Supercritical CO2 Extraction from Bacupari (Garcinia brasiliensis) and Leiteira (Tabernaemontana catharinensis) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Supercritical CO2 Extraction
2.3. Soxhlet Extraction
2.4. Oil Characterization
2.5. Statistical Analysis
3. Results
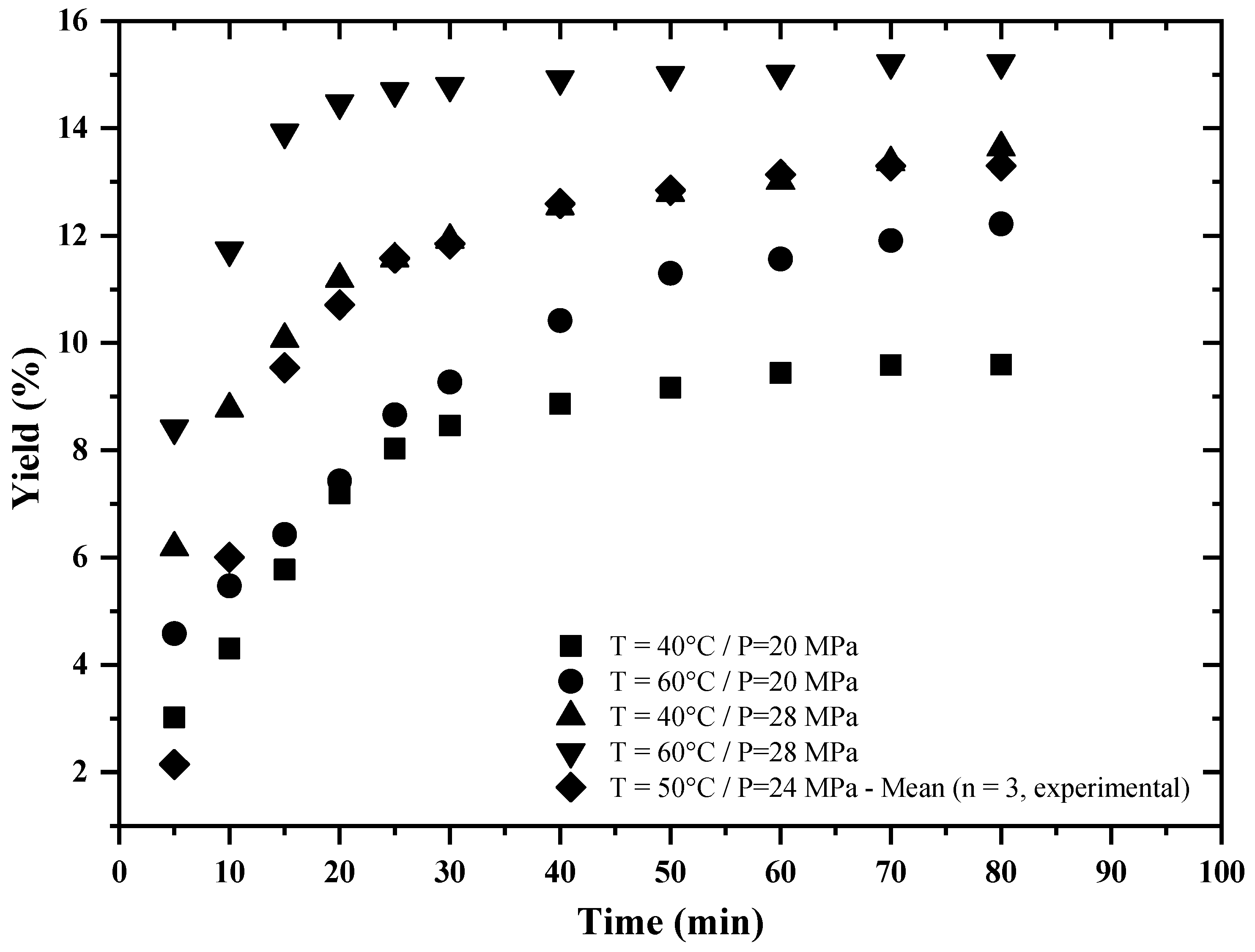
3.1. Extraction Yield
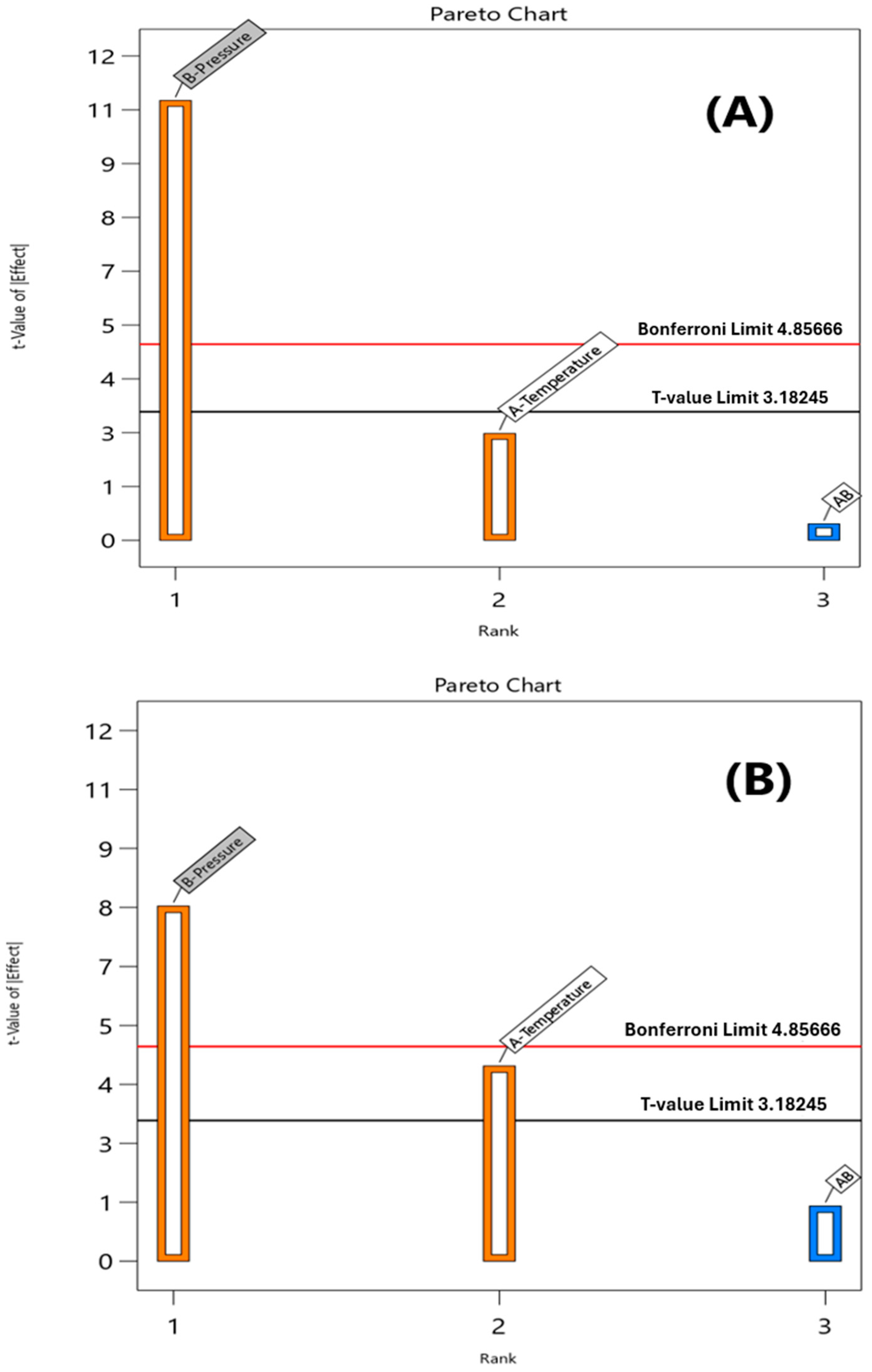
3.2. Statistical Analysis Results
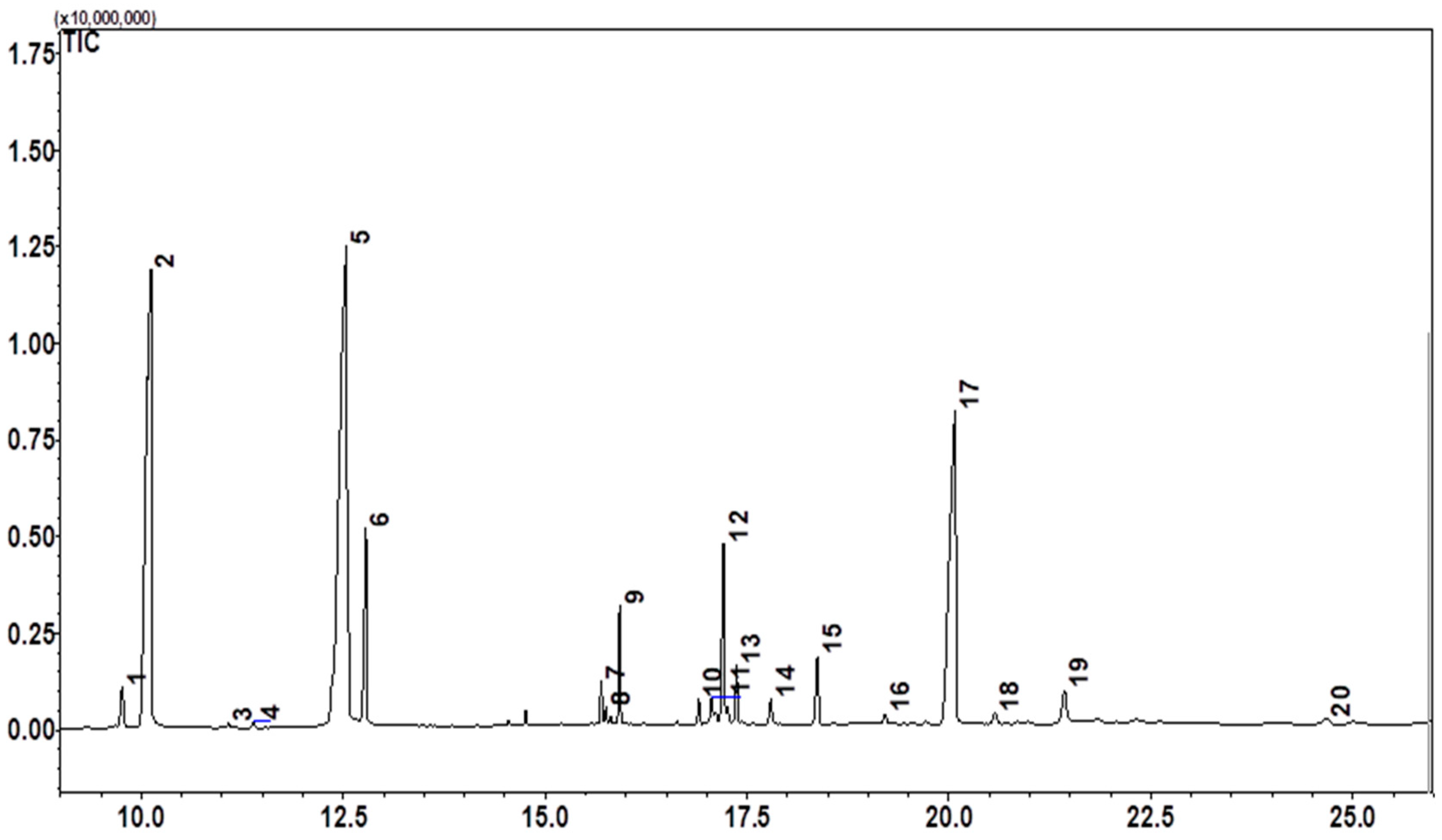
3.3. Chemical Profile of Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edris, A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Waseem, M.; Majeed, Y.; Nadeem, T.; Naqvi, L.H.; Khalid, M.A.; Sajjad, M.M.; Sultan, M.; Khayrullin, M.; Shariati, M.A.; Lorenzo, J.M. Conventional and advanced extraction methods of some bioactive compounds with health benefits of food and plant waste: A comprehensive review. Food Front. 2023, 4, 1681–1701. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Vilela, F.C.; da Rocha, C.Q.; Dias, D.F.; Cavalcante, G.P.; Freitas, L.A.; dos Santos, M.H.; Giusti-Paiva, A. Anti-inflammatory and antinociceptive effects of Garcinia brasiliensis. J. Ethnopharmacol. 2011, 133, 467–473. [Google Scholar] [CrossRef]
- da Silva, C.A.; Rosa, I.A.; de Souza, T.C.; Dos Santos, M.H. Evaluating four modes of extraction to analyze bioactive compounds in Garcinia brasiliensis (bacupari) by high-performance liquid chromatography diode-array detection (HPLC-DAD). Nat. Prod. Res. 2020, 35, 4073–4077. [Google Scholar] [CrossRef]
- Shahidi, F.; De Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Boskou, D. Olive Oil; AOCS Publishing: Champaign, IL, USA, 2006. [Google Scholar] [CrossRef]
- Silva, C.W.; Gonçalves, P.C.; Costa, C.Z.; Soares, K.L.; Batista, P.B.; Marques, S.; Júnior, M.R.M.; Junior, S.B.; Núñez, O.; Scherer, R. Chemical characterization of fruits of Garcinia brasiliensis Mart. Ciência Rural 2025, 55, e20240285. [Google Scholar] [CrossRef]
- Naidoo, C.M.; Naidoo, Y.; Dewir, Y.H.; Murthy, H.N.; El-Hendawy, S.; Al-Suhaibani, N. Major Bioactive Alkaloids and Biological Activities of Tabernaemontana Species (Apocynaceae). Plants 2021, 10, 313. [Google Scholar] [CrossRef]
- Boligon, A.A.; Piana, M.; Schawnz, T.G.; Pereira, R.P.; Rocha, J.B.T.; Athayde, M.L. Athayde, Chromatographic Analysis and Antioxidant Capacity of Tabernaemontana catharinensis. Nat. Prod. Commun. 2014, 9, 1934578X1400900119. [Google Scholar] [CrossRef]
- Camponogara, C.; Casoti, R.; Brusco, I.; Piana, M.; Boligon, A.A.; Cabrini, D.A.; Trevisan, G.; Ferreira, J.; Silva, C.R.; Oliveira, S.M. Tabernaemontana catharinensis leaves exhibit topical anti-inflammatory activity without causing toxicity. J. Ethnopharmacol. 2019, 231, 205–216. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioproc. Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Nicola, C.; Salvador, M.; Gower, A.E.; Moura, S.; Echeverrigaray, S. Chemical Constituents Antioxidant and Anticholinesterasic Activity of Tabernaemontana catharinensis. Sci. World J. 2013, 2013, 519858. [Google Scholar] [CrossRef]
- Sipeniece, E.; Mišina, I.; Qian, Y.; Grygier, A.; Sobieszczańska, N.; Sahu, P.K.; Rudzińska, M.; Patel, K.S.; Górnaś, P. Fatty Acid Profile and Squalene, Tocopherol, Carotenoid, Sterol Content of Seven Selected Consumed Legumes. Plant Foods Hum. Nutr. 2021, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Martín, Á.; Cocero, M.J. Supercritical Fluids. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–28. [Google Scholar] [CrossRef]
- Dixon, D.J.; Johnston, K.P. Supercritical Fluids. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.elsevier.com/books/supercritical-fluid-extraction/brenner/978-0-08-051817-6 (accessed on 9 June 2025).
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Yıldırım, M.; Erşatır, M.; Poyraz, S.; Amangeldinova, M.; Kudrina, N.O.; Terletskaya, N.V. Green Extraction of Plant Materials Using Supercritical CO2: Insights into Methods, Analysis, and Bioactivity. Plants 2024, 13, 2295. [Google Scholar] [CrossRef]
- Corrêa, G.; Souza, M.R.d.R.; Nascimento, E.S.; Bjerk, T.R.; Goncalves, J.E.; Moritz, C.M.F.; Sakai, O.A.; da Silva, E.A.; dos Santos, R.J.; da Silva, E.A.; et al. Supercritical CO2 Extraction of Natural Compounds from Capuchin (Tropaeolum majus) Leaves and Seeds. Processes 2024, 12, 1566. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Lopes, G.; Filho, A.C.; Postaue, N.; Belisário, C.; Paim, L.L.; Cardozo-Filho, L.; da Silva, C.; Ferreira-Pinto, L.; Favareto, R. Assessment of Yield, Flavonoid and Phytosterol Contents, and Fatty Acid Composition of Baru Almond Oil (Dipteryx alata Vogel) by Supercritical CO2 Extraction. Processes 2024, 12, 1729. [Google Scholar] [CrossRef]
- Mateus, L.S.; Dutra, J.M.; Favareto, R.; da Silva, E.A.; Ferreira Pinto, L.; da Silva, C.; Cardozo-Filho, L. Optimization Studies and Compositional Oil Analysis of Pequi (Caryocar brasiliense Cambess) Almonds by Supercritical CO2 Extraction. Molecules 2023, 28, 1030. [Google Scholar] [CrossRef] [PubMed]
- Stat-Ease. Design-Expert Software, version 12; Stat-Ease: Minneapolis, MN, USA, 2019.
- Pereira, C.G.; Leal, P.F.; Sato, D.N.; Meireles, M.A.A. Antioxidant and Antimycobacterial Activities of Tabernaemontana catharinensis Extracts Obtained by Supercritical CO2+ Cosolvent. J. Med. Food 2005, 8, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Marques, M.O.; Barreto, A.S.; Siani, A.C.; Fernandes, E.C.; Meireles, M.A. Extraction of indole alkaloids from Tabernaemontana catharinensis using supercritical CO2+ethanol: An evaluation of the process variables and the raw material origin. J. Supercrit. Fluids 2004, 30, 51–61. [Google Scholar] [CrossRef]
- Gunstone, F.D. Vegetable Oils in Food Technology; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Dutta, S.; Wu, K.C.-W.; Saha, B. Emerging strategies for breaking the 3D amorphous network of lignin. Catal. Sci. Technol. 2014, 4, 3785–3799. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef] [PubMed]





| Factors | Symbols | Units | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Temperature | T | °C | 40 | 50 | 60 |
| Pressure | P | MPa | 22 | 25 | 28 |
| Run | Temperature (°C) | Pressure (MPa) | Yield (wt%) |
|---|---|---|---|
| Bacupari Seeds | |||
| 1 | 40 | 20 | 11.4 |
| 2 | 60 | 20 | 12.2 |
| 3 | 40 | 28 | 14.2 |
| 4 | 60 | 28 | 14.8 |
| 5–7 | 50 | 24 | 13.1 ± 0.7 * |
| Ethanol Sohxlet (360 min) | Atmospheric | 17.4 ± 1.1 * | |
| Leiteira Seeds | |||
| 1 | 40 | 20 | 9.5 |
| 2 | 60 | 20 | 12.2 |
| 3 | 40 | 28 | 13.6 |
| 4 | 60 | 28 | 15.2 |
| 5–7 | 50 | 24 | 13.4 ± 0.4 * |
| Ethanol Soxhlet (360 min) | Atmospheric | 8.2 ± 0.6 * | |
| Terms | Sum of Squares | Degrees of Freedom | Mean Squares | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Bacupari Seeds | ||||||
| Model | 7.79 | 3 | 2.60 | 42.27 | 0.0059 | 0.977 |
| T | 0.4288 | 1 | 0.4288 | 6.98 | 0.0775 | |
| P | 7.29 | 1 | 7.29 | 118.67 | 0.0017 | |
| T.P | 0.0100 | 1 | 0.0100 | 0.1628 | 0.7136 | |
| Residual | 0.1843 | 3 | 0.0614 | |||
| Cor Total | 7.97 | 6 | ||||
| Leiteira Seeds | ||||||
| Model | 17.53 | 3 | 5.84 | 29.87 | 0.0098 | 0.968 |
| T | 3.81 | 1 | 3.81 | 19.49 | 0.0216 | |
| P | 12.60 | 1 | 12.60 | 64.43 | 0.0040 | |
| T.P | 0.3025 | 1 | 0.3025 | 1.55 | 0.3020 | |
| Residual | 0.5868 | 3 | 0.1956 | |||
| Cor Total | 18.11 | 6 | ||||
| ID | Retention Time | Math with the Library (%) | Compound | Chemical Class | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 9.760 | 100 | Palmitelaidic acid | Lipid—Unsaturated fatty acid | 1.16 |
| 2 | 10.118 | 100 | Palmitic Acid | Lipid—Saturated fatty acid | 25.84 |
| 4 | 11.386 | 99 | Heptadecanoic acid | Lipid—Saturated fatty acid | 0.19 |
| 5 | 12.534 | 96 | Oleic Acid | Lipid—Monounsaturated fatty acid | 35.05 |
| 6 | 12.779 | 100 | Stearic acid | Lipid—Saturated fatty acid | 5.25 |
| 8 | 15.752 | 99 | Hexadecanoic acid | Lipid—Saturated fatty acid | 0.35 |
| 9 | 15.922 | 98 | 1-Monopalmitin | Lipid—Monoglyceride | 1.94 |
| 10 | 16.902 | 99 | 2-linoleoylglycerol | Lipid—Monoglyceride | 0.55 |
| 11 | 17.058 | 98 | 2-Oleoylglycerol, | Lipid—Monoglyceride | 0.54 |
| 12 | 17.207 | 99 | 1-Monooleoylglycerol | Lipid—Monoglyceride | 4.30 |
| 13 | 17.371 | 97 | Glycerol monostearate | Lipid—Monoglyceride | 1.19 |
| 14 | 17.794 | 100 | Hexanedioic acid | Carboxylic acid—Dicarboxylic acid | 0.69 |
| 15 | 18.372 | 99 | Cholestane | Hydrocarbon—Steroid hydrocarbon | 0.79 |
| 17 | 20.074 | 100 | delta-Tocopherol | Phenolic compound—Vitamin E | 19.60 |
| 18 | 20.569 | 99 | Farnesol | Isoprenoid alcohol | 0.51 |
| 19 | 21.432 | 99 | beta-Tocopherol | Phenolic compound—Vitamin E | 1.62 |
| 20 | 24.679 | 98 | Stigmasterol | Steroid—Phytosterol (plant sterol) | 0.43 |
| Order | Retention Time | Math with the Library (%) | Compound | Chemical Class | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | 10.018 | 100 | Palmitic Acid | Lipid—Saturated fatty acid | 14.00 |
| 2 | 12.409 | 98 | Oleic Acid | Lipid—Monounsaturated fatty acid | 8.91 |
| 3 | 12.491 | 98 | 13-Octadecenoic acid | Lipid—Unsaturated fatty acid | 1.08 |
| 4 | 12.734 | 100 | Stearic acid | Lipid—Saturated fatty acid | 3.62 |
| 6 | 15.636 | 99 | Octacosane | Hydrocarbon—Alkane | 4.96 |
| 8 | 15.917 | 99 | Hexacosane | Hydrocarbon—Alkane | 3.64 |
| 10 | 17.183 | 96 | 1-Monooleoylglycerol | Lipid—Monoacylglycerol | 0.89 |
| 11 | 17.433 | 99 | Heneicosane | Hydrocarbon—Alkane | 3.76 |
| 12 | 17.814 | 100 | Hexanedioic acid | Carboxylic acid—Dicarboxylic acid | 29.17 |
| 13 | 18.372 | 99 | Cholestane | Hydrocarbon—Steroid hydrocarbon | 10.93 |
| 15 | 19.701 | 91 | gamma-Tocopherol | Phenolic compound—Vitamin E | 0.61 |
| 16 | 19.948 | 98 | delta-Tocopherol | Phenolic compound—Vitamin E | 1.48 |
| 17 | 21.767 | 92 | alpha-Tocopherol | Phenolic compound—Vitamin E | 1.84 |
| 18 | 24.074 | 100 | Campesterol | Steroid—Phytosterol (plant sterol) | 2.16 |
| 19 | 24.688 | 99 | Stigmasterol | Steroid—Phytosterol (plant sterol) | 4.99 |
| 20 | 26.018 | 100 | Stigmast-5-ene | Steroid—Phytosterol (plant sterol) | 7.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, G.d.S.; Conceição, M.A.; Hiranobe, C.T.; da Silva, C.; da Silva, E.A.; dos Santos, R.J.; Ferreira-Pinto, L. Supercritical CO2 Extraction from Bacupari (Garcinia brasiliensis) and Leiteira (Tabernaemontana catharinensis) Seeds. Sustain. Chem. 2025, 6, 35. https://doi.org/10.3390/suschem6040035
Lopes GdS, Conceição MA, Hiranobe CT, da Silva C, da Silva EA, dos Santos RJ, Ferreira-Pinto L. Supercritical CO2 Extraction from Bacupari (Garcinia brasiliensis) and Leiteira (Tabernaemontana catharinensis) Seeds. Sustainable Chemistry. 2025; 6(4):35. https://doi.org/10.3390/suschem6040035
Chicago/Turabian StyleLopes, Guilherme de Souza, Matheus Almeida Conceição, Carlos Toshiyuki Hiranobe, Camila da Silva, Erivaldo Antônio da Silva, Renivaldo José dos Santos, and Leandro Ferreira-Pinto. 2025. "Supercritical CO2 Extraction from Bacupari (Garcinia brasiliensis) and Leiteira (Tabernaemontana catharinensis) Seeds" Sustainable Chemistry 6, no. 4: 35. https://doi.org/10.3390/suschem6040035
APA StyleLopes, G. d. S., Conceição, M. A., Hiranobe, C. T., da Silva, C., da Silva, E. A., dos Santos, R. J., & Ferreira-Pinto, L. (2025). Supercritical CO2 Extraction from Bacupari (Garcinia brasiliensis) and Leiteira (Tabernaemontana catharinensis) Seeds. Sustainable Chemistry, 6(4), 35. https://doi.org/10.3390/suschem6040035






