Abstract
Various natural wastes can be promising for mining more valuable compounds if some specialized extraction techniques are adopted. Hydroxyapatite (HA) is a significant biomaterial that can be extracted from waste bovine bones by heating them at 700 °C and 900 °C. Based on this idea, we made a novel dicalcium phosphate (DCP) bone cement (BC) by extracting HA via the reaction with monocalcium phosphate monohydrate (MCPM) and trisodium citrate. The setting time, injectability, and compressive strength (CS) of this DCPBC were examined using various analytical techniques, such as X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM) attached with energy-dispersive X-ray (EDX) spectroscopy, and Fourier-transformed infrared spectroscopy (FTIR). The phase composition, surface morphology, and chemical compositions of HA and DCP were evaluated. A Gillmore needle apparatus was used to measure the initial and final setting times of the specimens. The CS values of the prepared specimens were determined using INSTRON Series IX. The in vitro dissolution behavior of all samples was evaluated by immersing them in simulated body fluid (SBF) over 7 days at 37 °C. The final setting times of samples 3, 4, and 5 were 20, 24, and 18 min, respectively. In addition, the CS value of sample 1 before immersion in SBF was much lower (1.23 MPa) compared to sample 5 (21.79 MPa) after 7 days of immersion. The CS of the DCP after 3 days of immersion was increased to 33.75 MPa. The in vitro results for the dissolution and bioactivity of HA showed the highest degradation rate after 1 day of immersion and then decreased with the increase in the immersion duration. The HA layer thickness was considerably improved with longer incubation times. The proposed injectable DCP bone cement may have potential in future orthopedic applications.
1. Introduction
Calcium phosphate-based bone cement (CPBC) has become attractive due to its distinct characteristics like strong setting reaction at low temperatures, bio-efficacy, osteoconduction, easy injectability, and moldability [1]. The properties of these materials can be easily altered, providing strong adaptation to the defect surface contour and in situ setting in the cavities of bone, which forms firm restorations [2]. Essentially, HA, as a common natural CP, is widely exploited in biomedical implantation, especially in the engineering of bone tissues. The inorganic components and their characteristics in human bone and teeth are comparable to artificial HA. Therefore, grafted bones made of synthetic HA could be a good biomaterial for repairing and substituting hard tissue. They show outstanding bioactivity, biocompatibility, osteoconductivity, and osteointegrity [3]. Human bone is well known for its excellent physiological properties like self-repairing and remodeling ability. However, such properties appear insufficient in the presence of any bone deficiency, especially for dental, orthopedic, and maxillofacial surgery, leading to wide-ranging defects in bone and tooth tissues.
CP-based cement (CPC) is the most widely used BC. These types of cement are made from a mixture of CP powder and aqueous medium [4], which are two categories depending on the pH values of the reactants and HA or DCP (brushite) [5]. The CPC must have the desired setting times, injectability, optimum mechanical strength, cell adhesion capacity, proliferations, and osteogenesis simulation. Recently, a high-performance CPC with injectability generated renewed interest [6]. It was implemented to enhance the osseous defect for possible clinical uses in oral implants, orthopedic implants, and graft fixations [7]. An injectable CPC was applied for correcting defective regions involving a thin cavity with inadequate or no accessibility, enabling a minimally invasive route [8]. The inorganic CPC made from an aqueous medium showed phase separation when injected for clinical use, limiting its widespread surgical use [7].
Hydraulic CPCs are divided into two categories: apatite cement and DCP dihydrate (DCPD) cement, which has the chemical formula CaHPO4·2H2O. The majority of investigations are made on apatite cement because of its closeness to human bone in terms of the existence of CP in bone, mechanical strength, and neutral pH value. In spite of all of apatite cement’s features, interest in DCPD cement is ever-growing [9]. DCPD is the metastable phase of CP under physiological conditions, making it more quickly resorbable compared to apatite cement [10]. Natural bones contain both inorganic (70%) and organic minerals (30%). Because 70% of natural bone minerals are 95% HA, HA thus became significant for biomedical uses [11]. Various biomaterials are made artificially to work properly in the aqueous bio-environment; since their discovery for human-related applications, this has been studied intensively worldwide [2]. Generally, CP ceramic can be depicted by its Ca-to-P proportion. Amongst all CP-based ceramics, HA with a composition of Ca10(PO4)6(OH)2 and Ca-to-P ratio of 1.667 and β-tricalcium phosphate (β-TCP) with a composition of β-Ca3(PO4)2 and Ca-to-P ratio of 1.5 were investigated in depth to determine their biological features [12].
As aforementioned, the main characteristics of CP cement as a potential bone-repairing biomaterial are now widely explored [5,9]. CP-based bone cements are easily injectable as a molded paste, harden in situ, and fit with irregular geometry (shape and size of bone defect). They can be effective for filling bone voids, principally in non-load-bearing regions like the cranio- and maxillofacial areas [13]. The novel DCPD bone cement, also called brushite, evolved from CP before HA, wherein DPCD has around 15-fold higher solubility compared to HA in a physiological pH environment [14]. DCPD cement was first discovered in 1989 and was made by mixing β-TCP and monocalcium phosphate monohydrate (MCPM) in water [15]. With MCPM (a major source of phosphate ions) as the main reactant, it dissolved rapidly and reduced the solution pH, where an acidic pH was needed because the DCPD-HA singular point was approximately 3.8 at 37 °C [16]. The higher Ca-to-P ratio in β-TCP compared to DCPD required MCP in the solution for the reaction to occur. Compared to HA, DCPD showed higher resorption and bone healing capacity in vivo [8]. Based on this idea, various alternative compositions to the original one were proposed. The substitution of MCPM with phosphoric acid is a popular strategy in which ortho-, poly-, and pyrophosphoric acids have been explored [17]. The main benefits of lower cement acidity and a controlled ion substitution process produce further impetus for HA-based research on DCPD-based bone cement development. In addition, the superior mechanical strength of HA over β-TCP is another reason for this research. Due to large unreacted component reinforcement in β-TCP-based cement making, the use of HA was suggested to prepare biphasic cement with improved mechanical strength.
Although using an HA-based composition to make DCPD cement shows many benefits, widespread studies are still lacking. Moreover, the reactivity of HA together with that of phosphoric acid remains unexplored. Therefore, a clear understanding of the implementation of HA as a DCPD cement reactant remains deficient. Some doubt still exists regarding the reaction capacity of HA with MCPM in CP-based cement because of its insolubility. Motivated by these considerations, we examined the possibility of making MCPM and HA-based cement useful for bone tissue engineering [18], wherein DCPD was formulated analogously to MCPM and β-TCP systems. So far, the reports on the synthesis and characterizations of HA- and MCPM-based DCPD cement are lacking. Thus, it is worth analyzing their setting times, compressive strength (CS) performance, and biodegradability [19].
CP-based cements have been employed for fixing artificial joints to bone stocks [20], wherein these cements were made by combining more than one ceramic powder that contained Ca. Next, the cement liquid was mixed for the reaction activation among the constituent powders, enabling phase conversion during cementation according to CPC’s setting times and stability. Earlier reports showed that the setting reaction can improve the overall characteristics of CPC [17]. CP-based systems are analogous to bone tissue compositions, thus enabling these materials to have high potential in damaged bone tissue replacement. CP-based cements composed of powders and liquids can undergo strong reactions together with setting followed by hardening [9]. Despite a few studies on CP-based bone cements, comprehensive knowledge of their potential as biomedical implants is still lacking. In this view, we extracted HA from waste bovine bones to make a series of brushite cement samples. The prepared cement specimens were thoroughly characterized to ascertain their setting times, structures, injection capacity, and mechanical properties.
2. Materials and Methods
2.1. Preparation of HA
Fresh bovine bone was acquired from the local shop in Iraq, chopped into tiny pieces after removing the meat and marrow parts, and then used to extract HA (Figure 1). In the extraction process, first, the tiny bone pieces were boiled for 4 h using deionized water (DW) to easily remove the fat and soft tissues from them. Next, they were immersed for 15 h in a mixture made of acetone to ether ratio of 3:1 for deprotonation. Later, these bones were oven-dried for 17 h at 120 °C, then for 2 h each at 200 °C and 700 °C. Finally, the dried bones were ground by a mortar and pestle before being subjected to calcination for 2 h at 900 °C to obtain HA. After heating at 900 °C, the color of the bone powder was changed from light grey to pure milky white, confirming the complete removal of organic components and crystallization of HA (As shown in Figure 2). The microstructures of the bone powder were examined by diverse tools. XRD analysis was performed (Philips 1730, Analytical B.V., Breda, The Netherlands) to determine the lattice structures, crystallinity, purity of phases, and particle size. FTIR spectra (Nicolet iS50 spectrometer, Thermo Fisher Scientific, Waltham, MA, USA) of the bone samples were recorded to study the chemical functional groups and bonding vibration. FESEM images of the samples were obtained on Oxford Instruments (Swift ED 3000, Abingdon, UK) to determine the surface morphologies (shape and size of particles) of the samples.
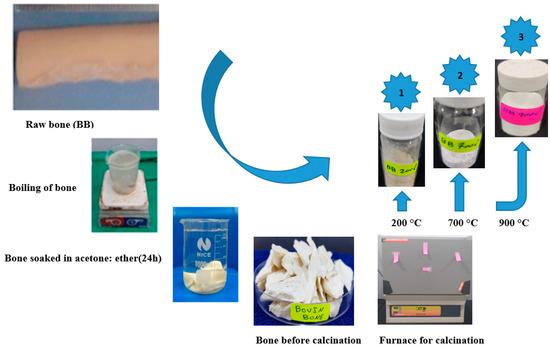
Figure 1.
Bovine bone powder’s preparation process.

Figure 2.
Appearance of bovine bone powder after heat treatment at 200, 700, and 900 °C.
2.2. Cement Preparation
Fast-setting DCPD cements normally have poor injectability due to the liquid–solid phase separation, which has limited their clinical use. For the present study, we chose to use trisodium citrate as a setting regulator in order to improve setting time. Citrate, which contains three carboxyl groups, is known to exert a calcium-chelating effect in solution. Citrate can also inhibit DCPD crystal growth, which is a surface-controlled process. by adsorbing to the surface and blocking potential growth sites. As expected, the addition of trisodium citrate proved effective for increasing the cement setting time, and we noted a dose-dependent effect. Higher sodium citrate concentrations could be used to adjust the setting time of MCPM/HA cements to a clinically useful range. Interestingly, trisodium citrate also seemed to have an effect on compressive strength. As shown in Figure 3 and to obtain the best mixture of the solid phase (MCPM/HA) and the liquid phase (trisodium citrate), different ratios were made to achieve the best ratio in cement preparation by optimizing the initial setting time and final setting time, as shown in Table 1, where the preferred cement preparation ratios were sample 4 and sample 5.
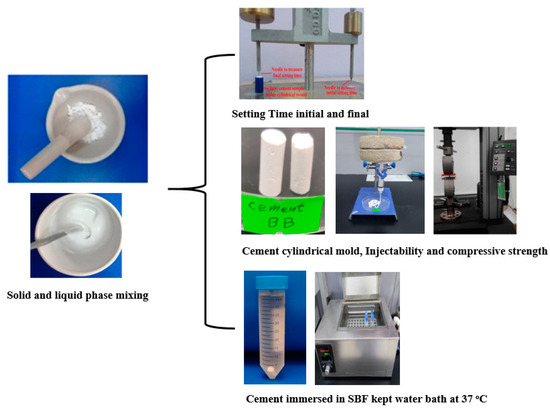
Figure 3.
Cement preparation and tests.

Table 1.
Various ratios of solid and liquid phases.
2.3. Setting Time
Gillmore needle (ASTM C266-89 [21]) was used to measure the initial and final setting time of the designed specimens. The solid powder and liquid were uniformly mixed for 2 min using a mortar to obtain the cement paste. Next, the resultant paste (DCPD cement paste or brushite) was poured into a split Teflon mold with diameter of 6 mm and width of 12 mm. A needle with diameter of 2.12 mm and weight of 113.4 g was put on the specimen. The initial setting time was recorded, and no surface impression on the paste was created by the needle. The final setting time was determined using a needle with diameter of 1.06 mm and weight of 453.6 g. Mean setting times were calculated from these measurements.
2.4. Injection Ability of Cement Specimen
A commercial syringe with aperture of 2 mm, a diameter of 13 mm, and volume of 10 mL was used to load the proposed paste. A vertical compressive load of 5 kg was applied on the plunger top for about 2 min, and the paste was injected until it became non-injectable. The injectability percent (Inj.%) was estimated via the following:
where WF, WA, and WE are the weight of the syringe when fully loaded, after the injection, and empty, respectively.
Inj.% = [(WF − WA)/(WF − WE)] × 100
2.5. Compressive Strength Test
DCPD cement samples, in the form of cylinder, were fabricated using the Teflon mold with diameter of 6 mm and height of 12 mm and left for hardening. After removing from the mold, these hardened specimens were polished with SiC sandpaper (800 grits) for smoothing in accordance with ASTM F451-99a [22]. Then, these samples were immersed in SBF solution (50 mL) for a duration of 24, 72, and 168 h. Later, these specimens were dried for a day, followed by CS measurement by INSTRON Series X1S Automated Materials Tester-Version 8.33.00 (Norwood, MA, USA) at a cross-head speed of 0.5 mm/min. The CS measurement was repeated 5 times to obtain the mean value.
2.6. In Vitro Ion Release Analysis
To evaluate the in vitro ion release from the prepared DCPD cement specimens, they were placed in Teflon mold with cylindrical shape with diameter of 6.0 mm and height of 12 mm, followed by setting at 25 °C for a day. The SBF solution was made by mixing various chemical reagents (MgCl26H2O, KCl, NaCl, CaCl2, K2HP4·3H2O, and Na2SO4 and DW) according to Kokobo’s condition. Next, they were immersed in the SBF solution at 37 °C for the duration of 168 h. Finally, the Ca2+ concentrations were determined using a Flame Atomic Absorption Spectrometer (Perkin Elmer Analyst 400, Waltham, MA, USA).
3. Results and Discussion
3.1. Phase Analysis of Calcined HA
The crystalline structures and phases of HA (calcined at 900 °C for 2 h) were determined by XRD analysis (see Figure 4) and tallied with ICDD PDF card No. 00-009-0432, showing the appearance of intense Bragg peaks at 26.801°, 28.601°, 32.026°, 33.424°, 34.165°, 40.722° 46.954°, and 52.271° corresponding to the lattice planes of (002), (210), (211), (300), (202), and (310), (222), and (213). These observed peaks were consistent with other reports. Table 2 shows the calculated lattice parameters and degree of crystallinity. Lattice parameters were calculated by using Unit Cell software (program UnitCell-method of TJB Holland and SAT Redfern 1995) of the bovine bone (BB) and calcined HA.
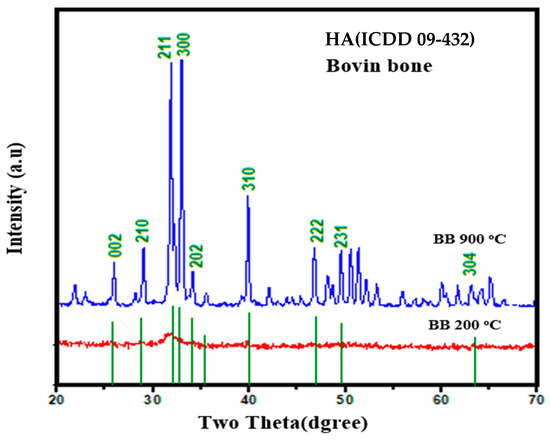
Figure 4.
XRD profiles of HA.

Table 2.
Lattice parameters and crystallinity of HA.
Figure 5 displays the room-temperature FTIR spectra of HA powder (in the range of 4000 to 400 cm−1) after calcination at 900 °C and 200 °C for 2 h. The spectra consisted of various characteristic IR peaks corresponding to the functional groups of HA, confirming the existence of PO43− and OH−. The IR bands at 609 and 1040 cm−1 were due to the stretching vibration modes of PO43−. The calcinations of BB powders at different temperatures were shown to produce CP compounds with the characteristic HA phases. In addition, the peaks due to the vibration of phosphate units appeared at 1084, 520, and 480 cm−1. IR peaks at 3390 and 627 cm−1 were due to the vibration modes of OH- groups. The peaks at 3078, 3585, and 3390 cm−1 verified the existence of water molecules in the HA phase together with OH- group vibration at 640 cm−1. Furthermore, the minor peaks about 1400–1600 cm−1 were due to the vibration of carbonate ions.
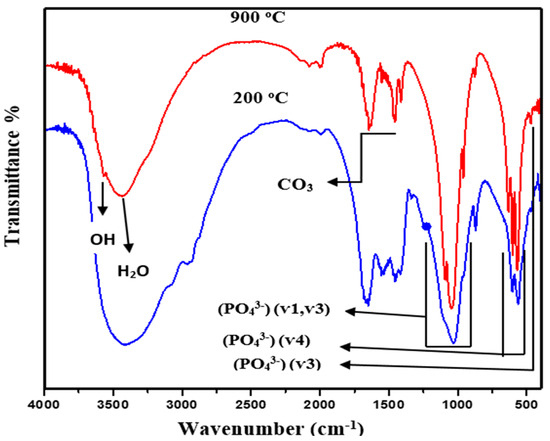
Figure 5.
FTIR spectra of calcined HA powder.
Figure 6 illustrates the FESEM image and EDX spectrum of calcined HA. The SEM morphology of HA powder mostly consisted of irregular and non-spherical particles with a mean diameter of a submicron. The microstructure of HA showed two distinct types of grains, such as small round shapes and large non-uniform crystallites (more elongated ones), with a tendency to align along their longer axis. The EDX spectrum of HA showed its appropriate elemental composition of Ca, O, and P, affirming the nucleation and growth of CaP-rich apatite layers.
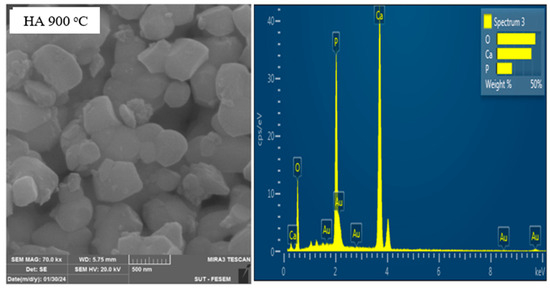
Figure 6.
FESEM image and EDX spectrum of calcined HA.
3.2. Structure and Morphology of DCPD Cement
The XRD measurement was performed to determine the phases of the studied brushite cement. The analysis of the XRD profiles (Figure 7) of the specimen revealed intense Bragg peaks at 21.13°, 29.51°, 30.75°, 34.32°, 37.224°, 41.77°, and 42.29° due to the reflection from the lattice planes of (12-1), (14-1), (121), (150), (141), (15-1), and (260), respectively, which matched with the standard crystalline brushite structure (ICDD 72-0713). The observed weak peak at 26.51° was due to the Bragg repletion from the lattice planes of (200) corresponding to the monetite phase (minor secondary phase) that existed in the cement specimen (JCDPS 71-1760). The lattice data obtained for pure brushite indeed verified the formation of monoclinic crystalline brushite with lattice parameters of a = 5.099 Å, b = 15.362 Å and c = 5.491 Å; α = β = 90° and γ =120°.
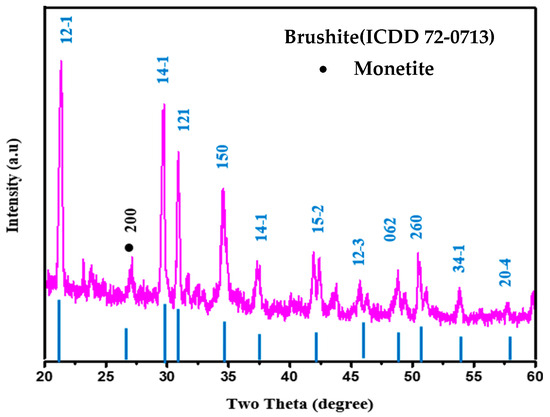
Figure 7.
XRD profiles of the prepared brushite cement.
Figure 8 presents the FTIR spectrum of the prepared brushite cement. The observed IR bands at 3547 and 3485 cm−1 corresponded to the stretching vibration modes of water molecules adsorbed by the specimen. The bending vibration mode of water molecules was probed at 1648 cm−1. Furthermore, the vibration modes of phosphate groups in the cement were observed at 1208, 1132, 1064, 981, 870, 658, 582, and 526 cm−1. Additionally, the bands at 1208–988 and 788–526 cm−1 were due to phosphate units’ ν3 and ν4 stretching and bending vibrations. The minor peaks at 981 and 870 cm−1 were due to the P-OH linkage stretching vibration present in the HPO4 unit. Table 3 elucidates the positions of FTIR bands and assignments of brushite cement.
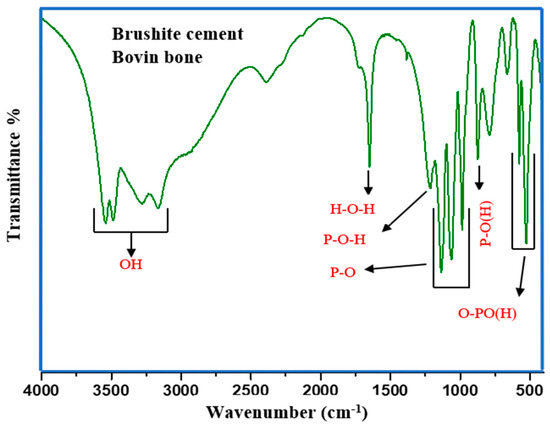
Figure 8.
FTIR spectrum of the prepared brushite cement.

Table 3.
FTIR bands position and assignments of brushite cement.
Figure 9 shows the FESEM image and EDX spectrum of brushite cement wherein the surface microstructures consisted of loosely packed tiny irregular grains with a nonuniform distribution.
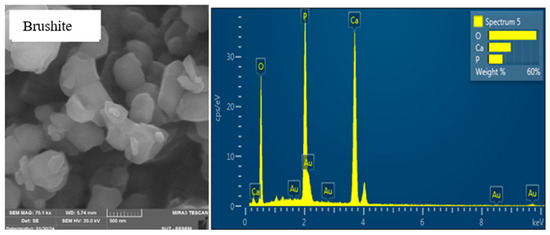
Figure 9.
FESEM image and EDX spectrum of brushite cement.
3.3. In Vitro Study (Setting Time and Injectability)
Owing to their easy dissolution in physiological fluids, brushite cement specimens are viable in clinical applications. One of the aims of the present work was to determine the influence of the setting reaction on the injectability of DCPD. Figure 8 shows the room-temperature setting times (both initial and final) of DCPD cement obtained by a Gilmore needle. The measured initial and final setting time for specimen 1 corresponded to 2.03 min and 5.3 min, respectively. The rapid setting time for the cement specimen is not useful for clinical practices. To overcome this problem, tri-sodium citrate contents were varied, achieving the setting (final) of 12.3 min (specimen 3). Generally, specimens 3, 4, and 5 revealed the corresponding final setting time of 20, 24, and 18 min (Figure 10). It was asserted that by adjusting the tri-sodium citrate contents, the setting times of DCPD can be improved, indicating the creation of a new composition of cement.
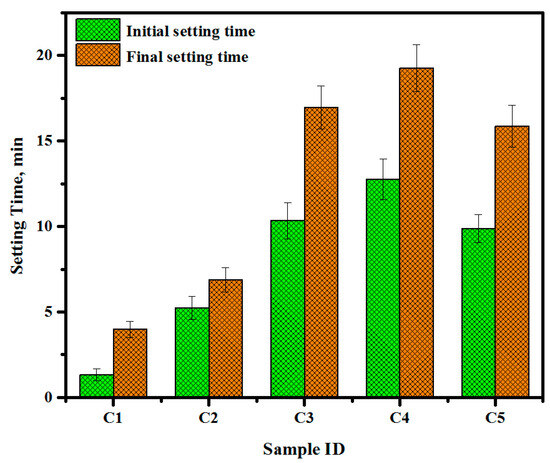
Figure 10.
Room-temperature setting times (initial and final) of DCPD cement.
The injection capacity of cement paste is significant for minimally invasive surgical practices for treating defective bone tissues. Improved degradability and enduring insertion in bone remodeling enable brushite cement specimens to be a potential candidate as bone-substitution material. Moreover, the low injection capacity and poor mechanical performance of brushite cements limit their applications in minimally invasive surgery. In fact, the phenomenon called filter-pressing, or the powder particles’ phase isolation from the liquid in the syringe during the injection of brushite cement, remains a major challenge. Thus, it is important to control the injection ability of brushite cement by evading the filter-pressing process. In the current work, the injection capacity of the cement paste was raised from 12.22 to 78% across specimens 1 to 5, as illustrated in Figure 11.
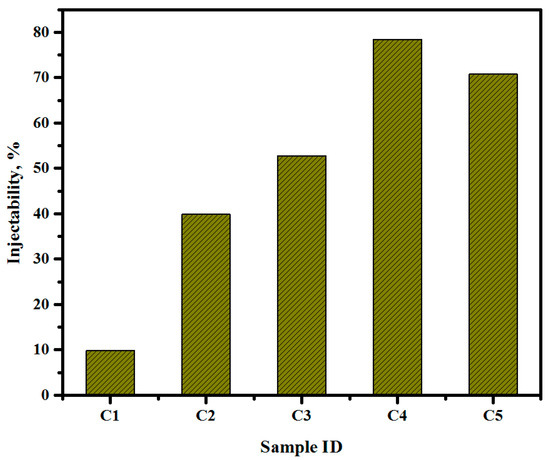
Figure 11.
Injectability of DCPD cement.
Table 4 and Figure 12 display the measured CS, mean value, and standard deviation (STD) of brushite when immersed in SBF solution for different durations of 0, 24, 72, and 168 h. The CS value of the pre-immersed cement specimen 1 was 1.23 MPa. However, the CS value of the post-immersed cement specimen C5 after 72 and 168 h increased to 33.75 and 21.79 MPa, respectively, indicating significant benefits as injectable paste in clinical applications for bone surgery.

Table 4.
Measured CS of brushite when immersed in SBF for different durations.
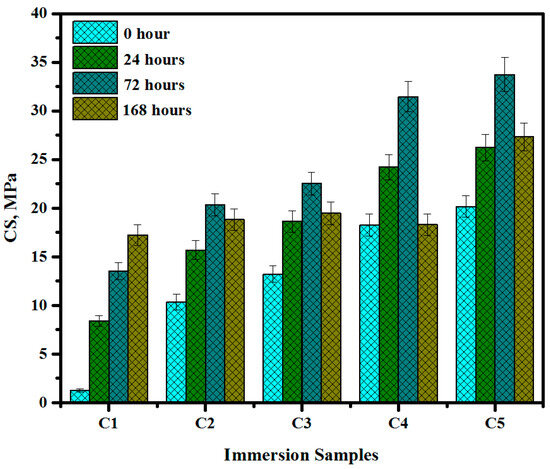
Figure 12.
The CS of brushite before and after immersion in SBF.
3.4. Release of Ions from Cement Specimen
Table 5 illustrates the release of Ca2+ ions from the proposed brushite cement specimen in the SBF solution after various durations of immersion. It was observed that the specimen dissolved immediately upon immersion in SBF solution. The concentration of released Ca2+ ions in SBF reached its peak after 72 hours of immersion. The decrease in the released Ca2+ content in SBF after 168 hours of immersion of the cement specimen may be attributed to the utilization of Ca, which could have contributed to the formation of apatite layers.

Table 5.
Ca2+ ions’ release in SBF solution after immerision for different durations.
3.5. Comparison of Injectable Dicalcium Phosphate with Previous Studies
CPC has been extensively studied, but its lack of osteoinductivity and inadequate mechanical properties limit its application. Conversely, strontium has shown promise in promoting bone formation and inhibiting bone resorption. In a study by Xu et al. [19], various proportions of tristrontium silicate were incorporated to develop a novel strontium-modified calcium phosphate cement (SMPC). According to the findings reported by the authors, SMPC exhibited superior injectability and shorter setting time compared to the CPC. Additionally, the results indicated that the addition of tristrontium silicate enhanced the CS of CPC, with specimens prepared with 5% SMPC, achieving a CS up to 6.0 MPa. In another study by the authors referenced in [23], the presence of Mg2+ ions was utilized to improve the degree of injectability, setting time, and mechanical properties of the brushite cement. The incorporation of Mg2+ ions led to a substantial increase in the CS of brushite cement, along with notable improvements in setting times. Furthermore, Ali et al. [18] conducted a study involving an array of dicalcium phosphate cement containing Sr2+ ions, focusing on the influence of Sr2+ doping on both the initial and final setting times, injectability, CS, porosity, and drug release. The authors reported that the utilizing of Sr2+ significantly enhanced the setting time, injectability, and CS of evaluated specimens. In comparison to previous studies [19,23,24,25], the use of trisodium citrate was found to significantly enhance the prepared specimens in terms of injectability, initial and final setting times, and CS, especially for C4 and C5 specimens.
In evaluating the long-term stability of bone cement in simulated body environments, several studies [26,27] have reported that specimens assessed after 2, 4, and 6 months show only marginal degradation after 6 months, along with increased bone density at the cement–bone interface and new bone formation primarily occurring within cement cracks. The cement was degraded by osteoclasts, accompanied by vascular invasion and bone ingrowth. However, a significant portion remained after 6 months, with a slight increase in the CS of the treated CPC. Meanz et al. [28] found that after 4 months, the cement was completely replaced by bone, with some residual cement fragments. In another study [29], bulk remained after 2 months, and the presence of both zinc and strontium resulted in the highest rate of new bone formation.
4. Conclusions
Based on the obtained results, the following conclusions were drawn:
- The bovine bone-extracted HA was shown to be a promising substitute for synthetic HA.
- The microstructure of the obtained HA revealed agglomeration and a non-uniform distribution of spherical grains of varied sizes and orientations.
- The crystal structures and phases of both HA and brushite cement were tallied with synthetic HA.
- The analysis results of the FTIR spectra showed various characteristic functional groups of the HA phase and structures.
- It was demonstrated that the prepared injectable dicalcium phosphate bone cement has high potential in orthopedic applications.
- The bovine bone-extracted HA-based injectable inorganic matrix showed excellent mechanical performance and setting times.
- The proposed cement paste is established to be efficient in the continual release of ions desirable for invasive surgical applications.
Author Contributions
Conceptualization, K.J.W. and A.T.S.; methodology, A.T.S.; software, K.J.W.; validation, K.J.W., A.T.S. and G.F.H.; formal analysis, K.J.W.; investigation, A.T.S.; resources, K.J.W.; data curation, K.J.W. and G.F.H.; writing—original draft preparation, K.J.W.; writing—review and editing, A.T.S. and G.F.H.; visualization, A.T.S.; supervision, A.T.S.; project administration, A.T.S.; funding acquisition, G.F.H. and A.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the University of Misan for their support and cooperation in conducting this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| BC | Bone cement. |
| CPBC | Calcium phosphate-based bone cement. |
| CPC | Calcium phosphate-based cement. |
| CS | Compressive strength. |
| DCP | Di-calcium phosphate. |
| DCPD | Di-calcium phosphate di-hydrate. |
| EDX | Energy dispersive X-ray. |
| FESEM | Field emission scanning electron microscopy. |
| FTIR | Fourier-transformed infrared spectroscopy. |
| HA | Hydroxyapatite. |
| MCPM | Monocalcium phosphate monohydrate. |
| XRD | X-ray diffraction. |
References
- Demir-Oğuz, Ö.; Boccaccini, A.R.; Loca, D. Injectable bone cements: What benefits the combination of calcium phosphates and bioactive glasses could bring? Bioact. Mater. 2023, 19, 217–236. [Google Scholar] [CrossRef]
- Burguera, E.F.; Xu, H.H.; Takagi, S.; Chow, L.C. High early strength calcium phosphate bone cement: Effects of dicalcium phosphate dihydrate and absorbable fibers. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 75, 966–975. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsiao, P.-C.; Chai, H.-J. Hydroxyapatite extracted from fish scale: Effects on MG63 osteoblast-like cells. Ceram. Int. 2011, 37, 1825–1831. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Burguera, E.F.; Xu, H.H.; Weir, M.D. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 77, 126–134. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and in vitro evaluations of injectable calcium phosphate cement doped with magnesium and strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Santa Cruz, G.; Goebel, W.S.; Chu, T.-M.G. Characterization of dicalcium phosphate dihydrate cements prepared using a novel hydroxyapatite-based formulation. Biomed. Mater. 2009, 4, 025016. [Google Scholar] [CrossRef]
- Deng, K.; Chen, H.; Dou, W.; Cai, Q.; Wang, X.; Wang, S.; Wang, D. Preparation and characterization of porous HA/β-TCP biphasic calcium phosphate derived from butterfish bone. Mater. Technol. 2022, 37, 1388–1395. [Google Scholar] [CrossRef]
- Bohner, M.; Theiss, F.; Apelt, D.; Hirsiger, W.; Houriet, R.; Rizzoli, G.; Gnos, E.; Frei, C.; Auer, J.A.; von Rechenberg, B. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials 2003, 24, 3463–3474. [Google Scholar] [CrossRef]
- Manalu, J.; Soegijono, B.; Indrani, D. Characterization of Hydroxyapatite Derived from Bovine Bone. Appl. Sci. 2015, 3, 2777. [Google Scholar]
- Monballiu, A.; Desmidt, E.; Ghyselbrecht, K.; Meesschaert, B. Phosphate recovery as hydroxyapatite from nitrified UASB effluent at neutral pH in a CSTR. J. Environ. Chem. Eng. 2018, 6, 4413–4422. [Google Scholar] [CrossRef]
- Engstrand, J.; Persson, C.; Engqvist, H. The effect of composition on mechanical properties of brushite cements. J. Mech. Behav. Biomed. Mater. 2014, 29, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-X.; Lv, Y.; Niu, Y.-R.; Zhao, X.-H.; Cao, D.-S.; Tang, J.; Sun, X.-C.; Chen, K.-Z. Physicochemical and biological properties of bovine-derived porous hydroxyapatite/collagen composite and its hydroxyapatite powders. Ceram. Int. 2017, 43, 16792–16798. [Google Scholar] [CrossRef]
- Hu, M.-H.; Chu, P.-Y.; Huang, S.-M.; Shih, B.-S.; Ko, C.-L.; Hu, J.-J.; Chen, W.-C. Injectability, processability, drug loading, and antibacterial activity of gentamicin-impregnated mesoporous bioactive glass composite calcium phosphate bone cement in vitro. Biomimetics 2022, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Tariq, U.; Haider, Z.; Chaudhary, K.; Hussain, R.; Ali, J. Calcium to phosphate ratio measurements in calcium phosphates using LIBS. In Proceedings of the Journal of Physics: Conference Series, Johor Bahru, Malaysia, 26–28 September 2017; p. 012015. [Google Scholar]
- Hsu, H.-C.; Tuan, W.-H.; Lee, H.-Y. In-situ observation on the transformation of calcium phosphate cement into hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 950–954. [Google Scholar] [CrossRef]
- Taha, A.; Akram, M.; Jawad, Z.; Alshemary, A.Z.; Hussain, R. Strontium doped injectable bone cement for potential drug delivery applications. Mater. Sci. Eng. C 2017, 80, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, L.; Tian, F.; Wang, C.; Wu, W.; Lu, B.; Yan, L.; Jia, S.; Hao, D. In vitro and in vivo evaluation of injectable strontium-modified calcium phosphate cement for bone defect repair in rats. Int. J. Mol. Sci. 2022, 24, 568. [Google Scholar] [CrossRef] [PubMed]
- Shahrezaei, M.; Shahrouzi, J.; Hesaraki, S.; Zamanian, A. The Effect of?-TCP Particle Size on Mechanical and Setting Properties of Calcium Phosphate Bone Cements. J. Arch. Mil. Med. 2014, 2, 16516. [Google Scholar] [CrossRef]
- ASTM-C266; Standard Test Method for Time of Setting of Hydraulic Cement Paste by Gillmore Needles. ASTM: West Conshohocken, PA, USA, 1989.
- ASTM F451-99a; Standard Specification for Acrylic Bone Cement. ASTM: West Conshohocken, PA, USA, 1999.
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Grafe, I.A.; Baier, M.; Nöldge, G.; Weiss, C.; Da Fonseca, K.; Hillmeier, J.; Libicher, M.; Rudofsky, G.; Metzner, C.; Nawroth, P. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine 2008, 33, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Apelt, D.; Theiss, F.; El-Warrak, A.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Fujishiro, T.; Belkoff, S.M.; Kobayashi, N.; Turner, A.S.; Seim, H.B., III; Zitelli, J.; Hawkins, M.; Bauer, T.W. Long-term evaluation of a calcium phosphate bone cement with carboxymethyl cellulose in a vertebral defect model. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Maenz, S.; Kunisch, E.; Mühlstädt, M.; Böhm, A.; Kopsch, V.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Enhanced mechanical properties of a novel, injectable, fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2014, 39, 328–338. [Google Scholar] [CrossRef]
- Ammann, P. Strontium ranelate: A novel mode of action leading to renewed bone quality. Osteoporos. Int. 2005, 16, S11–S15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).