Abstract
With increasing concerns about climate change and the depletion of fossil fuels, hemicellulose sugars from lignocellulosic biomass are gaining attention as sustainable feedstocks for producing biofuels and valuable chemicals. In this study, the extraction of hemicellulose sugars from corncob biomass was performed using hydrothermal pretreatment. Response Surface Methodology (RSM) with the Box–Behnken Design (BBD) was employed to optimize different parameters. The tested parameters included the corncob-to-water ratio (0.5:10, 1.5:10), time (30 to 90 min), and temperature (150 to 170 °C), to achieve the highest sugar yields (xylose, arabinose, and total sugars). The ANOVA results for the full quadratic polynomial model, which evaluates the effects of the three variables on xylose yield, indicate that the model is highly significant and provides a good fit to the data. This was evidenced by the minimal difference (0.003) between the predicted R2 and the adjusted R2. This study reports one of the highest recoveries of hemicellulosic sugars from corncobs and also evaluates degradation byproducts, offering a more efficient and comprehensive pretreatment approach that employs a lower temperature and a mild acid concentration (1%) compared with earlier research. The highest yields of xylose (103.49 mg/g), arabinose (26.75 mg/g), and total sugars (163.21 mg/g) were obtained at 160 °C and a corncob-to-water ratio of 0.5:10, after 90 min. Degradation products such as HMF and furfural in the hydrolysate were also analyzed by HPLC. The hydrolysate obtained from hydrothermal pretreatment contained oligomers that were converted into monomers through 1% H2SO4 hydrolysis. The highest yields after the acidic hydrolysis were 301.93 mg/g xylose, 46.96 mg/g arabinose, and 433.79 mg/g total sugars hydrolysis.
1. Introduction
The growing depletion of fossil fuels and their environmental and economic drawbacks have accelerated the shift toward biorefineries, with lignocellulosic biomass emerging as a crucial renewable resource [1]. Lignocellulosic biomass, derived from agricultural and forestry residues, is abundant, cost-effective, and rich in carbohydrates, making it a sustainable alternative for producing biofuels, biochemicals, and biomaterials [2]. As the global population increases and the demand for energy and materials rises, biorefineries provide a strategic solution by efficiently converting biomass into valuable products, minimizing harmful chemicals, and utilizing side streams [3]. This transition is essential for tackling climate change, lowering CO2 emissions, and fostering long-term environmental and economic sustainability [1,4]. Lignocellulosic biomass, produced abundantly at approximately 182 billion tons each year [1], is a complex natural composite primarily composed of cellulose (40–44%), hemicellulose (25–35%), and lignin (18–35%) [3]. Despite its broad availability, only a small fraction is effectively utilized due to the structural complexity and resistance of its tightly bound components, which obstruct efficient breakdown and processing [5]. The crystalline nature of cellulose, the intertwined matrix with hemicellulose, and the protective lignin barrier make the enzymatic, chemical, and physical hydrolysis and separation of components difficult and costly [6].
Pretreatment is a crucial step in converting lignocellulosic biomass into biofuels and valuable biochemicals, as it helps overcome the biomass’s natural resistance to degradation. This process involves breaking down hemicelluloses, modifying cellulose structure, and partially removing lignin to enhance cellulose accessibility for hydrolysis [6]. A variety of pretreatment methods have been developed, including liquid hot water (hydrothermal), enzymatic, dilute acid hydrolysis, mechanical processing, extrusion, steam explosion, organosolv treatment, sulfite pretreatment, and alkaline hydrolysis [7]. Hydrothermal treatment is particularly appealing among these approaches due to its efficiency, cost-effectiveness, and environmental safety, as it solely uses water and produces minimal toxins [8]. This method operates under high temperature and pressure, where water remains liquid. Under high pressure, hot water generates H3O+ (hydronium ions) through ionization, which helps to break down hemicelluloses partially [5,9]. The terms liquid hot water treatment, autohydrolysis, hot compressed water treatment, hydrothermolysis, aqueous extraction, aqueous fractionation, and hot water pretreatment all refer to the same process [3]. As a result of autohydrolysis, hemicellulose is solubilized into the liquid phase, also known as hydrolysate, while cellulose and lignin remain in the solid residue [10]. Researchers have utilized hydrothermal pretreatment on various lignocellulosic feedstocks such as pine wood, napiergrass, energycane, bagasse, poplar, and grapevine [10,11,12,13]. The hydrolysis extract from biomass contained various sugars and their oligomers, including glucose, cellobiose, xylose, galactose, arabinose, fructose, mannose, and rhamnose, as well as sugar-derived compounds such as furfural, 5-HMF, 5-methylfurfural, and organic acids like formic, levulinic, and lactic acids. Several studies have also explored the conversion of these sugars into high-value products (furfural and 5-HMF) under subcritical water conditions (150–250 °C) [14]. According to the U.S. Department of Energy, furfural is one of the top 30 bio-based platform chemicals and is obtained by the dehydration of xylose. Hydrothermal treatment alone seems inadequate for further hydrolysis of oligomeric sugars [4]. Consequently, many recent studies concentrate on two-step pretreatment methods to boost sugar yields, including the combination of hydrothermal with enzymatic hydrolysis and mechanochemical approaches with acid hydrolysis. However, the costs of enzymes, high energy input, long reaction times in mechanochemical processes, and the corrosive nature of concentrated acids limit these processes [15]. To overcome these challenges, the liquid phase, rich in oligomeric carbohydrates, can be subjected to dilute acid hydrolysis in the second step to yield monomeric sugars, furans, or levulinic acid, which are then transformed into biofuels or other high-value bio-based products after detoxification and fermentation, making hydrothermal pretreatment a key step in sustainable biorefinery development [10,16,17].
There has been a growing interest in utilizing corncob as a source of bioenergy and bio-based materials through eco-friendly methods, owing to the high yield of xylose and furfural [18]. Corn is one of the most widely grown crops around the world, with its cultivation covering all seven continents [19]. In 2020, the United States, Brazil, China, and Argentina collectively produced 64.63% of the global corn supply, according to the U.S. Department of Agriculture [20]. Currently, global corn production is about 1.23 billion metric tons per year [19]. Corncobs account for about 15% to 20% of the total corn residue [21]. Phojaroen Jiraporn et al. reported a yield of 0.488 kg/kg of fermentable sugar from corncob using a combined hydrothermal and mechanical pretreatment conducted at 180 °C for 30 min [22]. Maja Čolnik et al. studied the hydrolytic decomposition of corncob into sugars, yielding a maximum of 78.1 mg/g of xylose after 30 min at 200 °C [14]. Most existing studies on hydrothermal pretreatment of lignocellulosic biomass emphasize the use of solid products, while the resulting liquid stream has received relatively little attention [18]. Traditionally, parameter evaluation techniques concentrate on optimizing one variable at a time while keeping others constant. This approach limits the understanding of both individual effects and interactions among variables. Response Surface Methodology (RSM), when integrated with the Box–Behnken Design (BBD), offers a comprehensive approach to modeling and optimizing complex systems [23]. RSM identifies the relationships between variables and outcomes, enhancing process efficiency and reducing experimental error demands. DoE supports reliable and reproducible results through strategic experiment reduction [24].
This study presents a sustainable and innovative hydrothermal pretreatment of corncob that was conducted at different temperatures and times, followed by dilute sulfuric acid hydrolysis of the hydrolysate to convert oligomeric sugars into a high yield of monomers (xylose and arabinose) and minimal degradation products such as HMF and furfural. HPLC was used to analyze the types and yields of sugars and sugar derivatives such as furfural. Optimization of different parameters, including the corncob-to-water ratio, time, and temperature, was also performed using RSM with the BBD, to achieve the highest yield of sugars with minimal inhibitor production.
2. Materials and Methods
2.1. Chemicals
D-(+)-xylose (99%), D-(+)-glucose (≥99.5%), D-(+)-galactose (≥99%), D-(−)-arabinose (≥98%), D-(+)-mannose (≥99%), 5-hydroxymethyl-2-furaldehyde (5-HMF) (99%), and furfural (99%) for the HPLC standard, methanol (≥99.8%) and Glacial acetic acid for the mobile phase, and Sodium carbonate (99.95%) and 72% (w/w) sulfuric acid for pH were purchased. All chemicals were used without further purification.
2.2. Materials
Corncobs were collected from local fields at Mississippi State University and processed through a chipper to create pieces up to 1 cm in length, then ground and sieved through a 40–80 mesh sieve, and kept in a sealed plastic bag. The moisture content in corncobs was determined by oven-drying at a controlled temperature until a constant weight was achieved. Extractives were removed from corncob using a Soxhlet apparatus for 6 h with a 1:2 ethanol–benzene mixture. The de-extracted corncob biomass was dried in an oven for 24 h and stored in a sealed plastic bag until needed. The chemical composition of corncob was determined using the NREL/TP-510-42618 standard method [25]. Briefly, 0.33 g of corncob biomass was hydrolyzed with 3.1 mL (72%) H2SO4 for 1 h at 33 °C with continuous stirring. After that, the solution was diluted to (4%) H2SO4 concentration by adding 84 mL deionized water, transferred to a pressure tube, and placed in an autoclave for 1 h at 121 °C. After cooling, the pH was adjusted to 5.5 using Na2CO3. The solution was filtered with a 0.22 µm syringe and analyzed by high-performance liquid chromatography (HPLC) using an Agilent 1200 (Agilent Technologies, Santa Clara, CA, USA) instrument equipped with a refractive index detector and a Biorad HPX 87P column at 80 °C, utilizing water as the mobile phase. The sugars were quantified by comparing chromatographic peak areas to those of known sugar standards, and the results were expressed as a percentage of the dry weight of corncob.
2.3. Design of Experiment—Optimization of the Pretreatment Parameters
This study employed Response Surface Methodology (RSM) combined with the Box–Behnken Design (BBD) to design experiments and optimize parameters influencing sugar yield. The study aimed to determine the ideal operating conditions: temperature (T), time (M), and corncob-to-water ratio (R) to produce the highest sugar amount. Table 1 presents the BBD setup, which includes three variables at three levels (−1, 0, +1), where −1 indicates a low setting and +1 a high one. Based on the study [26], the BBD approach utilizes a second-order polynomial model that accurately represents linear, interaction, and quadratic effects. This model enables efficient exploration and optimization within the experimental space. Data analysis and graphical representation were performed using OriginPro 2021b software (9.8.5.212 version, OriginLab Corporation, Northampton, MA, USA). Model validation was performed through analysis of variance (ANOVA), with the model fit evaluated using the coefficient of determination (R2) and adjusted R2. The significance of individual and interaction effects was evaluated using F-tests and corresponding p-values.

Table 1.
Experimental design levels with three factors.
2.4. Extraction of Hemicellulosic Sugars by Hydrothermal Pretreatment
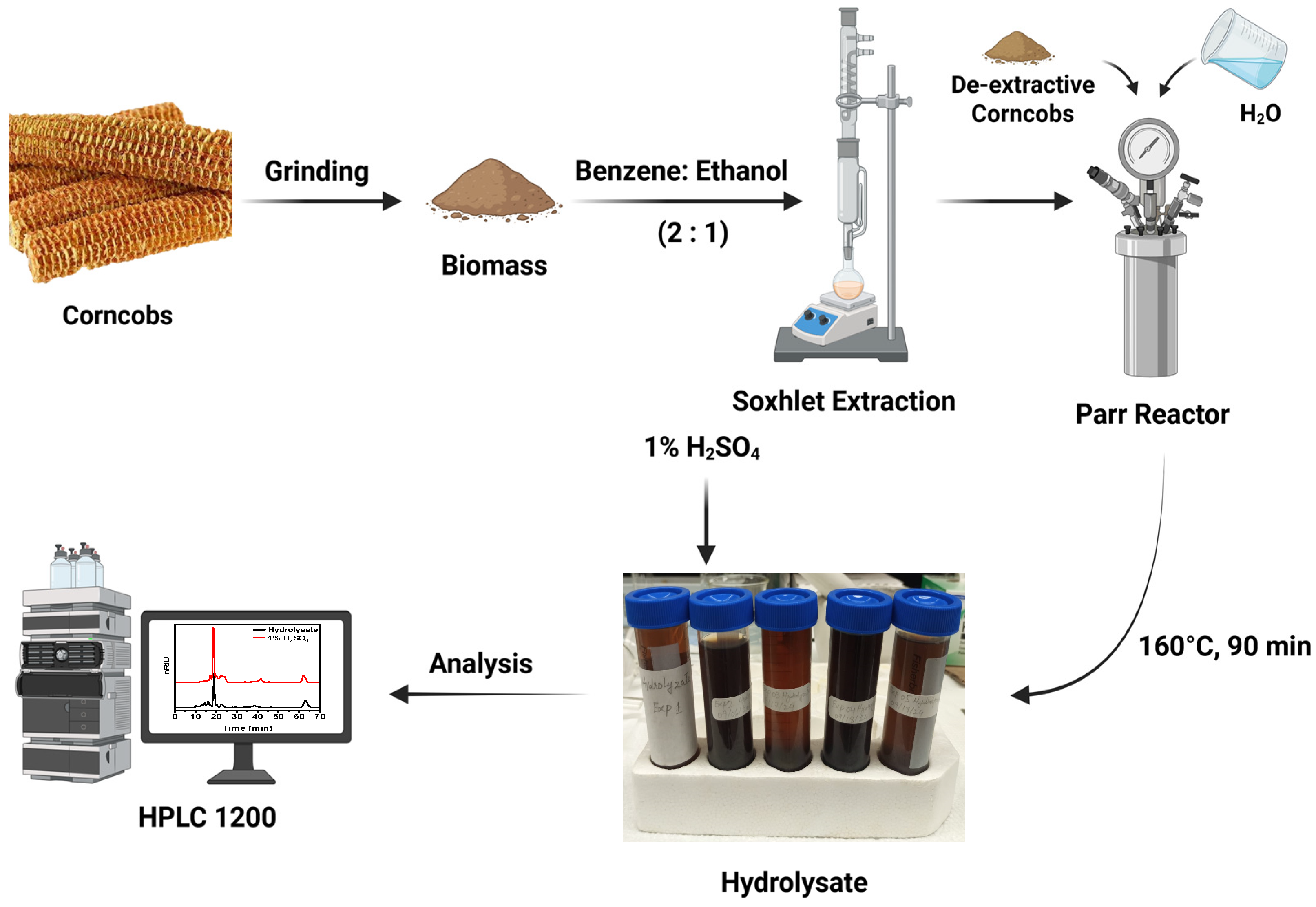
A 450 mL high-pressure Parr 5500 series batch reactor (Parr Instrument Company, Moline, IL, USA) was used for the hydrothermal pretreatment. De-extracted corncob was mixed with deionized water in various ratios (5, 10, 15 g of biomass to 100 mL H2O) in the reactor. The reactor was subsequently purged with nitrogen to eliminate air and then filled with nitrogen at a pressure of 800 psi. The pretreatment was conducted at different temperatures (150, 160, and 170 °C) for various time durations (30, 60, and 90 min) while maintaining a constant stirring speed of 500 rpm. This range was selected to extract more sugars and minimal degradation products. A total of 27 pretreatment experiments were performed to optimize the extraction conditions, as shown in Table 2. At the end of the pretreatment process, the reactor was immersed in cold water to stop the reaction. The hydrolysate was filtered to separate the solid residue of cellulose and lignin. The schematic diagram for the extraction of hydrolysate is shown in Scheme 1. Each experiment was conducted with three replicates, and the average value was then calculated and reported.

Table 2.
Pretreatment experiment conditions of hemicellulose extraction.
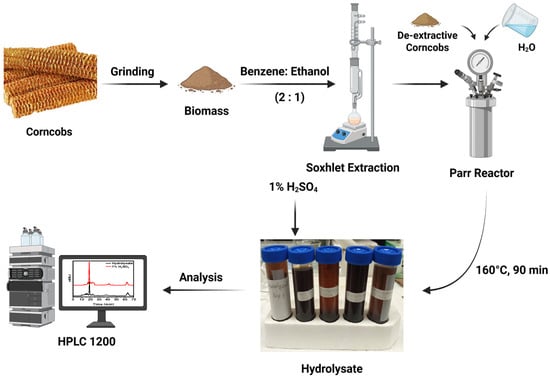
Scheme 1.
Schematic diagram for extracting hemicellulose from corncobs using hydrothermal treatment methods.
2.5. Acid Hydrolysis of Hydrolysate Oligomers and Sugar Analysis
The oligomeric sugars in the hydrolysates were hydrolyzed using different concentrations (1%, 2%, 4%) of 72% (w/w) H2SO4 to convert them into monomeric sugars. For 1% acidic hydrolysis, 20 mL of the hydrolysate was placed in a pressure tube, and 0.1 mL of 72% H2SO4 was added. The pressure tube was placed in an autoclave at 121 °C for 30 min. After cooling, the solution’s pH was neutralized using Na2CO3. The same procedure was followed for oligomer hydrolysis using 2% and 4% concentrations of 72% (w/w) H2SO4. The yields of sugars (glucose, xylose, galactose, arabinose, and mannose) before and after acidic hydrolysis were determined with high-performance liquid chromatography (HPLC) using an Agilent 1200 instrument equipped with a refractive index detector and a Biorad column HPX 87P at 80 °C, utilizing water as the mobile phase with a flow rate of 0.6 mL/min. The yields of HMF and furfural (furans) before and after acidic hydrolysis were determined using an HPLC Agilent 1100 instrument equipped with a UV detector and C18 column, employing methanol and water (1:5) with 5% acetic acid as the mobile phase, with a flow rate of 1 mL/min.
3. Results
3.1. Chemical Composition of Corncob
Table 3 shows the chemical composition of corncob, which indicates that it is primarily composed of 37.5% glucose, 29.6% xylose, 10.5 arabinose, and 11.2% insoluble lignin.

Table 3.
Chemical composition analysis of corncobs (%, dry basis).
3.2. Optimization of the Pretreatment Conditions
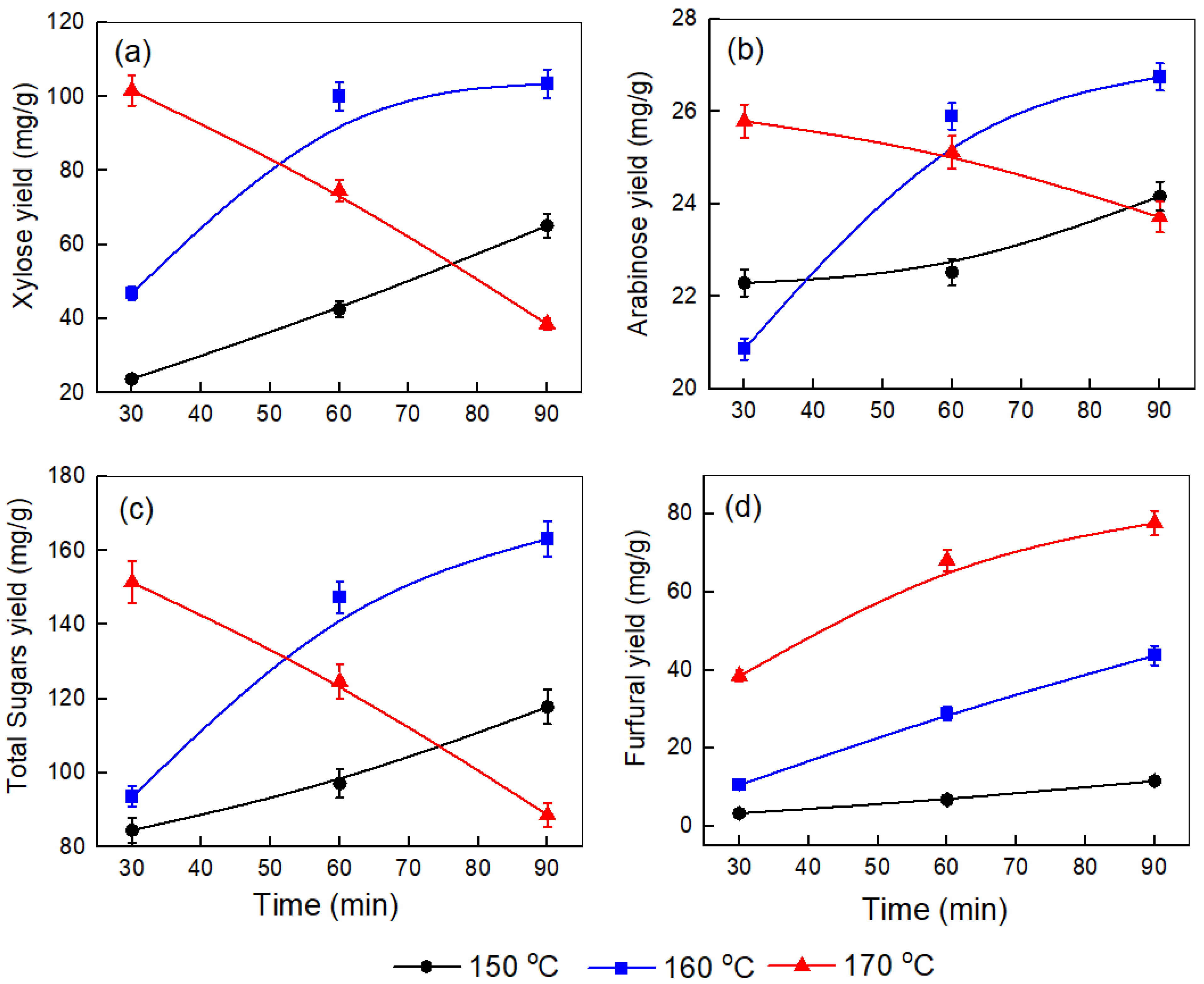
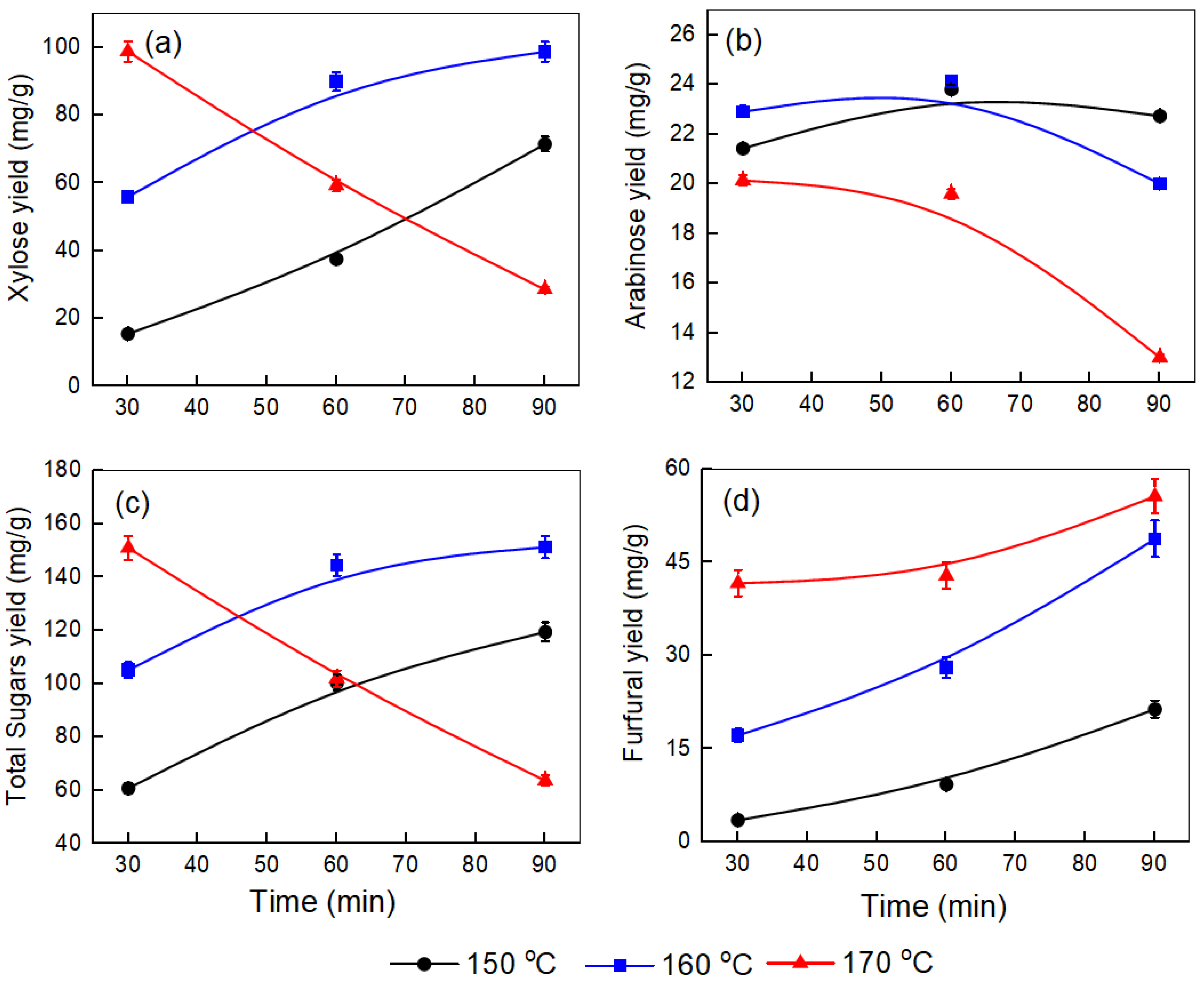
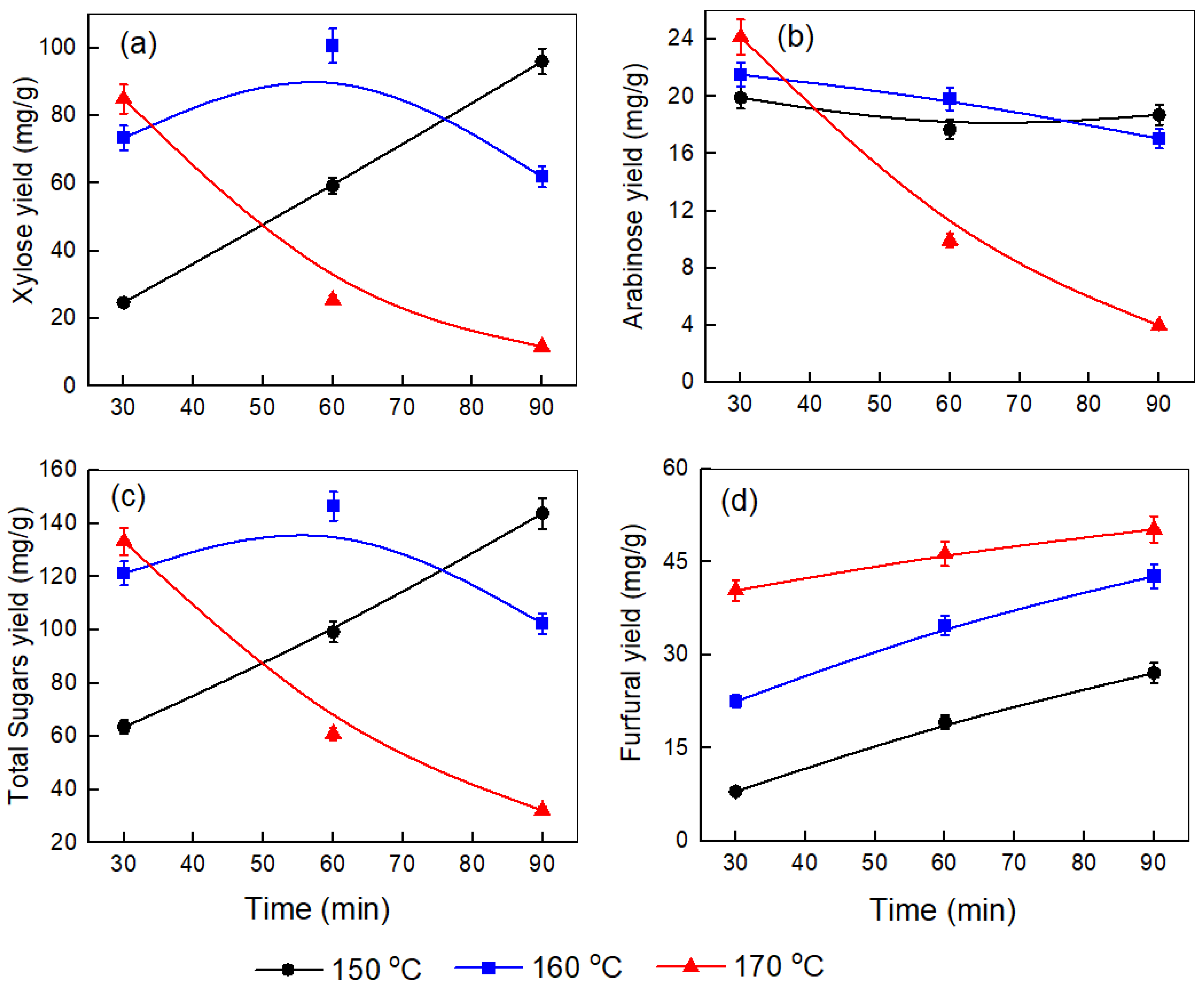
To maximize the yield of sugars, we optimized various pretreatment conditions, such as time, temperature, and the corncob-to-water ratio. Figure 1, Figure 2 and Figure 3 illustrate the effect of temperature (150–170 °C) and time (30–90 min) on the yields of xylose, arabinose, total sugars, and furfural produced at different corncob-to-water ratios (0.5:10, 1:10, and 1.5:10).
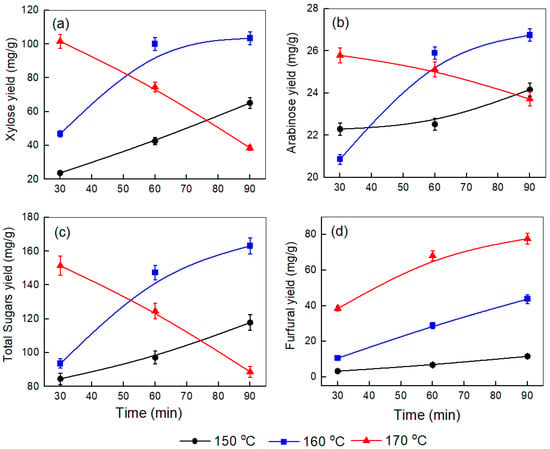
Figure 1.
Effect of temperature and time on the yields of (a) xylose, (b) arabinose, (c) total sugars, and (d) furfural produced after the pretreatment step at a corncob-to-water ratio of 0.5:10.
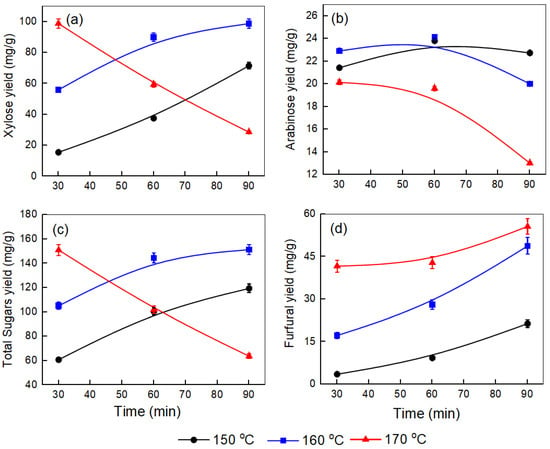
Figure 2.
Effect of temperature and time on the yields of (a) xylose, (b) arabinose, (c) total sugars, and (d) furfural produced after the pretreatment step at a corncob-to-water ratio of 1:10.
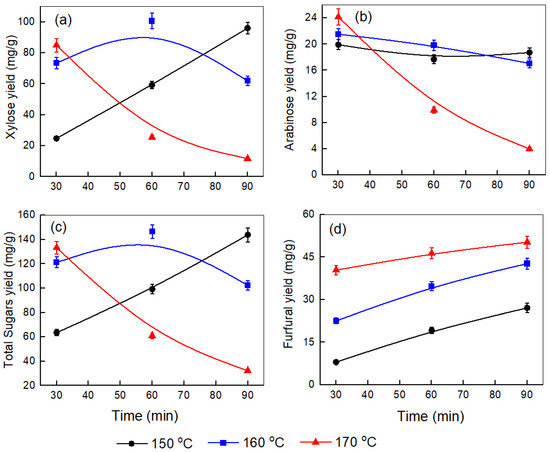
Figure 3.
Effect of temperature and time on the yields of (a) xylose, (b) arabinose, (c) total sugars, and (d) furfural produced after the pretreatment step at a corncob-to-water ratio of 1.5:10.
3.3. Acid Hydrolysis of Hydrolysate Oligomers
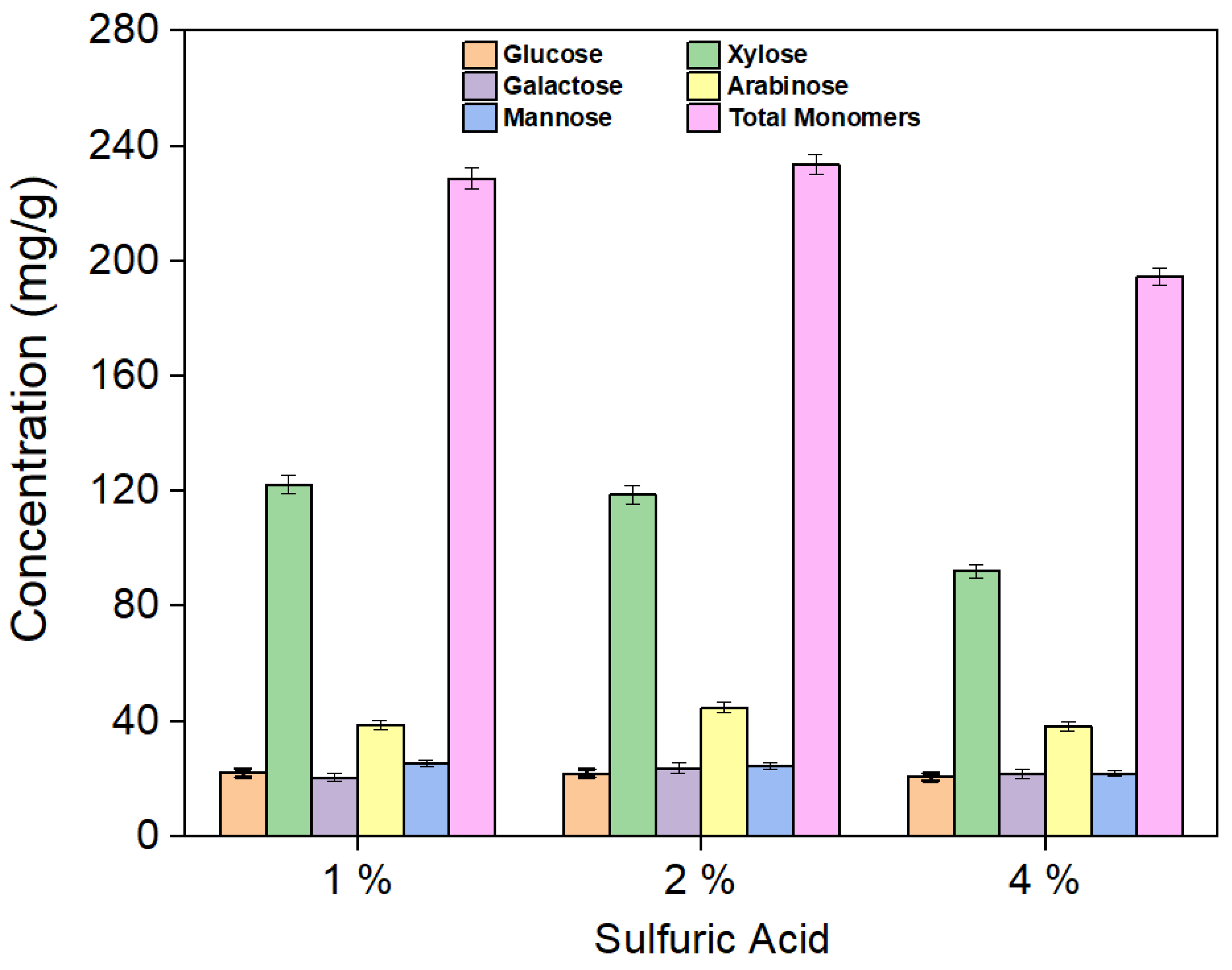
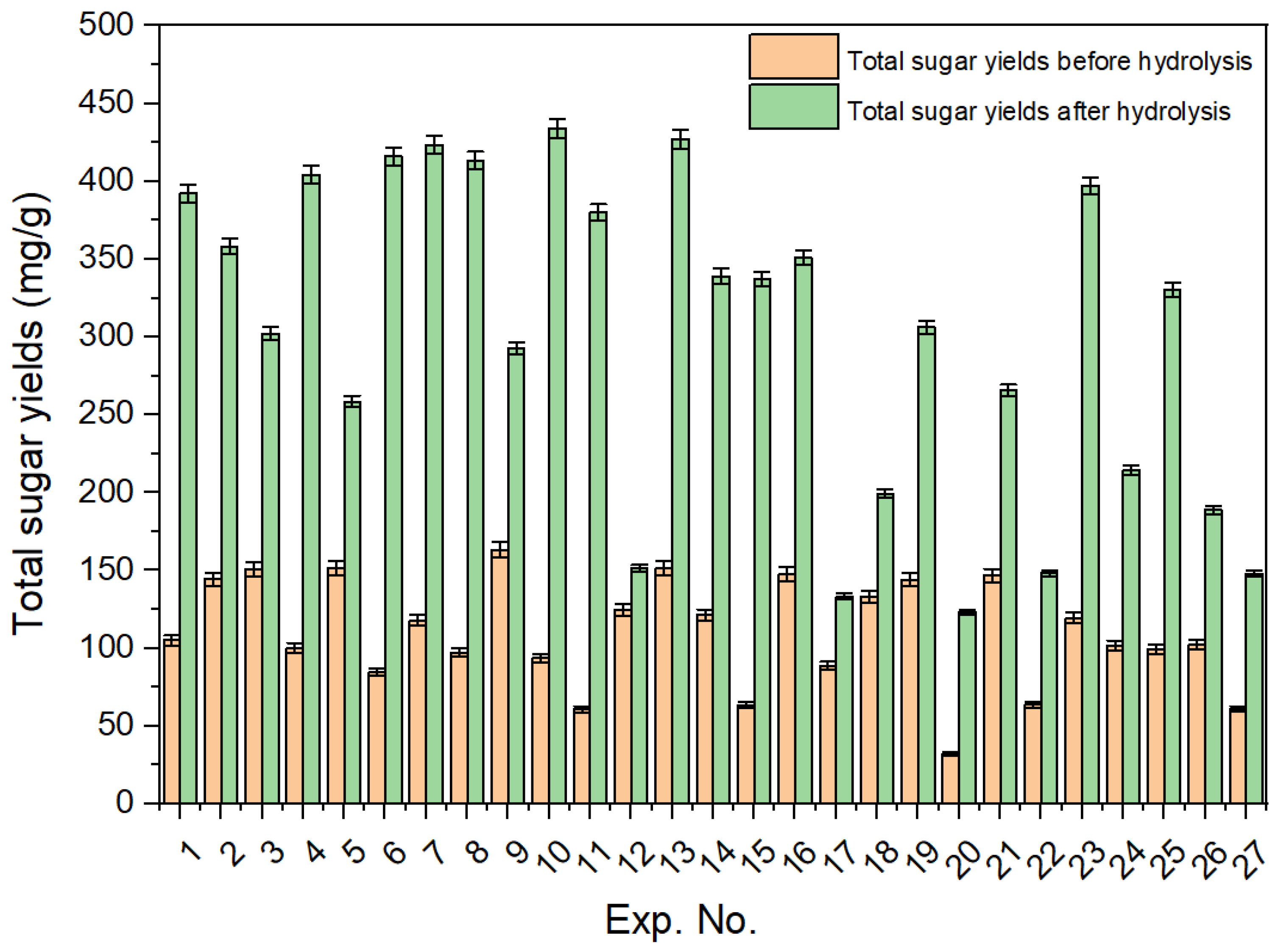
Oligomers, such as cellobiose and xylo-oligomers, were found in the hydrolysate obtained after hydrothermal pretreatment. To convert these oligomers into monomeric sugars, hydrolysis of the hydrolysate (Exp. 11) using 1%, 2%, and 4% sulfuric acid was performed, as shown in Figure 4. The total sugar yields obtained before and after 1% sulfuric acid hydrolysis for the 27 experiments are shown in Figure 5.
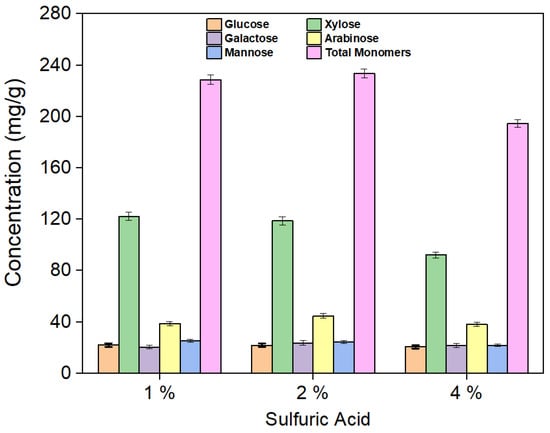
Figure 4.
Yield of sugars after 1%, 2%, and 4% H2SO4 hydrolysis of the hydrolysate (Exp. 11).
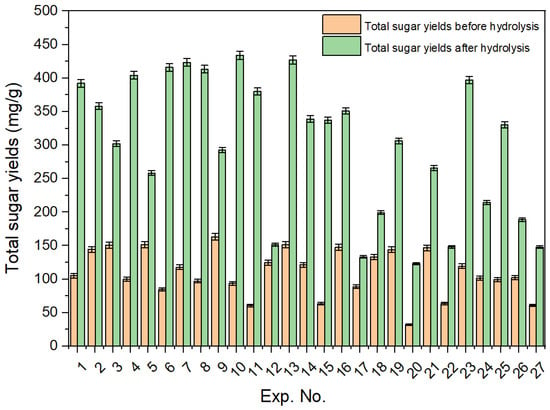
Figure 5.
Yields of hydrolysate total sugars before and after 1% sulfuric acid hydrolysis for the 27 experiments.
3.4. Optimization of Xylose Yield Using RSM
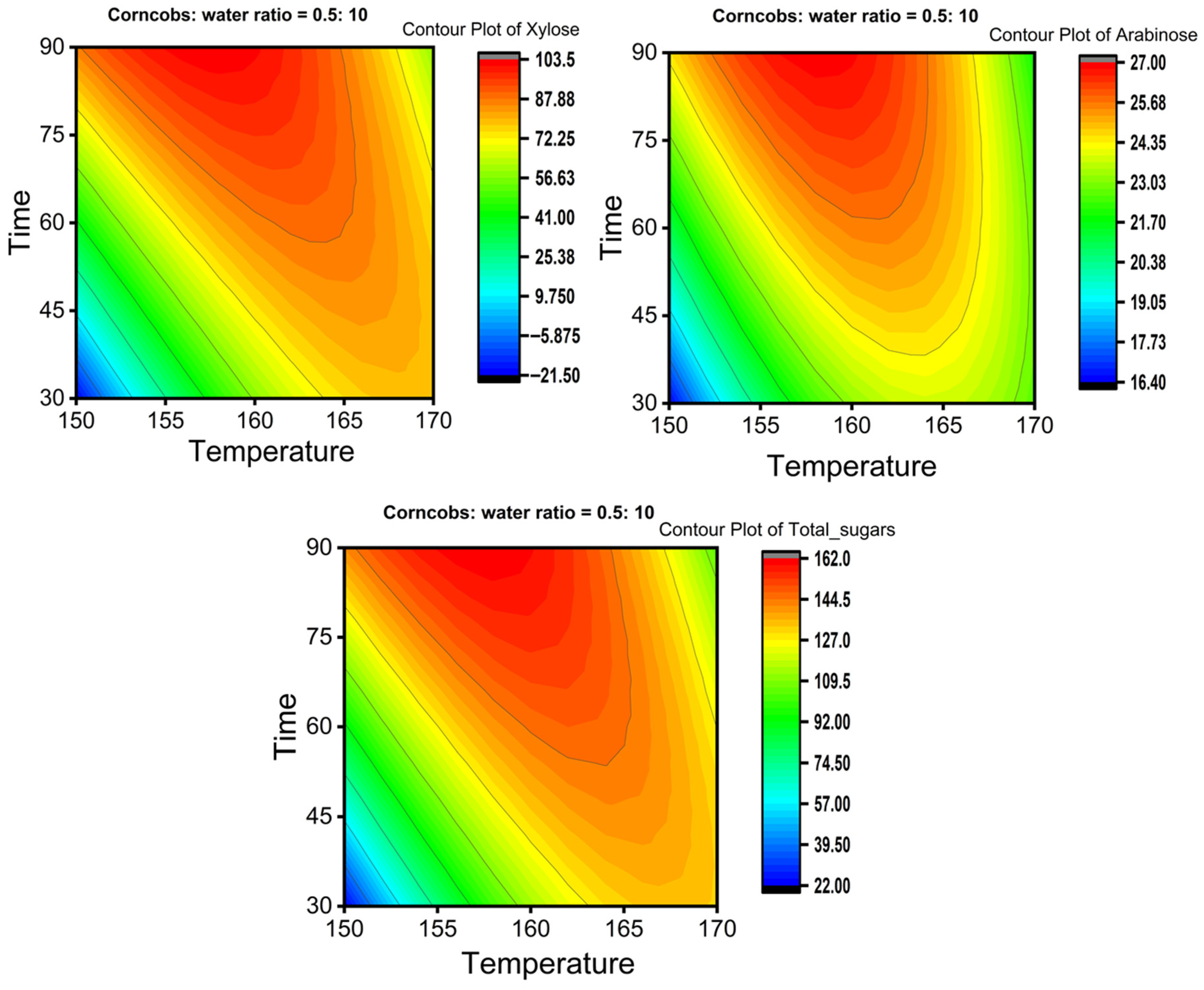
The Box–Behnken Design was employed to optimize xylose yield. Table 4, Table 5 and Table 6 present experimental and predicted yields, ANOVA results of the full quadratic polynomial model, and regression coefficients for xylose, respectively. Two-dimensional counter plots showing the effects of time and temperature at a corncob-to-water ratio of 0.5:10 for xylose, arabinose, and total sugars are illustrated in Figure 6.

Table 4.
Experimental yield with predicted yield of xylose.

Table 5.
Analysis of variance (ANOVA) of full quadratic polynomial model with statistics fit for xylose.

Table 6.
Regression coefficients of predicted full quadratic polynomial model for xylose.
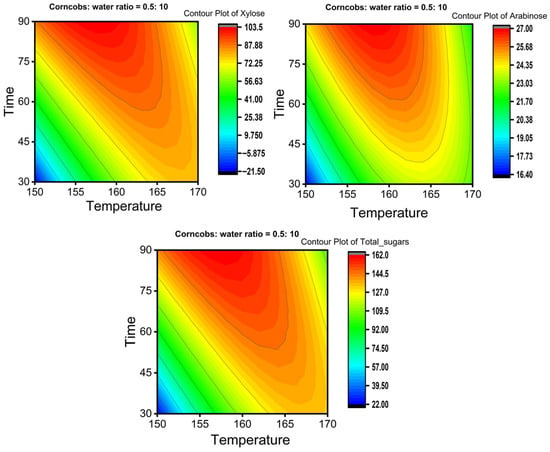
Figure 6.
Two-dimensional contour plots represent the effect of time and temperature on the yields of xylose, arabinose, and total sugar at a corncob-to-water ratio of 0.5:10.
4. Discussion
4.1. Optimization of the Pretreatment Conditions
In Figure 1a–c (0.5:10 corncob-to-water ratio), xylose, arabinose, and total sugar yields at 150 °C steadily increase over time, indicating that hemicellulose solubilization is mainly limited by diffusion and mass transfer. At 160 °C, there is a sharp rise to their maximum values after 90 min before leveling off, such as in the cases of xylose yield (103.49 mg/g), arabinose yield (26.75 mg/g), and total sugar yield (163.21 mg/g), reflecting accelerated hemicellulose depolymerization once the activation barrier is surpassed. This behavior aligns with kinetic models showing that first-order hemicellulose solubilization initially dominates until secondary pathways (sugar dehydration and condensation) become significant [27]. At 170 °C, the xylose and total sugar yield is high at the start, then decreases consistently, while the arabinose yield stays constant with time, showing that degradation processes dominate. Figure 1d also shows that the amount of furfural increases at all temperatures with longer times. These results suggest that higher temperatures and extended times promote sugar dehydration to furfural. Higher temperatures provide the energy needed to overcome the activation energy barrier for dehydration, while longer reaction times allow the process to proceed further [28].
Figure 2a displays trends similar to those in Figure 1a, but with different xylose yields at a corncob-to-water ratio of 1:10. At 150 °C, xylose levels gradually increase from 15.52 mg/g to 71.52 mg/g over 90 min. At 160 °C, the rise is more gradual, from 55.95 mg/g at 30 min to 98.75 mg/g at 90 min. Conversely, at 170 °C, a high yield of 98.78 mg/g is observed at 30 min, followed by a sharp drop to 28.52 mg/g at 90 min, indicating thermal degradation. Figure 2b shows that at 150 °C and 160 °C, arabinose yields initially increase, peaking around 60 min at approximately 23.80 mg/g and 24.12 mg/g, respectively. Then, they decrease after 90 min to roughly 22.73 mg/g and 20.01 mg/g, respectively. At 170 °C, the yield remains lower throughout, starting at 20.13 mg/g and dropping sharply to 13.01 mg/g within 90 min. Figure 2c illustrates a similar pattern for total sugars as in Figure 1c, but with slightly lower yields. At 150 °C, the total sugar yield rises from around 60.75 mg/g to 119.39 mg/g over 90 min. At 160 °C, the yield increases from approximately 105.02 mg/g at 30 min to about 151.25 mg/g after 90 min. At 170 °C, the yield initially begins at approximately 150.93 mg/g at 30 min and then drops sharply to about 63.67 mg/g by 90 min.
Figure 3a illustrates the more complex behavior of xylose yield at a 1.5:10 corncob-to-water ratio. At 150 °C, xylose levels increase over time from 24.75 mg/g to 96.07 mg/g. Notably, at 160 °C, the xylose yield rises from 73.43 mg/g at 30 min to a peak of 100.63 mg/g at 60 min, then sharply declines to 61.89 mg/g at 90 min. The 170 °C treatment shows the most significant dehydration, dropping from 84.96 mg/g to 11.72 mg/g over 90 min. Comparing these patterns, several key observations emerge: First, 160 °C generally offers optimal extraction conditions across biomass loadings. Second, higher biomass loadings (1:10 and 1.5:10) result in more concentrated systems, which may lead to insufficient water for complete hydrolysis of hemicellulose. This increases viscosity, restricting mixing and mass transfer and causing faster dehydration at longer treatment times, especially at higher temperatures, likely due to the conversion of released xylose into byproducts like furfural [29]. The optimal processing window appears narrower at higher loadings due to condensation reactions [19]. Third, the 0.5:10 ratio yields the highest overall xylose yield (103.49 mg/g at 160 °C, 90 min), indicating that lower solid-to-liquid ratios promote more efficient extraction by improving mass transfer and water penetration into the biomass [29,30]. Finally, at 170 °C, xylose dehydration is consistently significant across all loadings, intensifying with longer treatment durations. Xu et al. [31] observed a similar trend in xylose extraction between 110 °C and 190 °C over 30–150 min, achieving a maximum yield of 77.4% at 150 °C for 120 min. Initially, xylose levels increased with time and temperature, peaked, then decreased at higher temperatures (>150 °C) and longer durations due to the dehydration into furfural. Similarly, Si Lu et al. [32] studied corncob pretreatment at 130–160 °C for up to 120 min with a catalyst, observing consistent results: xylose yields rose early, confirming hydrolysis-induced release, with the highest yield of 88.77% at 160 °C after 20 min. It was also noted that at higher temperatures and with longer reaction times, the rate of xylose dehydration outpaced its formation times.
In Figure 3b, the negative impact of high temperature and extended processing time on arabinose yield is more evident. At 150 °C, the arabinose yield stays relatively constant at around 19–18 mg/g. When the temperature reaches 160 °C, the yield initially increases to 21.52 mg/g but then decreases to 17.05 mg/g after 90 min. At 170 °C, the yield sharply declines from 24.15 mg/g at 30 min to only 3.98 mg/g at 90 min, indicating a significant breakdown of arabinose under these harsher conditions. Comparing the three graphs (Figure 1b, Figure 2b and Figure 3b), it is clear that lower biomass loading (0.5), combined with a moderate temperature (160 °C) and extended reaction time (90 min), results in the highest arabinose yield. As biomass loading increases, the maximum arabinose yield decreases at all temperatures, and the negative effects of high temperature and longer times become more pronounced, especially at 170 °C. This suggests that higher solid-to-liquid ratios and excessive thermal severity accelerate arabinose degradation, thus limiting overall yield. Mosier et al. [33] found that arabinose yield from wheat straw increased with temperature and time up to an optimal point, but higher temperatures (170 °C) and longer durations led to rapid degradation of arabinose into furfural, particularly in water or strong acid conditions.
Figure 3c illustrates that at 150 °C, the total sugar yield rises with time to a maximum of 146.53 mg/g at 160 °C after 60 min, then declines to around 32.22 mg/g at 170 °C by 90 min. The total sugar amount decreases with higher temperature, longer processing time, and increased corncob loading. Similar patterns have been noted in previous research on corncob by Maja Colnik et al. [14] and Xu et al. [31]. Figure 2d and Figure 3d exhibit a similar trend to Figure 1d, confirming that higher temperatures and longer times favor the dehydration of sugars to furfural. Wang et al. [34] reported total sugar yields up to 81.6% (w/w) after KOH pretreatment of bagasse at 90 °C for 2 h under 5% solid loading, with enzymatic saccharification.
4.2. Acid Hydrolysis of Oligomers
Oligomers such as cellobiose, xylo-oligomers, and others were found in the hydrolysate obtained after hydrothermal pretreatment (Figure S1a). The total yields of all hydrolysate components (monomeric sugars and oligomers) after the hydrothermal pretreatment step are shown in Table S1. Hydrolysis of the hydrolysate was conducted using 1%, 2%, and 4% H2SO4 to convert oligomers into monomeric sugars. The yields of the produced sugars are shown in Figure 4. Hydrolysis experiments using 1% H2SO4 resulted in the highest xylose yield (122.3 mg/g) compared to both 2% (118.7 mg/g) and 4% (92.1 mg/g). While the total monomeric sugar yields were similar for both 1% and 2% acid hydrolysis, a noticeable decline was observed with the 4% acid treatment. At high acid concentrations, sugars tend to break down or convert into byproducts, and additional alkali is required to neutralize the solution. This not only increases the overall cost of the process but also causes corrosion issues. As a result, all hydrolysis experiments were conducted using 1% sulfuric acid. The yields of monomeric sugars in the hydrolysate after 1% H2SO4 treatment are listed in Table S2. Notably, Experiment 10 (corncob-to-water ratio 0.5:10, temperature 160 °C, and time 30 min) shows yields of 301.93 mg/g for xylose and 433.79 mg/g for total monomers after 1% acidic hydrolysis. A comparison of hydrolysate sugar yields before and after 1% H2SO4 hydrolysis for all experiments is shown in Figure 5. Additionally, Table S3 shows the amount of furan compounds before and after acidic hydrolysis, showing a notable increase in furfural yields. This confirms that some xylo-oligomers or xylose were converted into furfural upon the addition of 1% H2SO4.
4.3. RSM Analysis of Sugar Yields
Contour plots illustrate how changing parameters such as time, temperature, and corncob-to-water ratio influence the response of sugar yield. These visualizations also help reveal the interactions between parameters and their combined effects on the response. Figure 6 shows 2D contour plots of xylose, arabinose, and total sugar yields as affected by temperature and time, with the corncob-to-water ratio maintained at 5%. The figure indicates that the yields of xylose, arabinose, and total sugars increase over time from 30 min at 150 °C, reach their highest yields at 160 °C for 90 min, and then decrease as both time and temperature increase at 170 °C. For a single parameter effect, at constant temperature, yield rises with time up to a point and then declines, and vice versa (at constant time, varying temperature). This suggests there is an optimal extraction time for xylose, arabinose, and total sugars at each temperature, after which these sugars may degrade or convert into other byproducts.
4.4. Optimization of Xylose Yield by BBD
Table 4 illustrates the difference between experimental and predicted xylose yields. Each row details a specific variable combination along with the corresponding experimental and predicted yields, as well as the associated error. The predicted values closely align with the experimental results, with errors typically below ±2 mg/g, demonstrating a strong model fit. The xylose yield in these tests ranged from 15.51 mg/g to 103.49 mg/g. The highest experimental yield was observed in Exp. 10 (103.49 mg/g), with a small prediction error of 1.91 mg/g, obtained at 160 °C for 90 min and a corncob-to-water ratio of 0.5:10, indicating that these conditions are optimal. Notably, Experiments 13–15 (T = 160 °C, M = 60 min, R = 1:10) show near-perfect agreement between experimental and predicted yields (89.89 mg/g), suggesting robust model accuracy under these balanced conditions. Regression coefficients and T-values are presented in Table 6.
Table 5 presents a comprehensive ANOVA analysis, considering xylose yield as the response (dependent variable). The model’s significance was assessed using F- and p-values at a 95% confidence level. Response Surface Methodology (RSM) was employed to optimize these values for better model accuracy. Interaction terms with p-values above 0.05 were considered insignificant, while those below 0.05 were deemed statistically significant. The ANOVA results for the full quadratic polynomial model, which examines the effects of temperature (T), time (M), and corncob-to-water ratio (R), reveal that the model is highly significant and fits the data well, indicated by an R2 of 0.998 and an adjusted R2 of 0.996. According to fit statistics, a good model has a predicted R2 close to the adjusted R2, with a difference less than 0.2, which was only 0.003 for xylose yield. Among the linear terms, time (M) and ratio (R) are highly significant (p = 2.05 × 10−4 and 9.74 × 10−5), while temperature (T) is not significant (p = 0.07), though its quadratic term (TT) is highly significant (p = 7.8 × 10−7), indicating a strong nonlinear effect. Both time (M) and ratio (R) exhibit highly significant linear and quadratic effects (p < 0.001), with the quadratic term of R (RR) having the highest mean square (396.83). All two-way interactions (TM, TR, MR) are highly significant (p < 3.2 × 10−6), showing these factors influence the outcome together, especially TM, which has the largest F-value (1292.3). The model’s lack of fit (p = 3.05 × 1029) is also highly significant, possibly indicating model overfitting or excessive complexity; however, the high R2 values still suggest an overall excellent fit.
4.5. Optimization of Total Sugars by BBD
Table S4 compares the experimental and predicted total sugar yields under different combinations of temperature (T), time (M), and corncob-to-water ratio (R). The data reveal that the model predictions closely match the experimental results, with yields ranging from 57.75 mg/g to 160.20 mg/g and errors from about −3.2 to +3.2 mg/g, demonstrating good accuracy. The highest experimental total sugar yield was recorded in Std Order 10, where a temperature of 160 °C, a 90 min duration, and a cob-to-water ratio of 0.5:10 produced a yield of 160.21 mg/g. This indicates that these conditions are optimal for maximizing sugar yield. Regression coefficients and T-values are listed in Table S6.
Table S5 provides a comprehensive ANOVA analysis of total sugar yield, detailing the effects of T, M, and R, including their individual, quadratic, and interaction effects. The results indicate that R has a very significant influence on total sugars (F = 125.20), while time (M) is also significant (p = 0.037). Temperature (T), however, is not statistically significant on its own (p = 0.176). The quadratic terms for temperature (TT), time (MM), and corncob-to-water ratio (RR), along with all interaction terms (TM, TR, MR), show very low p-values, indicating high significance. The model’s high R2 of 0.996 and adjusted R2 of 0.989 demonstrate that it explains nearly all variability in total sugars. The extremely significant lack of fit (p = 1 × 10−29) confirms the model’s excellent fit. Overall, the findings emphasize that both R and M factors, including their interactions, are crucial in determining total sugar yield.
5. Conclusions
Hydrothermal pretreatment was used to extract hemicellulose sugars from corncob biomass. Several parameters, including the corncob-to-water ratio (0.5:10–1.5:10), temperature (150–170 °C), and time (30–90 min), were investigated to enhance sugar yield. Using RSM with the BBD, the process conditions were optimized, resulting in maximum xylose, arabinose, and total sugars of 103.49 mg/g, 26.75 mg/g, and 163.21 mg/g, respectively, at 160 °C, 90 min, and a 0.5:10 ratio. Trends showed that sugar content initially rose with temperature and time but decreased at higher levels due to the conversion into byproducts. A lower corncob-to-water ratio improved water penetration and sugar extraction, whereas prolonged heating and higher temperatures led to increased formation of furan compounds, particularly furfural. HPLC analysis confirmed that the hydrolysate produced from the hydrothermal pretreatment contained both oligomeric and monomeric sugars, and 1% H2SO4 was used to hydrolyze oligomers into monomers at 121 °C for 30 min. The yields of xylose, arabinose, and total sugars after acid treatment were 301.93 mg/g, 46.96 mg/g, and 433.79 mg/g, respectively, indicating the hydrolysis of oligomers. Additionally, acid hydrolysis slightly raised furans due to the dehydration of sugars. These findings highlight the industrial potential of hydrothermal pretreatment as a scalable, low-chemical process that can lower operational costs and be easily incorporated into existing biorefineries for sustainable fuel and chemical production.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/suschem6030027/s1: Figure S1. Spectra of hydrolysate analysis: (a) sugar chromatogram before 1% H2SO4 hydrolysis, (b) furans chromatogram before 1% H2SO4 hydrolysis, (c) sugar chromatogram after 1% H2SO4 hydrolysis, (d) furans chromatogram after 1% H2SO4 hydrolysis; Table S1. Yield of hydrolysate components after the hydrothermal pretreatment step; Table S2. Yield of hydrolysate monomeric sugars after 1% H2SO4 hydrolysis; Table S3. Yields of furfural and HMF in the hydrolysate before and after acid hydrolysis; Table S4. Experimental yield with predicted yield of total sugars; Table S5. Analysis of variance (ANOVA) of full quadratic polynomial model with statistics fit; Table S6. Regression coefficients of predicted full quadratic polynomial model.
Author Contributions
M.H.M.: Writing—original draft, methodology, investigation, formal analysis, I.E.: Writing—review and editing, validation, methodology, data curation, conceptualization; E.B.H.; Writing—review and editing, supervision, project administration, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the U.S. Department of Agriculture, National Institute of Food and Agriculture (USDA-NIFA), project award no. 2024-67021-42038. “Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy.”
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This manuscript is publication #SB1172 of the Sustainable Bioproducts, Mississippi State University. This publication is also a contribution of the Forest and Wildlife Research Center, Mississippi State.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Antonopoulou, G.; Papadopoulou, K.; Alexandropoulou, M.; Lyberatos, G. Liquid hot water treatment of woody biomass at different temperatures: The effect on composition and energy production in the form of gaseous biofuels. Sustain. Chem. Pharm. 2024, 38, 101485. [Google Scholar] [CrossRef]
- Cavailles, J.; Vaca-Medina, G.; Wu-Tiu-Yen, J.; Labonne, L.; Evon, P.; Peydecastaing, J.; Pontalier, P.-Y. Aqueous Pretreatment of Lignocellulosic Biomass for Binderless Material Production: Influence of Twin-Screw Extrusion Configuration and Liquid-to-Solid Ratio. Molecules 2024, 29, 3020. [Google Scholar] [CrossRef] [PubMed]
- Pelaez-Samaniego, M.R.; Mood, S.H.; Cisneros, J.F.; Fajardo-Seminario, J.; Yadama, V.; Garcia-Perez, T. Aqueous byproducts from biomass wet thermochemical processing: Valorization into fuels, chemicals, fertilizers, and biomaterials. Energy Convers. Manag. 2024, 307, 118360. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, L.; Zhang, W.; Lin, Y.; Liu, X.; Zhao, S.; Chang, C. Coupling process for preparing biomass-based furfural and levulinic acid from corncob: Extraction, green chemistry and techno-economic assessment. Bioresour. Technol. 2024, 394, 130301. [Google Scholar] [CrossRef]
- Wang, J.-X.; Wang, D.-M.; Xu, W.-L.; Zou, X.-J.; Zong, P.-J.; Zhang, H.-Z.; Shang, Y.-C.; Zhao, J.-L.; Wu, Y.-F.; Qiao, Y.-Y. Study on the hydrothermal gradient extraction of hemicellulose by a flow-through reactor. J. Energy Inst. 2024, 117, 101855. [Google Scholar] [CrossRef]
- Imman, S.; Laosiripojana, N.; Champreda, V. Effects of liquid hot water pretreatment on enzymatic hydrolysis and physicochemical changes of corncobs. Appl. Biochem. Biotechnol. 2018, 184, 432–443. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Sun, Z.-J.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol production. BioResources 2011, 6, 1576–1598. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Kruyeniski, J.; Area, M.C. Kinetic study of the extraction of hemicellulosic carbohydrates from sugarcane bagasse by hot water treatment. Ind. Crops Prod. 2015, 67, 1–6. [Google Scholar] [CrossRef]
- Liu, C.; Wyman, C.E. Partial flow of compressed-hot water through corn stover to enhance hemicellulose sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2005, 96, 1978–1985. [Google Scholar] [CrossRef]
- Santos, T.M.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rigual, V.; Rodriguez, F. Effect of autohydrolysis on Pinus radiata wood for hemicellulose extraction. Carbohydr. Polym. 2018, 194, 285–293. [Google Scholar] [CrossRef]
- Wells, J.M.; Drielak, E.; Surendra, K.; Khanal, S.K. Hot water pretreatment of lignocellulosic biomass: Modeling the effects of temperature, enzyme and biomass loadings on sugar yield. Bioresour. Technol. 2020, 300, 122593. [Google Scholar] [CrossRef]
- Sukhbaatar, B.; Kim, M.; Steele, P.; Ingram, L. Optimization of hot-compressed water pretreatment of bagasse and characterization of extracted hemicelluloses. Carbohydr. Polym. 2014, 101, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Čolnik, M.; Irgolič, M.; Perva, A.; Škerget, M. Hydrolytic Decomposition of Corncobs to Sugars and Derivatives Using Subcritical Water. Processes 2025, 13, 267. [Google Scholar] [CrossRef]
- Yu, Y.; Long, Y.; Wu, H. Near-complete recovery of sugar monomers from cellulose and lignocellulosic biomass via a two-step process combining mechanochemical hydrolysis and dilute acid hydrolysis. Energy Fuels 2016, 30, 1571–1578. [Google Scholar] [CrossRef]
- Rivas, S.; González-Muñoz, M.J.; Santos, V.; Parajó, J.C. Acidic processing of hemicellulosic saccharides from pine wood: Product distribution and kinetic modeling. Bioresour. Technol. 2014, 162, 192–199. [Google Scholar] [CrossRef]
- Nakasu, P.; Ienczak, L.; Costa, A.; Rabelo, S. Acid post-hydrolysis of xylooligosaccharides from hydrothermal pretreatment for pentose ethanol production. Fuel 2016, 185, 73–84. [Google Scholar] [CrossRef]
- Li, H.; Deng, A.; Ren, J.; Liu, C.; Lu, Q.; Zhong, L.; Peng, F.; Sun, R. Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef]
- Okeke, F.O.; Ahmed, A.; Imam, A.; Hassanin, H. A review of corncob-based building materials as a sustainable solution for the building and construction industry. Hybrid Adv. 2024, 6, 100269. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X. Research on corn production efficiency and influencing factors of typical farms: Based on data from 12 corn-producing countries from 2012 to 2019. PLoS ONE 2021, 16, e0254423. [Google Scholar] [CrossRef]
- Erickson, M.J.; Dobbins, C.; Tyner, W.E. The economics of harvesting corn cobs for energy. Crop Manag. 2011, 10, 1–8. [Google Scholar] [CrossRef][Green Version]
- Phojaroen, J.; Jiradechakorn, T.; Kirdponpattara, S.; Sriariyanun, M.; Junthip, J.; Chuetor, S. Performance Evaluation of Combined Hydrothermal-Mechanical Pretreatment of Lignocellulosic Biomass for Enzymatic Enhancement. Polymers 2022, 14, 2313. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.E.; Almeida, J.C.; Rocha, J.; Pereira, E. Application of Box-Behnken design to optimize the phosphorus removal from industrial wastewaters using magnetic nanoparticles. Environ. Sci. Pollut. Res. 2025, 32, 6804–6816. [Google Scholar] [CrossRef]
- Chen, C.; Luo, Z.; Tu, H.; Lin, X.; Pang, Y.; Huang, J.; Zhang, J.; Wang, X.; Cai, Q.; Wei, Z. Response surface methodology and Box-Behnken design optimization of Sulfaquinoxaline removal efficiency and degradation mechanisms by Bacillus sp. strain DLY-11. J. Hazard. Mater. 2025, 486, 136986. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Bubała, K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014, 160, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Ballachay, R.; Cai, G.; Cao, Y.; Trajano, H.L. Predicting xylose yield from prehydrolysis of hardwoods: A machine learning approach. Front. Chem. Eng. 2022, 4, 994428. [Google Scholar] [CrossRef]
- Zhou, Q.; Ding, A.; Zhang, L.; Wang, J.; Gu, J.; Wu, T.Y.; Gu, X.; Zhang, L. Furfural production from the lignocellulosic agro-forestry waste by solvolysis method—A technical review. Fuel Process. Technol. 2024, 255, 108063. [Google Scholar] [CrossRef]
- Ji, X.; Ma, H.; Tian, Z.; Lyu, G.; Fang, G.; Chen, J.; Saeed, H.A. Production of xylose from diluted sulfuric acid hydrolysis of wheat straw. BioResources 2017, 12, 7084–7095. [Google Scholar] [CrossRef]
- Wong, L.J.; San H’ng, P.; Abdullah, L.C.; Paridah, M.T.; Chin, K.L. Effect of Chemical Steeping on Yields of Glucose and Xylose from Dilute Acid Hydrolysis of Extract from Oil Palm Trunk. BioResources 2022, 17, 207–222. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, W.; Liu, G.; Liang, C.; Lu, S.; Qi, Z.; Hu, J.; Wang, Q.; Qi, W. Enhanced enzymatic hydrolysis of corncob by synthesized enzyme-mimetic magnetic solid acid pretreatment in an aqueous phase. ACS Omega 2019, 4, 17864–17873. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, Q.; Wang, X.; Liang, C.; Fu, J.; Xu, Z.; Wang, Z.; Yuan, Z.; Yue, J.; Qi, W. Preparation of reducing sugars from corncob by solid acid catalytic pretreatment combined with in situ enzymatic hydrolysis. Biomass Convers. Biorefin. 2021, 13, 12619–12629. [Google Scholar] [CrossRef]
- Kootstra, A.M.J.; Mosier, N.S.; Scott, E.L.; Beeftink, H.H.; Sanders, J.P. Differential effects of mineral and organic acids on the kinetics of arabinose degradation under lignocellulose pretreatment conditions. Biochem. Eng. J. 2009, 43, 92–97. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, X.; Feng, H.; Yao, X.; Bi, Y.; Yang, R.; Li, B. Potassium Hydroxide-Mediated Pretreatment of Sugarcane Bagasse: High-Efficiency Enzymatic Hydrolysis and Excellent Sugar Recovery. Appl. Biochem. Biotechnol. 2025, 197, 3907–3928. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).