Activation of Extracellular Signal-Regulated Kinase 2 and cAMP Response Element-Binding Protein in Cultured Neurons by the Macrocyclic Ellagitannin Oenothein B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Oenothein B and Reagents
2.2. Cell Cultures
2.3. Assessment of Cell Viability
2.4. Western Blot Analysis
2.5. Statistical Analysis
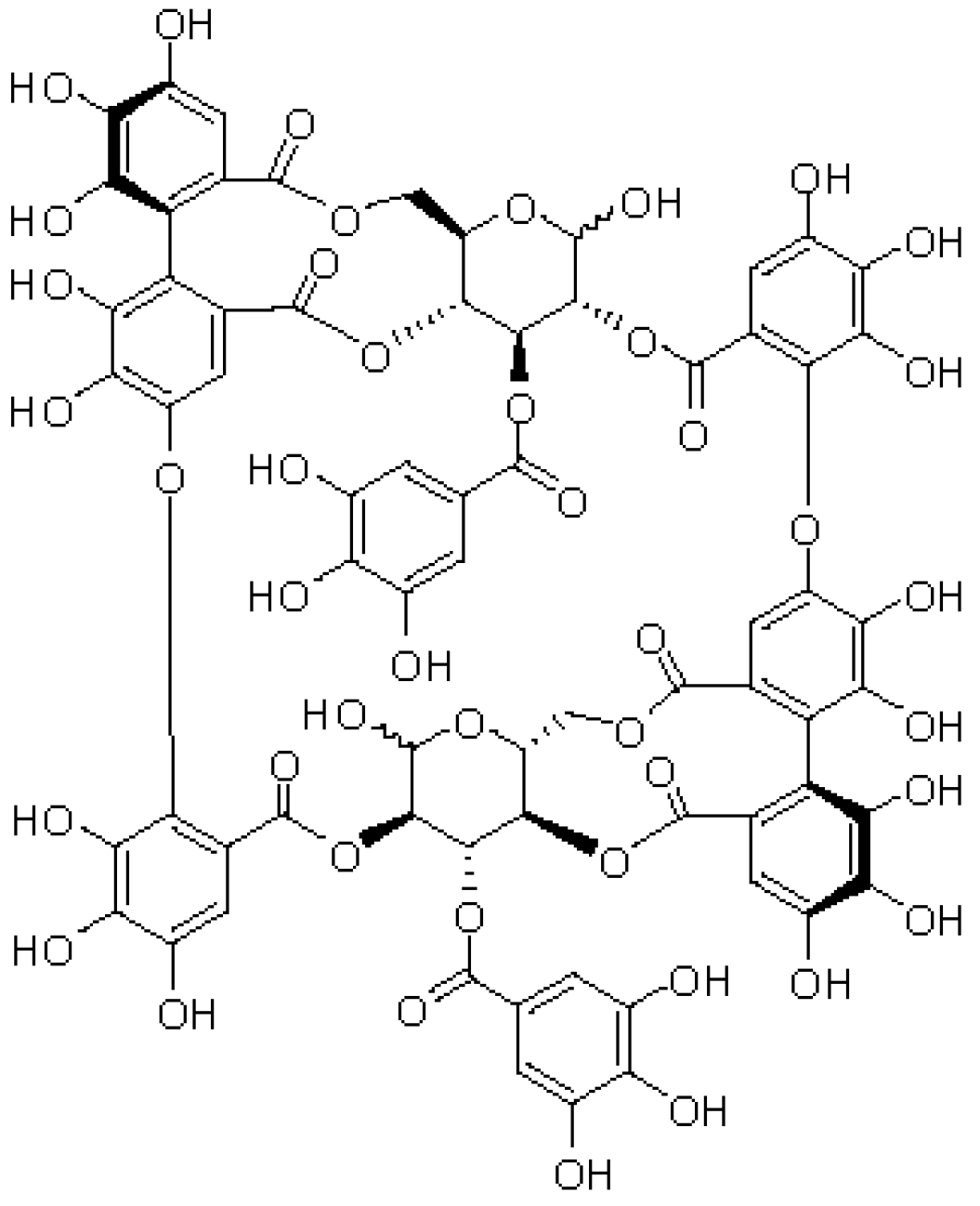
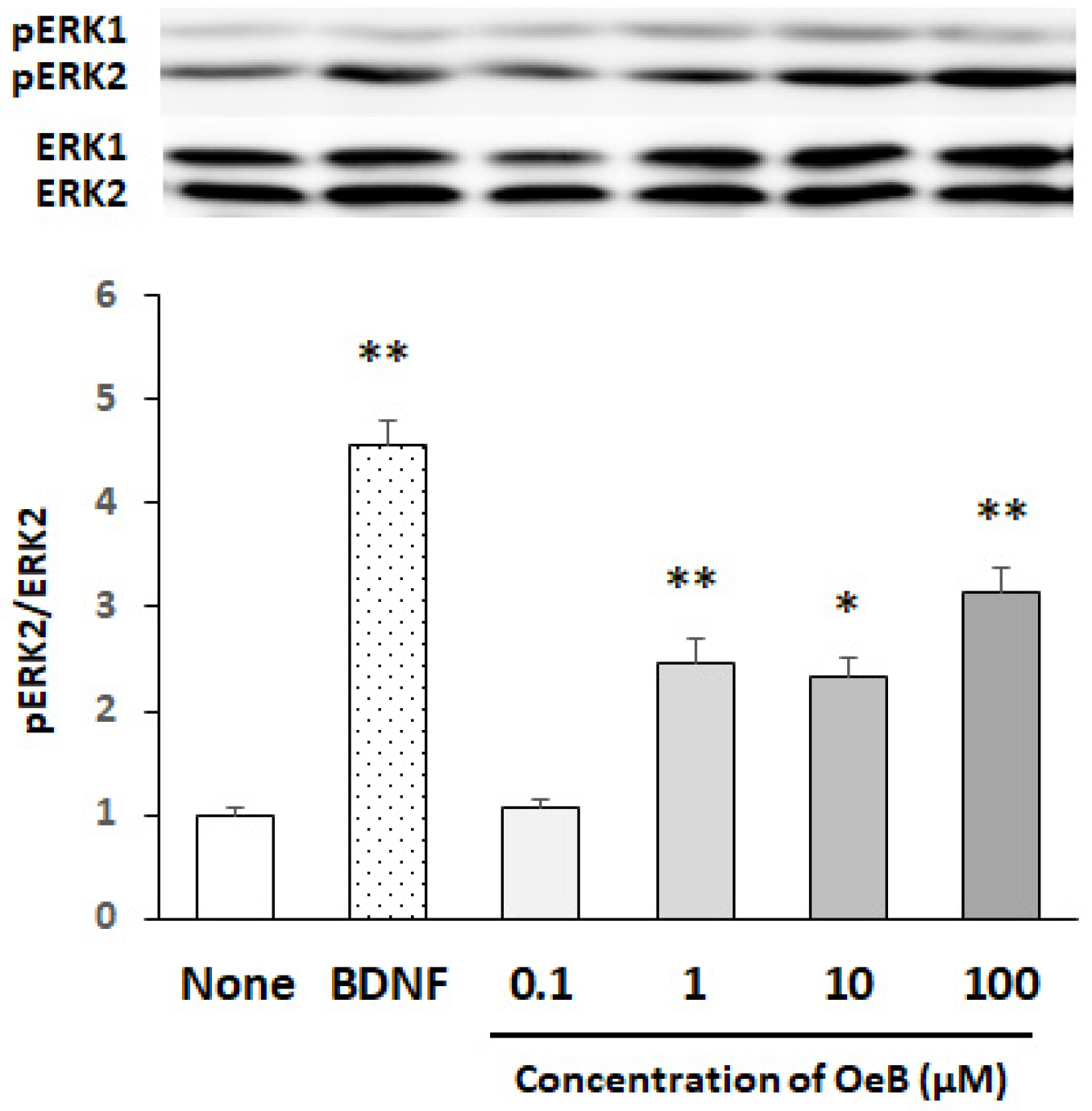
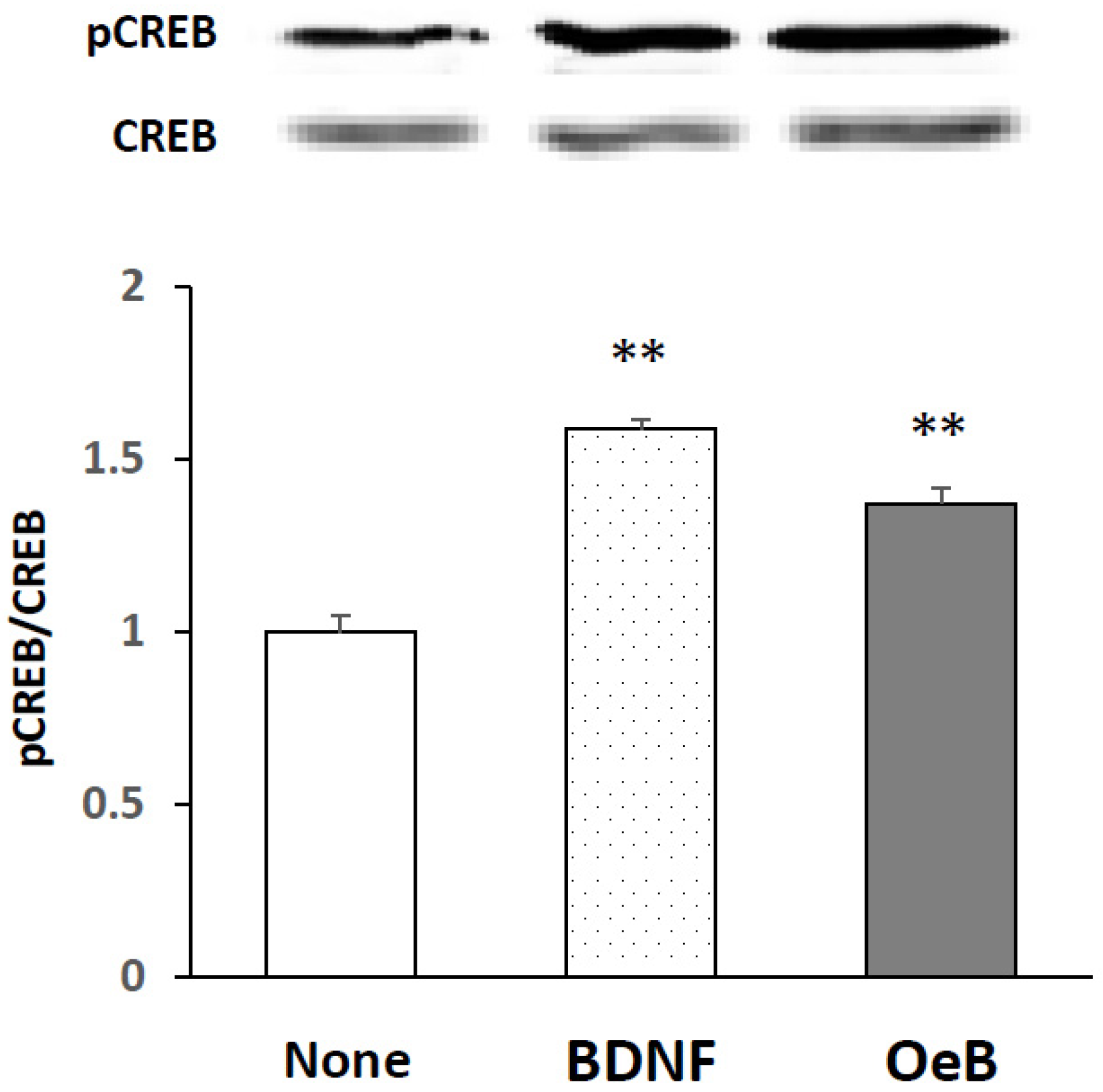
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 337, 1330–1336. [Google Scholar] [CrossRef]
- De Nicoló, S.; Tarani, L.; Ceccanti, M.; Maldini, M.; Natella, F.; Vania, A.; Chaldakov, G.N.; Fiore, M. Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Ding, M.L.; Ma, H.; Man, Y.G.; Lv, H.Y. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can. J. Physiol. Pharmacol. 2017, 95, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2016, 10, 23–42. [Google Scholar]
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H. Ellagitannins renewed the concept of tannin. In Chemistry and Biology of Ellagitannins; Quideau, S., Ed.; World Scientific: Hackensack, NJ, USA, 2009; pp. 1–54. [Google Scholar]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural features and biological properties of ellagitannins in the Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Makihata, N.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Nakajima, M.; Furukawa, Y. Oenothein B suppresses lipopolysaccharide (LPS)-induced inflammation in the mouse brain. Int. J. Mol. Sci. 2013, 14, 9767–9778. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Furukawa, Y.; Yoshimura, M.; Amakura, Y.; Nakajima, M.; Yoshida, T. Oenothein B, a bioactive ellagitannin, activates the extracellular signal-regulated kiase 2 signaling pathway in the mouse brain. Plants 2021, 10, 1030. [Google Scholar] [CrossRef]
- Samuels, I.S.; Karlo, J.C.; Faruzzi, A.N.; Pickeri, K.; Herrup, K.; Sweatt, J.D.; Saitt, S.C.; Landreth, G.E. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci. 2008, 28, 6983–6995. [Google Scholar] [CrossRef] [PubMed]
- Kida, S. A functional role for CREB as a positive regulator of memory formation and LTP. Exp. Neurobiol. 2012, 21, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.; Tavazoie, S.F.; Maloratsky, A.; Jacobs, K.M.; Harris, K.M.; Greenberg, M.E. CREB: A Major mediator of neuronal neurotrophin responses. Neuron 1997, 19, 1031–1047. [Google Scholar] [CrossRef] [Green Version]
- Tomas-Barberan, F.A.; Espin, J.C.; Garcia-Conesa, M.T. Bioavailability and metabolism of ellagic acid and ellagitannins. In Chemistry and Biology of Ellagitannins; Quideau, S., Ed.; World Scientific: Hackensack, NJ, USA, 2009; pp. 273–297. [Google Scholar]
- Beales, I.L.; Calam, J. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1beta and TNF-alpha requires tyrosine kinase activity, but not protein kinase C. Cytokine 1997, 9, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Jakiw, L.; Andrei, I.; Khlebnikov, A.I.; Blaskovich, C.L.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of oenothein B isolated from Epilobium angustifolium. J. Immunol. 2009, 183, 6754–6766. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.; Gruber, M.; Piskaty, C.; Woehs, F.; Renner, A.; Nagy, Z.; Kaltenboeck, A.; Wasserscheid, T.; Bazylko, A.; Kiss, A.K.; et al. Inhibition of NF-κB-dependent cytokine and inducible nitric oxide before synthesis by the macrocyclic ellagitannin oenothein B in TLR-stimulated RAW 264.7 macrophages. J. Nat. Prod. 2012, 75, 870–875. [Google Scholar] [CrossRef]
- Yoshimura, M.; Akiyama, H.; Kondo, K.; Sakata, K.; Matsuoka, H.; Amakura, Y.; Teshima, R.; Yoshida, T. Immunological effects of oenothein B, an ellagitannin dimer, on dendritic cells. Int. J. Mol. Sci. 2013, 14, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker constituents of the natural antioxidant eucalyptus leaf extract for the evaluation of food additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and characterization of activators of ERK/MAPK from citrus plants. Int. J. Mol. Sci. 2012, 13, 1832–1845. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Nakajima, M.; Furukawa, Y. Citrus flavonoid 3,5,6,7,8,3′,4′-heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol. Rep. 2019, 71, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Buscà, R.; Pouysségur, J.; Lenormand, P. ERK1 and ERK2 map kinases: Specific roles or functional redundancy? Front. Cell Dev. Biol. 2016, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Yang, X.; Hei, C.; Meli, Y.; Niu, J.; Sun, T.; Li, P.A. Rapamycin reduced ischemic brain damage in diabetic animals is associated with suppressions of mTOR and ERK1/2 signaling. Int. J. Biol. Sci. 2016, 12, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Furukawa, Y.; Watanabe, S.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M. Effect of citrus polymethoxyfavones on neuritogenesis in neuroblastoma cells. Biointerface Res. Appl. Chem. 2012, 2, 432–437. [Google Scholar]
- Okuyama, S.; Shimada, N.; Kaji, M.; Morita, M.; Miyoshi, K.; Minami, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Watanabe, S.; et al. Heptamethoxyflavone a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci. Lett. 2012, 528, 190–195. [Google Scholar] [CrossRef]
- Okuyama, S.; Fukata, T.; Nishigawa, Y.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M.; Furukawa, Y. Citrus flavonoid improves MK-801-induced locomotive hyperactivity: Possible relevance to schizophrenia. J. Func. Foods 2013, 5, 2002–2006. [Google Scholar] [CrossRef]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, on protection against memory impairment and neuronal cell death in a global cerebral ischemia mouse model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Miyazaki, K.; Yamada, R.; Amakura, Y.; Yoshimura, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Permeation of polymethoxyflavones into the mouse brain and their effect on MK-801-induced locomotive hyperactivity. Int. J. Mol. Sci. 2017, 18, 489. [Google Scholar] [CrossRef] [Green Version]
- Sawamoto, A.; Okuyama, S.; Yamamoto, K.; Amakura, Y.; Yoshimura, M.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules 2016, 21, 541. [Google Scholar] [CrossRef] [Green Version]
- Sawamoto, A.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yamada, T.; Yokogoshi, H.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone ameliorates depressive-like behavior and hippocampal neurochemical changes in chronic unpredictable mild stressed mice by regulating the brain-derived neurotrophic factor: Requirement for ERK activation. Int. J. Mol. Sci. 2017, 18, 2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sometami, A.; Nitta, A.; Nomoto, H.; Furukawa, Y.; Furukawa, S. 4-Methylcatechol stimulates phosphorylation of Trk family neurotrophin receptors and MAP kinases in cultured rat cortical neurons. J. Neurosci. Res. 2002, 77, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Miyazaki, K.; Sakai, S.; Yawo, H.; Nakata, N.; Moriguchi, S.; Fukunaga, K.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 2008, 578, 194–200. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer’s disease and Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The pathology of Parkinson’s Disease and potential benefit of dietary polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Ohizumi, Y. Beneficial effects of citrus-derived polymethoxylated flavones for central nervous system disorders. Nutrients 2021, 13, 145. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, S.; Yoshimura, M.; Amakura, Y.; Nakajima, M.; Furukawa, Y. Activation of Extracellular Signal-Regulated Kinase 2 and cAMP Response Element-Binding Protein in Cultured Neurons by the Macrocyclic Ellagitannin Oenothein B. NeuroSci 2022, 3, 387-394. https://doi.org/10.3390/neurosci3030028
Okuyama S, Yoshimura M, Amakura Y, Nakajima M, Furukawa Y. Activation of Extracellular Signal-Regulated Kinase 2 and cAMP Response Element-Binding Protein in Cultured Neurons by the Macrocyclic Ellagitannin Oenothein B. NeuroSci. 2022; 3(3):387-394. https://doi.org/10.3390/neurosci3030028
Chicago/Turabian StyleOkuyama, Satoshi, Morio Yoshimura, Yoshiaki Amakura, Mitsunari Nakajima, and Yoshiko Furukawa. 2022. "Activation of Extracellular Signal-Regulated Kinase 2 and cAMP Response Element-Binding Protein in Cultured Neurons by the Macrocyclic Ellagitannin Oenothein B" NeuroSci 3, no. 3: 387-394. https://doi.org/10.3390/neurosci3030028
APA StyleOkuyama, S., Yoshimura, M., Amakura, Y., Nakajima, M., & Furukawa, Y. (2022). Activation of Extracellular Signal-Regulated Kinase 2 and cAMP Response Element-Binding Protein in Cultured Neurons by the Macrocyclic Ellagitannin Oenothein B. NeuroSci, 3(3), 387-394. https://doi.org/10.3390/neurosci3030028





