Abstract
The persistent contamination of water bodies by organic compounds, heavy metals, and pathogenic microorganisms represents a critical environmental and public health concern worldwide. In this context, polymer composite materials have emerged as promising multifunctional platforms for advanced water purification. These materials combine the structural versatility of natural and synthetic polymers with the enhanced physicochemical functionalities of inorganic fillers, such as metal oxides and clay minerals. This review comprehensively analyzes recent developments in polymer composites designed to remove organic, inorganic, and biological pollutants from water systems. Emphasis is placed on key removal mechanisms, adsorption, ion exchange, photocatalysis, and antimicrobial action, alongside relevant synthesis strategies and material properties that influence performance, such as surface area, porosity, functional group availability, and mechanical stability. Representative studies are examined to illustrate contaminant-specific composite designs and removal efficiencies. Despite significant advancements, challenges remain regarding scalability, material regeneration, and the environmental safety of nanostructured components. Future perspectives highlight the potential of bio-based and stimuli-responsive polymers, hybrid systems, and AI-assisted material design in promoting sustainable, efficient, and targeted water purification technologies.
1. Introduction
The contamination of water bodies by organic, inorganic, and biological pollutants represents one of the most pressing environmental and public health challenges worldwide [1,2]. Industrial effluents, agricultural runoff, and municipal discharges introduce a wide range of contaminants, including synthetic dyes, heavy metals, pesticides, pharmaceuticals, and pathogenic microorganisms, threatening aquatic ecosystems and human health [3,4]. The persistence and complexity of these pollutants, many of which are toxic, bioaccumulative, or resistant to biodegradation, exacerbate the scarcity of safe drinking water and intensify the global water crisis [5,6].
Conventional wastewater treatment technologies, such as biological oxidation, coagulation with inorganic salts, and conventional filtration, can often not completely remove emerging contaminants or operate efficiently on a large scale [7]. These methods frequently present additional drawbacks, including high energy consumption, secondary waste generation, incomplete removal of recalcitrant species, and the use of non-biodegradable chemical reagents that may pose further environmental risks [8,9,10]. Although widely applied in desalination and purification, membrane-based processes still face challenges related to fouling, mechanical degradation, and cost–performance trade-offs [11]. Consequently, there is a growing demand for multifunctional, sustainable, and efficient materials capable of overcoming the limitations of conventional technologies [12].
Polymer composite materials have emerged as promising alternatives to address these challenges due to their structural versatility, tunable physicochemical properties, and compatibility with diverse functional fillers [13]. Natural and synthetic polymers provide a wide range of matrices that can be reinforced with inorganic components such as clays, metal oxides, carbon-based nanomaterials, and biochar [14,15]. This hybrid design enables the development of materials with enhanced adsorption capacity, ion-exchange properties, catalytic activity, antimicrobial performance, and mechanical stability [16]. Notably, biopolymers such as chitosan, alginate, cellulose, and mucilaginous polysaccharides from agricultural residues have gained increasing attention as eco-friendly alternatives for coagulants and adsorbents in wastewater treatment [17].
Recent advances have demonstrated the ability of polymer composites to selectively remove organic dyes, pharmaceuticals, and volatile organic compounds; capture toxic metal ions through chelation and electrostatic interactions; and inhibit or inactivate microorganisms via reactive oxygen species generation or metal ion release [18]. Furthermore, nanostructured systems, such as electrospun membranes, nanocomposite hydrogels, and thin-film composites, offer high surface area, hierarchical porosity, and controllable functionalization, directly contributing to improved purification performance [19,20].
This work analyzes polymer composite materials applied to water purification, focusing on removing organic, inorganic, and biological contaminants. Special emphasis on removal mechanisms, such as adsorption, ion exchange, photocatalysis, and antimicrobial action, as well as synthesis strategies and physicochemical characteristics that determine performance. Representative studies are examined to highlight advances, limitations, and knowledge gaps. Finally, future research directions are discussed. The main contribution of this review is establishing a comprehensive framework that correlates the composition, synthesis routes, and functional properties of polymer-based composites with their efficiency in removing organic, inorganic (including radionuclide), and biological contaminants. Beyond summarizing existing data, this work integrates these pollutant classes within a comparative approach, supported by figures and tables synthesizing key performance parameters. Furthermore, it provides novel insights into emerging research directions, including AI-assisted material design, multifunctional systems combining sensing and remediation, bio-based polymer–filler hybrids, and advanced manufacturing strategies (3D/4D printing), thereby offering a perspective for the sustainable development of next-generation polymer composites for water purification.
2. Polymer Composite Materials: Fundamentals
2.1. Definition and Composition
In general, a composite material can be defined as the combination of two or more distinct constituents, usually a matrix (continuous phase) and a reinforcement material (dispersed phase), to achieve superior or differentiated properties compared to those exhibited individually by each component [21]. The matrix, which constitutes the continuous phase, surrounds and holds the reinforcements together and may be polymeric, metallic, or ceramic [22,23]. In addition to providing shape to the composite, the matrix is responsible for distributing mechanical loads and protecting the reinforcements from physical and environmental damage [24].
The reinforcement, characterized as the dispersed phase, is incorporated into the matrix to enhance specific properties such as mechanical strength, stiffness, hardness, thermal stability, or electrical performance. It can take different morphologies, such as fibers, particles, whiskers, or platelets, and may be of natural or synthetic origin [25]. The synergistic interaction between matrix and reinforcement provides composite materials with unique characteristics, making them widely applicable in sectors that demand a high strength-to-weight ratio, durability, and performance tailored to the requirements of the final product.
Polymer composite materials are mixtures of polymers and reinforcing agents, created to enhance specific mechanical and thermal features. The polymers used as matrices may be natural or synthetic, depending on the desired traits of the final product. Synthetic polymers, such as polypropylene (PP) and polyethylene (PE), are common due to their high strength and low cost [26,27]. Natural polymers, such as polylactic acid (PLA) and chitin, are gaining popularity due to their biodegradable and environmentally friendly properties [26,28].
2.1.1. Matrix: Natural and Synthetic Polymers
The polymer matrix is a key component of a composite, since it contains the reinforcing materials and determines the final material’s strength and thermal stability. Natural polymers, like PLA and chitin, have been widely studied because of their low environmental impact, biodegradability, and renewability. However, synthetic polymers, such as PE and PP, are more common in industry due to their better mechanical properties, durability, and lower production costs. A rising interest in replacing synthetic materials with natural polymers is driven by increasing environmental concerns [26]. This movement is supported by research into using natural fibers as reinforcement, improving polymer composites’ mechanical and environmental properties [27]. These materials are crucial for making sustainable composites that satisfy the rising demand for eco-friendly products with reduced environmental footprints.
2.1.2. Reinforcement: Clays, Metal Oxides, Carbon-Based Nanomaterials, and Fibers
Reinforcing materials are essential for enhancing polymer composites’ mechanical and thermal properties. Common reinforcements include clays, metal oxides, carbon-based nanomaterials, and fibers, which can be natural or synthetic. Clays are popular because they increase rigidity and thermal resistance, especially when treated with modifying agents [27]. Organophilic clays also boost composites’ thermal stability and fire resistance, making them suitable for use in tough environments [26].
Metal oxides, such as TiO2, Fe2O3, and ZnO, are commonly used as reinforcements because of their catalytic and absorption properties, which boost the corrosion resistance and thermal stability of polymer composites [28]. These materials have been effectively employed to enhance the chemical and thermal resistance of polymer composites, making them more durable in extreme environmental conditions.
Carbon-based nanomaterials, such as carbon nanotubes (CNTs) and graphene, are especially valuable because of their high surface area and excellent electrical and thermal conductivity. The use of CNTs and graphene as reinforcements has been thoroughly studied, with results showing a significant increase in the mechanical and thermal properties of the composites [27]. These materials offer exceptional features useful in various applications, including electronics and high-performance materials.
Natural and synthetic fibers, like sisal, jute, and kenaf, are the most common reinforcements used in polymer composites. Natural fibers are more environmentally friendly and offer a favorable combination of low cost and good mechanical properties. However, they face challenges related to moisture absorption and adhesion to the polymer matrix. These issues can be addressed through chemical and hybrid modifications, exploring the potential of combining natural fibers with synthetic materials to improve adhesion and the overall properties of the composite [27,28].
Understanding the definition and composition of polymer composite materials is key to grasping their properties and potential uses. The selection of matrix and reinforcement materials is vital in shaping the final characteristics, such as mechanical strength, thermal stability, and moisture resistance. Recent research has demonstrated that combining natural fibers with synthetic materials can significantly enhance the properties of composites, making them more sustainable and effective [27].
2.2. Fabrication Techniques
Producing polymer composites involves a wide range of techniques, whose selection depends on the type of matrix, the reinforcement, and the desired final properties [29]. Traditionally, methods such as pultrusion, filament winding, hand lay-up, resin transfer molding (RTM), vacuum bagging, compression molding, and injection molding have been applied, particularly in the automotive, aerospace, and construction sectors, due to their versatility and suitability for large-scale processing [29,30]. More recently, advanced technologies such as surface coating, additive manufacturing, and magnetic pulse powder compaction have been incorporated, expanding the application field and improving material performance.
2.2.1. Conventional Methods
The fabrication of polymer composites has historically relied on well-established processing techniques that combine practicality, scalability, and cost-effectiveness. These conventional methods, widely adopted in industrial sectors such as aerospace, automotive, and construction, are characterized by their ability to produce components with consistent quality and diverse geometries [21]. Each technique presents advantages and limitations, depending on the matrix type, reinforcement, and final application, as briefly described below [22,23,25,31].
- •
- Pultrusion: Combines pulling and extrusion, in which fibers impregnated with resin are drawn through heated molds that define the final geometry (circular, rectangular, or structural profiles). This process enables high productivity and manufacturing constant cross-section parts with good mechanical strength.
- •
- Filament winding: Employs rotating mandrels for controlled deposition of resin-impregnated fibers in helical, circumferential, or polar patterns. It is particularly suited for hollow and cylindrical components, such as tanks and pipelines.
- •
- Hand lay-up: An open-mold technique where fibers and resin are manually placed into molds, generally with release agents and minimal pressure. It is simple, cost-effective, strongly dependent on operator skill, and offers limited dimensional control.
- •
- RTM: Involves injecting resin into closed molds containing pre-placed fiber reinforcements. It enables high production rates, good surface finish, and the fabrication of complex geometries, although it requires high tooling costs and strict process control.
- •
- Vacuum bagging: An enhanced version of hand lay-up, in which composite laminates are sealed in vacuum bags, ensuring compaction and improved interlaminar adhesion. When combined with autoclave curing, it produces high-performance structural components.
- •
- Compression molding: Is widely employed for thermoset and thermoplastic matrices using semi-cured compounds (BMC, SMC) or molten thermoplastics subjected to pressure and sometimes heat. It is commonly applied in the automotive industry due to its high productivity, dimensional accuracy, and short cycle times.
- •
- Injection molding: A well-established process in the plastics industry that allows the incorporation of short fibers or particles into polymer matrices. It enables rapid production of complex shapes, requiring reduced reinforcement dimensions and careful control to minimize residual stress.
2.2.2. Advanced Technologies
In recent years, the demand for high-performance, lightweight, and multifunctional polymer composites has driven the development of advanced fabrication technologies. Unlike conventional methods, which are well established and widely applied in large-scale production, these innovative approaches aim to overcome limitations related to design flexibility, property tailoring, and environmental constraints [22,23,25,31]. By integrating novel processing routes with precision engineering, these technologies represent an important step toward the next generation of polymer composites, particularly in sectors requiring complex geometries, enhanced durability, and sustainability.
- •
- Surface coating: Consists of depositing thin films on polymer matrices or non-polymeric substrates to improve corrosion resistance, tribological behavior, or biocompatibility. Techniques include plasma spraying, magnetron sputtering, electrochemical deposition, and sol–gel processing. Each approach offers specific advantages regarding thickness control, adhesion, and uniformity, although limitations such as high cost or susceptibility to cracking during drying may arise.
- •
- Additive manufacturing (3D/4D printing): Allows the layer-by-layer fabrication of complex composites by integrating CAD, lasers, and numerical control. While 3D printing is already consolidated, 4D and 5D variants add functionalities such as responsiveness to external stimuli and enhanced geometric freedom. This technique enables customization, rapid prototyping, and topological optimization, although it still faces material limitations and relatively low production speed.
- •
- Magnetic pulse powder compaction: Employs pulsed electromagnetic fields to consolidate polymeric or hybrid powders into dense structures. The process is fast, cost-effective, and suitable for simple parts, with applications in packaging, medical devices, and electronic components. However, it is limited by low energy efficiency and restrictions on part geometry.
Conventional methods still predominate in large-scale industrial production due to their maturity and reliability, whereas advanced technologies represent an evolutionary trend toward greater functionality, sustainability, and structural complexity. The choice of manufacturing route depends on the balance between cost, targeted properties, and technological feasibility, being a decisive factor in the life cycle of composite materials.
2.3. Characterization and Physicochemical Properties
Characterizing polymer composites is essential to understanding their structure, mechanical performance, and physicochemical stability. The application of advanced techniques enables the correlation between morphology, composition, and service behavior, providing valuable insights for optimizing formulations and manufacturing processes [21].
Structural and chemical analyses include Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy, which identify functional groups, monitor crosslinking reactions, and evaluate matrix–reinforcement interactions. Energy-dispersive X-ray spectroscopy (EDS), coupled with electron microscopy, complements these techniques by identifying the elemental composition [2,21,29].
Diffraction and thermal techniques are central in assessing stability and physicochemical transitions. X-ray diffraction (XRD) provides information on crystallinity and structural ordering. At the same time, thermal analyses such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) allow the determination of glass transition (Tg), melting (Tm), degradation temperatures, and thermal stability. These data are crucial for predicting behavior under different operating conditions [24,29,31].
Microscopy represents another critical approach. Scanning electron microscopy (SEM) is widely employed to investigate fracture morphology, reinforcement dispersion, and matrix–reinforcement interfacial quality. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) enable nanoscale analyses, revealing nanoparticle distribution, structural defects, and surface roughness features [2,17,21,22]. Figure 1 shows the SEM and TEM images of a new porous polymer (C-Daba), containing carboxyl groups in its structure, synthesized through a traditional Schiff-base condensation reaction [32].
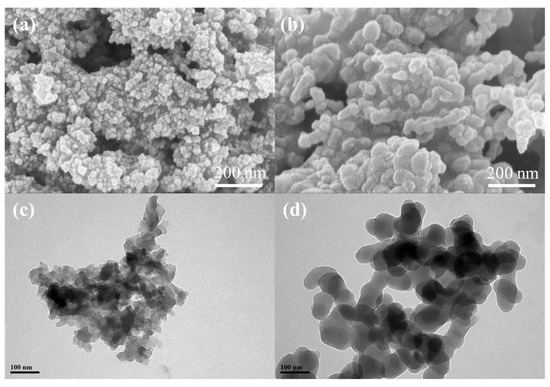
Figure 1.
(a,b) SEM and (c,d) TEM images of C-Daba. Reproduced with permission from [32].
Tensile, flexural, compression, and impact tests quantify mechanical properties such as elastic modulus, tensile strength, and toughness. Dynamic mechanical analysis (DMA) complements these tests by providing insights into viscoelastic behavior, dynamic transitions, and response to oscillatory loads [26,28,30].
Surface and physicochemical properties are explored through wettability, contact angle, and adsorption calorimetry, which evaluate surface energy and fluid interactions. Additionally, electrical and thermal conductivities can be measured by four-point probe methods and heat-flow analyses, respectively, broadening the understanding of composite functionalities [27,29].
These techniques provide an integrated view of physicochemical and structural properties, enabling the establishment of relationships between formulation, processing, and the final performance of polymer composites. This holistic approach is essential for developing advanced materials tailored to high-performance applications and contemporary environmental demands.
3. Removal of Organic Contaminants
Organic contaminants in wastewater represent a chemically diverse group of compounds derived from various sources, including industrial processes, agricultural runoff, domestic activities, and urban stormwater. These substances include naturally occurring and synthetic chemicals, many of which are recognized for their toxicity, persistence, and potential to bioaccumulate in the environment [33].
Discharging wastewater with a high organic load into natural water bodies can trigger a cascade of adverse environmental effects primarily driven by the elevated biochemical and chemical oxygen demand (BOD and COD, respectively). The microbial degradation of organic matter rapidly consumes dissolved oxygen, potentially leading to hypoxic or anoxic conditions that compromise aquatic life and reduce biodiversity [34]. Concurrently, associated nutrient inputs, such as nitrogen and phosphorus, can promote eutrophication, resulting in excessive algal blooms whose subsequent decomposition further exacerbates oxygen depletion [35]. Under low-oxygen conditions, anaerobic microbial pathways dominate, producing malodorous and potentially toxic compounds, including hydrogen sulfide, ammonia, and methane [36,37]. These changes alter aquatic community structure, degrade water quality, and may result in water unsuitable for recreational or potable uses.
Given their potential toxicity and environmental consequences, the effective removal of organic contaminants from wastewater is a critical aspect of environmental protection. This section discusses the types of organic pollutants, challenges, and current strategies for eliminating these compounds from wastewater, as well as some examples of polymer composite materials that can be applied as alternative treatment methods.
3.1. Types of Contaminants
Different types of organic contaminants can be detected depending on the wastewater source. Table 1 shows the commonly found organic contaminants for different wastewaters. Moreover, the wastewater source also dictates whether the organic compounds are readily degraded by conventional wastewater treatment methods or need special treatment processes. For example, the organic load of food and beverage wastewater is generally readily biodegradable since it mainly consists of sugars, soluble starches, ethanol, fatty acids, etc. [38]. In contrast, petroleum refining and the petrochemical industry generate complex effluents containing aliphatic and aromatic hydrocarbons (e.g., benzene, toluene, xylene), polycyclic aromatic hydrocarbons (PAHs), phenols, organic acids, and oil and grease. These compounds are hydrophobic and often exhibit high toxicity and low biodegradability [39].

Table 1.
Organic compounds found in different wastewater.
Among organic contaminants, persistent organic pollutants (POPs) are particularly concerning due to their toxicity, environmental persistence, carcinogenic potential, lipophilicity, long-range atmospheric transport, and tendency to bioaccumulate through the food chain [52,53]. This group includes compounds such as organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), polycyclic aromatic hydrocarbons (PAHs), polychlorinated naphthalenes (PCNs), polychlorinated biphenyls (PCBs), perfluoroalkyl and polyfluoroalkyl substances (PFAs), among others [53,54]. As shown in Table 1, these POPs are found in various wastewaters originating from industrial and domestic sources. POPs are known for their resistance to conventional wastewater treatment processes such as filtration, flocculation, coagulation, and activated sludge, necessitating the development and application of more advanced and targeted treatment technologies.
3.2. Removal Mechanisms
The removal of organic contaminants from wastewater can be performed by physical, chemical, and biological processes, often applied in combination depending on the characteristics of the wastewater. Physical methods, such as screening, sedimentation, flotation, and filtration, primarily target the separation of particulate and colloidal organics [55]. Chemical treatments, generally coagulation-flocculation, effectively remove dissolved and refractory organic compounds [56]. Biological processes, such as activated sludge systems, moving bed biofilm reactors (MBBRs), and membrane bioreactors (MBRs), rely on microbial communities to biodegrade the biodegradable fraction of organic matter [57]. In practice, wastewater treatment facilities combine these approaches to achieve higher removal efficiencies and meet discharge or reuse standards.
Conventional wastewater treatment generally relies on a primary clarification followed by activated sludge. In the clarification step, suspended solids and the hydrophobic organics adsorbed to them are separated from the wastewater by flocculation and coagulation. This stage requires the addition of coagulants and flocculants to destabilize colloidal particles, promoting their aggregation into larger flocs, which can then be removed through sedimentation, filtration, or dissolved air flotation [56]. Subsequently, the conventional activated sludge (CAS) mineralizes readily biodegradable carbon while sorbing part of the more hydrophobic or cationic compounds to sludge flocs [58].
Process variants that extend sludge-retention time, such as MBR, or employ attached biomass, such as MBBR or integrated fixed-film activated sludge (IFAS), can boost organic matter removal and elimination of some organic compounds that the conventional process would not remove. For example, Cesaro et al. (2013) compared an integrated fixed-film activated sludge membrane bioreactor (IFAS-MBR) with conventional activated sludge to remove 17 pharmaceutically active compounds and 22 trace organic pollutants [58]. The IFAS-MBR achieved the highest overall elimination, particularly for hormones (100% removal), other pharmaceuticals, and linear alkylbenzene sulfonates (65–100% removal). In contrast, the removal of nonylphenols and phthalates was limited to 10–30%. The superior performance of the IFAS-MBR is likely attributable to the presence of biofilm, which creates a range of redox conditions (aerobic, anoxic, and anaerobic) along its depth, thereby enhancing degradation pathways. Additionally, the higher sludge retention time promotes microbial acclimation to the target contaminants, further improving removal efficiency.
To increase the removal of phenolic compounds and phthalates, as well as many polar, recalcitrant species, such as certain PFAS, that escape essentially unchanged from biological treatment, advanced treatment methods can be applied [59,60,61]. Advanced treatment technologies, including adsorption (with activated carbon, for example), oxidation (ozonation, photocatalysis, Fenton and other radical-based advanced oxidation processes, AOPs), high-pressure membrane separations (nanofiltration and reverse osmosis), and hybrid treatments, provide higher removal of POPs than conventional methods [62,63]. For example, the addition of activated carbon (AC) at 10–20 mg/L in municipal wastewater removed 60–85% of a broad suite of drugs and endocrine disruptors [60,64]. Nanofiltration and reverse osmosis provide ≥90% rejection across polarity classes, including recalcitrant PFAS [65]. However, membrane separation processes demand higher energy inputs than biological or adsorptive methods and generate a concentrate stream that retains the pollutants, requiring further handling [65,66]. Hybrid configurations such as MBR or UF followed by AC adsorption integrate complementary mechanisms and have demonstrated >80–90% removal of pharmaceuticals, PFAS, and phenolics over multi-year pilots while consuming less energy or carbon than the corresponding single-step advanced processes [67,68].
Most advanced treatment technologies rely on engineered materials to achieve high removal efficiencies. Polymeric composite materials are rapidly emerging as a complementary strategy for removing organic contaminants from wastewater [69,70]. By tailoring surface chemistry and pore architecture at the nano- to meso-scale, these composites can be used as adsorbents and photocatalytic surfaces [71,72]. Recent studies have demonstrated their utility in synergistic removal approaches, particularly when integrated into membrane or filtration systems [73,74]. The following section will show examples of polymeric composite materials that can improve the removal efficiency of organic pollutants from wastewater.
3.3. Reported Application Studies
Polymeric composite materials introduce selectivity and/or reactivity directly into bead formats, nanoparticles, or membranes, potentially reducing footprint and enabling multi-function operation (separation plus adsorption or oxidation). These materials can have improved adsorptive and catalytic capacities.
Polymeric composite beads and nanoparticles can improve the adsorption of organic contaminants and their oxidation through photocatalysis. Wang et al. (2022), for example, demonstrate a multifunctional polymer–inorganic nanocomposite made of magnetic polymeric microspheres (Fe3O4/poly(N-isopropylacrylamide-co-methacrylic acid or Fe3O4/P(NIPAM-co-MAA)) with silver-decorated titanium dioxide nanoparticles (Ag-TiO2 NPs), in which carboxylated P(NIPAM-co-MAA) magnetic microspheres selectively adsorb cationic dyes while Ag-decorated TiO2 enables visible-light-driven photocatalytic degradation of antibiotics [75]. The nanocomposite exhibited a Langmuir maximum adsorption capacity of 150.4 mg/g for the cationic basic dye fuchsin (BF), reaching equilibrium within 30 min in the dark. In contrast, dark adsorption of ciprofloxacin (CIP) and norfloxacin (NFX) was negligible, with only 2% and 1% removal after 180 min, respectively. Under visible light irradiation, the composite photodegraded 47% of both CIP and NFX within 180 min. Reusability tests revealed a decline in BF adsorption efficiency from 87% to 36% and CIP photodegradation from 49% to 19% over five cycles. Moreover, the material is magnetically separable and demonstrates qualitative antibacterial activity against Escherichia coli.
Besides adsorption and photocatalysis, some polymeric composite materials have been studied for Fenton or photo-Fenton oxidation of organic pollutants. Overall, polymeric composites for Fenton degradation immobilize Fe-based catalytic sites on polymer carriers, enabling heterogeneous OH generation with photo-assisted Fe2+/Fe3+ cycling while simplifying catalyst recovery. Naumova et al. (2018), for example, demonstrated that polypropylene fibers modified with nano dispersed iron act as an efficient heterogeneous photo-Fenton catalyst under visible light, delivering 80–100% decolorization of both water-soluble dyes (eosin, brilliant green, rhodamine C) and fat-soluble dyes (blue, yellow, red) in aqueous and aqueous-organic media [76]. These composites can reach high degradation efficiencies but have durability and engineering challenges, such as polymer damage by ultraviolet light (UV) and reactive oxygen species, catalyst detachment/leaching, and the need for rigorous mineralization (TOC/COD) and real-wastewater validation alongside reactor-level optimization for industrial viability [76,77].
Mixed-matrix membranes have emerged as promising alternatives for wastewater treatment, combining the structural advantages of polymeric membranes with the functional properties of embedded adsorbents or catalysts. For example, among mixed-matrix membranes used as in-membrane adsorbers, polyvinylidene fluoride (PVDF) hollow-fiber membranes loaded with polymeric adsorber particles have demonstrated simultaneous uptake of pharmaceuticals such as diclofenac (DCF), sulfamethoxazole (SMX), and carbamazepine (CBZ) under dynamic flow [78]. At 5 mg/L in tap water, reported area-normalized capacities reached up to 13.7 g/m2 for DCF while co-adsorbing SMX or CBZ, illustrating the feasibility of coupling membrane separation with fast in-matrix adsorption. Building on the same concept, multi-channel polyethersulfone (PES) membranes with embedded powdered activated carbon (PAC) have been fabricated and tested for CBZ and paracetamol (PAR) removal, with additional evaluation of solvent regeneration [79]. In a crossflow filtration mode, the membrane with PAC removed 88.9% of CBZ and 92.2% of PAR after 8 h. The PES membrane with no GAC removed only 4.2% and 5.1% of CBZ and PAR, respectively. Cyclic regeneration with 50–100% ethanol at 20–40 °C restored a large fraction of initial retention over multiple cycles and highlighted practical trade-offs (e.g., higher ethanol improving desorption but potentially altering polymer hydrophilicity/permeability). Fouling with humic matter was also examined, and the authors caution that pharmaceuticals in real effluents occur at far lower concentrations than in the test solutions, underscoring the need to validate the membrane adsorbers under competitive dissolved organic carbon and mixed organics conditions.
Guo et al. (2023) developed polyethylene terephthalate (PET) ion track-etched membranes chemically grafted with propyl groups to enhance hydrophobicity for oil/water separation [80]. Channel sizes ranging from 310 to 1950 nm were tested, and the modification resulted in a maximum water contact angle of 140.8°. The membranes exhibited a high separation efficiency of 99.87%, a water flux of 12,158 L/m2·h, and stable long-term operation. The work demonstrates a cost-effective and efficient strategy for treating oily wastewater. Figure 2 presents the preparation route and application of the hydrophobic PET track-etched membrane. The process involves ion irradiation to generate tracks, chemical etching to form the membrane structure, and subsequent surface modification with propyltrichlorosilane. The schematic also illustrates the oil/water separation mechanism, supported by a photograph of a 9:1 oil/water emulsion and a microscopic image showing water droplets with diameters ranging from 0.5 to 10 μm.
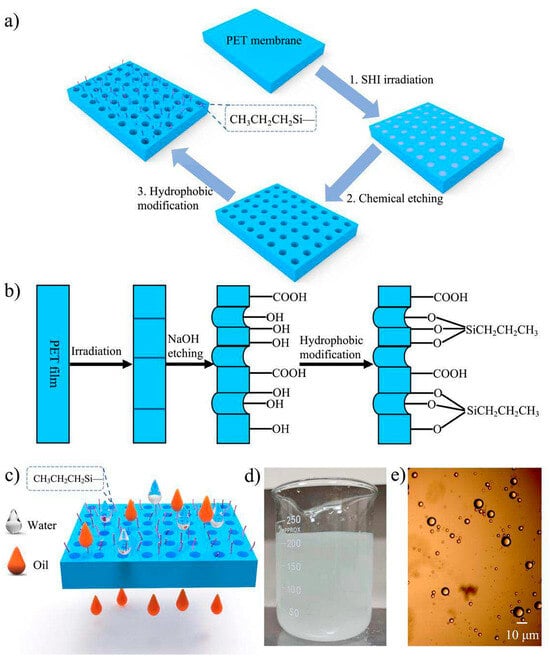
Figure 2.
(a) Preparation of hydrophobic PET TeM: SHI irradiation, chemical etching, and surface modification with propyltrichlorosilane. (b) Overall preparation and modification process. (c) Schematic of oil/water separation. (d) Photograph of 9:1 oil/water emulsion. (e) Microscopic image of emulsion droplets (0.5–10 μm). Reproduced with permission from [80].
Zhao et al. (2018) used a simple casting method to produce a gravity-driven oil/water separator using a PET nonwoven coated with poly(butyl methacrylate) (PBMA) and graphene [81]. The resulting surface exhibited hydrophobic and oleophilic properties, allowing continuous separation without external pressure. The coated nonwovens maintained over 95% separation efficiency for up to eight cycles. This low-cost and facile approach can be applied for oil spill remediation and removing organic pollutants from water surfaces.
Wang et al. (2020) fabricated cellulose acetate (CA) nanofiber membranes through electrospinning, followed by deacetylation to obtain a dual super-amphiphilic material [82]. The membranes displayed superhydrophilicity in oil and oleophobicity underwater, enabling efficient separation of free oil/water mixtures, emulsions, and even corrosive aqueous systems. Driven only by gravity, the membranes achieved ultrahigh fluxes up to 38,000 L/m2·h and separation efficiencies of 99.97%. They also exhibited excellent antifouling and reusability, making them promise for wastewater treatment in chemical, food, and textile industries and offshore oil spill cleanup. Figure 3 presents the separation performance of commercial cellulose acetate (c-CA) and deacetylated cellulose acetate (d-CA) membranes. The results include the separation flux for different oil/water mixtures, petroleum ether/water flux over successive cycles, and the corresponding separation efficiencies. Overall, the data demonstrate the superior flux and stability of the d-CA membranes compared with c-CA membranes under repeated operation.
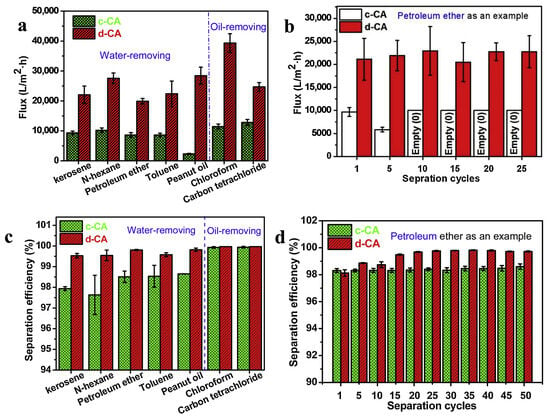
Figure 3.
(a) Separation flux of commercial cellulose acetate (c-CA) and deacetylated cellulose acetate (d-CA) membranes for different oil/water mixtures. (b) Separation flux of petroleum ether/water over successive cycles for c-CA and d-CA membranes. (c) Separation efficiency of c-CA and d-CA membranes for various oil/water mixtures. (d) Separation efficiency of petroleum ether/water at different cycles for c-CA and d-CA membranes. Reproduced with permission from [82].
Guo et al. (2024) reported the fabrication of sub-nanoporous polyetherimide (PEI) membranes via the track-UV method, which generates sub-nanometer gaps for selective ion transport [83]. The membranes exhibited remarkable monovalent/divalent ion selectivity, achieving a K+/Mg2+ ratio 8900 in electrodialysis tests, with a potassium flux of 0.49 mol/h·m2. A K+/Li+ selectivity of up to 6 was also achieved. These results highlight the potential of such membranes for highly selective ion separation, desalination, and energy conversion processes.
Li et al. (2016) designed cellulose acetate membranes reinforced with polyethylene terephthalate (PET) mesh using phase inversion [84]. Various fabrication parameters were optimized to enhance water flux and minimize internal concentration polarization. The optimized forward osmosis membrane exhibited a water flux of 3.47 L/m2·h and salt rejection of 95.48%, while in pressure-retarded osmosis mode, it reached 4.74 L/m2·h with 96.03% rejection. These results confirm the potential of PET mesh-enhanced CA membranes for applications such as desalination, water purification, food processing, and pharmaceutical uses.
Gu et al. (2021) prepared poly(vinylidene fluoride) (PVDF) microfiltration membranes that were modified by directly anchoring poly(vinyl alcohol) (PVA) chains using γ-ray irradiation without additional additives [85]. The resulting membranes gained hydrophilicity and underwater superoleophobicity, leading to oil rejection rates of 99.5% and fluxes of 690 L/m2·h·bar under ultralow pressure. Fouling was found to be almost fully reversible, with flux recovery increasing from 33% in pristine PVDF to 98% in the modified version.
Zhang et al. (2021) fabricated a porous cellulose acetate monolith reinforced with carboxylated multiwalled carbon nanotubes (OMWNTs) using a green phase separation method [86]. The material exhibited superhydrophobicity (water contact angle 155°) and superoleophilicity (~0°), along with a high porosity of 93.7% and specific surface area of 85.36 m2/g. It showed strong adsorption capacities for various oils and organic solvents (7.39–19.84 g/g) and retained performance under extreme pH (1–14) and temperature (−20 to 160 °C) conditions. Figure 4 illustrates the preparation and mechanical robustness of the porous CA/OMWNTs monolith. Table 2 provides an overview of the principal materials, fabrication strategies, and key performance metrics of polymeric and composite membranes designed for oil/water separation.
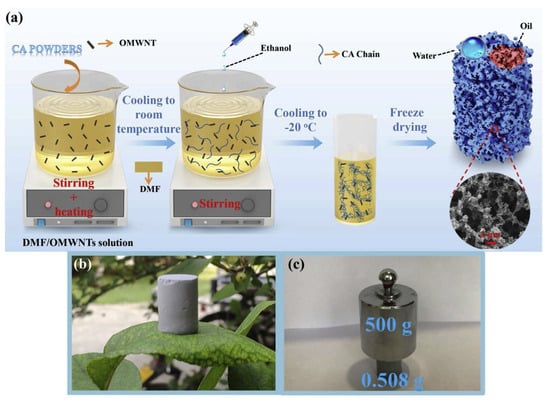
Figure 4.
(a) Schematic diagram of preparation procedure of porous CA/OMWNTs monolith; Digital images of CA/OMWNTs-1.5% monolith (b) standing on a leaf and (c) supporting weight without deformation. Reproduced with permission from [86].

Table 2.
Comparative overview of polymeric and composite membranes for oil/water separation and related applications.
Photocatalytic polymeric membranes extend the composite concept by embedding a catalytic phase (often TiO2) to combine separation with advanced oxidation. Shi et al. (2023), for example, fabricated a composite membrane by first producing a membrane from dopamine-modified PVDF powder, followed by depositing TiO2 nanoparticles via a liquid deposition method [87]. The composite membrane exhibited outstanding rejection performance for wastewater containing organic pollutants, achieving removal rates of approximately 99.0% for Congo red and Coomassie Brilliant Blue dyes, 98.2% for humic acid, and 86.7% for bovine serum albumin. Following filtration of a humic acid solution and subsequent 60 min of UV irradiation, the membrane recovered more than 87.2% of its initial water flux. Due to its favorable wetting characteristics and low underwater oil droplet adhesion, the membrane also demonstrated high rejection efficiency and excellent antifouling behavior during oil-water emulsion separation. This study indicates that photocatalytic polymeric membranes can remove a broad range of organic pollutants from wastewater and have antifouling capacity.
Yang et al. (2018) reported the fabrication of cellulose acetate (CA)-based SiO2/TiO2 hybrid microsphere composite aerogel films through a facile water vapor-induced phase inversion method combined with sol–gel reactions of tetrabutyl titanate and 3-aminopropyltrimethoxysilane [88]. The incorporation of SiO2 and TiO2 generated micro-nano hierarchical structures, imparting the films with high porosity (>76%), low density, and strong hydrophobic-oleophilic surface properties. These structural features enabled the efficient separation of surfactant-stabilized water-in-oil emulsions at ambient pressure, achieving fluxes up to 667 L/m2·h and separation efficiencies above 99.99 wt%. The films also showed excellent recyclability over multiple cycles and photocatalytic antifouling capacity under UV irradiation due to the TiO2 component, highlighting their dual functionality for emulsion separation and contaminant degradation.
Liu et al. (2015) presented the development of a pure inorganic ZnO-Co3O4 overlapped membrane via a low-cost and straightforward hydrothermal approach [89]. Constructed on a copper mesh substrate, the hierarchical micro/nano flower-like architecture provided the membrane with underwater superoleophobicity and under-oil superhydrophobicity. This dual superwetting behavior allowed gravity-driven separation of surfactant-free and surfactant-stabilized emulsions, including oil-in-water and water-in-oil systems, with oil concentrations in filtrates below 50 ppm and oil purities above 99.97%. In addition, the membrane exhibited outstanding flux (up to 300 L/m2·h depending on emulsion type), excellent antifouling resistance, and thermal stability up to 300 °C.
However, several studies have shown that their success hinges on wastewater composition (inorganics, alkalinity, DOC). Tran et al. (2022), for example, show that the presence of co-contaminants strongly modulates organic removal by altering both fouling and selectivity of PVDF-TiO2 photocatalytic membranes [90]. Most common inorganic ions did not hinder performance, but a trace amount of Cu2+ in the presence of bicarbonate (HCO3−) triggered inorganic fouling that collapsed flux and effectively shut down the membrane’s photo-induced properties. For organic matter, the membrane was highly resistant to sodium alginate but less so to humic acids. Humic acids were photo fragmented into smaller molecules that passed the membrane, lowering apparent rejection even though photo-filtration still sustained higher flux than dark filtration up to about 5 mg/L humic acid (and 50 mg/L alginate). The data indicate that specific ion pairs (e.g., Cu2+ with alkalinity) can negate photocatalytic benefits. At the same time, natural organic matter can reduce measured “removal” by converting to more permeable fragments. Thus, while photocatalytic polymeric membranes hold strong potential for simultaneous contaminant removal and fouling mitigation, their practical performance will depend on careful optimization for real wastewater conditions, including strategies to address competitive adsorption, light penetration limitations, and catalyst deactivation.
These studies show some insights regarding the application of polymeric composite materials for removing or degrading organic contaminants from wastewater. First, wastewater composition strongly influences the performance: results obtained in ultrapure or low-DOC waters often overestimate removal efficiencies for both catalytic and adsorptive composites, underscoring the need for studies on real wastewaters that also include transformation-product analysis. Second, regeneration and long-term durability are critical to practical deployment. While solvent-based regeneration protocols have been demonstrated, further work is needed to establish standardized multi-cycle procedures, monitor potential leachates (e.g., catalyst particles or polymer fragments), and assess mechanical integrity over extended use. Overall, current evidence supports polymeric composites as promising, modular add-on technologies that integrate selective adsorption or catalytic degradation into compact treatment units. Their transition to full-scale application will depend on robust demonstrations that quantify long-term performance, regeneration efficiency, and toxicity reduction under realistic wastewater conditions.
4. Removal of Inorganic Contaminants
4.1. Types of Contaminants
Inorganic pollutants in aquatic environments include heavy metals, nutrients, sediments, and industrial residues. The intensification of anthropogenic activities, particularly those linked to mining, agriculture, and related industrial operations, has accelerated the release of such contaminants into various water bodies. These pollutants are of particular concern due to their non-biodegradable nature and tendency to bioaccumulate, which leads to their persistence in ecosystems and a progressive increase in concentration [91]. As a result, they are strongly associated with severe health issues, including cancer and microbial-related diseases. Therefore, inorganic contaminants generally consist of byproducts from radiation, noise, heat, or light processes. Typical examples include heavy metals. These compounds are highly resistant to degradation and can persist for extended periods in the environment [92].
Contamination of water resources by toxic heavy metals has become a critical environmental concern. These non-biodegradable pollutants frequently enter agricultural soils and aquatic ecosystems through industrial discharges, chemical waste, and other anthropogenic activities. Given their persistence and hazardous effects, removing heavy metals from aquatic environments and ensuring their appropriate disposal is essential [93]. Water pollution by these elements is particularly concerning due to their high toxicity and strong bioconcentration potential. Therefore, effective remediation is relevant to both environmental and public health. Heavy metals accumulate in living organisms at elevated concentrations via ingestion or direct contact, posing significant risks to ecosystems and human health [94]. Despite their importance, detecting and effectively removing these contaminants remain significant challenges.
Among the most problematic metals found in polluted waters are arsenic (As), lead (Pb), chromium (Cr), cadmium (Cd), iron (Fe), and vanadium (V), all of which have been linked to severe health issues such as cancer, neurological damage, and systemic disorders, even at relatively low exposure levels in domestic water [95]. Additionally, cadmium (Cd), copper (Cu), lead (Pb), nickel (Ni), and zinc (Zn) are particularly notorious for their ability to accumulate in biological tissues, where they can trigger a wide range of diseases and dysfunctions. These metals are considered the most hazardous to environmental and human health, as they enter the body primarily through food and water consumption, leading to long-term health complications [96].
Industrial activities represent a primary source of heavy metal contamination in aquatic systems, posing significant environmental risks. The problem is exacerbated by increasing population density, intensifying water demand and pollution. Significant sources of inorganic pollutants are linked to mining, fossil fuel combustion, fertilizer application, municipal solid waste disposal, and various industrial discharges. The presence of these pollutants in water has been directly correlated with numerous diseases and comorbid health conditions. Their toxicity has been ranked as follows: Cd > Hg > Pb > Cu > Zn > Cr > Co > Fe [97]. The harmful effects of these metals extend to microbial metabolism and the nervous and cellular systems of humans and animals [98,99].
The degree of toxicity of heavy metals in water is influenced by several environmental parameters, such as pH, temperature, redox potential, and ionic composition of the medium [100]. Because these elements tend to bioaccumulate in aquatic systems, they have been extensively studied and monitored. Furthermore, their non-biodegradable nature leads to biomagnification across food chains.
In the literature, heavy metals are commonly defined as metallic or metalloid elements with a density above 4 g/cm3 [101], above 5 g/cm3 [102], or within the range of 3.5–7 g/cm3 [103], and they are considered toxic even at trace concentrations (µg/L). While heavy metals occur naturally in the environment, their levels are often exacerbated by the improper disposal of domestic, industrial, and agricultural effluents. Over recent decades, human exposure to these pollutants has intensified due to their extensive application in industrial processes [102]. Consequently, increasing urbanization and industrialization are the main drivers of heavy metal contamination in water systems.
4.2. Removal Mechanisms of Inorganic Pollutants
Different treatment methods have been developed to remove inorganic pollutants from industrial effluents, including coagulation/flocculation, ion exchange, flotation, membrane filtration, chemical precipitation, electrochemical processes, adsorption, and biological approaches. Each method presents specific advantages and drawbacks, and their applicability largely depends on the nature and concentration of the pollutant present [104].
For example, in coagulation/flocculation, heavy metals are converted into insoluble carbonates, sulfides, and hydroxides, forming colloidal particles with densities similar to water [105]. To enhance particle settling, coagulants are typically combined with flocculants, which aggregate the precipitated particles into larger, denser flocs that can be removed by filtration [104]. However, this method often results in high heavy metal content sludge, raising concerns about disposal and the extensive use of chemicals [106].
Furthermore, ion exchange relies on resins, usually composed of cross-linked polymer matrices functionalized with covalently bonded groups. Acidic resins, containing sulfonic acid groups, are generally more effective than basic resins, which incorporate carboxylic acids, for metal removal [104]. The flotation process involves the introduction of charged air bubbles into contaminated water. These bubbles attract and remove metallic ions through surface migration, making flotation effective for metals with varying chemical characteristics [107]. While this technique produces minimal sludge, its application is limited by high operational costs [104].
Membrane filtration operates primarily on the size exclusion principle and is suitable for separation via microfiltration, ultrafiltration, nanofiltration, or reverse osmosis [108,109]. This method provides high removal efficiency, compact modular systems, and ease of operation; however, its use is restricted by high installation costs, membrane fouling, and the need for periodic replacement [110].
Chemical precipitation, one of the most widely applied and cost-effective methods, relies on pH adjustment to convert dissolved metals into insoluble species. It is commonly employed to treat effluents containing Cu(II), Cd(II), Mn(II), and Zn(II) [111]. However, like coagulation/flocculation, it generates large amounts of sludge that are difficult to manage [112].
Electrochemical methods are generally applied as secondary treatments after precipitation or ion exchange. Electrons provide a versatile means of treatment by inducing coagulation, deposition, or flotation of metals through electron transfer [113]. Despite their potential, these techniques face limitations due to high energy requirements, operational costs, low mass transfer rates, and effluent heating.
Adsorption is widely regarded as one of the most efficient and environmentally sustainable approaches, offering low operational costs, limited fouling, and the possibility of adsorbent regeneration. It operates through interactions between metal ions and adsorbent functional groups, or via physical adsorption, with effectiveness largely determined by adsorbent surface area, pore size distribution, polarity, and functional groups [114,115].
Biological treatments, often used as secondary processes in activated sludge systems, represent an alternative for removing inorganic pollutants [116]. These rely on either biosorption, non-metabolic processes mediated by extracellular polymeric substances (EPS), or bioaccumulation, which involves metal uptake and transformation within microbial cells. While effective at removing metals even at high concentrations, biological systems may be inhibited by metal toxicity, which can deactivate microbial communities and limit practical application [117,118].
4.3. Reported Application Studies
Growing wastewater production has intensified the need for efficient and sustainable technologies to remove inorganic pollutants from aquatic environments. Adsorption has emerged as one of the most promising strategies, with a wide range of naturally occurring and synthetic adsorbents explored for this purpose. Nanomaterials (NMs) have gained considerable attention due to their high surface area, porosity, and chemical stability, significantly enhancing their adsorption performance. Nanoscale adsorbents exhibit faster adsorption kinetics and higher removal efficiencies than bulk materials. However, issues related to their synthesis and potential environmental impacts must still be addressed to ensure their safe large-scale application [119].
Among the advanced materials under investigation, metal–organic frameworks (MOFs) and their composites stand out due to their unique structural and catalytic properties. MOFs have been widely studied as adsorbents and photocatalysts for removing heavy metals and dyes from wastewater. More recently, carbonaceous derivatives of MOFs have been highlighted as highly effective sorbents, benefiting from large pore volume, high porosity, and excellent stability, which enhance their applicability in detoxification processes. Research has also focused on developing synthesis strategies for MOFs and their composites to optimize performance and enable multifunctional applications in water remediation [120,121].
Natural clay-based materials such as halloysite nanotubes (HNTs) offer another eco-friendly platform for water purification. Their tubular morphology, mesoporous interior, and modifiable surface make them highly suitable as supports for functional agents, including nanoparticles, polymers, surfactants, and enzymes. These functionalized HNT-based hybrids have improved adsorption capacity, catalytic activity, and selectivity toward persistent and non-persistent contaminants. While HNTs themselves are biocompatible, concerns about chemical modifiers’ environmental risks have been raised. Hence, recent research emphasizes the development of greener functionalization strategies to minimize secondary pollution and enhance the sustainability of HNT-based remediation technologies [122].
Biochar has also been widely investigated for its potential in removing emerging inorganic pollutants (EIPs) such as vanadium, antimony, thallium, mercury, fluoride, and rare earth elements. Its low cost, environmental compatibility, and adsorption efficiency make it a promising material. Recent advances have incorporated artificial intelligence tools to predict adsorption efficiency and optimize biochar design parameters, such as pyrolysis temperature and reaction pH, which strongly influence its performance. Machine learning models, particularly XGBoost, have shown high predictive accuracy, offering valuable insights for tailoring biochar properties to specific pollutants [123].
In addition, metallic nanoparticles are increasingly explored due to their large surface area, high reactivity, and adaptability. Strontium-based nanomaterials have attracted interest because of their structural similarity to magnesium and calcium, enabling their application in water treatment and biomedical fields. These materials have effectively removed heavy metals, inorganic ions, dyes, and antibiotics via degradation and adsorption. Hydrothermal and co-precipitation methods are the most widely employed synthesis techniques due to their simplicity, low toxicity, and reproducibility, further supporting the potential of Sr-based nanomaterials in future water treatment technologies [124].
Pandey et al. (2025) developed zerovalent iron (ZVI) encapsulated shellac polymer composite particles using the electrospraying technique to treat alkaline textile wastewater and oil-water separation [125]. The fabricated particles, with an average diameter of 6.7 ± 1.8 µm, achieved over 99% removal of methyl orange dye at pH values of 8, 10, and 12, maintaining stability and reusability for up to five cycles. The key innovation of this system lies in the controlled release of ZVI through the gradual dissolution of the shellac matrix under alkaline conditions, which prevents surface passivation and oxidation of the iron core. The polymer composite acts synergistically: the bio-based shellac provides a protective barrier that stabilizes the reactive metal and regulates ion release kinetics, while its amphiphilic nature enhances adsorption of both hydrophilic and hydrophobic contaminants. This combination of ZVI’s redox reactivity with the chemical versatility of the polymer matrix leads to a material that integrates high catalytic efficiency, chemical stability, and magnetic recoverability, illustrating how polymer composites enhance durability and selectivity in wastewater remediation.
Elkahlout et al. (2024) designed a polyacrylamide/silica/magnetic nanoparticle composite for the purification of produced water from oil extraction, which typically contains high levels of suspended solids and organic compounds [126]. The composite achieved removal efficiencies of 98% for turbidity, 93% for chemical oxygen demand, and 76% for total solids, demonstrating excellent stability and regenerability over multiple reuse cycles. The hybrid structure combines the hydrophilicity and flexibility of the polymer matrix, which facilitates contaminant diffusion and mass transport, with the mechanical strength and thermal stability of silica, and the magnetic recoverability provided by the iron nanoparticles. This multiphase design results in a larger active surface area, increased density of adsorption sites, and enhanced mechanical integrity, outperforming single-component materials. The improved performance arises from the synergistic interaction between the organic polymer and inorganic fillers, which stabilizes the nanoparticles, prevents aggregation, and preserves the accessibility of functional groups. Consequently, the composite exhibits higher removal efficiency, extended reusability, and reduced environmental risks, underscoring the critical role of polymer composites in developing sustainable and efficient water treatment technologies.
Yousefi et al. (2015) focused on the development of a polyaniline-based nanocomposite incorporating cobalt hexacyanoferrate (CoHCF) for the efficient removal of radioactive cesium (Cs+) from aqueous solutions [127]. The nanocomposite was synthesized through oxidative polymerization of aniline in the presence of CoHCF precursors, yielding core–shell particles with CoHCF embedded within the polyaniline matrix. Characterization by XRD, SEM, TEM, and TGA confirmed the crystalline nature of the composite, its enhanced thermal stability, and a uniform dispersion of nanoparticles. Batch adsorption experiments revealed that the composite exhibited a maximum sorption capacity of 181.8 mg/g at 60 °C, following Langmuir isotherm behavior and pseudo-second-order kinetics. The adsorption process was spontaneous and endothermic, with efficiency strongly dependent on pH, contact time, and CoHCF loading, thereby highlighting the material’s performance for cesium remediation in nuclear wastewater.
Anirudhan et al. (2010) investigated the preparation and application of a poly(methacrylic acid)-grafted chitosan/bentonite composite (PMAA-g-CTS/B) for the adsorption of thorium (Th4+) ions from aqueous and simulated seawater systems [128]. The graft copolymerization process yielded a porous, thermally stable composite with functional carboxyl groups introduced by methacrylic acid and structural reinforcement by bentonite. Extensive characterization using FTIR, XRD, XPS, SEM, TG/DTG, and surface area analysis confirmed all components’ successful synthesis and incorporation. Adsorption studies demonstrated that the composite exhibited optimal uptake at pH 5–6, with a maximum capacity of 110.5 mg/g at 30 °C, fitting well to the Langmuir isotherm model and pseudo-second-order kinetics. Moreover, regeneration tests showed stable adsorption–desorption performance across four cycles, underscoring the material’s applicability in the sustainable treatment of radioactive thorium-contaminated effluents.
Han et al. (2020) reported the design of a novel magnetic adsorbent based on trioctylamine-modified polystyrene microspheres incorporating Fe3O4 nanoparticles (Fe3O4/PS/TOA) for the selective removal of cerium (Ce3+) ions, used as a model for radioactive rare-earth elements [129]. The adsorbent was prepared by multiple permeation-deposition steps of Fe3O4 into porous PS microspheres, followed by swelling-deswelling with trioctylamine. Characterization with SEM, FTIR, TGA, and VSM confirmed the hierarchical core–shell structure, strong magnetic properties (saturation magnetization 19.78 emu/g), and stable chemical composition. Adsorption experiments revealed rapid uptake of Ce3+, with equilibrium achieved within 180 min and a maximum adsorption capacity of 39.7 mg/g, following the Langmuir isotherm and pseudo-second-order kinetics. The microspheres could be easily separated under an external magnetic field, combining high efficiency with operational simplicity and demonstrating potential for large-scale nuclear wastewater treatment. Figure 5 illustrates the synthesis route of Fe3O4/PS/TOA microabsorbents and their application in the adsorption of Ce3+ ions. The scheme depicts the permeation-deposition of Fe3O4 nanoparticles into porous polystyrene microspheres, followed by the swelling-deswelling process with trioctylamine, resulting in magnetic extractant microspheres. The final step highlights the selective adsorption of Ce3+, demonstrating the functional capability of the prepared material for radionuclide removal. Table 3 provides a comparative overview of recent advances in materials and treatment systems applied to the removal of inorganic contaminants from water.
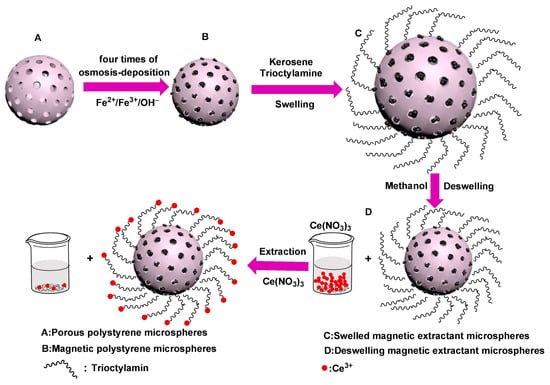
Figure 5.
Synthesis of Fe3O4/PS/TOA microabsorbents and their adsorption of Ce3+ ions. Reproduced with permission from [129].

Table 3.
Advanced materials for the removal of inorganic contaminants from water.
As shown in Table 3, the performance of these materials and the choice of the method varies depending on the contaminant type and operational conditions, underscoring the importance of treatment strategies to specific water quality challenges.
5. Removal of Biological Contaminants
Biological contaminants, such as bacteria, viruses, protozoa, and parasites, represent a significant risk to public health in water treatment systems [136]. Effective disinfection guarantees safe water for both human consumption and environmental release. Chlorination remains a conventional and widely applied method; however, its use can lead to the formation of disinfection by-products such as trihalomethanes and haloacetic acids, compounds linked to potential health risks [137].
To overcome the limitations of conventional disinfection methods, complementary treatment approaches such as membrane filtration have gained increasing attention [138,139,140]. Membrane technologies enable the efficient removal of microbial contaminants and facilitate compliance with stringent water quality standards [141]. Nevertheless, conventional polymeric membranes typically lack inherent antimicrobial properties, often leading to biofouling and consequently reducing their operational efficiency. This persistent challenge has driven recent research efforts toward developing composite membranes incorporating antimicrobial agents, thereby enhancing membrane performance and longevity [140,142]. Significant advances in antimicrobial polymers have been achieved over the past decades. These include the synthesis of novel polymeric structures and the functionalization of existing polymers with metallic nanoparticles. Such modifications endow the polymers with potent antimicrobial properties [143,144].
Despite these developments, the antimicrobial mechanisms underlying polymer action remain incompletely understood. The efficacy of antimicrobial polymers varies with polymer composition, structural attributes, and the specific target microorganism, complicating the establishment of a universal mode of action. Elucidating the molecular interactions between antimicrobial polymers and microbial membranes is challenging. This knowledge is essential for rationalizing design and optimizing materials intended for health, food safety, and environmental remediation.
Electrospun nanofiber membranes (NFMs) have shown considerable promise among innovative membrane technologies. NFMs enable precise control over pore size and distribution, parameters important for effective microorganism removal through size exclusion. Typically, bacteria found in water are larger than 0.2 μm, making microfiltration a highly effective physical barrier. For instance, polyacrylonitrile-based NFMs functionalized with cationic monomers such as 1-(1-vinylimidazolium)ethyl-3-vinylimidazolium dibromide and ethoxylated trimethylolpropane triacrylate demonstrated superior bacterial (E. coli) and viral (bacteriophage MS2) retention efficiencies of 99.9999% and 99.99%, respectively. These membranes, polymerized through potassium persulfate-induced reactions, exhibited enhanced hydrophobicity, mechanical strength, and uniform pore structure, outperforming commercial alternatives [139].
Another approach combined electrospinning with radiation-induced grafting to produce polyethersulfone (PES) NFMs functionalized with ionic liquids (PES-g-IL). These membranes displayed high adsorption capacities for bacteria, dyes, and heavy metals. They achieved nearly 99% E. coli elimination and demonstrated antimicrobial activity against S. aureus, underscoring their multifunctional potential in wastewater treatment [138]. The structural parameters of electrospun nanofibrous membranes, including fiber diameter, porosity, and thickness, directly influence microfiltration performance. A polyacrylonitrile nanofiber membrane supported on polyethylene terephthalate (PAN/PET) exhibited superior water flux and efficient E. coli removal, facilitated by an average pore size of 0.22 μm and porosity of up to 80% [145]. Figure 6 shows SEM images of the electrospun PAN/PET membrane after E. coli suspension filtration.
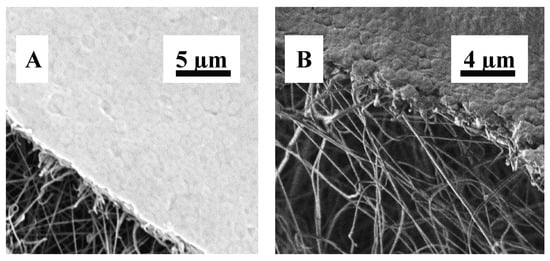
Figure 6.
SEM images of electrospun PAN/PET membrane after E. coli suspension filtration, from (A) surface and (B) cross-section views. Reproduced with permission from [145].
Multilayer filtration systems have also been innovated using electrospun poly(L-lactic acid) fibers integrated with biomass-derived graphene and silver-decorated graphene. These composite membranes removed contaminants over 87%, reduced total dissolved solids to potable levels, and exhibited excellent antibacterial performance attributable to silver nanoparticles’ high surface area and bactericidal activity [146].
Silver nanoparticles (AgNPs) are among the most widely applied antimicrobial agents in filtration membranes due to their potent bactericidal properties. However, the antimicrobial efficacy of AgNPs can be compromised by sulfidation and oxidation processes catalyzed by sulfur species commonly found in natural waters, which degrade their biofouling resistance. To address this limitation, Maharubin et al. (2019) developed an innovative system combining polysulfone membranes embedded with AgNPs and low-intensity electrofiltration technology [142]. The continuous application of an electric field using a carbon anode minimized silver oxidation, retained Ag+ ions in the aqueous medium, and prolonged antimicrobial activity. Membranes produced by wet phase inversion, containing either AgNPs or polyaniline nanofibers, were evaluated for biofilm resistance against Gram-positive (Staphylococcus aureus, ATCC 25923) and Gram-negative (Escherichia coli, ATCC 47076) bacteria. While electrical activation alone yielded limited antibiofilm effects, its integration with AgNPs significantly reduced biofilm formation, enhanced silver retention, and improved filtration performance compared to conventional membranes. This hybrid system shows high potential for sustainable biofouling control in water treatment.
In a related sustainable approach, mixed matrix polyethersulfone membranes incorporating AgNPs synthesized via green chemistry methods using Hibiscus rosa-sinensis extract have been developed for nanofiltration applications with enhanced antifouling performance. This biogenic synthesis route and advanced membrane fabrication improved permeability, selectivity, mechanical strength, hydrophilicity, and antimicrobial efficacy. These membranes also exhibited reduced protein adsorption and favorable surface charge modifications. Antifouling tests revealed that membranes containing 0.75% (w/w) AgNPs exhibited the lowest irreversible fouling and highest flow recovery, attributed to the combined antibacterial activity and optimized surface properties imparted by the nanoparticles. These findings highlight the promise of AgNPs as efficient nanofillers in fabricating high-performance, environmentally sustainable membranes for wastewater treatment [140].
Cellulose-based water filters represent a cost-effective alternative for removing particulate matter; however, size exclusion alone is insufficient for bacterial retention. To enhance antimicrobial performance, filters fabricated by layer-by-layer assembly combined polypropylene-polyethylene (PP/PE) fabrics, AgNPs, and cellulose. Functionalization of PP/PE fabrics with a higher density of AgNPs resulted in significant reductions of 96.7% and 97.9% in E. coli and S. aureus growth, respectively. Filters composed of AgNP@PP/PE layers combined with cellulose achieved inactivation efficiencies of approximately 99.4% and 98.7% for the same bacteria. The antimicrobial efficacy increased when AgNPs were synthesized at lower pH, with most microorganisms retained on the filter’s surface layers. The antimicrobial mechanism was attributed to physical adsorption and bacterial detoxification via direct contact without release of AgNPs into the water, as cellulose provided additional surface area for interaction [147].
Inorganic-organic hybrid materials such as clay-polymer composites have also demonstrated potential in water disinfection. Cationic starches modified with quaternary ammonium ethers were adsorbed onto bentonite, forming filter materials capable of reversing surface charge and enhancing bacterial adhesion and lysis. Composites containing commercial cationic starches (Topcat L-98 and L-95) achieved bacterial adsorption efficiencies greater than 93%, and cell death rates exceeding 85%. Compared to activated carbon, these composites exhibited superior microbial removal efficiency even after filtering multiple liters of water, making them competitive candidates for disinfection [148]. Additionally, polyethylene glycol-functionalized attapulgite composites embedded with green-synthesized silver nanoparticles were engineered to simultaneously remove micropollutants and bacteria such as Pseudomonas aeruginosa and Bacillus subtilis. These composites achieved near-complete microbial elimination without detectable silver release, conforming to WHO and Environmental Protection Agency safety standards [149].
Other nanocomposite membranes have further enhanced antibacterial properties. Polyvinylidene fluoride membranes modified with 4% TiO2 nanoparticles achieved the highest E. coli elimination among tested compositions, illustrating TiO2’s efficacy as an antimicrobial additive [150]. Similarly, polysulfone ultrafiltration membranes with silver nanoparticles inhibited biofilm formation and demonstrated good bactericidal activity against E. coli K12, Pseudomonas mendocina KR1, and MS2 bacteriophage. Their antimicrobial effect was primarily attributed to the sustained release of Ag+ ions [151].
Zinc oxide (ZnO) is another material with antibacterial properties that enhances membrane performance. Polysulfone ultrafiltration membranes coated with chitosan-ZnO composites were designed to improve selectivity and impart antifungal characteristics. Though less studied than bacterial fouling, fungal biofouling poses challenges in intermittently operated wastewater treatment systems where fungal growth is favored. Coatings with varying ZnO concentrations (0.1% to 0.4%) dispersed in chitosan increased antifungal activity against Saccharomyces cerevisiae, correlating positively with ZnO content [152].
Addressing the challenge of integrating disinfection with contaminant adsorption, multifunctional films composed of chitosan, zinc ions (Zn2+), and polyoxometalate were synthesized by freeze-drying. These films demonstrated broad-spectrum antibacterial activity against both Gram-positive (S. aureus, 99.80%) and Gram-negative (E. coli, 99.82%) bacteria, including antibiotic-resistant E. coli strains resistant to kanamycin (87.76%) and ampicillin (99.71%). The films’ efficacy was attributed to Zn2+ chelation with chitosan, which compromised bacterial membrane integrity and facilitated direct polyoxometalate contact, enhancing cell death. Furthermore, the films efficiently adsorbed tetracycline (75.2% adsorption rate) and maintained antimicrobial effectiveness after three reuse cycles. The addition of polyoxometalate amplified bactericidal properties through membrane destruction and reactive oxygen species generation, positioning these films as promising candidates for integrated wastewater treatment [153].
Multifunctional composite spheres combining copper-alginate matrices with AgNPs were also developed. The spheres, obtained by crosslinking alginate with varying Cu2+ concentrations (2%, 4%, and 8%), modification with Ag+, chemical reduction with NaBH4, and lyophilization, exhibited porous structures with well-dispersed AgNPs. The concentration of Cu2+ influenced AgNP content, catalytic activity, and antimicrobial efficacy. Antimicrobial and antifungal assays showed high efficacies for all materials, with enhanced activity in samples containing unreduced Ag+. These results underscore the importance of crosslinking control in optimizing composite functional properties [154].
In summary, integrating nanotechnology, polymer science, and surface engineering has propelled significant advancements in developing antimicrobial filtration membranes for wastewater treatment. Innovations in membrane composition and architecture, particularly through incorporating metal nanoparticles, ionic liquids, and natural or synthetic polymers, have enhanced microbial removal efficiency while mitigating challenges such as biofouling and membrane stability. Although many materials exhibit high antibacterial efficacy and adsorption capacity, a comprehensive understanding of their mechanisms of action remains essential to optimizing design and performance. Future research efforts should prioritize mechanistic studies, safety evaluations, and long-term performance assessments to enable large-scale implementation of these advanced materials in water treatment systems. Bridging these knowledge gaps and advancing material science will safeguard public health and ensure sustainable access to clean water.
6. Advantages and Limitations
Polymeric composites have emerged as promising materials for contaminant remediation owing to their structural versatility and multifunctionality [155]. Their main advantage lies in combining the intrinsic properties of polymers, such as flexibility, processability, and stability, with the functional features of fillers including carbon-based nanomaterials, clays, metallic oxides, and bio-based residues [156]. This synergy enables the development of materials with superior adsorption capacity, catalytic activity, and mechanical robustness, which are often difficult to achieve with single-component systems. Moreover, polymer matrices can be chemically modified or functionalized to promote selective interactions with specific pollutants, such as dyes, pharmaceuticals, and endocrine disruptors [157]. Despite these advantages, the environmental and health implications of nanostructured fillers such as AgNPs, TiO2, and metal–organic frameworks (MOFs) require careful consideration [158]. While these nanomaterials enhance membrane performance by providing antimicrobial activity, photocatalytic properties, and improved selectivity, their potential release into aquatic environments raises concerns regarding ecotoxicity, bioaccumulation, and long-term health effects. AgNPs may induce cytotoxicity and microbial resistance, TiO2 nanoparticles can generate reactive oxygen species under irradiation, and certain MOFs may leach toxic metal ions. Therefore, future developments in polymeric composites should balance performance improvements with rigorous evaluation of environmental safety, life-cycle impacts, and sustainable disposal strategies, ensuring that technological advances align with environmental protection and human health preservation [159].
From a sustainability standpoint, incorporating agro-industrial residues or natural fibers into polymeric matrices improves performance and contributes to the circular economy by reducing waste and valorizing renewable resources. Polymeric composites also present advantages in regeneration and reusability, as their structure can often withstand multiple cycles without significant loss of efficiency [160]. Moreover, the ability to design composites in different morphologies, membranes, beads, films, or aerogels broadens their applicability in batch and continuous flow systems [161].
Despite these benefits, important limitations remain regarding the practical deployment of polymer-based composites. The scalability of synthesis routes is still a significant challenge, especially when nanomaterials, multi-step functionalization, or costly precursors are required, which can hinder reproducibility and industrial adoption. In addition, questions about long-term stability are critical: under real environmental conditions, factors such as pH fluctuations, high ionic strength, and the presence of co-contaminants can reduce adsorption efficiency and compromise the integrity of polymer–filler interfaces. Over-extended use, polymer matrices may undergo physical aging, chemical degradation, or leaching of additives, potentially leading to the release of microplastics or toxic by-products. Regeneration processes also raise concerns, as they frequently demand aggressive chemical agents or energy-intensive treatments, which degrade material performance over cycles and generate secondary waste streams [162].
In addition to these factors, polymer composites’ regenerability, stability, cost, and environmental risks deserve particular attention. Many materials exhibit high removal efficiency under laboratory conditions but show limited regeneration capability and progressive deterioration after multiple reuse cycles. Chemical instability and mechanical fatigue can compromise long-term operation, while complex synthesis routes and high-cost precursors restrict large-scale production [163]. Furthermore, the potential leaching of nanofillers or degradation by-products introduces additional environmental risks, highlighting the need for greener synthesis routes, recyclable matrices, and standardized protocols to evaluate durability and safety [164]. Addressing these aspects is essential to advance the practical, economical, and sustainable implementation of polymer composites in real water treatment scenarios.
Finally, economic feasibility remains uncertain, since scaling up high-performance composites often entails higher costs than conventional sorbents such as activated carbon, making techno-economic analyses essential for future applications [165].
7. Future Trends and Research Perspectives
Future research in polymeric composites for contaminant remediation is expected to prioritize the design of multifunctional and sustainable systems. A key direction is the development of hybrid polymeric composites that combine synthetic polymers with bio-based fillers, nanostructured oxides, or carbon nanomaterials to achieve synergistic effects in adsorption, sensing, and catalytic degradation. For instance, composites integrating photocatalytic nanoparticles into polymer membranes may simultaneously separate and degrade organic pollutants under solar irradiation [166]. At the same time, bio-based hybrids such as chitosan-graphene or cellulose-metal oxide networks offer biodegradable and renewable alternatives.
Another emerging avenue involves the incorporation of stimuli-responsive polymers, capable of dynamically adjusting their structure or surface chemistry in response to pH, temperature, or light, thereby enhancing adaptability under variable environmental conditions [167]. Multifunctional systems that combine contaminant detection and removal within a single platform are also gaining momentum, enabling real-time monitoring alongside remediation. In parallel, the valorization of industrial and agricultural by-products as fillers in polymer matrices will align composite development with circular economy and sustainability goals [162].
On the technological front, advanced manufacturing techniques such as 3D and 4D printing are poised to revolutionize the fabrication of polymeric composites, enabling precise control of porosity, morphology, and hierarchical structures, which directly influence transport and adsorption mechanisms (Figure 7) [167]. In addition, coupling composites with renewable energy inputs in advanced oxidation processes (e.g., photocatalysis, electrocatalysis) may reduce energy demand and minimize the carbon footprint. Importantly, AI-assisted design and digital tools, including machine learning, molecular modeling, and data-driven screening, are expected to guide the rational selection of fillers, functional groups, and structural configurations, accelerating discovery and optimization. Finally, interdisciplinary approaches bridging environmental engineering, polymer chemistry, and material science will be essential to ensure efficiency, scalability, long-term stability, and economic viability of polymeric composite-based remediation technologies [159,166].
Figure 7.
Comparison between 3D and 4D printing techniques. Reproduced with permission from [168].
8. Conclusions
Polymeric composites represent a rapidly evolving class of materials with great potential for remediating organic, inorganic, and biological contaminants. Their tunable structures, multifunctionality, and ability to integrate a wide range of fillers make them superior to many conventional adsorbents and catalysts. Advances in developing biopolymer-based composites, nanostructured hybrids, and membrane-supported systems highlight their versatility for tackling persistent pollutants such as dyes, pharmaceuticals, and pesticides.
However, despite their remarkable laboratory-scale performance, several challenges persist. Issues such as synthesis scalability, material stability under realistic conditions, regeneration costs, and possible release of secondary pollutants must be addressed to ensure safe and effective deployment. Moreover, standardized methodologies for evaluating polymeric composites’ performance and environmental impact are still lacking, limiting direct comparison between studies.
Looking ahead, sustainable and multifunctional composites that integrate renewable fillers, green synthesis routes, and energy-efficient processes are expected to dominate the next generation of technologies. Polymeric composites’ success in environmental remediation will depend on their ability to balance technical performance, economic feasibility, and ecological safety. Ultimately, the convergence of polymer science, nanotechnology, and environmental engineering, coupled with strong collaboration between academia, industry, and policymakers, will be decisive in translating scientific advances into practical large-scale applications. In short, more studies should focus on
- •
- Scalability and standardization, aiming to develop cost-effective synthesis routes and establish unified methodologies for performance and environmental assessment.
- •
- Long-term stability and safety, evaluating the durability of polymer–filler interfaces under realistic conditions and the potential release of secondary pollutants.
- •
- Sustainable design, integrating renewable fillers, bio-based polymers, and green synthesis strategies to minimize ecological impact.
- •
- Multifunctional systems, coupling adsorption, sensing, and catalytic degradation within single platforms to enhance versatility.
- •
- AI-assisted and data-driven approaches, using machine learning, molecular modeling, and digital screening to accelerate the discovery and optimization of advanced composites.
- •
- Interdisciplinary collaboration.
Author Contributions
Conceptualization, C.R.S.d.O. and A.H.d.S.J.; methodology, C.R.S.d.O., J.M., É.F.R., C.E.D.O., R.S. and A.H.d.S.J.; validation, R.F.M., L.d.S. and A.d.S.; investigation, C.R.S.d.O., J.M., É.F.R., C.E.D.O., R.S. and A.H.d.S.J.; data curation, R.F.M., L.d.S. and A.d.S.; writing—original draft preparation, C.R.S.d.O., J.M., É.F.R., C.E.D.O., R.S. and A.H.d.S.J.; writing—review and editing, A.H.d.S.J., R.F.M., L.d.S. and A.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kubiak, A.; Stachowiak, M.; Cegłowski, M. Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review. Polymers 2023, 15, 4152. [Google Scholar] [CrossRef]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; da Silva, L.J.S.; da Silva, L.; Ulson de Souza, A.A.; de Souza, S.M.; de, A.G.U.; Borba, F.H.; da Silva, A. A Comprehensive Guide for Characterization of Adsorbent Materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Gómez Castaño, J.A.; Cifuentes, G.R. Evaluation of Turbidity and Color Removal in Water Treatment: A Comparative Study between Opuntia Ficus-Indica Fruit Peel Mucilage and FeCl3. Polymers 2023, 15, 217. [Google Scholar] [CrossRef]
- da Silva Júnior, A.H.; Mulinari, J.; de Oliveira, P.V.; de Oliveira, C.R.S.; Reichert Júnior, F.W. Impacts of Metallic Nanoparticles Application on the Agricultural Soils Microbiota. J. Hazard. Mater. Adv. 2022, 7, 100103. [Google Scholar] [CrossRef]
- Zhakina, A.K.; Muldakhmetov, Z.; Zhivotova, T.S.; Rakhimova, B.B.; Vassilets, Y.P.; Arnt, O.V.; Alzhankyzy, A.; Zhakin, A.M. Synthesis of a Polymer Composite Based on a Modified Aminohumic Acid Tuned to a Sorbed Copper Ion. Polymers 2023, 15, 1346. [Google Scholar] [CrossRef]
- da Silva Júnior, A.H.; de Oliveira, C.R.S.; Leal, T.W.; Mapossa, A.B.; Fiates, J.; Ulson de Souza, A.A.; Ulson de Souza, S.M.d.A.G.; da Silva, A. Organochlorine Pesticides Remediation Techniques: Technological Perspective and Opportunities. J. Hazard. Mater. Lett. 2024, 5, 100098. [Google Scholar] [CrossRef]
- da Silva Júnior, A.; de Oliveira, C.; Wolff Leal, T.; Pellenz, L.; de Souza, S.; de Souza, A.; Mapossa, A.; Tewo, R.; Rutto, H.; da Silva, L.; et al. Reviewing Perovskite Oxide-Based Materials for the Effective Treatment of Antibiotic-Polluted Environments: Challenges, Trends, and New Insights. Surfaces 2024, 7, 54–78. [Google Scholar] [CrossRef]
- da Silva Júnior, A.H.; de Oliveira, C.R.S.; Pellenz, L.; Moraes, P.A.D.; Marques, W.P.; Mazur, L.P.; Costa, T.G.; Horn Jr, A.; Guelli Ulson de Souza, S.M.d.A.; de Souza, A.A.U.; et al. Perovskite-Type Strontium Ferrite-Based Catalyst: Characterization and Antibiotic Degradation Approach. Process Saf. Environ. Prot. 2024, 187, 1403–1421. [Google Scholar] [CrossRef]
- da Silva Júnior, A.H.; de Oliveira, C.R.S.; Moraes, P.A.D.; Pellenz, L.; Guelli Ulson de Souza, S.M.d.A.; de Souza, A.A.U.; da Silva, L.; da Silva, A. Perovskite-Type Catalyst for Tetracycline Abatement under Dark Ambient over a Wide PH Range. J. Sol-Gel Sci. Technol. 2024, 110, 1–13. [Google Scholar] [CrossRef]
- de Medeiros Lima, S.V.; da Silva Júnior, A.H.; Quadri, M.B.; da Silva, A. Exploring a Perovskite-Type Catalyst for Diclofenac Photodegradation: A Comparative Investigation with TiO2. Water Air Soil Pollut. 2024, 235, 700. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Liu, T.; Chu, B. Cost-Effective Polymer-Based Membranes for Drinking Water Purification. Giant 2022, 10, 100099. [Google Scholar] [CrossRef]
- Malyar, Y.N.; Borovkova, V.S.; Kazachenko, A.S.; Fetisova, O.Y.; Skripnikov, A.M.; Sychev, V.V.; Taran, O.P. Preparation and Characterization of Di- and Tricarboxylic Acids-Modified Arabinogalactan Plasticized Composite Films. Polymers 2023, 15, 1999. [Google Scholar] [CrossRef]
- Saravanan, A.; Thamarai, P.; Kumar, P.S.; Rangasamy, G. Recent Advances in Polymer Composite, Extraction, and Their Application for Wastewater Treatment: A Review. Chemosphere 2022, 308, 136368. [Google Scholar] [CrossRef]
- Simelane, N.P.; Asante, J.K.O.; Ndibewu, P.P.; Mramba, A.S.; Sibali, L.L. Biopolymer Composites for Removal of Toxic Organic Compounds in Pharmaceutical Effluents—A Review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100239. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Gaona, S.; Ramón, J.; Valarezo, E. Porous Geopolymer/ZnTiO3/TiO2 Composite for Adsorption and Photocatalytic Degradation of Methylene Blue Dye. Polymers 2023, 15, 2697. [Google Scholar] [CrossRef] [PubMed]
- Neblea, I.E.; Chiriac, A.L.; Zaharia, A.; Sarbu, A.; Teodorescu, M.; Miron, A.; Paruch, L.; Paruch, A.M.; Olaru, A.G.; Iordache, T.V. Introducing Semi-Interpenetrating Networks of Chitosan and Ammonium-Quaternary Polymers for the Effective Removal of Waterborne Pathogens from Wastewaters. Polymers 2023, 15, 1091. [Google Scholar] [CrossRef] [PubMed]
- Mapossa, A.B.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Mhike, W. Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review. Polymers 2023, 15, 3443. [Google Scholar] [CrossRef] [PubMed]
- Aguilera Flores, M.M.; Valdivia Cabral, G.I.; Medellín Castillo, N.A.; Ávila Vázquez, V.; Sánchez Mata, O.; García Torres, J. Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater. Polymers 2022, 15, 37. [Google Scholar] [CrossRef]
- Podaru, I.A.; Stănescu, P.O.; Ginghină, R.; Stoleriu, Ş.; Trică, B.; Şomoghi, R.; Teodorescu, M. Poly(N-Vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels. Polymers 2022, 14, 4216. [Google Scholar] [CrossRef]
- Ortega, D.E.; Cortés-Arriagada, D.; Araya-Hermosilla, R. Computational Insights on the Chemical Reactivity of Functionalized and Crosslinked Polyketones to Cu2+ Ion for Wastewater Treatment. Polymers 2023, 15, 3157. [Google Scholar] [CrossRef]
- Lebedeva, O.V.; Sipkina, E.I. Polymer Composites and Their Properties. Proc. Univ. Appl. Chem. Biotechnol. 2022, 12, 192–207. [Google Scholar] [CrossRef]
- Wu, C.; Xu, F.; Wang, H.; Liu, H.; Yan, F.; Ma, C. Manufacturing Technologies of Polymer Composites—A Review. Polymers 2023, 15, 712. [Google Scholar] [CrossRef]
- Chohan, J.S.; Boparai, K.S.; Singh, R.; Hashmi, M.S. Manufacturing Techniques and Applications of Polymer Matrix Composites: A Brief Review. Adv. Mater. Process. Technol. 2022, 8, 884–894. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Ali, N.H.; Shihab, S.K.; Mohamed, M.T. Mechanical and Physical Characteristics of Hybrid Particles/Fibers-Polymer Composites: A Review. Mater. Today Proc. 2022, 62, 178–183. [Google Scholar] [CrossRef]
- Karim, M.R.A.; Tahir, D.; Haq, E.U.; Hussain, A.; Malik, M.S. Natural Fibres as Promising Environmental-Friendly Reinforcements for Polymer Composites. Polym. Polym. Compos. 2021, 29, 277–300. [Google Scholar] [CrossRef]
- Waghmare, S.; Shelare, S.; Aglawe, K.; Khope, P. A Mini Review on Fibre Reinforced Polymer Composites. Mater. Today Proc. 2022, 54, 682–689. [Google Scholar] [CrossRef]
- Bou Abdallah, G.; Ivanova, I.; Assih, J.; Diagana, C.; Dontchev, D. Composite Materials Based on Natural Fibres Applied for Structural Reinforcement. IOP Conf. Ser. Earth Environ. Sci. 2022, 960, 012007. [Google Scholar] [CrossRef]
- Asim, M.; Jawaid, M.; Saba, N.; Ramengmawii; Nasir, M.; Sultan, M.T.H. Processing of Hybrid Polymer Composites—A Review. In Hybrid Polymer Composite Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. ISBN 9780081007907. [Google Scholar]
- Al-Khazraji, M.S.; Bakhy, S.H.; Jweeg, M.J. Composite Sandwich Structures: Review of Manufacturing Techniques. J. Eng. Des. Technol. 2024, 22, 1616–1636. [Google Scholar] [CrossRef]
- Kashfipour, M.A.; Mehra, N.; Zhu, J. A Review on the Role of Interface in Mechanical, Thermal, and Electrical Properties of Polymer Composites. Adv. Compos. Hybrid Mater. 2018, 1, 415–439. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chen, Y.-J.; Chen, Y. Acid-Functionalized Porous Polymer as an Adsorbent for the Highly Efficient Removal of Ammonia Nitrogen from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2026, 178, 106390. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Zamora-Ledezma, C.; Chuya-Sumba, C.; De Sousa, F.B.; Whitehead, D.; Alexis, F.; Guerrero, V.H. Persistent Organic Pollutants: The Trade-off between Potential Risks and Sustainable Remediation Methods. J. Environ. Manag. 2021, 300, 113737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, S.; Song, W.; Wang, X.; Ding, J.; Lu, J. The Degradation of Dissolved Organic Matter in Black and Odorous Water by Humic Substance-Mediated Fe(II)/Fe(III) Cycle under Redox Fluctuation. J. Environ. Manag. 2022, 321, 115942. [Google Scholar] [CrossRef]
- Devlin, M.; Brodie, J. Nutrients and Eutrophication. Geogr. Environ. 2023, 75–100. [Google Scholar] [CrossRef]
- Meléndez-Plata, G.; Mastrogiacomo, J.R.A.; Castellanos, M.L.; Romero, J.P.; Hincapié, V.; Lizcano, H.; Acero, J.D.; Villegas-Torres, M.F.; Gómez, J.M.; Cruz, J.C.; et al. Malodorous Gases in Aquatic Environments: A Comprehensive Review from Microbial Origin to Detection and Removal Techniques. Processes 2025, 13, 1077. [Google Scholar] [CrossRef]
- Toledo, M.; Muñoz, R. Odour Prevention Strategies in Wastewater Treatment and Composting Plants: A Review. J. Environ. Manag. 2025, 375, 124402. [Google Scholar] [CrossRef] [PubMed]
- Valta, K.; Kosanovic, T.; Malamis, D.; Moustakas, K.; Loizidou, M. Overview of Water Usage and Wastewater Management in the Food and Beverage Industry. Desalin. Water Treat. 2015, 53, 3335–3347. [Google Scholar] [CrossRef]
- Kumar, L.; Chugh, M.; Kumar, S.; Kumar, K.; Sharma, J.; Bharadvaja, N. Remediation of Petrorefinery Wastewater Contaminants: A Review on Physicochemical and Bioremediation Strategies. Process Saf. Environ. Prot. 2022, 159, 362–375. [Google Scholar] [CrossRef]
- Schaider, L.A.; Rodgers, K.M.; Rudel, R.A. Review of Organic Wastewater Compound Concentrations and Removal in Onsite Wastewater Treatment Systems. Environ. Sci. Technol. 2017, 51, 7304–7317. [Google Scholar] [CrossRef]
- Shrivastava, V.; Ali, I.; Marjub, M.M.; Rene, E.R.; Soto, A.M.F. Wastewater in the Food Industry: Treatment Technologies and Reuse Potential. Chemosphere 2022, 293, 133553. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Islam, M.M.; Aidid, A.R.; Mohshin, J.N.; Mondal, H.; Ganguli, S.; Chakraborty, A.K. A Critical Review on Textile Dye-Containing Wastewater: Ecotoxicity, Health Risks, and Remediation Strategies for Environmental Safety. Clean. Chem. Eng. 2025, 11, 100165. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Ghimire, N.; Wang, S. Biological Treatment of Petrochemical Wastewater. In Petroleum Chemicals—Recent Insight; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-83880-402-2. [Google Scholar]
- Tian, X.; Song, Y.; Shen, Z.; Zhou, Y.; Wang, K.; Jin, X.; Han, Z.; Liu, T. A Comprehensive Review on Toxic Petrochemical Wastewater Pretreatment and Advanced Treatment. J. Clean. Prod. 2020, 245, 118692. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Yin, L.; Wang, R.; Li, P.; Wang, J.; Liu, K. Sustainable Utilization of Pulp and Paper Wastewater. Water 2023, 15, 4135. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Chandra, R. Environmental Pollutants of Paper Industry Wastewater and Their Toxic Effects on Human Health and Ecosystem. Bioresour. Technol. Rep. 2022, 20, 101250. [Google Scholar] [CrossRef]
- Šabić Runjavec, M.; Vuković Domanovac, M.; Meštrović, E. Removal of Organic Pollutants from Real Pharmaceutical Industrial Wastewater with Environmentally Friendly Processes. Chem. Pap. 2022, 76, 1423–1431. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Yabalak, E. Treatment of Agrochemical Wastewater by Thermally Activated Persulfate Oxidation Method: Evaluation of Energy and Reagent Consumption. J. Environ. Chem. Eng. 2021, 9, 105201. [Google Scholar] [CrossRef]
- Baumert, B.O.; Goodrich, J.A.; Hu, X.; Walker, D.I.; Alderete, T.L.; Chen, Z.; Valvi, D.; Rock, S.; Berhane, K.; Gilliland, F.D.; et al. Plasma Concentrations of Lipophilic Persistent Organic Pollutants and Glucose Homeostasis in Youth Populations. Environ. Res. 2022, 212, 113296. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Li, D.; Breivik, K.; Abbasi, G.; Li, Y.-F. What Do We Know about the Production and Release of Persistent Organic Pollutants in the Global Environment? Environ. Sci. Adv. 2023, 2, 55–68. [Google Scholar] [CrossRef]
- Ashraf, M.A. Persistent Organic Pollutants (POPs): A Global Issue, a Global Challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive Review of Industrial Wastewater Treatment Techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Lucas, M.S.; Teixeira, A.R.; Jorge, N.; Peres, J.A. Industrial Wastewater Treatment by Coagulation–Flocculation and Advanced Oxidation Processes: A Review. Water 2025, 17, 1934. [Google Scholar] [CrossRef]
- Chandran, P.; Suresh, S.; Balasubramain, B.; Gangwar, J.; Raj, A.S.; Aarathy, U.L.; Meyyazhagan, A.; Pappuswamy, M.; Sebastian, J.K. Biological Treatment Solutions Using Bioreactors for Environmental Contaminants from Industrial Waste Water. J. Umm Al-Qura Univ. Appl. Sci. 2023, 11, 185–207. [Google Scholar] [CrossRef]
- Cesaro, A.; Naddeo, V.; Belgiorno, V. Wastewater Treatment by Combination of Advanced Oxidation Processes and Conventional Biological Systems. J. Bioremediat. Biodegrad. 2013, 4, 208. [Google Scholar] [CrossRef]
- Choubert, J.M.; Martin Ruel, S.; Esperanza, M.; Budzinski, H.; Miège, C.; Lagarrigue, C.; Coquery, M. Limiting the Emissions of Micro-Pollutants: What Efficiency Can We Expect from Wastewater Treatment Plants? Water Sci. Technol. 2011, 63, 57–65. [Google Scholar] [CrossRef]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of Micropollutants in Municipal Wastewater: Ozone or Powdered Activated Carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Martin Ruel, S.; Esperanza, M.; Choubert, J.-M.; Valor, I.; Budzinski, H.; Coquery, M. On-Site Evaluation of the Efficiency of Conventional and Advanced Secondary Processes for the Removal of 60 Organic Micropollutants. Water Sci. Technol. 2010, 62, 2970–2978. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a Full-Scale Wastewater Treatment Plant Upgraded with Ozonation and Biological Post-Treatments: Abatement of Micropollutants, Formation of Transformation Products and Oxidation by-Products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef]
- Boehler, M.; Zwickenpflug, B.; Hollender, J.; Ternes, T.; Joss, A.; Siegrist, H. Removal of Micropollutants in Municipal Wastewater Treatment Plants by Powder-Activated Carbon. Water Sci. Technol. 2012, 66, 2115–2121. [Google Scholar] [CrossRef]
- Siegrist, H.; Joss, A. Review on the Fate of Organic Micropollutants in Wastewater Treatment and Water Reuse with Membranes. Water Sci. Technol. 2012, 66, 1369–1376. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Baresel, C.; Salem, M.; Roberts, R.; Malovanyy, A.; Lemström, H.; Esfahani, B. Approaching Breakthrough: Resource-Efficient Micropollutant Removal with MBR-GAC Configuration. Appl. Sci. 2024, 14, 7759. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Fång, J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710. [Google Scholar] [CrossRef]
- Naseem, K. Polymer-Based Composites for Wastewater Treatment. In Sodium Alginate-Based Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–159. ISBN 9780128235515. [Google Scholar]
- Sodha, V.; Patel, J.; Jha, S.; Parmar, M.; Gaur, R.; Shahabuddin, S. Polymer-Based Hybrid Composites for Wastewater Treatment. In Multifunctional Hybrid Semiconductor Photocatalyst Nanomaterials; Springer: Cham, Switzerland, 2023; pp. 349–389. ISBN 978-3-031-39481-2. [Google Scholar]
- Bhatt, P.; Joshi, S.; Urper Bayram, G.M.; Khati, P.; Simsek, H. Developments and Application of Chitosan-Based Adsorbents for Wastewater Treatments. Environ. Res. 2023, 226, 115530. [Google Scholar] [CrossRef]
- Khan, F.; Zahid, M.; Hanif, M.A.; Tabasum, A.; Mushtaq, F.; Noreen, S.; Mansha, A. Photocatalytic Polymeric Composites for Wastewater Treatment. In Aquananotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 467–490. ISBN 9780128211410. [Google Scholar]
- Cheng, L.; Zhou, Z.; Li, L.; Xiao, P.; Ma, Y.; Liu, F.; Li, J. PVDF/MOFs Mixed Matrix Ultrafiltration Membrane for Efficient Water Treatment. Front. Chem. 2022, 10, 985750, Corrected in Front. Chem. 2022, 10, 1067578. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Cevik, E.; Mustafa, A.; Qahtan, T.F.; Zeeshan, M.; Bozkurt, A. Emerging Developments in Polymeric Nanocomposite Membrane-Based Filtration for Water Purification: A Concise Overview of Toxic Metal Removal. Chem. Eng. J. 2024, 481, 148760. [Google Scholar] [CrossRef]
- Wang, J.; Sgarzi, M.; Němečková, Z.; Henych, J.; Licciardello, N.; Cuniberti, G. Reusable and Antibacterial Polymer-Based Nanocomposites for the Adsorption of Dyes and the Visible-Light-Driven Photocatalytic Degradation of Antibiotics. Glob. Chall. 2022, 6, 2200076. [Google Scholar] [CrossRef]
- Naumova, L.; Minakova, T.; Gorlenko, N.; Kurzina, I.; Vasenina, I. Oxidative Destruction of Organic Pollutants on the Polypropylene Fiber Modified by Nanodispersed Iron. Environments 2018, 5, 82. [Google Scholar] [CrossRef]
- Liang, L.; Ji, L.; Ma, Z.; Ren, Y.; Zhou, S.; Long, X.; Cao, C. Application of Photo-Fenton-Membrane Technology in Wastewater Treatment: A Review. Membranes 2023, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Uebele, S.; Goetz, T.; Ulbricht, M.; Schiestel, T. Mixed-Matrix Membrane Adsorbers for the Simultaneous Removal of Different Pharmaceutical Micropollutants from Water. ACS Appl. Polym. Mater. 2022, 4, 1705–1716. [Google Scholar] [CrossRef]
- Marx, J.; Back, J.; Hoiss, L.; Hofer, M.; Pham, T.; Spruck, M. Chemical Regeneration of Mixed-Matrix Membranes for Micropollutant Removal from Wastewater. Chem. Ing. Tech. 2023, 95, 1416–1427. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Liang, Z.; Zhang, Z.; Xie, J.; Gui, X.; Hou, B.; Mo, D.; Lu, L.; Yao, H. Hydrophobic Modified PET Ion Track-Etched Membrane for Oil/Water Separation. J. Water Process Eng. 2023, 54, 103997. [Google Scholar] [CrossRef]
- Zhao, J.; Han, P.; Quan, Q.; Shan, Y.; Zhang, T.; Wang, J.; Xiao, C. A Convenient Oil-Water Separator from Polybutylmethacrylate/Graphene-Deposited Polyethylene Terephthalate Nonwoven Fabricated by a Facile Coating Method. Prog. Org. Coat. 2018, 115, 181–187. [Google Scholar] [CrossRef]
- Wang, W.; Lin, J.; Cheng, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Dual Super-Amphiphilic Modified Cellulose Acetate Nanofiber Membranes with Highly Efficient Oil/Water Separation and Excellent Antifouling Properties. J. Hazard. Mater. 2020, 385, 121582. [Google Scholar] [CrossRef]
- Guo, Z.; Li, F.; Wu, X.; Liang, Z.; Junaid, M.; Xie, J.; Lu, L.; Duan, J.; Liu, J.; Yao, H. Efficient Ion Sieving and Ion Transport Properties in Sub-Nanoporous Polyetherimide Membranes. Desalination 2024, 573, 117192. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Hou, D.; Bai, Y.; Liu, H. Fabrication and Performance of PET Mesh Enhanced Cellulose Acetate Membranes for Forward Osmosis. J. Environ. Sci. 2016, 45, 7–17. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, B.; Fu, Z.; Li, J.; Yu, M.; Li, L.; Li, J. Poly (Vinyl Alcohol) Modification of Poly(Vinylidene Fluoride) Microfiltration Membranes for Oil/Water Emulsion Separation via an Unconventional Radiation Method. J. Memb. Sci. 2021, 619, 118792. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Wang, B.; Feng, Y.; Han, W.; Liu, C.; Shen, C. Superhydrophobic Cellulose Acetate/Multiwalled Carbon Nanotube Monolith with Fiber Cluster Network for Selective Oil/Water Separation. Carbohydr. Polym. 2021, 259, 117750. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, X.; Wu, Q.; Zhen, H.; Wang, S.; Dong, H.; Wang, J.; Li, Y. Enhance Organic Pollutants Removal of Wastewater by a PVDF/PDA-TiO2 Composite Membrane with Photocatalytic Property. J. Environ. Chem. Eng. 2023, 11, 110389. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.; Ling, J.; Li, N.; Wang, D.; Yue, F.; Xu, S. Cellulose Acetate-Based SiO2/TiO2 Hybrid Microsphere Composite Aerogel Films for Water-in-Oil Emulsion Separation. Appl. Surf. Sci. 2018, 435, 609–616. [Google Scholar] [CrossRef]
- Liu, N.; Lin, X.; Zhang, W.; Cao, Y.; Chen, Y.; Feng, L.; Wei, Y. A Pure Inorganic ZnO-Co3O4 Overlapped Membrane for Efficient Oil/Water Emulsions Separation. Sci. Rep. 2015, 5, 9688. [Google Scholar] [CrossRef]
- Tran, D.-T.; Mendret, J.; Méricq, J.-P.; Faur, C.; Brosillon, S. Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components. Membranes 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, M.; Sondhi, S.; Kaur, K.; Sharma, D.; Sharma, S.; Utreja, D. Removal of Inorganic Pollutants from Wastewater: Innovative Technologies and Toxicity Assessment. Sustainability 2023, 15, 16376. [Google Scholar] [CrossRef]
- Popiolski, A.S.; Demaman Oro, C.E.; Steffens, J.; Bizzi, C.A.; Santos, D.; Alessio, K.O.; Flores, É.M.M.; Duarte, F.A.; Dallago, R.M. Microwave-Assisted Extraction of Cr from Residual Tanned Leather: A Promising Alternative for Waste Treatment from Tannery Industry. J. Environ. Chem. Eng. 2022, 10, 6–11. [Google Scholar] [CrossRef]
- Popiolski, A.S.; Dallago, R.M.; Steffens, J.; Mignoni, M.L.; Venquiaruto, L.D.; Santos, D.; Duarte, F.A. Ultrasound-Assisted Extraction of Cr from Residual Tannery Leather: Feasibility of Ethylenediaminetetraacetic Acid as the Extraction Solution. ACS Omega 2018, 3, 16074–16080. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of Co-Contaminated Soil with Heavy Metals and Pesticides: Influence Factors, Mechanisms and Evaluation Methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Elemike, E.E.; Onwudiwe, D.C.; Onyango, M.S. Metal Oxide-Cellulose Nanocomposites for the Removal of Toxic Metals and Dyes from Wastewater. Int. J. Biol. Macromol. 2020, 164, 2477–2496. [Google Scholar] [CrossRef] [PubMed]
- El Saidy, N.R.; El-Habashi, N.; Saied, M.M.; Abdel-Razek, M.A.S.; Mohamed, R.A.; Abozeid, A.M.; El-Midany, S.A.; Abouelenien, F.A. Wastewater Remediation of Heavy Metals and Pesticides Using Rice Straw and/or Zeolite as Bioadsorbents and Assessment of Treated Wastewater Reuse in the Culture of Nile Tilapia (Oreochromis Niloticus). Environ. Monit. Assess. 2020, 192, 779. [Google Scholar] [CrossRef]
- Buaisha, M.; Balku, S.; Yaman, Š.Ö. Heavy Metal Removal Investigation in Conventional Activated Sludge Systems. Civ. Eng. J. 2020, 6, 470–477. [Google Scholar] [CrossRef]
- Ma, Y.; Egodawatta, P.; Mcgree, J.; Liu, A.; Goonetilleke, A. Science of the Total Environment Human Health Risk Assessment of Heavy Metals in Urban Stormwater. Sci. Total Environ. 2016, 557–558, 764–772. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, J.; Fu, Q.; Li, T.; Gao, S.; Wang, R.; Zhao, S.; Zhu, B. The Boom Era of Emerging Contaminants: A Review of Remediating Agricultural Soils by Biochar. Sci. Total Environ. 2024, 931, 172899. [Google Scholar] [CrossRef]
- Eom, H.; Kang, W.; Kim, S.; Chon, K.; Lee, Y.G.; Oh, S.E. Improved Toxicity Analysis of Heavy Metal-Contaminated Water via a Novel Fermentative Bacteria-Based Test Kit. Chemosphere 2020, 258, 127412. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Sorouraddin, S.M.; Farajzadeh, M.A.; Okhravi, T. Development of Dispersive Liquid-Liquid Microextraction Based on Deep Eutectic Solvent Using as Complexing Agent and Extraction Solvent: Application for Extraction of Heavy Metals. Sep. Sci. Technol. 2020, 55, 2955–2966. [Google Scholar] [CrossRef]
- Akindele, E.O.; Omisakin, O.D.; Oni, O.A.; Aliu, O.O.; Omoniyi, G.E.; Akinpelu, O.T. Heavy Metal Toxicity in the Water Column and Benthic Sediments of a Degraded Tropical Stream. Ecotoxicol. Environ. Saf. 2020, 190, 110153. [Google Scholar] [CrossRef] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Ghernaout, D.; Al-ghonamy, A.I.; Boucherit, A.; Ghernaout, B.; Naceur, M.W.; Messaoudene, N.A.; Aichouni, M.; Mahjoubi, A.A.; Elboughdiri, N.A. Brownian Motion and Coagulation Process. Am. J. Environ. Prot. 2015, 4, 1–15. [Google Scholar] [CrossRef]
- Yan, L.; Yin, H.; Zhang, S.; Leng, F.; Nan, W.; Li, H. Biosorption of Inorganic and Organic Arsenic from Aqueous Solution by Acidithiobacillus Ferrooxidans BY-3. J. Hazard. Mater. 2010, 178, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.R.; Lazaridis, N.K.; Matis, K.A. Study of Flotation Conditions for Cadmium(II) Removal from Aqueous Solutions. Process Saf. Environ. Prot. 2015, 94, 203–211. [Google Scholar] [CrossRef]
- Bortoluzzi, A.C.; Demaman Oro, C.E.; dos Santos, M.S.N.; Mignoni, M.L.; Dallago, R.M.; Steffens, J.; Tres, M.V. Combination of Chemical Coagulation and Membrane-Based Separation for Dairy Wastewater Treatment. J. Food Sci. Technol. 2023, 60, 84–91. [Google Scholar] [CrossRef]
- Mulinari, J.; Rigo, D.; Demaman Oro, C.E.; de Meneses, A.C.; Zin, G.; Eleutério, R.V.; Tres, M.V.; Dallago, R.M. Multienzyme Immobilization on PVDF Membrane via One-Step Mussel-Inspired Method: Enhancing Fouling Resistance and Self-Cleaning Efficiency. Membranes 2024, 14, 208. [Google Scholar] [CrossRef]
- Oro, C.E.D.; Dos Santos, M.S.N.; Dallago, R.M.; Tres, M.V. Membrane Applications in the Dairy Industry. Biointerface Res. Appl. Chem. 2021, 12, 5012–5020. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste Biomass Adsorbents for Copper Removal from Industrial Wastewater—A Review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.C.; Lee, I.H.; Chern, J.M. Heavy Metal Extraction from PCB Wastewater Treatment Sludge by Sulfuric Acid. J. Hazard. Mater. 2010, 177, 881–886. [Google Scholar] [CrossRef]
- Mores, R.; Cunha Junior, A.; Antes, F.G.; Di Luccio, M.; Oro, C.E.D.; Tres, M.V.; Steffens, C.; Steffens, J.; Kunz, A.; Dallago, R.M. Electrochemical Precipitation of Struvite from Wastewater: A Sustainable Approach for Nitrogen Recovery. Separations 2025, 12, 108. [Google Scholar] [CrossRef]
- Caovilla, M.; Oro, C.E.D.; Mores, R.; Venquiaruto, L.D.; Mignoni, M.L.; Di Luccio, M.; Treichel, H.; Dallago, R.M.; Tres, M.V. Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal. Separations 2025, 12, 39. [Google Scholar] [CrossRef]
- Ojedokun, A.T.; Bello, O.S. Sequestering Heavy Metals from Wastewater Using Cow Dung. Water Resour. Ind. 2016, 13, 7–13. [Google Scholar] [CrossRef]
- Maal-bared, R. Operational Impacts of Heavy Metals on Activated Sludge Systems: The Need for Improved Monitoring. Environ. Monit. Assess 2020, 192, 560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shukla, P. Microalgal Multiomics-Based Approaches in Bioremediation of Hazardous Contaminants. Environ. Res. 2024, 247, 118135. [Google Scholar] [CrossRef]
- Meyer, L.; Guyot, S.; Chalot, M.; Capelli, N. The Potential of Microorganisms as Biomonitoring and Bioremediation Tools for Mercury-Contaminated Soils. Ecotoxicol. Environ. Saf. 2023, 262, 115185. [Google Scholar] [CrossRef]
- Sahu, D.; Pervez, S.; Karbhal, I.; Tamrakar, A.; Mishra, A.; Verma, S.R.; Deb, M.K.; Ghosh, K.K.; Pervez, Y.F.; Shrivas, K.; et al. Applications of Different Adsorbent Materials for the Removal of Organic and Inorganic Contaminants from Water and Wastewater—A Review. Desalin. Water Treat. 2024, 317, 100253. [Google Scholar] [CrossRef]
- Sundararaman, S.; Annam Renita, A.; Prabu, D.; Aravind Kumar, J.; Anish, M.; Jayaprabakar, J.; Thandaiah Prabu, R.; Sathish, T.; Baigenzhenov, O. Elucidation of Synthesis Routes and Adsorptive Mechanisms in Removal of Water Contaminants by MOF-Derived Carbon Materials. Mater. Sci. Eng. B 2025, 315, 118093. [Google Scholar] [CrossRef]
- Liu, K.-G.; Bigdeli, F.; Sharifzadeh, Z.; Gholizadeh, S.; Morsali, A. Role of Metal-Organic Framework Composites in Removal of Inorganic Toxic Contaminants. J. Clean. Prod. 2023, 404, 136709. [Google Scholar] [CrossRef]
- Deb, A.K.; Hassan, M.; Biswas, B.; Naidu, R.; Xi, Y.; Rahman, M.M. Functionalization of Halloysite Nanotubes as Remediation Agents for Organic and Inorganic Contaminants: Insights into Synthesis Routes, Removal Techniques, and Eco-Friendly Perspectives. Sci. Total Environ. 2025, 989, 179771. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, S.; Zhu, X.; Chen, B.; Rao, Z.; Wu, N.; Idris, A.M. Machine Learning-Aided Biochar Design for the Adsorptive Removal of Emerging Inorganic Pollutants in Water. Sep. Purif. Technol. 2025, 362, 131421. [Google Scholar] [CrossRef]
- Bashir, M.; Batool, M.; Arif, N.; Tayyab, M.; Zeng, Y.-J.; Nadeem Zafar, M. Strontium-Based Nanomaterials for the Removal of Organic/Inorganic Contaminants from Water: A Review. Coord. Chem. Rev. 2023, 492, 215286. [Google Scholar] [CrossRef]
- Pandey, K.; Poddar, D.; Yoo, H.-M. Zerovalent Iron Encapsulated Shellac Polymer Composite Particles for Alkaline Wastewater Treatment and Oil–Water Separation. J. Ind. Eng. Chem. 2025, 148, 380–390. [Google Scholar] [CrossRef]
- Elkahlout, Z.I.; AlMomani, F.; Furlan, R. Treatment of Produced Water Using Modifying Magnetic Nanoparticles/Polymer Composite: Enhanced Treatment Efficiency. J. Water Process Eng. 2024, 68, 106489. [Google Scholar] [CrossRef]
- Yousefi, T.; Torab-Mostaedi, M.; Moosavian, M.A.; Mobtaker, H.G. Potential Application of a Nanocomposite:HCNFe@polymer for Effective Removal of Cs (I) from Nuclear Waste. Prog. Nucl. Energy 2015, 85, 631–639. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rijith, S.; Tharun, A.R. Adsorptive Removal of Thorium(IV) from Aqueous Solutions Using Poly(Methacrylic Acid)-Grafted Chitosan/Bentonite Composite Matrix: Process Design and Equilibrium Studies. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 368, 13–22. [Google Scholar] [CrossRef]
- Han, Q.; Du, M.; Guan, Y.; Luo, G.; Zhang, Z.; Li, T.; Ji, Y. Removal of Simulated Radioactive Cerium (III) Based on Innovative Magnetic Trioctylamine-Polystyrene Composite Microspheres. Chem. Phys. Lett. 2020, 741, 137092. [Google Scholar] [CrossRef]
- Nguegang, B.; Ambushe, A.A. Insight into the Chemical and Biochemical Mechanisms Governing Inorganic Contaminants Removal by Selective Precipitation and Neutralization in Acid Mine Drainage Treatment Using MgO: A Comparative Study. J. Water Process Eng. 2024, 59, 104924. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Niazi, Z.; Heidari, K.; Afarinandeh, A.; Samadi Kazemi, M.; Haghighat, G.A.; Vasseghian, Y.; Rezania, S.; Barghi, A. Nickel and Iron-Based Metal-Organic Frameworks for Removal of Organic and Inorganic Model Contaminants. Environ. Res. 2022, 212, 113164. [Google Scholar] [CrossRef]
- Kundu, S.; Naskar, M.K. Carbon-Layered Double Hydroxide Nanocomposite for Efficient Removal of Inorganic and Organic Based Water Contaminants—Unravelling the Adsorption Mechanism. Mater. Adv. 2021, 2, 3600–3612. [Google Scholar] [CrossRef]
- Esquivel, S.E.; Aranda, F.L.; Vellojin, J.P.; Pérez-Zurita, D.; Montoya-Rodriguez, D.; Villegas-Escobar, N.; Meléndrez, M.; Sánchez, J.; Palacio, D.A. Combining Experimental and Molecular Computational Approaches for Enhanced Removal of Organic and Inorganic Pollutants via Diafiltration Using Chitosan with Permanent Cationic Charges. Int. J. Biol. Macromol. 2025, 320, 145738. [Google Scholar] [CrossRef]
- Salih, S.S.; Shihab, M.A.; Kadhom, M.; Albayati, N.; Ghosh, T.K. Simultaneous Removal of Organic and Inorganic Pollutants onto Chitosan-Coated Pumice Adsorbent. Water Sci. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Grabowski, J.; Tokarz, A.; Nowak, D. Application of Multilayer Bed for Removal of Organic and Inorganic Compounds from Aqueous Solutions. J. Water Process Eng. 2022, 50, 103243. [Google Scholar] [CrossRef]
- Shah, A.; Arjunan, A.; Baroutaji, A.; Zakharova, J. A Review of Physicochemical and Biological Contaminants in Drinking Water and Their Impacts on Human Health. Water Sci. Eng. 2023, 16, 333–344. [Google Scholar] [CrossRef]
- Leonard, L.T.; Vanzin, G.F.; Garayburu-Caruso, V.A.; Lau, S.S.; Beutler, C.A.; Newman, A.W.; Mitch, W.A.; Stegen, J.C.; Williams, K.H.; Sharp, J.O. Disinfection Byproducts Formed during Drinking Water Treatment Reveal an Export Control Point for Dissolved Organic Matter in a Subalpine Headwater Stream. Water Res. X 2022, 15, 100144. [Google Scholar] [CrossRef]
- Zheng, X.; Ni, C.; Xiao, W.; Liang, Y.; Li, Y. Ionic Liquid Grafted Polyethersulfone Nanofibrous Membrane as Recyclable Adsorbent with Simultaneous Dye, Heavy Metal Removal and Antibacterial Property. Chem. Eng. J. 2022, 428, 132111. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Functionalized Electrospun Nanofibrous Microfiltration Membranes for Removal of Bacteria and Viruses. J. Memb. Sci. 2014, 452, 446–452. [Google Scholar] [CrossRef]
- Bashir, N.; Afzaal, M.; Khan, A.L.; Nawaz, R.; Irfan, A.; Almaary, K.S.; Dabiellil, F.; Bourhia, M.; Ahmed, Z. Green-Synthesized Silver Nanoparticle-Enhanced Nanofiltration Mixed Matrix Membranes for High-Performance Water Purification. Sci. Rep. 2025, 15, 1001. [Google Scholar] [CrossRef]
- García-Ávila, F.; Zambrano-Jaramillo, A.; Velecela-Garay, C.; Coronel-Sánchez, K.; Valdiviezo-Gonzales, L. Effectiveness of Membrane Technologies in Removing Emerging Contaminants from Wastewater: Reverse Osmosis and Nanofiltration. Water Cycle 2025, 6, 357–373. [Google Scholar] [CrossRef]
- Maharubin, S.; Zhou, Y.; Tan, G.Z. Integration of Silver Nanoparticles and Microcurrent for Water Filtration. Sep. Purif. Technol. 2019, 212, 57–64. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric Antimicrobial Membranes Enabled by Nanomaterials for Water Treatment. J. Memb. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Dilxat, D.; Xie, D.; Wang, J.; Habibul, N.; Zhang, H.-C.; Sheng, G.-P.; Wang, Y. Molecular Design of Ultrafiltration Membranes with Antibacterial Properties for the Inactivation of Antibiotic-Resistant Bacteria. J. Memb. Sci. 2024, 690, 122131. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun Nanofibrous Membranes for High Flux Microfiltration. J. Memb. Sci. 2012, 392–393, 167–174. [Google Scholar] [CrossRef]
- John, J.F.; Jagannathan, M.; Rajendran, A.R.; Mohanapriya, P.; Natarajan, T.S.; Dhinasekaran, D. Sustainable Multilayer Biomass Carbon and Polymer Hybrid Column as Potential Antibacterial Water Filter. Chemosphere 2022, 286, 131691. [Google Scholar] [CrossRef]
- Ghodake, G.; Shinde, S.; Saratale, G.D.; Kadam, A.; Saratale, R.G.; Kim, D.-Y. Water Purification Filter Prepared by Layer-by-Layer Assembly of Paper Filter and Polypropylene-Polyethylene Woven Fabrics Decorated with Silver Nanoparticles. Fibers Polym. 2020, 21, 751–761. [Google Scholar] [CrossRef]
- Undabeytia, T.; Posada, R.; Nir, S.; Galindo, I.; Laiz, L.; Saiz-Jimenez, C.; Morillo, E. Removal of Waterborne Microorganisms by Filtration Using Clay–Polymer Complexes. J. Hazard. Mater. 2014, 279, 190–196. [Google Scholar] [CrossRef]
- Goswami, S.; Dutta, D.; Pandey, S.; Chattopadhyay, P.; Lalhmunsiama; Dubey, R.; Tiwari, D. Novel Fibrous Ag(NP) Decorated Clay-Polymer Composite: Implications in Water Purification Contaminated with Predominant Micro-Pollutants and Bacteria. J. Environ. Manag. 2024, 359, 121063. [Google Scholar] [CrossRef] [PubMed]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the Self Cleaning, Antibacterial and Photocatalytic Properties of TiO2 Entrapped PVDF Membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone Ultrafiltration Membranes Impregnated with Silver Nanoparticles Show Improved Biofouling Resistance and Virus Removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef]
- Raval, H.D.; Gohel, S.; Magiya, U. Chitosan-Zinc Oxide Supramolecular Assembly over Polysulfone Ultrafiltration Membrane for Antifungal Membrane Surface Formation. Inorg. Chem. Commun. 2025, 177, 114351. [Google Scholar] [CrossRef]
- Ji, Z.; Liang, M.; Wang, C.; Ma, M.; Tian, J.; Su, Y.; Chang, H.; Li, M. High-Efficiency Broad-Spectrum Antibacterial Activity of Chitosan/Zinc Ion/Polyoxometalate Composite Films for Water Treatment. Langmuir 2024, 40, 26997–27009. [Google Scholar] [CrossRef] [PubMed]
- Benali, F.; Boukoussa, B.; Issam, I.; Mokhtar, A.; Iqbal, J.; Hachemaoui, M.; Habeche, F.; Cherifi, Z.; Hacini, S.; Patole, S.P.; et al. Assessment of AgNPs@Cu@Alginate Composite for Efficient Water Treatment: Effect of the Content of Cu(II) Crosslinking Agent. J. Polym. Environ. 2023, 31, 4170–4183. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Bukvić, M.; Milojević, S.; Gajević, S.; Đorđević, M.; Stojanović, B. Production Technologies and Application of Polymer Composites in Engineering: A Review. Polymers 2025, 17, 2187. [Google Scholar] [CrossRef]
- Komisarz, K.; Majka, T.M.; Pielichowski, K. Chemical and Physical Modification of Lignin for Green Polymeric Composite Materials. Materials 2022, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Abeykoon, C.; Karim, N. Investigation into the Effects of Fillers in Polymer Processing. Int. J. Light. Mater. Manuf. 2021, 4, 370–382. [Google Scholar] [CrossRef]
- Moutik, B.; Summerscales, J.; Graham-Jones, J.; Pemberton, R. Quality Assessment of Life Cycle Inventory Data for Fibre-Reinforced Polymer Composite Materials. Sustain. Prod. Consum. 2024, 49, 474–491. [Google Scholar] [CrossRef]
- Gulbalkan, H.C.; Haslak, Z.P.; Altintas, C.; Uzun, A.; Keskin, S. Assessing CH4/N2 Separation Potential of MOFs, COFs, IL/MOF, MOF/Polymer, and COF/Polymer Composites. Chem. Eng. J. 2022, 428, 131239. [Google Scholar] [CrossRef]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef]
- Joseph, B.; Krishnan, S.; Kavil, S.V.; Pai, A.R.; James, J.; Kalarikkal, N.; Thomas, S. Green Chemistry Approach for Fabrication of Polymer Composites. Sustain. Chem. 2021, 2, 254–270. [Google Scholar] [CrossRef]
- Sajan, S.; Philip Selvaraj, D. A Review on Polymer Matrix Composite Materials and Their Applications. Mater. Today Proc. 2021, 47, 5493–5498. [Google Scholar] [CrossRef]
- Tao, Y.; Hadigheh, S.A.; Wei, Y. Recycling of Glass Fibre Reinforced Polymer (GFRP) Composite Wastes in Concrete: A Critical Review and Cost Benefit Analysis. Structures 2023, 53, 1540–1556. [Google Scholar] [CrossRef]
- Schubel, P.J. Cost Modelling in Polymer Composite Applications: Case Study—Analysis of Existing and Automated Manufacturing Processes for a Large Wind Turbine Blade. Compos. Part B Eng. 2012, 43, 953–960. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent Advancements in the Application of New Monomers and Membrane Modification Techniques for the Fabrication of Thin Film Composite Membranes: A Review. React. Funct. Polym. 2021, 166, 105015. [Google Scholar] [CrossRef]
- Huang, X.; Panahi-Sarmad, M.; Dong, K.; Li, R.; Chen, T.; Xiao, X. Tracing Evolutions in Electro-Activated Shape Memory Polymer Composites with 4D Printing Strategies: A Systematic Review. Compos. Part A Appl. Sci. Manuf. 2021, 147, 106444. [Google Scholar] [CrossRef]
- Hassan, M.; Mohanty, A.K.; Wang, T.; Dhakal, H.N.; Misra, M. Current Status and Future Outlook of 4D Printing of Polymers and Composites-A Prospective. Compos. Part C Open Access 2025, 17, 100602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).