Indwelling Vascular Access Ports: Application, Advantages, and Management in Nonhuman Primates
Abstract
1. Introduction
2. Indications and Advantages of Vascular Access Port Placement
| Year | Citation | Purpose | Animal |
|---|---|---|---|
| 1985 | Garner and Laks, “Chronic catheter for BP measurement” [16] | Arterial VAP used to monitor cardiac output and blood pressure monitoring | Dog |
| 1986 | Bailie et al., “VAP implantation in swine” [17] | Simplify venous access in miniature pigs via jugular vein VAP | Miniature Pig |
| 1987 | Mann et al., “BP measurement in dog” [18] | Using arterial VAP to monitor blood pressure in dogs | Dog |
| 1988 | Garner et al., “BP measurement in rats” [19] | Arterial VAP for blood pressure monitoring in rats | Rat |
| 1991 | Perry-Clark and Meunier, “VAP in rabbits” [20] | Jugular VAP for chronic infusions and venous sampling | New Zealand White Rabbit |
| 1994 | Wojnicki et al., “VAPs in Rhesus” [21] | Jugular VAP for blood sampling and drug administration in drug abuse model | Rhesus Macaque |
| 1994 | Bacher et al., “CSF sampling in Rhesus” [7] | Chronic port access to CSF for intrathecal drug monitoring | Rhesus Macaque |
| 1995 | Rockar et al., “CSF retrieval in dogs” [8] | Development of port placement technique for CSF sampling | Dog |
| 1995 | Kwei et al., “Intestinal and portal VAP” [22] | Portal and intestinal VAPs for drug absorption studies | Dog |
| 1996 | Landi et al., “VAP infection in monkeys” [23] | Evaluation of infection after VAP placement for venous blood sampling | Cynomolgus Macaque |
| 1998 | Kissinger et al., “Bile collection in dogs” [6] | Implanted catheter placed within biliary tree for bile collection | Dog |
| 1999 | Cowart et al., “Optimizing VAP in pigs” [24] | Optimizing VAPs in young pigs to minimize complications | Pig |
| 2002 | Henry et al., “VAP in cats” [25] | Jugular vein VAP for blood sampling in cats | Cat |
| 2003 | Gilberto et al., “Alternative CSF sampling method” [9] | Improved technique for subcutaneous port placement for CSF sampling | Rhesus Macaque |
| 2004 | Kunta et al., “Intestinal VAP in rabbits” [26] | Venous, portal, and intestinal placed VAPs for drug metabolism studies | Rabbit |
| 2009 | Graham et al., “Novel technique for VAP placement” [27] | New percutaneous saphenous vein VAP technique versus conventional jugular/femoral vein | NHP (Cynomolgus, Rhesus, Baboon) |
| 2011 | Graham et al., “Long-term portal VAP” [10] | Portal vein VAP for islet cell transplantation | NHP (Cynomolgus, Rhesus) |
| 2011 | Aubert et al., “VAP use in feline blood donors” [28] | Application of VAP for frequent use in blood donor animals | Cat |
| 2013 | Farrow et al., “Jugular VAP placement for sampling in cats” [29] | Evaluation of implanted VAPs for long-term blood sampling in cats | Cat |
| 2015 | Guérios et al., “Surgical placement of VAPs in dogs and cats” [30] | Technique for placement and management of VAPs in dogs and cats | Dog and Cat |
| 2020 | Mutch et al., “Long term management of VAPs” [15] | Management of VAPs for long-term sampling and fluid/drug administration | NHP (Cynomolgus, Rhesus) |
| 2022 | Pálek et al., “IV access in experimental surgery” [31] | Suitability of VAPs in the external jugular vein for use in experimental surgery | Pig |
| 2023 | Ehrmann et al., “Vascular access button for chronic access in rabbits” [32] | Long-term vascular access technique for chronic blood sampling and drug administration | Rabbit |
3. Surgical Technique
3.1. Location Considerations
- Nonhuman primates. Conventional VAP implantation sites in NHPs include the jugular or femoral veins using a cutdown approach with a tunneled catheter to the dorsum of the animal where a second incision is made to create a pocket for the port head [21,23,36]. The necessity of two separate incisions and long tunnel increases procedural invasiveness and has a relatively high rate of infections, mechanical or thrombotic occlusions, and dehiscence or erosion have been reported [21,23,36,37,38,39]. Moreover, this approach requires that animals be restrained, typically using manual or chemical restraint, to position them for VAP access.
- 2.
- Pigs. Port and catheter placement in pigs has been extensively reviewed with the most common site for placement being the external jugular vein [17,24,40,41]. Special consideration must be given to location of the port head, because pigs may persistently rub surgical sites which can lead to trauma, infection, and wound breakdown. Given this, port heads are often placed on the dorsum of the pig, typically at the neck or chest wall.
- 3.
- Dogs. The external jugular vein, femoral vein, and lateral saphenous vein have all been utilized for VAP placement in dogs. The external jugular vein is used with preference, with most studies reporting a <5% complication rate [25,30,42]. However, the factors driving this preference are not fully clear as comparison between the external jugular vein and the lateral saphenous vein sites for VAP placement demonstrated higher complications in JV placements as compared to SV placements. Catheter tip malposition occurred in 17.4% of JV placements versus 0% of SV cases [43] and 30.4% of dogs implanted with a JV VAP developed a seroma; increased seroma risk may be related to the more extensive dissection used for JV placement [28,30].
- 4.
- 5.
- Rabbits. The external jugular vein or the femoral vein are the primary sites for VAP placement [20,45]. To avoid disruption of the port head, it is usually placed on the dorsum. The position of the port head in this species can be affected by the need for restraint. Placement of the port head on the neck dorsally may require manipulation of the ears during sterile prep, making access difficult during restraint, so mid-back alongside the vertebral column is alternatively used [32].
3.2. Anesthesia and Surgical Prep Considerations
3.3. General Surgical Principles
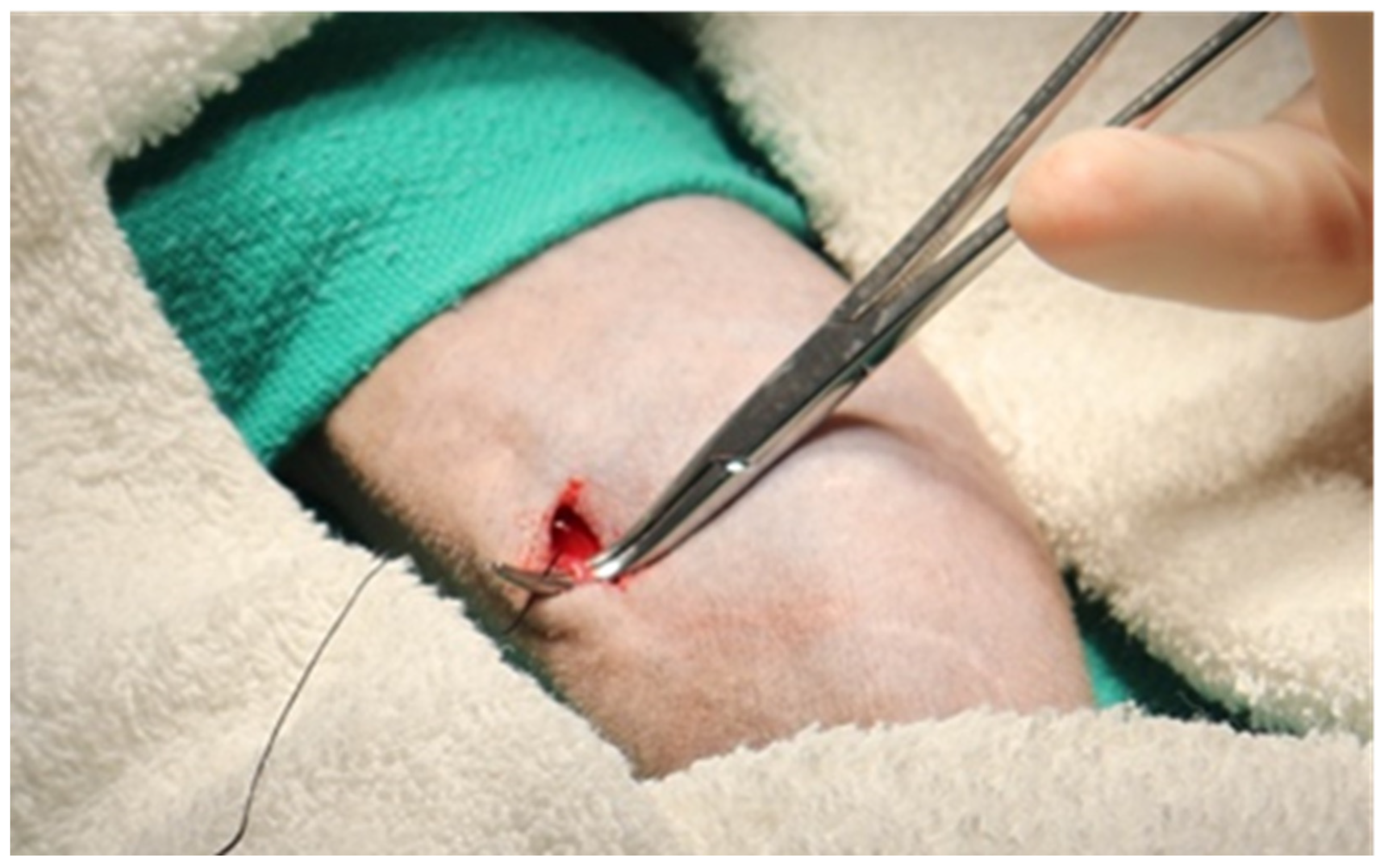
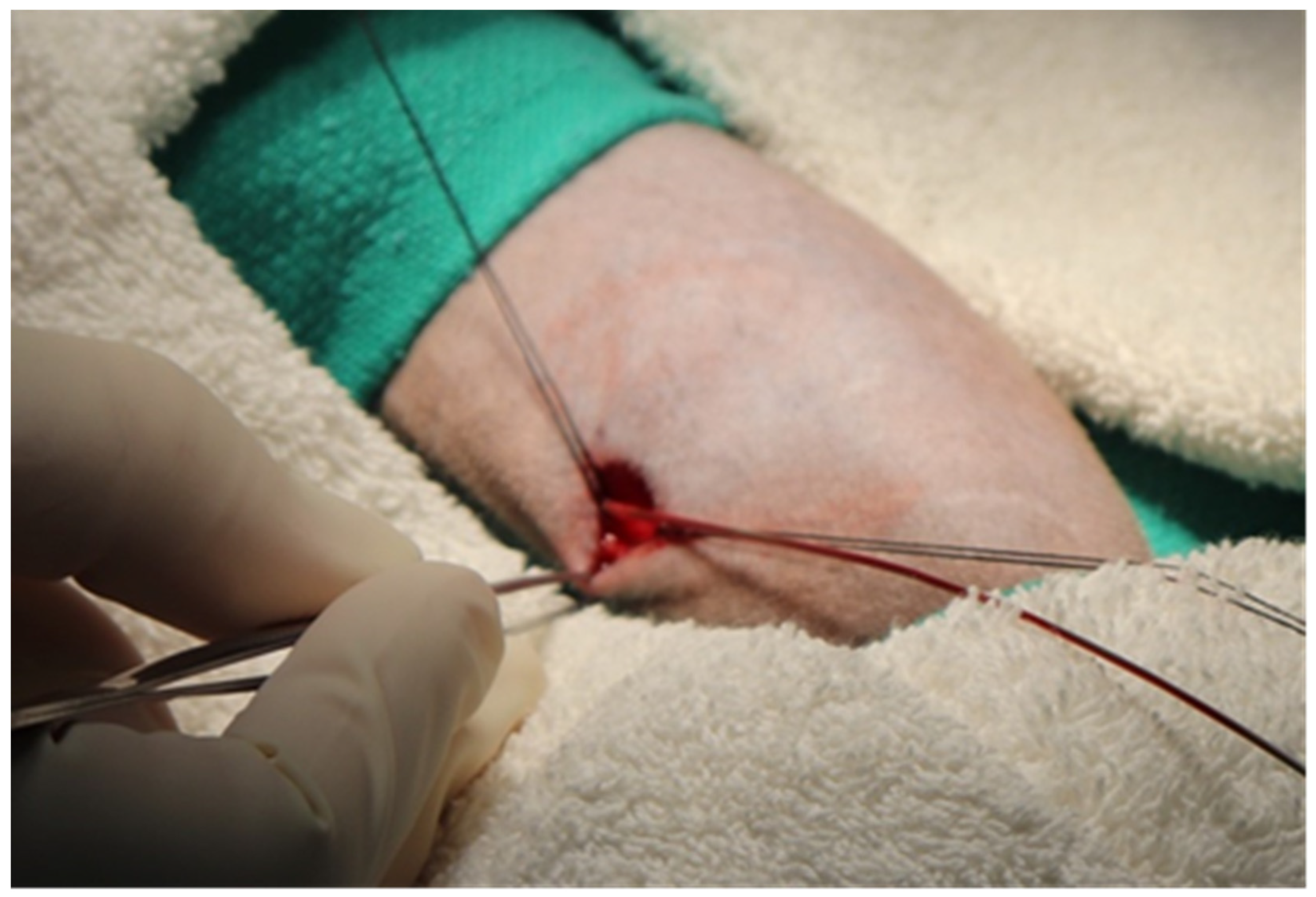
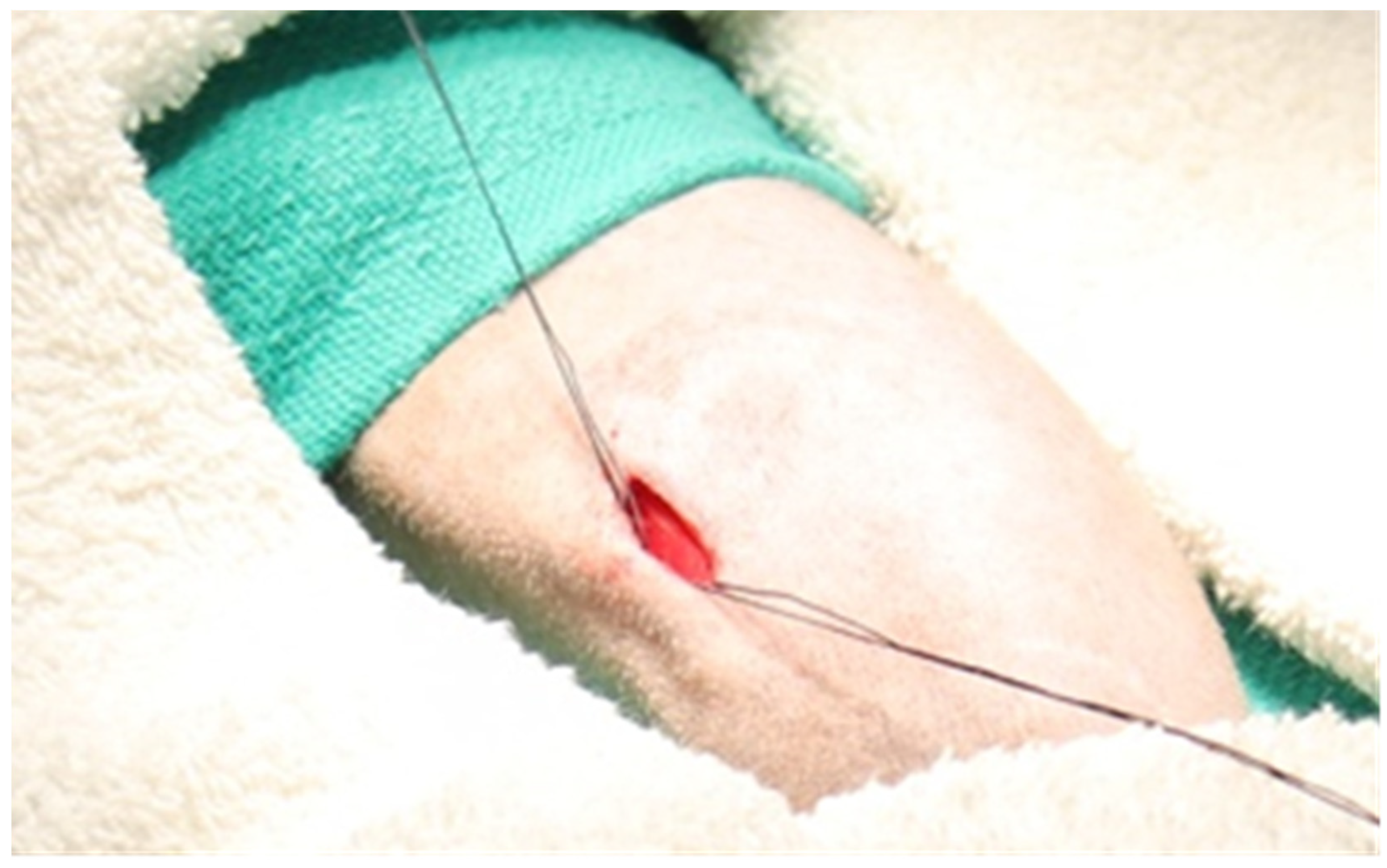
3.4. Single-Incision Peripheral Insertion Technique
4. Postoperative Care and Management
5. Vascular Access Port Use and Maintenance
6. Complications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyves, J.; Ensminger, W.; Niederhuber, J.; Liepman, M.; Cozzi, E.; Doan, K.; Dakhil, S.; Wheeler, R. Totally implanted system for intravenous chemotherapy in patients with cancer. Am. J. Med. 1982, 73, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Bow, E.J.; Kilpatrick, M.G.; Clinch, J.J. Totally Implantable Venous Access Ports Systems for Patients Receiving Chemotherapy for Solid Tissue Malignancies: A Randomized Controlled Clinical Trial Examining the Safety, Efficacy, Costs, and Impact on Quality of Life. J. Clin. Oncol. 1999, 17, 1267. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Boyd, K.; Paul, J.; McCartney, E.; Ritchie, M.; Mellon, D.; Kelly, L.; Dixon-Hughes, J.; Moss, J. Hickman catheter and implantable port devices for the delivery of chemotherapy: A phase II randomised controlled trial and economic evaluation. Br. J. Cancer 2016, 114, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Zaghal, A.; Khalife, M.; Mukherji, D.; El Majzoub, N.; Shamseddine, A.; Hoballah, J.; Marangoni, G.; Faraj, W. Update on totally implantable venous access devices. Surg. Oncol. 2012, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Niederhuber, J.E.; Ensminger, W.; Gyves, J.W.; Liepman, M.; Doan, K.; Cozzi, E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982, 92, 706–712. [Google Scholar] [PubMed]
- Kissinger, J.T.; Garver, E.M.; Schnell, M.A.; Schantz, J.D.; Coatney, R.W.; Meunier, L.V.D. A new method to collect bile and access the duodenum in conscious dogs. J. Am. Assoc. Lab. Anim. Sci. 1998, 37, 89–93. [Google Scholar]
- Bacher, J.D.; Balis, F.M.; McCully, C.L.; Godwin, K.S. Cerebral subarachnoid sampling of cerebrospinal fluid in the rhesus monkey. Lab. Anim. Sci. 1994, 44, 148–152. [Google Scholar]
- Rockar, R.A.; Sadanaga, K.K.; Burkett, D.E.; Mitroka, J.G.; Bonner, R.A.; Weinstein, M.J. Cerebrospinal fluid retrieval in the conscious dog: A methods development study. J. Investig. Surg. 1995, 8, 85–94. [Google Scholar] [CrossRef]
- Gilberto, D.B.; Zeoli, A.H.; Szczerba, P.J.; Gehret, J.R.; Holahan, M.A.; Sitko, G.R.; Johnson, C.A.; Cook, J.J.; Motzel, S.L. An alternative method of chronic cerebrospinal fluid collection via the cisterna magna in conscious rhesus monkeys. J. Am. Assoc. Lab. Anim. Sci. 2003, 42, 53–59. [Google Scholar]
- Graham, M.L.; Mutch, L.A.; Rieke, E.F.; Dunning, M.; Zolondek, E.K.; Schutten, M.M.; Hering, B.J.; Schuurman, H.-J. Long-term hepatic vascular access in the nonhuman primate for recurrent portal vein infusion. J. Investig. Surg. 2011, 24, 59–66. [Google Scholar] [CrossRef]
- Mao, W.; Guo, S.; Ye, D.; Cao, Y.; Jiang, X.; Huang, Y.; Yuan, H.; Jiao, L. Surgical application of an implantable biliary access device in the treatment of refractory recurrent cholangiolithiasis. Quant. Imaging Med. Surg. 2023, 13, 3333–3342. [Google Scholar] [CrossRef]
- Pino, M.G.; Ganguly, R.; Rich, K.A.; Fox, A.; Mattox, L.; Keckley, E.; Joseph, M.; Malbrue, R.; Youngblood, B.; Krishna, V.; et al. Continual cerebrospinal fluid sampling in the neonatal domestic piglet for biomarker and discovery studies. J. Neurosci. Methods 2022, 366, 109403. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, M. Clinical Indications, Preoperative Assessment, Set-up and Organizational Aspects. In Totally Implantable Venous Access Devices: Management in Mid- and Long-Term Clinical Setting; Niederhuber, J.E., Di Carlo, I., Biffi, R., Eds.; Springer: Milan, Italy, 2012; pp. 37–42. [Google Scholar] [CrossRef]
- Gurkan, S.; Seber, S.; Gur, O.; Yetisyigit, T.; Okan Donbaloglu, M.; Ozkaramanli Gur, D. Retrospective evaluation of totally implantable venous access port devices: Early and late complications. J. BUON 2015, 20, 338–345. [Google Scholar]
- Mutch, L.A.; Klinker, S.T.; Janecek, J.J.; Niewinski, M.N.; Lee, R.M.; Graham, M.L. Long-Term Management of Vascular Access Ports in Nonhuman Primates Used in Preclinical Efficacy and Tolerability Studies. J. Investig. Surg. 2020, 33, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.; Laks, M.M. New implanted chronic catheter device for determining blood pressure and cardiac output in conscious dog. Am. J. Physiol.-Heart Circ. Physiol. 1985, 249, H681–H684. [Google Scholar] [CrossRef] [PubMed]
- Bailie, M.; Wixson, S.; Landi, M. Vascular-access-port implantation for serial blood sampling in conscious swine. Lab. Anim. Sci. 1986, 36, 431–433. [Google Scholar]
- Mann, W.; Landi, M.; Horner, E.; Woodward, P.; Campbell, S.; Kinter, L. A simple procedure for direct blood pressure measurements in conscious dogs. Lab. Anim. Sci. 1987, 37, 105–108. [Google Scholar] [PubMed]
- Garner, D.; McGivern, R.; Jagels, G.; Laks, M.M. A new method for direct measurement of systolic and diastolic pressures in conscious rats using Vascular-Access-Ports. Lab. Anim. Sci. 1988, 38, 205–207. [Google Scholar]
- Perry-Clark, L.; Meunier, L. Vascular access ports for chronic serial infusion and blood sampling in New Zealand white rabbits. Lab. Anim. Sci. 1991, 41, 495–497. [Google Scholar]
- Wojnicki, F.; Bacher, J.D.; Glowa, J.R. Use of subcutaneous vascular access ports in rhesus monkeys. Lab. Anim. Sci. 1994, 44, 491–494. [Google Scholar]
- Kwei, G.Y.; Gehret, J.R.; Novak, L.B.; Drag, M.D.; Goodwin, T. Chronic catheterization of the intestines and portal vein for absorption experimentation in beagle dogs. Lab. Anim. Sci. 1995, 45, 683–685. [Google Scholar] [PubMed]
- Landi, M.; Schantz, J.; Jenkins, E.; Warnick, C.; Kissinger, J. ICLAS proceedings: A survey of vascular access port infection in cynomolgus monkeys (Macaca fascicularis). Scand. J. Lab. Anim. Science. Suppl. 1996. [Google Scholar]
- Cowart, R.P.; Payne, J.T.; Turk, J.R.; Tyler, J.W.; Casteel, A.W. Factors optimizing the use of subcutaneous vascular access ports in weaned pigs. J. Am. Assoc. Lab. Anim. Sci. 1999, 38, 67–70. [Google Scholar]
- Henry, C.; Russell, L.; Tyler, J. Comparison of hematologic and biochemical values for blood samples obtained via jugular venipuncture and via vascular access ports in cats. J. Am. Vet. Med. Assoc. 2002, 220, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Kunta, J.R.; Lee, S.-H.; Perry, B.A.; Lee, Y.-H.; Sinko, P.J. Differentiation of gut and hepatic first-pass loss of verapamil in intestinal and vascular access-ported (IVAP) rabbits. Drug Metab. Dispos. 2004, 32, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L.; Rieke, E.F.; Dunning, M.; Mutch, L.A.; Craig, A.M.; Zolondek, E.K.; Hering, B.J.; Schuurman, H.J.; Bianco, R.W. A novel alternative placement site and technique for totally implantable vascular access ports in non-human primates. J. Med. Primatol. 2009, 38, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Aubert, I.; Abrams-Ogg, A.C.; Sylvestre, A.M.; Dyson, D.H.; Allen, D.G.; Johnstone, I.B. The use of vascular access ports for blood collection in feline blood donors. Can. J. Vet. Res. 2011, 75, 25–34. [Google Scholar]
- Farrow, H.A.; Rand, J.S.; Burgess, D.M.; Coradini, M.; Vankan, D.M. Jugular vascular access port implantation for frequent, long-term blood sampling in cats: Methodology, assessment, and comparison with jugular catheters. Res. Vet. Sci. 2013, 95, 681–686. [Google Scholar] [CrossRef]
- Guérios, S.D.; Silva, D.M.; Souza, C.H.; Bacon, N.J. Surgical placement and management of jugular vascular access ports in dogs and cats: Description of technique. Rev. Colomb. Cienc. Pecu. 2015, 28, 265–271. [Google Scholar] [CrossRef][Green Version]
- Pálek, R.; Rosendorf, J.; Šarčevič, S.; Ševčík, J.; Brzoň, O.; Kepková, L.; Krystl, P.; Brousil, M.; Červenková, L.; Třeška, V. Permanent intravenous access in experimental surgery–our experience. Rozhl. Chir. Mesic. Ceskoslovenske Chir. Spol. 2022, 101, 577–583. [Google Scholar]
- Ehrmann, J.; Johnson, W.; de Castro, A.; Donnelly, M. Implantation of a Vascular Access Button for Chronic Blood Sampling and Drug Administration in the Rabbit. Surgeries 2023, 4, 141–151. [Google Scholar] [CrossRef]
- Bertone, S.A.; Fisher, M.C.; Mortensen, J.E. Quantitative skin cultures at potential catheter sites in neonates. Infect. Control. Hosp. Epidemiol. 1994, 15, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.M.; Wagener, M.M.; Miller, J.M.; Muder, R.R. Risk of infection due to central venous catheters: Effect of site of placement and catheter type. Infect. Control. Hosp. Epidemiol. 1998, 19, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, K.; Lathyris, D.; Blot, S.; Apostolidou-Kiouti, F.; Koulenti, D.; Haidich, A.B. Cumulative Evidence of Randomized Controlled and Observational Studies on Catheter-Related Infection Risk of Central Venous Catheter Insertion Site in ICU Patients: A Pairwise and Network Meta-Analysis. Crit. Care Med. 2017, 45, e437–e448. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, D.C.; Thompson, T.E.; Emerson, T.E., Jr. A simple technique which maintains vascular patency after catheterization. Lab. Anim. Sci. 1990, 40, 643–644. [Google Scholar]
- Graham, M.L.; Rieke, E.F.; Wijkstrom, M.; Dunning, M.; Aasheim, T.C.; Graczyk, M.J.; Pilon, K.J.; Hering, B.J. Risk factors associated with surgical site infection and the development of short-term complications in macaques undergoing indwelling vascular access port placement. J. Med. Primatol. 2008, 37, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kinsora, J.J., Jr.; Christoffersen, C.L.; Swalec, J.M.; Juneau, P.L. The novel use of vascular access ports for intravenous self-administration and blood withdrawal studies in squirrel monkeys. J. Neurosci. Methods 1997, 75, 59–68. [Google Scholar] [CrossRef]
- Swindle, M.M.; Nolan, T.; Jacobson, A.; Wolf, P.; Dalton, M.J.; Smith, A.C. Vascular access port (VAP) usage in large animal species. Contemp. Top. Lab. Anim. Sci. 2005, 44, 7–17. [Google Scholar]
- Chuang, M.; Orvieto, M.; Laven, B.; Gerber, G.; Wardrip, C.; Ritch, C.; Shalhav, A. Comparison of external catheters with subcutaneous vascular access ports for chronic vascular access in a porcine model. Contemp. Top. Lab. Anim. Sci. 2005, 44, 24–27. [Google Scholar]
- Swindle, M.M.; Smith, A.C.; Goodrich, J.A. Chronic cannulation and fistulization procedures in swine: A review and recommendations. J. Investig. Surg. 1998, 11, 7–20. [Google Scholar] [CrossRef]
- Culp, W.T.; Mayhew, P.D.; Reese, M.S.; Duda, L.; Glassman, M.M.; Brown, D.C. Complications associated with use of subcutaneous vascular access ports in cats and dogs undergoing fractionated radiotherapy: 172 cases (1996–2007). J. Am. Vet. Med. Assoc. 2010, 236, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.N.; Grier, C.K.; Yoshikawa, H.; Ringwood, P.B. Complications associated with the use of vascular access ports in dogs receiving external beam radiation therapy. J. Am. Vet. Med. Assoc. 2008, 233, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.I.; Bliss, J.M.; Herbst, L.H. Use of vascular access ports in the cat. Lab. Anim. Sci. 1995, 45, 110–114. [Google Scholar] [PubMed]
- Huynh, M.; Boyeaux, A.; Pignon, C. Assessment and care of the critically ill rabbit. Vet. Clin. Exot. Anim. Pract. 2016, 19, 379–409. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.S.; Dunlop, C.I.; Wagner, A.E.; Wertz, E.M.; Golden, A.E.; Demme, W.C. Complications and mortality associated with anesthesia in dogs and cats. J. Am. Anim. Hosp. Assoc. 1999, 35, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Barker, K.; Odom, T.; Lewis, K.; Giordano, P.; Walsh, V.; Chambers, J.P. A survey of dog and cat anaesthesia in a sample of veterinary practices in New Zealand. N. Z. Vet. J. 2018, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Spofford, N.; Yang, M.; Saito, E.; Lambert, L.; Faunt, K. Development and implementation of veterinary anesthesia medical quality standards for primary care. Vet. Anaesth. Analg. 2022, 49, 233–242. [Google Scholar] [CrossRef]
- Klonner, M.E.; Springer, S.; Braun, C. Complications secondary to endotracheal intubation in dogs and cats: A questionnaire-based survey among veterinary anaesthesiologists. Vet. Anaesth. Analg. 2023, 50, 220–229. [Google Scholar] [CrossRef]
- Bernal, J.; Adrian, S.; Burkart, H.; Laffins, M. Guideline for Vascular Access Port Use and Maintenance in Large Animals for Biomedical Research. Surgeries 2022, 3, 219–228. [Google Scholar] [CrossRef]
- Abee, C.R.; Mansfield, K.; Tardif, S.D.; Morris, T. Nonhuman Primates in Biomedical Research: Biology and Management; Academic Press: Cambridge, MA, USA, 2012; Volume 1. [Google Scholar]
- Authier, S.; Chaurand, F.; Legaspi, M.; Breault, C.; Troncy, E. Comparison of three anesthetic protocols for intraduodenal drug administration using endoscopy in rhesus monkeys (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2006, 45, 73–79. [Google Scholar]
- Fish, R.; Danneman, P.J.; Brown, M.; Karas, A. Anesthesia and Analgesia in Laboratory Animals; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Fowler, K.A.; Huerkamp, M.J.; Pullium, J.K.; Subramanian, T. Anesthetic protocol: Propofol use in Rhesus macaques (Macaca mulatta) during magnetic resonance imaging with stereotactic head frame application. Brain Res./Brain Res. Protoc. 2001, 7, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Young, J.T.; Vlasova, R.M.; Howell, B.R.; Knickmeyer, R.C.; Morin, E.; Kuitchoua, K.I.; Lubach, G.R.; Noel, J.; Hu, X.; Shi, Y.; et al. General anaesthesia during infancy reduces white matter micro-organisation in developing rhesus monkeys. Br. J. Anaesth. 2021, 126, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Lauer, S.K.; Baldwin, C.J.; Evans, R.B.; Andreasen, C.B.; Kinyon, J.M.; Swanson, E. Evaluation of the use of subcutaneous implantable vascular access ports in feline blood donors. J. Am. Vet. Med. Assoc. 2007, 230, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E. The surgical infection prevention project: Processes, outcomes, and future impact. Surg. Infect. 2006, 7, s-17–s-26. [Google Scholar] [CrossRef]
- Valentini, F.; Fassone, F.; Pozzebon, A.; Gavazza, A.; Lubas, G. Use of totally implantable vascular access port with mini-invasive Seldinger technique in 12 dogs undergoing chemotherapy. Res. Vet. Sci. 2013, 94, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kurul, S.; Saip, P.; Aydin, T. Totally implantable venous-access ports: Local problems and extravasation injury. Lancet Oncol. 2002, 3, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-H.; Mammadov, K.; Hickethier, T.; Borggrefe, J.; Hellmich, M.; Maintz, D.; Kabbasch, C. Fibrin sheaths in central venous port catheters: Treatment with low-dose, single injection of urokinase on an outpatient basis. Ther. Clin. Risk Manag. 2017, 13, 111–115. [Google Scholar] [CrossRef]
- Aldrighetti, L.; Caterini, R.; Ronzoni, M.; Jannello, A.; Ferla, G. Role of totally implantable systems for long-term vascular access in the treatment of the neoplastic patient. Minerva Chir. 1995, 50, 447–454. [Google Scholar]
- Clari, M.; Spoto, M.; Franceschi, G.; Acuto, M.; Tonella, S.; Caristia, S.; Buratti, G.; Gaboardi, S.; Rasero, L.; Campagna, S.; et al. Short Versus Long Timing of Flushing of Totally Implantable Venous Access Devices When Not Used Routinely: A Systematic Review and Meta-analysis. Cancer Nurs. 2021, 44, 205–213. [Google Scholar] [CrossRef]
- Ponec, D.; Irwin, D.; Haire, W.D.; Hill, P.A.; Li, X.; McCluskey, E.R. Recombinant Tissue Plasminogen Activator (Alteplase) for Restoration of Flow in Occluded Central Venous Access Devices: A Double-Blind Placebo-Controlled Trial—The Cardiovascular Thrombolytic to Open Occluded Lines (COOL) Efficacy Trial. J. Vasc. Interv. Radiol. 2001, 12, 951–955. [Google Scholar] [CrossRef]
- Baskin, J.L.; Pui, C.-H.; Reiss, U.; Wilimas, J.A.; Metzger, M.L.; Ribeiro, R.C.; Howard, S.C. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar] [CrossRef]
- Gebarski, S.S.; Gebarski, K.S. Chemotherapy port “Twiddler’s syndrome”. A need for preinjection radiography. Cancer 1984, 54, 38–39. [Google Scholar] [CrossRef]
- Ince, M.E.; Ozkan, G.; Ors, N.; Yildirim, A.K.; Doganci, S. Complications and pitfalls of central venous port catheters: Experience with 782 patients with cancer. Ir. J. Med. Sci. 2020, 189, 1371–1377. [Google Scholar] [CrossRef]
- Barbetakis, N.; Asteriou, C.; Kleontas, A.; Tsilikas, C. Totally implantable central venous access ports. Analysis of 700 cases. J. Surg. Oncol. 2011, 104, 654–656. [Google Scholar] [CrossRef]
- Chang, L.; Tsai, J.S.; Huang, S.J.; Shih, C.C. Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am. J. Infect. Control. 2003, 31, 34–39. [Google Scholar] [CrossRef]
- Ji, L.; Yang, J.; Miao, J.; Shao, Q.; Cao, Y.; Li, H. Infections Related to Totally Implantable Venous-Access Ports: Long-Term Experience in One Center. Cell Biochem. Biophys. 2015, 72, 235–240. [Google Scholar] [CrossRef] [PubMed]


| Medication | Dosage | Purpose | Duration | Route |
|---|---|---|---|---|
| Ketamine | 3–15 mg/kg | Light sedation (brief handling): 3–9 mg/kg Moderate sedation and immobilization: 10–15 mg/kg | 30 min | IM |
| Midazolam | 0.1–0.5 mg/kg | Moderate sedation and immobilization | 30–45 min | IM/IV |
| Tiletamine/ Zolazepam (Telazol) | 3–6 mg/kg | Anesthetic for minor procedures | Up to 60 min | IM |
| Ketamine + Midazolam | 4–15 mg/kg ketamine + 0.05–0.2 mg/kg midazolam | Moderate sedation and immobilization | 30–45 min | IM/IV |
| Ketamine + Dexmedetomidine | 2.5–5 mg/kg IV bolus, then 0.01–0.06 mg/kg/min CRI | Light surgical anesthesia | Continuous | IV |
| Propofol | 2–8 mg/kg 0.2–0.6 mg/kg/min | General or deep surgical anesthesia: bolus dose followed by continuous infusion | Bolus: up to 10 min Continuous | IV |
| Isoflurane | 0.5–5% | General anesthesia induction: 3–5% Anesthesia maintenance: 0.5–3% | Continuous | Inhaled |
| Medication | Dosage | Duration | Class | Route |
|---|---|---|---|---|
| Lidocaine (1–2%) | 2–4 mg/kg (maximum 5 mg/kg) | Up to 2 h | Local | Local |
| Bupivacaine (0.5% or 0.25%) | 1–2 mg/kg (maximum 4 mg/kg) | Up to 8 h | Local | Local |
| Buprenorphine | 0.01–0.03 mg/kg BID-QID | 12–24 h | Opioid | IM |
| Carprofen | 4.4 mg/kg SID or 2.2 mg/kg BID | Up to 24 h | NSAID | PO/SC |
| Ketoprofen | 2 mg/kg IM/IV BID or 5 mg/kg SID | Up to 24 h | NSAID | IM/IV |
| Meloxicam | 0.2 mg/kg loading dose, then 0.1 mg/kg SID | Up to 24 h | NSAID | PO/SC |
| Ibuprofen | 7–15 mg/kg BID | 4–12 h | NSAID | PO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppler, S.H.; Leishman, D.J.; Graham, M.L. Indwelling Vascular Access Ports: Application, Advantages, and Management in Nonhuman Primates. Surgeries 2023, 4, 446-460. https://doi.org/10.3390/surgeries4030044
Oppler SH, Leishman DJ, Graham ML. Indwelling Vascular Access Ports: Application, Advantages, and Management in Nonhuman Primates. Surgeries. 2023; 4(3):446-460. https://doi.org/10.3390/surgeries4030044
Chicago/Turabian StyleOppler, Scott H., David J. Leishman, and Melanie L. Graham. 2023. "Indwelling Vascular Access Ports: Application, Advantages, and Management in Nonhuman Primates" Surgeries 4, no. 3: 446-460. https://doi.org/10.3390/surgeries4030044
APA StyleOppler, S. H., Leishman, D. J., & Graham, M. L. (2023). Indwelling Vascular Access Ports: Application, Advantages, and Management in Nonhuman Primates. Surgeries, 4(3), 446-460. https://doi.org/10.3390/surgeries4030044







