Towards Sustainable Scaling-Up of Nanomaterials Fabrication: Current Situation, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Scalable Synthesis Routes of Nanomaterials
2.1. Top-Down Methods
2.1.1. Mechanical/Mechanochemical Milling
2.1.2. Solid-State Segregation
2.2. Bottom-Up Techniques
2.2.1. Liquid Phase Techniques
Supercritical Fluids
- static supercritical fluid (SSF) process;
- rapid expansion of supercritical solutions (RESS);
- particles from gas-saturated solutions (PGSS);
- precipitation from compressed antisolvent (PCA);
- aerosol solvent extraction system (ASES);
- supercritical antisolvent process (SAS);
- solution enhanced dispersion by supercritical fluids (SEDS);
- supercritical antisolvent process with enhanced mass transfer (SAS-EM);
- hydrothermal synthesis under supercritical conditions via flow reactor (HTSSF);
- hydrothermal synthesis under supercritical conditions via batch reactor (HTSSB);
- supercritical fluids drying (SCFD);
- supercritical fluid extraction emulsions (SFEE).
Solvothermal and Hydrothermal
Sonochemical
Sol-Gel
2.3. Vapor Phase Technique
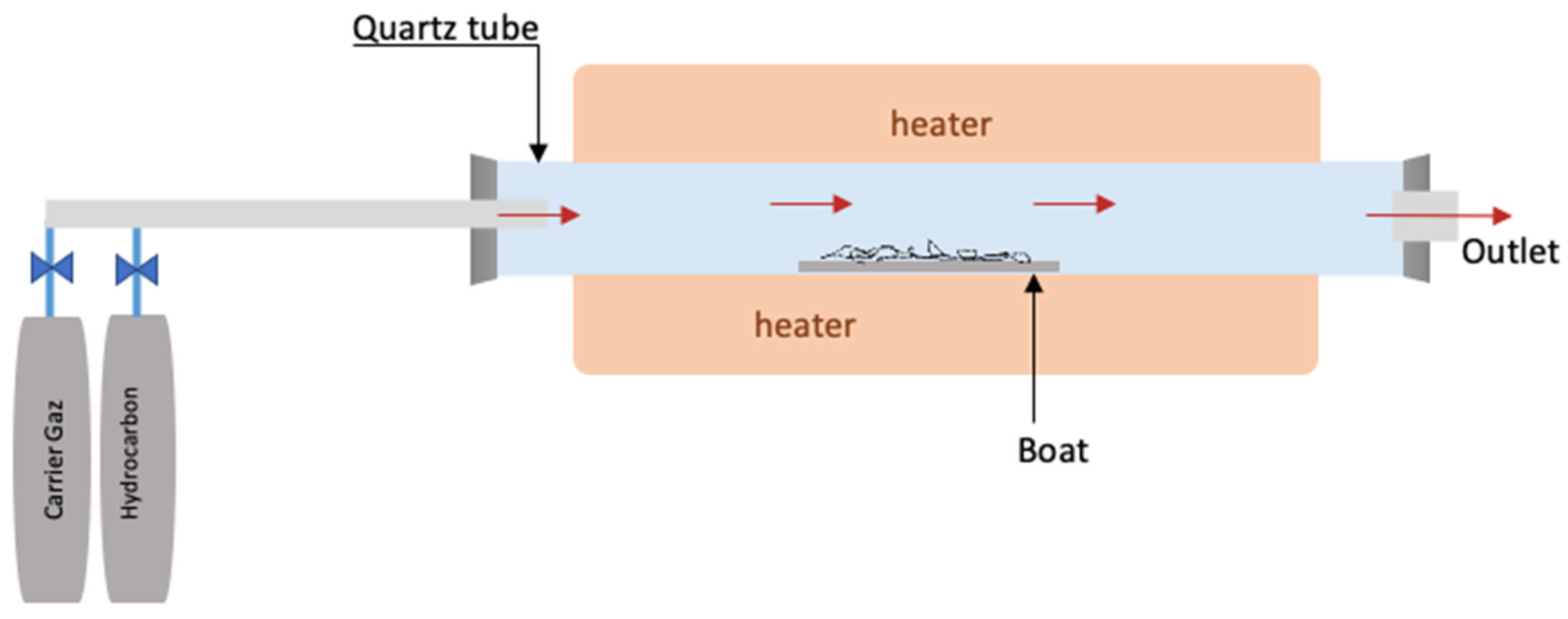
2.3.1. Chemical Vapor Deposition (CVD)
- Development of a rapid method for the growth of metal nanoparticles on nanowires using the plasma-enhanced CVD technique [150].
- Preparation of Ni nanoparticles with sizes varying from 2 to 6 nm (depending on the nanowire substrate temperature).
2.3.2. Arc Discharge Technique
2.3.3. Plasma Process
2.4. Hybrid Techniques
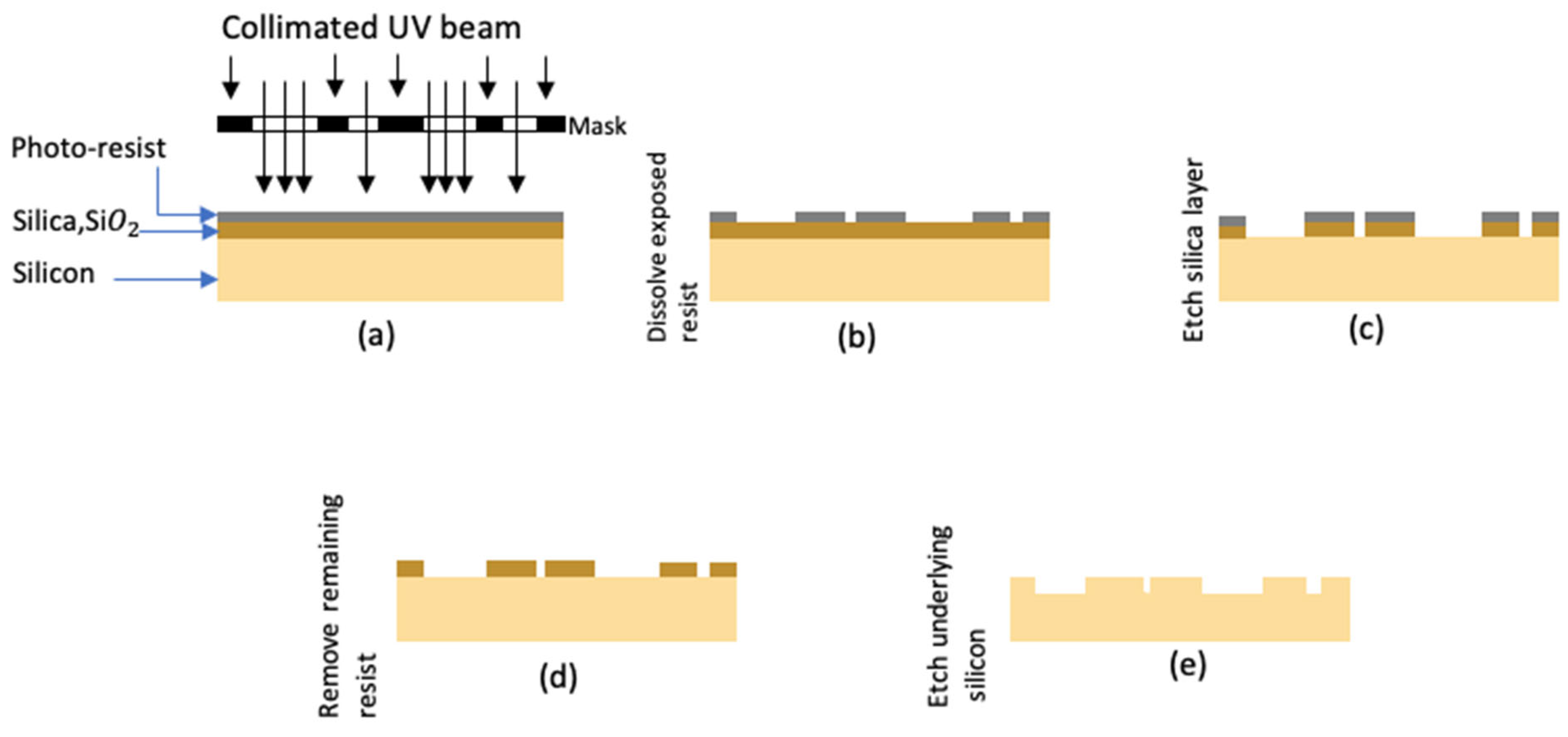
2.4.1. Photolithography
2.4.2. Scanning Probe Microscopy
2.4.3. Template Fabrication
3. Emerging Technologies for Scalable Nanomaterial Synthesis
3.1. Computer-Aided Tools
3.2. Additive Manufacturing and 3D Printing Approaches

3.3. Ionic Liquids
4. Challenges Facing the Development of Nanomaterial Production and Future Perspectives
- Safety challenges: Various studies showed the effects of exposure to the nanomaterials on human health, and it is easy to penetrate the body [204]. Inhaled nanomaterials can cause tissue damage and subsequent systemic effects, in addition to impairing the ability of macrophages to phagocytose and clear particles, and this may contribute to inflammatory reactions [205,206]. Another risk is the ability of the particles to move through the blood to vital organs, which can cause cardiovascular and other extrapulmonary effects [207,208]. As we are going toward large-scale production of nanomaterials in many industries, it is just a matter of time before gradual as well as accidental releases of nanomaterials will occur, hence the challenge of inventing safer processes [209].
- Environmental Impact: The effect of the environmental impact is a matter of concern in the design of production methods of nanomaterials; the principles of green chemistry present a framework for that design. It is believed that the top-down techniques generate more waste than bottom-up [210]. On the other hand, various bottom-up techniques use and/or generate toxins, while others require high energy consumption [211].
- Reproducibility: nanomaterials applications required the conservation of the same properties as in the laboratory, for example in nano-lubrication, the MoS2 and WS2 shapes, size, and other properties are crucial in this application [212], or the carbon nanotubes which only have a significant impact if they are produced with uniform properties [213,214]. However, a slight variation of a parameter in the synthesis will result in a change in the product’s properties. This cannot be done with the majority of top-down techniques that are unable to control surface structure.
- The physical stability of nanomaterials can be affected both during and after production. Therefore, it is essential to characterize both the processes and the nanomaterials themselves. To reduce physical alterations, it is crucial to identify and analyze key manufacturing parameters during the development phase [215].
- Stronger Academia-Industry Collaboration: Bridging the gap between research and application requires closer partnerships between academic institutions and industrial stakeholders. Such collaborations can align research objectives with real-world needs, accelerate technology transfer, and foster innovation in scalable production methods.
- Development of Scalable, Green Manufacturing Technologies: Future research should prioritize environmentally friendly and energy-efficient synthesis techniques. Projects like SHYMAN, which combines academic and industrial expertise to scale up hydrothermal processes while reducing CO2 emissions and costs, exemplify this direction [153].
- Integration of Machine Learning and Artificial Intelligence: AI and ML are emerging as transformative tools in nanomaterial research. These technologies can optimize synthesis parameters, predict material properties, and accelerate the discovery of novel nanostructures. For instance, ML algorithms can analyze vast datasets from experimental and simulation studies to identify patterns and correlations that would be difficult to detect manually. AI-driven platforms can also enable autonomous laboratories, where robotic systems guided by ML models conduct experiments, analyze results, and refine synthesis protocols in real time. Incorporating these tools into nanomaterial development pipelines can significantly enhance reproducibility, efficiency, and innovation.
- Standardization and Regulatory Frameworks: Establishing standardized protocols for nanomaterial characterization, safety assessment, and environmental impact evaluation will enhance reproducibility and facilitate regulatory approval. This is crucial for building public trust and ensuring safe integration into consumer products.
- Investment in Pilot-Scale Demonstrations: Initiatives such as ADDNANO, SHYMAN, and other projects have shown that pilot-scale demonstrations are vital for validating laboratory findings under industrial conditions. Continued investment in such initiatives will be key to overcoming scale-up barriers and optimizing performance [32,153,216].
- Focus on Societal Impact: Nanomaterials hold immense potential to address global challenges, from reducing carbon emissions to enabling sustainable technologies. Future research should emphasize applications that contribute to societal well-being, aligning technological advancement with environmental and ethical considerations [217].
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, G.A. Introduction to Nanotechnology and Its Applications to Medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef]
- Byrappa, K.; Ohara, S.; Adschiri, T. Nanoparticles Synthesis Using Supercritical Fluid Technology—Towards Biomedical Applications. Adv. Drug Deliv. Rev. 2008, 60, 299–327. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Zhang, L.; Webster, T.J. Nanotechnology and Nanomaterials: Promises for Improved Tissue Regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Taniguchi, N. On the Basic Concept of Nanotechnology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who Should Be Given the Credit for the Discovery of Carbon Nanotubes? Carbon N. Y. 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Xu, J.; Sun, X.; Zhao, X.; Li, H.; Liu, Y.; Tian, J.; Hao, X.; Kong, X.; et al. The Development of Microscopic Imaging Technology and Its Application in Micro- and Nanotechnology. Front. Chem. 2022, 10, 931169. [Google Scholar] [CrossRef] [PubMed]
- Yamal, G.; Sharmila, P.; Rao, K.; Pardha-Saradhi, P. Inbuilt Potential of YEM Medium and Its Constituents to Generate Ag/Ag2O Nanoparticles. PLoS ONE 2013, 8, e61750. [Google Scholar] [CrossRef]
- Cappy, A.; Stievenard, D.; Vuillaume, D. Nanotechnology: The Next Industrial Revolution? In Proceedings of the Gallium Arsenide Applications Symposium. GAAS 2002, Monterey, CA, USA, 20–23 October 2002. [Google Scholar]
- Nolly, C.; Ikpo, C.O.; Ndipingwi, M.M.; Ekwere, P.; Iwuoha, E.I. Pseudocapacitive Effects of Multi-Walled Carbon Nanotubes-Functionalised Spinel Copper Manganese Oxide. Nanomaterials 2022, 12, 3514. [Google Scholar] [CrossRef]
- Bayer, B.C.; Castellarin-Cudia, C.; Blume, R.; Steiner, S.A., III; Ducati, C.; Chu, D.; Goldoni, A.; Knop-Gericke, A.; Schlögl, R.; Cepek, C.; et al. Tantalum-oxide catalysed chemical vapour deposition of single- and multi-walled carbon nanotubes. RSC Adv. 2013, 3, 4086–4092. [Google Scholar] [CrossRef]
- Nanotechnology Market Research Report: Market Size, Industry Outlook, Market Forecast, Demand Analysis, Market Share, Market Report 2019–2025. Available online: https://www.industryarc.com/Report/15022/nanotechnology-market.html (accessed on 25 February 2020).
- Prabhakar, P.K.; Khurana, N.; Vyas, M.; Sharma, V.; Batiha, G.E.-S.; Kaur, H.; Singh, J.; Kumar, D.; Sharma, N.; Kaushik, A.; et al. Aspects of Nanotechnology for COVID-19 Vaccine Development and Its Delivery Applications. Pharmaceutics 2023, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, M.; Jafaryar, M. Performance of Energy Storage Unit Equipped with Vase-Shaped Fins Including Nanoparticle Enhanced Paraffin. J. Energy Storage 2023, 58, 106416. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Q.; Zhao, J.; Ali, H.M.; Said, Z.; Liu, C. Experimental Study on Sodium Acetate Trihydrate/Glycerol Deep Eutectic Solvent Nanofluids for Thermal Energy Storage. J. Mol. Liq. 2023, 372, 121164. [Google Scholar] [CrossRef]
- Marques, C.; Leal-Júnior, A.; Kumar, S. Multifunctional Integration of Optical Fibers and Nanomaterials for Aircraft Systems. Materials 2023, 16, 1433. [Google Scholar] [CrossRef]
- Manikandan, V.; Min, S.C. Roles of Polysaccharides-Based Nanomaterials in Food Preservation and Extension of Shelf-Life of Food Products: A Review. Int. J. Biol. Macromol. 2023, 252, 126381. [Google Scholar] [CrossRef] [PubMed]
- Biener, J.; Wittstock, A.; Baumann, T.F.; Weissmüller, J.; Bäumer, M.; Hamza, A.V. Surface Chemistry in Nanoscale Materials. Materials 2009, 2, 2404–2428. [Google Scholar] [CrossRef]
- Epelle, E.I.; Desongu, K.S.; Obande, W.; Adeleke, A.A.; Ikubanni, P.P.; Okolie, J.A.; Gunes, B. A Comprehensive Review of Hydrogen Production and Storage: A Focus on the Role of Nanomaterials. Int. J. Hydrogen Energy 2022, 47, 20398–20431. [Google Scholar] [CrossRef]
- Kong, F.; Ning, W.; Wang, A.; Liu, Y.; Tian, M. Convenient Solvothermal Synthesis of Nanoscale 0-2D Bi without Surfactants and Templates. J. Alloys Compd. 2018, 737, 484–489. [Google Scholar] [CrossRef]
- Heremans, J.; Thrush, C.M. Thermoelectric Power of Bismuth Nanowires. Phys. Rev. B 1999, 59, 12579–12583. [Google Scholar] [CrossRef]
- Robertson, J.M.; Wittekoek, S.; Popma, T.J.A.; Bongers, P.F. Preparation and Optical Properties of Single Crystal Thin Films of Bismuth Substituted Iron Garnets for Magneto-Optic Applications. Appl. Phys. 1973, 2, 219–228. [Google Scholar] [CrossRef]
- Yang, F.Y.; Liu, K.; Hong, K.; Reich, D.H.; Searson, P.C.; Chien, C.L. Large Magnetoresistance of Electrodeposited Single-Crystal Bismuth Thin Films. Science 1999, 284, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, J.; Ning, W.; Mallouk, T.E.; Chan, M.H.W. Surface Superconductivity in Thin Cylindrical Bi Nanowire. Nano Lett. 2015, 15, 1487–1492. [Google Scholar] [CrossRef]
- McMahon, R.E.; Wang, L.; Skoracki, R.; Mathur, A.B. Development of Nanomaterials for Bone Repair and Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 387–397. [Google Scholar] [CrossRef]
- Langer, R. Drug Delivery and Targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and Targeted Systems for Cancer Therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Bhanja, P.; Bhaumik, A. Porous Nanomaterials as Green Catalyst for the Conversion of Biomass to Bioenergy. Fuel 2016, 185, 432–441. [Google Scholar] [CrossRef]
- Akram, H.; Mateos-Pedrero, C.; Gallegos-Suárez, E.; Guerrero-Ruíz, A.; Chafik, T.; Rodríguez-Ramos, I. Effect of Electrolytes Nature and Concentration on the Morphology and Structure of MoS2 Nanomaterials Prepared Using One-Pot Solvothermal Method. Appl. Surf. Sci. 2014, 307, 319–326. [Google Scholar] [CrossRef]
- Deorsola, F.A.; Russo, N.; Blengini, G.A.; Fino, D. Synthesis, Characterization and Environmental Assessment of Nanosized MoS2 Particles for Lubricants Applications. Chem. Eng. J. 2012, 195–196, 1–6. [Google Scholar] [CrossRef]
- FINAL PROJECT REPORT Project Acronym: SHYMAN Project Title: Sustainable Hydrothermal Manufacturing of Nano-Materials Grant Agreement No: 280983 Call Topic: NMP.2011.1.4-1, Large-Scale Green and Economical Synthesis of Nanoparticles and Nanostructures, Project Duration: 48 Months Deliverable. Available online: https://cordis.europa.eu/docs/results/280/280983/final1-final-public-report-for-shyman-publishable.pdf (accessed on 12 May 2025).
- Waleka, E.; Stojek, Z.; Karbarz, M. Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics 2021, 13, 654. [Google Scholar] [CrossRef]
- Verma, V.; Ryan, K.M.; Padrela, L. Production and Isolation of Pharmaceutical Drug Nanoparticles. Int. J. Pharm. 2021, 603, 120708. [Google Scholar] [CrossRef]
- Patra, J.K.; Fraceto, L.F.; Das, G.; Campos, E.V.R. (Eds.) Green Nanoparticles: Synthesis and Biomedical Applications (Nanotechnology in the Life Sciences); Springer: Cham, Switzerland, 2020. [Google Scholar]
- Sharma, M.; Nehra, J.; Kumar, S.; Alvi, P.A. First Principle Study of Electronic Properties of Fe-Doped SnO2 Nanoparticles. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2019. [Google Scholar]
- Khurana, K.; Rani, N.; Jaggi, N. Plasmon Resonance-Induced Photoluminescence Enhancement of CdS Quantum Dots Thin Films on Fabricated Au/Ag/Cu Metallic Thin Films. Physica B Condens. Matter 2022, 631, 413717. [Google Scholar] [CrossRef]
- Kumar, A.; Jayeoye, T.J.; Mohite, P.; Singh, S.; Rajput, T.; Munde, S.; Eze, F.N.; Chidrawar, V.R.; Puri, A.; Prajapati, B.G.; et al. Sustainable and Consumer-Centric Nanotechnology-Based Materials: An Update on the Multifaceted Applications, Risks and Tremendous Opportunities. Nano-Structures Nano-Objects 2024, 38, 101148. [Google Scholar] [CrossRef]
- Aflatouni, F. Advancements in Nanotechnology: Revolutionizing Medicine and Electronics. Int. J. Innov. Comput. Sci. IT Res. 2025, 10, 16–24. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Chandraprabha, M.N.; Hari Krishna, R.; Samrat, K.; Sakunthala, A.; Sasikumar, M. Optical and Antibacterial Activity of Biogenic Core-Shell ZnO@TiO2 Nanoparticles. J. Indian Chem. Soc. 2022, 99, 100361. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics 2024, 13, 121. [Google Scholar] [CrossRef]
- Nascimento Júnior, J.A.C.; Santos, A.M.; Oliveira, A.M.S.; Santos, A.B.; Araújo, A.A.d.S.; Frank, L.A.; Serafini, M.R. Use of Nanotechnology Applied to Sunscreens: Technological Prospection Based on Patents. J. Drug Deliv. Sci. Technol. 2024, 91, 105245. [Google Scholar] [CrossRef]
- Dima, O.; Didilescu, A.C.; Manole, C.C.; Pameijer, C.; Călin, C. Synthetic Composites versus Calcium Phosphate Cements in Bone Regeneration: A Narrative Review. Ann. Anat. 2024, 255, 152273. [Google Scholar] [CrossRef]
- Radu, R.D.; Drăgănescu, D. Present and Future of ZrO2 Nanostructure as Reservoir for Drug Loading and Release. Coatings 2023, 13, 1273. [Google Scholar] [CrossRef]
- Martins, A.F.N.; Diniz, F.B.; Rodrigues, A.R. Reaction between Fe3+ and Aniline in the Synthesis of PANI-γFe2O3 and PANI-Fe3O4 Nanocomposites: Mechanistic Studies and Evaluation of Parameters. Nano-Structures Nano-Objects 2025, 42, 101477. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Global Health J. 2023, 7, 70–78. [Google Scholar] [CrossRef]
- Khakbiz, M.; Shakibania, S.; Ghazanfari, L.; Zhao, S.; Tavakoli, M.; Chen, Z. Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications. Nanotechnol. Rev. 2023, 12, 20220523. [Google Scholar] [CrossRef]
- Moiseeva, E.O.; Skribitsky, V.A.; Finogenova, Y.A.; German, S.V.; Shpakova, K.E.; Sergeev, I.S.; Terentyeva, D.A.; Sindeeva, O.A.; Kulikov, O.A.; Lipengolts, A.A.; et al. Ultrasmall maghemite nanoparticles as MRI contrast agent: Unique combination of aggregation stability, low toxicity, and tumor visualization. Nanomedicine 2023, 65, 102811. [Google Scholar] [CrossRef]
- Pech-Pech, I.E.; Gervasio, D.F.; Pérez-Robles, J.F. Nanoparticles of Ag with a Pt and Pd Rich Surface Supported on Carbon as a New Catalyst for the Oxygen Electroreduction Reaction (ORR) in Acid Electrolytes: Part 2. J. Power Sources 2015, 276, 374–381. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Watanabe, F.; Honma, T.; Nakamura, I.; Poly, S.S.; Kawaguchi, T.; Tsuji, T.; Murayama, H.; Tokunaga, M.; Fujitani, T. Continuous-Flow Synthesis of Pd@Pt Core-Shell Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126607. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, J.; Gao, L.; Zang, S.; Chen, L.; Wang, L.; Mo, L. CuO/CeO2 Catalysts Prepared by Modified Impregnation Method for Ethyl Acetate Oxidation. Chem. Eng. J. 2023, 471, 144667. [Google Scholar] [CrossRef]
- Jin, F.; Yin, H.; Feng, R.; Niu, W.; Zhang, W.; Liu, J.; Du, A.; Yang, W.; Liu, Z. Charge Transfer and Vacancy Engineering of Fe2O3 Nanoparticle Catalysts for Highly Selective N2 Reduction Towards NH3 Synthesis. J. Colloid Interface Sci. 2023, 647, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Nishu; Kumar, S. Smart and Innovative Nanotechnology Applications for Water Purification. Hybrid Adv. 2023, 3, 100044. [Google Scholar] [CrossRef]
- Ferdous, A.R.; Shah, S.N.A.; Shah, S.S.; Aziz, M.A. Advancements in Nanotechnology Applications: Transforming Catalysts, Sensors, and Coatings in Petrochemical Industries. Fuel 2024, 371, 132020. [Google Scholar] [CrossRef]
- Nouri-Mashiran, M.; Taghavi, L.; Fataei, E.; Ebrahimzadeh-Rajaei, G.; Ramezani, M. Green Synthesis of ZnO Nanoparticles and Comparison of 2,4-Dinitrophenol Removal Efficiency Using Photocatalytic, Sonocatalytic, and Adsorption Processes. Main Group Chem. 2022, 21, 559–575. [Google Scholar] [CrossRef]
- Vakhshouri, M.; Najafzadehkhoee, A.; Talimian, A.; Pernia, C.L.; Poyato, R.; Gallardo-López, Á.; Gutiérrez-Mora, F.; Prnova, A.; Galusek, D. Al2O3/Y3Al5O12 (YAG)/ZrO2 Composites by Single-Step Powder Synthesis and Spark Plasma Sintering. J. Eur. Ceram. Soc. 2024, 44, 7180–7188. [Google Scholar] [CrossRef]
- K, A.; Devarajan, Y. Nanomaterials-Based Wastewater Treatment: Addressing Challenges and Advancing Sustainable Solutions. Bionanoscience 2025, 15, 1–14. [Google Scholar] [CrossRef]
- Zhu, Q.; Chua, M.H.; Ong, P.J.; Lee, J.J.C.; Chin, K.L.O.; Wang, S.; Kai, D.; Ji, R.; Kong, J.; Dong, Z.; et al. Recent Advances in Nanotechnology-Based Functional Coatings for the Built Environment. Mater. Today Adv. 2022, 15, 100270. [Google Scholar] [CrossRef]
- Bansal, R.; Barshilia, H.C.; Pandey, K.K. Nanotechnology in Wood Science: Innovations and Applications. Int. J. Biol. Macromol. 2024, 262, 130025. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Mofidian, R.; Hosseini, S.S.; Miansari, M. Investigation of Mechanical Properties of Granular γ-Alumina Using Experimental Nanoindentation and Nanoscratch Tests. SN Appl. Sci. 2023, 5, 164. [Google Scholar] [CrossRef]
- Łach, Ł.; Svyetlichnyy, D. Recent Progress in Heat and Mass Transfer Modeling for Chemical Vapor Deposition Processes. Energies 2024, 17, 3267. [Google Scholar] [CrossRef]
- Rathod, S.; Preetam, S.; Pandey, C.; Bera, S.P. Exploring Synthesis and Applications of Green Nanoparticles and the Role of Nanotechnology in Wastewater Treatment. Biotechnol. Rep. 2024, 41, e00830. [Google Scholar] [CrossRef]
- Srinivasa Rao, S.; Reddy Parne, S.; Nagaraju, P.; Vaddadi, V.S.C.S.; Vijayakumar, Y.; Edla, D.R. Synthesis and Characterization of Spray Deposited Nanostructured WO3 Thin Films for Ammonia Sensing Applications. Inorg. Chem. Commun. 2022, 144, 109892. [Google Scholar] [CrossRef]
- Serrano-Bayona, R.; Chu, C.; Liu, P.; Roberts, W.L. Flame Synthesis of Carbon and Metal-Oxide Nanoparticles: Flame Types, Effects of Combustion Parameters on Properties and Measurement Methods. Materials 2023, 16, 1192. [Google Scholar] [CrossRef]
- Zhang, H.; Goh, B.H.H.; Chong, C.T.; Zhang, Y.; Lee, C.T.; Gao, Y.; Tian, B.; Tran, M.-V.; Yasin, M.F.M.; Ng, J.-H. A Review of Flame Aerosol Synthesis Technology for the Synthesis of Nanoparticles and Functional Energy Materials. J. Solid State Chem. 2024, 336, 124774. [Google Scholar] [CrossRef]
- Kumaresan, L.; Shanmugavelayutham, G.; Surendran, S.; Sim, U. Thermal Plasma Arc Discharge Method for High-Yield Production of Hexagonal AlN Nanoparticles: Synthesis and Characterization. J. Korean Ceram. Soc. 2022, 59, 338–349. [Google Scholar] [CrossRef]
- Kumaresan, L.; Harshini, K.S.; Amir, H.; Shanmugavelayutham, G.; Viswanathan, C. Single-Step Synthesis of Mn3N2, MnxON and Mn3O4 Nanoparticles by Thermal Plasma Arc Discharge Technique and Their Comparative Study as Electrode Material for Supercapacitor Application. J. Alloys Compd. 2023, 942, 169121. [Google Scholar] [CrossRef]
- Zhakypov, A.S.; Nemkayeva, R.R.; Yerlanuly, Y.; Tulegenova, M.A.; Kurbanov, B.Y.; Aitzhanov, M.B.; Markhabayeva, A.A.; Gabdullin, M.T. Synthesis and In Situ Oxidation of Copper Micro- and Nanoparticles by Arc Discharge Plasma in Liquid. Sci. Rep. 2023, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Cui, Q.; Wu, Y.; Shen, C.; Shen, Q.; Chen, F. Enhancement of the Load Bearing Capacity of AlN/Mo Composites via Grain Boundary Modifications Using Dispersed Ni Nanoparticles. Ceram. Int. 2024, 50, 12690–12700. [Google Scholar] [CrossRef]
- Zain Ul Abidin, M.; Ikram, M.; Moeen, S.; Nazir, G.; Kanoun, M.B.; Goumri-Said, S. A Comprehensive Review on the Synthesis of Ferrite Nanomaterials via Bottom-Up and Top-Down Approaches Advantages, Disadvantages, Characterizations and Computational Insights. Coord. Chem. Rev. 2024, 520, 216158. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-Edge Advances in Tailoring Size, Shape, and Functionality of Nanoparticles and Nanostructures: A Review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Sonawane, L.D.; Mandawade, A.S.; Bhoye, L.N.; Ahemad, H.I.; Tayade, S.S.; Aher, Y.B.; Gite, A.B.; Nikam, L.K.; Shinde, S.D.; Jain, G.H.; et al. Sol-gel and hydrothermal synthesis of CeO2 NPs: Their physio-chemical properties and applications for gas sensor with photocatalytic activities. Inorg. Chem. Commun. 2024, 164, 112313. [Google Scholar] [CrossRef]
- Patel, M.; Mishra, S.; Verma, R.; Shikha, D. Synthesis of ZnO and CuO nanoparticles via Sol gel method and its characterization by using various technique. Discover Mater. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Nawaz, M.; Ullah, H.; Uddin, I.; Shad, S.; Eldin, E.; Alshgari, R.A.; Bahajjaj, A.A.A.; Arifeen, W.U.; Javed, M.S. Synthesis and Characterization of Ni Nanoparticles via the Microemulsion Technique and Its Applications for Energy Storage Devices. Materials 2023, 16, 325. [Google Scholar] [CrossRef] [PubMed]
- Morán, D.; Gutiérrez, G.; Mendoza, R.; Rayner, M.; Blanco-López, C.; Matos, M. Synthesis of controlled-size starch nanoparticles and superparamagnetic starch nanocomposites by microemulsion method. Carbohydr. Polym. 2023, 299, 120223. [Google Scholar] [CrossRef] [PubMed]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of Chemical Synthesis in the Synthesis of Nanomaterial and Nanoparticles by the Chemical Deposition Method: A Review. BioNanoScience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Puri, C.; Kaur, B.; Golia, S.S.; Zargar, R.A.; Arora, M. Luminescent Nanocrystalline Metal Oxides. In Metal Oxide Nanocomposite Thin Films for Optoelectronic Device Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 65–100. [Google Scholar] [CrossRef]
- Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Meng, X.; Pan, Z.; Li, Y.; Qian, H.; Zhu, X.; Yang, X.; Zhang, X. Advancements in nanohydroxyapatite: Synthesis, biomedical applications and composite developments. Regen. Biomater. 2025, 12, rbae129. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of Mixing and Milling in Mechanochemical Synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Calderón Bedoya, P.A.; Botta, P.M.; Bercoff, P.G.; Fanovich, M.A. Influence of the milling materials on the mechanochemical synthesis of magnetic iron oxide nanoparticles. J. Alloys Compd. 2023, 939, 168720. [Google Scholar] [CrossRef]
- Fernandez-Diaz, L.; Castillo, J.; Sasieta-Barrutia, E.; Arnaiz, M.; Cabello, M.; Judez, X.; Terry, A.; Otaegui, L.; Morant-Miñana, M.C.; Villaverde, A. Mixing methods for solid state electrodes: Techniques, fundamentals, recent advances, and perspectives. Chem. Eng. J. 2023, 464, 142469. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Bin Emran, T.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Singha, P.; Asaithambi, N.; Singh, S.K. Ultrasound-assisted rapid biological synthesis and characterization of silver nanoparticles using pomelo peel waste. Food Chem. 2022, 385, 132602. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Faro, M.J.L.; Irrera, A. Silicon Nanowires Synthesis by Metal-Assisted Chemical Etching: A Review. Nanomaterials 2021, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Krishnia, L.; Thakur, P.; Thakur, A. Synthesis of Nanoparticles by Physical Route. In Synthesis and Applications of Nanoparticles; Springer: Singapore, 2022; pp. 45–59. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, K.; Shi, J.; Wu, F.; Zhu, X.; Dong, W.; Xie, A. Metal/nitrogen co-doped hollow carbon nanorods derived from self-assembly organic nanostructure for wide bandwidth electromagnetic wave absorption. Compos. B Eng. 2022, 228, 109424. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Zidan, A.; El-Mageed, A.I.A.A. Preparation methods of different nanomaterials for various potential applications: A review. J. Mol. Struct. 2023, 1281, 135148. [Google Scholar] [CrossRef]
- Behrens, S.H.; Breedveld, V.; Mujica, M.; Filler, M.A. Process Principles for Large-Scale Nanomanufacturing. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Luther, W. Industrial Application of Nanomaterials—Chances and Risks: Technology Analysis; Future Technologies Division of VDI Technologiezentrum GmbH: Düsseldorf, Germany, 2004. [Google Scholar]
- Virji, M.A.; Stefaniak, A.B. A Review of Engineered Nanomaterial Manufacturing Processes and Associated Exposures. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 103–125. [Google Scholar] [CrossRef]
- Daraio, C.; Jin, S. Synthesis and Patterning Methods for Nanostructures Useful for Biological Applications; Springer: New York, NY, USA, 2012; pp. 27–44. [Google Scholar]
- Zhong, W.-H. Nanoscience and Nanomaterials: Synthesis, Manufacturing and Industry Impacts; Destech Publications: Lancaster, PA, USA, 2012. [Google Scholar]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials; World Scientific: Singapore, 2011. [Google Scholar]
- Klar, E. Powder Metallurgy. In Metals Handbook Desk Edition; ASM International: Almere, The Netherlands, 1998. [Google Scholar]
- Edelstein, A.S.; Cammaratra, R.C. Nanomaterials: Synthesis, Properties and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Letfullin, R.R.; George, T.F. Introduction to Nanomedicine. In Computational Nanomedicine and Nanotechnology; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–61. [Google Scholar]
- Eugene, A.; Mariagoretti, U.; Samuel, A. A Review on Synthetic Methods of Nanostructured Materials. Chem. Res. J. 2017, 2, 97–123. [Google Scholar]
- Shah, P.S.; Hanrath, T.; Johnston, K.P.; Korgel, B.A. Nanocrystal and nanowire synthesis and dispersibility in supercritical fluids. J. Phys. Chem. B 2004, 108, 9574–9587. [Google Scholar] [CrossRef]
- Elizondo, E.; Veciana, J.; Ventosa, N. Nanostructuring molecular materials as particles and vesicles for drug delivery, using compressed and supercritical fluids. Nanomedicine 2012, 7, 1391–1408. [Google Scholar] [CrossRef]
- Bensebaa, F. Nanoparticle Technologies: From Lab to Market; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Saidi, M.Z.; Pasc, A.; El Moujahid, C.; Canilho, N.; Badawi, M.; Delgado-Sanchez, C.; Celzard, A.; Fierro, V.; Peignier, R.; Kouitat-Njiwa, R.; et al. Improved tribological properties, thermal and colloidal stability of poly-α-olefins based lubricants with hydrophobic MoS2 submicron additives. J. Colloid Interface Sci. 2020, 562, 91–101. [Google Scholar] [CrossRef]
- Lu, X.; Ziegler, K.J.; Ghezelbash, A.; Johnston, K.P.; Korgel, B.A. Synthesis of germanium nanocrystals in high temperature supercritical fluid solvents. Nano Lett. 2004, 4, 969–974. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of metal oxide nanoparticles in supercritical water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [PubMed]
- Tighe, C.J.; Cabrera, R.Q.; Gruar, R.I.; Darr, J.A. Scale up production of nanoparticles: Continuous supercritical water synthesis of Ce-Zn oxides. Ind. Eng. Chem. Res. 2013, 52, 5522–5528. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. REVIEW SUMMARY Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef]
- Auxéméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous supercritical solvothermal preparation of nanostructured ceria-zirconia as supports for dry methane reforming catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Fernandez, C.A.; Wai, C.M. Nanoparticle size Continuous Tuning of Silver Nanoparticle Size in a Water-in-Supercritical Carbon Dioxide Microemulsion. Small 2006, 2, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Talebi, B.; Moradi, M. Solvothermal synthesis of CMTS quaternary semiconductor nanoparticles with a symmetric kesterite structure: The role of the autoclave filling factor. Nano-Structures Nano-Objects 2023, 35, 101008. [Google Scholar] [CrossRef]
- Akram, H.; Mateos-Pedrero, C.; Gallegos-Suarez, E.; Chafik, T.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of surfactant concentration on the morphology of MoxSy nanoparticles prepared by a solvothermal route. Green Process. Synth. 2017, 6, 161–171. [Google Scholar] [CrossRef]
- Kolen’Ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovnir, K.; Lebedev, O.I.; et al. Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Lou, W.; Chen, M.; Wang, X.; Liu, W. Novel single-source precursors approach to prepare highly uniform Bi2S3 and Sb2S3 nanorods via a solvothermal treatment. Chem. Mater. 2007, 19, 872–878. [Google Scholar] [CrossRef]
- Ku, K.; Lee, S.-W.; Park, J.; Kim, N.; Chung, H.; Han, C.-H.; Kim, W. Large-scale solvothermal synthesis of fluorescent carbon nanoparticles. Nanotechnology 2014, 25, 395601. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J.; Jiang, R. Large-scale synthesis of silver nanowires via a solvothermal method. J. Mater. Sci. Mater. Electron. 2011, 22, 6–13. [Google Scholar] [CrossRef]
- Patil, G.P.; Jadhav, C.D.; Lyssenko, S.; Minnes, R. Hydrothermally synthesized copper telluride nanoparticles: First approach to flexible solid-state symmetric supercapacitor. Chem. Eng. J. 2024, 498, 155284. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Jiang, G.; Liu, H.; Yang, J.; Li, Y. Continuous supercritical hydrothermal synthesis of stabilized ZrO2 nanocomposites: Doping mechanism of typical metals and transition elements. Mater. Today Chem. 2024, 35, 101902. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, H.; Lu, M.; Liu, B.; Zhao, Y.; Ou, Y.; Wang, Y.; Tao, J.; Zou, T.; Huang, Y.; et al. Machine learning guided hydrothermal synthesis of thermochromic VO2 nanoparticles. Ceram. Int. 2023, 49, 30794–30800. [Google Scholar] [CrossRef]
- Dunne, P.W.; Starkey, C.L.; Munn, A.S.; Tang, S.V.; Luebben, O.; Shvets, I.; Ryder, A.G.; Casamayou-Boucau, Y.; Morrison, L.; Lester, E.H. Bench- and pilot-scale continuous-flow hydrothermal production of barium strontium titanate nanopowders. Chem. Eng. J. 2016, 289, 433–441. [Google Scholar] [CrossRef]
- Dunne, P.W.; Munn, A.S.; Starkey, C.L.; Lester, E.H. The sequential continuous-flow hydrothermal synthesis of molybdenum disulphide. Chem. Commun. 2015, 51, 4048–4050. [Google Scholar] [CrossRef] [PubMed]
- Karnan, M.; Nanda, O.P.; Durai, L.; Badhulika, S. One-step hydrothermal synthesis of Bi2CuO4 nanoflakes: An excellent electrode material for symmetric supercapacitors. J. Energy Storage 2023, 63, 106993. [Google Scholar] [CrossRef]
- Malarde, D.; Johnson, I.D.; Godfrey, I.J.; Powell, M.J.; Cibin, G.; Quesada-Cabrera, R.; Darr, J.A.; Carmalt, C.J.; Sankar, G.; Parkin, I.P.; et al. Direct and continuous hydrothermal flow synthesis of thermochromic phase pure monoclinic VO2 nanoparticles. J. Mater. Chem. C 2018, 6, 11731–11739. [Google Scholar] [CrossRef]
- Suslick, K.S. Applications of Ultrasound to Materials Chemistry. MRS Bull. 1995, 20, 29–34. [Google Scholar] [CrossRef]
- Zhou, S.M.; Feng, Y.S.; Zhang, L.D. Sonochemical synthesis of large-scale single crystal CdS nanorods. Mater. Lett. 2003, 57, 2936–2939. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Design process for nanomaterials. J. Mater. Sci. 2013, 48, 3605–3622. [Google Scholar] [CrossRef]
- Schodek, D.; Ferreira, P.; Ashby, M. Nanomaterials, Nanotechnologies and Design; Elsevier Ltd.: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Kim, H.S.; Cho, K.; Song, H.W.; Kim, J.H.; Lee, J.W.; Lee, J.S.; Min, B.; Kim, S.H.; Kim, S.S. Sonochemical Synthesis and Photocurrent of HgTe NANOPARTICLES. Key Eng. Mater. 2004, 277–279, 961–965. [Google Scholar] [CrossRef]
- Hu, C.G.; Li, Y.; Liu, J.P.; Zhang, Y.; Bao, G.; Buchine, B.; Wang, Z. Sonochemical synthesis of ferromagnetic core-shell Fe3O4-FeP nanoparticles and FeP nanoshells. Chem. Phys. Lett. 2006, 428, 343–347. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, H.; Hibino, M.; Honma, I.; Ichihara, M. Synthesis of MnO2 nanoparticles confined in ordered mesoporous carbon using a sonochemical method. Adv. Funct. Mater. 2005, 15, 381–386. [Google Scholar] [CrossRef]
- Vinodgopal, K.; He, Y.; Ashokkumar, M.; Grieser, F. Sonochemically prepared platinum-ruthenium bimetallic nanoparticles. J. Phys. Chem. B 2006, 110, 3849–3852. [Google Scholar] [CrossRef]
- Bahlawan, H.; Poganietz, W.R.; Spina, P.R.; Venturini, M. Cradle-to-gate life cycle assessment of energy systems for residential applications by accounting for scaling effects. Appl. Therm. Eng. 2020, 171, 115062. [Google Scholar] [CrossRef]
- Ugemuge, N.; Parauha, Y.R.; Dhoble, S.J. Synthesis and luminescence study of silicate-based phosphors for energy-saving light-emitting diodes. In Energy Materials: Fundamentals to Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 445–480. [Google Scholar] [CrossRef]
- Kamat, P.V. Composite semiconductor nanoclusters. Stud. Surf. Sci. Catal. 1997, 103, 237–259. [Google Scholar] [CrossRef]
- Fendler, J.H. Colloid Chemical Approach to Nanotechnology. Korean J. Chem. Eng. 2001, 18, 1–13. [Google Scholar] [CrossRef]
- Patil, A.B.; Panda, R.N. Synthesis, characterizations and magnetic properties of nanoscale CoVxFe2-xO4 (0.0 ≤ x ≤ 0.9) materials synthesized via sol-gel autocombustion route. Mater. Chem. Phys. 2023, 307, 128215. [Google Scholar] [CrossRef]
- Tsuzuki, T. Nanotechnology commercialisation. In Nanotechnology Commercialisation; Pan Stanford Publishing Pte. Ltd.: Singapore, 2013; pp. 73–138. [Google Scholar]
- Solinas, S.; Piccaluga, G.; Morales, M.P.; Serna, C.J. Sol-gel formation of γ-Fe2O3/SiO2 nanocomposites. Acta Mater. 2001, 49, 2805–2811. [Google Scholar] [CrossRef]
- Pechini, M.P. Method Of Preparing Idead and Alkaline Earth Titanates And Niobates and Coating Method Using The Same. U.S. Patent US3330697A, 11 July 1967. [Google Scholar]
- Lu, C.H.; Lin, Y.; Wang, H.C. Chromium-ion doped spinel lithium manganate nanoparticles derived from the sol-gel process. J. Mater. Sci. Lett. 2003, 22, 615–618. [Google Scholar] [CrossRef]
- Solunke, A.; Barote, V.K.; Sonawane, B.; Shirsath, S.E.; Kadam, R.; Shinde, V.S. Sol-gel synthesis of Fe-rich cobalt ferrite nanoparticles and influence of pH concentration. Mater. Today Proc. 2023, 92, 1225–1230. [Google Scholar] [CrossRef]
- Swihart, M.T. Vapor-phase synthesis of nanoparticles. Curr. Opin. Colloid Interface Sci. 2003, 8, 127–133. [Google Scholar] [CrossRef]
- El Kasmi, A.; Arshad, M.F.; Waqas, M.; Monguen, C.K.F.; Azar, F.-Z.; Wu, L.-N.; Tian, Z.-Y. Insights into catalytic oxidation mechanism of CO over Cu catalyst: Experimental and modeling study. Mater. Res. Bull. 2023, 166, 112343. [Google Scholar] [CrossRef]
- El Kasmi, A.; Tian, Z.Y.; Vieker, H.; Beyer, A.; Chafik, T. Innovative CVD synthesis of Cu2O catalysts for CO oxidation. Appl. Catal. B 2016, 186, 10–18. [Google Scholar] [CrossRef]
- Boateng, E.; Thiruppathi, A.R.; Hung, C.K.; Chow, D.; Sridhar, D.; Chen, A. Functionalization of graphene-based nanomaterials for energy and hydrogen storage. Electrochim. Acta 2023, 452, 142340. [Google Scholar] [CrossRef]
- Okumura, M.; Nakamura, S.; Tsubota, S.; Nakamura, T.; Azuma, M.; Haruta, M. Chemical vapor deposition of gold on Al2O3, SiO2, and TiO2 for the oxidation of CO and of H2. Catal. Lett. 1998, 51, 53–58. [Google Scholar] [CrossRef]
- Ishida, T.; Nagaoka, M.; Akita, T.; Haruta, M. Deposition of gold clusters on porous coordination polymers by solid grinding and their catalytic activity in aerobic oxidation of alcohols. Chem. Eur. J. 2008, 14, 8456–8460. [Google Scholar] [CrossRef]
- LaLonde, A.D.; Norton, M.G.; Zhang, D.; Gangadean, D.; Alkhateeb, A.; Padmanabhan, R.; McIlroy, D.N. A rapid method for growth of metal nanoparticles on nanowire substrates. J. Nanopart. Res. 2006, 8, 99–104. [Google Scholar] [CrossRef]
- Lee, S.H.; Deshpande, R.; Parilla, P.A.; Jones, K.M.; To, B.; Mahan, A.H.; Dillon, A.C. Crystalline WO3 nanoparticles for highly improved electrochromic applications. Adv. Mater. 2006, 18, 763–766. [Google Scholar] [CrossRef]
- Shukla, U. Carbon Nanotubes: Potentially Revolutionary Impact of Nanomaterials. J. Nanosci. Res. Rep. 2023, 5, 1–7. [Google Scholar]
- Final Report Summary—BUONAPART-E (Better Upscaling and Optimization of Nanoparticle and Nanostructure Production by Means of Electrical Discharges) | Report Summary | BUONAPART-E | FP7 | CORDIS | European Commission. Available online: https://cordis.europa.eu/project/id/280765/reporting (accessed on 26 February 2020).
- Vollath, D. Plasma Synthesis of Nanoparticles. KONA Powder Part. J. 2007, 25, 39–55. [Google Scholar] [CrossRef]
- Tong, L.; Reddy, R.G. The processing of nanopowders by thermal plasma technology. JOM 2006, 58, 62–66. [Google Scholar] [CrossRef]
- Vollath, D.; Sickafus, K.E. Synthesis of nanosized ceramic oxide powders by microwave plasma reactions. Nanostruct. Mater. 1992, 1, 427–437. [Google Scholar] [CrossRef]
- Vollath, D. A Cascaded Microwave Plasma Source for Synthesis of Ceramic Nanocomposite Powders. MRS Proc. 1994, 347, 629. [Google Scholar] [CrossRef]
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Dzur, B. Plasma puts heat into spherical powder production. Metal Powder Rep. 2008, 63, 12–15. [Google Scholar] [CrossRef]
- Bapat, A.; Anderson, C.; Perrey, C.R.; Carter, C.B.; A Campbell, S.; Kortshagen, U. Plasma synthesis of single-crystal silicon nanoparticles for novel electronic device applications. Plasma Phys. Control. Fusion 2004, 46, B97–B109. [Google Scholar] [CrossRef]
- Chuncheng, H.; Zuolin, C.; Yansheng, Y.; Zhikun, Z. Preparation and mechanical properties of Fe3Al nanostructured intermetallics. J. Nanopart. Res. 2002, 4, 107–110. [Google Scholar] [CrossRef]
- Barankin, M.D.; Creyghton, Y.; Schmidt-Ott, A. Synthesis of nanoparticles in an atmospheric pressure glow discharge. J. Nanopart. Res. 2006, 8, 511–517. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Müller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Wu, N.; Aapro, M.; Jestilä, J.S.; Drost, R.; García, M.M.; Torres, T.; Xiang, F.; Cao, N.; He, Z.; Bottari, G.; et al. Precise Large-Scale Chemical Transformations on Surfaces: Deep Learning Meets Scanning Probe Microscopy with Interpretability. J. Am. Chem. Soc. 2024, 147, 1240–1250. [Google Scholar] [CrossRef]
- Mao, Y.T.; Kuo, K.C.; Tseng, C.E.; Huang, J.-Y.; Lai, Y.-C.; Yen, J.-Y.; Lee, C.-K.; Chuang, W.-L. Research on three dimensional machining effects using atomic force microscope. Rev. Sci. Instrum. 2009, 80, 065105. [Google Scholar] [CrossRef]
- Versen, M.; Klehn, B.; Kunze, U.; Reuter, D.; Wieck, A. Nanoscale devices fabricated by direct machining of GaAs with an atomic force microscope. Ultramicroscopy 2000, 82, 159–163. [Google Scholar] [CrossRef]
- Xu, S.; Lei, Y. Template-Assisted Fabrication of Nanostructured Arrays for Sensing Applications. ChemPlusChem 2018, 83, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Björk, M.T.; Liddle, J.A.; Sönnichsen, C.; Boussert, B.; Alivisatos, A.P. Integration of colloidal nanocrystals into lithographically patterned devices. Nano Lett. 2004, 4, 1093–1098. [Google Scholar] [CrossRef]
- Malaquin, L.; Kraus, T.; Schmid, H.; Delamarche, E.; Wolf, H. Controlled particle placement through convective and capillary assembly. Langmuir 2007, 23, 11513–11521. [Google Scholar] [CrossRef]
- Seemann, L.; Stemmer, A.; Naujoks, N. Local surface charges direct the deposition of carbon nanotubes and fullerenes into nanoscale patterns. Nano Lett. 2007, 7, 3007–3012. [Google Scholar] [CrossRef]
- Fan, Z.; Ho, J.C.; Jacobson, Z.A.; Yerushalmi, R.; Alley, R.L.; Razavi, H.; Javey, A. Wafer-scale assembly of highly ordered semiconductor nanowire arrays by contact printing. Nano Lett. 2008, 8, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Stępniowski, W.; Salerno, M. Fabrication of nanowires and nanotubes by anodic alumina template-assisted electrodeposition. In Nanowires and Nanotubes; Elsevier: Amsterdam, The Netherlands, 2014; pp. 321–357. [Google Scholar]
- Iriarte, G.F. Large Scale Synthesis of Silicon Nanowires. J. Nanopart. Res. 2011, 13, 1737–1745. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Ross, C.A.; Thomas, E.L.; Smith, H.; Vancso, G. Templated Self-Assembly of Block Copolymers: Effect of Substrate Topography. Adv. Mater. 2003, 15, 1599–1602. [Google Scholar] [CrossRef]
- Huck, W.; Samuelson, L. Functionalized Surfaces and Nanostructures for Nanotechnological Applications. Nanotechnology 2003, 14, 1. [Google Scholar] [CrossRef]
- Hachhach, M.; Akram, H.; Hanafi, M.; Achak, O.; Chafik, T. Simulation and Sensitivity Analysis of Molybdenum Disulfide Nanoparticle Production Using Aspen Plus. Int. J. Chem. Eng. 2019, 2019, 3953862. [Google Scholar] [CrossRef]
- Hachhach, M.; Akram, H.; Achak, O.; Chafik, T. Simulation of the Synthesis Route of MoS2 at Laboratory Scale: Comparison between Simulated and Experimental Results. In Proceedings of the 2017 International Renewable and Sustainable Energy Conference, IRSEC 2017, Tangier, Morocco, 4–7 December 2017; pp. 9–11. [Google Scholar] [CrossRef]
- Larbi, F.; García, A.; del Valle, L.J.; Hamou, A.; Puiggalí, J.; Belgacem, N.; Bras, J. Simulation Basis for a Techno-Economic Evaluation of Chitin Nanomaterials Production Process Using Aspen Plus® Software. Data Brief 2018, 20, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Boo Chen Qing, E.; Kirzner Chong Kai Wen, J.; Seh Liang, L.; Ying, L.Q.; Jie, L.Q.; Mubarak, N. Pilot Study of Synthesis of Nanocrystalline Cellulose Using Waste Biomass via ASPEN plus Simulation. Mater. Sci. Energy Technol. 2020, 3, 364–370. [Google Scholar] [CrossRef]
- Hervy, M.; Evangelisti, S.; Lettieri, P.; Lee, K.Y. Life Cycle Assessment of Nanocellulose-Reinforced Advanced Fibre Composites. Compos. Sci. Technol. 2015, 118, 154–162. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, G.; Zimmermann, B.; Weil, M. Nanotoxicity and Life Cycle Assessment: First Attempt towards the Determination of Characterization Factors for Carbon Nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012029. [Google Scholar] [CrossRef]
- Gavankar, S.; Suh, S.; Keller, A.F. Life Cycle Assessment at Nanoscale: Review and Recommendations. Int. J. Life Cycle Assess. 2012, 17, 295–303. [Google Scholar] [CrossRef]
- Hachhach, M.; Akram, H.; El Kasmi, A.; Hanafi, M.; Achak, O.; Chafik, T. Life Cycle Assessment of Large-Scale Production of MoS2 Nanomaterials through the Solvothermal Method. J. Nanopart. Res. 2022, 24, 181. [Google Scholar] [CrossRef]
- Lu, M.; Ji, H.; Zhao, Y.; Chen, Y.; Tao, J.; Ou, Y.; Wang, Y.; Huang, Y.; Wang, J.; Hao, G. Machine Learning-Assisted Synthesis of Two-Dimensional Materials. ACS Appl. Mater. Interfaces 2023, 15, 1871–1878. [Google Scholar] [CrossRef]
- Chen, X.; Lv, H. Intelligent Control of Nanoparticle Synthesis on Microfluidic Chips with Machine Learning. NPG Asia Mater. 2022, 14, 1–20. [Google Scholar] [CrossRef]
- Alfathia Fadhilah, R.; Roil Bilad, M. Techno-Economic Analysis in The Production of Copper Nanoparticles with Chemical Reduction Methods Using L-Ascorbic Acid. Indones. J. Digit. Bus. 2022, 2, 31–40. [Google Scholar] [CrossRef]
- Agboola, A.E.; Pike, R.W.; Hertwig, T.A.; Lou, H.H. Conceptual Design of Carbon Nanotube Processes. Clean Technol. Environ. Policy 2007, 9, 289–311. [Google Scholar] [CrossRef]
- Arteaga-Díaz, S.J.; Meramo, S.; González-Delgado, Á.D. Computer-Aided Modeling, Simulation, and Exergy Analysis of Large-Scale Production of Magnetite (Fe3O4) Nanoparticles via Coprecipitation. ACS Omega 2021, 6, 30666–30673. [Google Scholar] [CrossRef] [PubMed]
- Hachhach, M.; Akram, H.; Hanafi, M.; Achak, O.; Chafik, T. Process Design and Economic Assessment of Large-Scale Production of Molybdenum Disulfide Nanomaterials. Chem. Prod. Process Model. 2023, 18, 355–368. [Google Scholar] [CrossRef]
- GadelHak, Y.; Muhammad, A.; El-Azazy, M.; El-Shafie, A.S.; Shibl, M.F.; Mahmoud, R. Computer-Aided Design of Large-Scale Nanomaterials Synthesis Processes: A Detailed Review. ChemBioEng Rev. 2024, 11, e202300075. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Moreno-Sader, K.; González-Delgado, Á.D. Computer-Aided Simulation and Exergy Analysis of TiO2 Nanoparticles Production via Green Chemistry. PeerJ 2019, 2019, e8113. [Google Scholar] [CrossRef] [PubMed]
- Peplow, M. Google AI and Robots Join Forces to Build New Materials. Nature 2023. [Google Scholar] [CrossRef]
- Lu, M.; Ji, H.; Chen, Y.; Gao, F.; Liu, B.; Long, P.; Deng, C.; Wang, Y.; Tao, J. Machine Learning Assisted Layer-Controlled Synthesis of MoS2. J. Mater. Chem. C Mater. 2024, 12, 8893–8900. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, T. Ink-Based Additive Manufacturing for Electrochemical Applications. Heliyon 2024, 10, e33023. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Chang, X.; Gao, W.; Kong, L.; Yan, B.; Chen, G. Revolutionizing Catalytic Water Treatment: A Critical Review on the Role of 3D Printed Catalysts. Sep. Purif. Technol. 2025, 363, 132194. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, X.; Guo, R.; Yuan, X.; Chen, H.; Abrahams, I.; Zhang, D. 3D Printing of Anisotropic Polymer Nanocomposites with Aligned BaTiO3 Nanowires for Enhanced Energy Density. Mater. Adv. 2020, 1, 14–19. [Google Scholar] [CrossRef]
- Ramanathan, A.; Feng, S.; Saji Kumar, A.; Thummalapalli, S.V.; Sobczak, M.T.; Bick, L.R.; Song, K.; Yang, S. Light-Driven Nanonetwork Assembly of Gold Nanoparticles via 3D Printing for Optical Sensors. ACS Appl. Nano Mater. 2024, 7, 27998–28007. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.J.; Hadrawi, S.K.; Kianfar, E. Synthesis and Modification of Nanoparticles with Ionic Liquids: A Review. Bionanoscience 2023, 13, 760–783. [Google Scholar] [CrossRef]
- Pereira, J.; Souza, R.; Moita, A. A Review of Ionic Liquids and Their Composites with Nanoparticles for Electrochemical Applications. Inorganics 2024, 12, 186. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids. Nanomaterials 2021, 11, 3270. [Google Scholar] [CrossRef] [PubMed]
- Vucetic, N.; Virtanen, P.; Nuri, A.; Shchukarev, A.; Mikkola, J.-P.; Salmi, T. Tuned Bis-Layered Supported Ionic Liquid Catalyst (SILCA) for Competitive Activity in the Heck Reaction of Iodobenzene and Butyl Acrylate. Catalysts 2020, 10, 963. [Google Scholar] [CrossRef]
- Vucetic, N.; Virtanen, P.; Shchukarev, A.; Salmi, T.; Mikkola, J.-P. Competing Commercial Catalysts: Unprecedented Catalyst Activity and Stability of Mizoroki-Heck Reaction in a Continuous Packed Bed Reactor. Chem. Eng. J. 2022, 433, 134432. [Google Scholar] [CrossRef]
- El-Kady, M.M.; Ansari, I.; Arora, C.; Rai, N.; Soni, S.; Verma, D.K.; Singh, P.; El Din Mahmoud, A. Nanomaterials: A Comprehensive Review of Applications, Toxicity, Impact, and Fate to Environment. J. Mol. Liq. 2023, 370, 121046. [Google Scholar] [CrossRef]
- Lam, C.-W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary Toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days after Intratracheal Instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar] [CrossRef]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.; Webb, T.R. Comparative Pulmonary Toxicity Assessment of Single-Wall Carbon Nanotubes in Rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar] [CrossRef]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.M.; Verbruggen, A.; Nemery, B. Passage of Intratracheally Instilled Ultrafine Particles from the Lung into the Systemic Circulation in Hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef]
- Hoet, P.H.M.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and Unknown Health Risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- OECD. Opportunities and Risks of Nanotechnologies Report in Co-Operation with the OECD International Futures Programme; OECD: Paris, France, 2005. [Google Scholar]
- Dhingra, R.; Naidu, S.; Upreti, G.; Sawhney, R. Sustainable Nanotechnology: Through Green Methods and Life-Cycle Thinking. Sustainability 2010, 2, 3323–3338. [Google Scholar] [CrossRef]
- Akram, H.; Achak, O.; Haffane, S.; Elmoujahid, C.; Elmesbahi, A.; Elmessoudi, D.; Chafik, T.; Bensemlali, S.; Elmouakibi, A. Comparison of Synthesis Routes of Inorganic Fullerene-Like Nano-Additives for Wind Turbine Lubrication: Application of Life Cycle Assessment Approach. In Proceedings of the 2014 International Renewable and Sustainable Energy Conference (IRSEC), Ouarzazate, Morocco, 17–19 October 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Gulzar, H.; Masjuki, M.A.; Kalam, M.; Varman, N.W.M.; Zulkifli, R.A.; Mufti, R.; Zahid, M.H. Tribological Performance of Nanoparticles as Lubricating Oil Additives. J. Nanopart. Res. 2016, 18, 1–25. [Google Scholar] [CrossRef]
- Grobert, N. Nanotubes—Grow or Go? Mater. Today 2006, 9, 64. [Google Scholar] [CrossRef]
- Sengupta, J. Carbon Nanotube Fabrication at Industrial Scale. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 172–194. [Google Scholar]
- Saleh, T.A. Large-Scale Production of Nanomaterials and Adsorbents. Interface Sci. Technol. 2022, 34, 167–197. [Google Scholar] [CrossRef]
- Final Report Summary—ADDNANO (The Development and Scale-Up of Innovative Nanotechnology-Based Processes into the Value Chain of the Lubricants Market)|Report Summary|ADDNANO|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/229284/reporting (accessed on 26 February 2020).
- Nano-Enhanced Industrial Materials: Building the Next European Industrial Revolution | Results Pack | CORDIS | European Commission. Available online: https://cordis.europa.eu/article/id/401228-nanoenhanced-industrial-materials-building-the-next-european-industrial-revolution (accessed on 28 April 2025).
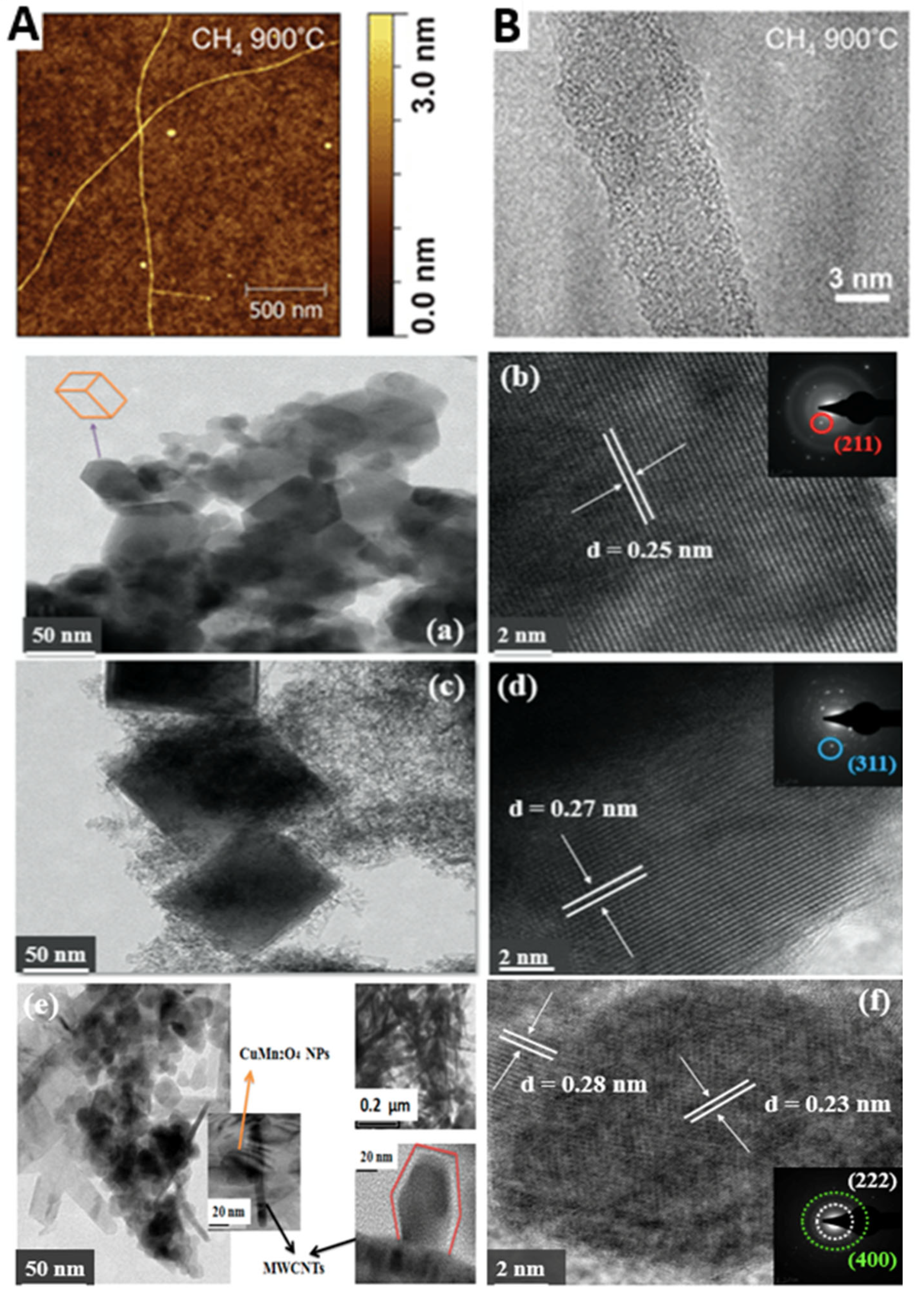



| Application Area | Materials/Compounds | Uses/Functions |
|---|---|---|
| Pharmaceutical | IM/PVP, HA/PVP [33] | Implant strengtheners, Formulated insolubles [34,35] |
| Electronics | St-Fe/SnO2, Ag/Cu/ITO, YAG/ZrO2-Ce [36,37] | Conductors/magnets, Printable inks (e.g., RFID), Laser lenses [38,39] |
| Healthcare | Ag, TiO2/ZnO, Fe2O3/Fe3O4 [40,41,42] | Antimicrobial [43], Sunscreens [44], Pigments [35] |
| Medical | YAG/ZrO2-Ce, Fe2O3/Fe3O4, HA/CaPO4 [45,46,47] | Cell signaling, MRI contrast agents, Artificial bone agents [48,49,50] |
| Catalysts | Fe2O3/Mo-Fe, Cu/CuO, CeO, Ag/Pd/Pt [51,52,53,54] | CNTs synthesis, Polymerization enhancers, Combustion additives, Generic metals [55,56] |
| Materials | SiO2, YAG/ZrO2, ZnO/Cd2SnO4 [57,58,59] | Scratch resistance, Strength enhancers, Smart material coatings [60,61,62] |
| Bottom Up | Top Down | Other Methods | |
|---|---|---|---|
| Vapor/Aerosol Phase Synthesis | Liquid Phase Synthesis (Wet Method) | Solid Phase Synthesis (Mechanical) | |
| Chemical vapor deposition [63] -Thermally-activated (TA) -Plasma-enhanced (PE) -Flame-assisted (FA) -Electrochemical (EC) -Laser-assisted (LA) -Metal-organic (MO) -Metal-catalyzed (MC) -Aerosol-assisted (AA) -Direct-liquid (DL) -Atom layer (AL) -Template-assisted (TA) Physical vapor deposition [64,65] -Evaporation/MBE -Sputtering Spray pyrolysis -Tubular reactor -Vapor flame reactor -Emulsion combustion Flame [66,67] -Flame aerosol -Flame spray -Flame pyrolysis Other [68,69,70,71] -Arc discharge -Submerged arc discharge -Solid-Vapor synhesis | Chemical pro-precipitation [72] -Microwave assisted -Metalorganic -Solvothermal/hydrothermal -Sonication assisted -Polyol -Template Sol-Gel [73,74,75,76] -Pchimi method -Reverse micelle Microemulsion [77,78] -Micelle -Reverse micelle Electrochemical deposition [79] -Cathodic deposition -Anodization Other [80,81,82] -Electrospraying -Sonochemical -Precipitation, Freeze drying -Plasma, Microwave, Radiation, Electric field | Mechanical milling/solid-state phase segregation [83,84,85] | Biologically assisted [64,86,87] -Intercellular -Extracellular Hybrid or product-specific methods -HIPOC -CaNaCAT Nano-fabrication (patterning/manipulation) -Lithography/etching [88,89] -Self-assembly/template assisted [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachhach, M.; Bayou, S.; El Kasmi, A.; Saidi, M.Z.; Akram, H.; Hanafi, M.; Achak, O.; El Moujahid, C.; Chafik, T. Towards Sustainable Scaling-Up of Nanomaterials Fabrication: Current Situation, Challenges, and Future Perspectives. Eng 2025, 6, 149. https://doi.org/10.3390/eng6070149
Hachhach M, Bayou S, El Kasmi A, Saidi MZ, Akram H, Hanafi M, Achak O, El Moujahid C, Chafik T. Towards Sustainable Scaling-Up of Nanomaterials Fabrication: Current Situation, Challenges, and Future Perspectives. Eng. 2025; 6(7):149. https://doi.org/10.3390/eng6070149
Chicago/Turabian StyleHachhach, Mouad, Sanae Bayou, Achraf El Kasmi, Mohamed Zoubair Saidi, Hanane Akram, Mounir Hanafi, Ouafae Achak, Chaouki El Moujahid, and Tarik Chafik. 2025. "Towards Sustainable Scaling-Up of Nanomaterials Fabrication: Current Situation, Challenges, and Future Perspectives" Eng 6, no. 7: 149. https://doi.org/10.3390/eng6070149
APA StyleHachhach, M., Bayou, S., El Kasmi, A., Saidi, M. Z., Akram, H., Hanafi, M., Achak, O., El Moujahid, C., & Chafik, T. (2025). Towards Sustainable Scaling-Up of Nanomaterials Fabrication: Current Situation, Challenges, and Future Perspectives. Eng, 6(7), 149. https://doi.org/10.3390/eng6070149





