Abstract
This review sheds some light on the emerging niche of the reuse of spent adsorbents in electrochemical devices. Reuse and repurposing extend the adsorbent’s life cycle, remove the need for long-term storage, and generate additional value, making it a highly eco-friendly process. Main adsorbent-type materials are overviewed, emphasising desired properties for initial adsorption and subsequent conversion to electroactive material step. The effects of the most frequent regeneration procedures are compared to highlight their strengths and shortcomings. The latest efforts of repurposing and reuse in supercapacitors, fuel cells, and batteries are analysed. Reuse in supercapacitors is dominated by materials that, after a regeneration step, lead to materials with high surface area and good pore structure and is mainly based on the conversion of organic adsorbents to some form of conductive carbon adlayer. Additionally, metal/metal-oxide and layered-double hydroxides are also being developed, but predominantly towards fuel cell and battery electrodes with respectable oxygen reduction characteristics and significant capacities, respectively. Repurposed adsorbents are being adopted for peroxide generation as well as direct methanol fuel cells. The work puts forward electrochemical devices as a valuable avenue for spent adsorbents and as a puzzle piece towards a greener and more sustainable future.
1. Introduction
Research focusing on environmental protection and more efficient energy solutions increasingly turns to advances in materials science [1]. Being a skilled scientist in finding solutions, structures, and performances of new materials implies a deep understanding of the key interactions expected from newly synthesised materials. Synthesis methods tend to become more complex, especially in the light of new metal–organic structures or MXens [2]. However, truly sustainable materials must rely on naturally available and inexpensive resources [3]. The same is expected for the selection of materials in environmental remediation [4].
Adsorption is most often examined among the environmental techniques, due to its efficiency and simple interpretation of results [5]. The kinetics and thermodynamics of the process can be easily established [6]. Moreover, for surface optimisation, different phases with functional groups can be tailored to support a variety of applications. However, the idea of a large adsorption capacity for various pollutants in the environment is so much in focus that they are often applied in experimental procedures that will increase capacities even though they are not applicable in real conditions, such as a significant reduction in the adsorbent mass or application of high temperatures or inadequate acidity. The selection of an experimental design can mask the true nature of the process. The next problem with adsorption studies is that the possibilities for testing flow systems are often limited, and this often results in unexpected outcomes. This leads to a central problem in using adsorption as a remediation method: how to manage spent adsorbents? The management of spent pollutant adsorbents, such as porous silicates, aluminosilicates, and carbon-based materials, poses a significant challenge in environmental remediation. These materials, widely used for their exceptional capacity to trap pollutants, often become secondary waste once saturated with contaminants, raising concerns about their disposal and potential environmental impact. The storage of spent adsorbents is not an option in any sustainable system; therefore, the idea of efficient regeneration has long been part of adsorption studies. Again, efficiency is primary, and different organic solvents or thermal methods are suggested for regeneration [7]. The eventual regeneration of organic phases using ionic liquids is considered in the recent literature, which could enable the closing of the cycle, i.e., obtaining pollutants, such as pesticides, in their original form [8]. Despite the expense, ionic liquids are attractive because they can be tailored to selectively desorb pollutants while preserving the adsorbent structure waste precursor [9]. On the other hand, thermal methods are often associated with significant energy consumption when there is a limited share of renewable energy sources in the electricity supply.
In recent years, repurposing these waste adsorbents into functional materials has received considerable attention, offering a viable alternative to conventional regeneration or disposal. Transformative processes such as electrochemical oxidation and thermal degradation can modify the chemical and structural properties of these materials, resulting in composites with potential applications in energy technologies. Due to their high conductivity, large surface area, and catalytic activity, composites derived from waste adsorbents may be used in supercapacitors and as catalysts for oxygen reduction in fuel cells [10].
This review explores this novel field of adsorbent regeneration and repurposing, adding value to typical environmental remediation procedures and evolving towards green energy. Understanding the principles of electrochemical and thermal transformation processes enables the production of materials of value for energy storage and conversion systems. The challenges and future directions of this sustainable approach are also discussed, emphasising the role of both theoretical and experimental methods for future studies.
2. Pollutant Adsorbents: Advanced Precursors in Disguise
Pollutant adsorbents, as insoluble porous materials, facilitate the removal of liquid or gaseous contaminants through surface binding interactions. They are classified into organic, inorganic, and hybrid types and are available in various forms, including nano- and microparticles and membranes. Their origins span from natural to synthetic, with materials existing in raw form or subjected to physical and/or chemical modifications, as well as being structured into frameworks and composite systems. From an adsorption perspective, when choosing the best adsorbent, several parameters must be considered: the adsorbent should be selective, have a large adsorption capacity, be easily regenerable, be durable, be stable under relevant conditions, be inexpensive, be locally available, and be capable of being shaped for optimal mechanical and dynamic properties. While it is difficult to find an adsorbent that meets all of these criteria, a balance between these factors must be found. Frequently utilised materials for pollutant adsorption include carbon-based materials (activated carbon, biochar, and carbon nanomaterials), siliceous compounds, zeolites, clay minerals, biomass, metal–organic frameworks (MOFs), and a range of polymers, including biopolymers, synthetic fibres, geopolymers, hydrogels, and aerogels, among others [11,12,13,14,15].
2.1. Porous Structure, Chemical Composition, and Adsorption Interactions
The use of adsorbents in environmental remediation, through selective adsorption of pollutants, requires materials with precisely defined characteristics. These include suitable pore structure parameters, the chemical composition of the surface and internal framework, and adequate mechanical properties such as strength, compressibility, and the shape and size of the particles.
Understanding the impact of adsorbent pore size on the competition mechanisms involving natural organic matter is essential for selecting the most suitable adsorbent. Targeted adsorbates are a part of a complex mixture of organic compounds with varying size, molecular weight, and functionality, commonly present in surface water and, to a lesser extent, in groundwater. When adsorbent pores are large enough for micropollutants but exclude larger organic matter, pore blockage dominates. If both micropollutants and organic matter fit, direct competition for adsorption sites prevails. Thus, widening the pore size distribution of highly microporous adsorbents can help reduce pore blockage caused by organic matter and lessen its negative effect on the adsorption of trace micropollutants [16].
Apart from pore size, the surface functional groups play a crucial role in adsorption, especially for pollutants larger than the pore openings of the adsorbent. If the porous structure remains largely unchanged across the sample series, the effect of functional groups on adsorption properties can be studied without major interference from variations in pore size distribution. Various electrostatic forces, including orientation, induction, and dispersion forces, govern dominant intermolecular interactions in adsorption. A common misconception in the literature is the separation of hydrophobic interactions from electrostatic ones, despite their same origin [17]. Thus, it is of utmost importance to bring to light the basic adsorption mechanisms specific to each system. Physical adsorption is witnessed in each system dealing with pollutant removal. Chemical adsorption, although investigated in papers testing adsorption thermodynamics, as summarised in a review by Mohammed et al. [18], cannot be established at ambient temperatures and mild conditions seen in adsorption studies.
2.2. Adsorbents in Water Treatment and Remediation
In the context of water purification, the most commonly utilised adsorbents include zeolites, clay-based minerals, bio-derived materials, and activated carbon, due to their high surface area and strong affinity for various contaminants. Metal–organic frameworks (MOFs) have gained recognition as innovative and highly versatile materials, finding applications across a wide range of fields, including water purification.
Zeolites are widely used as adsorbents owing to their microporosity, well-defined pore structure, thermal stability, and the ability to tailor their properties through post-synthetic modifications. Their shape- and size-selectivity make even minor changes in cation distribution or framework structure highly influential to their performance as adsorbents [19]. Zeolites are considered promising materials for pesticide adsorption. In particular, zeolite Y (FAU) has been suggested as an effective adsorbent for the removal of simazine, the second most frequently detected pesticide in surface and groundwater in the USA, Australia, and Europe. FAU applicability is supported by low cost, commercial availability, uniform pore size (~0.74 nm), acid sites capable of hydrogen bonding with nitrogen atoms, and high thermal stability [20]. The FAU-type demonstrated superior adsorption performance for neonicotinoid pesticides compared to both BEA and MFI types. Pesticide uptake increased as the aluminium content decreased, with maximum efficiency observed in FAU samples having a Si/Al ratio ≥ 30. This is attributed to a higher number of isolated proton-donor sites, which are more accessible to pesticide molecules. While BEA and MFI zeolites also exhibited adsorption potential, their performance was still lower than that of the FAU-type [21]. The high content of aluminium and silicon in fly ash, a byproduct of coal combustion, serves as a valuable source of aluminosilicate, which is essential for synthesising zeolites and zeolitic materials. In the research by Andrunik et al. [22]., two distinct adsorbents were used: zeolite type X and a zeolite-carbon composite incorporating zeolite type X. To enhance the sorption efficiency of 2,4-dichlorophenoxyacetic acid, 2-methyl-4-chlorophenoxyacetic acid, carbendazim, and simazine, the zeolite materials were functionalised with cationic and non-ionic surfactants. The results emphasise the significant potential of fly ash-based zeolites and zeolite-carbon composites for effectively removing pesticides from water, owing to their rapid adsorption and efficient regeneration without a loss of adsorption capacity.
Clays, hydrous aluminosilicates, are favourite adsorbents due to their low cost, abundance, high specific surface area, excellent adsorption properties, non-toxic nature, and potential for ion exchange. Exchangeable ions make them effective for water treatment, especially for ion-ion interaction with ionisable pollutants. Moreover, in addition to ion-dipole interaction for polar contaminants, the surface charge of alumosilicates enables ion-induced dipole interaction to emerge also as a mechanism for pollutant retention. Clays are especially effective for metal cations due to their high cation exchange capacity, surface area, and pore volume. The adsorption of heavy metals involves mechanisms like chemical bonding, surface complexation, and ion exchange. Pre-treatment of clays can enhance their adsorption capacity by increasing surface area, pore volume, and the number of acid sites, making them more effective at adsorbing organic compounds [23]. Combining clay minerals with layered double hydroxides (LDHs) consisting of divalent and trivalent cations with exchangeable interlayers of ions and water molecules can be anticipated to address issues of aggregation and poor porosity. Nowadays, LDH-modified montmorillonite, mica, illite, and chlorite to form composite adsorbents were prepared for the removal of atrazine and 4-chloro-2-methylphenoxyacetic acid, with promising results [24].
Bio-derived materials include natural polymers [25], plant-based materials, agricultural waste products [26], and biochar [27], which are renewable and environmentally friendly. Biochars produced via thermochemical treatment of biomass are increasingly investigated for applications in energy storage, carbon sequestration, and soil and water remediation [28]. In adsorption, biochars offer sustainability due to renewable precursors, low production costs, and potential for regeneration [29] and repurposing. The surface area, porosity, and surface chemistry depend on precursor type and processing conditions [30]. Common conversion methods include pyrolysis, microwave-assisted, and hydrothermal carbonisation, relying on agricultural, forestry, sludge, or other waste precursors [31]. Recently, invasive plants have also gained interest as abundant, low-cost biomass sources for adsorbent preparation [32]. However, their pristine adsorption capacity often requires activation to enhance their properties through physical or chemical activation [33]. Generally, all carbon-rich organic materials are considered carbonised substances, which can serve as precursors for activated carbon. Raw materials include agricultural residues, livestock and industrial byproducts, while commercially, wood, anthracite and bituminous coal, lignite, peat, shells, and coconut are most commonly used. Activation can be carried out physically or chemically. Chemical activation is generally more advantageous, as it requires lower temperatures and shorter processing times and achieves higher carbon efficiency, while also producing a more developed porous structure. Various chemicals are used as activators, including alkaline compounds (KOH, NaOH, CaCl2, K2CO3), acids (H3PO4, H2SO4), transition metal salts like ZnCl2, and other agents. Comparisons between physical mixing and impregnation methods using alkali metals show that physically mixed samples yield activated carbon with higher porosity than those produced via impregnation [34]. Activation processes significantly increase the internal surface area, enabling the material to retain small molecules effectively. In water and wastewater treatment, the primary forms are granular and powdered activated carbon, which differ in particle size and structure. Both are widely used in drinking water treatment for the removal of taste and odour compounds, synthetic organic chemicals, and dissolved natural organic matter. Particle size plays a significant role in adsorption kinetics, influencing the rate at which contaminants are removed from water [35]. During prolonged industrial-scale use, adsorbents often exhibit a reduction in specific surface area and pore volume. This decline arises from the accumulation of adsorbates within pores, deposition of suspended solids or biofilms on the surface, and gradual structural degradation caused by repeated adsorption-regeneration cycles. Zieliński et al. [36] reported the development of granular activated carbon dedicated to the removal of pesticides from water, ultimately producing an industrial-scale adsorbent that effectively reduced acetochlor sulfonic acid concentrations in treated water from approximately 0.4 µg/L to below 0.1 µg/L. Over 11 months of operation, a decline in adsorption efficiency was observed, attributed to a reduction in specific surface area and pore volume, as well as to the competitive adsorption of natural organic matter, which occupies active sites and reduces the availability for pesticide adsorption. The performance of granular activated carbon in removing commonly used agricultural pesticides, such as atrazine, malathion, and parathion, can be further enhanced through the use of biological activated carbon, a process that combines physical adsorption with the biodegradation of pollutants and organic compounds by microbial biofilms [37]. In biologically activated carbon technology, activated carbon serves as a support material for microorganisms. Under suitable temperature and nutrient conditions, microorganisms grow and reproduce on the carbon surface. This system enables simultaneous adsorption of contaminants and their biodegradation [38].
Metal–organic frameworks (MOFs) are highly porous crystalline materials composed of metal ions or clusters coordinated to organic ligands. Tunable pore structures and adaptable surface functionalities make them promising adsorbents and catalysts for water purification [39]. A recent review by Ihsanullah [40] highlights the growing interest in MOF-based materials for the removal of various waterborne pollutants. MOFs have demonstrated effective removal capabilities for a wide range of contaminants, including persistent organic pollutants [39], dyes [41,42], heavy metals [43], and pesticides [44]. Their adsorption properties can be finely tuned either during synthesis or through post-synthetic modification. For example, amino-functionalised MOFs have shown excellent performance in the removal of metal ions [45], while nitrogen-containing functional groups improve dispersibility and increase the number of active adsorption sites, particularly in dye removal applications [46]. Moreover, composites combining MOFs with conducting polymers have proven highly efficient for the adsorption of emerging contaminants in complex aqueous matrices [47], emphasising their potential in advanced water treatment technologies.
3. Environmental Challenges of Spent Adsorbents
When adsorbents become saturated with pollutants, their capacity to remove contaminants diminishes, leading to the generation of spent adsorbents that contain concentrated hazardous substances. Proper management of these materials is crucial because improper handling or disposal of spent adsorbents can cause the release of adsorbed pollutants back into the environment, resulting in secondary contamination of the environment. This risk is especially critical for toxic or persistent contaminants.
Mitigating secondary pollution and transfer of pollutants from one phase to another, waste management strategies offer sustainable approaches to extending the utility of adsorbents [48]. It should be noted that most of the adsorption studies are conducted in the same manner—adsorbent loading, contact time, and efficiency with respect to adsorbate concentration are evaluated, without a fundamental understanding that adsorption isotherms assemble all information about the adsorption process. Additionally, no detailed insight can be drawn from adsorption kinetics and thermodynamics, lacking rigorous physicochemical analysis. Therefore, there is a rising need for bold and novel propositions on analysing adsorption in environmental studies.
Various strategies are employed to minimise secondary pollution, including regenerating spent adsorbents for reuse in adsorption processes. A particularly attractive method of handling spent adsorbents is their conversion through appropriate treatments into new materials for use in other applications—repurposing.
3.1. Regeneration
Beyond adsorption capacity, the sustainability of this process hinges on the effective regeneration of saturated adsorbents to restore their performance and minimise waste [49]. Regeneration focuses on restoring the adsorption functionality by eliminating the retained contaminants in several cycles [48]. Among regeneration strategies, chemical, electrochemical, thermal, and biological methods are often explored.
3.1.1. Chemical and Electrochemical Methods
Chemical desorption may seem like the first choice, as adsorbate–adsorbent interaction relies on a whole range of intermolecular forces. Acid solutions are used for metal desorption [50], while base solvents may be used for organics. A proposition of a combination of solvents (NaOH followed by H2SO4) for the regeneration of activated carbon in a pilot plant regime is given in the publication by Li et al. [51]. Inorganic salts, adding to the ionic strength in the solution, can assist the removal of ionic species from the adsorbent, for instance, antibiotics from MXene adsorbents [52], dyes from sawdust [53], or in combination with other solvents, as in the recovery of chromium-saturated attapulgite [54]. Organic solvents could also be used to regenerate an adsorbent. However, although efficient, they are not environmentally acceptable. Acetone, alcohols, and their water mixtures can extract dyes [55], while methanol is the solvent of choice for the regeneration of phenol adsorbents [56] and even for the adsorption of complex heterocyclic compounds such as benzotriazole [57]. The spent modified activated carbon, employed for adsorptive diesel fuel desulfurization, was regenerated using a solvent extraction method to assess its durability. The results showed that the regeneration efficiency of various solvents decreased in the following order: iso-octane > ethanol > methanol > acetonitrile [58]. To effectively compare chemical regeneration with other techniques, several key issues need to be clarified, such as whether a given regenerant performs equally well in continuous flow, fixed-bed systems as it does in batch processes. Moreover, the potential for regenerant reuse and the recovery of valuable desorbed compounds requires further investigation to accurately assess the cost-efficiency and long-term sustainability of chemical regeneration as a viable alternative to thermal methods [32].
Oxidation-based regeneration, both chemical and physicochemical, is widely employed to restore saturated adsorbents by degradation of adsorbates. Chemical oxidation includes H2O2, O3, or KMnO4. These methods are quite effective for organic contaminants and dyes but may suffer from incomplete mineralisation, operational costs, and scalability. Physicochemical oxidation includes advanced oxidation processes, which combine oxidants with UV light, ultrasound, or catalysts (TiO2, Fe2+/Fe3+ in Fenton and Fenton-like reactions) to generate highly reactive hydroxyl and superoxide radicals. An iron nanocatalyst added to activated carbon-saturated adsorbents enables efficient regeneration over 90% in the Fenton system [59]. Homogeneous Fenton reagent is efficiently applied for the regeneration of the adsorbent used for the removal of organochlorine pollutants [60]. This process can be boosted at elevated temperatures, as suggested in the literature [61] for granular carbons loaded with chlorobenzenes. However, although Fenton reagents in solid or liquid phase introduce significant cost to regeneration, they often leave a regeneration efficiency of 70% [62]. This type of adsorbent/catalyst system enables sustainable pollutant removal, while special attention should be paid to adsorbent/catalyst stability in highly oxidising environments [63].
3.1.2. Biological and Other Regeneration Methods
Biological methods are slow, and complete biodegradability of the adsorbates may be unreachable, along with large-scale processing [51]. Microbial regeneration of spent activated carbon relies on the combined action of adsorption, through which pollutants are retained on the adsorbent surface, and biodegradation, by which microorganisms degrade the adsorbates, thereby freeing the surface and restoring the adsorptive capacity of the activated carbon. Kinetic parameters can be acquired from different models in bioregeneration, taking into account microbial growth, adsorbent stability, and efficiency of the process [64]. Diatomite adsorbents of cationic dye and chromium ion were regenerated in a prolonged period using Lysinibacillus fusiformis, reaching over 95% and 71%, respectively [65]. Cultivated Thiobacillus ferrooxidans was applied for restoring the adsorption efficiency of mordenite for Fe/Mn contaminants in water [66], achieving a removal efficiency of 85% for iron and only 30% for manganese.
Another interesting regeneration method is ultrasonic treatment of coffee waste hydrochar, which achieved complete regeneration in the first cycle and over 70% up to the fourth cycle [67]. Sonochemical regeneration treatment of clay adsorbent [68] resulted in better performance in comparison to the pristine adsorbent. The desorption of trichloroethane from granular activated carbon restores surface active sites and may serve as an alternative to chemical treatment [69]. Ultrasound can assist activation as well as regeneration of biochars [70]. The combination of sono/thermal methods significantly improved regeneration efficiency and reduced mass loss of granular activated carbon [71]. A similar method was employed for powdered activated carbon loaded with chlorphenols [72]. An important finding relies on a rise in oxygen functional group content on regenerated samples, enabling efficient reuse.
Additionally, hybrid techniques such as microwave-UV advanced oxidation, electro-oxidation, and electrochemical, coupled with thermal degradation, are also explored. A combination of microwave, ozone, and UV irradiation was found to be highly efficient in the regeneration of tetracycline adsorbent, preventing VOC emission [73].
3.1.3. Regeneration of PFAS Adsorbent
Special attention must be given to per- and polyfluoroalkyl substances (PFAS) contamination and associated loaded adsorbents. Due to strong C-F bonds, PFAS are highly resistant to degradation and complete removal from adsorbents. The risk of incomplete removal of PFAS adsorbates and their potential release may be accompanied by loss of adsorbent stability over regeneration cycles. A hydrothermal alkaline treatment under high temperature/pressure is proposed for the regeneration of the PFAS adsorbent. Almost complete degradation of perfluorooctanesulfonate on activated carbon was achieved, without formation of fluoro-organic intermediates [74]. Gagliano et al. [75] investigated microwaves for activated carbon saturated with perfluorooctanoic and perfluorooctane sulfonic acid under varied conditions (up to 500 W and 12 min). Regeneration over 90% needs temperatures of 600 °C, but after several cycles, regeneration remained at 65%. Due to the complexity of adsorbent systems loaded with PFAS, sophisticated methods are explored, such as modified supercritical CO2 extraction [76]. It was found to be quite efficient, reaching over 99% desorption of perfluorooctanoic acid within 1 h at 100 °C. Volatile fluorinated byproduct release can be overcome with organic additives and acid modifiers. By combining foam fractionation to concentrate PFAS from groundwater and landfill leachate and electrochemical oxidation, degradation of PFAS can be achieved. The system efficiency depends on PFAS structure, as long-chain molecules undergo over 85% while short-chain degradation was set at 31% [77]. The electrochemical approach is a method of choice alongside adsorption for PFAS removal. When combined with ion exchange with different salts, it leads to regeneration, which can produce chlorate and perchlorate with NaCl. The addition of H2O2 mitigated byproducts and enhanced perfluorooctanoic acid degradation and defluorination. Sulphate and bicarbonate show consistent efficiency, while Cl− and OH− are less efficient due to competitive anode interactions. Methanol improved ion exchange regeneration but reduced PFAS decomposition. A Na2SO4 solution was found to be an optimal salt for regeneration and degradation without introducing additional chemicals [78].
3.1.4. Thermal Degradation
Thermal degradation is widely employed for the regeneration and modification of spent adsorbents through processes such as pyrolysis, calcination, and controlled combustion. These methods remove adsorbed pollutants but may alter the physicochemical properties of the adsorbent to enhance its reusability. An insightful review on engineering-activated carbon regeneration was recently given by Baghirzade et al. [79]. Pyrolysis, conducted in an inert environment, enables organic matter graphitisation at temperatures going up to 700 °C. The high-temperature treatment can improve the textural properties of materials [80]. Calcination involves heating in air or combined atmospheres at temperatures over 600 °C, resulting in the complete or partial burn-off of adsorbates. Controlled combustion, on the other hand, enables the complete oxidation of organic residues [81], but it may affect the adsorbent structure. The atmosphere influences the pore development and surface functional groups governing potential reuses of resulting materials. Xiao et al. [82] studied thermal degradation of different PFCAs, PFSAs, and PFECA in a range of atmospheres (N2, O2, CO2, and air) and found that PFCAs and PFECAs decompose even at a modest 200 °C, while PFSAs require a temperature above 450 °C. The use of activating agents such as KOH, ZnCl2, or H3PO4 during thermal treatment promotes porosity development and a rise in specific surface. Thus, thermal modification of adsorbents may lead to improved adsorption capacities or result in a composite material where the pristine adsorbent is covered with an activated carbon layer.
Among the reported regeneration strategies, thermal treatment remains widely applied due to its effectiveness, despite energy demand and potential structural degradation of the adsorbent. Chemical regeneration using solvents or oxidants enables sufficient recovery but leads to secondary waste and prevents large-scale implementation. The search for sustainable regeneration requires biological or catalytic approaches, although still limited to laboratory-grade methods. The thermally regenerated adsorbents are usually converted into functional carbon materials. The combination of post-adsorption thermal treatment with secondary, energy-related applications provides a reasonable ground for future investigations.
3.2. Repurposing in Energy-Related Applications
The prevailing idea is that regeneration is expected with complex and expensive materials. However, it often requires a significant amount of energy/chemicals, which can introduce additional environmental concerns. Repurposing, on the other hand, offers different applications. The reuse of spent adsorbents in secondary applications (e.g., as catalysts, fertilisers, cement additives, secondary adsorbents, or biofuels) is also discussed as a pathway toward circular economy integration. The solution is to either generate value-added materials or prepare composite materials for targeted applications [48]. Recycling extends the life cycle of adsorbents and enhances resource efficiency. However, unlike regeneration, this approach relies on innovative ideas and technologies that will address both efficiency and safety.
Among various types of adsorbents, those applied for the removal of metal ions are first considered, most commonly used for the adsorption of Cr(VI), Hg(II), Pb(II), Cd(II), Cu(II), Fe(III), and Co(II). Adsorption is the method of choice for metal adsorbents due to its suitability, cost, and efficiency. However, metal-loaded adsorbents need attention to prevent secondary pollution and leaching, with no alternative for degradation, recycling, or repurposing. Some prospective applications of spent adsorbents rely on their catalytic activity, energy-related applications, and even forensic practice [83].
Studies have shown that carbonaceous adsorbents utilised for heavy metal removal can be effectively converted into catalytic materials. Amino-Brønsted acid precursor functional biocarbons were prepared by the hydrothermal-ammonia carbonisation of biomass and subsequent mechanochemical modification with L-cysteine. Amino-carbons modified retained Ni2+ metal with a significant capacity over 300 mg g−1, while spent adsorbents were treated with hydrogen peroxide. The resulting oxidation of Brønsted –SH sites produced catalysts for carbohydrate dehydration-oxidation reactions [84].
The Cr adsorbents can be reused as highly efficient catalysts for treating sulphur-containing volatile organic compounds (methyl mercaptan, CH3SH). The adsorbed Cr is calcinated and reduced to chromium (III) oxide and immobilised as a chromium(III) sulphide, eliminating methyl mercaptan. Although Cr offers active sites for catalysis, excessive Cr(VI) levels promote coke buildup and deactivation [85].
The review by the Kandasubramanian group explores the reuse of spent adsorbents as antimicrobial and construction materials, catalysts, and fertilisers [86]. For the investigated post-adsorption application, textural properties and surface functionalities are selected as dominant features governing efficiency. Cement formation is seen as one of the routes for immobilisation of toxic metals, while transition metal loading benefits subsequent catalytic activity. Enhancing surface development with thermal treatment may add a possibility for further adsorption.
For soil-compatible adsorbents, like biochars derived from spent adsorbents, there is an interesting option for additives or fertilisers. If enriched with nutrients removed from water sources, biochar may act as a soil amendment [87]. Added value may be seen in altering cation sorption and release from biochar and soil particles [88]. The possibility of this usage from spent adsorbents relies on the acidity of the soil and whether cationic species would readily emerge from loaded adsorbents due to the negatively charged carbon surface over a wide pH range. Interestingly, several studies addressed the performance of spent adsorbents and found them to be efficient additives to agricultural production [89,90]. Usage in soil is evidenced by the Siddique group review covering fertilisers, composting, and other agricultural features [91]. The perspective of this use must be accompanied by semi-empirical and DFT methods to assess adsorption energy, model interaction, and the foreseen release rate of nutrients.
Applications gaining affirmation in repurposing are those related to energy conversion and storage. Among these, supercapacitors, fuel cells, and batteries are the most studied and are discussed in more detail further. Their typical electrochemical responses are illustrated in Figure 1.
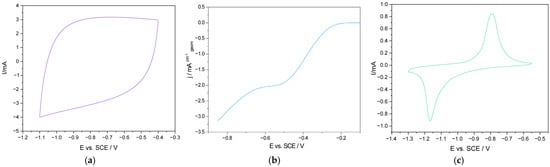
Figure 1.
Responses of repurposed materials in energy storage applications. (a) Typical slightly distorted rectangular CV shape of a supercapacitor type material; (b) double stepped oxygen reduction LSV curve attributed to partial (2e−) and full (4e−) mechanisms; (c) CV curve with protruding maxima associated with Faradaic processes connected to ion de/intercalation.
3.2.1. Adsorbents to Materials in Supercapacitors
Thermally treated carbonaceous adsorbents often exhibit hierarchically porous structures with abundant micropores and mesopores, which promote ion diffusion and charge storage [92]. Additionally, the retention or incorporation of heteroatoms (N, O, S) during pyrolysis can introduce surface functional groups that enhance catalytic activity, especially for oxygen reduction reactions (ORR) in fuel cells [93]. Furthermore, the increased thermal stability of such composites makes them suitable for repeated energy cycling. Therefore, thermally modified spent adsorbents serve not only as recyclable materials but also as high-value composites for sustainable energy technologies.
Porous carbons are promising for supercapacitor anodes, and a secondary high-temperature carbonisation was used to gain a surface area of over 3300 m2/g, with a conductivity of 69 S/m. The resulting capacitance reached 200 F/g at 1 A/g, while the symmetric supercapacitor device achieved 43.2 Wh/kg at 625 W/kg [94]. Dried digested sludge, a by-product of anaerobic digestion, can serve as an adsorbent for pollutants such as methylene blue, which contains heteroatoms N, S, and Cl [95]. Consequently, it is of interest to explore its treatment via pyrolysis/activation to produce self-doped porous carbon suitable for use as supercapacitor electrodes. The resulting material features O- and N-doped hierarchical porosity and a high surface area (2100 m2/g) with partial graphitisation, enabling efficient ion transport and enhanced conductivity. Resulting electrodes show capacitance of 245 F/g at 1 A/g, with cycling stability over 98% after 2000 cycles [94]. Electrode materials from electric double-layer capacitors generate activated carbon waste. Recycling this waste offers a sustainable approach, possibly through steam physical activation. The removal of residual electrolytes and organics while maintaining structural integrity with enhanced porosity leads to 103 F/g at 0.5 A/g. A strong rate capability and cycling stability outperform calcined carbon [96].
The carbonisation of acetamiprid-loaded zeolitic adsorbents under argon at 700 °C can produce nitrogen-rich carbon composites for supercapacitor electrodes [10]. The resulting nitrogen-doped carbon layer, bonded via C-Si linkage, is a result of a two-stage process. A stable Faradaic capacitance, with values up to 610 F/g based on the carbon adlayer, was achieved, along with excellent cycling stability over 1000 cycles. To define a route for converting pollutant-loaded adsorbents into high-value electroactive materials, a detailed analysis of steps in thermal treatment coupled with mass spectrometry in different atmospheres can resolve the composition of the layer that remains attached to the adsorbent in each step [97]. If an adsorbent can act as a catalyst in the thermal degradation of pollutants, both regeneration and repurposing are facilitated. In the case of Y zeolite loaded with acetamiprid, it was reported that low-temperature regeneration at 300 °C is highly efficient. The zeolite-catalysed process lowers degradation temperatures, enabling efficient recovery and retaining 65% capacity after 10 cycles. A final 700 °C treatment fully restores performance [97], while pyrolysis leads to an electroactive composite [10].
The effective route for converting metal-saturated adsorbents into functional electrode materials may find its way into energy storage applications. A favourable example of electrochemical repurposing involves the hydrothermal treatment of Ni-loaded biochar used for water remediation. This material, prepared at 90 °C, achieved a capacitance of 387 F/g while maintaining 79% of capacity after cycling. The enhanced performance was attributed to the formation of flower-like nickel hydroxide structures dispersed on carbon and to Ni-C interactions that improved electrochemical activity and thermal stability [98]. A recent study demonstrated that sewage sludge contaminated with Cu(II), obtained after flocculation using a carboxyl-functionalised starch derivative, can be directly transformed into Cu-doped carbons through carbonisation. The capacitance of the resulting materials showed a direct correlation with the Cu(II) removal efficiency, which reached up to 99.5% at an optimal flocculant dosage. The carbonised product achieved a specific capacitance of 390 F/g at 1 A/g and retained over 96% of its capacity after 2500 cycles [99].
Shoeb et al. [100] repurposed Sb-enriched waste adsorbent (RGO-Au-Ag2O/PIn@SbOx) in a symmetric supercapacitor device, which provided an energy density of 40.66 Wh/kg with a respectable power density of 1000 W/kg. The prepared device worked for over 12,000 cycles with 82% capacitance retention.
Similarly, Mashkoor et al. [101] used functionalised CNT decorated with Sm/Co-LDH for antimony removal. Expanded adsorbent was electrochemically tested as a supercapacitor material and displayed extraordinary specific capacitances of 850 F/g at 2 A/g. A prepared symmetric device based on fCNT-Sm/Co-LDH@SbOx reached an energy density of 78 Wh/kg with a power density of 833 W/kg. Even more remarkably, 92% capacitance retention after 10,000 cycles was reported.
3.2.2. Adsorbents to Materials for Fuel Cells
Besides the supercapacitor electrode, this approach can be used in the preparation of materials for the ORR process. Natural silicates are often applied for the adsorption of organic pollutants, such as methylene blue dye. Bentonite stands out as the best adsorbent, and thermal treatment of dye-loaded adsorbent at 700 °C in an inert atmosphere was applied. The nitrogen-doped carbon/silicate composite acted as an oxygen reduction catalyst in alkaline media via a two-electron pathway, generating HO2−, which degraded 70% of MB in 1 h [102]. The thermal treatment/electrodegradation approach converts spent adsorbents into reusable, functional materials for environmental remediation.
Zaher et al. [103] used the spent adsorbents (Co-Fe LDH/CV and Co-Fe LDH/Cellulose/CV) as catalysts for the half-reaction of electro-oxidation in direct-methanol fuel cells. In part due to stability in an alkaline medium (NaOH), the waste material was repurposed as an active catalyst towards methanol oxidation. Co-Fe LDH/CV displayed a remarkable 75% efficiency and a current density of 76 mA/cm2 at 50 mV/s in 1 M methanol.
Similarly, Abdel-Hady et al. [104] showed that calcination at different temperatures of MB-saturated Zn-Co-Fe/LDH adsorbent leads to catalytically active materials. Calcination at 400 °C led to the largest pore radius (6.4 nm) and offered the best catalytic performance for methanol oxidation, reaching current densities of 41 mA/cm2 at 50 mV/s and 3 M methanol. A study showed the viability of repurposing spent LDH residue as a catalyst in direct methanol fuel cells (DMFCs).
3.2.3. Adsorbents to Materials in Batteries
An interesting example of repurposing spent adsorbents was reported in a study where an algae-derived adsorbent, previously saturated with lead ions, was successfully utilised as an anode material for lithium-ion batteries. After a simple one-step alkali treatment, the material exhibited significantly improved electrochemical performance, achieving a reversible capacity of 358 mAh g−1 after 100 cycles. This enhancement was attributed to the increased surface area and improved Li+ diffusion and electron transport [105].
Another study demonstrated the development of nanolignocellulose-based hydrogels with high adsorption capacities for Mn(II), Zn(II), and Pb(II), attributed to their 3D porous structure and abundant hydroxyl groups. The adsorption capacities reached 78 mg/g for Mn(II), 89 mg/g for Zn(II), and 123 mg/g for Pb(II). After metal adsorption, the spent hydrogels were transformed via in situ deposition and high-temperature carbonisation, resulting in carbon aerogels embedded with metal oxides. Among these, the manganese-containing material exhibited the best electrochemical performance, with a reversible lithium-ion storage capacity of 436 mAh/g. The porous carbon structure acted as a buffer matrix, enhancing the lithium storage behaviour [106].
In the study by Ma and Liu, low-cost Fe3O4/carbon composites synthesised from orange peel were employed as adsorbents for Cr(VI) from contaminated water. The spent adsorbents, loaded with Cr(VI), were then repurposed as anode materials for potassium-ion batteries. The composites exhibited a K-storage capacity exceeding 300 mAh/g at a current density of 0.1 A/g, with performance improving alongside the Fe3O4 content due to enhanced chemisorption of Cr(VI). Additionally, the carbon matrix provided good electrical conductivity, resulting in excellent rate capability [107].
Adsorption of organic dyes has been shown to improve the electrochemical performance of Fe3O4-polypyrrole nanocomposites used as supercapacitor electrodes. While the pure nanocomposite exhibited higher capacitance, dye-modified versions demonstrated superior cyclic stability, with the methyl orange-loaded composite retaining 85% of its initial capacitance after 1000 cycles [108].
4. Perspectives
Let us return to the beginning—to the fundamental interactions. Functional and sustainable adsorption can only serve as an initial step in the creation of advanced materials tailored for cutting-edge energy solutions. Achieving this requires a synergy between theoretical modelling and experimental design. The theoretical chemistry methods often investigate adsorbate–adsorbent interactions to resolve the mechanism and enable adsorbent optimisation [3,109,110] with typical findings presented in Figure 2 (unpublished results). An interaction of PFAS (PFOA) with the graphene surface is modelled using DFTB+ software v23.1 in Figure 2a. It is based on an approximate version of Density Functional Theory (DFT). The appropriate Slater–Koster files were used for creating the Hamiltonian. Similarly, pesticide encapsulation in zeolite FAU voids enables efficient investigation of the adsorption mechanism targeting polar groups, see Figure 2b. Interactions between the acetamiprid molecule and the FAU zeolite fragment were modelled using the semiempirical programme MOPAC.
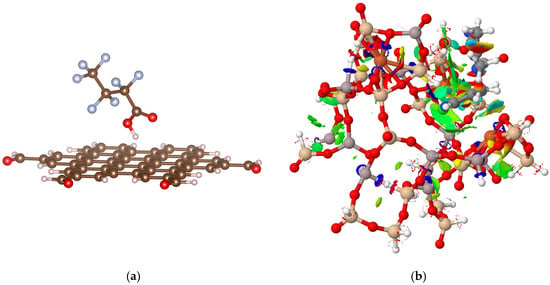
Figure 2.
(a) An interaction of PFOA with the graphene surface and (b) acetamiprid pesticide within the FAU zeolite cage.
The study by Zhang et al. [111] demonstrates how DFT can clarify the deactivation mechanisms of porous carbons, influenced by their pore structure and functional groups. Adsorption technology based on porous carbon offers a promising and cost-effective method for recovering or removing valuable volatile organic compounds (VOCs). Combining experimental investigations with DFT examines the adsorption and regeneration of ethyl acetate on carbon-fibre adsorbents. The DFT revealed that suitable basic functional groups enhance acetate adsorption, while physisorption in micropores primarily contributes to adsorption capacity. A strong chemisorption hinders complete regeneration, with only 81% capacity retention after six cycles.
Liu et al. [112]. used DFT to investigate the interaction mechanism between ciprofloxacin and durian peel biochars. Biochar functionalised with oxygen groups exhibited significantly higher adsorption energy compared to pristine biochar. This enhancement is mainly due to π-π interactions and hydrogen bonding between the oxygenated groups and ciprofloxacin. An increasing amount of oxygen-containing functional groups on biochar can improve the adsorption capacity for drugs.
Although still relatively rare, semi-empirical and density functional theory (DFT) methods have begun to explore the structural, electronic, and chemical properties of spent or degraded adsorbents. An example is the work of Shi et al. [113], where polyaniline (PANI) adsorbents were used for metal removal from wastewater. This study employs DFT to examine the structural and electronic properties of PANI adsorbents loaded with transition metals after use. Bonding analyses, including Wiberg bond order, IRI, and LOL, demonstrate strong coordination between metal ions and imine nitrogen atoms, supporting the stability of the spent material. Electrostatic potential maps of PANI/metal models show only positive potentials, due to positive charge delocalisation and redistribution. These materials can be reutilised for the adsorption of anionic dyes and pharmaceutical contaminants. This conclusion is supported by an experimental study of Fe(III)-loaded PANI. Fukui function and ADCH charge analyses reveal that electron transfer and the presence of reducing groups enhance chemical robustness and resistance to oxidation. Additional evaluations of conductivity, excited states, and surface charge suggest promising applications of spent PANI adsorbents in energy storage, catalysis, and environmental remediation.
While numerous studies investigate the adsorption and regeneration performance of materials using a combination of experimental techniques and DFT calculations, most of them focus their theoretical modelling on pristine surfaces. DFT is commonly employed to elucidate adsorption mechanisms, active sites, or the influence of functional groups on adsorbents. However, there is a notable lack of studies where DFT calculations are performed on spent or repurposed adsorbents, materials that have undergone structural or chemical changes due to pollutant exposure. This represents a critical gap, as understanding the altered electronic and adsorption properties of used adsorbents could provide valuable insights into their reuse potential and long-term performance. Comprehensive theoretical studies on versatile saturated adsorbents are necessary for a deeper understanding of the material functionalities and their efficient repurposing, especially for energy-related applications.
5. Conclusions
Ultimately, both regeneration and recycling offer meaningful contributions to sustainable waste handling. The decision to employ one approach over the other depends on multiple factors, such as the physical and chemical characteristics of adsorbents, the types of contaminants involved, resource availability, and environmental priorities. An evaluation that considers technical feasibility, environmental impact, and cost-effectiveness is essential to selecting the most appropriate strategy. Continued research and innovation are needed to improve the sustainability and scalability of both approaches, leading to more environmentally responsible waste management practices. To assist this route, semi-empirical and DFT methods need to be employed, intrinsic interactions in the adsorption system resolved, and then novel applications of spent adsorbent sought.
Among adsorbent materials, the most promising for repurposing in electrochemical applications are those that enable tailored porosity, surface chemistry, and redox properties. These criteria can be fulfilled by a range of advanced materials, including zeolite-templated carbons, MOF- and COF-derived carbons, as well as layered and framework-derived mixed oxides. Although some of them are currently expensive, they may nevertheless represent a viable long-term solution if effectively reused and repurposed.
Author Contributions
Conceptualization, M.M.-R. and D.B.-B.; methodology, A.J.; software, B.N.V.; validation, N.G., S.U.-M. and A.J.L.; formal analysis, A.J.L., A.J. and M.R.; investigation, A.J. and M.R.; data curation, A.J. and M.R.; writing—original draft preparation, N.G., S.U.-M. and A.J.L.; writing—review and editing, A.J., B.N.V., M.M.-R. and D.B.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (grant numbers 451-03-137/2025-03/200146, 451-03-136/2025-03/200146, 451-03-137/2025-03/200161, and AIRES project no. 003417039). and supported by the Science Fund of the Republic of Serbia, GRANT No 17990, Advanced electrochemical treatment of PFAS contaminated water: Novel Materials and Mechanisms—ALTER.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Sangmesh, B.; Karuppasamy, K.S.K. Renewable and Sustainable Clean Energy Development and Impact on Social, Economic, and Environmental Health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Farasati Far, B.; Rabiee, N.; Iravani, S. Environmental Implications of Metal–Organic Frameworks and MXenes in Biomedical Applications: A Perspective. RSC Adv. 2023, 13, 34562–34575. [Google Scholar] [CrossRef]
- Milojević-Rakić, M.; Gavrilov, N.; Janošević Ležaić, A.; Uskoković-Marković, S.; Nedić Vasiljević, B.; Bajuk-Bogdanović, D. Complementary: Green Catalysis over Red Soil for Pollutant Removal. Appl. Clay Sci. 2024, 262, 107601. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Muliwa, A.M.; Makgato, S.S.; Onwudiwe, D.C. Research Progress on Green Adsorption Process for Water Pollution Control Applications. Hybrid Adv. 2025, 8, 100338. [Google Scholar] [CrossRef]
- Milojević-Rakić, M.; Bajuk-Bogdanović, D. (Eds.) Zeolites and Porous Materials: Insight into Catalysis and Adsorption Processes; MDPI: Basel, Switzerland, 2023; ISBN 978-3-0365-8155-2. [Google Scholar]
- Busetty, S. Environmental Treatment Technologies: Adsorption. In Handbook of Environmental Materials Management; Springer International Publishing: Cham, Switzerland, 2019; pp. 1367–1397. [Google Scholar]
- Fouda-Mbanga, B.G.; Onotu, O.; Tywabi-Ngeva, Z. Advantages of the Reuse of Spent Adsorbents and Potential Applications in Environmental Remediation: A Review. Green Anal. Chem. 2024, 11, 100156. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, X.; Dong, H.; Liang, Y.; Huo, Z.; Qian, K.; Yang, F. Applications of Ionic Liquids in the Field of Agriculture: A Review. Agriculture 2023, 13, 2279. [Google Scholar] [CrossRef]
- Roy, S.; Ahmaruzzaman, M. Ionic Liquid Based Composites: A Versatile Materials for Remediation of Aqueous Environmental Contaminants. J. Environ. Manag. 2022, 315, 115089. [Google Scholar] [CrossRef]
- Popadić, D.; Krstić, J.; Janošević Ležaić, A.; Popović, M.; Milojević-Rakić, M.; Ignjatović, L.; Bajuk-Bogdanović, D.; Gavrilov, N. Acetamiprid’s Degradation Products and Mechanism: Part II—Inert Atmosphere and Charge Storage. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 308, 123772. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.; Chen, D. Adsorbent Materials for Ammonium and Ammonia Removal: A Review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva, A.H., Jr.; da Silva, L.J.S.; da Silva, L.; de Souza, A.A.U.; Ulson, S.M.D.A.G.; Borba, F.H.; da Silva, A. A Comprehensive Guide for Characterization of Adsorbent Materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Pellenz, L.; da Silva, L.J.S.; Mazur, L.P.; Figueiredo, G.M.; de Borba, F.H.; Ulson de Souza, A.A.; Guelli Ulson de Souza, S.M.A.; da Silva, A. Functionalization of Graphene with Nitrogen-Based Groups for Water Purification via Adsorption: A Review. J. Water Process Eng. 2022, 48, 102873. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A Review on the Influence of Chemical Modification on the Performance of Adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V.L. Competitive Adsorption in Natural Water: Role of Activated Carbon Pore Size. Water Res. 1999, 33, 1209–1219. [Google Scholar] [CrossRef]
- Hammer, M.U.; Anderson, T.H.; Chaimovich, A.; Shell, M.S.; Israelachvili, J. The Search for the Hydrophobic Force Law. Faraday Discuss. 2010, 146, 299. [Google Scholar] [CrossRef] [PubMed]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Sannino, F.; Ruocco, S.; Marocco, A.; Esposito, S.; Pansini, M. Cyclic Process of Simazine Removal from Waters by Adsorption on Zeolite H-Y and Its Regeneration by Thermal Treatment. J. Hazard. Mater. 2012, 229–230, 354–360. [Google Scholar] [CrossRef]
- Milojević-Rakić, M.; Popadić, D.; Janošević Ležaić, A.; Jevremović, A.; Nedić Vasiljević, B.; Uskoković-Marković, S.; Bajuk-Bogdanović, D. MFI, BEA and FAU Zeolite Scavenging Role in Neonicotinoids and Radical Species Elimination. Environ. Sci. Process Impacts 2022, 24, 265–276. [Google Scholar] [CrossRef]
- Andrunik, M.; Skalny, M.; Gajewska, M.; Marzec, M.; Bajda, T. Comparison of Pesticide Adsorption Efficiencies of Zeolites and Zeolite-Carbon Composites and Their Regeneration Possibilities. Heliyon 2023, 9, e20572. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Liu, X.; Ding, J.; Zhang, N.; Li, Z.; Zhao, M.; Meng, F.; Meng, Z. Removal of Pesticides by Layered Double Hydroxide Modified Different Clay Minerals and Site Energy Analysis. Chem. Eng. Sci. 2024, 287, 119803. [Google Scholar] [CrossRef]
- Gomez-Maldonado, D.; Erramuspe, I.B.V.; Peresin, M.S. Natural Polymers as Alternative Adsorbents and Treatment Agents for Water Remediation. Bioresources 2019, 14, 10093–10160. [Google Scholar] [CrossRef]
- Ahmad, F.A. The Use of Agro-Waste-Based Adsorbents as Sustainable, Renewable, and Low-Cost Alternatives for the Removal of Ibuprofen and Carbamazepine from Water. Heliyon 2023, 9, e16449. [Google Scholar] [CrossRef] [PubMed]
- Hama Aziz, K.H.; Kareem, R. Recent Advances in Water Remediation from Toxic Heavy Metals Using Biochar as a Green and Efficient Adsorbent: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100495. [Google Scholar] [CrossRef]
- Mohtasim, M.S.; Das, B.K. Advancement of Biocarbon Materials in Sustainable Thermal and Electrochemical Energy Storage with Future Outlooks. Renew. Sustain. Energy Rev. 2025, 218, 115779. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of Adsorption and Functionalization of Biochar for Pesticides: A Review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Wang, J.; Zhao, X.; Zhao, Y.; Qian, J.; Wang, T. Pyrolysis and Hydrothermal Carbonization of Biowaste: A Comparative Review on the Conversion Pathways and Potential Applications of Char Product. Sustain. Chem. Pharm. 2023, 33, 101106. [Google Scholar] [CrossRef]
- Stojanović, J.; Milojević-Rakić, M.; Bajuk-Bogdanović, D.; Ranđelović, D.; Otašević, B.; Malenović, A.; Janošević Ležaić, A.; Protić, A. Carbonization of Invasive Plant Species—Novel Route for Removal of Active Pharmaceutical Ingredients via Adsorption. Processes 2024, 12, 2149. [Google Scholar] [CrossRef]
- Pathy, A.; Pokharel, P.; Chen, X.; Balasubramanian, P.; Chang, S.X. Activation Methods Increase Biochar’s Potential for Heavy-Metal Adsorption and Environmental Remediation: A Global Meta-Analysis. Sci. Total Environ. 2023, 865, 161252. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Insights into Chemical Regeneration of Activated Carbon for Water Treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]
- Zieliński, B.; Miądlicki, P.; Przepiórski, J. Development of Activated Carbon for Removal of Pesticides from Water: Case Study. Sci. Rep. 2022, 12, 20869. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.A.; Salimi Beni, A.; Azhdarpoor, A.; Moeini, Z. The Application of Granular and Biological Activated Carbon Columns in Removal of Organochlorine and Organophosphorus Pesticides in a Water Treatment Plant. J. Water Process Eng. 2023, 56, 104383. [Google Scholar] [CrossRef]
- Jin, P.; Jin, X.; Wang, X.; Feng, Y.; Wang, X.C. Biological Activated Carbon Treatment Process for Advanced Water and Wastewater Treatment. In Biomass Now—Cultivation and Utilization; InTech: London, UK, 2013. [Google Scholar]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent Advances in Application of Metal-Organic Frameworks (MOFs) as Adsorbent and Catalyst in Removal of Persistent Organic Pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah, I. Applications of MOFs as Adsorbents in Water Purification: Progress, Challenges and Outlook. Curr. Opin. Environ. Sci. Health 2022, 26, 100335. [Google Scholar] [CrossRef]
- Sağlam, S.; Türk, F.N.; Arslanoğlu, H. Use and Applications of Metal-Organic Frameworks (MOF) in Dye Adsorption: Review. J. Environ. Chem. Eng. 2023, 11, 110568. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Q.; Huang, B.; Qi, J.; Wang, R.; Zhou, Q.; Chen, Q.; Zhu, L.; Jin, J.; Kong, Y. Removal Performance and Adsorption Kinetics of Dyes by a Co-Based Metal Organic Framework. Microporous Mesoporous Mater. 2023, 360, 112665. [Google Scholar] [CrossRef]
- Mubarak, A.S.; Salih, S.S.; Kadhom, M.; Ghosh, T.K. Removal of Heavy Metals from Contaminated Water Using Metal-Organic Frameworks (MOFs): A Review on Techniques and Applications. Mater. Sci. Eng. B 2025, 315, 118105. [Google Scholar] [CrossRef]
- Li, J.; Lv, Q.; Bi, L.; Fang, F.; Hou, J.; Di, G.; Wei, J.; Wu, X.; Li, X. Metal-Organic Frameworks as Superior Adsorbents for Pesticide Removal from Water: The Cutting-Edge in Characterization, Tailoring, and Application Potentials. Coord. Chem. Rev. 2023, 493, 215303. [Google Scholar] [CrossRef]
- Rahmani, A.; Shabanloo, A.; Zabihollahi, S.; Salari, M.; Leili, M.; Khazaei, M.; Alizadeh, S.; Nematollahi, D. Facile Fabrication of Amino-Functionalized MIL-68(Al) Metal–Organic Framework for Effective Adsorption of Arsenate (As(V)). Sci. Rep. 2022, 12, 11865. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.-D.; Li, D.-D.; Leng, F.; Yu, J.-H.; Jia, M.-J.; Xu, J.-Q. A Metal–Organic Framework with Rich Accessible Nitrogen Sites for Rapid Dye Adsorption and Highly Efficient Dehydrogenation of Formic Acid. Dalton Trans. 2022, 51, 8695–8704. [Google Scholar] [CrossRef] [PubMed]
- Jevremović, A.; Savić, M.; Janošević Ležaić, A.; Krstić, J.; Gavrilov, N.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Ćirić-Marjanović, G. Environmental Potential of Carbonized MOF-5/PANI Composites for Pesticide, Dye, and Metal Cations—Can They Actually Retain Them All? Polymers 2023, 15, 4349. [Google Scholar] [CrossRef]
- Faheem, M.; Azher Hassan, M.; Du, J.; Wang, B. Harnessing Potential of Smart and Conventional Spent Adsorbents: Global Practices and Sustainable Approaches through Regeneration and Tailored Recycling. Sep. Purif. Technol. 2025, 354, 128907. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, N.; Dalal, A.; Panghal, P.; Sharma, A.K.; Kumar, S. Cutting-Edge Regeneration Technologies for Saturated Adsorbents: A Systematic Review on Pathways to Circular Wastewater Treatment System. Environ. Monit. Assess. 2025, 197, 215. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Choi, J.W.; Mtei, K.M.; Hosseini-Bandegharaei, A.; Sillanpää, M. Adsorption and Desorption Processes of Toxic Heavy Metals, Regeneration and Reusability of Spent Adsorbents: Economic and Environmental Sustainability Approach. Adv. Colloid. Interface Sci. 2024, 329, 103196. [Google Scholar] [CrossRef]
- Li, Q.; Qi, Y.; Gao, C. Chemical Regeneration of Spent Powdered Activated Carbon Used in Decolorization of Sodium Salicylate for the Pharmaceutical Industry. J. Clean. Prod. 2015, 86, 424–431. [Google Scholar] [CrossRef]
- Ghani, A.A.; Shahzad, A.; Moztahida, M.; Tahir, K.; Jeon, H.; Kim, B.; Lee, D.S. Adsorption and Electrochemical Regeneration of Intercalated Ti3C2Tx MXene for the Removal of Ciprofloxacin from Wastewater. Chem. Eng. J. 2021, 421, 127780. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye Using Tectona Grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Liu, S.; Wang, C. Enhanced Removal of Chromium(III) for Aqueous Solution by EDTA Modified Attapulgite: Adsorption Performance and Mechanism. Sci. Total Environ. 2020, 720, 137391. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-J.; Lin, H.-C.; Yu, W.-T.; Chern, J.-M. Chemical Regeneration of Activated Carbon Used for Dye Adsorption. J. Taiwan Inst. Chem. Eng. 2011, 42, 305–311. [Google Scholar] [CrossRef]
- Cooney, D.O.; Nagerl, A.; Hines, A.L. Solvent Regeneration of Activated Carbon. Water Res. 1983, 17, 403–410. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Soltaninejad, Y.; Esmaeili, S.; Babaei, A.A. Preparation, Characterization, and Application of Modified Magnetic Biochar for the Removal of Benzotriazole: Process Optimization, Isotherm and Kinetic Studies, and Adsorbent Regeneration. Water Sci. Technol. 2022, 85, 3036–3054. [Google Scholar] [CrossRef]
- Hamad, K.I.; Humadi, J.I.; Abdulkareem, H.A.; Al-Salihi, S.; Farhan, O.I. Efficient Immobilization of Acids into Activated Carbon for High Durability and Continuous Desulfurization of Diesel Fuel. Energy Sci. Eng. 2023, 11, 3662–3679. [Google Scholar] [CrossRef]
- Chiu, C.-A.; Hristovski, K.; Huling, S.; Westerhoff, P. In-Situ Regeneration of Saturated Granular Activated Carbon by an Iron Oxide Nanocatalyst. Water Res. 2013, 47, 1596–1603. [Google Scholar] [CrossRef]
- Toledo, L.C.; Silva, A.C.B.; Augusti, R.; Lago, R.M. Application of Fenton’s Reagent to Regenerate Activated Carbon Saturated with Organochloro Compounds. Chemosphere 2003, 50, 1049–1054. [Google Scholar] [CrossRef]
- Sánchez-Yepes, A.; Santos, A.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T.; Lorenzo, D. Sustainable Reuse of Toxic Spent Granular Activated Carbon by Heterogeneous Fenton Reaction Intensified by Temperature Changes. Chemosphere 2023, 341, 140047. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Yang, Z.; Tan, D. Regeneration Performance of Spent Granular Activated Carbon for Tertiary Treatment of Dyeing Wastewater by Fenton Reagent and Hydrogen Peroxide. J. Mater. Cycles Waste Manag. 2017, 19, 256–264. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Nath, K.; Bhakhar, M.S. Microbial Regeneration of Spent Activated Carbon Dispersed with Organic Contaminants: Mechanism, Efficiency, and Kinetic Models. Environ. Sci. Pollut. Res. 2011, 18, 534–546. [Google Scholar] [CrossRef]
- Gong, X.; Tian, W.; Wang, L.; Bai, J.; Qiao, K.; Zhao, J. Biological Regeneration of Brewery Spent Diatomite and Its Reuse in Basic Dye and Chromium (III) Ions Removal. Process Saf. Environ. Prot. 2019, 128, 353–361. [Google Scholar] [CrossRef]
- Noor, R.; Septiyani, E.; Zevi, Y.; Arifianingsih, N.N. Bioregeneration of Saturated Natural Mordenite to Reduce Iron and Manganese in Groundwater. E3S Web Conf. 2020, 148, 02003. [Google Scholar] [CrossRef]
- Khataee, A.; Kayan, B.; Kalderis, D.; Karimi, A.; Akay, S.; Konsolakis, M. Ultrasound-Assisted Removal of Acid Red 17 Using Nanosized Fe3O4-Loaded Coffee Waste Hydrochar. Ultrason. Sonochem. 2017, 35, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, C.; Su, X.; Xue, X. Regeneration of Spent Bleaching Clay by Ultrasonic Irradiation and Its Application in Methylene Blue Adsorption. Clay Min. 2020, 55, 24–30. [Google Scholar] [CrossRef]
- Lim, J.-L.; Okada, M. Regeneration of Granular Activated Carbon Using Ultrasound. Ultrason. Sonochem. 2005, 12, 277–282. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhao, Z.; Musoke, F.S.N.; Wu, X. Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment. Microorganisms 2022, 10, 1593. [Google Scholar] [CrossRef]
- Shi, K.; Xu, Z.; Wang, Y.; Fu, W.; Chen, B. Study on Regeneration Characteristics of Granular Activated Carbon Using Ultrasonic and Thermal Methods. Environ. Sci. Pollut. Res. 2024, 31, 26580–26591. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Li, X.; Zhou, Z.; Wei, B. Ultrasonic–Thermal Regeneration of Spent Powdered Activated Carbon. Sustainability 2023, 15, 9060. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, T.; Zhang, G.; Zheng, Y.; Wang, P. Effect and Mechanism of Microwave-Activated Ultraviolet-Advanced Oxidation Technology for Adsorbent Regeneration. Environ. Sci. Pollut. Res. 2018, 25, 290–298. [Google Scholar] [CrossRef]
- Soker, O.; Hao, S.; Trewyn, B.G.; Higgins, C.P.; Strathmann, T.J. Application of Hydrothermal Alkaline Treatment to Spent Granular Activated Carbon: Destruction of Adsorbed PFASs and Adsorbent Regeneration. Environ. Sci. Technol. Lett. 2023, 10, 425–430. [Google Scholar] [CrossRef]
- Gagliano, E.; Falciglia, P.P.; Zaker, Y.; Karanfil, T.; Roccaro, P. Microwave Regeneration of Granular Activated Carbon Saturated with PFAS. Water Res. 2021, 198, 117121. [Google Scholar] [CrossRef]
- Didenko, T.; Lau, A.; Purohit, A.L.; Feng, J.; Pinkard, B.; Ateia, M.; Novosselov, I.V. Regeneration of PFAS-Laden Granular Activated Carbon by Modified Supercritical CO2 Extraction. Chemosphere 2025, 370, 143986. [Google Scholar] [CrossRef]
- Smith, S.J.; Lauria, M.; Ahrens, L.; McCleaf, P.; Hollman, P.; Bjälkefur Seroka, S.; Hamers, T.; Arp, H.P.H.; Wiberg, K. Electrochemical Oxidation for Treatment of PFAS in Contaminated Water and Fractionated Foam—A Pilot-Scale Study. ACS EST Water 2023, 3, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Zeidabadi, F.A.; Esfahani, E.B.; McBeath, S.T.; Mohseni, M. Managing PFAS Exhausted Ion-Exchange Resins Through Effective Regeneration/Electrochemical Process. Water Res. 2024, 255, 121529. [Google Scholar] [CrossRef] [PubMed]
- Sonmez Baghirzade, B.; Zhang, Y.; Reuther, J.F.; Saleh, N.B.; Venkatesan, A.K.; Apul, O.G. Thermal Regeneration of Spent Granular Activated Carbon Presents an Opportunity to Break the Forever PFAS Cycle. Environ. Sci. Technol. 2021, 55, 5608–5619. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Picheau, E.; Amar, S.; Derré, A.; Pénicaud, A.; Hof, F. An Introduction to the Combustion of Carbon Materials. Chem. A Eur. J. 2022, 28, e202200117. [Google Scholar] [CrossRef]
- Xiao, F.; Sasi, P.C.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Soli, D. Thermal Stability and Decomposition of Perfluoroalkyl Substances on Spent Granular Activated Carbon. Environ. Sci. Technol. Lett. 2020, 7, 343–350. [Google Scholar] [CrossRef]
- Velempini, T.; Ahamed, M.E.H.; Pillay, K. Heavy-Metal Spent Adsorbents Reuse in Catalytic, Energy and Forensic Applications- a New Approach in Reducing Secondary Pollution Associated with Adsorption. Results Chem. 2023, 5, 100901. [Google Scholar] [CrossRef]
- Guo, H.; Inoue, Y.; Isoda, Y.; Honma, T.; Smith, R.L. Upcycling of Spent Functional Biocarbon Adsorbents to Catalysts for the Conversion of C5/C6 Carbohydrates into Platform Chemicals. RSC Sustain. 2023, 1, 554–562. [Google Scholar] [CrossRef]
- He, D.; Zhang, L.; Zhao, Y.; Mei, Y.; Chen, D.; He, S.; Luo, Y. Recycling Spent Cr Adsorbents as Catalyst for Eliminating Methylmercaptan. Environ. Sci. Technol. 2018, 52, 3669–3675. [Google Scholar] [CrossRef]
- Nighojkar, A.; Sangal, V.K.; Dixit, F.; Kandasubramanian, B. Sustainable Conversion of Saturated Adsorbents (SAs) from Wastewater into Value-Added Products: Future Prospects and Challenges with Toxic per- and Poly-Fluoroalkyl Substances (PFAS). Environ. Sci. Pollut. Res. 2022, 29, 78207–78227. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar Application to Low Fertility Soils: A Review of Current Status, and Future Prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar Amendment Improves Crop Production in Problem Soils: A Review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Vigneshwar, S.S.; Swetha, A. Sustainable and Eco-Friendly Approach for Phosphorus Recovery from Wastewater by Hydrothermally Carbonized Microalgae: Study on Spent Bio-Char as Fertilizer. J. Water Process Eng. 2020, 38, 101567. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.-L. Hierarchically Structured Porous Materials: Synthesis Strategies and Applications in Energy Storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Tricase, A.; Muhyuddin, M.; Erable, B.; Atanassov, P.; Pant, D.; Santoro, C.; Bollella, P. Bio- and Electrocatalysts for Oxygen Reduction Reaction in Neutral Media: From Mechanisms to Practical Applications. J. Power Sources 2025, 646, 237267. [Google Scholar] [CrossRef]
- Chi, W.; Wang, G.; Qiu, Z.; Li, Q.; Xu, Z.; Li, Z.; Qi, B.; Cao, K.; Chi, C.; Wei, T.; et al. Secondary High-Temperature Treatment of Porous Carbons for High-Performance Supercapacitors. Batteries 2023, 10, 5. [Google Scholar] [CrossRef]
- Aoulad El Hadj Ali, Y.; Ahrouch, M.; Ait Lahcen, A.; Demba N’diaye, A.; El Yousfi, F.; Stitou, M. Dried Sewage Sludge as an Efficient Adsorbent for Pollutants: Cationic Methylene Blue Removal Case Study. Nanotechnol. Environ. Eng. 2021, 6, 17. [Google Scholar] [CrossRef]
- Dong, S.; Li, Z.; Yi, Z.; Li, H.; Tian, Y.; Liu, S. Recycling of Activated Carbons from Spent Supercapacitors to Refabricate Improved Supercapacitors. J. Energy Storage 2024, 102, 114182. [Google Scholar] [CrossRef]
- Popadić, D.; Gavrilov, N.; Krstić, J.; Nedić Vasiljević, B.; Janošević Ležaić, A.; Uskoković-Marković, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. Spectral Evidence of Acetamiprid’s Thermal Degradation Products and Mechanism. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 301, 122987. [Google Scholar] [CrossRef]
- Li, D.; Ma, J.; Xu, H.; Xu, X.; Qiu, H.; Cao, X.; Zhao, L. Recycling Waste Nickel-Laden Biochar to Pseudo-Capacitive Material by Hydrothermal Treatment: Roles of Nickel-Carbon Interaction. Carbon Res. 2022, 1, 16. [Google Scholar] [CrossRef]
- Tan, Z.; Yu, F.; Liu, L.; Jia, X.; Lv, Y.; Chen, L.; Xu, Y.; Shi, Y.; Guo, X. Cu-Doped Porous Carbon Derived from Heavy Metal-Contaminated Sewage Sludge for High-Performance Supercapacitor Electrode Materials. Nanomaterials 2019, 9, 892. [Google Scholar] [CrossRef]
- Shoeb, M.; Mashkoor, F.; Naved Khan, M.; Kim, B.-J.; Jeong, C. Waste to Energy Strategy: Graphene-Supported Au-Ag2O PolyIndole Nanocomposites for Antimony Adsorption and Their Sequential Utilization in Supercapacitors Device. Sep. Purif. Technol. 2025, 354, 128656. [Google Scholar] [CrossRef]
- Mashkoor, F.; Shoeb, M.; Khan, M.N.; Jeong, C. CNT Supported Sm/Co-LDH for Antimony Adsorption and Subsequent Application in Supercapacitor to Prevent Secondary Pollution. J. Alloys Compd. 2024, 981, 173557. [Google Scholar] [CrossRef]
- Popadić, D.; Gavrilov, N.; Ignjatović, L.; Krajišnik, D.; Mentus, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. How to Obtain Maximum Environmental Applicability from Natural Silicates. Catalysts 2022, 12, 519. [Google Scholar] [CrossRef]
- Zaher, A.; Kamal, W.; Essam, D.; Yousry, E.M.; Mahmoud, R. Repurposing Co-Fe LDH and Co-Fe LDH/Cellulose Micro-Adsorbents for Sustainable Energy Generation in Direct Methanol Fuel Cells. J. Water Process Eng. 2024, 62, 105317. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Mahmoud, R.; Hafez, S.H.M.; Mohamed, H.F.M. Hierarchical Ternary ZnCoFe Layered Double Hydroxide as Efficient Adsorbent and Catalyst for Methanol Electrooxidation. J. Mater. Res. Technol. 2022, 17, 1922–1941. [Google Scholar] [CrossRef]
- Yu, J.; Tang, T.; Cheng, F.; Huang, D.; Martin, J.L.; Brewer, C.E.; Grimm, R.L.; Zhou, M.; Luo, H. Exploring Spent Biomass-Derived Adsorbents as Anodes for Lithium Ion Batteries. Mater. Today Energy 2021, 19, 100580. [Google Scholar] [CrossRef]
- An, Y.; Zhang, W.; Zhang, X.; Zhong, Y.; Ding, L.; Hao, Y.; White, M.; Chen, Z.; An, Z.; Wang, X. Adsorption Recycling and High-Value Reutilization of Heavy-Metal Ions from Wastewater: As a High-Performance Anode Lithium Battery. Langmuir 2023, 39, 12324–12335. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C. Turning Waste into Treasure: Reuse of Contaminant-Laden Adsorbents (Cr(vi)-Fe3O4/C) as Anodes with High Potassium-Storage Capacity. J. Colloid Interface Sci. 2021, 582, 1107–1115. [Google Scholar] [CrossRef]
- Goswami, B.; Das, C.; Mahanta, D. Effect of Dye-Adsorption on Fe3O4-Polypyrrole Nanocomposite as Electrode Material in Electrochemical Capacitors. J. Energy Storage 2021, 44, 103429. [Google Scholar] [CrossRef]
- Ranković, M.; Jevremović, A.; Janošević Ležaić, A.; Arsenijević, A.; Rupar, J.; Dobričić, V.; Nedić Vasiljević, B.; Gavrilov, N.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Can Zeolite-Supporting Acridines Boost Their Anticancer Performance? J. Funct. Biomater. 2023, 14, 173. [Google Scholar] [CrossRef]
- Jevremović, A.; Stanojković, A.; Arsenijević, D.; Arsenijević, A.; Arzumanyan, G.; Mamatkulov, K.; Petrović, J.; Nedić Vasiljević, B.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Mitigating Toxicity of Acetamiprid Removal Techniques—Fe Modified Zeolites in Focus. J. Hazard. Mater. 2022, 436, 129226. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Pan, H.; Zeng, X.; Li, G.; Liu, H.; Kong, J.; Zhao, H.; An, T. Experimental and DFT Investigations on Adsorption–Regeneration Performance and Deactivation Mechanism over Engineered Carbon Fiber: Role of Pore Structure and Functional Groups. Environ. Sci. Nano 2023, 10, 2790–2798. [Google Scholar] [CrossRef]
- Liu, X.; Tai, L.; Huang, P.; Ma, W.; Jin, F. Unveiling Adsorption Mechanisms and Regeneration Challenges of Durian Peel Biochar for Ciprofloxacin Removal: Batch Experiments and Dft Study. J. Water Process Eng. 2025, 75, 107991. [Google Scholar] [CrossRef]
- Shi, B.; Fu, X.; Zhao, C.; Li, M.; Rao, Y.; Komarneni, S.; Yang, H. Insight into Properties and Reutilization Potential of Spent Polyaniline Adsorbents Containing Transition Metals through DFT Calculations. Sep. Purif. Technol. 2024, 335, 126182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).