Abstract
Synthetic nitrogen fertilizer is the backbone of modern agriculture, helping to feed ~50% of the world’s population. However, the current industrial production, distribution, and use of nitrogen fertilizers are built on an unsustainable foundation of fossil resources, and are energy-intensive, environmentally polluting, and inefficient in their usage. With the rapidly declining cost of renewable electricity, such as solar and wind, it is time to develop and implement the decentralized production and application of nitrogen fertilizer with nonthermal plasma technologies. Such locally sourced production at the farm site, using only air and water as feedstock, circumvents the need for the extensive capital investment and infrastructure required for synthetic nitrogen fertilizer production and storage, as well as the complex and costly distribution networks. It will be adaptive to the intermittency of the solar/wind electricity supply, leave no carbon footprint, and also have the advantage of being easily switched on/off, immediately responding to weather changes and local conditions, such as soil, climate, crops, and farming business models, for precision agriculture.
1. Background Information
1.1. Current Unsustainable System for Nitrogen Fertilizers
The availability of nitrogen fertilizers (N-fertilizers) is crucial for achieving an optimal agricultural yield, because nitrogen is the most common limiting nutrient for plants. Ammonia and nitric acid are the primary raw materials used to produce almost all nitrogen-based synthetic fertilizers, and their demand is closely tied to global population growth and food demand. However, unless environmentally friendly alternatives are adopted, current methods established on the foundation of the Haber–Bosch process (HB process) for producing ammonia and its derivatives could lead to carbon emissions of over 1300 million tons of CO2 per year by 2050, and significantly impact the world’s energy infrastructure [1].
Industrial nitrogen fixation was first commercialized as the thermal-plasma-based Birkeland–Eyde process to produce nitrogen oxides (NOx) and nitric acid about a century ago, which was economically outcompeted by the centralized, large-scale HB process, using an ultra-pure nitrogen and hydrogen feed (mainly from natural gas and some coal) at high temperature and pressure (>450 °C, 150–350 atm) to catalytically synthesize ammonia that dominates the production of N-fertilizers today. As the result, although nearly 50% of the nitrogen found in the human body has passed through the HB process [2], the production of ammonia is unsustainable, because making this single chemical consumes approximately 2% of the world’s annual energy supply and accounts for 1.4% of the annual global carbon emissions, higher than any another chemical [1,3]; this does not include methane emitted from such facilities, which is thought to be underestimated by as much as 50 times, and the number increases further if CO2 emissions associated with natural gas extraction are included [4,5].
Unfortunately, the production of almost all synthetic N-fertilizers relies on the HB process. Urea is produced from synthetic ammonia and carbon dioxide, and urea manufacturers are always located adjacent to the site where the ammonia is manufactured. The industrial production of ammonium nitrate, the dominant form of nitrate and ammonium fertilizer accounting for 43% of all N-fertilizers [6], entails the acid–base reaction of ammonia with nitric acid. The nitric acid is industrially produced via the Ostwald process that oxidizes ammonia, in the presence of a noble metal catalyst such as platinum with 10% rhodium (top two most expensive metals), to nitric oxide (NO), and, in a second step, NO is further oxidized to yield nitrogen dioxide (NO2), which is absorbed by water to form nitric acid while reducing a portion of NO2 back to NO that must be recycled. Evidently, the production of nitrate is a detour—nitrogen in N2 (oxidation state 0) is first reduced to ammonia (oxidation state -3), and then the ammonia is oxidized to NO and NO2 (oxidation state +2 and +4, respectively), where extensive energy and fossil fuels are consumed [7]. Furthermore, the tail gas stream in an Ostwald process contains nitrogen oxides (NOx, mainly NO and NO2), a gas much more harmful than CO2 to the climate and environment. To dispose of this pollutant, the industry usually catalytically (again using a noble metal catalyst such as platinum or palladium) reduces the NOx back to harmless nitrogen gas based on the reaction of NOx with a fossil fuel, such as hydrogen, methane, butane, propane, or light naphtha [8].
Today, the HB process alone is responsible for the production of over 170 million tons of ammonia, and emits over 300 million tons of CO2 per year [1,3]. However, the emission of greenhouse gases and pollutants does not just end there. Over 86% of global ammonia produced is applied as N-fertilizer in a variety of chemical forms, among which only about 50% is used by crops, and the rest is lost to the atmosphere via volatilization and denitrification or to ground water through leaching; in some cases, the loss can go up to 70% due to the excessive amount, the low plant population, poor application methods, etc. [9].
Further, global ammonia production has been exclusively established at centralized large scales to be economical, requiring massive capital and infrastructure for H2 feedstock generation and ammonia storage, and demanding an extensive, costly, and hazardous distribution system [10]. The fossil-fuel-based transportation for the distribution system was not accounted for in the CO2 emission stated above. Consequently, although the HB process has approached the theoretical limit after continuous development and optimization for more than a century, holding an unbeatable position in terms of energy efficiency despite having great environmental flaws, there is a large variation in the prices and inequality in the accessibility to N-fertilizers globally. For instance, in Sub-Saharan Africa, where sunshine is abundant, low crop yields are associated with low fertilizer use due to high prices [11].
From a systematic engineering view, the current industrial production, distribution, and use of N-fertilizers were built on an unsustainable foundation of fossil resources, and are energy-intensive, inefficient, and environmentally polluting.
1.2. Endeavor to Decarbonize N-Fertilizer Production
The production of N-fertilizer utilizing renewable resources to decarbonize the process has received attention on a global scale driven by the fast declining cost of solar and wind electricity, and sustainability concerns were further incentivized by public policy measures such as a carbon tax [12,13]. In general, research and development endeavors for decarbonizing nitrogen fixation follow either the nitrogen reduction route to fix nitrogen in the form of ammonia (NH3) or the oxidation path to produce nitrogen oxides (NOx).
Current efforts in the ammonia route focus on decarbonizing the production of ammonia, not just for fertilizer, but as a platform chemical and energy vector, striving to reduce the specific energy input while increasing the ammonia production rate to achieve Haber–Bosch parity [14]. As a transitional step, the Generation 1 technology for sustainable ammonia production, referred to as “blue ammonia”, involves the use of carbon sequestration or offsets to bring the net carbon impact of the ammonia production to near-zero [4]. Recently, two major technologies were extensively researched. The Generation 2 technology still produces ammonia from the HB process, but uses “green” hydrogen from water electrolysis instead of fossil resources, requiring an enormous amount of specialized water and being limited by the energy and economic efficiency of the electrolysis equipment. Further, the HB process must run continuously, and is poorly adaptive to the day–night cycle of solar energy or the intermittency of wind energy [4]. Generation 3 technologies bypass the HB process through the electrocatalytic nitrogen reduction reaction (eNRR) of N2 to NH3 with a promising energy efficiency, which has recently gained increasing attention [4]. However, the low ammonia production rate and system stability, the requirement for expensive ultra-dry and oxygen-free organic solvents, or pure nitrogen and hydrogen feedstocks, and platinum and lithium metal requirements are significant drawbacks of this pathway [4,15]. Other methods include the plasma-catalysis of gaseous H2 and N2 to NH3, metallocomplex nitrogen fixation, photochemical synthesis, and bio-catalysis, all of which are currently at the lab research stage, hardly to be practically applicable, scaled up, and economically competitive with the HB process [13,16,17].
In the oxidation path to fix nitrogen in the form of nitrogen oxides (NOx), air NTP plasma technologies should be the focus, practically because the free atmospheric air is the predominant source of chemically inert N2. It is noteworthy that the Birkeland–Eyde process to produce NOx is the first thermal-plasma-based N2-oxidation concept, which only utilizes around 3–4% of the applied energy in chemical reactions, the remaining applied energy being wasted as heat [18]. The theoretical energy efficiency of thermal plasma is 0.86 MJ/mol of nitrogen oxide (NO), which could be achieved under hypothetical conditions of 20–30 Bars, 3000–3500 K, and a 107 K/s cooling rate [19], higher than that of the HB process for N fixation into ammonia (0.48 MJ/mol ammonia produced). The renewed interest in plasma-based N fixation focuses on nonthermal or warm plasma, which has a lower theoretical energy cost than the HB process, and is projected to become a highly competitive alternative, if the actual energy cost can be significantly reduced [7]. In the case of NOx production, nonthermal plasma (NTP) has a much lower theoretical limit of energy consumption (0.2 MJ/mol in vacuum) than the HB process [20].
However, comparing the energy efficiency of different technologies for the production of ammonia or NOx could be misleading. Most of the studies in the oxidation path estimated the energy efficiency based on the end product of NOx gas (mainly the mixture of NO2 and NO, e.g., in [15,21,22,23,24,25,26,27]), which cannot be directly used as an N-fertilizer. NO needs to be further oxidized to NO2, which then dissolves in water to form nitric acid, but part of the NO2 would be reacted back to NO. Further, the nitrate fertilizer in the form of nitric acid is highly acidic, limiting its use to alkaline soil or otherwise requiring neutralization before use. For the production and application of N-fertilizers, we need to take a holistic view at the cost of practical end uses, as well as the cost to our health and environment.
1.3. Booming Renewable Electricity and Its Intermittency
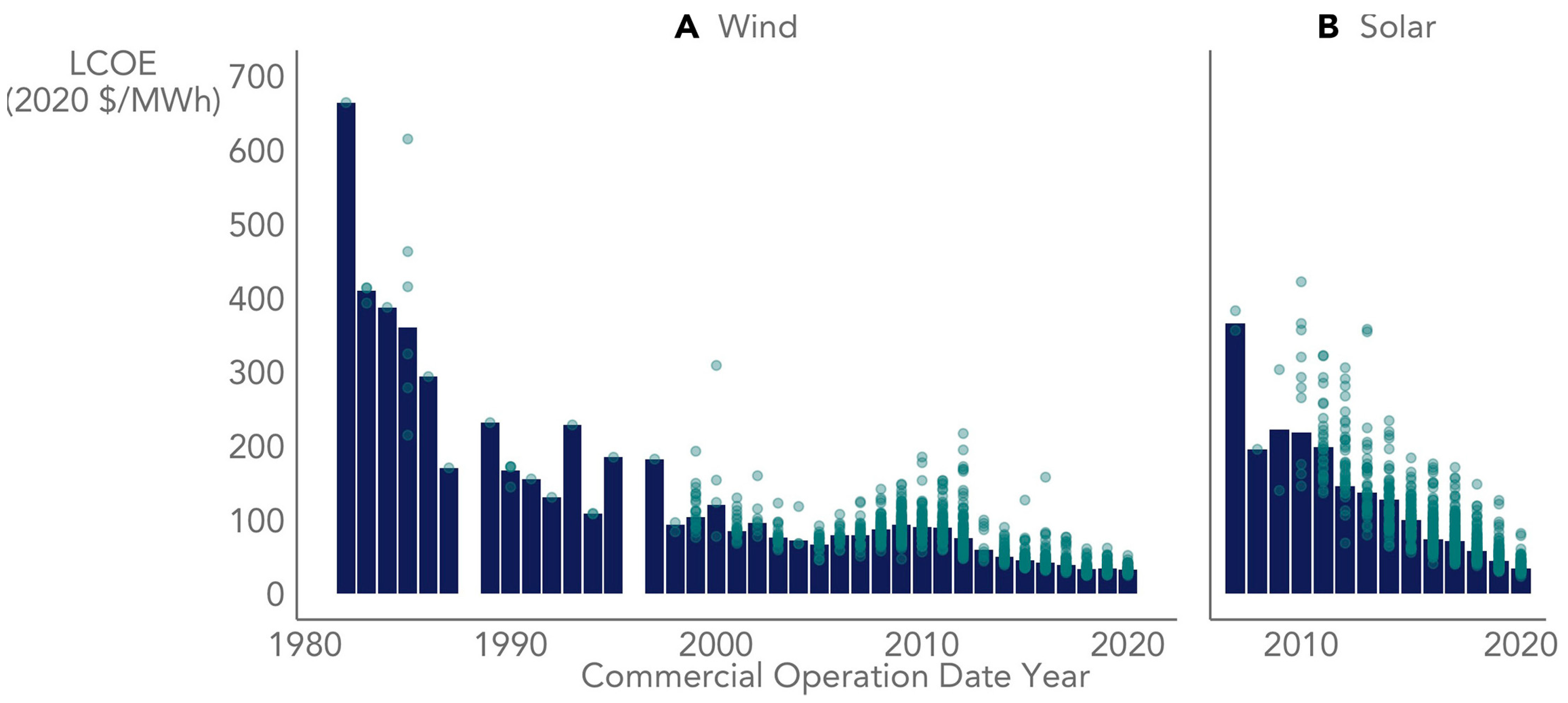
The industry of renewable electricity is fast expanding and the cost of solar/wind electricity is rapidly declining (Figure 1). A U.S. Department of Energy study found that renewable electricity generation from technologies that are commercially available today, in combination with a more flexible electric system, is more than adequate to supply 80% of total U.S. electricity generation in 2050 while meeting the electricity demand on an hourly basis in every region of the country. This study explores the implications and challenges of very high renewable electricity generation levels—from 30% up to 90%, focusing on 80%, of all U.S. electricity generation—in 2050. At such high levels of renewable electricity generation, the unique characteristics of some renewable resources, specifically the geographical distribution and variability and uncertainty in output, pose challenges to the operability of the nation’s electric system [28].
Figure 1.
Historical non-normalized levelized cost of electricity (LCOE) of utility-scale wind (A) and solar (B) energy in the United States [29].
Critics of renewable energy frequently highlight that technologies such as wind and solar only generate electricity when specific natural conditions are met—wind blowing or sun shining. They argue that the widespread adoption of renewable energy is hindered until cost-effective electricity storage technologies are developed. The challenge in integrating variable electricity sources arises from the grid’s historical design centered on large, controllable generators. Modern grid operators employ a three-phase planning approach to ensure power plants generate sufficient electricity precisely when needed to consistently and reliably meet demand. Due to the limited storage capacity within the grid, maintaining a continuous balance between the electricity supply and demand is critical to preventing potential blackout events or other systemic failures. Consequently, our modern power grid has been established with a significant overcapacity in order to satisfy the peak load demand in a narrow window of time [30]. Intermittent renewables are challenging because they disrupt the conventional methods for planning the daily operation of the electric grid.
In this respect, the decentralized production of N-fertilizers with NTP technologies are adaptive to the intermittency of renewable electricity and the need of the power grid by “storing” electricity into valuable chemicals, and have a high potential to change the landscape of N-fertilizers, because the production process can be easily switched on and off.
2. Perspective: Decarbonizing N-Fertilizer with NTP Technologies
2.1. Decentralized N-Fertilizer Production and Application Based on NTP
Nitrogen (N) is a critical element essential for optimal plant growth and development. Plants utilize nitrogen primarily in the forms of nitrate (NO3−) and ammonium (NH4+). Ammonium ions readily bind to negatively charged soil particles, preventing their leaching. Throughout the growing season, soil micro-organisms convert ammonium into nitrate, the primary form of nitrogen absorbed by plants [31]. However, microbial processes like denitrification also convert nitrate and nitrite (NO2−) into gaseous nitrogen forms, predominantly nitrogen (N2) and nitrous oxide (N2O) [32]. Nitrous oxide is a potent, long-lived greenhouse gas with 300 times the warming potential of CO2 [33]. Nitrate, being negatively charged, dissolves in water and moves through the soil with the soil water movement. During rainfall, nitrate can percolate downward through the soil profile, potentially entering tiles or drainage channels and becoming lost from agricultural production. This leaching process is the primary mechanism causing nitrogen loss from coarse-textured sandy soils. Nitrite is a less common form of nitrogen in the soil, and it is typically produced by the breakdown of organic matter or the action of nitrifying bacteria. Nitrite is also absorbed by plant roots, but it is less readily available to plants than nitrate and could be toxic to plants, especially at higher concentrations [34,35,36]. Each form of N-fertilizers has specific properties that determine when, where, and how various fertilizer materials can be used [31].
We need to re-examine our current system for N-fertilizer production and distribution established on the foundation of the HB process. A recent spike in the prices of N-fertilizers (in U.S., the farmer-paid price for anhydrous ammonia increased from $487/ton in 2020 to $1516/ton as of March 2022), due to the disruption of the natural gas supply chain, intensifies the alarm on this fragile and unsustainable system [37].
Given that over 86% of the global ammonia production involves either the direct application to soil as ammonia fertilizer or conversion into other forms such as ammonium, nitrate, and urea, it results in a complex distribution network, uneven access to nitrogen fertilizers, and significant application losses [9]. Therefore, there is an urgent and practical need to develop an N-fertilizer production system that utilizes renewable electricity (solar or wind) and NTP technologies for a sustainable decentralized production and application in the future. Such a system would utilize water, air, and electricity as the primary inputs to synthesize an aqueous mixture of nitrate and ammonium suitable for the direct application to crops. This approach facilitates local production at the farm level, allowing installation either independently or integrated with existing irrigation systems. It can operate effectively with an intermittent solar or wind electricity supply, resulting in zero carbon emissions. Furthermore, it can enable rapid startup and shutdown, promptly responding to weather fluctuations and local agricultural conditions such as the soil type, climate, crop varieties, and farming practices, for precision agriculture.
Prospectively, the implementation of such an N-fertilizer system will require the demonstration of the following: (1) a scalable modular system that can deliver sufficient aqueous N-fertilizer according to the demand of an agricultural land, small or large, (2) a sufficiently low electric energy cost to be economically competitive, and (3) evidence that the N-fertilizer product can be directly applied to grow crops/vegetables and totally replace synthetic N-fertilizers.
2.2. Plasma-Activated Water and the Applications in Agriculture
Plasma-activated water (PAW), commonly derived from the interaction of atmospheric plasma with water in a batch mode, has attracted widespread attention, with applications ranging from microbial decontamination in the food industry to medical wound healing. Importantly, it is considered a supplement to chemical fertilizers in agriculture because it is rich in fixed nitrogen in the forms of nitrate and nitrite. Other reported benefits of PAW in agriculture include enhancing seed germination, increasing rooting speed, promoting plant growth, enhancing drought tolerance and resistance to abiotic stresses, and controlling plant diseases and pests, due to the presence of reactive oxygen and nitrogen species (RONS), such as hydroxyl radicals (·OH), hydrogen peroxide (H2O2), ozone (O3), and nitrate/nitrite (NO3− and NO2−) [38,39,40,41]. Although there are reported negative effects of high-concentration PAW on plants [39,42], a recent study concluded that PAW application, even at high amounts, has no negative influence on the physicochemical properties of soil and it can be safely applied in sustainable, environmentally friendly agriculture [43].
Although the benefits of PAW for agriculture production deserve further research, PAW is not a well-defined product; different methods of generating PAW lead to different contents of reactive chemical species, and most of them are transient, which will ultimately affect the activity of the resulting PAW for different applications. To the prospect of this article, the fixed N content in PAW is insufficient to replace synthetic N-fertilizers.
2.3. On-Site Production of Liquid N-Fertilizer
As discussed in Section 2.1, the decentralized N-fertilizer production utilizing air, water, and renewable electricity holds significant promise for the on-site production of liquid N-fertilizer. This approach circumvents the need for the extensive capital investment and infrastructure required for ammonia production and storage, as well as the complex and costly distribution networks associated with synthetic N-fertilizers. Moreover, it eliminates the necessity for downstream separations or additional processes to obtain nitrate, ammonium, or urea, as the desired product would be a blend of nitrate and ammonium directly suitable for application to crops and vegetables.
The key to achieving such production capabilities lies in the development of nitrogen fixation technologies based on NTP in continuous reactors that can achieve a high productivity of nitrate and ammonium at sufficiently low electricity costs to be economically viable for agriculture. Recent research has increasingly focused on understanding the interaction between plasma and water, aiming to advance the fundamental knowledge at the largely unexplored interface of NTP and water for engineering development [44,45,46]. Despite advancements in energy efficiency in nitrogen fixation using NTP technologies, few reported developments have successfully balanced a high productivity with the low electricity costs required for continuous operation and practical applicability in agricultural settings.
2.3.1. Recent Development of a Continuous NTP Reaction System for N-Fertilizer
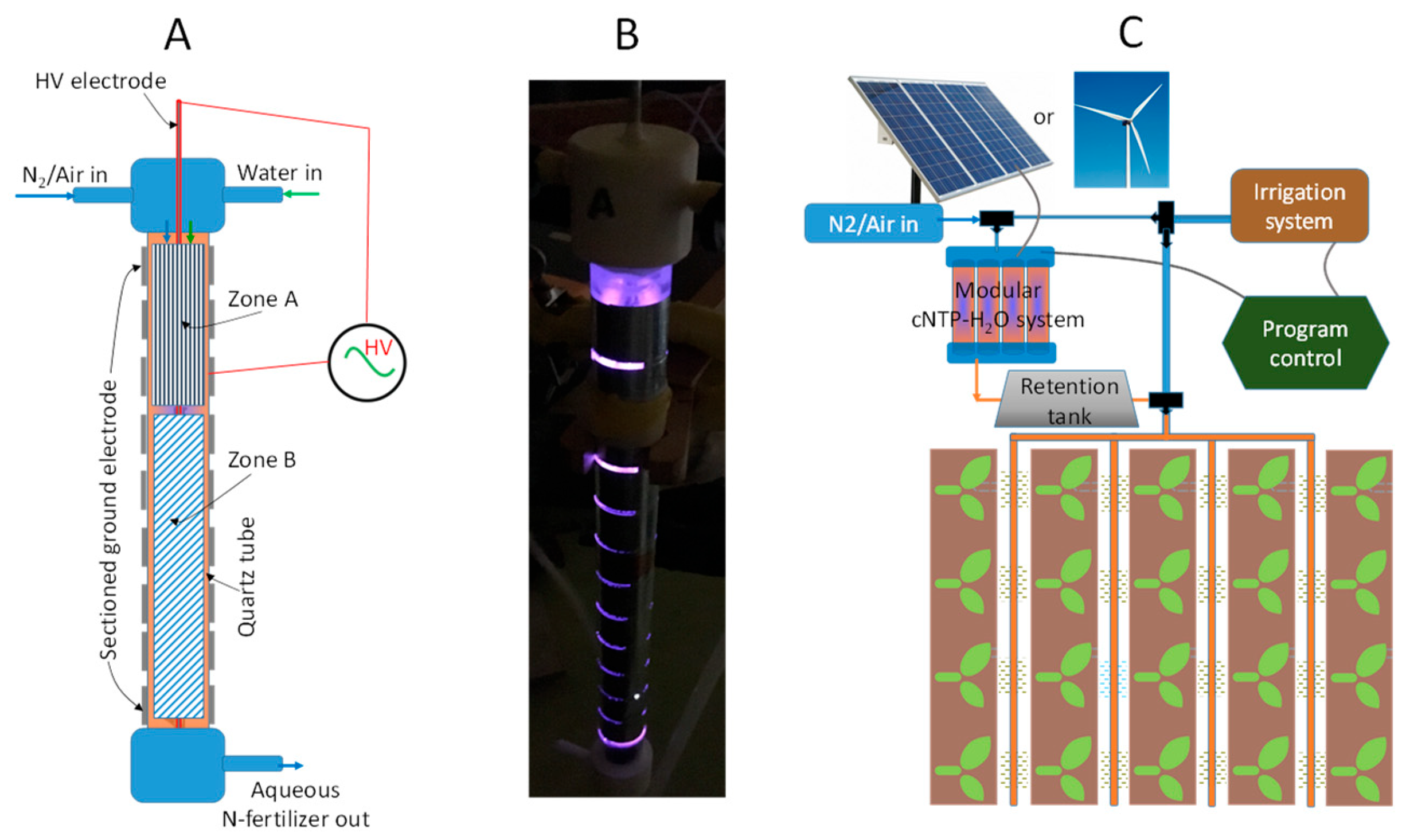
The author has recently developed a continuous NTP reaction system (denoted as cNTP-H2O hereafter) for the distributed on-site production and delivery of N-fertilizer [47], as presented in Figure 2. This design enables a high rate of water-flow-through operation, continuously delivering an aqueous N-fertilizer, mainly in the forms of nitrate and ammonium with a trace of nitrite.
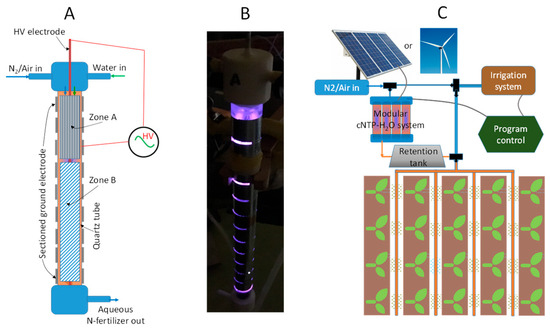
Figure 2.
(A) Schematic unit-cell design of the cNTP-H2O system; (B) photo image showing a discharging unit-cell against dark background; and (C) vision of integrating modular cNTP-H2O system with irrigation.
The modular cNTP-H2O system consists of uniquely designed unit-cells, featuring a two-zone, configurable high-voltage (HV) electrode and ground electrode to generate dielectric barrier discharge (DBD) plasma, as schematically presented in Figure 2A. Figure 2B shows the photo image of a discharging unit-cell against a dark background during operation. It is envisaged that the system can be scaled up by the parallel connection of a number of unit-cells and coupled to an irrigation line as illustrated in Figure 2C. Importantly, this invention establishes an innovative platform for research and development, which will allow us to configure the reactor (e.g., the shape, geometry, and metal materials for the two zones of HV electrode) and tune the product composition of nitrate/nitrite and ammonium, aiming to maximize the productivity of the N-fertilizer while minimizing the energy cost.
Interest in exploring the interaction between plasma and water has grown due to its potential to enhance the efficiency of nitrogen fixation, producing an aqueous N-fertilizer. Redox reactions induced by NTP occur not only at the plasma/air and plasma/water interfaces, but also in the volume of the aqueous phase [48]. Both the oxidation and reduction of nitrogen can be achieved using NTP with water, despite thermodynamic or kinetic limitations at ambient conditions, leading to the production of both NOx and ammonium [49]. Although this is still a largely unexplored area, good discussions on the mechanisms and pathways for the formation of reactive oxygen and nitrogen species (RONS) leading to the production of NOx and ammonium can be found in recent publications [44,45,46]. However, most highly reactive plasma species have a very short half-life in the order of a few nanoseconds to a few milliseconds. Therefore, it is important to consider not only the generation of these reactive species, but also their efficient delivery into liquid products when designing an NTP reaction system.
In the design of the cNTP-H2O system, both water and air simultaneously flow through plasma discharge zones, intensifying the interfacial reactions to enhance mass transfer and tune the production of fixed nitrogen continuously. In addition to common RONS generated in air plasma, the presence of water in the plasma also generates solvated electrons, hydrogen radicals (H•), hydroxyl radicals (•OH), perhydroxyl radicals (HOO•), and hydrogen peroxide (H2O2), which are among the strongest reduction or oxidation agents for nitrogen fixation [48,50,51]. The presence of water helps quench the RONS produced by NTP, rapidly converting them into more stable N-containing compounds. This quenching effect helps prevent the back reactions of RONS. Water also acts as an absorption medium in situ for the fixed N, effectively removing it from the gas phase where back reactions are more likely to occur, shifting the equilibrium towards product formation.
For the cNTP-H2O system, we designed multiple plasma discharge regimes within one unit-cell, which make efficient utilization of the applied power and reduce energy consumption. As an example, the generation of RONS and reactive hydrogen species can be greatly enhanced as the glow discharge acts as an excitation pre-treatment in Zone A prior to the filament discharge in Zone B. The designed turbulent flows of air and water transiently pass through the multiple plasma discharge regimes, undergoing intensified contact and mixing, while the produced NOx and ammonium are absorbed by water in situ, further driving reactions toward a higher yield of fixed nitrogen.
Different configurations of the cNTP-H2O system were tested and key results are summarized in Table 1, which also includes preliminary economic estimations. Table 1 shows the significant progress of improving the energy efficiency and production rate of fixed nitrogen by configuring the cNTP-H2O system and operation parameters.

Table 1.
Test results of the cNTP-H2O system with preliminary economic estimations.
There is potential to significantly improve the performance of the cNTP-H2O system through systematic optimization. A major challenge arises from balancing minimizing the energy cost and maximizing the production rate, which is a complex function of a multitude of factors including the plasma discharge type, the reactor geometry/configuration, the discharge operating parameters, and the presence of catalysts. Two targets that could be potentially achieved after the optimization for practical implementation are also listed in Table 1. Target 1 has a high optimized production rate, and Target 2 has a high optimized energy efficiency. If one of the targets is achieved, solar panels with a surface area of only about 1% of a land area will produce sufficient N-fertilizer for the land. Although the specific energy cost is still higher than that of the HB process, they are potentially viable at the farm site without the emissions and distribution costs associated with the HB process.
2.3.2. Implications and Significance of the cNTP-H2O System
The implications for further development are listed below:
- Hydrogen is the limiting reactant in the reduction pathway to make ammonium. This limiting reactant could be supplemented by H2 gas or ethanol, and potentially by methane, which can be generated at the farm site via anaerobic digestion; however, the use of an extra hydrogen source or enriched N2 or O2 input will inevitably increase the cost for the production of the N-fertilizer.
- Compared to the reduction pathway for making ammonium, the oxidation pathway to produce an aqueous N-fertilizer rich in nitrate from air and water is advantageous; the key is to minimize the energy cost, and the content of nitrite to avoid toxicity to plants.
- Using air as a feed gas to the cNTP-H2O system, the fixed N in the product is dominantly in the form of nitrate, with less than 10 ppm of nitrite in all the cases listed in Table 1, which can be directly utilized by plants. Importantly, the nitrate concentration reached an unprecedented 380 ppm (Entry G in Table 1), ideal for fertigation.
- It appeared that the metal material of the electrode that was in contact with reactants affected the performance. Therefore, catalysts can be added onto the electrode (e.g., coating/embedding catalysts onto the electrode), which may potentially improve the reaction kinetics and product yield significantly.
- With the non-equilibrium DBD plasma, the cNTP-H2O system runs at non-equilibrium steady states; thus, the NTP thermodynamics and kinetics, transport processes, and chemical reaction kinetics all influence the production rate and product composition. Therefore, it is possible, based on the cNTP-H2O platform, to further improve the production rate and specific electric energy consumption through the optimization of the process parameters and configurations.
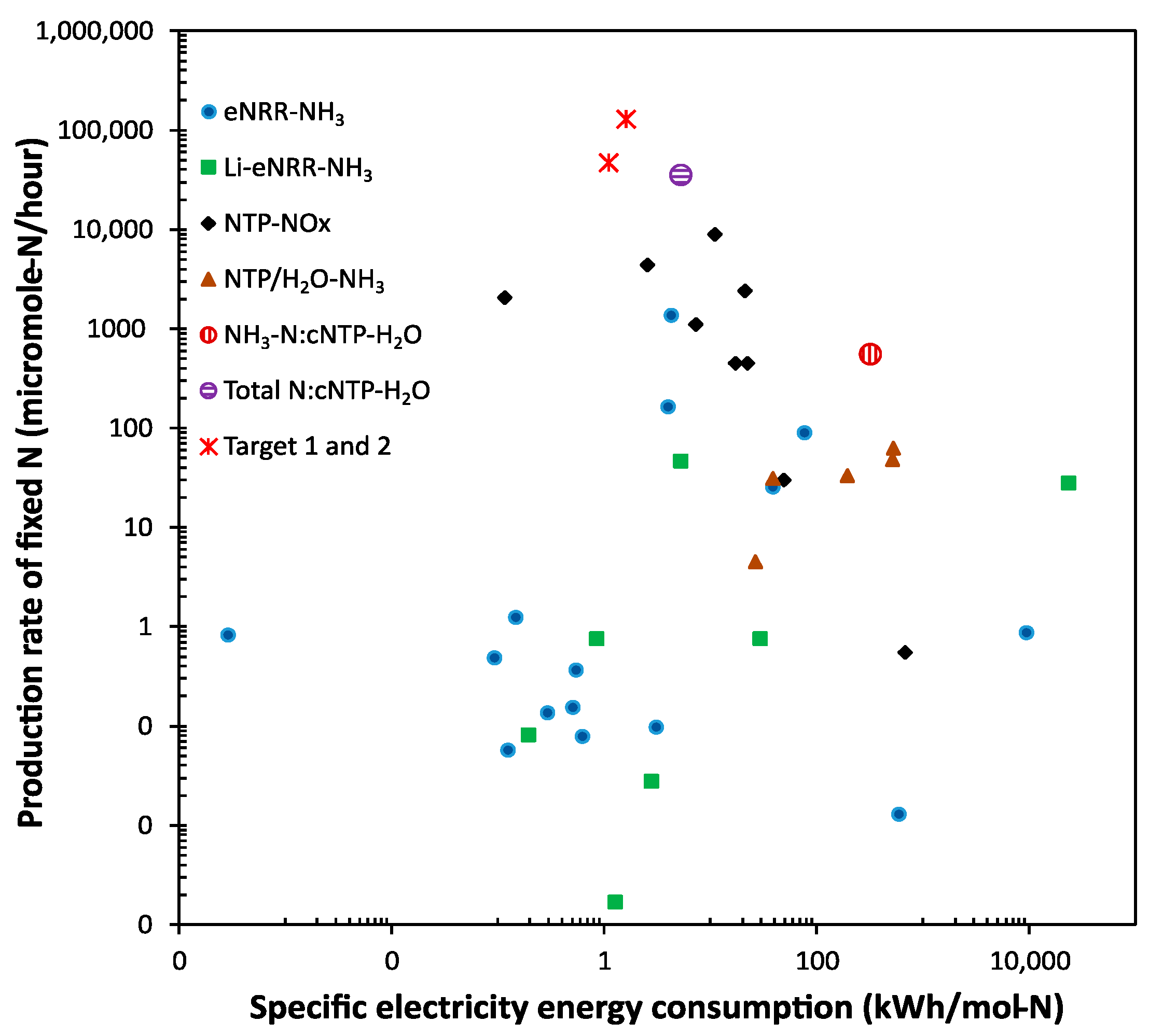
The significance of the results can be revealed by benchmarking against published data, as shown in Figure 3. Because there are different technologies using different feedstocks for nitrogen fixation (e.g., air, N2, or artificially mixed N2/O2 with various ratios as the nitrogen source; and pure H2 or water as the hydrogen source), preventing straightforward and fair comparisons, we benchmark against four categories of promising technologies: (1) the electrocatalytic nitrogen reduction of N2 to NH3 (eNRR-NH3) [15,56,57,58,59,60,61,62,63,64,65,66,67], (2) the lithium-mediated electrocatalytic nitrogen reduction of N2 to NH3 (Li-eNRR-NH3) [68,69,70,71,72,73,74], (3) the plasma-driven NOx production (NTP-NOx) [15,21,22,23,24,25,26], and (4) the plasma-driven NH3 production from N2 and water (NTP/H2O-NH3) [75,76,77,78,79].
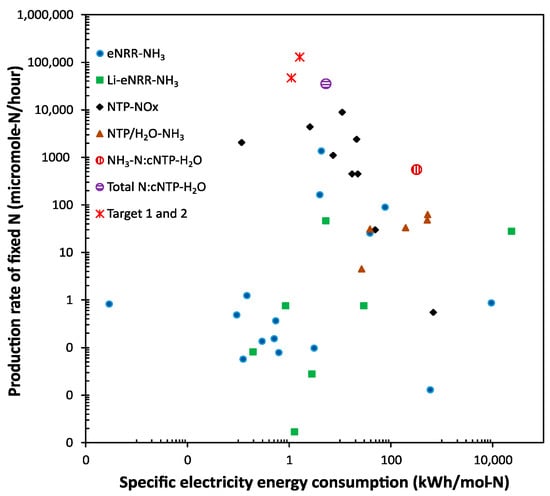
Figure 3.
Benchmarking the performance of the cNTP-H2O system against literature data.
It should be pointed out that: (1) although the research effort worldwide focuses on reducing the specific energy consumption to achieve Haber–Bosch parity, both a high production rate and low specific energy consumption are required for practical implementation; (2) we had to use the logarithmic scale for the plot in Figure 3 in order to capture the large variation in both the production rate and specific energy consumption reported in the literature; (3) except for the NTP-NOx category, most other technologies require N2 gas instead of air as feedstock, potentially increasing the production cost; and, (4) even in the NTP-NOx category, the artificial mixture of N2/O2 with various ratios, instead of air, were commonly used to improve the NOx yield, and the reported yields were based on the measurement of the produced NOx gas, not ready to be used as N-fertilizer. Nonetheless, Figure 3 provides an overview of the significant status of the cNTP-H2O system.
For the eNRR-NH3 and Li-eNRR-NH3 technologies to make ammonia, although some achieved a very low specific energy consumption, the production rates were too low for practical applications; the only promising one in the eNRR-NH3 category used a hybrid method, in which NTP was first used to produce NOx, then the NOx was fed to an eNRR cell to make ammonia [15]. Furthermore, a low system stability, the requirement for expensive ultra-dry and oxygen-free organic solvents, or the demand for pure nitrogen and hydrogen feedstocks, and platinum and lithium metal, result in low levels of technology readiness [4,15].
Generally, the NTP-NOx technologies have a higher production rate and moderate specific energy consumption, but most of these studies reported NO as the major product. Although a recent study in this category [26] claimed a record-low specific energy consumption of 0.42 MJ per mol of fixed N (mainly NO) by using a pulsed plasma jet, the production rate is very low (~480 μg-N/min).
Comparing the cNTP-H2O result (Entry C in Table 1) with those reported using NTP/H2O-NH3 technologies and N2 gas as the feed for ammonia production, a much higher production rate was achieved using the cNTP-H2O system, although the specific energy consumption is at the higher end of this category (but it is at the lower end considering the total fixed N). In terms of the total fixed N using air as the feed, we achieved the highest production rate compared with others in the category of NTP-NOx, and the specific energy consumption is at the lower end (Entry G in Table 1). Importantly, the product of the cNTP-H2O system is an aqueous mixture of nitrate and ammonium, which can be directly applied to plants.
Another important consideration in the development of the cNTP-H2O system is practicality. Although the concentration of the fixed N in the aqueous N-fertilizer product (Entry G in Table 1) is about 87 mg-N/L, mainly in the form of NO3−, the pH is nearly neutral, which can be directly applied to plants without neutralization. This is because tap water has a buffering effect due to its carbonate hardness (KH) and general hardness (GH). Especially, the GH measures the concentration of calcium, magnesium, and other mineral ions present in the water, ranging from 10 to over 300 mg/L depending on the locations. In fact, nitric acid is used to dissolve the mineral deposition in irrigation systems [80], and calcium/magnesium nitrates are excellent fertilizers. Further, the pH of the aqueous N-fertilizer could be tuned by adjusting the process parameters of the cNTP-H2O system according to specific applications, e.g., for citrus trees that prefer slightly acidic soil, or for alkaline soil.
3. Conclusions and Prospects
With the advancement in the fundamental sciences of nonthermal plasma and engineering development in recent years, the implementation of the decentralized on-site production of N-fertilizer is urgently needed and practically feasible. It has taken more than a century of continuous development and optimization for the HB process to achieve its current level of energy efficiency of approximately 0.5 MJ/mol, which is near its theoretical limit. However, we must point out that the HB process is just one step in the extensive chain of the synthetic N-fertilizer production and distribution entailing heavy emissions and pollutions, which could be largely bypassed by implementing the on-site production of the N-fertilizer using NTP technologies.
We would like to point out the following for accelerating the implementation:
- (1)
- Minimizing the electric energy cost with NTP technologies without sacrificing productivity should be the priority, because this is directly related to the economic competitiveness. This requires the fundamental research of plasma physics and chemistry, and the reaction kinetics and engineering, especially at the NTP/water interface. Further, the design of the electric power supply directly converting solar or wind electricity to high voltage for better efficiency, although it is out of the scope of this article, is an important aspect for the on-site N-fertilizer production that will certainly improve the performance and energy efficiency. For example, nanosecond-pulsed high voltage has the potential to significantly reduce plasma energy consumption [81], but this type of power supply is expensive. We call on the development of efficient and inexpensive power supplies dedicated to N-fertilizer production with NTP technologies.
- (2)
- The involvement of agronomists, plant scientists, and soil scientists is crucial for the implementation of the on-site N-fertilizer production and application. As for any disruptive technologies, the new technologies and N-fertilizer products require testing and validation, involving field tests, nutrient uptake studies, soil health monitoring, crop management and improvement, the optimization of fertilizer application, farmer education and training, and an economic and environmental impact assessment. In some cases, the implementation may require the adjustment of current agricultural practices.
- (3)
- In the early stage of a new technology, process modeling and techno-economic analysis (TEA) can help to assess potential economic feasibilities, bottlenecks, and operation targets for process improvement, and identify further research and development effort requirements. Life cycle analysis (LCA) will evaluate the reduction of greenhouse gas emissions and the environmental impact, resource efficiency, the impact on the ecosystem and human health, and long-term sustainability. Results from the TEA and LCA will also help mitigate socioeconomic and behavioral challenges in adopting new technologies, facilitating the effort of technology transfer for real-world applications.
Opportunities for the on-site N-fertilizer production with NTP technologies are already here.
Agrivoltaics is an innovative approach that combines solar energy production with agriculture on the same land, offering dual land use, a symbiotic relationship, and resource efficiency. Agrivoltaics is wide-spreading globally, and there are more than 300 identified agrivoltaic projects in the United States, representing over 2.8 GW of solar capacity [82]. Assuming only 600 h of usage of the solar capacity for a crop-growing season and that the energy cost of the on-site N-fertilizer production reaches 0.5 MJ/mol-N of Haber–Bosch parity, the 2.8 GW solar capacity would produce 0.17 million tons of fixed N, equivalent to 0.5 million tons of ammonium nitrate fertilizer on site, cutting down the CO2 emission from the production alone by 0.8 million tons [83].
The NTP-produced N-fertilizer can be combined with animal waste management to treat animal manure, resulting in antimicrobial effects and a higher N content compared to untreated manure, and reducing ammonia and methane emissions [84,85].
Controlled Environment Agriculture (CEA) is an advanced approach to food production that uses technology to create optimal growing conditions for crops. We expect that CEA is ideal for starting the implementation of NTP-produced N-fertilizer, because of its investment in emerging technologies and the need to use nitrate N-fertilizer [86]. A current trend in new CEA facilities is to implement micro-grids of renewable electricity [87], thus enabling local N-fertilizer production with NTP technologies.
Funding
The author would like to thank the support of the U.S. Department of Agriculture S-1075 Multistate Project, and the University of Tennessee Institute of Agriculture (UTIA) AgResearch Seed Grant.
Conflicts of Interest
The author has a patent pending related to the cNTP-H2O system.
References
- Lim, J.; Fernández, C.A.; Lee, S.W.; Hatzell, M.C. Ammonia and Nitric Acid Demands for Fertilizer Use in 2050. ACS Energy Lett. 2021, 6, 3676–3685. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Gielen, D.; Bennaceur, K.; Kerr, T.; Tam, C.; Tanaka, K.; Taylor, M.; Taylor, P. Tracking Industrial Energy Efficiency and CO2 Emissions; IEA: Paris, France, 2007. [Google Scholar]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Zhou, X.; Passow, F.H.; Rudek, J.; von Fisher, J.C.; Hamburg, S.P.; Albertson, J.D. Estimation of methane emissions from the U.S. ammonia fertilizer industry using a mobile sensing approach. Elem. Sci. Anthr. 2019, 7, 19. [Google Scholar] [CrossRef]
- Ahlgren, S.; Baky, A.; Bernesson, S.; Nordberg, Å.; Norén, O.; Hansson, P.-A. Ammonium nitrate fertiliser production based on biomass—Environmental effects from a life cycle perspective. Bioresour. Technol. 2008, 99, 8034–8041. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Jardali, F.; Bogaerts, A.; Lefferts, L. From the Birkeland–Eyde process towards energy-efficient plasma-based NOX synthesis: A techno-economic analysis. Energy Environ. Sci. 2021, 14, 2520–2534. [Google Scholar] [CrossRef]
- Searles, R. Pollution from Nitric Acid Plants. Platin. Met. Rev. 1973, 17, 57–63. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Bonilla Cedrez, C.; Chamberlin, J.; Guo, Z.; Hijmans, R.J. Spatial variation in fertilizer prices in Sub-Saharan Africa. PLoS ONE 2020, 15, e0227764. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Engelmann, Y.; van ‘t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-driven catalysis: Green ammonia synthesis with intermittent electricity. Green Chem. 2020, 22, 6258–6287. [Google Scholar] [CrossRef]
- Fernandez, C.A.; Hatzell, M.C. Editors’ Choice—Economic Considerations for Low-Temperature Electrochemical Ammonia Production: Achieving Haber-Bosch Parity. J. Electrochem. Soc. 2020, 167, 143504. [Google Scholar] [CrossRef]
- Sun, J.; Alam, D.; Daiyan, R.; Masood, H.; Zhang, T.; Zhou, R.; Cullen, P.J.; Lovell, E.C.; Jalili, A.; Amal, R. A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy Environ. Sci. 2021, 14, 865–872. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Ammonia, 4. Green Ammonia Production. In Ullmann’s Encyclopedia of Industrial Chemistry; Verlag Chemie: Hoboken, NJ, USA, 2020; pp. 1–20. [Google Scholar]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. Process Intensif. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Patil, B.S.; Wang, Q.; Hessel, V.; Lang, J. Plasma N2-fixation: 1900–2014. Catal. Today 2015, 256, 49–66. [Google Scholar] [CrossRef]
- Rusanov, V.D.; Fridman, A.A.; Sholin, G.V. The physics of a chemically active plasma with nonequilibrium vibrational excitation of molecules. Sov. Phys. Uspekhi 1981, 24, 447–474. [Google Scholar] [CrossRef]
- Li, S.; Medrano, J.A.; Hessel, V.; Gallucci, F. Recent Progress of Plasma-Assisted Nitrogen Fixation Research: A Review. Processes 2018, 6, 248. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Process. Polym. 2017, 14, 1600207. [Google Scholar] [CrossRef]
- Nakaso, T.; Harigai, T.; Kusumawan, S.A.; Shimomura, T.; Tanimoto, T.; Suda, Y.; Takikawa, H. Multi-spark discharge system for preparation of nutritious water. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2018; Volume 1929. [Google Scholar]
- Ogawa, K.; Oh, J.-S.; Gaur, N.; Hong, S.-H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D.; Ito, M.; Szili, E.J. Modulating the concentrations of reactive oxygen and nitrogen species and oxygen in water with helium and argon gas and plasma jets. Jpn. J. Appl. Phys. 2019, 58, SAAB01. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Comparative study of discharge schemes for production rates and ratios of reactive oxygen and nitrogen species in plasma activated water. J. Phys. D Appl. Phys. 2019, 52, 385202. [Google Scholar] [CrossRef]
- Uchida, G.; Takenaka, K.; Takeda, K.; Ishikawa, K.; Hori, M.; Setsuhara, Y. Selective production of reactive oxygen and nitrogen species in the plasma-treated water by using a nonthermal high-frequency plasma jet. Jpn. J. Appl. Phys. 2018, 57, 0102B4. [Google Scholar] [CrossRef]
- Vervloessem, E.; Gorbanev, Y.; Nikiforov, A.; De Geyter, N.; Bogaerts, A. Sustainable NOx production from air in pulsed plasma: Elucidating the chemistry behind the low energy consumption. Green Chem. 2022, 24, 916–929. [Google Scholar] [CrossRef]
- Muzammil, I.; Lee, D.H.; Dinh, D.K.; Kang, H.; Roh, S.A.; Kim, Y.-N.; Choi, S.; Jung, C.; Song, Y.-H. A novel energy efficient path for nitrogen fixation using a non-thermal arc. RSC Adv. 2021, 11, 12729–12738. [Google Scholar] [CrossRef] [PubMed]
- Hand, M.M.B.S.; DeMeo, E.; Reilly, J.M.; Mai, T.; Arent, D.; Porro, G.; Meshek, M.; Sandor, D. Renewable Electricity Futures Study; DOE, Ed.; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Bolinger, M.; Wiser, R.; O’Shaughnessy, E. Levelized cost-based learning analysis of utility-scale wind and solar in the United States. iScience 2022, 25, 104378. [Google Scholar] [CrossRef]
- Sovacool, B.K. The intermittency of wind, solar, and renewable electricity generators: Technical barrier or rhetorical excuse? Util. Policy 2009, 17, 288–296. [Google Scholar] [CrossRef]
- Mengel, D.B. Types and Uses of Nitrogen Fertilizers for Crop Production; Purdue University and U.S. Department of Agriculture cooperating: Cooperative Extension work in Agriculture and Home Economics, State of Indiana. 2020. Available online: https://www.extension.purdue.edu/extmedia/AY/AY-204.html (accessed on 11 April 2024).
- Takai, K. The Nitrogen Cycle: A Large, Fast, and Mystifying Cycle. Microbes Environ. 2019, 34, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann Bot 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Osawa, T. Nitrite toxicities in vegetable crops I. Effect of nitrite and pH levels in nutrient solution on growth of vegetable crops. J. Jpn. Soc. Hortic. Sci. 1971, 40, 395–400. [Google Scholar] [CrossRef]
- Oke, O.L. Nitrite Toxicity to Plants. Nature 1966, 212, 528. [Google Scholar] [CrossRef]
- Schnitkey, G.; Paulson, N.; Zulauf, C.; Swanson, K.; Colussi, J.; Baltz, J. Weekly Farm Economics: Nitrogen Fertilizer Prices and Supply in Light of the Ukraine-Russia Conflict. Farmdoc Dly. 2022, 12, 45. [Google Scholar]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Dushkov, V.Y.; Budnik, M.I.; Belyakov, A.M.; Kulik, K.N.; et al. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161. [Google Scholar] [CrossRef]
- Subramanian, P.S.G.; J, A.; P, L.; Rao, H.; Shivapuji, A.M.; Girard-Lauriault, P.-L.; Rao, L. Plasma-activated water from DBD as a source of nitrogen for agriculture: Specific energy and stability studies. J. Appl. Phys. 2021, 129, 090401. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Kong, T.-K.; Lin, C.-M.; Chen, H.-L. The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Appl. Sci. 2021, 11, 5304. [Google Scholar] [CrossRef]
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357. [Google Scholar] [CrossRef]
- Huang, Z.; Xiao, A.; Liu, D.; Lu, X.; Ostrikov, K. Plasma-water-based nitrogen fixation: Status, mechanisms, and opportunities. Plasma Process. Polym. 2022, 19, 2100198. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Addy, M.; Cheng, Y.; Zhang, Y.; Anderson, E.; Zhou, N.; Schiappacasse, C.; Hatzenbeller, R.; Fan, L.; et al. In situ plasma-assisted atmospheric nitrogen fixation using water and spray-type jet plasma. Chem. Commun. 2018, 54, 2886–2889. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, Y. Sustainable nitrogen fixation by plasma-liquid interactions. Cell Rep. Phys. Sci. 2023, 4, 101618. [Google Scholar] [CrossRef]
- Ye, X. Non-Thermal Plasma Reaction Assembly for Continuous Aqueous Nitrogen-Based Fertilizer Production and Method Thereof. University of Tennessee Research Foundation: Provisional Application for Patent UTRF 24035-01 (8597-3017-1), 12 December 2023. [Google Scholar]
- Volkov, A.G.; Bookal, A.; Hairston, J.S.; Roberts, J.; Taengwa, G.; Patel, D. Mechanisms of multielectron reactions at the plasma/water interface: Interfacial catalysis, RONS, nitrogen fixation, and plasma activated water. Electrochim. Acta 2021, 385, 138441. [Google Scholar] [CrossRef]
- Sakakura, T.; Murakami, N.; Takatsuji, Y.; Haruyama, T. Nitrogen Fixation in a Plasma/Liquid Interfacial Reaction and Its Switching between Reduction and Oxidation. J. Phys. Chem. C 2020, 124, 9401–9408. [Google Scholar] [CrossRef]
- Rumbach, P.; Bartels, D.M.; Sankaran, R.M.; Go, D.B. The solvation of electrons by an atmospheric-pressure plasma. Nat. Commun. 2015, 6, 7248. [Google Scholar] [CrossRef]
- Liu, J.; He, B.; Chen, Q.; Li, J.; Xiong, Q.; Yue, G.; Zhang, X.; Yang, S.; Liu, H.; Liu, Q.H. Direct synthesis of hydrogen peroxide from plasma-water interactions. Sci. Rep. 2016, 6, 38454. [Google Scholar] [CrossRef]
- Manley, T.C. The electric characteristics of the ozonator discharge. Trans. Electrochem. Soc. 1943, 84, 12. [Google Scholar] [CrossRef]
- Xia, Y.; Kwon, H.; Wander, M. Developing county-level data of nitrogen fertilizer and manure inputs for corn production in the United States. J. Clean. Prod. 2021, 309, 126957. [Google Scholar] [CrossRef]
- Ramasamy, V.; Feldman, D. U.S. Solar Photovoltaic System and Energy Storage Cost Benchmarks: Q1 2021; G. National Renewable Energy Lab. (NREL): Golden, CO, USA, 2021. [Google Scholar]
- RAYmaps. How to Calculate the Surface Area Required by Solar Panels. Available online: https://www.raymaps.com/index.php/how-to-calculate-the-area-required-by-solar-panels/ (accessed on 10 June 2024).
- Lamichhane, P.; Adhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 045026. [Google Scholar] [CrossRef]
- Kumari, S.; Pishgar, S.; Schwarting, M.E.; Paxton, W.F.; Spurgeon, J.M. Synergistic plasma-assisted electrochemical reduction of nitrogen to ammonia. Chem. Commun. 2018, 54, 13347–13350. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, D.; Azofra, L.M.; Harb, M.; Cavallo, L.; Jalili, R.; Mitchell, D.R.G.; Chatti, M.; MacFarlane, D.R. MoS2 Polymorphic Engineering Enhances Selectivity in the Electrochemical Reduction of Nitrogen to Ammonia. ACS Energy Lett. 2019, 4, 430–435. [Google Scholar] [CrossRef]
- Shi, M.-M.; Bao, D.; Wulan, B.-R.; Li, Y.-H.; Zhang, Y.-F.; Yan, J.-M.; Jiang, Q. Au Sub-Nanoclusters on TiO2 toward Highly Efficient and Selective Electrocatalyst for N2 Conversion to NH3 at Ambient Conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Lim, A.; Yoon, C.; Jang, J.H.; Ham, H.C.; Han, J.; Nam, S.; Kim, D.; Sung, Y.-E.; Choi, J.; et al. Electrochemical Synthesis of NH3 at Low Temperature and Atmospheric Pressure Using a γ-Fe2O3 Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 10986–10995. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, L.X.; Chen, G.F.; Yang, X.; Wang, H. Ammonia Synthesis Under Ambient Conditions: Selective Electroreduction of Dinitrogen to Ammonia on Black Phosphorus Nanosheets. Angew. Chem. Int. Ed. Engl. 2019, 58, 2612–2616. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Zhang, Q.; Meng, F.-L.; Zhong, H.-X.; Shi, M.-M.; Zhang, Y.; Yan, J.-M.; Jiang, Q.; Zhang, X.-B. Electrochemical Reduction of N2 under Ambient Conditions for Artificial N2 Fixation and Renewable Energy Storage Using N2/NH3 Cycle. Adv. Mater. 2017, 29, 1604799. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, L.; Liu, X.; Tang, C.; Xu, W.; Chen, S.; Song, L.; Zheng, Y.; Qiao, S.-Z. Nitrogen Vacancies on 2D Layered W2N3: A Stable and Efficient Active Site for Nitrogen Reduction Reaction. Adv. Mater. 2019, 31, 1902709. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, L.; Hu, L.; Chen, G.; Xin, H.; Feng, X. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat. Commun. 2018, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon-Nanotube-Based Electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Bao, D.; Shi, M.-M.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Amorphizing of Au Nanoparticles by CeOx–RGO Hybrid Support towards Highly Efficient Electrocatalyst for N2 Reduction under Ambient Conditions. Adv. Mater. 2017, 29, 1700001. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Cullen, D.A.; Karakalos, S.G.; Liu, K.; Zhang, H.; Zhao, S.; Xu, H.; More, K.L.; Wang, G.; Wu, G. Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes. Nano Energy 2018, 48, 217–226. [Google Scholar] [CrossRef]
- Lazouski, N.; Chung, M.; Williams, K.; Gala, M.L.; Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 2020, 3, 463–469. [Google Scholar] [CrossRef]
- Lazouski, N.; Schiffer, Z.J.; Williams, K.; Manthiram, K. Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction. Joule 2019, 3, 1127–1139. [Google Scholar] [CrossRef]
- Lee, H.K.; Koh, C.S.L.; Lee, Y.H.; Liu, C.; Phang, I.Y.; Han, X.; Tsung, C.-K.; Ling, X.Y. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci. Adv. 2018, 4, eaar3208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Azofra, L.M.; Ali, M.; Kar, M.; Simonov, A.N.; McDonnell-Worth, C.; Sun, C.; Zhang, X.; MacFarlane, D.R. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ. Sci. 2017, 10, 2516–2520. [Google Scholar] [CrossRef]
- Andersen, S.Z.; Čolić, V.; Yang, S.; Schwalbe, J.A.; Nielander, A.C.; McEnaney, J.M.; Enemark-Rasmussen, K.; Baker, J.G.; Singh, A.R.; Rohr, B.A.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoo, C.-Y.; Kim, J.-N.; Yoon, H.C.; Han, J.-I. Electrochemical Synthesis of Ammonia from Water and Nitrogen in Ethylenediamine under Ambient Temperature and Pressure. J. Electrochem. Soc. 2016, 163, F1523. [Google Scholar] [CrossRef]
- Chen, G.-F.; Cao, X.; Wu, S.; Zeng, X.; Ding, L.-X.; Zhu, M.; Wang, H. Ammonia Electrosynthesis with High Selectivity under Ambient Conditions via a Li+ Incorporation Strategy. J. Am. Chem. Soc. 2017, 139, 9771–9774. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Schiappacasse, C.; Zhou, N.; Addy, M.; Cheng, Y.; Zhang, Y.; Anderson, E.; Chen, D.; Wang, Y.; Liu, Y.; et al. Plasma in situ gas—Liquid nitrogen fixation using concentrated high-intensity electric field. J. Phys. D Appl. Phys. 2019, 52, 494001. [Google Scholar] [CrossRef]
- Toth, J.R.; Abuyazid, N.H.; Lacks, D.J.; Renner, J.N.; Sankaran, R.M. A Plasma-Water Droplet Reactor for Process-Intensified, Continuous Nitrogen Fixation at Atmospheric Pressure. ACS Sustain. Chem. Eng. 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Hawtof, R.; Ghosh, S.; Guarr, E.; Xu, C.; Mohan Sankaran, R.; Renner, J.N. Catalyst-free, highly selective synthesis of ammonia from nitrogen and water by a plasma electrolytic system. Sci. Adv. 2019, 5, eaat5778. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Vervloessem, E.; Nikiforov, A.; Bogaerts, A. Nitrogen Fixation with Water Vapor by Nonequilibrium Plasma: Toward Sustainable Ammonia Production. ACS Sustain. Chem. Eng. 2020, 8, 2996–3004. [Google Scholar] [CrossRef]
- Indumathy, B.; Ananthanarasimhan, J.; Rao, L.; Yugeswaran, S.; Ananthapadmanabhan, P.V. Catalyst-free production of ammonia by means of interaction between a gliding arc plasma and water surface. J. Phys. D Appl. Phys. 2022, 55, 395501. [Google Scholar] [CrossRef]
- Chant, J. How Nitric Acid is used in the Horticulture Industry. Available online: https://www.monarchchemicals.co.uk/Information/News-Events/795-/How-Nitric-Acid-is-used-in-the-Horticulture-Industry (accessed on 2 June 2024).
- Huiskamp, T. Nanosecond pulsed streamer discharges Part I: Generation, source-plasma interaction and energy-efficiency optimization. Plasma Sources Sci. Technol. 2020, 29, 023002. [Google Scholar] [CrossRef]
- DOE, Agrivoltaics: Solar and Agriculture Co-Location. Office of Energy Efficiency & Renewable Energy, Department of Energy. Available online: https://www.energy.gov/eere/solar/agrivoltaics-solar-and-agriculture-co-location (accessed on 4 May 2024).
- Mares, B.P.F.A.J.W. Greenhouse Gas Index for Products in 39 Industrial Sectors: Nitrogenous Fertilizer Manufacturing. NAICS CODE 325311. Resources for the Future. 2022. Available online: https://media.rff.org/documents/WP_22-16_M8.pdf (accessed on 11 May 2024).
- Mousavi, H.; Cottis, T.; Pommeresche, R.; Dörsch, P.; Solberg, S.Ø. Plasma-Treated Nitrogen-Enriched Manure Does Not Impose Adverse Effects on Soil Fauna Feeding Activity or Springtails and Earthworms Abundance. Agronomy 2022, 12, 2314. [Google Scholar] [CrossRef]
- Nyvold, M.; Dörsch, P. Complete elimination of methane formation in stored livestock manure using plasma technology. Front. Sustain. Food Syst. 2024, 8, 1370542. [Google Scholar] [CrossRef]
- Ragaveena, S.; Edward, A.S.; Surendran, U. Smart controlled environment agriculture methods: A holistic review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 887–913. [Google Scholar] [CrossRef]
- Uddin, M.; Mo, H.; Dong, D.; Elsawah, S.; Zhu, J.; Guerrero, J.M. Microgrids: A review, outstanding issues and future trends. Energy Strategy Rev. 2023, 49, 101127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).