Abstract
This paper presents the results of testing the adhesion of geopolymer coatings and varnishes with ceramic additives to concrete and steel substrates. The measurement method used and described in this article was the pull-off method. The pull-off method test provides an easy way to evaluate the degree of adhesion of coatings to metal surfaces. The pull-off device provides values for the peel stress, which not only allows a quick determination of the adhesion of the coating to the substrate, but also makes it easier to compare the adhesion of several coatings to each other. However, this method requires appropriate preparation, so an attempt was made to determine its suitability for geopolymer layers. The results of testing the adhesion of a geopolymer layer to a geopolymer substrate and a concrete substrate are presented. As a result of this study, a higher adhesion strength of the geopolymer layer to the geopolymer substrate was found in comparison to geopolymer coatings applied on conventional concrete. Adhesion tests were also conducted for steel substrates to which both geopolymer and acrylic lacquer were applied.
1. Introduction
The effectiveness of a protective coating, especially its anti-corrosion effect, is strongly dependent on its tightness and the strength of its adhesion to the component. Proper adhesion of the coating to the substrate is necessary for the coating to fulfill its protective function. This is why great importance is attached to the preparation of the substrate before applying the coating. Before painting, the surface is sanded with abrasive papers in order to increase its roughness. In addition, the substrate is cleaned of any impurities that could reduce the adhesion of the coating. In order to increase the adhesion of the paint system to the surface of the element, primers are also used before applying the topcoat. The loss of adhesion of the coating results in the loss or deterioration of its protective properties and damage to the element (e.g., due to corrosion). It is especially important to prevent this from happening if the element is working in an aggressive environment, where the destruction will proceed at a much faster pace. In order to make sure that the paint coating has been applied correctly and its adhesion force to the substrate is sufficient, coating adhesion tests are conducted using the notch grid method and the peel-off method. The results of these tests enable to evaluate the coating, confirming its proper adhesion and thus the effectiveness of its operation in the future, or they signal that the given coating is characterized by insufficient adhesion which may lead to its falling off and exposure of the element to harmful factors [1,2,3,4]. In order to make sure that the paint coating has been applied correctly and its adhesion force to the substrate is sufficient, tests of coating adhesion are carried out using the notch grid method and the peel-off method. The results of these tests make it possible to evaluate the coating, confirming its proper adhesion and thus the effectiveness of its operation in the future, or they signal that a given coating is characterized by insufficient adhesion which may lead to its falling off and exposure of the element to harmful factors [5,6]. The number of materials that can be used as coatings/protective layers is basically unlimited due to the multitude of variants of various types of coatings. Recently, coatings made of inorganic aluminosilicate polymers called geopolymers have attracted great interest [7].
By analyzing the surface of the specimens after detachment, three types of cracking due to the applied force can be observed. The first type is a cohesive fracture. Cohesion results from intermolecular forces in the material being torn or crushed. If the fracture occurs inside the applied coating, it is called cohesive fracture. In the case of cohesive fracture, the same material is present on both the surface of the specimen and the surface of the stamp. Both these surfaces will be covered with the same coating material [8,9]. A schematic of cohesive cracking is shown in Figure 1.
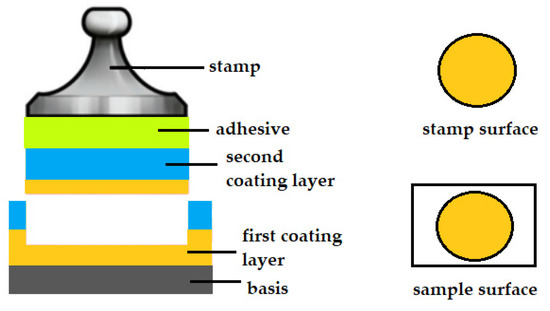
Figure 1.
Schematic of a cohesive fracture.
The second type of specimen rupture is an adhesive fracture. Adhesion is the interaction between molecules that are in the surface layers of two bodies adjacent to each other. Adhesion rupture indicates a low bonding ability between the surfaces of two materials. An adhesive fracture occurs between two layers (i.e. between the base and the applied coating or between the first and second coatings). In the case of an adhesive fracture, there will be a different material on the surface of the stamp than on the surface of the sample. The coating layer will be on the surface of the stamp and the second coating layer will be on the surface of the specimen or the surface of the specimen will remain uncoated [10,11]. The example of an adhesive fracture is shown in Figure 2.
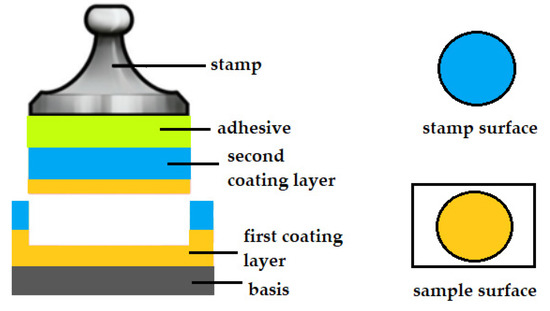
Figure 2.
Schematic of an adhesive fracture.
During the adhesion test, the adhesive layer may be torn internally by the application of force or the adhesive may separate completely from the coating. For the test to be considered valid, the coating must cover at least half of the surface of the stamps. If the surface of the test stamp is not coated at all, or is coated but less than 50%, the test is faulty [10,12]. A schematic of the separation of the adhesive from the coating is shown in Figure 3.
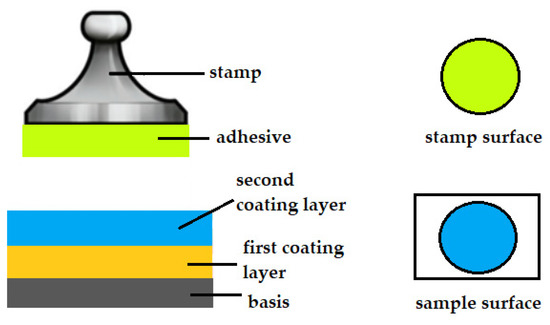
Figure 3.
Schematic of the separation of the adhesive from the coating.
Geopolymers are inorganic materials whose development has been very dynamic in recent years. Geopolymer materials can be synthesized at elevated temperature or at ambient temperature by alkaline activation of industrial wastes (fly ash, slag) or materials of geological origin (metakaolin, volcanic tuff) [13]. Worldwide, access to starting materials (raw materials) is very common, and the demand for such materials also seems to be growing, hence their wide potential application and attractiveness. The production of geopolymers is very economical and also safe for humans and the environment. These materials are also suitable for immobilization of hazardous waste (radioactive waste, asbestos) or toxic waste (mercury, lead, arsenic) [14,15,16].
Geopolymer materials are a group of modern construction materials that show a range of properties that allow them to replace common engineering materials such as traditional concretes and building composites [17,18]. This group of binders is characterized by such properties as high compressive strength [19,20,21,22,23,24,25], resistance to acids, chlorides and sulfates [26,27], thermal resistance (up to about 800 °C) [28] or good frost resistance [29,30], among others. Moreover, there is no corrosion of steel reinforcement in geopolymers and the material shows good adhesion properties with steel [31].
Geopolymers appear to be a promising material for making them into protective coatings on various types of surfaces (including metals) due to their excellent mechanical, chemical and thermal properties. Geopolymer mortar has the right consistency for various application methods. It can be applied by trowel or paint roller. After application, it is left for 4 h. After drying, the surface strength of the grout is over 2 MPa, which is comparable to commonly used epoxy resins. The action of a chemically aggressive environment causes weight loss of Portland cement based concretes. Using a 10% aqueous solution of organic and inorganic acids, material degradation can be observed. Uncoated concrete can lose up to 70% of its basic mass when exposed to inorganic acids such as hydrochloric or nitric acid. The degradation is mainly related to the breakdown of the C-S-H phase and the formation of soluble calcium salts. Concrete coated with geopolymer coating, on the other hand, shows much better resistance in corrosive environments. The weight loss is estimated to be about 2% [32]. These coatings are attracting more and more attention due to the lack of UV degradation problem or lack of susceptibility to fire. Attempts have already been made to produce such coating on metal components [33]. Temuujin et al. fabricated geopolymer coatings on steel by focusing on the effect of Si:Al ratio on the adhesion strength of the applied coating to the substrate. They investigated both coatings produced from metakaolin-based geopolymers and also fly ash-based geopolymers. In both cases they found that the adhesion strength of the coating to steel depended on its chemical composition, and the highest adhesion strength was observed for compositions containing high amounts of silica. The effect of surface preparation on the adhesion of the coating was also observed. The coatings adhered better to rough surfaces than to a polished steel surface [34,35].
Deshmukh produced fly ash-based geopolymer coatings on steel using NaOH as an activator. The coatings with the highest sodium silicate content obtained the best adhesion to steel. Deshmukh studied the thermal stability and corrosion resistance of the produced geopolymer coatings. The coatings showed thermal stability up to about 500 °C; however, it was noted with increasing temperature the coatings gradually darkened. Beyond 500 °C the coatings were not stable and started to fall off from the coated steel. Electrochemical measurements showed a strong corrosion resistance of the coated material [33]. Khan also produced geopolymer coatings on steel using fly ash as a precursor but they did not contain sodium silicate in their composition. Again, the coatings adhered well to the steel and exhibited thermal stability. Thus, it can be concluded that geopolymer coatings are an interesting alternative, and the results obtained confirm their beneficial effect on improving corrosion resistance and thermal resistance [36].
This paper presents the results of testing the strength of adhesion of geopolymers applied to steel, conventional concrete and geopolymer. Additionally, the results of testing the adhesion of varnish layers to steel are presented, and the effect of ceramic additives introduced to varnishes as corrosion inhibitors (influence on the adhesion of layers) is evaluated. As stated in the introduction, the adhesion of protective coatings is of crucial importance for, among others, proper operation and protection of a material against corrosion. Therefore, the best possible materials and coating application methods should be developed. Geopolymers are relatively new materials that can be used as protective coatings. However, this requires further development and research in this area. There are already known cases of their use in industry, but not on a mass production scale.
2. Materials and Methods
Geopolymer were made based on fly ash, which came from the Heat Power Plant (Skawina, Poland) and river sand (Świętochłowice, Poland). Lach et al., in their works, also investigated the same type of fly ash [7]. Table 1 shows the oxide composition of the ash determined using the XRF method.

Table 1.
Oxide composition of fly ash.
The alkaline activator was a solution of 14 M sodium hydroxide and R-145 sodium water glass with a molar modulus of 2.5 and a density of about 1.45 g/cm3. An aqueous solution of sodium hydroxide of a given concentration was mixed with water glass at a ratio of 1:2.5 by weight. The solution was prepared according to the scheme: Technical hydroxide flakes were solubilized in water, and then an aqueous solution of sodium silicate was added. The components were mixed and allowed to reach a constant concentrate.
The same amount of sand and fly ash (1:1 weight ratio) was used to make each sample. The hydraulic additives used in this study were additionally building gypsum and Portland cement CEM 52.5. The solid components fly ash and sand were mixed dry until a homogeneous mixture was obtained, then the alkali solution was added and mixed thoroughly. The mixing was carried out in a laboratory mixer, for about 15 min.
2.1. Geopolymer Layers on Steel, Concrete and Geopolymer Bases
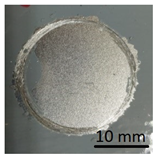
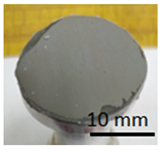
The prepared geopolymer masses were applied to different types of substrates: concrete, geopolymer and steel. An example of the appearance of the samples and how they were made is shown in the figures below (Figure 4 and Figure 5). The layers of geopolymer were applied manually with a special applicator. The thickness of these layers was 2 mm.

Figure 4.
Geopolymer cubes before coating.
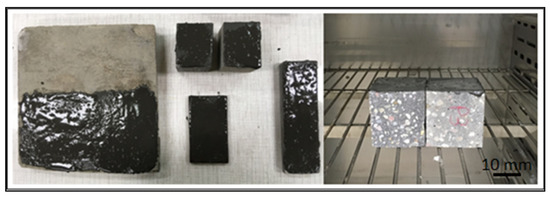
Figure 5.
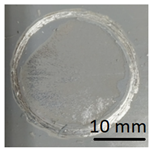
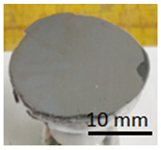
Concrete and geopolymer materials coated with a geopolymer layer and placed in a dryer.
After applying the geopolymer layer, the samples were placed in an oven and cured at 75 °C for 24 h (Figure 5).
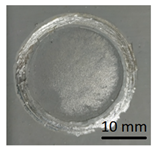
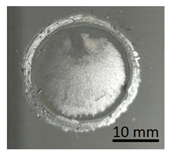
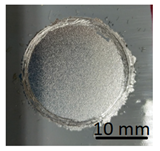
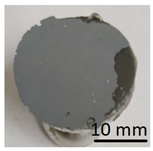
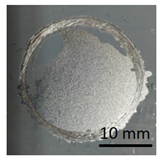
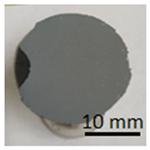
In preparation for testing the geopolymer coatings protecting the steel, DC01 steel specimens of dimensions 100 mm × 150 mm × 0.8 mm were prepared and painted on one side with geopolymer under identical conditions for each batch (temperature 23 ± 2 °C and humidity 50 ± 5%). The test tiles were flat, without deformations and complied with the requirements of ISO 1514. Before testing, the samples were conditioned for at least 16 h under identical conditions (temperature of 23 ± 2 °C and humidity of 50 ± 5%). An example of the specimen appearance (after the test) is shown in Figure 6.
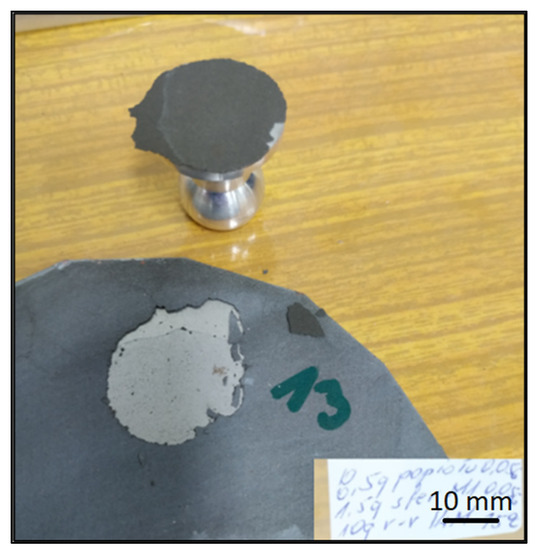
Figure 6.
Geopolymer layer on DC01 steel-after peel-off test.
2.2. Tests of Varnish Layers with the Addition of Ceramics on Steel
The same type of coating was used for the six coats, which was a two-component acrylic lacquer-semi-matte and gloss. Additionally, volcanic tuff was introduced to four of the coatings. Volcanic tuff was introduced to evaluate the effect of ceramic particles on the adhesion of the varnish layers. This material was chosen because of its proven corrosion inhibiting effect. The volcanic tuff used came from the Kowalska Góra field (Poland) and its main composition is sanidine and silica. Three samples were made for each type of coating. The samples after sticking the measuring stamps are shown in Figure 7.
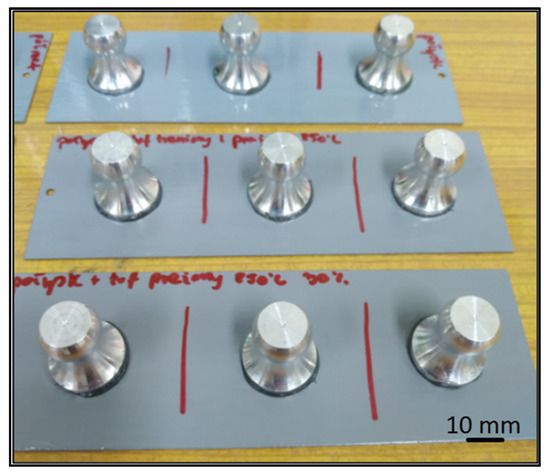
Figure 7.
Stamps on a metal base.
Table 2 gives the characteristics of the coatings that were tested in the pull-off test.

Table 2.
Types of varnish coatings applied to steel slabs.
Table 3 shows the characteristics of the geopolymer coated samples tested by the pull-off method.

Table 3.
Types of geopolymer coated samples tested using the pull-off method.
2.3. Test Method
The device used to perform the pull-off measurements was the DeFelsko’s PosiTest AT-A pull-off device (ATA20, DeFelsko Corporation, Ogdensburg, New York, USA). The AT-A model is an automatic meter that uses a hydraulic cylinder. It measures the force or stress required to peel a specific area of the coating from the substrate using hydraulic pressure. The value of the force or stress, depending on which option is selected in the settings, is displayed on the device and describes the adhesion of the coating to the substrate. The instrument gives a choice of units such as psi, Niutons, MPa or N/mm2. When making measurements for this thesis the unit [N/mm2] was chosen and the results will be reported in this unit. According to ISO 4624, ASTM D4541 and D7234, the PosiTest device evaluates the peel strength of a coating by determining the highest tensile force it can withstand before rupture. The PosiTest AT-A device is shown in Figure 8 and the pull-off test scheme is shown in Figure 9.

Figure 8.
Sensor for measuring the adhesion of coatings DeFelsko PosiTest AT-A.
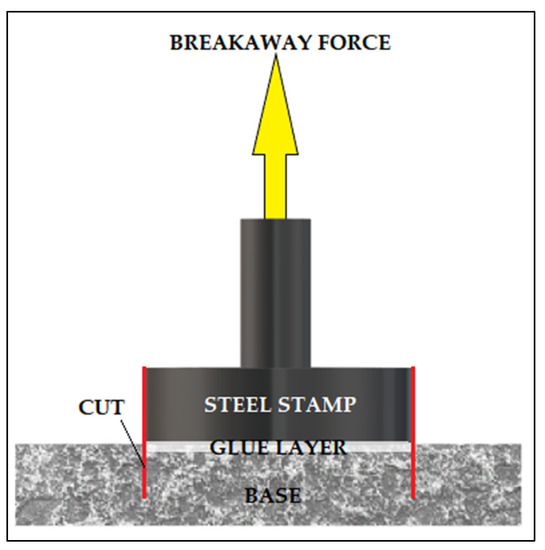
Figure 9.
Pull-Off test scheme.
3. Results
The following are the results of testing the adhesion of geopolymer and varnish layers to various substrates. The results should be evaluated both by considering the numerical force values measured with the pull-off tester and by visually evaluating the peeled off coatings (surfaces).
3.1. Varnish Coatings
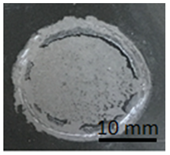
After the pull-off test, the surface of the samples and the surface of the stamps were analyzed to determine the type of cracks present. Table 4 shows the appearance of the stamps and the surface of the varnished specimens after the pull-off test.

Table 4.
Varnish coatings (without additives) applied to the DC01 metal slab-semi-mat, after pull-off test.
For the three samples made for the semi-matt coating without additives, the fractures were both adhesive and cohesive in character. For sample number C.1, adhesive fracture between the coating and the substrate occurred on more than half of the sample surface. For sample number C.2, however, adhesive rupture occurred on an area of less than 50% of its surface. Sample number C.3 had approximately 90% adhesion detachment. The remaining area for each of these three samples was cohesive rupture within the coating. Such a varied appearance of the surface of the samples after the test may be indicative of a non-homogeneous preparation of the substrate before coating. The substrate may have had different roughness in the areas concerned, which affected the adhesion properties of the coating.
Table 5 shows the stamp and surface of the samples coated with semi-matt varnish after the pull-off test. In this case, etched and heated at 850 °C, the tuff was added to the varnish before it was applied to the substrate.

Table 5.
Varnish coatings (with added etched and heated at 850 °C tuff) applied to the DC01 metal slab-semi-mat, after pull-off test.
For specimens C.4, C.5 and C.6 coated with a semi-matt coating with an addition of tuff etched and heated at 850 °C, the fracture was approximately 85% adhesive between the coating and the substrate and approximately 15% cohesive within the coating. For these samples, cohesive fracture is observed mainly at the edges of the area to which the stamp was adhered.
Table 6 shows the appearance of the stamps and the substrate after the pull-off test, for layers of varnish, with the addition of heated tuff at 850 °C.

Table 6.
Varnish coatings (with addition of heated tuff at 850 °C) applied to the DC01 metal slab-semi-mat, after pull-off test.
For specimens C.7, C.8 and C.9 with a varnish coating containing heated tuff at 850 °C, only cohesive cracks were observed within the coating. However, the coating layer that remained intact on the surface side was very thin. The translucent metal substrate DC01 can be observed in the figures.
Table 7 shows the stamps and surfaces of the gloss-coated specimens after the pull-off test.

Table 7.
Varnish coatings (without additives) applied to the DC01 metal slab-gloss, after pull-off test.
Samples numbered C.10, C.11 and C.12 showed mainly adhesive detachment between the substrate and the applied coating.
Table 8 shows the appearance of the stamps and substrate after the pull-off test, for varnish layers (gloss), with the addition of etched and heated at 850 °C tuff.

Table 8.
Varnish coatings (with added etched and heated at 850 °C tuff) applied to the DC01 metal slab-gloss, after pull-off test.
The samples coated with gloss varnish with etched and heated tuff (C.13, C.14 and C.15) detached both adhesively and cohesively. Additionally, in their case, areas were observed where the coating could not be detached due to insufficient adhesive strength. The problem with partial non-adhesion of the adhesive to the coating may have been caused by irregular roughness of the coating or insufficient cleaning of the coating in some areas. Sample number C.15 had about 70% adhesive breakage, while for samples C.13 and C.14 the proportion of cohesive breakage area was much higher than adhesive breakage.
Table 9 shows the appearance of the stamps and the substrate after the pull-off test, for layers of varnish, with the addition of heated tuff at 850 °C.

Table 9.
Varnish coatings (with addition of heated tuff at 850 °C) applied to the DC01 metal slab-gloss, after pull-off test.
The samples coated with a glossy varnish with added tuff heated at 850 °C detached adhesively.
Figure 10 shows the average results from the pull-off test carried out for the different variants of coatings varnished on the DC01 steel base.
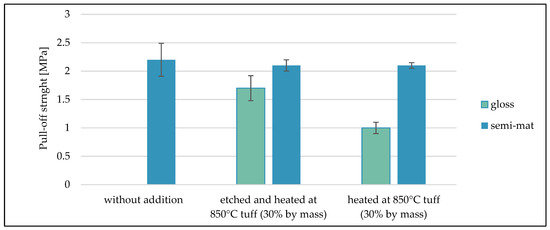
Figure 10.
Summary of average pull-off test results for varnish coatings.
For samples (C.10, C.11, C.12) coated with glossy varnish without any additives, the measurement failed because the obtained value of breaking stress was too low for the device to register the measurement. The highest values of breaking stress were obtained for samples coated with semi-matt varnish without tuff addition. The average pull-off stress value was in the range of 2.2 MPa. Similar values of rupture stresses were achieved by coatings made of semi-matt varnishes with tuff addition. These values were about 2.1 MPa. The lowest average pull-off strength value was achieved by coatings made of glossy varnish with tuff addition (about 1.0 MPa).
3.2. Geopolymer Coatings
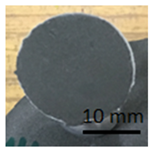
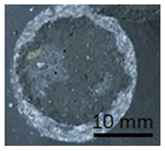
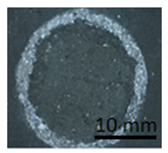
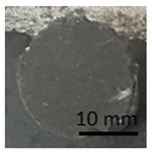
The samples in Table 10 below show the geopolymer coating on DC01 steel. Samples G.1 and G.2 were not cut with the cutting instrument before detachment. It can be seen that the coating was also detached outside the cutting area, particularly in the case of sample G.1. Adhesion damage occurred in all examples shown in the figures.

Table 10.
Geopolymer coating on DC01 metal slab, after pull-off test.
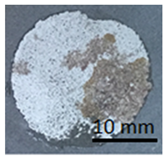
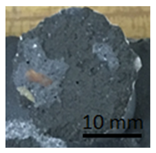
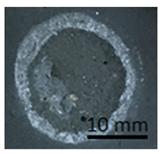
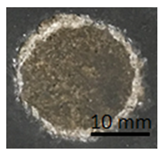
Table 11 shows samples with a geopolymer layer on a geopolymer concrete substrate. The geopolymer material is characterized by a rather high porosity. Due to the fact that the coating was applied by hand, it was impossible to obtain a perfectly smooth surface. All samples were cut out with a cutting tool. The applied coating has "peeled off". The same coating is present on both the stamp surface and the base surface. In addition, in samples G.7 and G.9, a section of the substrate was also torn out (visible as a lighter area on the stamp surface and on the base). This damage can be described as cohesive damage, as the pull-out occurred within the coating and substrate, rather than at the interface between the material layers.

Table 11.
Geopolymer coating on a geopolymer slab, after pull-off test.
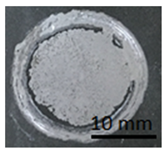
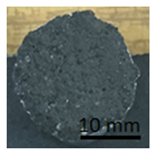
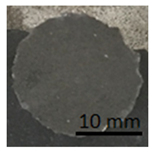
Table 12 shows samples with a geopolymer layer on the surface from a concrete slab. In each case shown in Table 12, it can be seen that the tested layer remained on the punch and on the concrete surface. The character of the rupture can be described as adhesive and cohesive failure.

Table 12.
Geopolymer coating on concrete slabs, after pull-off test.
Figure 11 shows the average results of the pull-off test carried out for geopolymer coatings on different types of substrate.
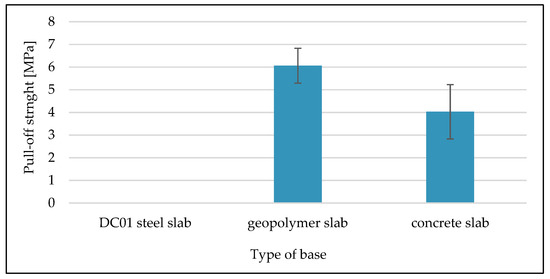
Figure 11.
Results of pull-off method tests for applied geopolymer coatings.
The average breaking strength of the geopolymer coating on the geopolymer concrete substrate was about 6 MPa, while on the concrete slab substrate it was about 4 MPa. The pull-off strength of the geopolymer coating applied to the steel slab was not successfully tested. The reason for this is that the coating-substrate bond is too weak. As a result, the measuring equipment did not record any stress value.
4. Conclusions
Pull-off testing is an attractive method and provides an easy way to evaluate the adhesive strength of coatings to metal surfaces. The pull-off device provides values of the peel stress, which not only allows a quick determination of the adhesion of the coating to the substrate, but also makes it easier to compare the adhesion of several coatings among themselves. However, based on the tests performed, it seems that this method is suitable for testing various types of paint coatings. Conversely, it is not appropriate for testing the adhesion of fragile coatings such as geopolymer coatings.
A number of practical conclusions were reached from the study:
- Automatic pull-off devices do not allow the measurement of stress for coatings with very low adhesion to the substrate (for which the value of pull-of is less than 1 MPa).
- Pull-off adhesion testing of coatings is very complicated and time-consuming. Before performing a pull-off test, aspects such as proper preparation of the coating surface and test stamps, adhesive selection, adhesive curing time and incision (or not) of the area around the stamps must be considered.
- The pull-off test provides a more objective and more detailed assessment of the coating’s degree of adhesion to the surface. This is possible because the breakaway stress values are automatically provided by the pull-off device, and observation of the specimen surface allows the percentage of adhesive and cohesive breakage to be determined.
- The test result is correct when the entire surface of the punch is covered with a tear-off coating.
Author Contributions
Conceptualization, M.Ł., A.B., G.R. and K.O.; methodology, M.Ł., investigation, G.R. and K.O.; resources, M.Ł.; writing—original draft preparation, M.Ł. and A.B.; writing—review and editing, M.Ł. and K.P.; supervision, K.P. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work has been financed by the Polish National Agency for Academic Exchange under the International Academic Partnership Programme within the framework of the grant: E-mobility and sustainable materials and technologies EMMAT (PPI/APM/2018/1/00027).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Adraider, Y.; Pang, Y.X.; Nabhani, F.; Hodgson, S.N.; Sharp, M.C.; Al-Waidh, A. Laser-induced deposition of alumina ceramic coating on stainless steel from dry thin films for surface modification. Ceram. Int. 2014, 40, 6151–6156. [Google Scholar] [CrossRef]
- Singh, L.; Chawla, V.; Grewal, J.S. A Review on Detonation Gun Sprayed Coatings. J. Miner. Mater. Charact. Eng. 2012, 11, 243–265. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Sinha, A.S.K.; Singh, I.B. Effect of sintering temperatures on corrosion and wear properties of Sol-Gel alumina coatings on surface pre-treated mild steel. Corros. Sci. 2008, 50, 639–649. [Google Scholar] [CrossRef]
- Zhang, G.; Xie, Q.; Ma, C.; Zhang, G. Permeable epoxy coating with reactive solvent for anticorrosion of concrete. Prog. Org. Coat. 2018, 117, 29–34. [Google Scholar] [CrossRef]
- Kasala, S.; Vidyavathy, M. Advanced ceraminc coatings on stainless steel: A review of research, methods, materials, applications and opportunities. Int. J. Adv. Eng. Technol. 2016, 7, 126–141. [Google Scholar]
- Pavan, C.M.; Narendra, B.B.R. Review of Ceramic Coating on Mild Steel Methods, Applications and Opportunities. Int. J. Adv. Sci. Res. Eng. 2018, 4, 44–49. [Google Scholar]
- Łach, M.; Pławecka, K.; Bąk, A.; Lichocka, K.; Korniejenko, K.; Cheng, A.; Lin, W.-T. Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams. Materials 2021, 14, 5090. [Google Scholar] [CrossRef] [PubMed]
- Szeląg, M.; Fic, S. Analysis of cluster crack development in cement slurry modified with microsilica. Build. Arch. 2015, 4, 117–127. [Google Scholar]
- Hejwowski, T.; Łabacz-Kęcik, A. Microstructure and wear resistance of coatings sprayed by flame-powder mixtures. Weld. Technol. Rev. 2012, 84, 359. [Google Scholar]
- Rudawska, A. Selected Issues of Forming Adhesive Joints Homogeneous and Hybrid; Lublin University of Technology: Lublin, Poland, 2013; pp. 1–44. [Google Scholar]
- Izdebska-Szanda, I.; Baliński, A. Nvestigations of adhesion and cohesion properties of chemically modified sodium silicates. Odlewnictwo–Nauka i Praktyka 2005, 7, 3–7. [Google Scholar]
- Mirski, Z.; Piwowarczyk, T. Metal gluing. Przegląd Spaw. 2003, 75, 10–12. [Google Scholar]
- Mikuła, J.; Łach, M. Eco-Friendly Solutions for Production. In Modern Eco-Friendly Composite Materials; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2014; pp. 13–32. [Google Scholar]
- Guo, B.; Pan, D.; Liu, B.; Volinsky, A.A.; Fincan, M.; Du, J.; Zhang, S. Immobilization mechanism of Pb in fly ash-based geopolymer. Constr. Build. Mater. 2017, 134, 123–130. [Google Scholar] [CrossRef]
- Nath, S.K. Fly ash and zinc slag blended geopolymer: Immobilization of hazardous materials and development of paving blocks. J. Hazard. Mater. 2020, 387, 121673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taki, K.; Mukherjee, S.; Patel, A.K.; Kumar, M. Reappraisal review on geopolymer: A new era of aluminosilicate binder for metal immobilization. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100345. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Lach, M.; Mikula, J. Alkali activation of waste clay bricks: Influence of the silica modulus, SiO2/Na2O, H2O/Na2O molar ratio, and liquid/solid ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction-recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Yazdi, M.A.; Liebscher, M.; Hempel, S.; Yang, S.; Mechtcherine, V. Correlation of microstructural and mechanical properties of geopolymers produced from fly ash and slag at room temperature. Constr. Build. Mater. 2018, 191, 330–341. [Google Scholar] [CrossRef]
- Ramujee, K.; PothaRaju, M. Mechanical Properties of Geopolymer Concrete Composites. Mater. Today Proc. 2017, 4, 2937–2945. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Mechanical Behaviour and Microstructural Investigation of Geopolymer Concrete after Exposure to Elevated Temperatures. Arab. J. Sci. Eng. 2020, 45, 3843–3861. [Google Scholar] [CrossRef]
- Li, J.; Sun, P.; Li, J.; Lv, Y.; Ye, H.; Shao, L.; Du, D. Synthesis of electrolytic manganese residue-fly ash based geopolymers with high compressive strength. Constr. Build. Mater. 2020, 248, 118489. [Google Scholar] [CrossRef]
- Łach, M.; Kluska, B.; Janus, D.; Kabat, D.; Pławecka, K.; Korniejenko, K.; Guigou, M.D.; Choińska, M. Effect of Fiber Reinforcement on the Compression and Flexural Strength of Fiber-Reinforced Geopolymers. Appl. Sci. 2021, 11, 10443. [Google Scholar] [CrossRef]
- Huseien, G.H.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive strength and microstructure of assorted wastes incorporated geopolymer mortars: Effect of solution molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Krzywoń, R.; Dawczyński, S. Strength Parameters of Foamed Geopolymer Reinforced with GFRP Mesh. Materials 2021, 14, 689. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Yang, T.; Liu, C.; Zhang, Z. Increasing mechanical strength and acid resistance of geopolymers by incorporating different siliceous materials. Constr. Build. Mater. 2018, 175, 411–421. [Google Scholar] [CrossRef]
- Özcan, A.; Karakoç, M.B. Evaluation of sulfate and salt resistance of ferrochrome slag and blast furnace slag-based geopolymer concretes. Struct. Concr. 2019, 20, 1607–1621. [Google Scholar] [CrossRef]
- Luhar, S.; Chaudhary, S.; Luhar, I. Thermal resistance of fly ash based rubberized geopolymer concrete. J. Build. Eng. 2018, 19, 420–428. [Google Scholar] [CrossRef]
- Zhao, R.; Yuan, Y.; Cheng, Z.; Wen, T.; Li, J.; Li, F.; Ma, Z.J. Freeze-thaw resistance of Class F fly ash-based geopolymer concrete. Constr. Build. Mater. 2019, 222, 474–483. [Google Scholar] [CrossRef]
- Degirmenci, F.N. Freeze-Thaw and fire resistance of geopolymer mortar based on natural and waste pozzolans. Ceram. Silik. 2018, 62, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020. [Google Scholar]
- Sikora, S.; Gapys, E.; Michalowski, B.; Horbanowicz, T.; Hynowski, M. Geopolymer coating as a protection of concrete aganist chemical attack and corrosion. E3S Web Conf. 2018, 49, 00101. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, K.; Parsai, R.; Anshul, A.; Singh, A.; Bharadwaj, P.; Gupta, R.; Mishra, D.; Amritphale, S.S. Studies on fly ash based geopolymeric material for coating on mild steel by paint brush technique. Int. J. Adhes. Adhes. 2017, 75, 139–144. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Riessen, A. Preparation of metakaolin based geopolymer coatings on metal substrates as thermal barriers. Appl. Clay Sci. 2009, 46, 265–270. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Riessen, A. Fly ash based geopolymer thin coatings on metal substrates and its thermal evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Azizli, K.; Sufian, S.; Man, Z. Sodium Silicate-Free Geopolymers as Coating Materials: Effects of Na/Al and Water/Solid Ratios on Adhesion Strength. Ceram. Int. 2015, 41, 2794–2805. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).