Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) are the group of drugs most commonly used in medicine. They are available over the counter to treat fevers and pains of various origins. The clinical and pharmaceutical analysis of these drugs requires effective analytical procedures for drug quality control, pharmacodynamic and pharmacokinetic studies. This article presents the spectrophotometric method that was used to analyze selected drugs from the NSAID group. The conditions for the determination of selected coxibs and oxicams in the UV range with the use of microplates have been developed. The presented procedure has been validated in accordance with the requirements, guaranteeing reliable results. The obtained results give the basis for the conclusion that the method can be successfully used in the quality control of pharmaceutical preparations with a small amount of available sample.
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a group of drugs most commonly used in human and veterinary medicine. Many of them are available over the counter, most often to treat fever and moderate pain. NSAIDs include chemical compounds of various structures and therapeutic applications. However, they share certain characteristics, i.e., identical basic pharmacological properties, similar basic mechanisms of action as well as similar side effects. In addition, all drugs in this group are acidic in nature (pK values in the range 3.0–5.0). NSAID molecules can contain both hydrophilic (carboxyl or enol groups) and lipophilic (e.g., aromatic ring) groups. Due to their acidic nature, they are present in gastric juice in a protonated form. Also in the small intestine, there are favorable conditions for the absorption of weak acids due to the environment prevailing there. NSAIDs may exist in an ionized form in plasma. They are characterized by a very high level of plasma protein binding (>97%) and values of the volume of distribution ranging from 0.1 to 1.0. Most NSAIDs are metabolized in the liver through oxidation and conjugation of inactive metabolites which are excreted in the urine or bile. The metabolism of NSAIDs may be disturbed in some disease states, then accumulation of the drug may occur even at normal doses [1,2,3]. Disease entities in which NSAIDs are used include pain accompanying infection, metabolic and neoplastic diseases, systemic diseases of connective tissue, degenerative joint diseases, neurovascular disorders and other diseases accompanied by inflammatory and pain symptoms from the skeletal system [4,5]. The mechanism of action of this group of drugs is associated with the inhibition of the transformation of eicosanoids (prostanoids, leukotrienes), which are products of transformation of polyunsaturated fatty acids. The substrate for this synthesis is arachidonic acid, which is released from cell membranes by phospholipase A2. The activity of this enzyme is stimulated by calmodulin, bradykinin, thrombin and calcium, while inhibition is caused by glucocorticosteroids.
The NSAID group also includes oxicams and coxibs (Figure 1), substances whose mechanism of action is to inhibit COX prostaglandin cyclooxygenase. There are two isoenzymes: COX-1 (a cellular enzyme responsible for the synthesis of prostaglandins necessary for physiological processes) and COX-2 (appears in cells where the inflammatory process takes place and determines the formation of prostaglandins during this process) [6,7].
Figure 1.
Chemical structures of analyzed compounds.
Taking the chemical structure into account, oxicams are classified as derivatives of enolic acids. Drugs belonging to this family include piroxicam, tenoxicam and meloxicam—drugs with strong, long-lasting anti-inflammatory and analgesic effects that are relatively well tolerated [2,3]. Oxicams are quickly absorbed from the gastrointestinal tract and are over 90% bound to plasma proteins. They are metabolized mainly to glucuronides and excreted in feces and urine. The half-life of oxicams, ranging from 30–40 h, allows for once-a-day dosing of the drug.
Drugs belonging to the coxib group are selective for the COX-2 isoform. The development of drugs that selectively inhibit the inducible cyclooxygenase isoform allowed for a significant reduction in side effects while maintaining full anti-inflammatory and analgesic activity. It has been shown that these drugs have an anti-inflammatory effect comparable to non-selective cyclooxygenase inhibitors, and at the same time they are almost completely devoid of adverse effects on the gastrointestinal tract [4,8]. Studies that assessed the condition of the gastric mucosa by using gastroscopy have shown that the use of coxib is associated with a much lower incidence of ulcer complications than other NSAIDs. At the same time, selective COX-2 inhibitors are not without side effects. They are known to slow ulcer healing and can under certain conditions impair kidney function. Two generations of drugs can be distinguished within the group of coxibs: the 1st generation includes rofecoxib, celecoxib and valdecoxib, while the 2nd generation includes etoricoxib and lumiracoxib. The 1st generation inhibits COX-2 2 to 35 times more than COX-1. In turn, the second generation inhibits COX-2 that is 106 to 515 times stronger than COX-1. Celecoxib is a drug of the diarylpyrazole group, which is used to relieve symptoms of rheumatoid arthritis and osteoporosis as an analgesic. Administration of celecoxib has also been shown to inhibit the development of colorectal adenomas. Contraindication to use is caused by an allergy to sulfonamides or acetylsalicylic acid, and caution should be exercised in patients with asthma, liver and kidney damage and allergy sufferers. Etoricoxib, belonging to the NSAIDs from the dipyridine derivative group, is used in the symptomatic treatment of osteoarthritis, rheumatoid arthritis, symptoms of joint inflammation, pain treatment and in the acute phase of gout.
The widespread use of NSAIDs causes the creation of new, more effective combinations, and at the same time obliges increasing the quality control of these substances and therapeutic preparations so that they are as free as possible from contamination that may remain from the production process or arise during the decomposition of active substances or auxiliary. Clinical and pharmaceutical drug analysis requires effective analytical procedures to evaluate the quality of pharmacodynamic and pharmacokinetic studies. A review of relevant publications shows the use of various analytical methods.
The most widely used techniques of instrumental analysis are optical methods. Each molecule has the ability to absorb its characteristic portion of energy provided by electromagnetic radiation at a given wavelength. This makes it possible to identify functional groups in the molecule. The basis for the use of the spectrophotometric method is to determine the wavelength that has been absorbed and to determine the degree of intensity of radiation passing through the tested sample [9]. Spectrophotometric methods are a commonly used method, among others, in the study of biochemical processes (monitoring enzymatic reactions, determination of reaction products, measurement of the reaction rate) [10,11,12,13,14] or for quick monitoring of enzymatic reactions, both for measuring the reaction rate and determining reaction products [15]. Direct analyses are possible only when the wavelength characteristic for the determined component is not disturbed by other components or by background absorption [16,17].
The available literature includes spectroscopic methods used in the analysis of selected compounds. Tenoxicam in various pharmaceutical forms was determined spectrophotometrically with the use of organic or inorganic reagents (such as alizarin or potassium iodide) at visible range [18,19,20]. Methylene blue solution and orthophosphate(V) buffer (pH 8) were used for the determination of meloxicam in tablets, capsules and suppositories, and the assays were carried out at the maximum absorption of 653.5 nm [21]. Another author’s developed method involved the use of a 0.1 mol/L NaOH solution and the chosen wavelength was 362 nm [22]. Taha et al. carried out the determination of meloxicam in pharmaceuticals using a solution of 7-chloro-4-nitrobenz-2-oxy-1,3-diazole (NBD-Cl) and acetone, at λ = 535 nm [20]. UV spectrophotometric methods were used to analyze celecoxib and tizanidine hydrochloride in a 0.1 mol/L sodium hydroxide solution at an absorption maximum of 251.2 nm [23,24]. Two spectrophotometric methods (at 253.2 or 509.2 nm) have also been developed for the quantification of celecoxib in capsules [23,25]. Etoricoxib was analyzed using a different solvent system (90% methanol and NaOH aqueous solution) [23]. Two methods have been developed: direct for drug analysis in tablets and indirect for determining drug concentration in human plasma (extraction to acetonitrile and ethyl acetate) [23,26].
Separation methods, i.e., liquid chromatography (HPLC), gas chromatography, thin-layer chromatography and electrophoresis occupy a permanent place in this industry. These methods, especially HPLC, are widely used, however, they generally require complex and expensive equipment [27,28,29,30].
The aim of the presented study was to determine the conditions of quantitative microanalysis of selected drugs from the group of oxicams and coxibs with the use of the spectrophotometric method in the UV range. Optimization of the conditions and validation of the developed analytical procedure will enable the determination of the content of tenoxicam, meloxicam, celecoxib and etoricoxib in pharmaceutical preparations using very small amounts of solutions on OptiPlate plates. The presented procedure is a rapid and economical (low0cost and non-polluting conditions) method for quantitative determination of selected drugs that can be successfully applied in laboratories.
2. Materials and Methods
2.1. Reagents and Apparatus
The tests were performed using Spectrophotometer VICTOR™ X4 Multilabel Plate Reader (PerkinElmer) with OptiPlate 96 quartz bottom plates. All weights were made using an analytical balance with accuracy of 1 μg (Radwag, Poland). Methanol of analytical grade was purchased from POCH Gliwice, Poland. Standard substances were purchased from: tenoxicam (T0909, Sigma-Aldrich, Poznań, Poland), meloxicam (PHR1799, Supelco, Poland), celecoxib (PHR1683, Sigma-Aldrich, Poznań, Poland) and etoricoxib (31097, Supelco, Poland).
2.2. Pharmaceutical Preparations
The analyzed pharmaceutical preparations included: Tilcotil (1), tablets containing 20 mg of tenoxicam (No F0140, Roche, France); Tilcotil (2), tablets containing 20 mg of tenoxicam (No F0141, Roche, France); Meloxicam, tablets containing 7.5 mg of meloxicam (No 0010605, Grodzkie Zakłady Farmaceutyczne “Polfa” Sp z.o.o., Poland); Mobic, tablets containing 7.5 mg of meloxicam (No 902471, Boehringer Ingelheim, Germany); Mobic, tablets containing 15 mg of meloxicam (No 103123, Boehringer Ingelheim, Germany); Opokan, tablets containing 7.5 mg of meloxicam (No 01AF0918, Aflofarm Farmacja Polska Sp. z.o.o., Poland); Celebrex, capsules containing 200 mg of celecoxib (No 93870, Pfizer); Celebrex (1), capsules containing 100 mg of celecoxib (No 1507660DH, Pfizer); Celebrex (2), capsules containing 100 mg of celecoxib (No CD5629, Pfizer); Acroxia, tablets containing 120 mg of etoricoxib (No 0283210, Merck Sharp&Dohme); Acroxia, tablets containing 90 mg of etoricoxib (No NA43020, Merck Sharp&Dohme); Acroxia, tablets containing 60 mg of etoricoxib (No 0282500, Merck Sharp&Dohme); Acroxia MSD, tablets containing 60 mg of etoricoxib, (No NA44450, Merck Sharp&Dohme).
2.3. Solutions for Analysis
A series of standard solutions of individual substances (tenoxicam, meloxicam, celecoxib and etericoxib) were prepared in methanol by weighing the appropriate amounts of the substance into 10.0 mL flasks in order to obtain 0.02% (m/v) solutions. The obtained solutions were diluted at a further stage of the research, obtaining successively solutions with a concentration of 0.001 and 0.0005 % (m/v).
Preparation solutions: The appropriate masses of tablets or capsules, ground in a mortar, were weighed and dissolved in methanol to obtain solutions with the appropriate concentrations. The contents were shaken for 15 min and the filtered extracts were analyzed.
2.4. Conditions for the Determination of Substances
The prepared standard solutions of the analyzed substances and the solutions of preparations were subjected to spectrophotometric analysis on OptiPlates with a quartz bottom at a wavelength of λ = 260 nm (Figure 2).
Figure 2.
A sample image of spectrophotometric assay obtained by a VICTORTMX4 microplate reader.
2.5. Validation of the Method
A criteria for drug quality must be defined and measurable. The process that enables verification of the correctness of the selected method is validation. The validation process allows us to assess whether a given measurement method with its analytical parameters can be used in the routine laboratory. Thanks to that, the results obtained in specific studies thanks to a specific procedure are appropriate in relation to the intended purpose. Parameters taken into account during method validation are: specificity and selectivity, precision expressed as direct and indirect precision, accuracy, linearity, limits of detection and quantification and the range of the method [31].
2.5.1. Precision
Precision determines the degree of agreement between individual analysis results. A measure of precision is the value of the standard deviation (SD) or the relative standard deviation (RSD,%). Precision includes two concepts: repeatability and reproducibility. Repeatability expresses the precision of determinations made in short time interval, by the same analyst and under the same conditions. Reproducibility allows us to assess whether the method leads to the same results when performed by different analysts, using solvents from various manufacturers, while maintaining the parameters required in the description of the method.
2.5.2. Accuracy
Accuracy allows us to determine the compatibility between the actual value (content, concentration) and the value resulting from the analysis. The measure of the accuracy of an analytical method is the value of its systematic error. Accuracy can also be expressed as the percent recovery of the analyte added to the sample.
2.5.3. Linearity
Linearity defined as the ability to obtain analytical measurement results directly proportional to the concentration (content) of the analyte in the sample is usually maintained within a certain concentration range. The linear range of the method is determined by plotting the so-called calibration curve (graphical representation of the relationship Y = f(c), where Y is the measured value, and c—the concentration value of the determined component) and calculation of the correlation coefficient, r.
2.5.4. Selectivity and Specificity
The determination of the selectivity of the method consists of establishing the conditions for determining one component (or several components) in the presence of others. A perfectly selective method for one substance is defined as specific.
The sensitivity of the method is a parameter that defines the change of the measured value due to the change of the analyte content in the test sample. It is expressed as the slope of the calibration curve and numerically equal to the slope of the straight line. The sensitivity of the analytical method is higher the greater the change in the measured value is, leading to a small change in the analyte concentration.
2.5.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
The limit of detection is the smallest amount of a test substance in a sample that can be detected but not necessarily determined with adequate accuracy. This parameter can be determined according to the formula:
where: a is the slope of the stright line, Se is the standard deviation of the estimate. The limit of quantification is the smallest amount of test substance in a sample that can be quantified with sufficient precision and accuracy. It is determined by the formula:
where: a is the slope of the straight line and Se is the standard deviation of the estimate.
2.5.6. The Range of the Method
The range of the method is the interval between the minimum and maximum content of the active substance in the test sample for which the analytical method has a definite linearity, accuracy and precision.
2.6. Determination of Active Substances in Selected Pharmaceutical Preparations
In order to determine the content of the analyzed substances in pharmaceutical preparations, Optiplte 96 microplates were used. Each well was filled with 100 μL of standard solutions (0.001% (m/v) concentration for tenoxicam and etoricoxib and 0.0005% (m/v) for meloxicam and celecoxib) and 100 mL of test solutions, and then 200 μL of methanol was added to each solution. After putting the plate in the spectrophotometer, the absorbance values of all the solutions were measured at 260 nm. Measurements were made in triplicate, using the mean absorbance values for calculations.
3. Results and Discussion
A new procedure for the determination of tenoxicam, meloxicam, etoricoxib and celecoxib in pharmaceutical preparations has been developed. The analysis was performed with the spectrophotometric method using Optiplate 96 microplates. For quantitative determinations, absorbance values obtained for standard and test solutions for individual components were used. In the preliminary stage of the work, the developed method was validated by establishing the parameters of accuracy, linearity, quantification and detection limits and precision.
In order to determine the precision, solutions of individual standard substances and solvent (methanol) were placed on the Optilate 96 plate in the following proportions: 100 µL of the substance solution and 200 µL of methanol. Then the absorbance value was measured at λ = 260 nm. The obtained results of absorbance were the basis for determining the intra-day precision. One week later, solutions of individual substances were prepared in an analogous manner, the absorbance was measured and the inter-day precision was determined. The received values are summarized in Table 1.

Table 1.
Results of precision with statistical evaluation.
The percentage of recovery was established by adding a known amount of the analyte (80, 100 and 120%) to the test sample and then determining with the developed procedure (Table 2).

Table 2.
Results of the accuracy of the method with statistical evaluation.
On the basis of the measured absorbance values at the wavelength λ = 260 nm for increasing concentrations of tenoxicam, meloxicam, etoricoxib and celecoxib, the dependences of absorbance on the concentration of the substance were plotted (Figure 3). They were found to be linear in the tested concentration range, and the parameters of the calibration curve are presented in Table 3.
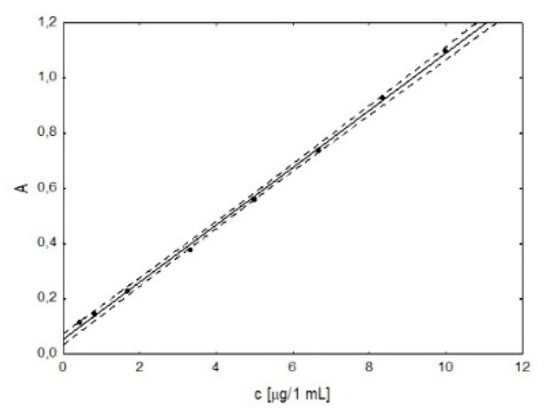
Figure 3.
An example plot of absorbance (A) versus concentration (c) for meloxicam solutions.

Table 3.
Linear regression parameters.
Based on the determined linearity parameters, the LOD and LOQ for individual substances were calculated, and the results are presented in Table 4.

Table 4.
Limit of Detection (LOD) and Limit of Quantification (LOQ) values.
Based on the obtained results, it was found that the developed method is characterized by a wide range of linearity (2.0–5.0 μg/mL for tenoxicam, 1.3–10.0 μg/mL for meloxicam, 1.9–5.0 μg/mL for etoricoxib, 1.4–5.0 μg/mL for celecoxib) for all analyzed substances with a correlation coefficient (r) ranging from 0.9946 to 0.9994, good precision (RSD ranging from 1.51 to 2.81%) and the appropriate accuracy (an average recovery value of 98%). Thus, it meets the requirements for analytical procedures and can be used to determine the content of selected substances in pharmaceutical products.
Six pharmaceutical preparations containing tenoxicam and meloxicam, and seven preparations containing etoricoxib and celecoxib were tested. Ten measurements were made for each of the preparations. The obtained absorbance values were used to determine the content of active substance in individual preparations. Statistical evaluation was performed for each series of results and the obtained values are summarized in Table 5.

Table 5.
Results of the determination of selected active substances with statistical evaluation.
In the case of the analysis of selected pharmaceutical preparations, the content of the active ingredient ranges from 64% to 100.07% in relation to the value declared by the manufacturers. The lower content of active ingredients in relation to the declared values is probably related to the fact that these drugs were expired. In these preparations, the active substance could be partially degraded, and thus its content in the tablet/capsule was reduced. Comparing the expiry dates of the pharmaceutical preparations used in the analysis with the content in the tablet/capsule obtained in the tests, it can be noticed that preparations with a shorter expiry date contained a smaller amount of the active substance compared to drugs with a longer expiry date. The preparations analyzed within the expiry date contained an amount of the active substance in accordance with the declaration on the packaging.
The obtained results allowed us to conclude that the developed method is simple and accurate, and the small volumes of the solutions used (a maximum of 300 μL) and the quick analysis time (a measurement time of approximately 0.1 s/well) allow them to be used in laboratories for routine analyses of chosen NSAIDs.
4. Conclusions
The developed new procedure for the quantification of tenoxicam, meloxicam, etoricoxib and celecoxib in pharmaceutical preparations with the UV spectrophotometric method using microplates meets the requirements for validation of analytical methods. Due to the simplicity of determination and speed of analysis, it can be helpful in routine analyses and quality control of pharmaceutical preparations. Spectrophotometric and chromatographic methods with UV detection are analytical techniques widely used in analytical laboratories. The HPLC methods described in the literature always involve sample pretreatment and large amounts of various components in the mobile phase. HPLC with MS detection makes procedures more complicated and accessible to few parties. The described spectrophotometric methods also often include sample pretreatment (derivatizaton). The aim of this work was to develop a fast and economical method to be used among others in quality control laboratories. Even if the results obtained with different techniques are equally accurate, the significant advantages of our methodology, compared to other existing methods, are its low cost, non-polluting conditions, simplicity of implementation and small sample volume needed for analysis. Therefore, the method can be used for routine quality control of selected drugs in pure substance or commercial samples.
Author Contributions
Conceptualization, M.S.; investigation, P.G.; investigation, P.G., M.S., M.D.; writing—original draft preparation, P.G.; writing—review and editing, M.S. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gouda, A.A.; El-Sayed, M.I.K.; Amin, A.S.; El Sheikh, R. Spectrophotometric and spectrofluorometric methods for the determination of non-steroidal anti-inflammatory drugs: A review. Arab. J. Chem. 2013, 6, 145–163. [Google Scholar] [CrossRef]
- Starek, M.; Krzek, J. A review of analytical techniques for determination of oxicams, nimesulide and nabumetone. Talanta 2009, 77, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Zejc, A.; Gorczyca, M. Chemia Leków; PZWL: Warszawa, Poland, 2008. [Google Scholar]
- Castellsague, J.; Riera-Guardia, N.; Calingaert, B.; Varas-Lorenzo, C.; Fourrier-Reglat, A.; Nicotra, F.; Sturkenboom, M.; Perez-Gutthann, S. Safety of non-steroidal anti-inflammatory drugs (SOS) Project: Individual NSAIDs and upper gastrointestinal complications. Drug Saf. 2012, 35, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal Toxicity With Celecoxib vs Nonsteroidal Anti-inflammatory Drugs for Osteoarthritis and Rheumatoid Arthritis. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef]
- Fokunang, C.N.; Fokunang, E.T.; Frederick, K.; Ngameni, B.; Ngadjui, B. Overview of non-steroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol. 2018, 4, 5–13. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Varga, Z.; Kriska, M.; Kristova, V.; Petrová, M. Analysis of non-steroidal anti-inflammatory drug use in hospitalized patients and perception of their risk. Interdiscip. Toxicol. 2013, 6, 141–144. [Google Scholar] [CrossRef][Green Version]
- Parmar, A.; Sharma, S. Derivative UV-vis absorption spectra as an invigorated spectrophotometric method for spectral resolution and quantitative analysis: Theoretical aspects and analytical applications: A review. TrAC Trends Anal. Chem. 2016, 77, 44–53. [Google Scholar] [CrossRef]
- Talluri, M.K.; Bairwa, M.K.; Dugga, H.H.T.; Srinivas, R. Development and Validation Of RP-HPLC and ultraviolet spectrophotometric methods of analysis for simultaneous determination of paracetamol and lornoxicam in pharmaceutical dosage forms. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 129–140. [Google Scholar] [CrossRef]
- Sahoo, S.; Giri, R.; Patil, S.; Behera, A.; Mohapatra, R. Development of Ultraviolet Spectrophotometric Method for Analysis of Lornoxicam in Solid Dosage Forms. Trop. J. Pharm. Res. 2012, 11, 269–273. [Google Scholar] [CrossRef]
- Elbashir, A.A.; Suliman, F.O.; Aboul-Enein, H.Y. The Application of 7-Chloro-4-nitrobenzoxadiazole (NBD-Cl) for the Analysis of Pharmaceutical-Bearing Amine Group Using Spectrophotometry and Spectrofluorimetry Techniques. Appl. Spectrosc. Rev. 2011, 46, 222–241. [Google Scholar] [CrossRef]
- Erk, N.; Altuntas, T.G. Comparison of derivative spectrophotometric and liquid chromatographic methods for the determination of rofecoxib. Die Pharm. 2004, 59, 453–456. [Google Scholar]
- Moreira, T.S.; Pierre, M.B.R.; Fraga, C.A.M.; Sousa, V.P. Development and validation of HPLC and UV spectrophotometric methods for the determination of lumiracoxib in tablets. Rev. Ciênc. Farm. Básica Appl. 2008, 29, 267–275. [Google Scholar]
- Rojas, F.S.; Pavón, J.C. Spectrophotometry: Biochemical Applications. Encycl. Anal. Sci. 2005, 366–372. [Google Scholar] [CrossRef]
- Calatayud, J.M. Spectrophotometry: Pharmaceutical Applications. Encycl. Anal. Sci. 2005, 373–383. [Google Scholar] [CrossRef]
- Redasani, V.K.; Patel, P.R.; Marathe, D.Y.; Chaudhari, S.R.; Shirkhedkar, A.A.; Surana, S.J. A review on derivative uv-spectrophotometry analysis of drugs in pharmaceutical formulations and biological samples review. J. Chil. Chem. Soc. 2018, 63, 4126–4134. [Google Scholar] [CrossRef]
- El-Ries, M.A.; Mohamed, G.; Khalil, S.; El-Shall, M. Spectrophotometric and potentiometric determination of piroxicam and tenoxicam in pharmaceutical preparations. Chem. Pharm. Bull. 2003, 51, 6–10. [Google Scholar] [CrossRef]
- Amin, A.S. Spectrophotometric determination of piroxicam and tenoxicam in pharmaceutical formulations using alizarin. J. Pharm. Biomed. Anal. 2002, 29, 729–736. [Google Scholar] [CrossRef]
- Taha, E.A.; Salama, N.N.; Fattah, L.E.-S.A. Spectrofluorimetric and Spectrophotometric Stability-Indicating Methods for Determination of Some Oxicams Using 7-Chloro-4-nitrobenz-2-oxa-1,3-diazole (NBD-Cl). Chem. Pharm. Bull. 2006, 54, 653–658. [Google Scholar] [CrossRef]
- Zawilla, N.H.; Mohammad, M.A.-A.; El Kousy, N.M.; Aly, S.M.E.-M. Determination of meloxicam in bulk and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2003, 32, 1135–1144. [Google Scholar] [CrossRef]
- Hassan, E.M. Spectrophotometric and fluorimetric methods for the determination of meloxicam in dosage forms. J. Pharm. Biomed. Anal. 2002, 27, 771–777. [Google Scholar] [CrossRef]
- Starek, M. Review of the applications of different analytical techniques for coxibs research. Talanta 2011, 85, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Sankar, D.G. UV spectrophotometric methods for the determination of celecoxib and tizanidine hydrochloride. Indian J. Pharm. Sci. 2001, 63, 521–523. [Google Scholar]
- Pillai, S.; Singhvi, I. Spectrophotometric estimation of celecoxib from capsule formulation. Asian J. Chem. 2006, 18, 1560–1562. [Google Scholar]
- Vadnerkar, G.; Jain, S.K.; Jain, D. Determination of etoricoxib in bulk drug, dosage form and human plasma by UV-spectrophotometry. Asian J. Chem. 2006, 18, 2895–2901. [Google Scholar]
- Antonoaea, P.; Cârje, A.G.; Ciurba, A.; Todoran, N.; Vlad, A.R.; Muntean, D.L. Validation of High Performance Liquid Chromatography Methods for Determination of Meloxicam and Tenoxicam from Transdermal Therapeutic Systems. Acta Med. Marisiensis 2017, 63, 178–182. [Google Scholar] [CrossRef]
- Semreen, M.H.; Aboul-Enein, H.Y. LC-UV Method Development And Validation for the Non Steroidal Anti-Inflammatory Agent Tenoxicam. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 720–729. [Google Scholar] [CrossRef]
- Jayasagar, G.; Kumar, M.K.; Chandrasekhar, K.; Prasad, P.S.; Rao, Y.M. Validated HPLC method for the determination of celecoxib in human serum and its application in a clinical pharmacokinetic study. Die Pharm. 2002, 57, 619–621. [Google Scholar]
- Topalli, S.; Chandrashekhar, T.G.; Annapurna, M.M. Validated RP-HPLC Method for the Assay of Etoricoxib (A Non-Steroidal Anti-Inflammatory Drug) in Pharmaceutical Dosage Forms. E J. Chem. 2012, 9, 832–838. [Google Scholar] [CrossRef]
- Pawlaczyk, J.; Zając, M. Walidacja Metod Analizy Chemicznej; Wyd. Naukowe AM: Poznań, Poland, 2005. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
