Process Simulation of a Commercial Pervaporation Unit for Ethanol–Water Separation
Abstract
1. Introduction
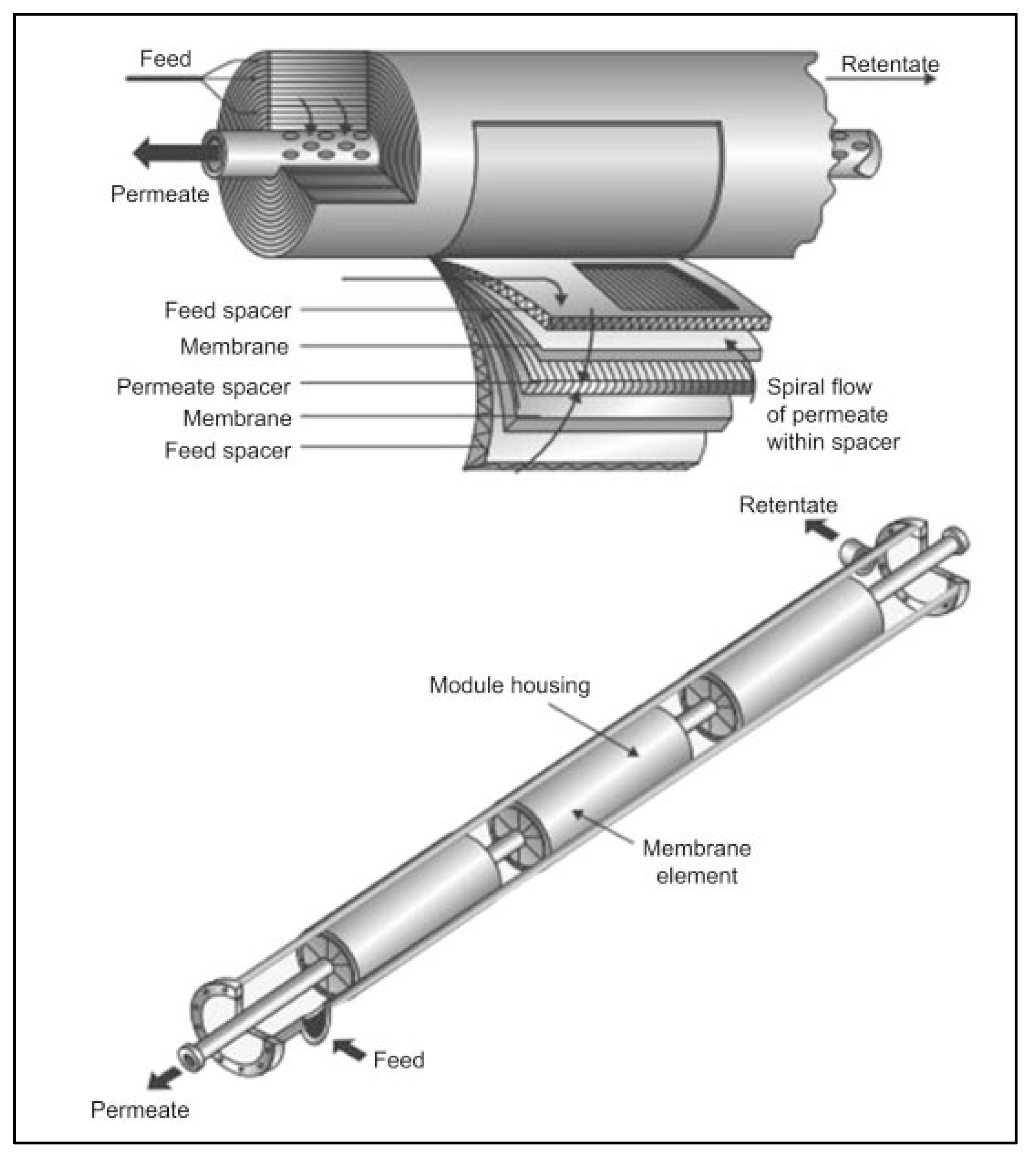
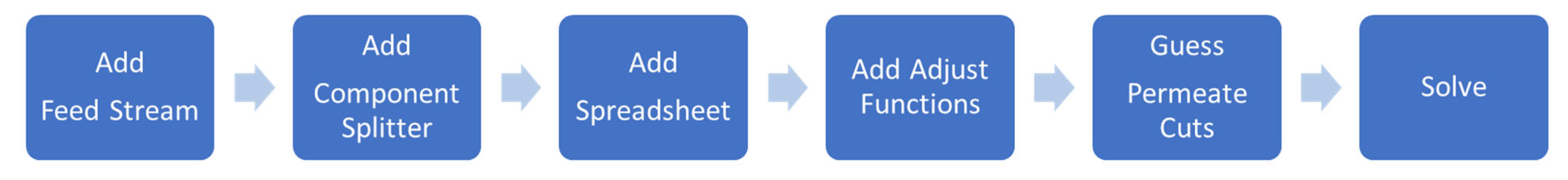
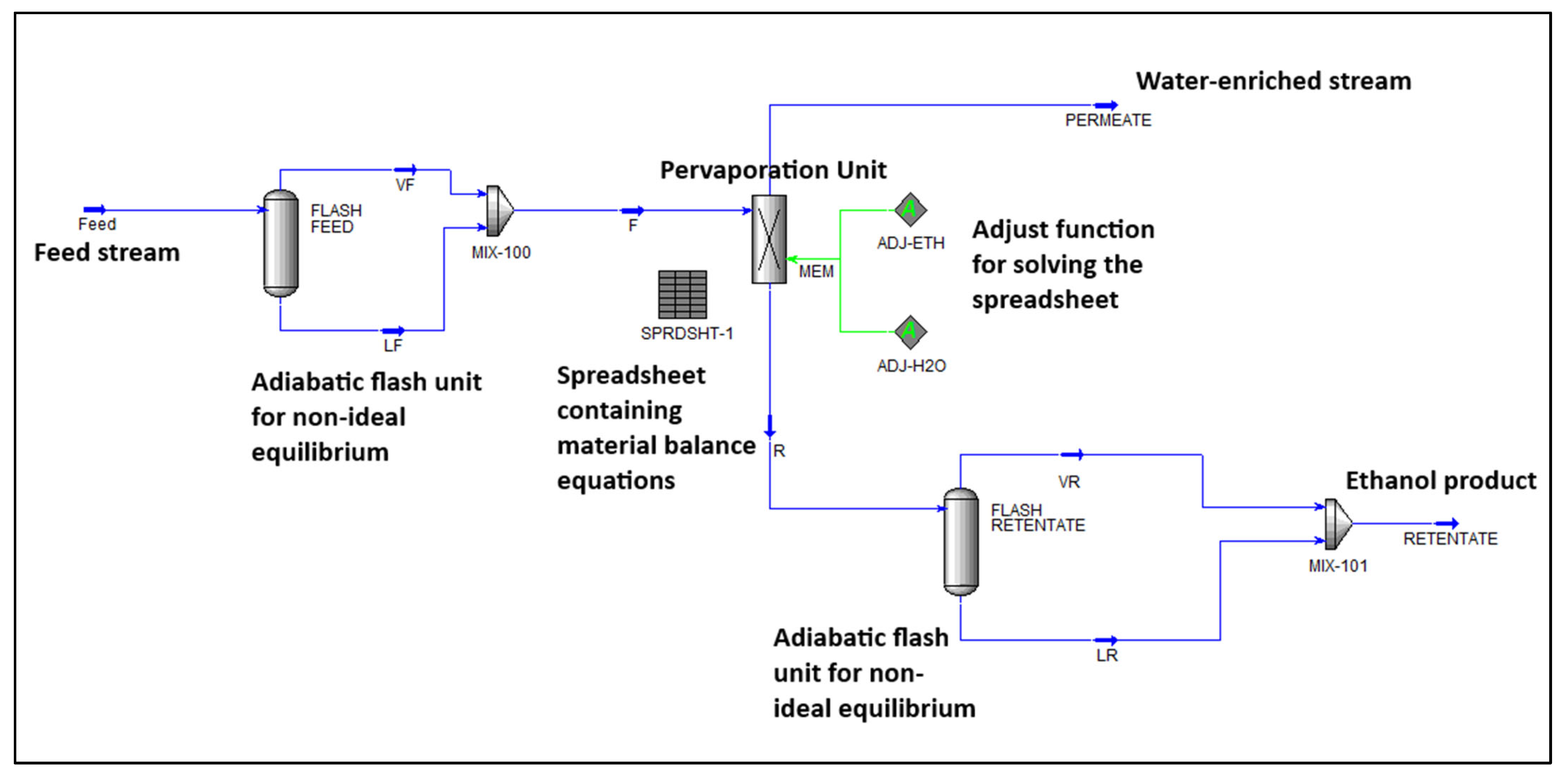
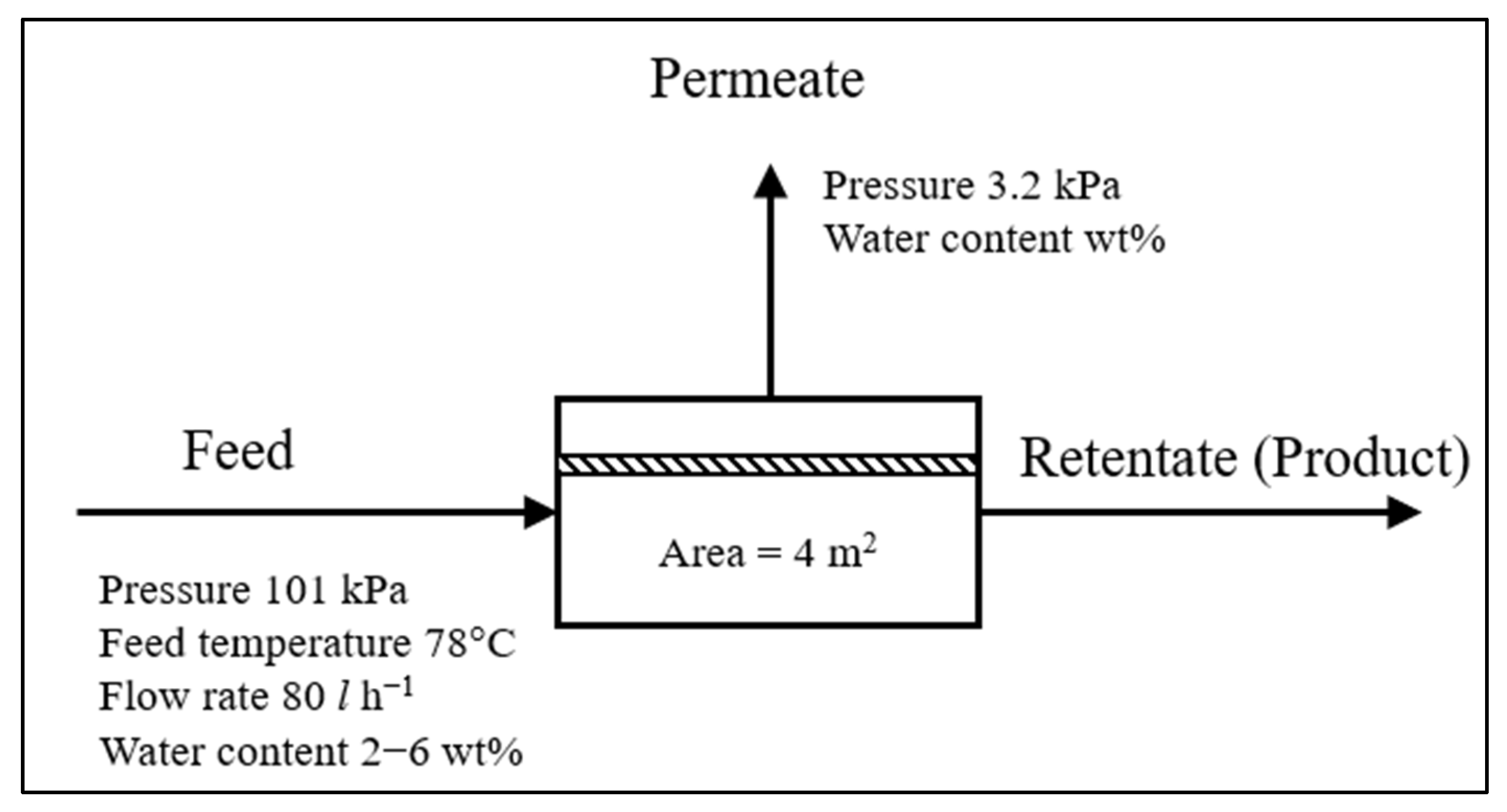
2. Methodology
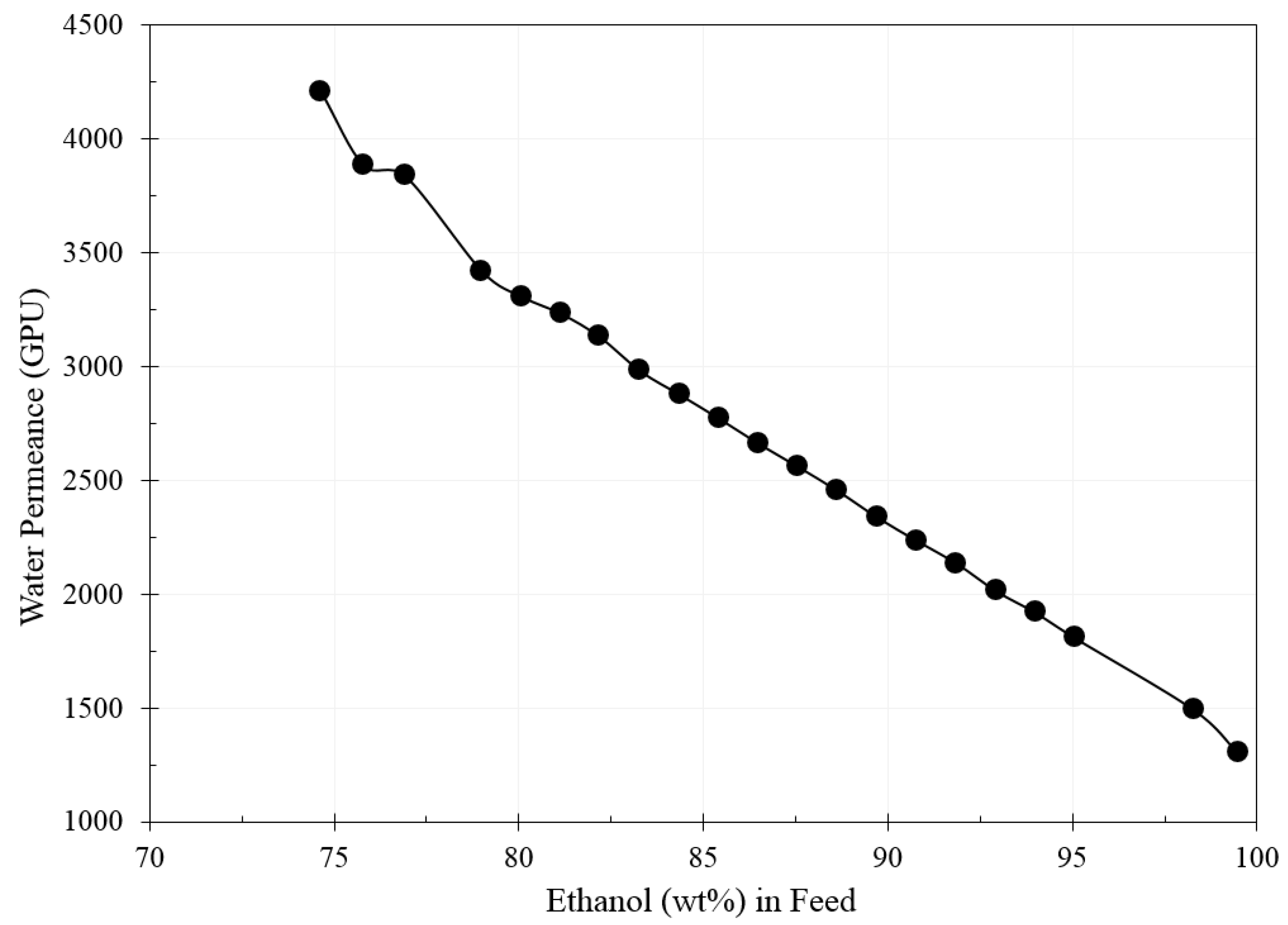
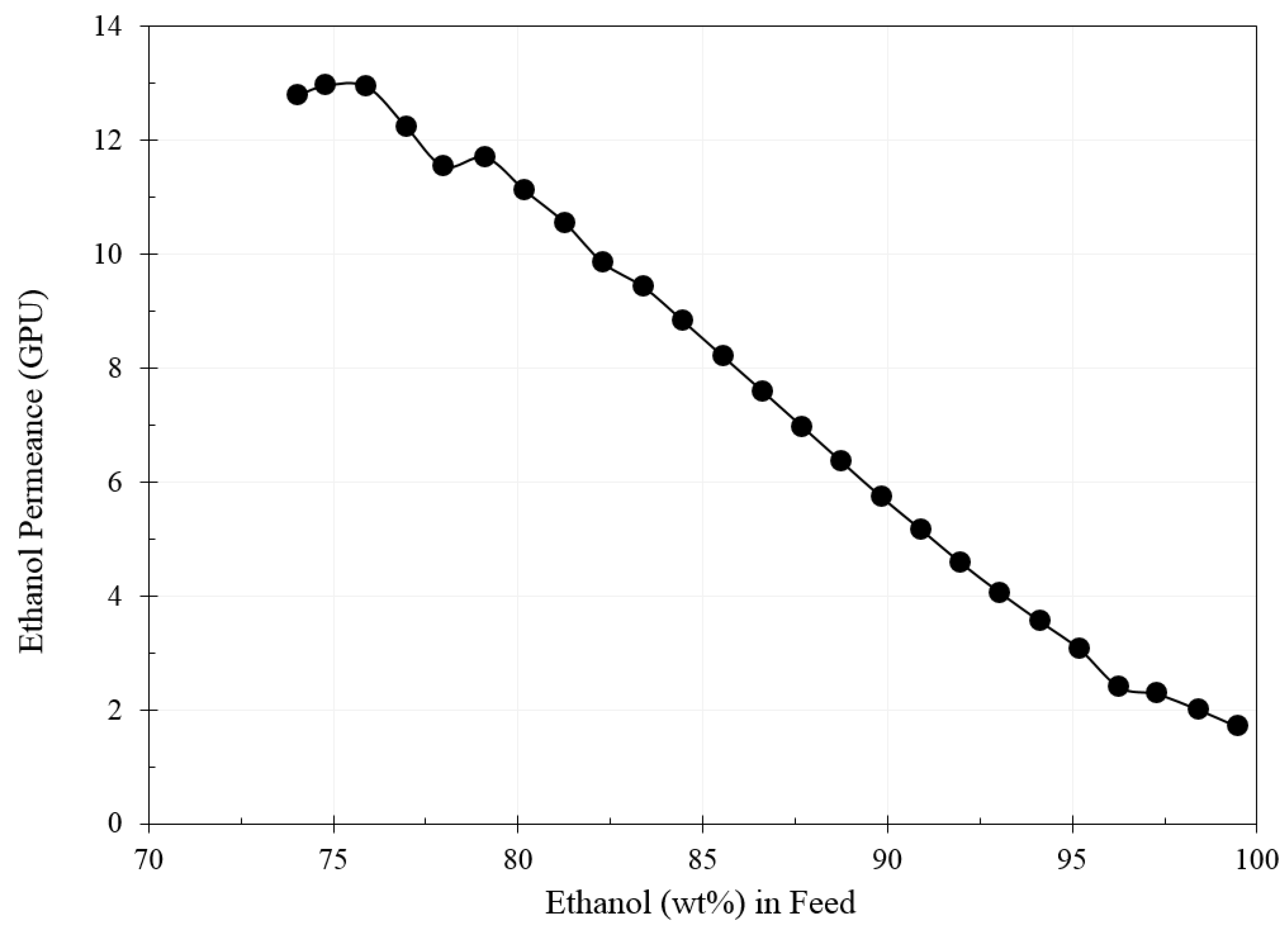
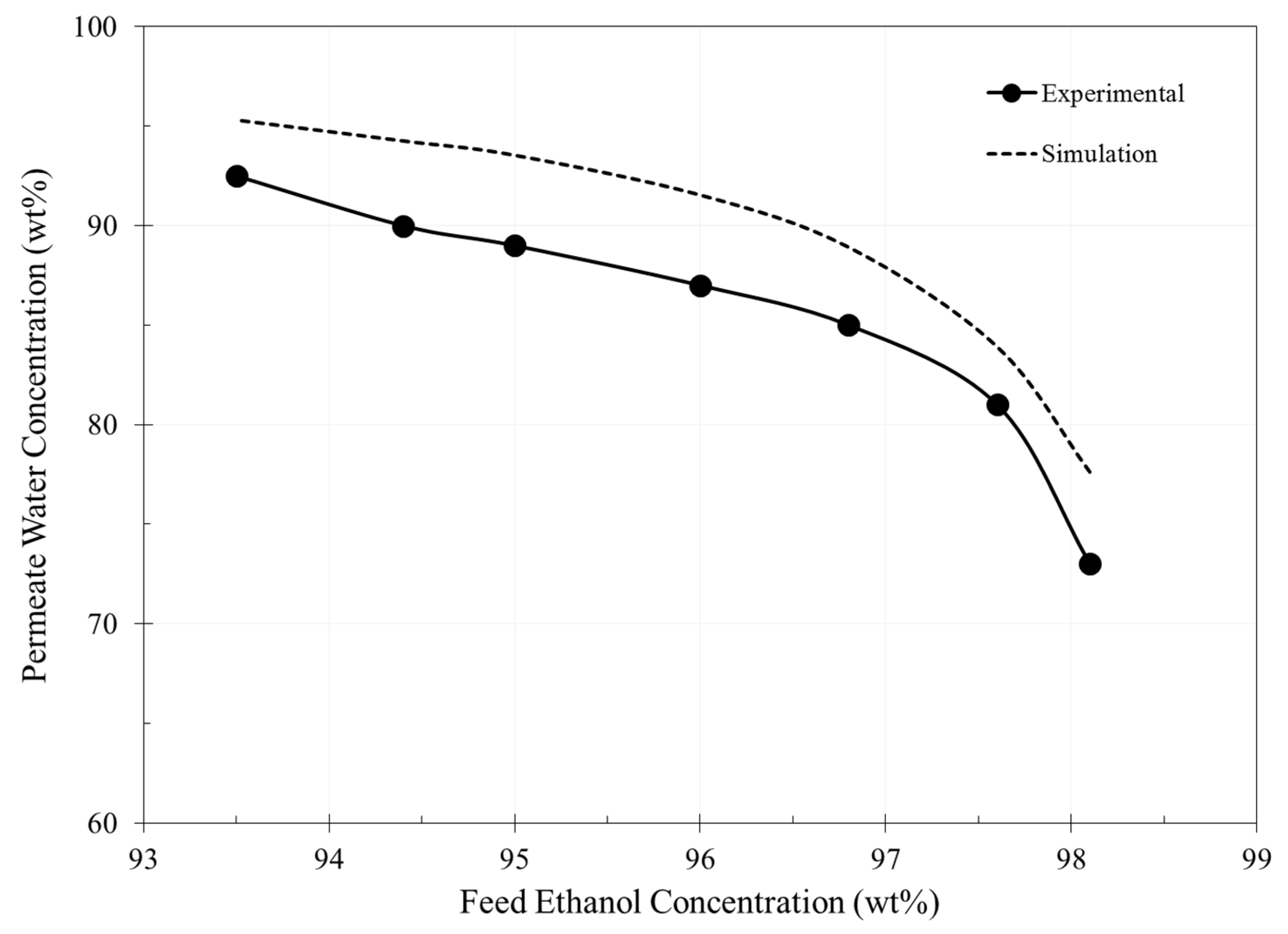
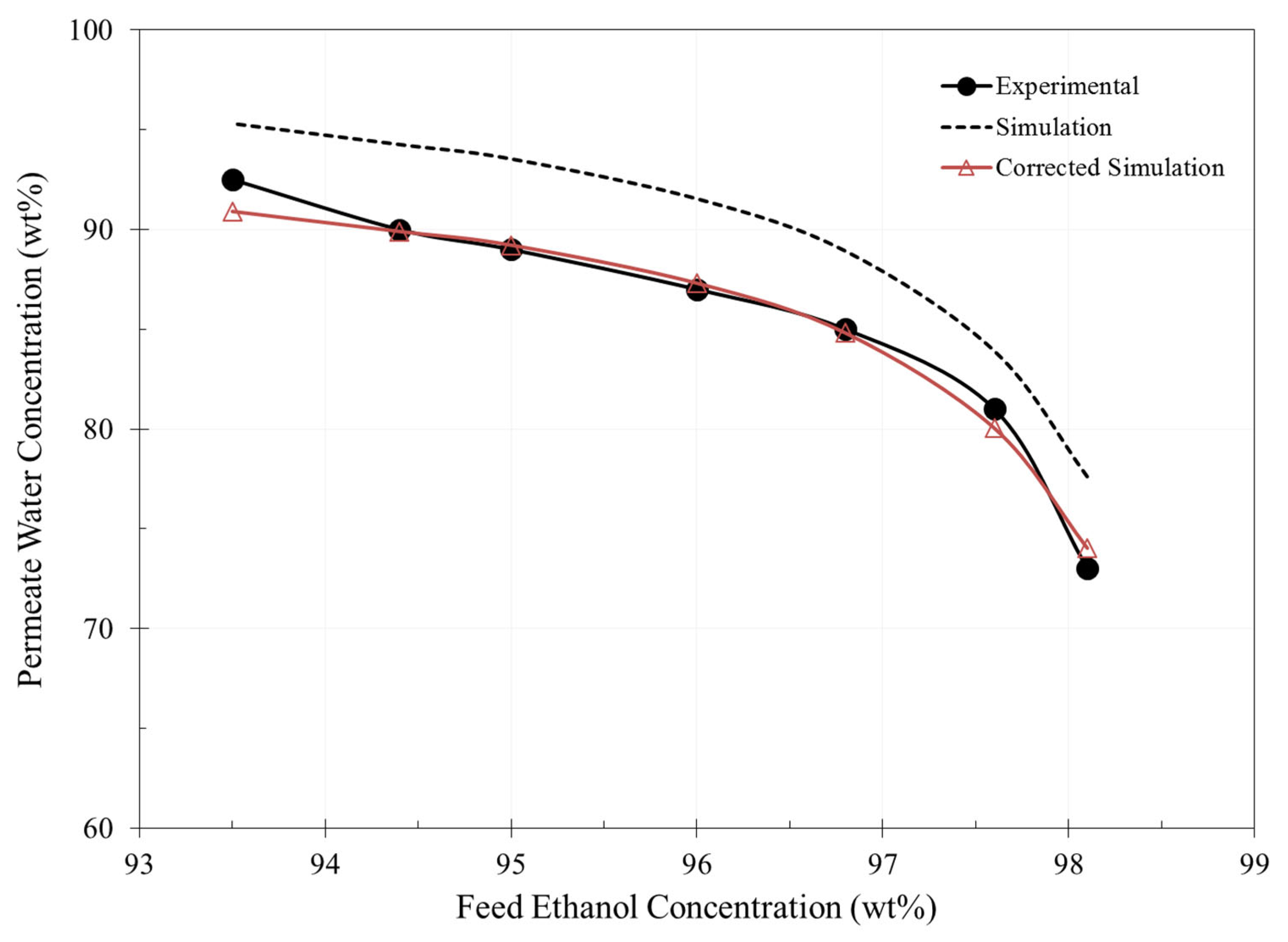
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Latif, M.; Wan Isahak, W.; Samsuri, A.; Hasan, S.; Manan, W.; Yaakob, Z. Recent advances in the technologies and catalytic processes of ethanol production. Catalysts 2023, 13, 1093. [Google Scholar] [CrossRef]
- Lachenmeier, D. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Freitag, W.; Stoye, D. Paints, Coatings and Solvents; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cardinali, V.; de Souza, T.; da Costa, R.; Pinto, G.; Roque, L.; Vidigal, L.; Frez, G.; Pérez-Rangel, N.; Coronado, C. Exploring possible pathways for green hydrogen-based transportation in Brazil: Fuel cells, hydrogen engines and dual-fuel combustion. Int. J. Hydrogen Energy 2025, 120, 238–253. [Google Scholar] [CrossRef]
- Kunwer, R.; Pasupuleti, S.R.; Bhurat, S.S.; Gugulothu, S.K.; Rathore, N. Blending of ethanol with gasoline and diesel fuel—A review. Mater. Today Proc. 2022, 69, 560–563. [Google Scholar] [CrossRef]
- Zentou, H.; Abidin, Z.; Yunus, R.; Awang Biak, D.; Korelskiy, D. Overview of alternative ethanol removal techniques for enhancing bioethanol recovery from fermentation broth. Processes 2019, 7, 458. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, H.; Zhou, Z.; Fan, X. Distillation-pervaporation hybrid process for ethanol dehydration: Process optimization and economic evaluation. Chem. Ing. Tech. 2025, 97, 311–323. [Google Scholar] [CrossRef]
- Kang, Q.; Huybrechts, J.; Van der Bruggen, B.; Baeyens, J.; Tan, T.; Dewil, R. Hydrophilic membranes to replace molecular sieves in dewatering the bio-ethanol/water azeotropic mixture. Sep. Purif. Technol. 2014, 136, 144–149. [Google Scholar] [CrossRef]
- Ong, Y.; Tan, S. Pervaporation separation of a ternary azeotrope containing ethyl acetate, ethanol and water using a buckypaper supported ionic liquid membrane. Chem. Eng. Res. Des. 2016, 109, 116–126. [Google Scholar] [CrossRef]
- Yuan, H.-K.; Ren, J.; Ma, X.-H.; Xu, Z.-L. Dehydration of ethyl acetate aqueous solution by pervaporation using PVA/PAN hollow fiber composite membrane. Desalination 2011, 280, 252–258. [Google Scholar] [CrossRef]
- Yave, W. Separation performance of improved PERVAP™ membrane and its dependence on operating conditions. J. Membr. Sci. Res. 2019, 5, 216–221. [Google Scholar] [CrossRef]
- Koczka, K.; Mizsey, P.; Fonyo, Z. Rigorous modelling and optimization of hybrid separation processes based on pervaporation. Cent. Eur. J. Chem. 2007, 5, 1124–1147. [Google Scholar] [CrossRef]
- Basile, A.; Favvas, E. Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Davis, R. Simple gas permeation and pervaporation membrane unit operation models for process simulators. Chem. Eng. Technol. 2002, 25, 717–722. [Google Scholar] [CrossRef]
- Schwarz, C. Effect of variation between different experimental VLE data sets on thermodynamic model and separation predictions: NRTL correlation of the ethanol + water system. Ind. Eng. Chem. Res. 2024, 63, 10721–10734. [Google Scholar] [CrossRef]
- Chang, J.; Yoo, J.; Ahn, S.; Lee, K.; Ko, S. Simulation of pervaporation process for ethanol dehydration by using pilot test results. Korean J. Chem. Eng. 1998, 15, 28–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqaheem, Y.; Alkandari, S.H. Process Simulation of a Commercial Pervaporation Unit for Ethanol–Water Separation. Eng 2025, 6, 136. https://doi.org/10.3390/eng6070136
Alqaheem Y, Alkandari SH. Process Simulation of a Commercial Pervaporation Unit for Ethanol–Water Separation. Eng. 2025; 6(7):136. https://doi.org/10.3390/eng6070136
Chicago/Turabian StyleAlqaheem, Yousef, and Sharifah H. Alkandari. 2025. "Process Simulation of a Commercial Pervaporation Unit for Ethanol–Water Separation" Eng 6, no. 7: 136. https://doi.org/10.3390/eng6070136
APA StyleAlqaheem, Y., & Alkandari, S. H. (2025). Process Simulation of a Commercial Pervaporation Unit for Ethanol–Water Separation. Eng, 6(7), 136. https://doi.org/10.3390/eng6070136





