AI-Driven Predictive Modeling of Nanoparticle-Enhanced Solvent-Based CO2 Capture Systems: Comprehensive Review and ANN Analysis
Abstract
1. Introduction
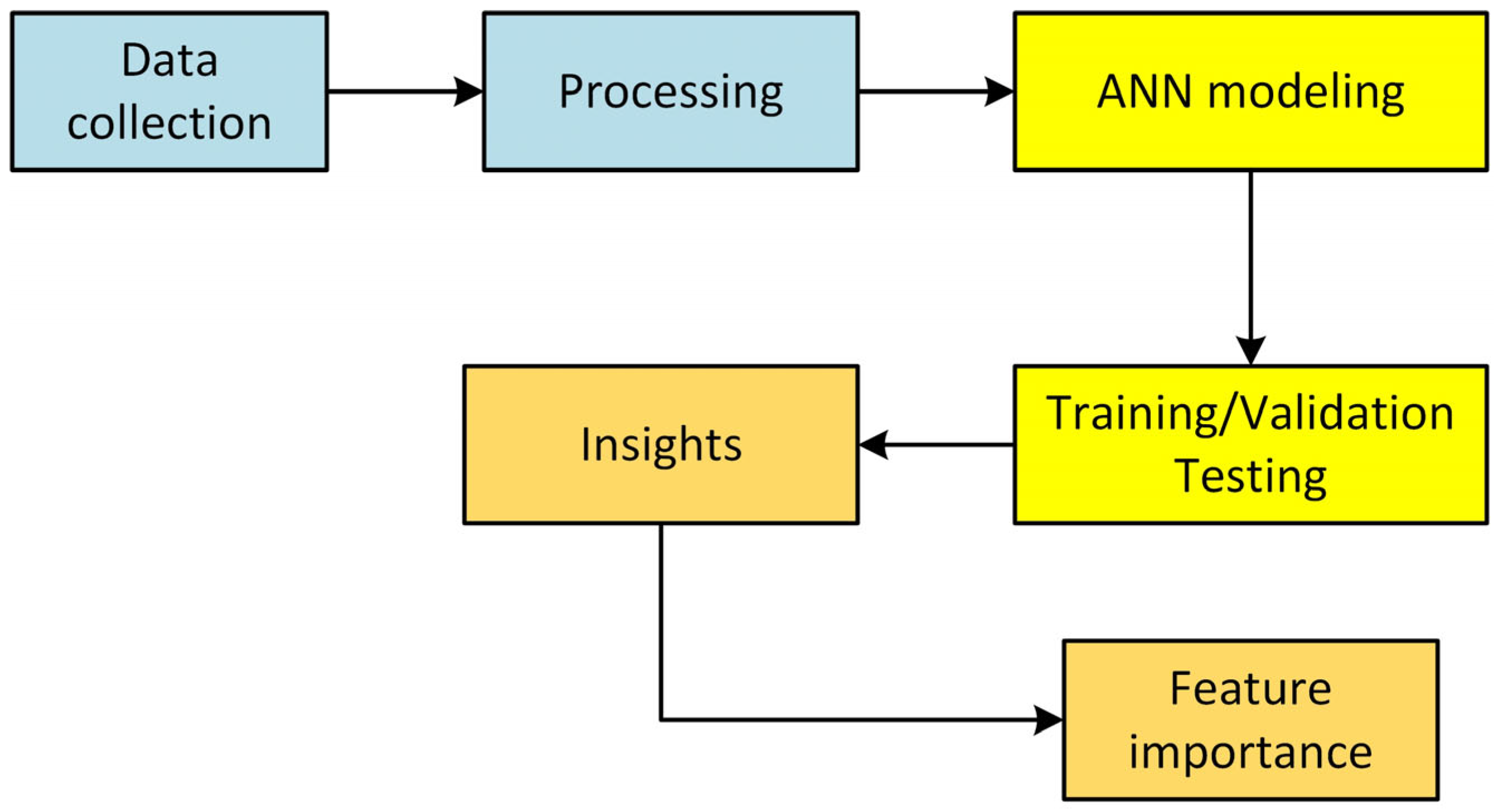
2. Methodology
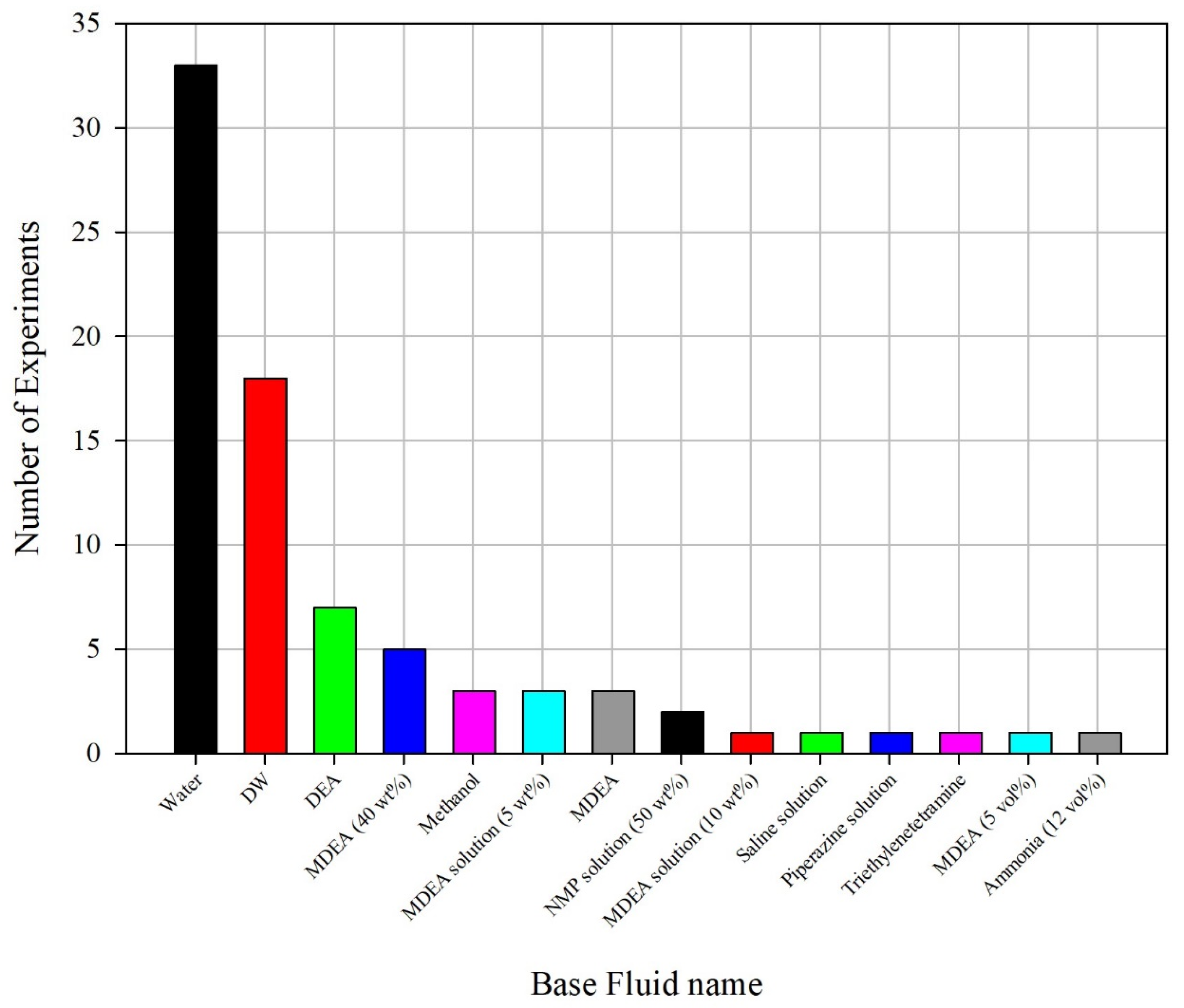
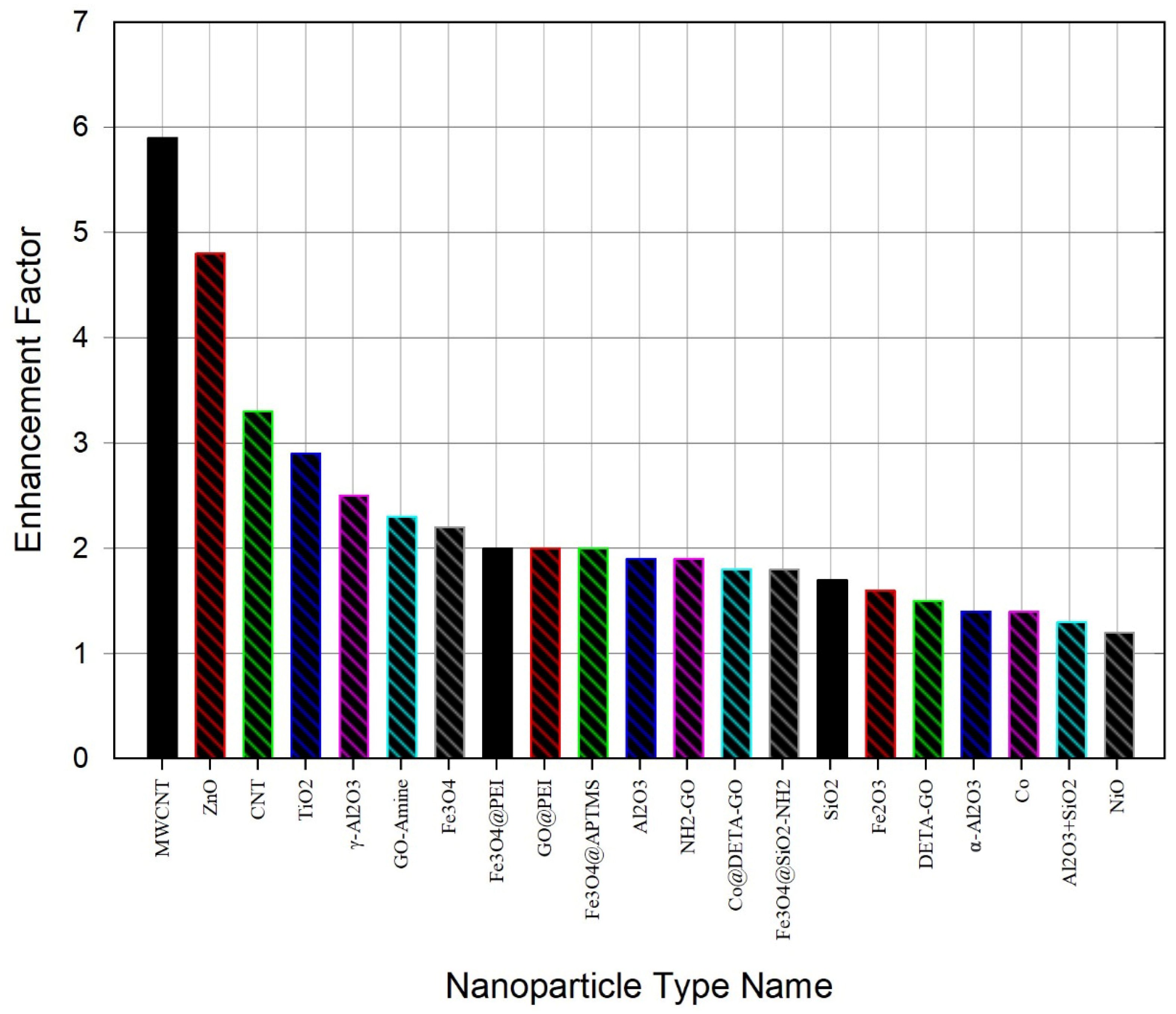
2.1. Data Extraction and Preparation
2.2. ANN Model Architecture
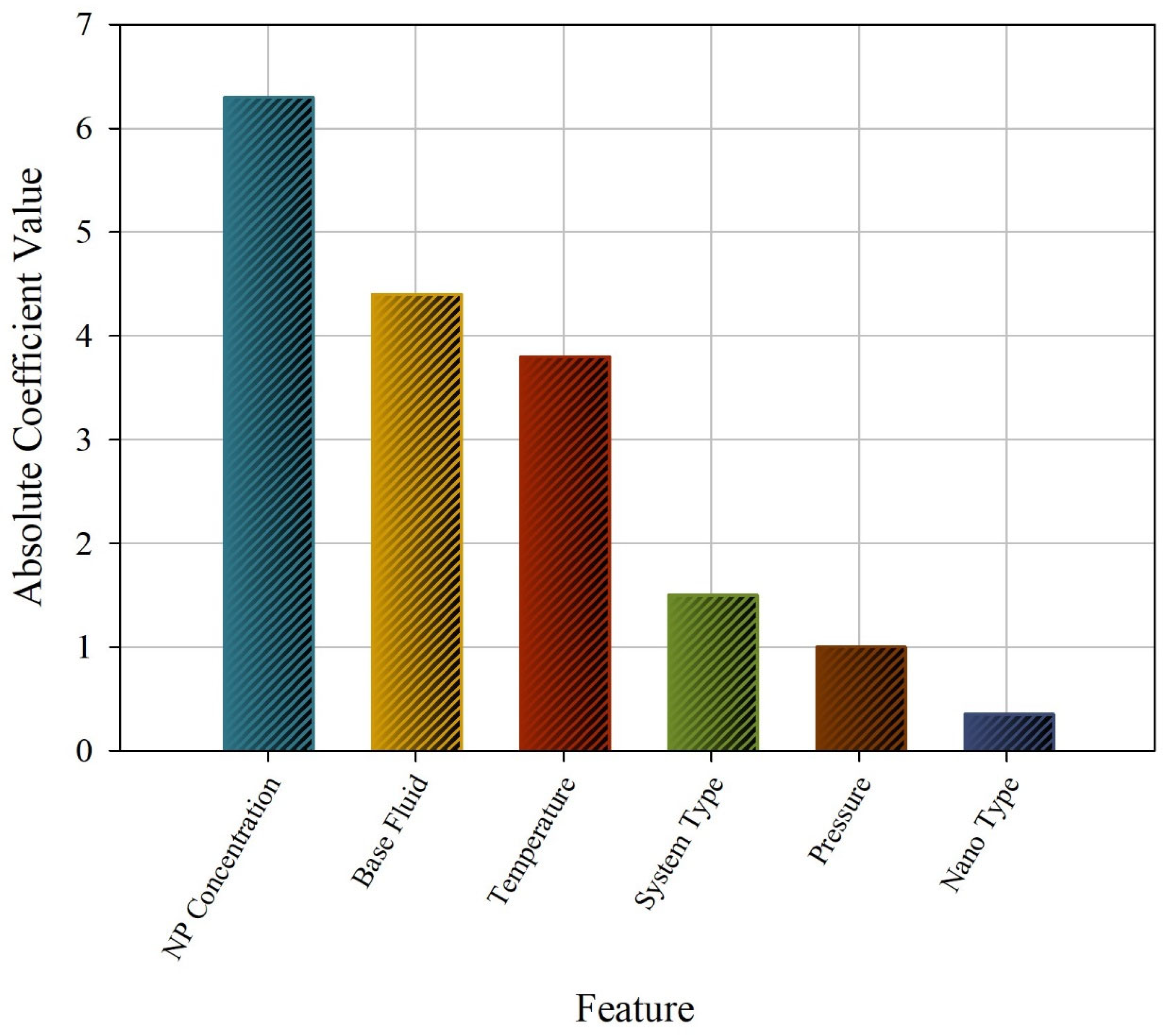
2.3. Additional Analyses
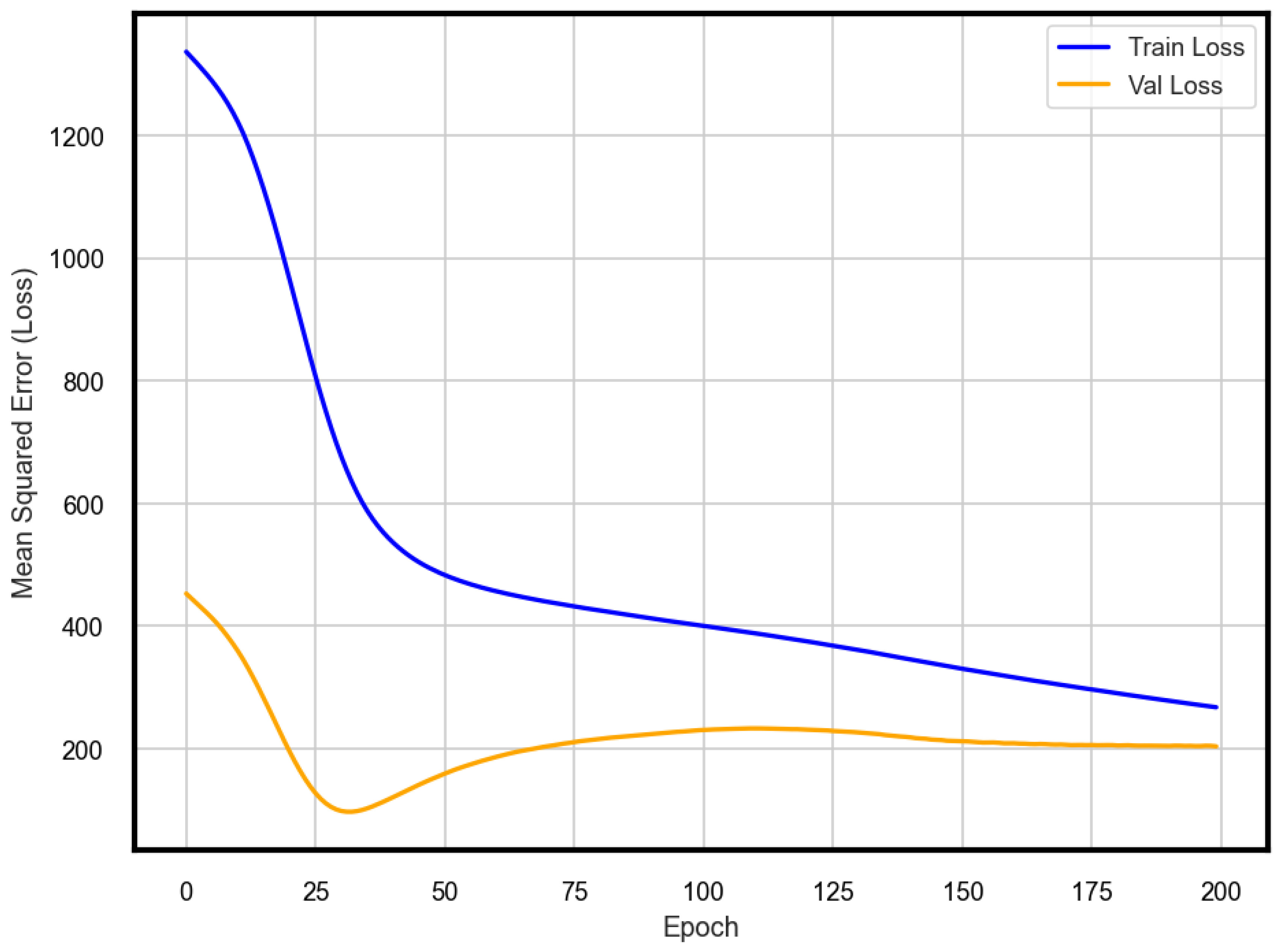
2.4. Model Validation
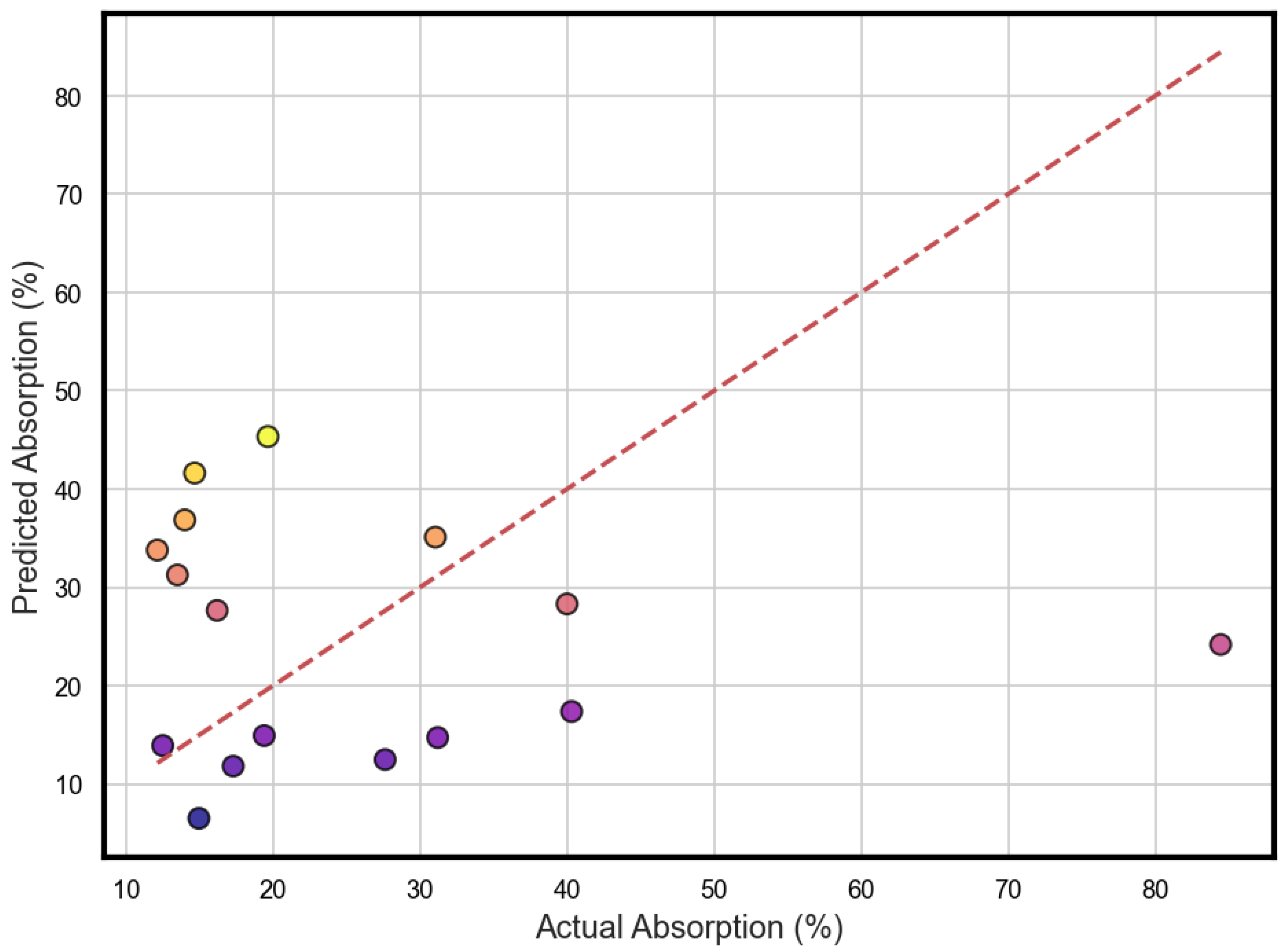
3. Results and Discussion
3.1. Limits and How Broadly It Applies
3.2. Model Comparison
4. Present and Future Directions
- Material Innovation: Explore advanced nanomaterials such as MOFs and MXenes with tailored surface functionalization, particularly for amine-group modifications shown to improve EF by 10–19% in equilibrium cells.
- Standardization: Develop standardized absorption measurement protocols to ensure consistency across studies and datasets.
- Pilot-Scale Validation: Conduct pilot-scale testing under real operating conditions to assess scalability and economic feasibility.
- Circular Economy Integration: Investigate the integration of nanoparticle-enhanced CO2 capture systems into circular economy frameworks for sustainable deployment.
- This multi-pronged strategy will help bridge laboratory research with industrial implementation, advancing the practical relevance and long-term impact of nanoparticle-enhanced CO2 capture technologies.
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANN | Artificial Neural Network |
| DEA | Diethanolamine |
| MDEA | Methyldiethanolamine |
| DW | Distilled Water |
| RMSE | Root Mean Square Error |
| MAE | Mean Absolute Error |
| R2 | Coefficient of Determination |
| CO2 | Carbon Dioxide |
References
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Abanades, J.C.; Rubin, E.S.; Anthony, E.J. Emerging CO2 capture systems. Energy Environ. Sci. 2015, 8, 1077–1092. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Koumpouras, G.; Yeung, H. Dynamic modelling and analysis of post-combustion CO2 chemical absorption process for coal-fired power plants. Fuel 2010, 89, 2791–2801. [Google Scholar] [CrossRef]
- Tan, L.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Fa, M.; Yang, Y.; Wang, Y.; Li, F.; Lai, L.S.; Yeap, S.P. Potential of a 1-Amino-2-propanol/Sulfolane Biphasic Solution for CO2 Capture: Performance and Mechanism Study. Energy Fuels 2024, 38, 17726–17740. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, P.; Li, Y.; Teng, L.; Zhu, J. Hydrodynamic characteristics and mass transfer performance of rotating packed bed for CO2 removal by chemical absorption: A review. J. Nat. Gas Sci. Eng. 2020, 79, 103373. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, B.; Du, M. How Digital Economy Mitigates Urban Carbon Emissions: The Green Facilitative Power of Industrial Coagglomeration. Appl. Econ. 2025, 1–19. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.; Pineda, I.T.; Kang, Y.T. Review of nanoabsorbents for capture enhancement of CO2 and its industrial applications with design criteria. Renew. Sustain. Energy Rev. 2021, 138, 110524. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Baena-Moreno, F.M.; Pan, Z.; Zhang, R.; Zhou, H.; Zhang, Z. Review and perspectives of CO2 absorption by water- and amine-based nanofluids. Energy Fuels 2023, 37, 8883–8901. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, J.; Chen, F.; Li, H.; Zhang, W.; Qi, W. Progress in enhancement of CO2 absorption by nanofluids: A mini review of mechanisms and current status. Renew. Energy 2018, 118, 527–535. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Park, A.-H.A.; Fang, M. Review of liquid nanoabsorbents for enhanced CO2 capture. Nanoscale 2019, 11, 17137–17156. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Bhattacharya, P.; Phelan, P.E.; Prasher, R. Enhanced mass transport in nanofluids. Nano Lett. 2006, 6, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Aghel, B.; Janati, S.; Alobaid, F.; Almoslh, A.; Epple, B. Application of nanofluids in CO2 absorption: A review. Appl. Sci. 2022, 12, 3200. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Zaeri, M.; Mehdipour, M.; Keshavarz, P. Synthesis of Different Modified Magnetic Nanoparticles for Selective Physical/Chemical Absorption of CO2 in a Bubble Column Reactor. J. Environ. Chem. Eng. 2020, 8, 104195. [Google Scholar] [CrossRef]
- Rahmatmand, B.; Keshavarz, P.; Ayatollahi, S. Study of absorption enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 nanoparticles in water and amine solutions. J. Chem. Eng. Data 2016, 61, 1378–1387. [Google Scholar] [CrossRef]
- Zarei, F.; Jahromi, F.B.; Elhambakhsh, A.; Keshavarz, P. Enhanced CO2 absorption and reduced regeneration energy consumption using modified magnetic NPs. Energy 2023, 278, 127776. [Google Scholar] [CrossRef]
- Irani, V.; Maleki, A.; Tavasoli, A. CO2 absorption enhancement in graphene-oxide/MDEA nanofluid. J. Environ. Chem. Eng. 2019, 7, 102782. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Mousavi, S.M.; Ghasemi, M.N.; Esmaeilzadeh, F.; Keshavarz, P.; Asadi, K.; Haghighi, R.K.; Wang, X. Absorption increment of various physical/chemical CO2 absorbents using CeO2/SiO2/TiO2 nanocomposite. Chem. Pap. 2022, 76, 4817–4834. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Darabi, M.; Soroush, E.; Mesbah, M. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep. Purif. Technol. 2019, 210, 920–926. [Google Scholar] [CrossRef]
- Golkhar, A.; Keshavarz, P.; Mowla, D. Investigation of CO2 removal by silica and CNT nanofluids in microporous hollow fiber membrane contactors. J. Membr. Sci. 2013, 433, 17–24. [Google Scholar] [CrossRef]
- Cao, Y.; Rehman, Z.U.; Ghasem, N.; Al-Marzouqi, M.; Abdullatif, N.; Nakhjiri, A.T.; Ghadiri, M.; Rezakazemi, M.; Marjani, A.; Pishnamazi, M.; et al. Intensification of CO2 absorption using MDEA-based nanofluid in a hollow fibre membrane contactor. Sci. Rep. 2021, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Xu, R.; Lee, W.; Choi, C.K.; Kang, Y.T. CO2 absorption performance enhancement by dodecane nanoemulsion absorbents. J. CO2 Util. 2019, 30, 18–27. [Google Scholar] [CrossRef]
- Zandahvifard, M.J.; Elhambakhsh, A.; Ghasemi, M.N.; Esmaeilzadeh, F.; Parsaei, R.; Keshavarz, P.; Wang, X. Effect of modified Fe3O4 magnetic NPs on the absorption capacity of CO2 in water, wettability alteration of carbonate rock surface, and water-oil interfacial tension for oilfield applications. Ind. Eng. Chem. Res. 2021, 60, 3421–3434. [Google Scholar] [CrossRef]
- Ghasem, N. Chemical Absorption of CO2 Enhanced by Nanoparticles Using a Membrane Contactor: Modeling and Simulation. Membranes 2019, 9, 150. [Google Scholar] [CrossRef]
- Darabi, M.; Rahimi, M.; Dehkordi, A.M. Gas absorption enhancement in hollow fiber membrane contactors using nanofluids: Modeling and simulation. Chem. Eng. Process. 2017, 119, 7–15. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, J.; Jiang, Z. Numerical investigation of carbon dioxide capture using nanofluids via machine learning. J. Clean. Prod. 2024, 450, 141916. [Google Scholar] [CrossRef]
- Feng, L.; Zhong, K.; Liu, J.; Ghanbari, A. Applying supervised intelligent scenarios to numerical investigate carbon dioxide capture using nanofluids. J. Clean. Prod. 2022, 381, 135088. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Keshavarz, P. Enhanced CO2 capture efficiency applying amine-based nano magnetite/sulfinol-M nano solvents at high pressures. Environ. Sci. Pollut. Res. 2021, 28, 3455–3464. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Keshavarz, P. Investigation of carbon dioxide absorption using different functionalized Fe3O4 magnetic nanoparticles. Energy Fuels 2020, 34, 7198–7208. [Google Scholar] [CrossRef]
- Haghtalab, A.; Mohammadi, M.; Fakhroueian, Z. Absorption and solubility measurement of CO2 in water-based ZnO and SiO2 nanofluids. Fluid Phase Equilib. 2015, 392, 33–42. [Google Scholar] [CrossRef]
- Pineda, I.T.; Lee, J.W.; Jung, I.; Kang, Y.T. CO2 absorption enhancement by methanol-based Al2O3 and SiO2 nanofluids in a tray column absorber. Int. J. Refrig. 2012, 35, 1402–1409. [Google Scholar] [CrossRef]
- Qu, C.; Wang, J.; Yin, H.; Lu, G.; Li, Z.; Feng, Y. Condensate oil-tolerant foams stabilized by an anionic–sulfobetaine surfactant mixture. ACS Omega 2019, 4, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, H.; Ghaemi, A.; Nasiri, M.; Heydarifard, M. Experimental investigation of the effect of nano heavy metal oxide particles in Piperazine solution on CO2 absorption using a stirrer bubble column. Energy Fuels 2018, 32, 2037–2052. [Google Scholar] [CrossRef]
- Li, S.H.; Ding, Y.; Zhang, X.S. Enhancement on CO2 bubble absorption in MDEA solution by TiO2 nanoparticles. Adv. Mater. Res. 2013, 631, 127–134. [Google Scholar] [CrossRef]
- Taheri, M.; Mohebbi, A.; Hashemipour, H.; Rashidi, A.M. Simultaneous absorption of carbon dioxide (CO2) and hydrogen sulfide (H2S) from CO2–H2S–CH4 gas mixture using amine-based nanofluids in a wetted wall column. J. Nat. Gas Sci. Eng. 2016, 28, 410–417. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2013, 53, 206–211. [Google Scholar] [CrossRef]
- Mohammaddoost, H.; Azari, A.; Ansarpour, M.; Osfouri, S. Experimental investigation of CO2 removal from N2 by metal oxide nanofluids in a hollow fiber membrane contactor. Int. J. Greenh. Gas Control 2018, 69, 60–71. [Google Scholar] [CrossRef]
- Peyravi, A.; Keshavarz, P.; Mowla, D. Experimental investigation on the absorption enhancement of CO2 by various nanofluids in hollow fiber membrane contactors. Energy Fuels 2015, 29, 8135–8142. [Google Scholar] [CrossRef]
- Zare, P.; Keshavarz, P.; Mowla, D. Membrane absorption coupling process for CO2 capture: Application of water-based ZnO, TiO2, and multi-walled carbon nanotube nanofluids. Energy Fuels 2019, 33, 1392–1403. [Google Scholar] [CrossRef]
- Preston, D.J.; Lu, Z.; Song, Y.; Zhao, Y.; Wilke, K.L.; Antao, D.S.; Louis, M.; Wang, E.N. Heat Transfer Enhancement During Water and Hydrocarbon Condensation on Lubricant Infused Surfaces. Sci. Rep. 2018, 8, 540. [Google Scholar] [CrossRef]
- Lashgarinejad, A.; Hosseini, S.S.; Irani, V.; Ghasemi, M.H.; Mohammadpour, R.; Tavasoli, A. Enhancement of CO2 absorption and heat transfer properties using amine functionalized magnetic graphene oxide/MDEA nanofluid. J. Iran. Chem. Soc. 2023, 20, 1629–1642. [Google Scholar] [CrossRef]
- Irani, V.; Tavasoli, A.; Vahidi, M. Preparation of amine functionalized reduced graphene oxide/methyl diethanolamine nanofluid and its application for improving the CO2 and H2S absorption. J. Colloid Interface Sci. 2018, 527, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Irani, V.; Tavasoli, A.; Maleki, A.; Vahidi, M. Polyethyleneimine-functionalized HKUST-1/MDEA nanofluid to enhance the absorption of CO2 in gas sweetening process. Int. J. Hydrogen Energy 2018, 43, 5610–5619. [Google Scholar] [CrossRef]
- Vahidi, M.; Tavasoli, A.; Rashidi, A.M. Preparation of amine functionalized UiO-66, mixing with aqueous N-Methyldiethanolamine and application on CO2 solubility. J. Nat. Gas Sci. Eng. 2016, 28, 651–659. [Google Scholar] [CrossRef]
- Sodeifian, G.; Niazi, Z. Prediction of CO2 absorption by nanofluids using artificial neural network modeling. Int. Commun. Heat Mass Transf. 2021, 123, 105193. [Google Scholar] [CrossRef]
- Li, X.; Qin, J.; Wu, X.; Wei, C.; Xu, L. Designing back propagation neural network to predict CO2 mass transfer enhancement factor of TiO2-MEA/MDEA blended amine nanofluids. DeCarbon 2023, 2, 100021. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S.; Moayedi, H. Artificial intelligence in the field of nanofluids: A review on applications and potential future directions. Powder Technol. 2019, 353, 276–301. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Totten, J.D.; Johnston, B.F.; Seib, F.P. Microfluidic-Assisted Silk Nanoparticle Tuning. Nanoscale Adv. 2019, 1, 873–883. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA Nanoparticles Formed by Single- Or Double-Emulsion With Vitamin E-TPGS. J. Vis. Exp. 2013, 51015. [Google Scholar] [CrossRef] [PubMed]
- Lancel, M.; Gomez, C.; Port, M.; Amara, Z.; McCall, R.L.; Sirianni, R.W.; Wongpinyochit, T.; Totten, J.D.; Johnston, B.F.; Seib, F.P. Performances of Homogeneous and Heterogenized Methylene Blue on Silica Under Red Light in Batch and Continuous Flow Photochemical Reactors. Front. Chem. Eng. 2021, 3, 873–883. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Kumar, R.; Trivedi, J.; Sheng, J.J.; Sharma, T. Stable Silica Nanofluids of an Oilfield Polymer for Enhanced CO2 Absorption for Oilfield Applications. Energy Fuels 2018, 32, 12730–12741. [Google Scholar] [CrossRef]
- Heidari, S.; Esmaeilzadeh, F.; Rafati, R.; Haddad, A.S. Experimental and modeling of CO2 absorption in a bubble column using a water-based nanofluid containing co-doped SiO2 nanoparticles. Model. Earth Syst. Environ. 2024, 10, 3229–3241. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, S.; Malof, J.; Padilla, W. Deep Inverse Photonic Design: A Tutorial. Photon. Nanostruct. Fundam. Appl. 2022, 52, 101070. [Google Scholar] [CrossRef]
- Alavekar, A.; Lanjewar, T.; Vyawahare, A.; Patil, M.P. Artificial Intelligence in Carbon Capture: Modeling CO2 Absorption Using Amine Blends. J. Phys. Conf. Ser. 2024, 2763, 12020. [Google Scholar] [CrossRef]
- Su, Z.; Yang, L.; Zhao, N.; Song, J.; Li, X.; Wu, X. Experimental and neural network prediction of the cyclic stability and light absorption characteristics of supercritical CO2 based CNTs nanofluids. Appl. Therm. Eng. 2024, 241, 122347. [Google Scholar] [CrossRef]
- Delfani, S.; Esmaeili, M.; Karami, M. Application of artificial neural network for performance prediction of a nanofluid-based direct absorption solar collector. Sustain. Energy Technol. Assess. 2019, 36, 100559. [Google Scholar] [CrossRef]
- Devakki, B.; Thomas, S. Experimental Investigation on Absorption Performance of Nanofluids for CO2 Capture. Int. J. Air-Cond. Refrig. 2020, 28, 2050017. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, M.; Tan, Z.; Yu, S.; Liu, J.; Jiang, G. Particle Coating-Dependent Interaction of Molecular Weight Fractionated Natural Organic Matter: Impacts on the Aggregation of Silver Nanoparticles. Environ. Sci. Technol. 2015, 49, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Dalal, C.; Jana, N.R. Colloidal Nanobioconjugate With Complementary Surface Chemistry for Cellular and Subcellular Targeting. Langmuir 2018, 34, 13461–13471. [Google Scholar] [CrossRef] [PubMed]
- Kartohardjono, S.; Shabanindita, S.; Harianja, M.; Dixon, A.O.; Yuliusman, Y.; Saksono, N. N2O Absorption Through Super Hydrophobic Hollow Fiber Membrane Contactor. Environ. Prog. Sustain. Energy 2018, 38, 362–366. [Google Scholar] [CrossRef]
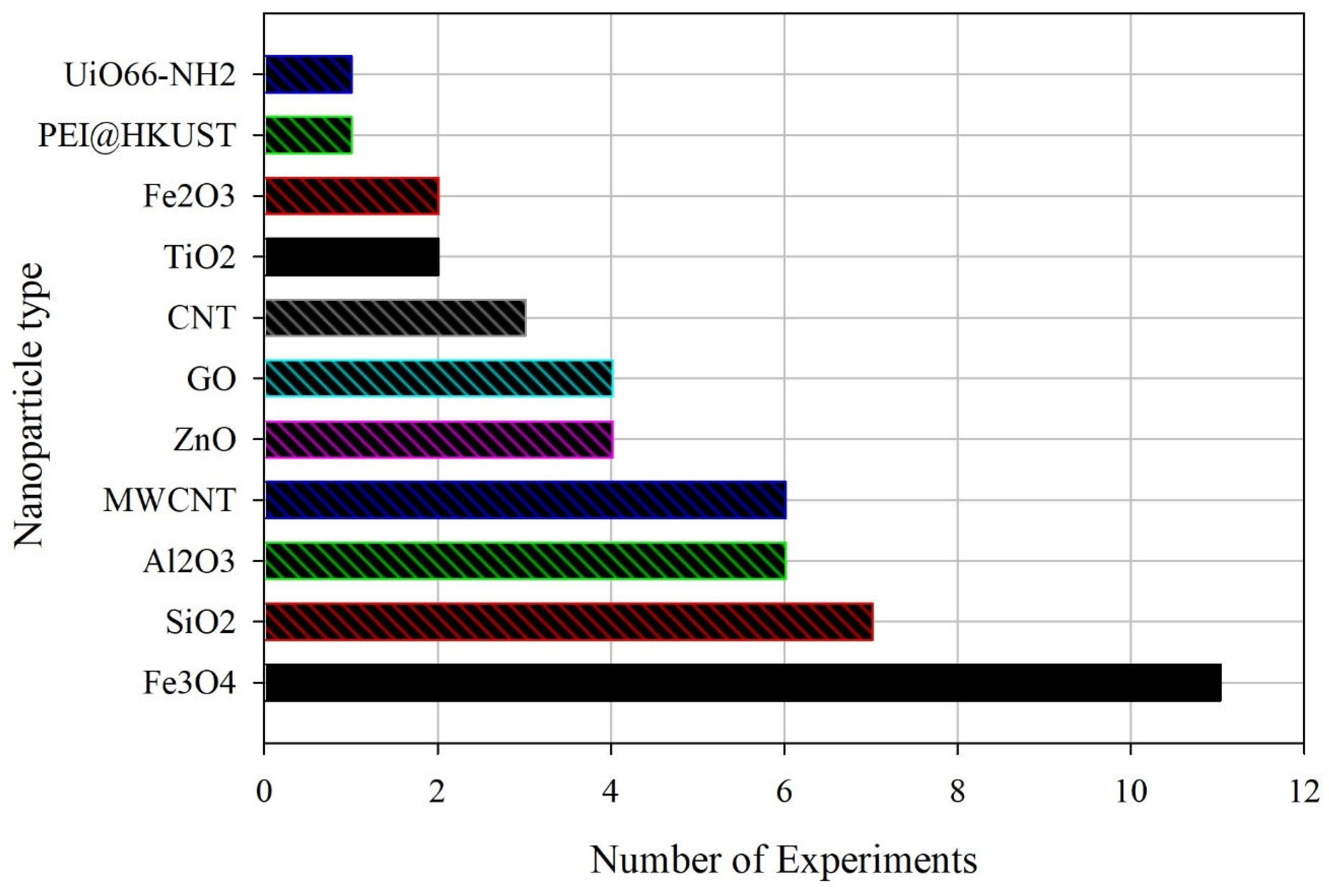
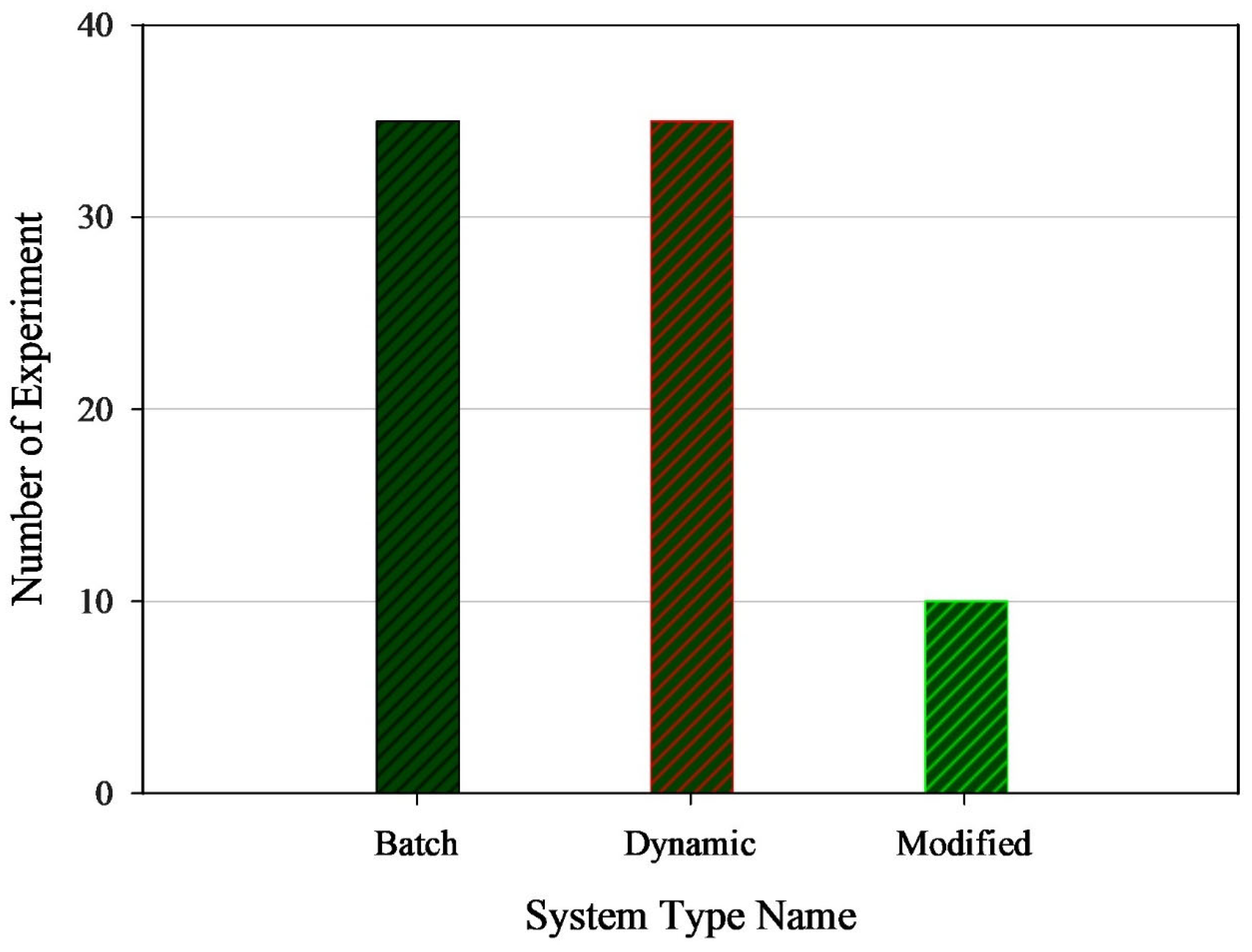



| Nanoparticles Used | System Type | Solvent Type | Enhancement Factor (EF) | Modeling/Analysis Approach | Limitation/How This Study Differs |
|---|---|---|---|---|---|
| Fe3O4@SiO2-NH2 | Batch Reactor | Water | 34.2% | Experimental only | Focused on single nanoparticle type and single system; no predictive modeling used. |
| ZnO | Membrane Contactor | Water | 130% | Experimental only | Optimized for one reactor type; no integration across multiple configurations. |
| SiO2 | Packed Column | DEA | 40% | Experimental only | Did not consider nanoparticle concentration, surface functionalization, or ANN prediction. |
| NH2-GO | Equilibrium Cell | Water | 19% | Experimental only | Limited to small-scale equilibrium studies; lacks generalizable modeling. |
| TiO2 | Bubble Column | MDEA | 11.5% | Experimental + Mechanistic Model | Mechanistic models struggle to capture nonlinear interactions across diverse datasets. |
| CNT | Hollow Fiber Membrane | Water | 32% | Experimental only | Focused on one nanoparticle; does not evaluate cross-system predictive capability. |
| Al2O3 | Wetted Wall Column | DEA | 33% | Experimental only | Limited solvent variability; no integration of data-driven prediction methods. |
| This Study | Integrated Framework | Multiple (Water, Amine, Mixed) | Up to 130% | ANN-based meta-analysis | First, to integrate 312 literature datasets across multiple reactor types, apply ANN predictive modeling, conduct feature importance analysis, and provide generalizable design insights. |
| Nanoparticle (NP) | Base Fluid | Optimal NP Loading | EF (%) | Ref |
|---|---|---|---|---|
| SiO2 | DW | 0.1 wt% | 7 | [32] |
| ZnO | DW | 0.1 wt% | 14 | [32] |
| Fe3O4− | DW | 0.02 wt% | 25.07 | [31] |
| Fe3O4− | DW | 0.1 wt% | 31.04 | [31] |
| Fe3O4@SiO2-NH2 | DW | 0.1 wt% | 34.23 | [31] |
| Fe3O4− | MDEA | 0.02 wt% | 6.78 | [31] |
| Fe3O4− | MDEA | 0.1 wt% | 12.13 | [31] |
| Nanoparticle (NP) | Base Fluid (BF) | Device | Optimal NP Loading | EF (%) | Ref |
|---|---|---|---|---|---|
| Al2O3 | Methanol | Tray Column | 0.05 vol% | 9.4 | [33] |
| SiO2 | Methanol | Tray Column | 0.05 vol% | 9.7 | [34] |
| ZnO | DEA | Bubble column | 0.1 wt% | 33.3 | [35] |
| ZnO | Piperazine | Bubble column | 0.1 wt% | 17 | [35] |
| TiO2 | MDEA | Bubble column | 0.8 wt% | 11.54 | [36] |
| Al2O3 | DEA | Wetted wall column | 0.05 wt% | 33 | [37] |
| Al2O3 | NaCl | Bubble Column | 0.01 vol% | 12.5 | [38] |
| SiO2 | DEA | Wetted wall column | 0.05 wt% | 40 | [37] |
| Nanoparticle | Base Fluid | LFR (mL/min) | Optimal NP Loading | EF (%) | Ref |
|---|---|---|---|---|---|
| CNT | DW | 166.7 | 0.05 wt% | 32 | [27] |
| SiO2 | DW | 166.7 | 0.05 wt% | 16 | [27] |
| Al2O3 | DW | 1600 | 0.2 wt% | 125 | [39] |
| Fe3O4 | DW | 116.7 | 0.15 wt% | 43.8 | [40] |
| CNT | DW | 116.7 | 0.1 wt% | 38 | [40] |
| Al2O3 | DW | 116.7 | 0.05 wt% | 3 | [40] |
| SiO2 | DW | 116.7 | 0.05 wt% | 25.9 | [40] |
| ZnO | DW | 10 | 0.15 wt% | 130 | [41] |
| MWCNT | DW | 10 | 0.15 wt% | 60 | [41] |
| TiO2 | DW | 10 | 0.15 wt% | 60 | [41] |
| Fe3O4 | DW | 10 | 0.025 wt% | 40.31 | [42] |
| Fe3O4@ | DW | 10 | 0.05 wt% | 84.45 | [42] |
| Nanoparticle | Base Fluid | Optimal NP Loading | EF (%) | Ref |
|---|---|---|---|---|
| GO | MDEA | 0.2 wt% | 10.4 | [43] |
| PEI@GO | MDEA | 0.1 wt% | 15 | [18] |
| NH2-GO reduced | MDEA | 0.1 wt% | 16.2 | [43] |
| NH2-GO magnetic | MDEA | 0.1 wt% | 19 | [44] |
| PEI@HKUST | MDEA | 0.2 wt% | 16 | [45] |
| UiO66-NH2 | MDEA | 0.1 wt% | 10 | [46] |
| Model | R2 | RMSE (%) | MAE (%) |
|---|---|---|---|
| ANN | 0.92 | 4.2 | 3.1 |
| Random Forest | 0.88 | 5.6 | 4.3 |
| Linear Regression | 0.76 | 7.9 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasem, N. AI-Driven Predictive Modeling of Nanoparticle-Enhanced Solvent-Based CO2 Capture Systems: Comprehensive Review and ANN Analysis. Eng 2025, 6, 226. https://doi.org/10.3390/eng6090226
Ghasem N. AI-Driven Predictive Modeling of Nanoparticle-Enhanced Solvent-Based CO2 Capture Systems: Comprehensive Review and ANN Analysis. Eng. 2025; 6(9):226. https://doi.org/10.3390/eng6090226
Chicago/Turabian StyleGhasem, Nayef. 2025. "AI-Driven Predictive Modeling of Nanoparticle-Enhanced Solvent-Based CO2 Capture Systems: Comprehensive Review and ANN Analysis" Eng 6, no. 9: 226. https://doi.org/10.3390/eng6090226
APA StyleGhasem, N. (2025). AI-Driven Predictive Modeling of Nanoparticle-Enhanced Solvent-Based CO2 Capture Systems: Comprehensive Review and ANN Analysis. Eng, 6(9), 226. https://doi.org/10.3390/eng6090226







