Peri-Operative Nursing of Patients with Malignant Hyperthermia: A Narrative Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Research Question Definition
- What are the main barriers and knowledge gaps in perioperative nursing management of MH?
- What international practices can guide future improvements in MH prevention and management by perioperative teams?
2.3. Key
2.4. Inclusion Criteria and Screening
2.5. Information Sources and Search Strategy
2.6. Data Extraction and Synthesis
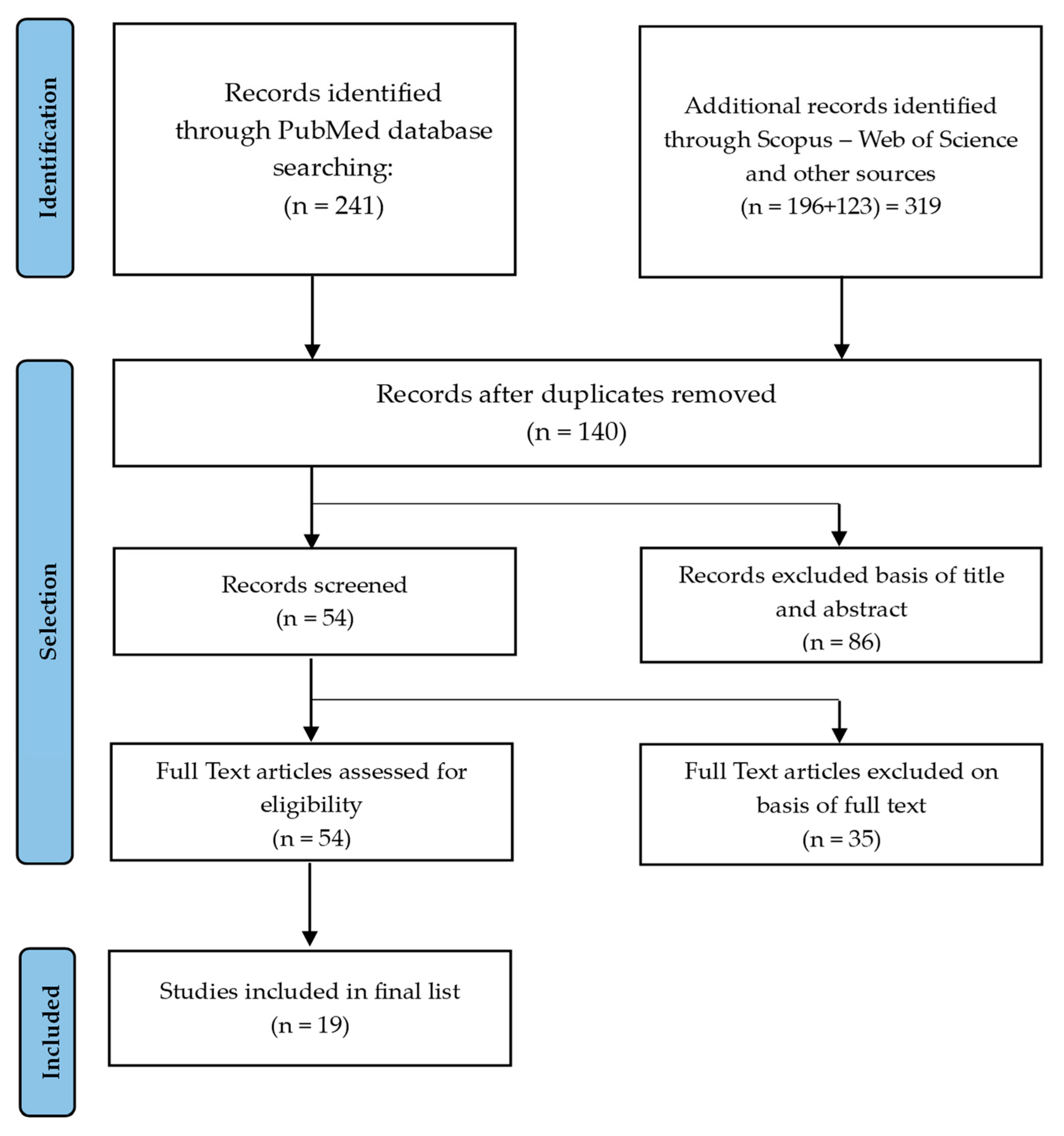
3. Results
3.1. Characteristics and Emerging Themes of Included Studies
3.2. The Nurse’s Role in Prevention
3.3. Preoperative Assessment
3.4. Staff Training
3.5. Alternative Approaches to Prevention
3.6. Activated Carbonl Filters
4. Discussion
4.1. Perspectives for Clinical Practice
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Guidelines for Safe Surgery 2009: Safe Surgery Saves Lives; World Health Organization: Geneva, Switzerland, 2009; Available online: https://www.who.int/publications/i/item/9789241598552 (accessed on 26 June 2025).
- Hopkins, P.M.; Rüffert, H.; Snoeck, M.M.; Girard, T.; Glahn, K.P.; Ellis, F.R.; Müller, C.R.; Urwyler, A.; European Malignant Hyperthermia Group. European Malignant Hyperthermia Group Guidelines for Investigation of Malignant Hyperthermia Susceptibility. Br. J. Anaesth. 2015, 115, 531–539. [Google Scholar] [CrossRef]
- Hopkins, P.M.; Girard, T.; Dalay, S.; Jenkins, B.; Thacker, A.; Patteril, M.; McGrady, E. Malignant hyperthermia 2020: Guideline from the Association of Anaesthetists. Anaesthesia 2021, 76, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Rüffert, H.; Bastian, B.; Bendixen, D.; Girard, T.; Heiderich, S.; Hellblom, A.; Hopkins, P.M.; Johannsen, S.; Snoeck, M.M.; Urwyler, A.; et al. Consensus guidelines on perioperative management of malignant hyperthermia suspected or susceptible patients from the European Malignant Hyperthermia Group. Br. J. Anaesth. 2021, 126, 120–130. [Google Scholar] [CrossRef]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef]
- Larach, M.G.; Brandom, B.W.; Allen, G.C.; Gronert, G.A.; Lehman, E.B. Malignant hyperthermia deaths related to inadequate temperature monitoring, 2007–2012. Anesth. Analg. 2014, 119, 1359–1366. [Google Scholar] [CrossRef]
- Martin, S.D.; Rosenberg, H. Malignant hyperthermia: Crisis management and perioperative team training. Curr. Opin. Anaesthesiol. 2023, 36, 232–238. [Google Scholar] [CrossRef]
- Riazi, S.; Kraeva, N.; Hopkins, P.M. Malignant hyperthermia in the post-genomics era: New perspectives on an old concept. Can. J. Anaesth. 2018, 65, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, S.; Schuster, F. Maligne Hyperthermie—Pathophysiologie, Diagnostik und Therapie [Malignant Hyper-thermia—Update on Pathophysiology, Diagnostics and Treatment]. Anästhesiol. Intensivmed. Notfallmed. Schmerzther. 2019, 54, 527–537. [Google Scholar] [CrossRef]
- Hopkins, P.M.; Ellis, F.R.; Halsall, P.J. Malignant hyperthermia: Pharmacology of triggering. Br. J. Anaesth. 2015, 115, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.M.; Foulds, N.; Millar, J.S.; Sutherland, P.D.; Pappachan, V.J.; Holden, S.; Mein, R.; Hopkins, P.M.; Jungbluth, H. RYR1-Related Malignant Hyperthermia with Marked Cerebellar Involvement—A Paradigm of Heat-Induced CNS Injury? Neuromuscul. Disord. 2015, 25, 138–140. [Google Scholar] [CrossRef]
- Safety Committee of Japanese Society of Anesthesiologists. JSA Guideline for the Management of Malignant Hyperthermia Crisis 2016. J. Anesth. 2017, 31, 307–317. [Google Scholar] [CrossRef]
- Jurkat-Rott, K.; Holzherr, B.; Fauler, M.; Lehmann-Horn, F. The pathogenesis of malignant hyperthermia. Anaesthesia 2010, 65, 545–552. [Google Scholar] [CrossRef]
- White, R.; Schiemann, A.H.; Burling, S.M.; Bjorksten, A.; Bulger, T.; Gillies, R.; Hopkins, P.M.; Kamsteeg, E.-J.; Machon, R.G.; Massey, S.; et al. Functional analysis of RYR1 variants in patients with confirmed susceptibility to malignant hyperthermia. Br. J. Anaesth. 2022, 129, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Kraeva, N.; Riazi, S. Exertional rhabdomyolysis and malignant hyperthermia susceptibility: Is there a link? Br. J. Anaesth. 2020, 125, e326–e328. [Google Scholar] [CrossRef]
- Broman, M.; Islander, G.; Müller, C.R. Malignant Hyperthermia, a Scandinavian Update. Acta Anaesthesiol. Scand. 2015, 59, 951–961. [Google Scholar] [CrossRef]
- Biesecker, L.G.; Dirksen, R.T.; Girard, T.; Hopkins, P.M.; Riazi, S.; Rosenberg, H.; Stowell, K.; Weber, J. Genomic screening for malignant hyperthermia susceptibility. Anesthesiology 2020, 133, 1277–1282. [Google Scholar] [CrossRef]
- Galli, L.; Orrico, A.; Lorenzini, S.; Censini, S.; Falciani, M.; Covacci, A.; Tegazzin, V.; Sorrentino, V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum. Mutat. 2006, 27, 830. [Google Scholar] [CrossRef]
- Brandom, B.W.; Bina, S.; Wong, C.A.; Wallace, T.; Visoiu, M.; Isackson, P.J.; Vladutiu, G.D.; Sambuughin, N.; Muldoon, S.M. Ryanodine Receptor Type 1 Gene Variants in the Malignant Hyperthermia-Susceptible Population of the United States. Anesth. Analg. 2013, 116, 1078–1086. [Google Scholar] [CrossRef]
- Nelson, T.E.; Flewellen, E.H.; Britt, B.A. Clinical aspects, diagnosis, and management of malignant hyperthermia. Anesth. Analg. 1983, 62, 555–564. [Google Scholar] [CrossRef]
- Murayama, T.; Kurebayashi, N.; Ogawa, H.; Yamazawa, T.; Oyamada, H.; Suzuki, J.; Kanemaru, K.; Oguchi, K.; Iino, M.; Sakurai, T. Genotype-phenotype correlations of malignant hyperthermia and central core disease mutations in the central region of the RYR1 channel. Hum. Mutat. 2016, 37, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.M. Malignant hyperthermia: Advances in clinical management and diagnosis. Br. J. Anaesth. 2011, 107, 118–129. [Google Scholar] [CrossRef]
- Schneiderbanger, D.; Johannsen, S.; Roewer, N.; Schuster, F. Management of Malignant Hyperthermia: Diagnosis and Treatment. Ther. Clin. Risk Manag. 2014, 10, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, S.; Müller, C.R.; Zorzato, F.; Muntoni, F.; Sewry, C.; Treves, S.; Voermans, N.C.; Böske, J.; Løseth, S.; Wappler, F.; et al. Comprehensive diagnostic approach for malignant hyperthermia susceptibility. Neuromuscul. Disord. 2023, 33, 143–153. [Google Scholar] [CrossRef]
- Pinyavat, T.; Riazi, S.; Deng, J.; Slessarev, M.; Cuthbertson, B.H.; Ibarra Moreno, C.A.; Jerath, A. Malignant Hyperthermia. Crit. Care Med. 2024, 52, 1934–1940. [Google Scholar] [CrossRef]
- Sheikh, M.M.; Riaz, A.; Umair, H.M.; Waqar, M.; Muneeb, A. Succinylcholine-Induced Masseter Muscle Rigidity Successfully Managed with Propofol and Laryngeal Mask Airway: A Case Report and Brief Review. Cureus 2020, 12, e9376. [Google Scholar] [CrossRef]
- Frassanito, L.; Sbaraglia, F.; Piersanti, A.; Vassalli, F.; Lucente, M.; Filetici, N.; Zanfini, B.A.; Catarci, S.; Draisci, G. Real Evidence and Misconceptions about Malignant Hyperthermia in Children: A Narrative Review. J. Clin. Med. 2023, 12, 3869. [Google Scholar] [CrossRef]
- de Mello, J.M.; Andrade, P.V.; Santos, J.M.; Oliveira, A.S.B.; Vainzof, M.; do Amaral, J.L.G.; Almeida da Silva, H.C. Predictive factors of the contracture test for diagnosing malignant hyperthermia in a Brazilian population sample: A retrospective observational study. Braz. J. Anesthesiol. 2023, 73, 145–152. [Google Scholar] [CrossRef]
- Simões, C.M. Malignant hyperthermia: New knowledge changing perspectives. Braz. J. Anesthesiol. 2023, 73, 125–127. [Google Scholar] [CrossRef]
- Assolari, F.; Mancin, S.; Lopane, D.; Dacomi, A.; Coldani, C.; Tomaiuolo, G.; Cattani, D.; Palomares, S.M.; Cangelosi, G.; Mazzoleni, B. Advanced Practice Nursing in Surgery: A Scoping Review of Roles, Responsibilities, and Educational Programs. Int. Nurs. Rev. 2025, 72, e13045. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Mancin, S.; Zarrella, A.; Petrelli, F.; Cosmai, S.; Cattani, D.; Lopane, D.; Scollo, S.; Morales Palomares, S.; Sguanci, M.; Amendola, A.; et al. Diabetes, Chronic Kidney Disease, and Vascular Ulcers: Prevention Strategies and Clinical Implications. Diabetology 2025, 6, 10. [Google Scholar] [CrossRef]
- Cangelosi, G.; Palomares, S.M.; Pantanetti, P.; De Luca, A.; Biondini, F.; Nguyen, C.T.T.; Mancin, S.; Sguanci, M.; Petrelli, F. COVID-19, Nutrients and Lifestyle Eating Behaviors: A Narrative Review. Diseases 2024, 12, 193. [Google Scholar] [CrossRef]
- Sguanci, M.; Mancin, S.; Piredda, M.; De Marinis, M.G. Protocol for Conducting a Systematic Review on Diagnostic Accuracy in Clinical Research. MethodsX 2024, 12, 102569. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Gallegos, E.; Hennen, B. Malignant Hyperthermia Preparedness Training: Using Cognitive Aids and Emergency Checklists in the Perioperative Setting. J. PeriAnesthesia Nurs. 2022, 37, 24–28. [Google Scholar] [CrossRef]
- An, X.; Wang, Q.; Qiu, Y.; Zhu, Z.; Ma, Z.; Hua, W.; Li, X. Nursing Interventions of Intraoperative Malignant Hyperthermia in Patients with Scoliosis: A Report of 3 Cases. J. Neurosci. Nurs. 2020, 52, 66–71. [Google Scholar] [CrossRef]
- Ebbitt, L.; Johnson, E.; Herndon, B.; Karrick, K.; Johnson, A. Suspected Malignant Hyperthermia and the Application of a Multidisciplinary Response. Healthcare 2020, 8, 328. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Godsch, C.; Sessler, D.I.; Volk, T.; Kreuer, S.; Hüppe, T. Residual Volatile Anesthetics after Workstation Preparation and Activated Charcoal Filtration. Acta Anaesthesiol. Scand. 2020, 64, 759–765. [Google Scholar] [CrossRef]
- Soenarto, R.; Auerkari, A.; Peddyandhari, F.; Lunaesti, C.; Rahyussalim, A. Perioperative Management in a Malignant Hyperthermia Susceptible Patient. Bali J. Anesthesiol. 2019, 3, 178. [Google Scholar] [CrossRef]
- Lewellen, D.B. Malignant Hyperthermia Crisis: Preparing Your Endoscopy Staff. Gastroenterol. Nurs. 2019, 42, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sicat, A.; Bennett, C.; Homer, K.; Shimi, S.; Spafford, R.; Han, G.; Rollison, S. Malignant Hyperthermia: How the Lack of Regular Simulation Training Threatens Patient Safety. Clin. Simul. Nurs. 2025, 98, 101651. [Google Scholar] [CrossRef]
- Kaur, H.; Katyal, N.; Yelam, A.; Kumar, K.; Srivastava, H.; Govindarajan, R. Malignant Hyperthermia. Mo. Med. 2019, 116, 154–159. [Google Scholar] [PubMed]
- Schaad, S. Simulation-Based Training: Malignant Hyperthermia. AORN J. 2017, 106, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Bashaw, M. Integrating Simulations Into Perioperative Education for Undergraduate Nursing Students. AORN J. 2016, 103, 175–183. [Google Scholar] [CrossRef]
- Denholm, B.G. Using Informatics to Improve the Care of Patients Susceptible to Malignant Hyperthermia. AORN J. 2016, 103, 365–379. [Google Scholar] [CrossRef]
- Denholm, B.G. Using a Vulnerability Theoretical Model to Assess the Malignant Hyperthermia Susceptible Population. Adv. Emerg. Nurs. J. 2015, 37, 209–222. [Google Scholar] [CrossRef]
- Sousa, C.S.; Cunha, A.L.M. Knowledge of Nursing Professionals of a Surgical Center Regarding Malignant Hyperthermia. Rev. Gaúcha Enferm. 2014, 35, 43–48. [Google Scholar] [CrossRef][Green Version]
- Seifert, P.C.; Wahr, J.A.; Pace, M.; Cochrane, A.B.; Bagnola, A.J. Crisis Management of Malignant Hyperthermia in the OR. AORN J. 2014, 100, 189–202.e1. [Google Scholar] [CrossRef]
- Hirshey Dirksen, S.J.; Van Wicklin, S.A.; Mashman, D.L.; Neiderer, P.; Merritt, D.R. Developing Effective Drills in Preparation for a Malignant Hyperthermia Crisis. AORN J. 2013, 97, 329–353. [Google Scholar] [CrossRef]
- Hutton, D. Emergency Preparedness Case Study. Plast. Surg. Nurs. 2012, 32, 80–83. [Google Scholar] [CrossRef]
- Birgenheier, N.; Stoker, R.; Westenskow, D.; Orr, J. Activated Charcoal Effectively Removes Inhaled Anesthetics from Modern Anesthesia Machines. Anesth. Analg. 2011, 112, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Noble, K.A. Malignant Hyperthermia: Hot Stuff! J. PeriAnesthesia Nurs. 2007, 22, 341–345. [Google Scholar] [CrossRef]
- Neacsu, A. Malignant Hyperthermia. Nurs. Stand. 2006, 20, 51–57. [Google Scholar] [CrossRef]
- Adam, M.P.; Feldman, J.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Amemiya, A. (Eds.) GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 1993–2025. [Google Scholar]
- Ramsey, L.B.; Prows, C.A.; Tang Girdwood, S.; Van Driest, S. Current Practices in Pharmacogenomics. Pediatr. Clin. N. Am. 2023, 70, 995–1011. [Google Scholar] [CrossRef]
- Mungunsukh, O.; Deuster, P.; Muldoon, S.; O’Connor, F.; Sambuughin, N. Estimating Prevalence of Malignant Hyperthermia Susceptibility Through Population Genomics Data. Br. J. Anaesth. 2019, 123, e461–e463. [Google Scholar] [CrossRef]
- Kathiresan, N.; Ramachandran, S.; Kulanthaivel, L. Next-Generation Sequencing to Study the DNA Interaction. Methods Mol. Biol. 2024, 2719, 249–264. [Google Scholar] [CrossRef]
- Levy, S.E.; Boone, B.E. Next-Generation Sequencing Strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a025791. [Google Scholar] [CrossRef]
- Riazi, S.; Larach, M.G.; Hu, C.; Wijeysundera, D.; Massey, C.; Kraeva, N. Malignant Hyperthermia in Canada: Characteristics of Index Anesthetics in 129 Malignant Hyperthermia Susceptible Probands. Anesth. Analg. 2014, 118, 381–387. [Google Scholar] [CrossRef]
- Kim, D.C. Malignant hyperthermia. Korean J. Anesthesiol. 2012, 63, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Glahn, K.P.E.; Bendixen, D.; Girard, T.; Hopkins, P.M.; Johannsen, S.; Rüffert, H.; Snoeck, M.M.; Urwyler, A.; European Malignant Hyperthermia Group. Availability of Dantrolene for the Management of Malignant Hyperthermia Crises: European Malignant Hyperthermia Group Guidelines. Br. J. Anaesth. 2020, 125, 133–140. [Google Scholar] [CrossRef]
- Glahn, K.P.E.; Girard, T.; Hellblom, A.; Hopkins, P.M.; Johannsen, S.; Rüffert, H.; Snoeck, M.M.; Urwyler, A.; European Malignant Hyperthermia Group. Recognition and Management of a Malignant Hyperthermia Crisis: Updated 2024 Guideline from the European Malignant Hyperthermia Group. Br. J. Anaesth. 2025, 134, 221–223. [Google Scholar] [CrossRef]
- Brandom, B.W. Update on Dantrolene in the Treatment of Anesthetic Induced Malignant Hyperthermia. SOJ Anesthesiol. Pain Manag. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Saito, J.; Akabane, M.; Ishikawa, Y.; Iwahashi, K.; Nakamura, H.; Yamatani, A. Retrospective survey of compounded medications for children in Japan. Eur. J. Pharm. Biopharm. 2020, 155, 122–127. [Google Scholar] [CrossRef]
- Dasta, J.F.; McLaughlin, T.P.; Mody, S.H.; Piech, C.T. Daily Cost of an Intensive Care Unit Day: The Contribution of Mechanical Ventilation. Crit. Care Med. 2005, 33, 1266–1271. [Google Scholar] [CrossRef]
- Graf, J.; Mühlhoff, C.; Doig, G.S.; Reinartz, S.; Bode, K.; Dujardin, R.; Koch, K.C.; Roeb, E.; Janssens, U. Health Care Costs, Long-Term Survival, and Quality of Life Following Intensive Care Unit Admission after Cardiac Arrest. Crit. Care 2008, 12, R92. [Google Scholar] [CrossRef]
- Kaier, K.; Heister, T.; Motschall, E.; Hehn, P.; Bluhmki, T.; Wolkewitz, M. Impact of mechanical ventilation on the daily costs of ICU care: A systematic review and meta regression. Epidemiol. Infect. 2019, 147, e314. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.M.; Kuszajewski, M.L.; Merritt, D.R.; Muckler, V.C. High-Fidelity Simulation Training for Nurse Anesthetists Managing Malignant Hyperthermia: A Quality Improvement Project. Clin. Simul. Nurs. 2019, 26, 72–80. [Google Scholar] [CrossRef]
- Dunn, J. Subordination by design: Rethinking power, policy, and autonomy in perioperative nursing. Nurs. Inq. 2025, 32, e70043. [Google Scholar] [CrossRef]
- Xie, A.; Duff, J.; Munday, J. Perioperative nursing shortages: An integrative review of their impact, causal factors, and mitigation strategies. J. Nurs. Manag. 2024, 2024, 2983251. [Google Scholar] [CrossRef] [PubMed]
- Boehm, O.; Baumgarten, G.; Hoeft, A. Preoperative patient assessment: Identifying patients at high risk. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Lomuscio, S.; Capogna, E.; Sironi, S.; Sguanci, M.; Morales Palomares, S.; Cangelosi, G.; Ferrara, G.; Mancin, S.; Amodeo, A.; Destrebecq, A.; et al. Debriefing methodologies in nursing simulation: An exploratory study of the Italian settings. Nurs. Rep. 2024, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Okatta, A.U.; Amaechi, E.C.; Elendu, T.C.; Ezeh, C.P.; Elendu, I.D. The impact of simulation-based training in medical education: A review. Medicine 2024, 103, e38813. [Google Scholar] [CrossRef]
- Higham, H. Simulation past, present and future—A decade of progress in simulation-based education in the UK. BMJ Simul. Technol. Enhanc. Learn. 2020, 7, 404–409. [Google Scholar] [CrossRef]
- Duff, J.P.; Morse, K.J.; Seelandt, J.; Gross, I.T.; Lydston, M.; Sargeant, J.; Dieckmann, P.; Allen, J.A.; Rudolph, J.W.; Kolbe, M. Debriefing methods for simulation in healthcare: A systematic review. Simul. Healthc. 2024, 19 (Suppl. S1), S112–S121. [Google Scholar] [CrossRef]
- Hardy, J.B.; Gouin, A.; Damm, C.; Compère, V.; Veber, B.; Dureuil, B. The use of a checklist improves anaesthesiologists’ technical and non-technical performance for simulated malignant hyperthermia management. Anaesth. Crit. Care Pain Med. 2018, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sakata, D.J.; Orr, J.A. Manufacturer’s response to “Residual volatile anesthetics after workstation preparation and activated charcoal filtration”. Acta Anaesthesiol. Scand. 2020, 64, 1382. [Google Scholar] [CrossRef]
- Popova, L.; Carabetta, V.J. The use of next-generation sequencing in personalized medicine. Methods Mol. Biol. 2025, 2866, 287–315. [Google Scholar] [CrossRef]
- Caudle, K.E.; Gammal, R.S.; Whirl-Carrillo, M.; Hoffman, J.M.; Relling, M.V.; Klein, T.E. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am. J. Health Syst. Pharm. 2016, 73, 1977–1985. [Google Scholar] [CrossRef][Green Version]
- Kraeva, N.; Sapa, A.; Dowling, J.J.; Riazi, S. Malignant Hyperthermia Susceptibility in Patients with Exertional Rhabdomyolysis: A Retrospective Cohort Study and Updated Systematic Review. Can. J. Anaesth. 2017, 64, 736–743. [Google Scholar] [CrossRef]
- Litman, R.S.; Smith, V.I.; Larach, M.G.; Mayes, L.; Shukry, M.; Theroux, M.C.; Watt, S.; Wong, C.A. Consensus Statement of the Malignant Hyperthermia Association of the United States on Unresolved Clinical Questions Concerning the Management of Patients with Malignant Hyperthermia. Anesth. Analg. 2019, 128, 652–659. [Google Scholar] [CrossRef]
- Normandin, P.A.; Benotti, S.A. New October 2018 Malignant Hyperthermia Guidelines: Is Your Emergency Department Prepared? J. Emerg. Nurs. 2019, 45, 214–217. [Google Scholar] [CrossRef]
- Bin, X.; Wang, B.; Tang, Z. Malignant Hyperthermia: A Killer If Ignored. J. Perianesth. Nurs. 2022, 37, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Daly, C.; Aboelsaod, E.M.; Gardner, L.; Hobson, S.J.; Riasat, K.; Shepherd, S.; Robinson, R.L.; Bilmen, J.G.; Gupta, P.K.; et al. Genetic epidemiology of malignant hyperthermia in the UK. Br. J. Anaesth. 2018, 121, 944–952. [Google Scholar] [CrossRef]
- Mullins, M.F. Malignant Hyperthermia: A Review. J. Perianesth. Nurs. 2018, 33, 582–589. [Google Scholar] [CrossRef]
- Cieniewicz, A.; Trzebicki, J.; Mayzner-Zawadzka, E.; Kostera-Pruszczyk, A.; Owczuk, R. Malignant Hyperthermia—What Do We Know in 2019? Anaesthesiol. Intensive Ther. 2019, 51, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sguanci, M.; Mancin, S.; Morales Palomares, S.; Cangelosi, G.; Parozzi, M.; Piredda, M.; De Marinis, M.G. The role of Clinical Nurse Specialist and the safety management in operating theatre during the COVID-19 pandemic: An integrative scoping review. Perioper. Care Oper. Room Manag. 2024, 37, 100437. [Google Scholar] [CrossRef]
- von Vogelsang, A.C.; Swenne, C.L.; Gustafsson, B.Å.; Falk Brynhildsen, K. Operating theatre nurse specialist competence to ensure patient safety in the operating theatre: A discursive paper. Nurs. Open 2019, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sandelin, A.; Kalman, S.; Gustafsson, B.Å. Prerequisites for safe intraoperative nursing care and teamwork—Operating theatre nurses’ perspectives: A qualitative interview study. J. Clin. Nurs. 2019, 28, 2635–2643. [Google Scholar] [CrossRef]
- Singh, K.; Alomari, A.M.A.; Sayed, H.M.A.; Mannethodi, K.; Kunjavara, J.; Joy, G.V.; Hassan, N.; Martinez, E.; Lenjawi, B.A. Barriers and Solutions to the Gap between Theory and Practice in Nursing Services: A Systematic Review of Qualitative Evidence. Nurs. Forum 2024, 2024, 7522900. [Google Scholar] [CrossRef]
- Bester, E.; van Wyk, N.C.; Maree, C. Development of collaboration guidelines for nursing education and related healthcare services. Health SA 2024, 29, 2496. [Google Scholar] [CrossRef]
- Rossiter, R.; Robinson, T.; Cox, R.; Collison, L.; Hills, D. Mentors supporting nurses transitioning to primary healthcare roles: A practice improvement initiative. SAGE Open Nurs. 2024, 10, 23779608241231174. [Google Scholar] [CrossRef]
- Ferrara, G.; Morales Palomares, S.; Parozzi, M.; Petrelli, F.; Cangelosi, G. The Internet of Things in the Nutritional Management of Patients with Chronic Neurological Cognitive Impairment: A Scoping Review. Healthcare 2024, 13, 23. [Google Scholar] [CrossRef]
- Al-Kahtani, M.S.; Khan, F.; Taekeun, W. Application of Internet of Things and Sensors in Healthcare. Sensors 2022, 22, 5738. [Google Scholar] [CrossRef]
- Sguanci, M.; Palomares, S.M.; Cangelosi, G.; Petrelli, F.; Sandri, E.; Ferrara, G.; Mancin, S. Artificial Intelligence in the Management of Malnutrition in Cancer Patients: A Systematic Review. Adv. Nutr. 2025, 16, 100438. [Google Scholar] [CrossRef]
- Javidan, A.P.; Li, A.; Lee, M.H.; Forbes, T.L.; Naji, F. A Systematic Review and Bibliometric Analysis of Applications of Artificial Intelligence and Machine Learning in Vascular Surgery. Ann. Vasc. Surg. 2022, 85, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Sacchini, F.; Mancin, S.; Cangelosi, G.; Palomares, S.M.; Caggianelli, G.; Gravante, F.; Petrelli, F. The role of artificial intelligence in diabetic retinopathy screening in type 1 diabetes: A systematic review. J. Diabetes Complicat. 2025, 39, 109139. [Google Scholar] [CrossRef] [PubMed]
- Warraich, H.J.; Tazbaz, T.; Califf, R.M. FDA Perspective on the Regulation of Artificial Intelligence in Health Care and Biomedicine. JAMA 2025, 333, 241–247. [Google Scholar] [CrossRef] [PubMed]

| Publications Year | Frequency (n = 19) | Percentage (%) |
|---|---|---|
| 2022 | 1 | 5.3 |
| 2020 | 3 | 15.8 |
| 2019 | 3 | 15.8 |
| 2017 | 1 | 5.3 |
| 2016 | 2 | 10.5 |
| 2015 | 1 | 5.3 |
| 2014 | 2 | 10.5 |
| 2013 | 1 | 5.3 |
| 2012 | 1 | 5.3 |
| 2011 | 1 | 5.3 |
| 2007 | 1 | 5.3 |
| 2006 | 1 | 5.3 |
| Country | ||
| USA | 14 | 73.7 |
| Others | 5 | 26.3 |
| Type of Study | ||
| Case Report | 10 | 52.6 |
| Simulation | 4 | 21.0 |
| Literature Review | 2 | 10.5 |
| Instrumental Test | 2 | 10.5 |
| Guidelines | 1 | 5.3 |
| First Author/Year | Type of Study | Country | Sample | Outcomes/Key Points | Main Results |
|---|---|---|---|---|---|
| Gallegos et al. [33], 2022 | Evidence-Based Practice Project | USA | 13 perioperative staff | Use of Stanford Emergency Manual in MH crisis | Cognitive aid checklists improved staff performance and reduced omission of critical treatment steps in simulation. |
| An et al. [34], 2020 | Case Report | China | 3 patients with MH during surgery | Application of MHCGS scoring for diagnosis and management | Multidisciplinary approach, prompt recognition, risk assessment, and timely intervention led to successful rescue. |
| Ebbitt et al. [35], 2020 | Case Report | USA | 22-year-old male | Importance of multidisciplinary protocols and MH trolleys | Preoperative assessment and protocol adherence enabled prompt recognition and effective treatment, preventing complications. |
| Müller-Wirtz et al. [36], 2020 | Instrumental Test | Germany | Not specified | MH prevention via anesthesia equipment protocols | 10 min circuit flush at ≥10 L/min ensures safety in elective cases; activated charcoal filters for emergencies. |
| Soenarto et al. [37], 2019 | Case Report | Indonesia | 16-year-old female | Regular perioperative MH crisis simulation | Team-wide MH knowledge, written protocols, and simulation are fundamental for preparedness and safety. |
| Lewellen [38], 2019 | Case Report | USA | Endoscopy staff | Preparation and institutional MH policies | Hospital-wide MH preparedness includes ongoing education, annual updates, MH point persons, and regular drills. |
| Shawn et al. [39], 2019 | Quality Improvement Project | USA | 16 nurses | MH simulation training for knowledge and self-confidence | Simulation improved knowledge and technical/non-technical skills required to manage MH crisis efficiently. |
| Normandin et al. [40], 2019 | Guidelines | USA | 1 emergency room case | Training in MH recognition and response for staff and administrators | Emphasizes avoidance of succinylcholine, regular MH drills, alert bracelets, adherence to guidelines. |
| Schaad [41], 2017 | Simulation-Based Training | USA | OR and ICU staff | Annual simulation-based training on MH for staff competency | Simulation-based training enhances team performance, safety, and communication. |
| Bashaw [42], 2016 | Case Report | USA | 9 nursing students | Teamwork and simulation in management of MH | Simulation allows safe practice of critical scenarios, improving performance and teamwork. |
| Denholm [43], 2016 | Literature Review | USA | Not specified | Role of nursing informatics and data management in MH | IT supports clinical decision-making; collaboration among nurses, managers, and informatics specialists enhances preparedness. |
| Denholm [44], 2015 | Literature Review | USA | Not specified | Use of vulnerability models to assess MH risk in populations | Strategies for prevention: temperature monitoring, dantrolene, education. Advanced nurses reduce patient vulnerability. |
| Sousa et al. [45], 2014 | Descriptive Exploratory Study | Spain | 96 nurses | Assessment of nurses’ knowledge regarding MH | Nurses scored > 80% on basic MH knowledge, but showed deficits (14.3–42.9%) in diagnosis/treatment competencies. |
| Seifert et al. [46], 2014 | Case Report | USA | 16-year-old male | Team training and simulation in MH emergency management | Simulation and checklists on anesthesia machines are essential for emergency preparedness. |
| Hirshey Dirksen et al. [47], 2013 | Case Report | USA | 25-year-old male | Development of assessment tools for MH risk | Regular mock drills and team preparation are essential to improve emergency response. |
| Hutton [48], 2012 | Case Report | USA | 49-year-old male | Management of perioperative emergencies and team roles | Early recognition, protocol implementation, and specific team role assignments are crucial for optimal outcomes. |
| Birgenheier et al. [49], 2011 | Instrumental Test | USA | Not specified | Efficacy of activated charcoal filters for MH prevention | Charcoal filters reduce anesthetic concentrations < 5 ppm within 2 min, maintain safe levels for at least 60 min. |
| Noble [50], 2007 | Case Report | USA | 21-year-old male | Emphasis on preparation and education in MH management | Prognosis improved through perioperative teamwork; education and preparation are key. |
| Neacsu [51], 2006 | Case Report | USA | Not specified | Management, nursing care, and screening for MH susceptibility | Structured nursing models and enhanced communication improve pre- and post-operative care and patient outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruta, F.; Della Monica, A.; Dal Mas, F.; Bolgeo, T.; Notarnicola, I.; Procacci, C.; Ferrara, P.; Masini, A.; Mancin, S.; Cangelosi, G.; et al. Peri-Operative Nursing of Patients with Malignant Hyperthermia: A Narrative Literature Review. Surgeries 2025, 6, 78. https://doi.org/10.3390/surgeries6030078
Ruta F, Della Monica A, Dal Mas F, Bolgeo T, Notarnicola I, Procacci C, Ferrara P, Masini A, Mancin S, Cangelosi G, et al. Peri-Operative Nursing of Patients with Malignant Hyperthermia: A Narrative Literature Review. Surgeries. 2025; 6(3):78. https://doi.org/10.3390/surgeries6030078
Chicago/Turabian StyleRuta, Federico, Annalisa Della Monica, Francesca Dal Mas, Tatiana Bolgeo, Ippolito Notarnicola, Cataldo Procacci, Paolo Ferrara, Alice Masini, Stefano Mancin, Giovanni Cangelosi, and et al. 2025. "Peri-Operative Nursing of Patients with Malignant Hyperthermia: A Narrative Literature Review" Surgeries 6, no. 3: 78. https://doi.org/10.3390/surgeries6030078
APA StyleRuta, F., Della Monica, A., Dal Mas, F., Bolgeo, T., Notarnicola, I., Procacci, C., Ferrara, P., Masini, A., Mancin, S., Cangelosi, G., Parozzi, M., & Sacchini, F. (2025). Peri-Operative Nursing of Patients with Malignant Hyperthermia: A Narrative Literature Review. Surgeries, 6(3), 78. https://doi.org/10.3390/surgeries6030078










