Turbulent Flame Propagation in Hydrogen-Air and Methane-Air Mixtures in the Field of Synthetic Turbulence: Direct Numerical Simulation
Abstract
1. Introduction
2. Materials and Methods
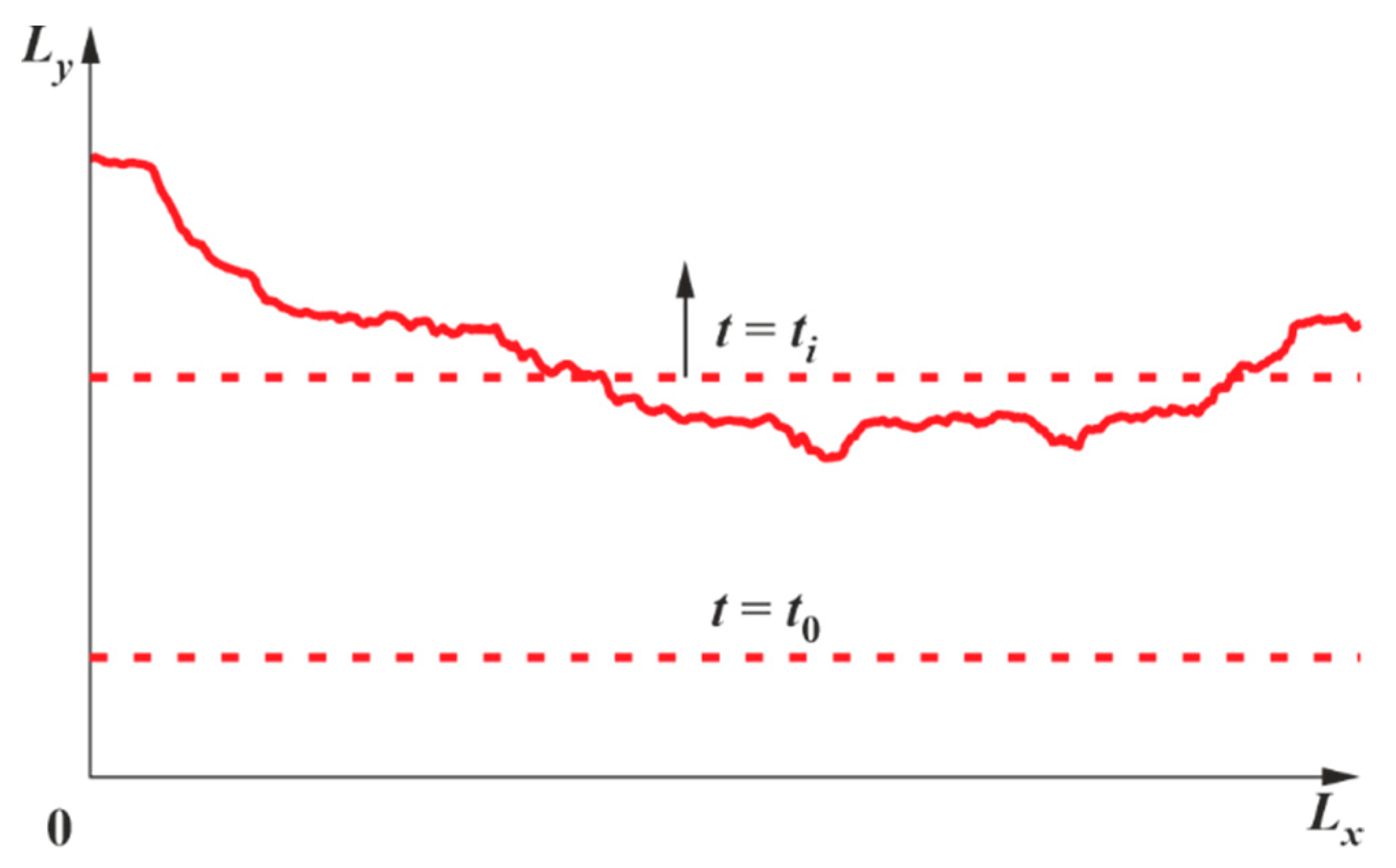
2.1. Mathematical Statement of the Problem
- (i)
- the flow domain is simple; turbulence is homogeneous, isotropic, and statistically stationary (forced);
- (ii)
- the effect of gravity is negligible, and the pressure is constant (). These assumptions greatly simplify the problem as the solution of the momentum equation is not required;
- (iii)
- radiative heat transfer is negligible, and heat flux is due to solely the molecular thermal conductivity;
- (iv)
- thermal diffusivity is negligible;
- (v)
- Fick’s law with a binary diffusion coefficient is applicable to the diffusion fluxes.
2.2. Numerical Method
3. Results and Discussion
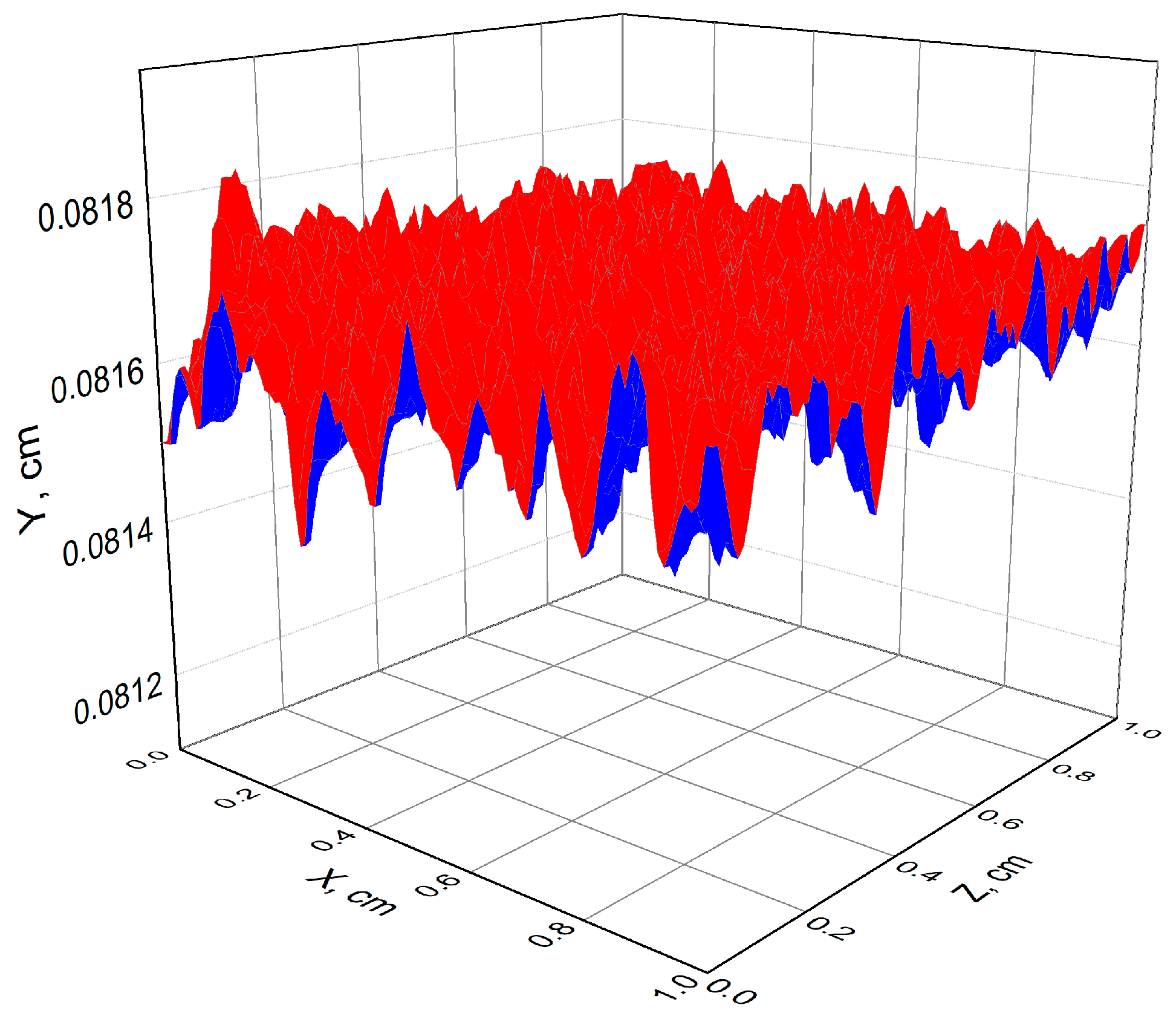
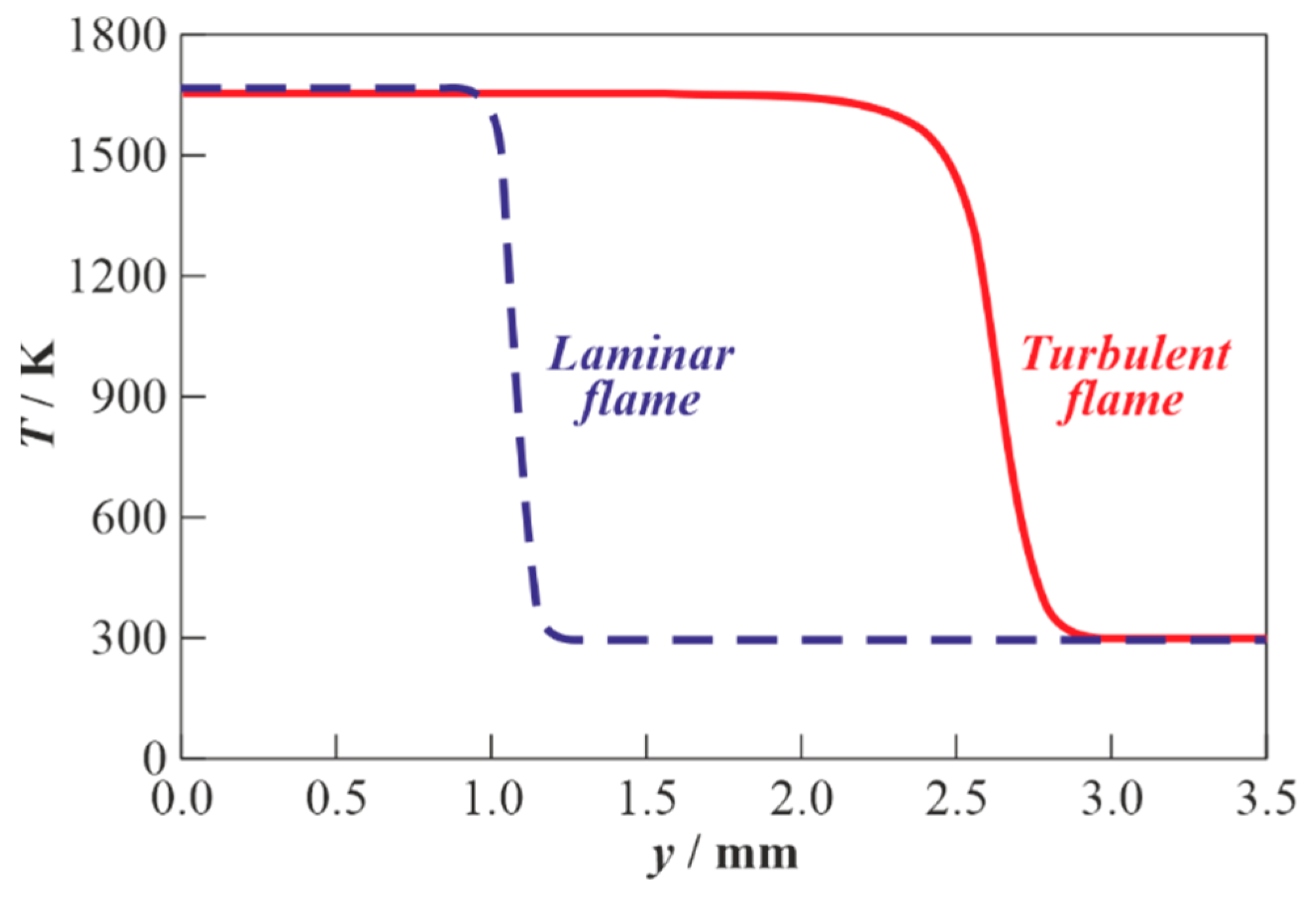
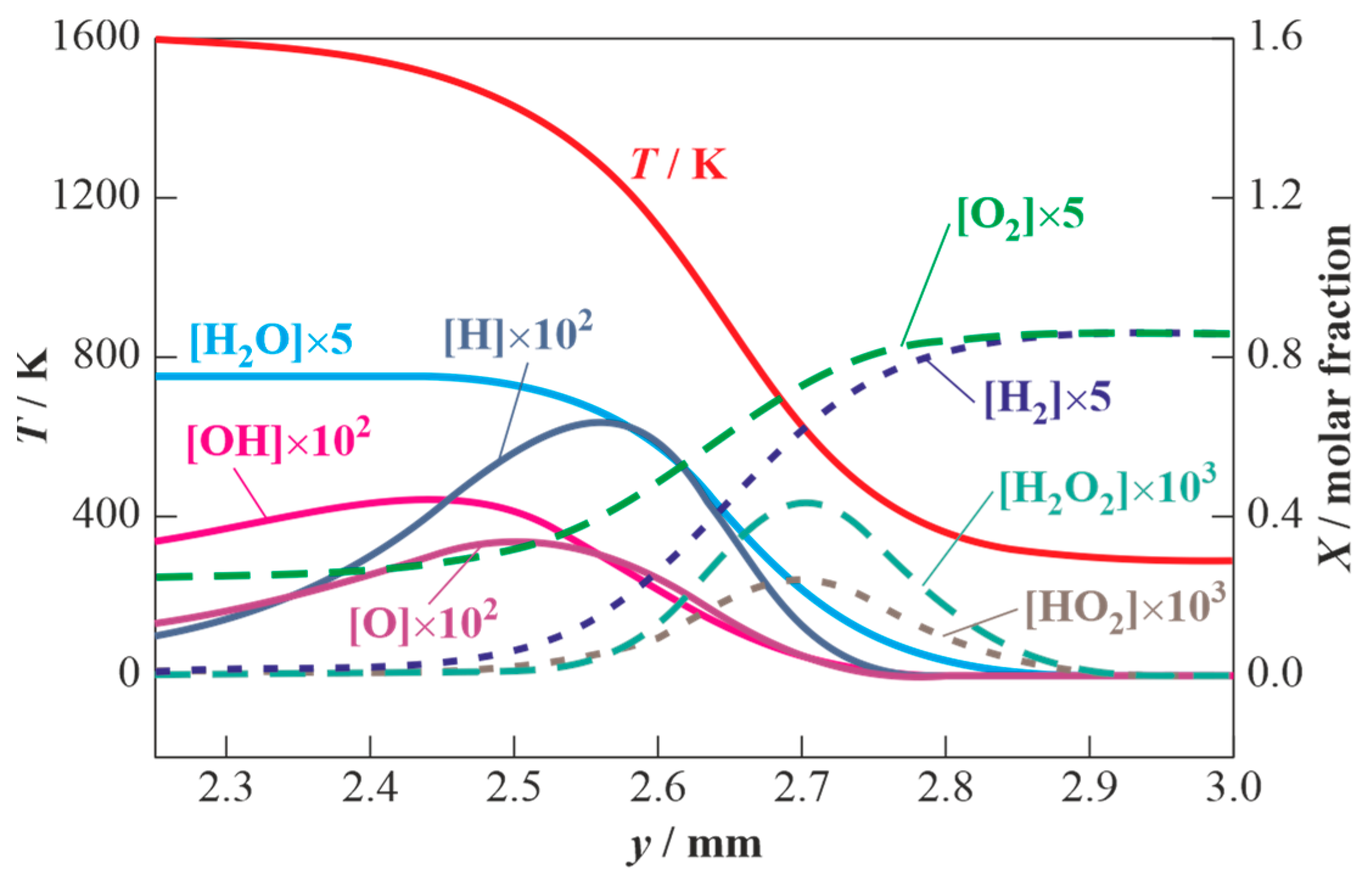
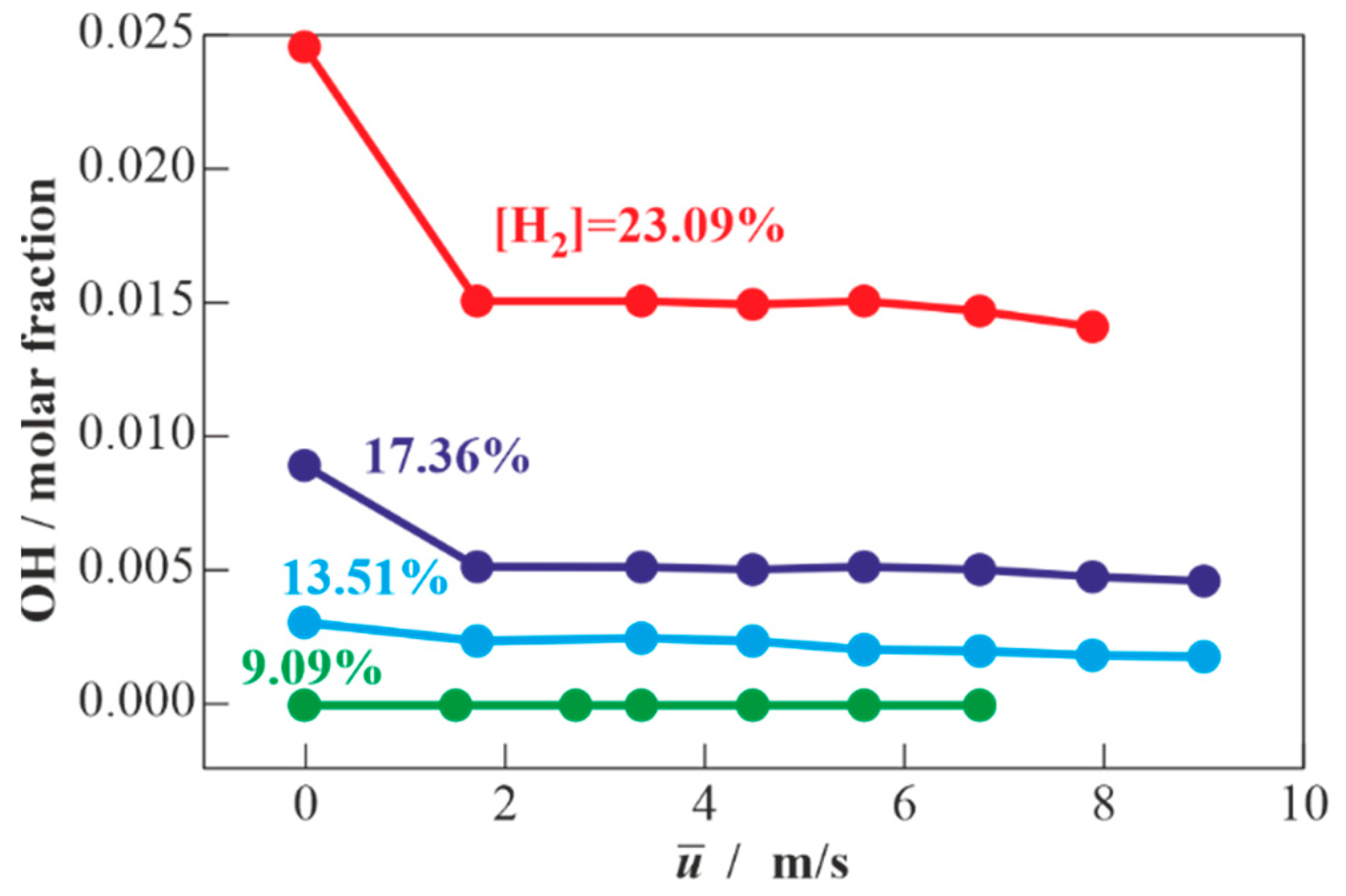
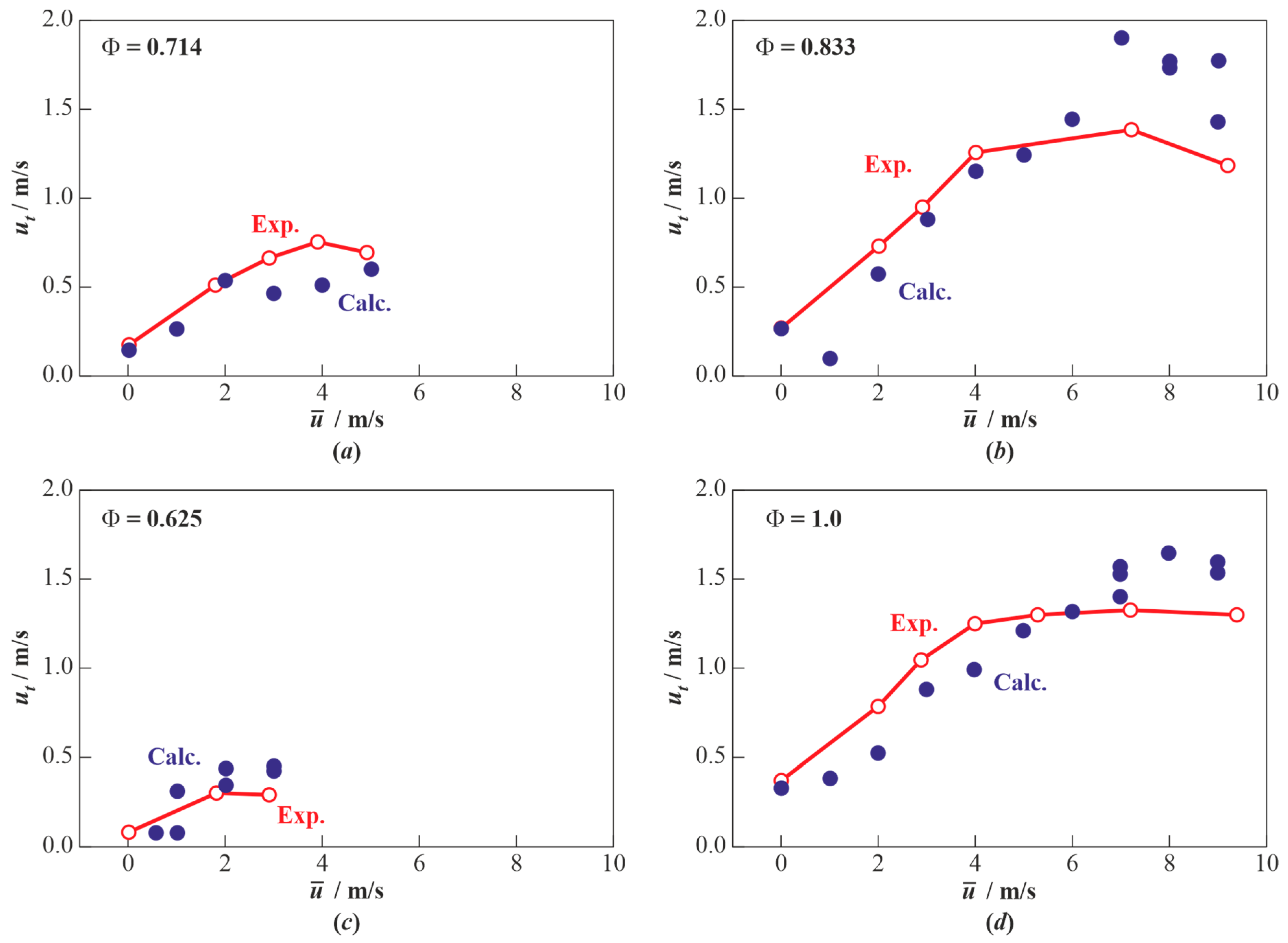
3.1. Results of Calculations for Hydrogen-Air Mixtures and Discussion
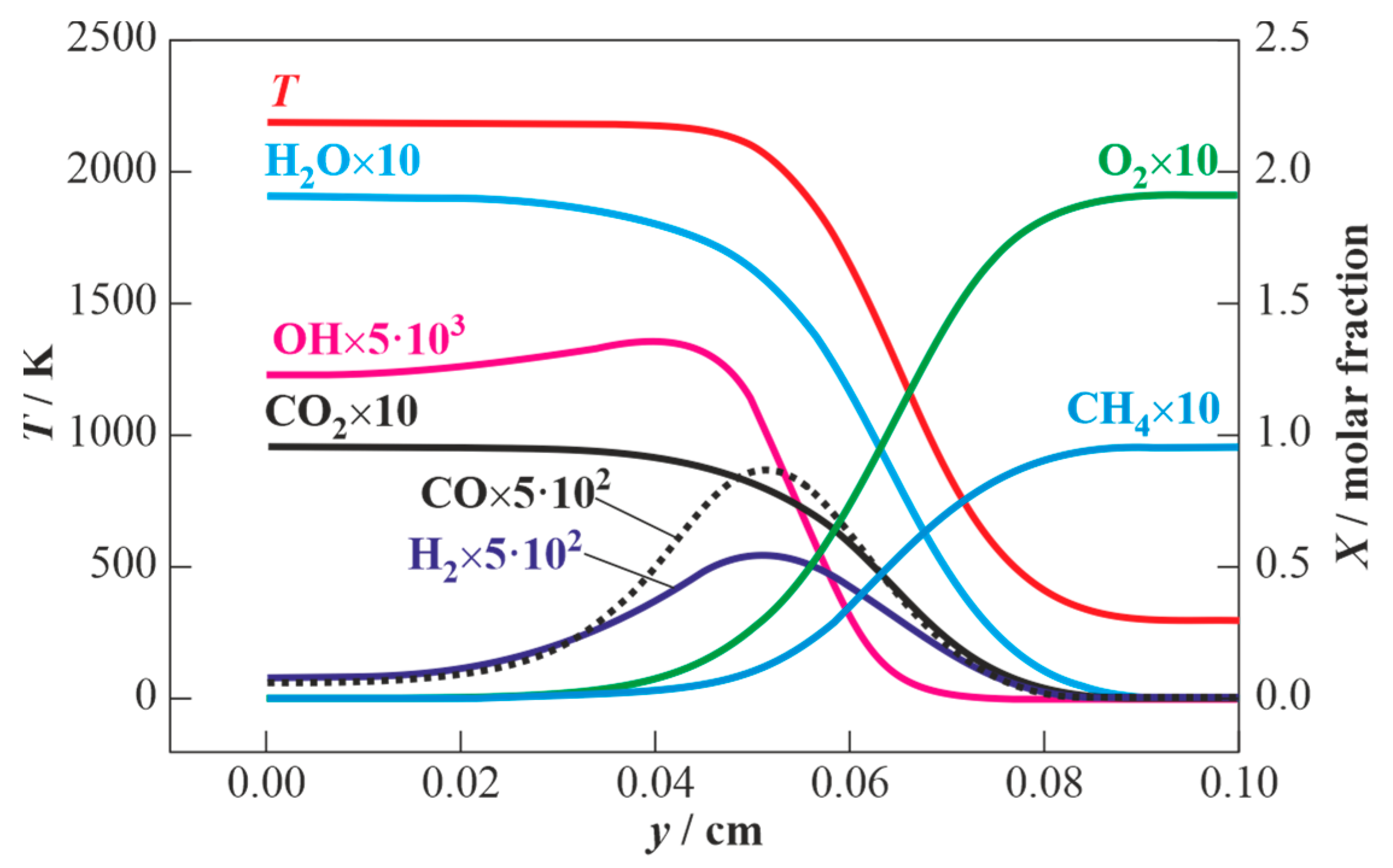
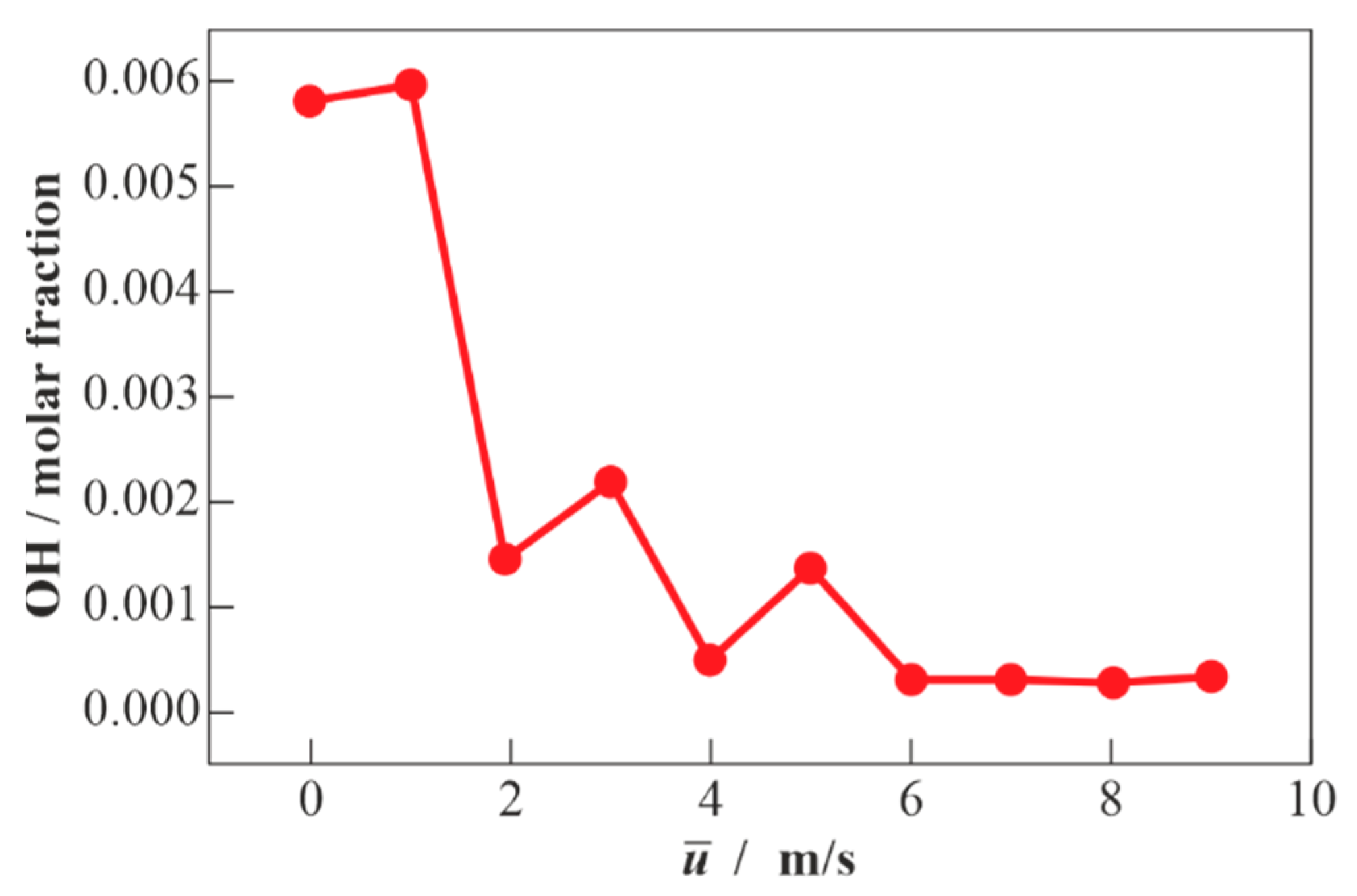
3.2. Results of Calculations for Methane-Air Mixtures and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orszag, S.A. Analytical theories of turbulence. J. Fluid Mech. 1970, 41, 363–386. [Google Scholar] [CrossRef]
- Leonard, A.D.; Hill, J.C. Direct numerical simulation of turbulent flows with chemical reaction. J. Sci. Comput. 1988, 3, 25–43. [Google Scholar] [CrossRef]
- Bell, J.B.; Day, M.S.; Grcar, J.F. Numerical simulation of premixed turbulent methane combustion. Proc. Combust. Inst. 2002, 29, 1987–1993. [Google Scholar] [CrossRef]
- Echekki, T.; Chen, J.H. Direct numerical simulation of autoignition in nonhomogeneous hydrogen–air mixtures. Combust. Flame 2003, 134, 169–191. [Google Scholar] [CrossRef]
- Bell, J.B.; Cheng, R.K.; Day, M.S.; Shepherd, I.G. Numerical simulation of Lewis number effects on lean premixed turbulent flames. Proc. Combust. Inst. 2006, 31, 1309–1317. [Google Scholar] [CrossRef]
- Aspden, A.J.; Day, M.S.; Bell, J.B. Three-dimensional direct numerical simulation of turbulent lean premixed methane combustion with detailed kinetics. Combust. Flame 2016, 166, 266–283. [Google Scholar] [CrossRef]
- Lamioni, R.; Lapenna, P.E.; Berger, L.; Kleinheinz, K.; Attili, A.; Pitsch, H.; Creta, F. Pressure-induced hydrodynamic instability in premixed methane-air slot flames. Combust. Sci. Techn. 2020, 192, 1998–2009. [Google Scholar] [CrossRef]
- Lapenna, P.E.; Lamioni, R.; Creta, F. Subgrid modeling of intrinsic instabilities in premixed flame propagation. Proc. Combust. Inst. 2021, 38, 2001–2011. [Google Scholar] [CrossRef]
- Trisjono, P.; Pitsch, H. Systematic analysis strategies for the development of combustion models from DNS: A review. Flow Turbul. Combust 2015, 95, 231–259. [Google Scholar] [CrossRef]
- Driscoll, J.F.; Chen, J.H.; Skiba, A.W.; Carter, C.D.; Hawkes, E.R.; Wang, H. Premixed flames subjected to extreme turbulence: Some questions and recent answers. Prog. Energy Combust. Sci. 2020, 76, 100802. [Google Scholar] [CrossRef]
- Domingo, P.; Vervisch, L. Recent developments in DNS of turbulent combustion. Proc. Combust. Inst. 2022, in press. [Google Scholar] [CrossRef]
- Da Silva, C.B.; Malico, I.; Coelho, P.J. Radiation statistics in homogeneous isotropic turbulence. New J. Phys. 2009, 11, 093001. [Google Scholar] [CrossRef]
- Basevich, V.Y.; Volodin, V.P.; Kogarko, S.M.; Peregudov, N.I. Calculations of turbulent flame in two-dimensional approximation. Khim. Fiz. 1982, 1, 1130–1137. [Google Scholar]
- Karpov, V.P.; Severin, E.S. Effects of molecular transport coefficients on the rate of turbulent combustion. Combust. Explos. Shock Waves 1980, 16, 41–46. [Google Scholar] [CrossRef]
- Bradley, D.; Lawes, M.; Mansour, M.S. Correlation of turbulent burning velocities of ethanol–air, measured in a fan-stirred bomb up to 1.2 MPa. Combust. Flame 2011, 158, 123–138. [Google Scholar] [CrossRef]
- Kobayashi, H. Experimental study of high-pressure turbulent premixed flames. Exp. Therm. Fluid Sci. 2002, 26, 375–387. [Google Scholar] [CrossRef]
- Kraichnan, R.H. Diffusion by a random velocity field. Phys. Fluids 1970, 13, 22–31. [Google Scholar] [CrossRef]
- Heinz, S. A review of hybrid RANS-LES methods for turbulent flows: Concepts and applications. Prog. Aerosp. Sci. 2020, 114, 100597. [Google Scholar] [CrossRef]
- Basevich, V.Y.; Belyaev, A.A.; Frolov, S.M.; Basara, B. Direct numerical simulation of turbulent combustion of gases in two-dimensional approximation. Combust. Explos. 2017, 10, 4–10. [Google Scholar]
- Basevich, V.Y.; Belyaev, A.A.; Frolov, S.M.; Frolov, F.S. Direct numerical simulation of turbulent combustion of hydrogen–air mixtures of various compositions in a two-dimensional approximation. Russ. J. Phys. Chem. B 2019, 13, 75–85. [Google Scholar] [CrossRef]
- Frolov, S.M.; Ivanov, V.S.; Basara, B.; Suffa, M. Numerical simulation of flame propagation and localized preflame autoignition in enclosures. J. Loss Prev. Process Ind. 2013, 26, 302–309. [Google Scholar] [CrossRef]
- Zimont, V.L. Theory of turbulent combustion of a homogeneous fuel mixture at high Reynolds numbers. Combust. Explos. Shock Waves 1979, 15, 305–311. [Google Scholar] [CrossRef]
- Guelder, Ö.L. Turbulent premixed flame propagation models for different combustion engines. Proc. Comb. Inst. 1990, 23, 743–750. [Google Scholar] [CrossRef]
- Bradley, D. How fast can we burn? Proc. Combust. Inst. 1992, 24, 247–253. [Google Scholar] [CrossRef]
- Peters, N. The turbulent burning velocity for large scale and small-scale turbulence. J. Fluid Mech. 1999, 384, 107–132. [Google Scholar] [CrossRef]
- Williams, F.A. The Combustion Theory; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Loitsyanskii, L.G. Mechanics of Liquids and Gases; Drofa Publs: Moscow, Russia, 2003. [Google Scholar]
- Godunov, S.K.; Ryaben’kiy, V.S. Finite-Difference Schemes; Nauka: Moscow, Russia, 1977. [Google Scholar]
- Douglas, J.; Rachford, H.H. On the numerical solution of heat conduction problems in two and three space variables. Trans. Amer. Math. Soc. 1956, 82, 421–439. [Google Scholar] [CrossRef]
- Basevich, V.Y.; Belyaev, A.A.; Posvyanskii, V.S.; Frolov, S.M. Mechanisms of the oxidation and combustion of normal paraffin hydrocarbons: Transition from C1–C10 to C11–C16. Russ. J. Phys. Chem. B 2013, 7, 161–169. [Google Scholar] [CrossRef]
- Burcat, A. Ideal Gas Thermodynamic Data in Polynomial Form for Combustion and Air Pollution Use. Lab. Chem. Kinetics. Available online: http://garfield.chem.elte.hu/Burcat/burcat.html (accessed on 15 January 2023).
- Reid, C.; Prausnitz, J.; Sherwood, T. The Properties of Gases and Liquids, 3rd ed.; McGraw Hill: London, UK, 1977. [Google Scholar]
- Basevich, V.Y.; Kogarko, S.M. Hydrocarbon formation in turbulent combustion of a methane–air mixture. Combust. Explos. Shock Waves 1985, 21, 514–518. [Google Scholar] [CrossRef]


| , cm/s | , cm | , s |
|---|---|---|
| 100–200 | 0.74 | |
| 200–300 | 0.64 | |
| 300–900 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basevich, V.Y.; Belyaev, A.A.; Frolov, F.S.; Frolov, S.M. Turbulent Flame Propagation in Hydrogen-Air and Methane-Air Mixtures in the Field of Synthetic Turbulence: Direct Numerical Simulation. Eng 2023, 4, 748-760. https://doi.org/10.3390/eng4010045
Basevich VY, Belyaev AA, Frolov FS, Frolov SM. Turbulent Flame Propagation in Hydrogen-Air and Methane-Air Mixtures in the Field of Synthetic Turbulence: Direct Numerical Simulation. Eng. 2023; 4(1):748-760. https://doi.org/10.3390/eng4010045
Chicago/Turabian StyleBasevich, Valentin Y., Andrey A. Belyaev, Fedor S. Frolov, and Sergey M. Frolov. 2023. "Turbulent Flame Propagation in Hydrogen-Air and Methane-Air Mixtures in the Field of Synthetic Turbulence: Direct Numerical Simulation" Eng 4, no. 1: 748-760. https://doi.org/10.3390/eng4010045
APA StyleBasevich, V. Y., Belyaev, A. A., Frolov, F. S., & Frolov, S. M. (2023). Turbulent Flame Propagation in Hydrogen-Air and Methane-Air Mixtures in the Field of Synthetic Turbulence: Direct Numerical Simulation. Eng, 4(1), 748-760. https://doi.org/10.3390/eng4010045







