionics and watching spaceflight crews think: Generation-after-next research tools in functional neuroimaging. Aviat. Space Environ. Med. 2005, 76, B208–B212. [Google Scholar] [PubMed]
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.M.A.; van der Flier, W.M.; Stam, C.J.; Hillebrand, A.; Scheltens, P.; van Straaten, E.C.W. Alzheimer’s disease: The state of the art in resting-state magnetoencephalography. Clin. Neurophysiol. 2017, 128, 1426–1437. [Google Scholar] [CrossRef]
- Dai, Z.; He, Y. Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer’s disease. Neurosci. Bull. 2014, 30, 217–232. [Google Scholar] [CrossRef]
- Lopez-Sanz, D.; Bruna, R.; de Frutos-Lucas, J.; Maestu, F. Magnetoencephalography applied to the study of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2019, 165, 25–61. [Google Scholar] [CrossRef]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Perico, C.; Dubois, B.; Escudero, J.; Fernandez, A.; Frisoni, G.; et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol. Aging 2020, 85, 58–73. [Google Scholar] [CrossRef]
- Maestu, F.; Fernandez, A. Role of magnetoencephalography in the early stages of Alzheimer disease. Neuroimaging Clin. N. Am. 2020, 30, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.; Silk, A.; Hansen, L. Are rises in electro-magnetic field in the human environment, interacting with multiple environmental pollutions, the tripping point for increases in neurological deaths in the Western world? Med. Hypotheses 2019, 127, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.H.W.; Fahnle, M. A short review on the influence of magnetic fields on neurological diseases. Front. Biosci. 2021, 13, 181–189. [Google Scholar] [CrossRef]
- Riancho, J.; de la Torre, J.R.S.; Paz-Fajardo, L.; Limia, C.; Santurtun, A.; Cifra, M.; Kourtidis, K.; Fdez-Arroyabe, P. The role of magnetic fields in neurodegenerative diseases. Int. J. Biometeorol. 2021, 65, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bragin, D.E.; Statom, G.L.; Hagberg, S.; Nemoto, E.M. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J. Neurosurg. 2015, 122, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ge, H.; Zhao, H.; Zou, Y.; Chen, Y.; Feng, H. Electromagnetic fields for the regulation of neural stem cells. Stem Cells Int. 2017, 2017, 9898439. [Google Scholar] [CrossRef] [PubMed]
- van Belkum, S.M.; Bosker, F.J.; Kortekaas, R.; Beersma, D.G.M.; Schoevers, R.A. Treatment of depression with low-strength transcranial pulsed electromagnetic fields: A mechanistic point of view. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Gogulski, J.; Ross, J.M.; Talbot, A.; Cline, C.C.; Donati, F.L.; Munot, S.; Kim, N.; Gibbs, C.; Bastin, N.; Yand, J.; et al. Personalized repetitive transcranial magnetic stimulation for depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 351–360. [Google Scholar] [CrossRef]
- Petrosino, N.J.; Cosmo, C.; Berlow, Y.A.; Zandvakilli, A.; van’t Wout-Frank, M.; Philip, N.S. Transcranial magnetic stimulation for post-traumatic stress disorder. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211049921. [Google Scholar] [CrossRef]
- Bashir, S.; Uzair, M.; Abualait, T.; Arshad, M.; Khallaf, R.A.; Niaz, A.; Thani, Z.; Yoo, W.K.; Tunez, I.; Demirtas-Tatlidede, A.; et al. Effects of transcranial magnetic stimulation on neurobiological changes in Alzheimer’s disease. Mol. Med. Rep. 2022, 25, 109. [Google Scholar] [CrossRef]
- Zhi, W.; Zou, Y.; Ma, L.; He, S.; Guo, Z.; Zhao, X.; Hu, X.; Wang, L. 900 MHZ electromagnetic field exposure relieved AD-like symptoms on APP/PS1 mice: A potential non-invasive strategy for AD treatment. Biochem. Biophys. Res. Commun. 2023, 658, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Dufor, T.; Lohof, A.M.; Sherrard, R.M. Magnetic stimulation as a therapeutic approach for brain modulation and repair: Underlying molecular and cellular mechanisms. Int. J. Mol. Sci. 2023, 24, 16456. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Costantini, E.; Reale, M.; Americo, P. Wound repair and extremely low frequency-electromagnetic field: Insight from in vitro study and potential clinical application. Int. J. Mol. Sci. 2021, 22, 5037. [Google Scholar] [CrossRef] [PubMed]
- Collard, J.-F.; Hinsenkamp, M. Cellular processes involved in human epidermal cells exposed to extremely low frequency fields. Cell Signal. 2015, 27, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimdamavandi, S.; Mobasheri, H. Application of a static magnetic field as a complementary aid to healing in an in vitro wound model. J. Wound Care. 2019, 28, 40–52. [Google Scholar] [CrossRef]
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Extremely low frequency electromagnetic field and wound healing: Implication of cytokines as biological mediators. Eur. Cytokine Netw. 2013, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ekici, Y.; Aydogan, C.; Balcik, C.; Haberal, N.; Kirnap, M.; Moray, G.; Haberal, M. Effect of static magnetic field on experimental dermal wound strength. Indian J. Plast. Surg. 2012, 45, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Cheing, G.L.Y.; Li, X.; Huang, L.; Kwan, R.L.C.; Cheung, K.K. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics 2014, 35, 161–169. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.G.; Deng, K.Q.; Yun, P.; Gong, T. Therapeutic effects of static magnetic field on wound healing in diabetic rats. J. Diabetes Res. 2017, 2017, 6305370. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Hart, D.A. Influence of comorbidities, neuropathy, vasculopathy, and diabetes on healing response quality. Adv. Wound Care 2013, 2, 410–421. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Schizas, N.; Li, J.; Ahmed, M.; Ostenson, C.-G.; Salo, P.; Hewitt, C.; Hart, D.A.; Ackermann, P.W. Type 2 diabetes impairs tendon repair after injury in a rat model. J. Appl. Physiol. 2012, 113, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Lou, L.; Jiao, L.; Hu, J.; Zhang, P.; Wang, Z.; Xu, W.; Geng, X.; Song, H. Effects of low-frequency pulsed electromagnetic fields on plateau frostbite healing in rats. Wound Repair Regen. 2016, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Glascott, M.W.; Brown, E.W.; Dorsey, K.; Laber, C.H.; Conley, K.; Ray, J.D.; Moores, L.C.; Netchaev, A. Selecting an optimal Faraday cage to minimize noise in electrochemical experiments. Anal. Chem. 2022, 94, 11983–11989. [Google Scholar] [CrossRef]
- Hansson, H.A. Purkinje nerve cell changes caused by electric fields-ultrastructural studies on long-term effects on rabbits. Med. Biol. 1981, 59, 103–110. [Google Scholar] [PubMed]
- Akdag, M.Z.; Dasdag, S.; Alsen, F.; Isik, B.; Yilmaz, F. Effect of ELF magnetic fields on lipid peroxidation, sperm count, p53, and trace elements. Med. Sci. Monit. 2006, 12, BR366–BR371. [Google Scholar] [PubMed]
- Caprani, A.; Richert, A.; Flaud, P. Experimental evidence of a potentially increased thrombo-embolic disease risk by domestic electromagnetic field exposure. Bioelectromagnetics 2004, 25, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Influence of space environments in system physiologic and molecular integrity: Redefining the concept of human health beyond the boundary conditions of Earth. J. Biomed. Sci. Eng. 2019, 12, 400–408. [Google Scholar] [CrossRef]
- Hart, D.A. Human heterogeneity and survival of the species: How did it arise and being sustained?- The conundrum facing researchers. J. Biomed. Sci. Eng. 2021, 14, 212–221. [Google Scholar] [CrossRef]
- Waliszewski, P.; Skwarek, R.; Jeromin, L.; Minikowski, H. On the mitochondrial aspect of reactive oxygen species action in external magnetic fields. J. Photochem. Photobiol. B 1999, 52, 137–140. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Falone, S.; Mijit, M.; Tatone, C.; Amicarelli, F.; Di Emido, G. Role of mitochondria in the oxidative stress induced by electromagnetic fields: Focus on reproductive systems. Oxid. Med. Cell Longev. 2018, 2018, 5076271. [Google Scholar] [CrossRef]
- Toda, T.; Ito, M.; Takeda, J.-I.; Masuda, A.; Mino, H.; Hattori, N.; Mohri, K.; Ohno, K. Extremely low-frequency pulses of faint magnetic field induce mitophagy to rejuvenate mitochondria. Commun. Biol. 2022, 5, 453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ito, M.; Zhang, S.; Toda, T.; Takeda, J.-I.; Ogi, T.; Ohno, K. Extremely low-frequency electromagnetic field induces acetylation of heat shock proteins and enhances protein folding. Ecotoxicol. Environ. Saf. 2023, 264, 115482. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.V.; Osipova, E.A. Molecular biological effects of weak low-frequency magnetic fields: Frequency-amplitude efficiency windows and possible mechanisms. Int. J. Mol. Sci. 2023, 24, 10989. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, D.A. The Influence of Magnetic Fields, Including the Planetary Magnetic Field, on Complex Life Forms: How Do Biological Systems Function in This Field and in Electromagnetic Fields? Biophysica 2024, 4, 1-21. https://doi.org/10.3390/biophysica4010001
Hart DA. The Influence of Magnetic Fields, Including the Planetary Magnetic Field, on Complex Life Forms: How Do Biological Systems Function in This Field and in Electromagnetic Fields? Biophysica. 2024; 4(1):1-21. https://doi.org/10.3390/biophysica4010001
Chicago/Turabian StyleHart, David A. 2024. "The Influence of Magnetic Fields, Including the Planetary Magnetic Field, on Complex Life Forms: How Do Biological Systems Function in This Field and in Electromagnetic Fields?" Biophysica 4, no. 1: 1-21. https://doi.org/10.3390/biophysica4010001
APA StyleHart, D. A. (2024). The Influence of Magnetic Fields, Including the Planetary Magnetic Field, on Complex Life Forms: How Do Biological Systems Function in This Field and in Electromagnetic Fields? Biophysica, 4(1), 1-21. https://doi.org/10.3390/biophysica4010001





