One-Part Alkali-Activated Binder Produced from Tungsten-Molybdenum (W-Mo) Tailings
Abstract
1. Introduction
2. Experimental Procedures
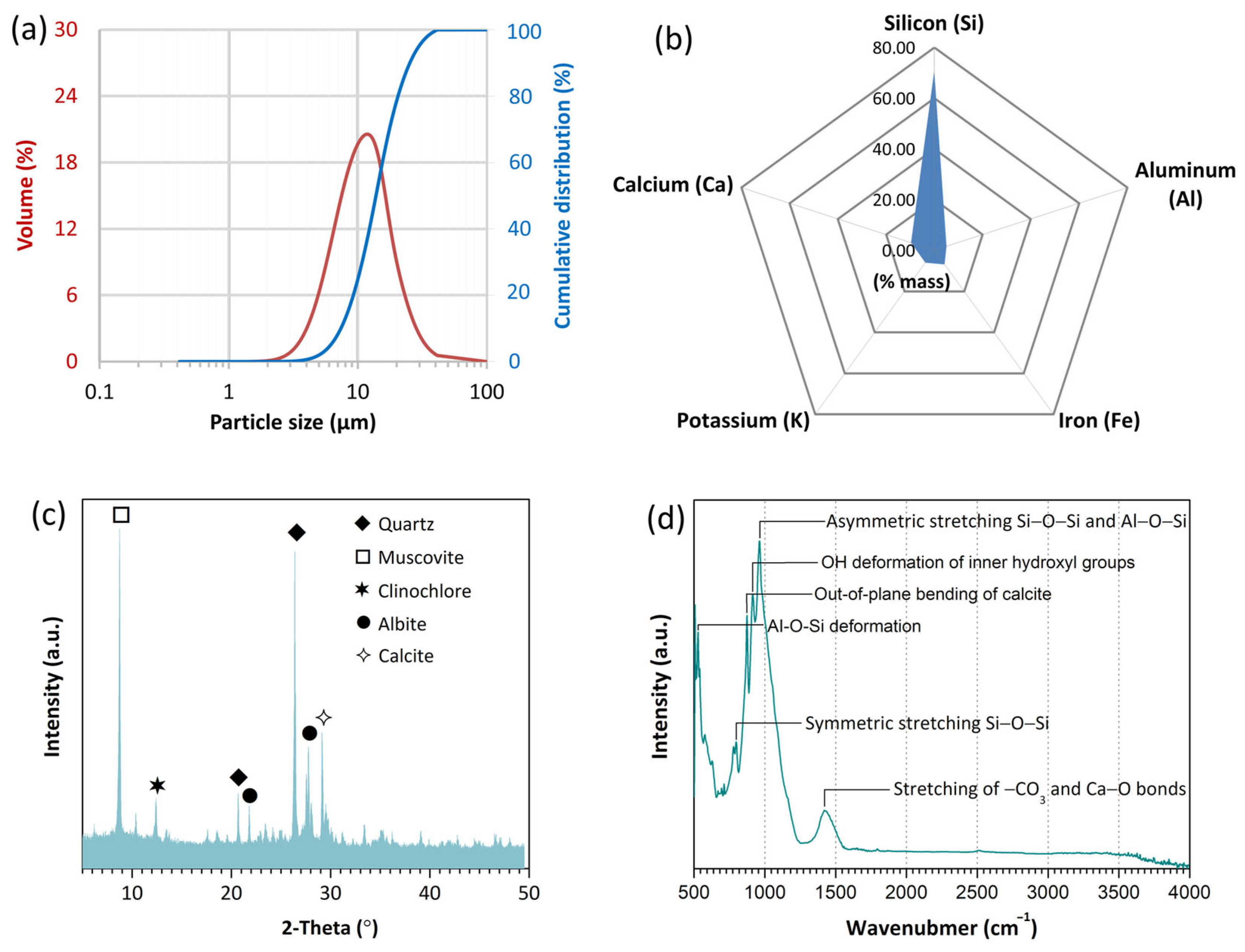
2.1. Materials
2.2. Mix Proportions and Mixing
2.3. Testing Methods and Characterization
3. Results
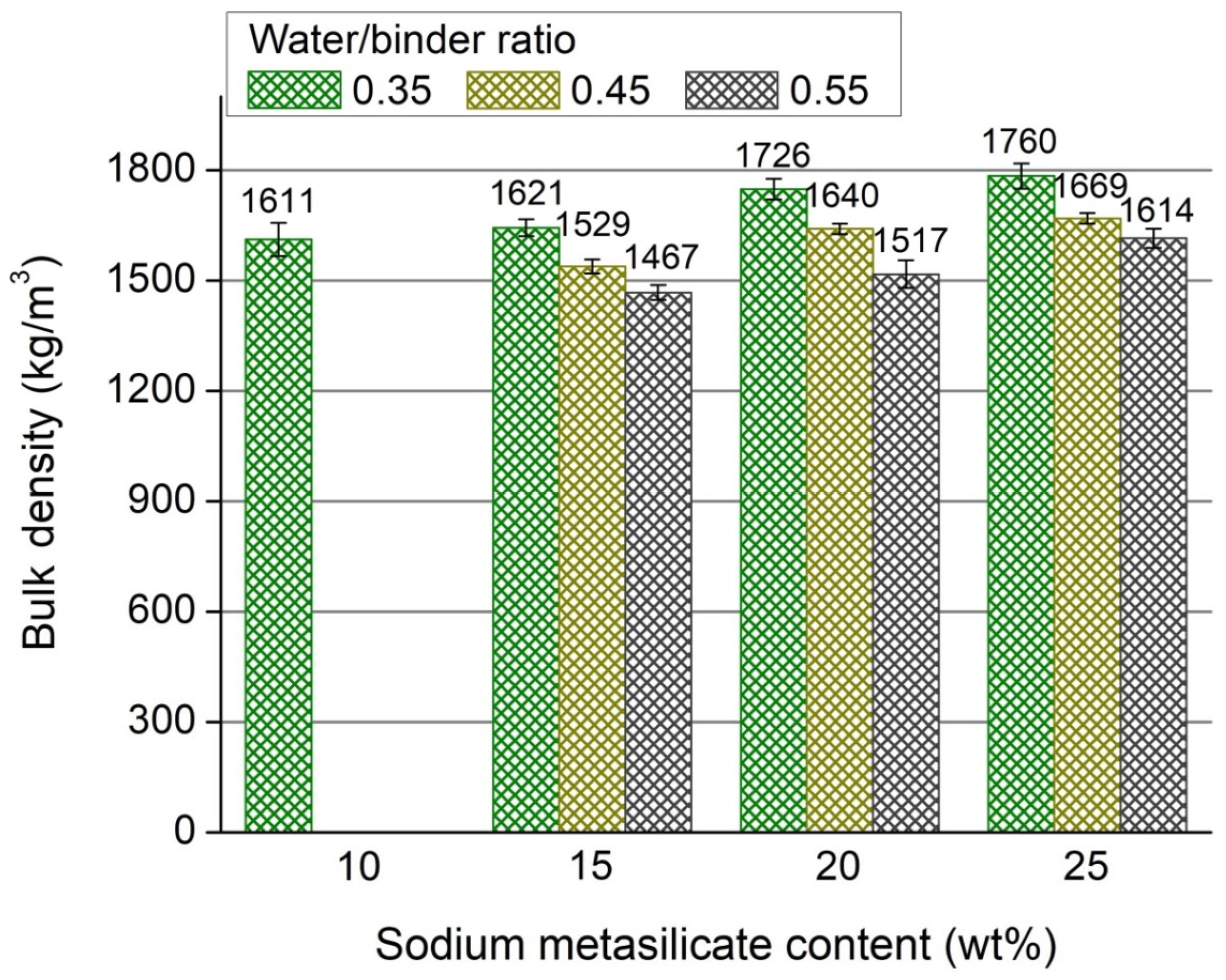
3.1. Bulk Density
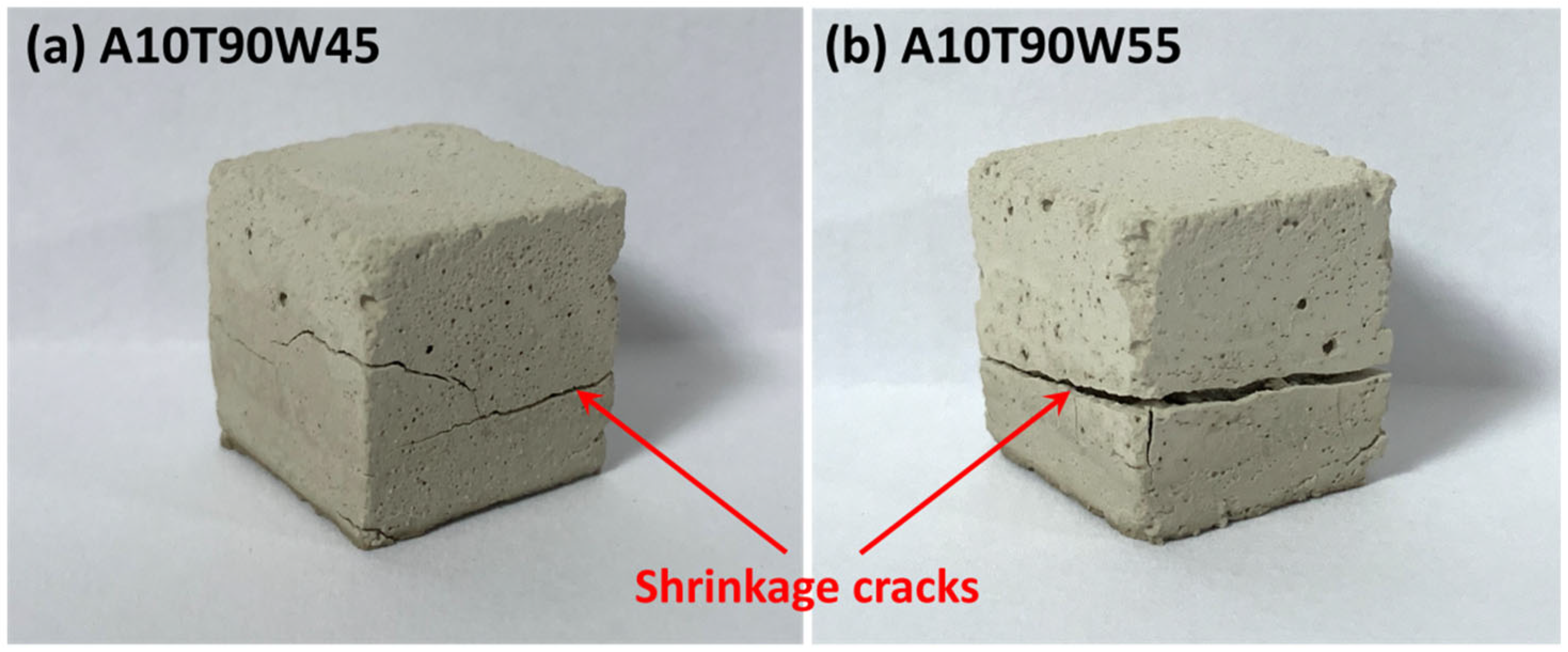
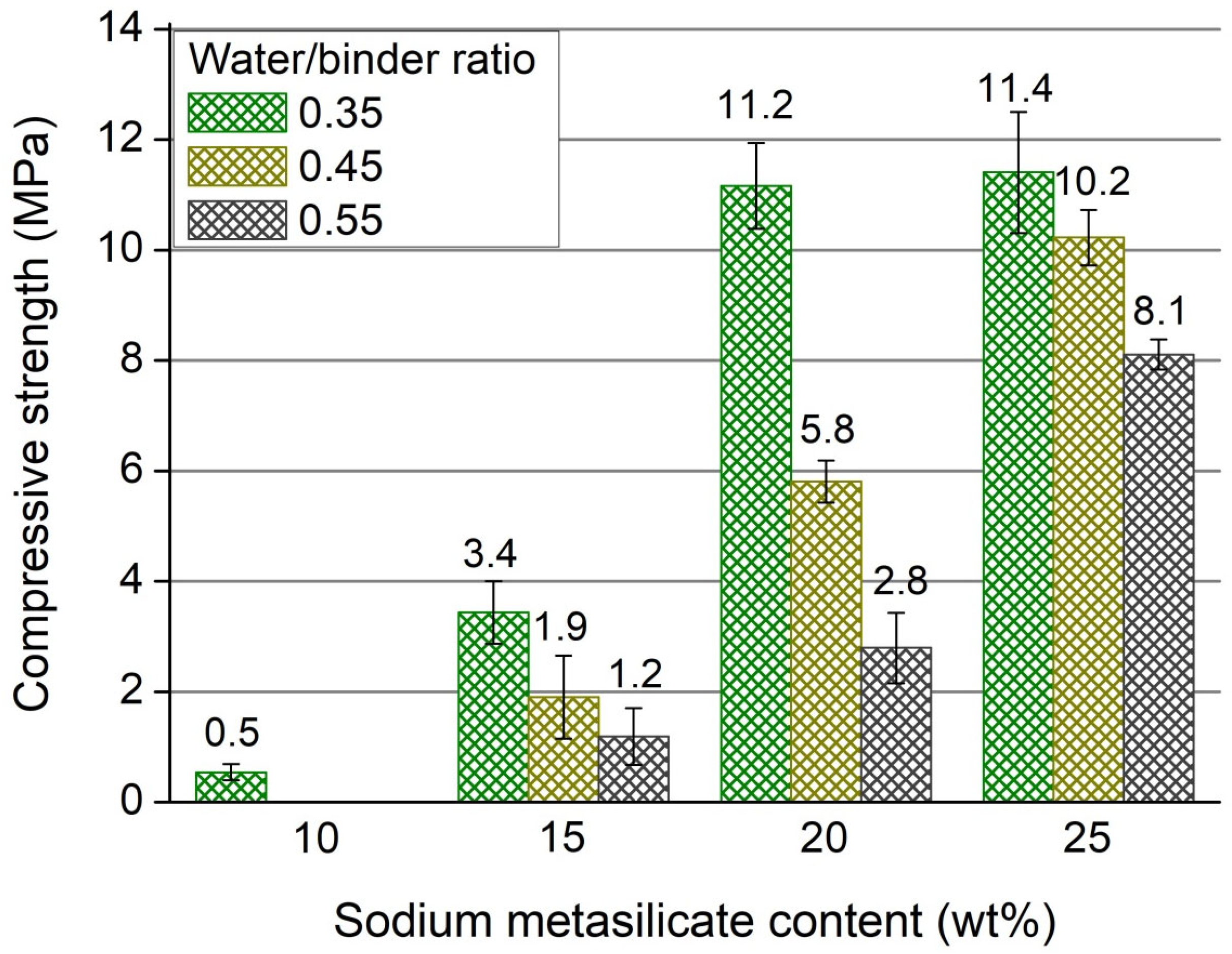
3.2. Unconfined Compressive Strength
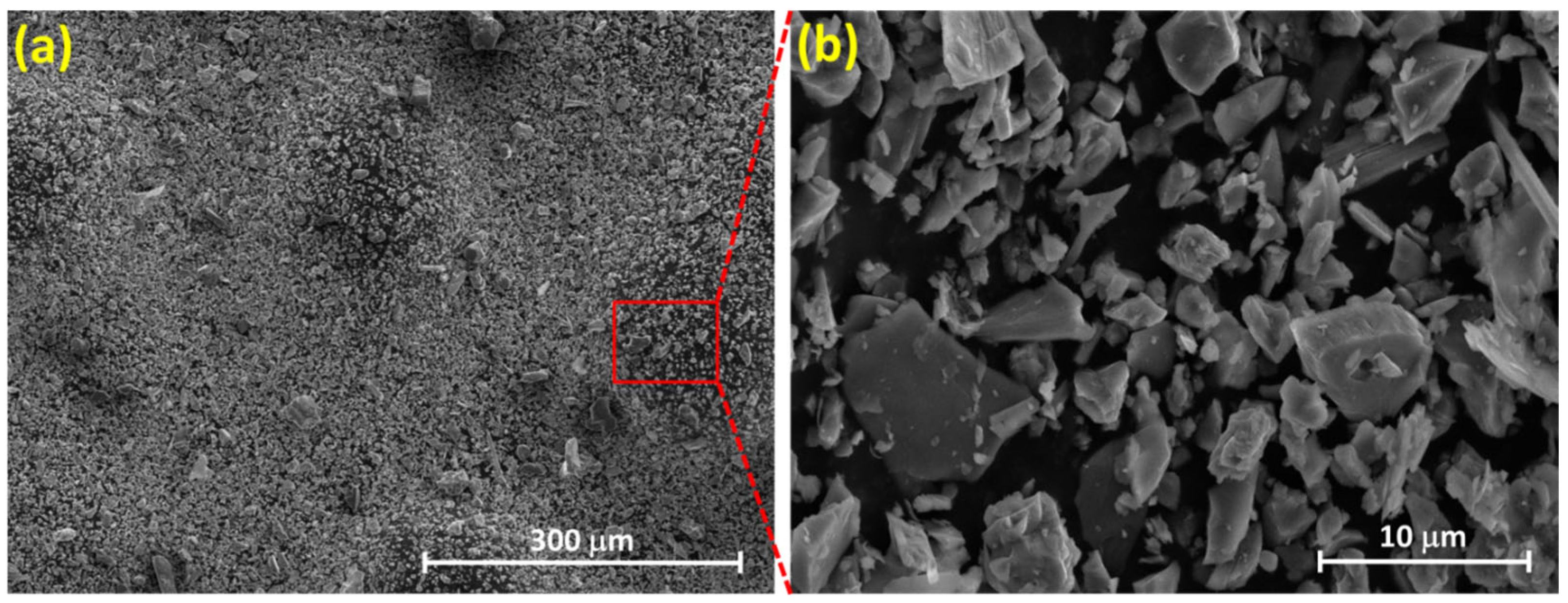
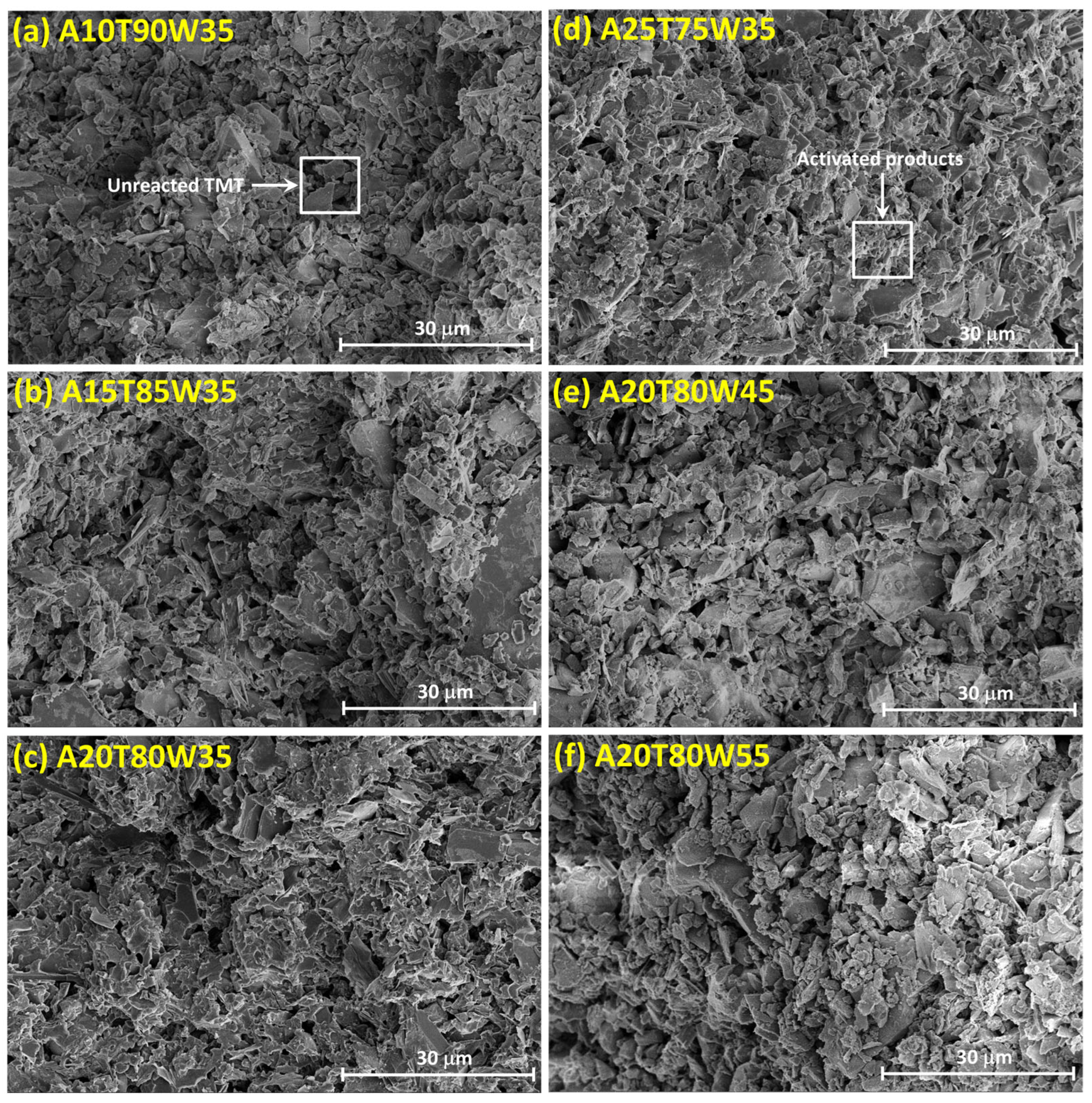
3.3. Microstructure
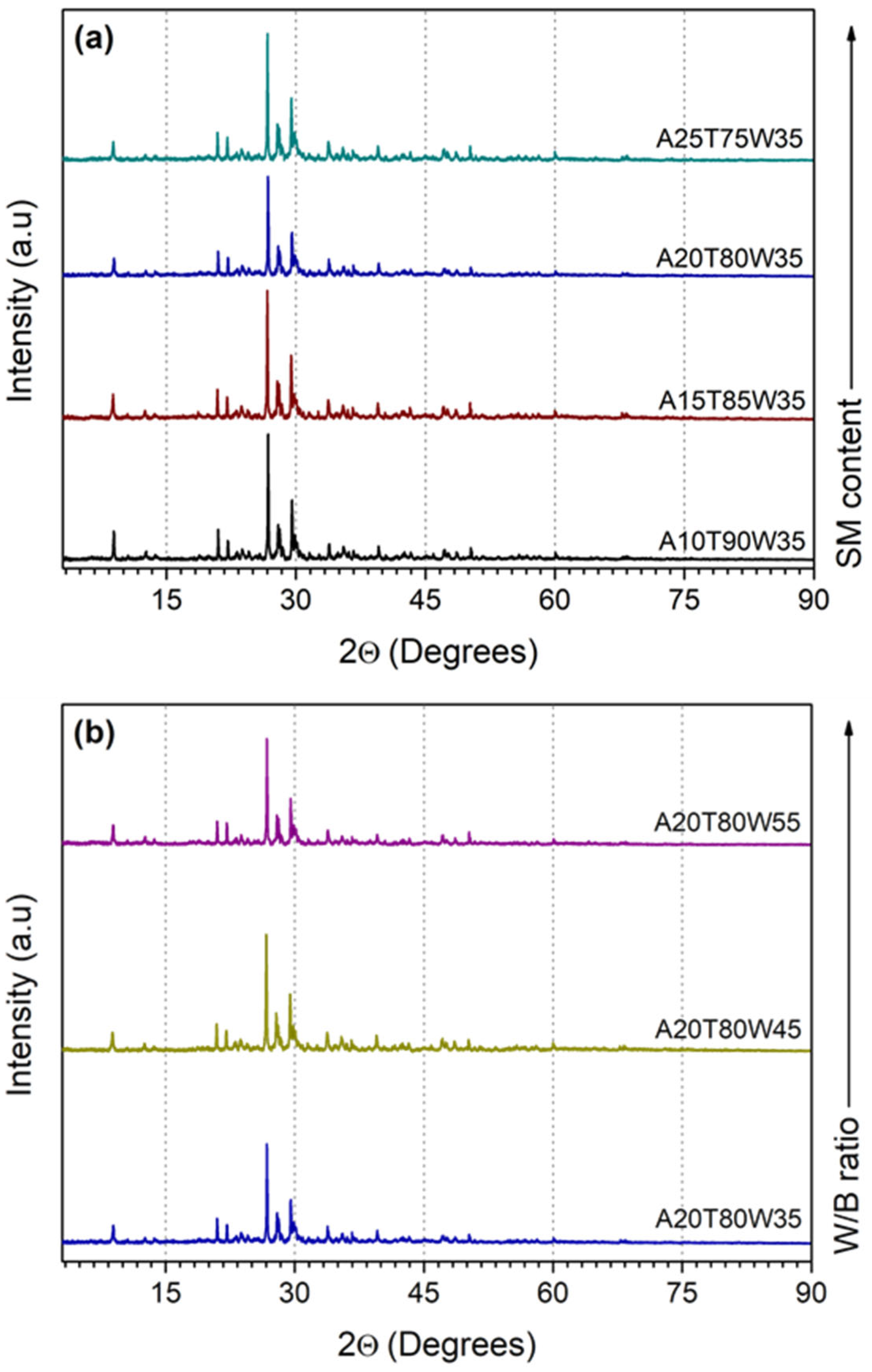
3.4. XRD Patterns
3.5. FTIR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singla, Y.K.; Maughan, M.R.; Arora, N.; Dwivedi, D.K. Enhancing the wear resistance of iron-based alloys: A comprehensive review of alloying element effects. J. Manuf. Process. 2024, 120, 135–160. [Google Scholar] [CrossRef]
- Lutton Cwalina, K.; Demarest, C.R.; Gerard, A.Y.; Scully, J.R. Revisiting the effects of molybdenum and tungsten alloying on corrosion behavior of nickel-chromium alloys in aqueous corrosion. Curr. Opin. Solid State Mater. Sci. 2019, 23, 129–141. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.-H.; Wu, C.-B.; Zhao, Y.; Zu, G.-Q.; Zhu, W.-W.; Ran, X. A short review on the role of alloying elements in duplex stainless steels. Tungsten 2023, 5, 419–439. [Google Scholar] [CrossRef]
- Luo, N.; Yang, W.; Feng, L.; Huang, S.; Huang, P.; Wei, M. Recent advances in utilizing molybdenum and tungsten carbides for fischer-tropsch synthesis. Mol. Catal. 2024, 553, 113683. [Google Scholar] [CrossRef]
- Kotsidi, M.; Gorgolis, G.; Pastore Carbone, M.G.; Paterakis, G.; Anagnostopoulos, G.; Trakakis, G.; Manikas, A.C.; Pavlou, C.; Koutroumanis, N.; Galiotis, C. Graphene nanoplatelets and other 2D-materials as protective means against the fading of coloured inks, dyes and paints. Nanoscale 2023, 15, 5414–5428. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, W.; Liang, W.; Yan, T.; Li, T.; Zhang, L.; Li, S. Inorganic nanomaterial lubricant additives for base fluids, to improve tribological performance: Recent developments. Friction 2022, 10, 645–676. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries, January 2024. Available online: https://pubs.usgs.gov/publication/mcs2024 (accessed on 25 November 2024).
- Peng, K.; Yang, H.; Ouyang, J. Tungsten tailing powders activated for use as cementitious material. Powder Technol. 2015, 286, 678–683. [Google Scholar] [CrossRef]
- Reutova, N.V.; Reutova, T.V.; Dreeva, F.R.; Shevchenko, A.A. Long-term impact of the Tyrnyauz tungsten–molybdenum mining and processing factory waste on environmental pollution and children’s population. Environ. Geochem. Health 2022, 44, 4557–4568. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, R.; Wang, X.; Yan, Z. Soil tungsten contamination and health risk assessment of an abandoned tungsten mine site. Sci. Total Environ. 2022, 852, 158461. [Google Scholar] [CrossRef]
- Chechel, L.P.; Zamana, L.V.; Abramova, V.A. Formation of waters of tungsten-ore areas under the influence of natural and anthropogenic factors (Eastern Transbaikalia, Russia). Appl. Geochem. 2023, 154, 105687. [Google Scholar] [CrossRef]
- Bolan, S.; Wijesekara, H.; Ireshika, A.; Zhang, T.; Pu, M.; Petruzzelli, G.; Pedron, F.; Hou, D.; Wang, L.; Zhou, S.; et al. Tungsten contamination, behavior and remediation in complex environmental settings. Environ. Int. 2023, 181, 108276. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, T.; Li, Z.; Hao, X.; Lu, A. Preparation and characterization of ceramic substrate from tungsten mine tailings. Constr. Build. Mater. 2015, 77, 139–144. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, B.; Zuo, W.; Jiang, K.; Chen, H.; Ku, J. Effect of sintering temperature on structure and properties of porous ceramics from tungsten ore tailings. Mater. Chem. Phys. 2022, 287, 126315. [Google Scholar] [CrossRef]
- Alfonso, P.; Tomasa, O.; Garcia-Valles, M.; Tarragó, M.; Martínez, S.; Esteves, H. Potential of tungsten tailings as glass raw materials. Mater. Lett. 2018, 228, 456–458. [Google Scholar] [CrossRef]
- Maruthupandian, S.; Chaliasou, A.; Kanellopoulos, A. Recycling mine tailings as precursors for cementitious binders—Methods, challenges and future outlook. Constr. Build. Mater. 2021, 312, 125333. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, Y.J.; Choi, O.; Lee, K.M.; Lachemi, M. Utilization of tailings from tungsten mine waste as a substitution material for cement. Constr. Build. Mater. 2009, 23, 2481–2486. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, S.; Yilmaz, E. Microstructure and mechanical behavior of cemented gold/tungsten mine tailings-crushed rock backfill: Effects of rock gradation and content. J. Environ. Manag. 2023, 339, 117897. [Google Scholar] [CrossRef]
- Luo, T.; Yi, Y.; Liu, F.; Sun, Q.; Pan, X.; Hua, C. Early-age hydration and strength formation mechanism of composite concrete using molybdenum tailings. Case Stud. Constr. Mater. 2022, 16, e01101. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Mischinenko, V. Rubberized geopolymer composites: Effect of filler surface treatment. J. Environ. Chem. Eng. 2021, 9, 105601. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Investigations of tungsten mine waste geopolymeric binder: Strength and microstructure. Constr. Build. Mater. 2008, 22, 2212–2219. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Investigations on mix design of tungsten mine waste geopolymeric binder. Constr. Build. Mater. 2008, 22, 1939–1949. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Tungsten mine waste geopolymeric binder: Preliminary hydration products investigations. Constr. Build. Mater. 2009, 23, 200–209. [Google Scholar] [CrossRef]
- Li, S.; Shou, K.; Wang, L.; Liu, Z. The effect of alkali concentration on the properties of activated tungsten tailings. Environ. Sci. Pollut. Res. 2023, 30, 34623–34635. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Adhesion characterization of tungsten mine waste geopolymeric binder. Influence of OPC concrete substrate surface treatment. Constr. Build. Mater. 2008, 22, 154–161. [Google Scholar] [CrossRef]
- Silva, I.; Castro-Gomes, J.P.; Albuquerque, A. Effect of immersion in water partially alkali-activated materials obtained of tungsten mine waste mud. Constr. Build. Mater. 2012, 35, 117–124. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Influence of sodium carbonate addition on the thermal reactivity of tungsten mine waste mud based binders. Constr. Build. Mater. 2010, 24, 56–60. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- GOST 5802; Mortars. Test Methods. State Committee of the USSR for Construction Affairs: Moscow, Russia, 1986.
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Kankia, M.U.; Amran, M.; Gora, A.M. Long-term strength development of fly ash-based one-part alkali-activated binders. Materials 2021, 14, 4160. [Google Scholar] [CrossRef]
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Haruna, A. Compressive strength and workability of high calcium one-part alkali activated mortars using response surface methodology. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012018. [Google Scholar] [CrossRef]
- Xu, L.-Y.; Qian, L.-P.; Huang, B.-T.; Dai, J.-G. Development of artificial one-part geopolymer lightweight aggregates by crushing technique. J. Clean. Prod. 2021, 315, 128200. [Google Scholar] [CrossRef]
- Wan-En, O.; Yun-Ming, L.; Cheng-Yong, H.; Li Ngee, H.; Al Bakri Abdullah, M.M.; Bin Khalid, M.S.; Kai Loong, F.; Shee-Ween, O.; Pei Seng, T.; Yong Jie, H.; et al. Towards greener one-part geopolymers through solid sodium activators modification. J. Clean. Prod. 2022, 378, 134370. [Google Scholar] [CrossRef]
- Ibrahim, M.; Wan Ibrahim, W.M.; Abdullah, M.M.A.B.; Nabialek, M.; Putra Jaya, R.; Setkit, M.; Ahmad, R.; Jeż, B. Synthesis of Metakaolin Based Alkali Activated Materials as an Adsorbent at Different SM/NaOH Ratios and Exposing Temperatures for Cu2+ Removal. Materials 2023, 16, 1221. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Ramey, D.; Waterman, B.; Ormsby, S. Utilization of aluminum sludge (AS) to enhance mine tailings-based geopolymer. J. Mater. Sci. 2015, 50, 1370–1381. [Google Scholar] [CrossRef]
- Ouffa, N.; Benzaazoua, M.; Belem, T.; Trauchessec, R.; Lecomte, A. Alkaline dissolution potential of aluminosilicate minerals for the geosynthesis of mine paste backfill. Mater. Today Commun. 2020, 24, 101221. [Google Scholar] [CrossRef]
- Colombani, J. The Alkaline Dissolution Rate of Calcite. J. Phys. Chem. Lett. 2016, 7, 2376–2380. [Google Scholar] [CrossRef]
- Perumal, P.; Piekkari, K.; Sreenivasan, H.; Kinnunen, P.; Illikainen, M. One-part geopolymers from mining residues—Effect of thermal treatment on three different tailings. Miner. Eng. 2019, 144, 106026. [Google Scholar] [CrossRef]
- Ojo, E.B.; Mustapha, K.; Teixeira, R.S.; Savastano, H. Development of unfired earthen building materials using muscovite rich soils and alkali activators. Case Stud. Constr. Mater. 2019, 11, e00262. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Zhang, Z.; Wu, Q.; Du, J. Partial replacement of metakaolin with thermally treated rice husk ash in metakaolin-based geopolymer. Constr. Build. Mater. 2019, 221, 527–538. [Google Scholar] [CrossRef]
- Chen, W.; Peng, R.; Straub, C.; Yuan, B. Promoting the performance of one-part alkali-activated slag using fine lead-zinc mine tailings. Constr. Build. Mater. 2020, 236, 117745. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Sulavko, S.N.; Yavna, V.A.; Kochur, A.G. A study of the structural and spectral characteristics of free and bound water in kaolinite. Opt. Spectrosc. 2016, 121, 357–363. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Zhang, T.; Fernández, C.A. Alkali-activated copper tailings-based pastes: Compressive strength and microstructural characterization. J. Mater. Res. Technol. 2020, 9, 6557–6567. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Ren, Z.; Liu, S. Optimizing raw material composition and alkali content in low silicon-aluminum tailings-based geopolymers. Constr. Build. Mater. 2023, 404, 133339. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.; Zhang, R. The effect of pore behavior and gel structure on the mechanical property at different initial water content. Constr. Build. Mater. 2021, 309, 125146. [Google Scholar] [CrossRef]


| Specimen Code | Dry Activator (wt%) | W-Mo Tailings (wt%) | Water/Binder Ratio | Product |
|---|---|---|---|---|
| A10T90W35 | 10 | 90 | 0.35 | Paste |
| A10T90W45 1 | 10 | 90 | 0.45 | Mortar |
| A10T90W55 1 | 10 | 90 | 0.55 | Mortar |
| A15T85W35 | 15 | 85 | 0.35 | Paste |
| A15T85W45 | 15 | 85 | 0.45 | Mortar |
| A15T80W55 | 15 | 85 | 0.55 | Mortar |
| A20T80W35 | 20 | 80 | 0.35 | Paste |
| A20T80W45 | 20 | 80 | 0.45 | Mortar |
| A20T80W55 | 20 | 80 | 0.55 | Mortar |
| A25T75W35 | 25 | 75 | 0.35 | Paste |
| A25T75W45 | 25 | 75 | 0.45 | Mortar |
| A25T75W55 | 25 | 75 | 0.55 | Mortar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazorenko, G.; Wang, Y.; Fedotov, A.; Kasprzhitskii, A. One-Part Alkali-Activated Binder Produced from Tungsten-Molybdenum (W-Mo) Tailings. Eng 2024, 5, 3148-3160. https://doi.org/10.3390/eng5040165
Lazorenko G, Wang Y, Fedotov A, Kasprzhitskii A. One-Part Alkali-Activated Binder Produced from Tungsten-Molybdenum (W-Mo) Tailings. Eng. 2024; 5(4):3148-3160. https://doi.org/10.3390/eng5040165
Chicago/Turabian StyleLazorenko, Georgy, Yanshuai Wang, Alexandr Fedotov, and Anton Kasprzhitskii. 2024. "One-Part Alkali-Activated Binder Produced from Tungsten-Molybdenum (W-Mo) Tailings" Eng 5, no. 4: 3148-3160. https://doi.org/10.3390/eng5040165
APA StyleLazorenko, G., Wang, Y., Fedotov, A., & Kasprzhitskii, A. (2024). One-Part Alkali-Activated Binder Produced from Tungsten-Molybdenum (W-Mo) Tailings. Eng, 5(4), 3148-3160. https://doi.org/10.3390/eng5040165











