Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Collection and Sample Preparation
2.2. Reagents
2.3. Experimental Design
2.4. Determination of Extraction Yield
2.5. Determination of Total Phenolic Content
2.6. Determination of Condensed Tannin Content
2.7. Determination of Antioxidant Activity
2.7.1. DPPH Free Radical Scavenging Effect
2.7.2. ABTS Radical Cation Scavenging Effect
2.7.3. Oxygen Radical Absorbance Capacity
2.8. Quantitative Analysis of Extracts by HPLC-DAD Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Factor Testing
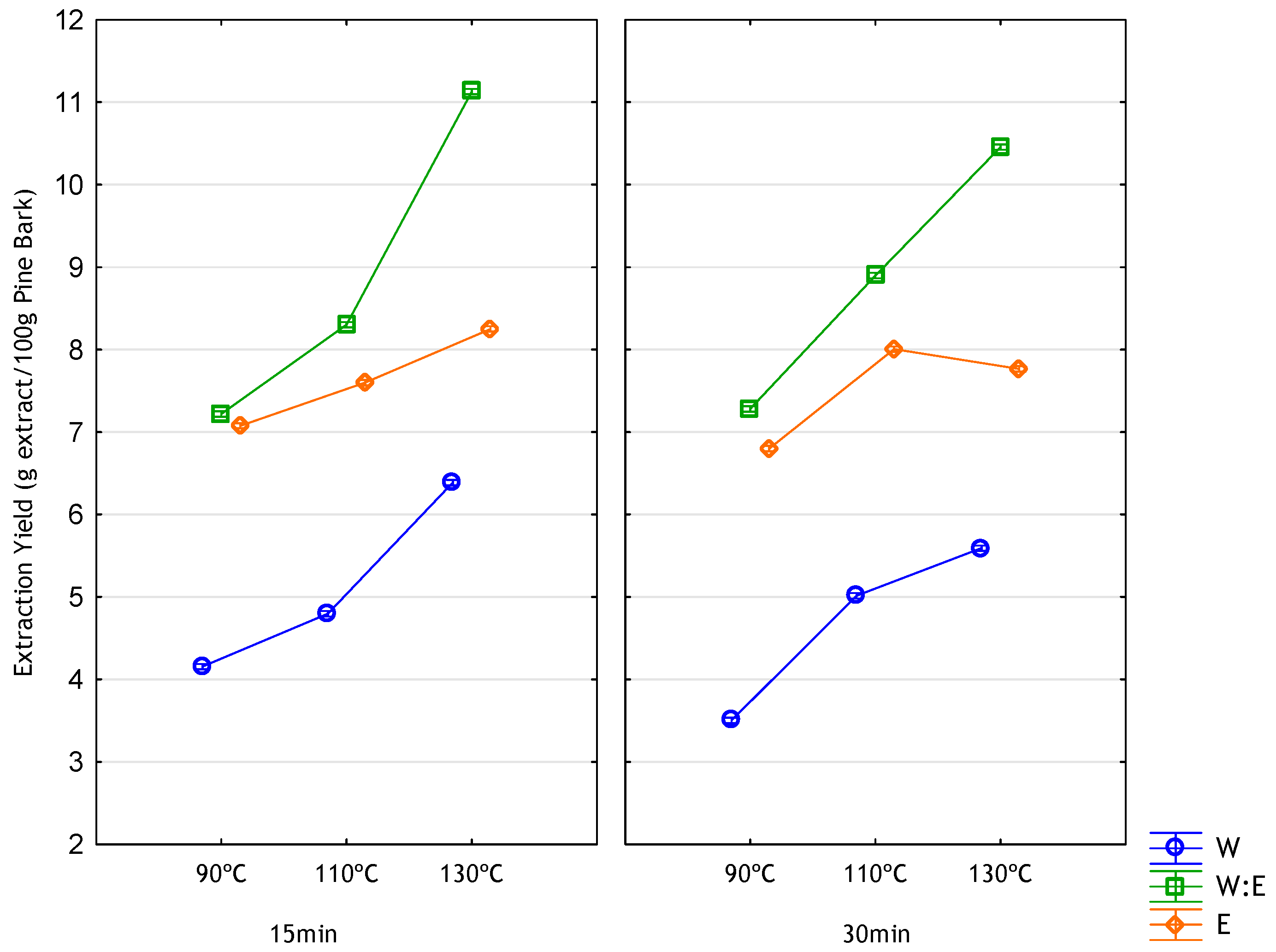
3.2. Extraction Yield
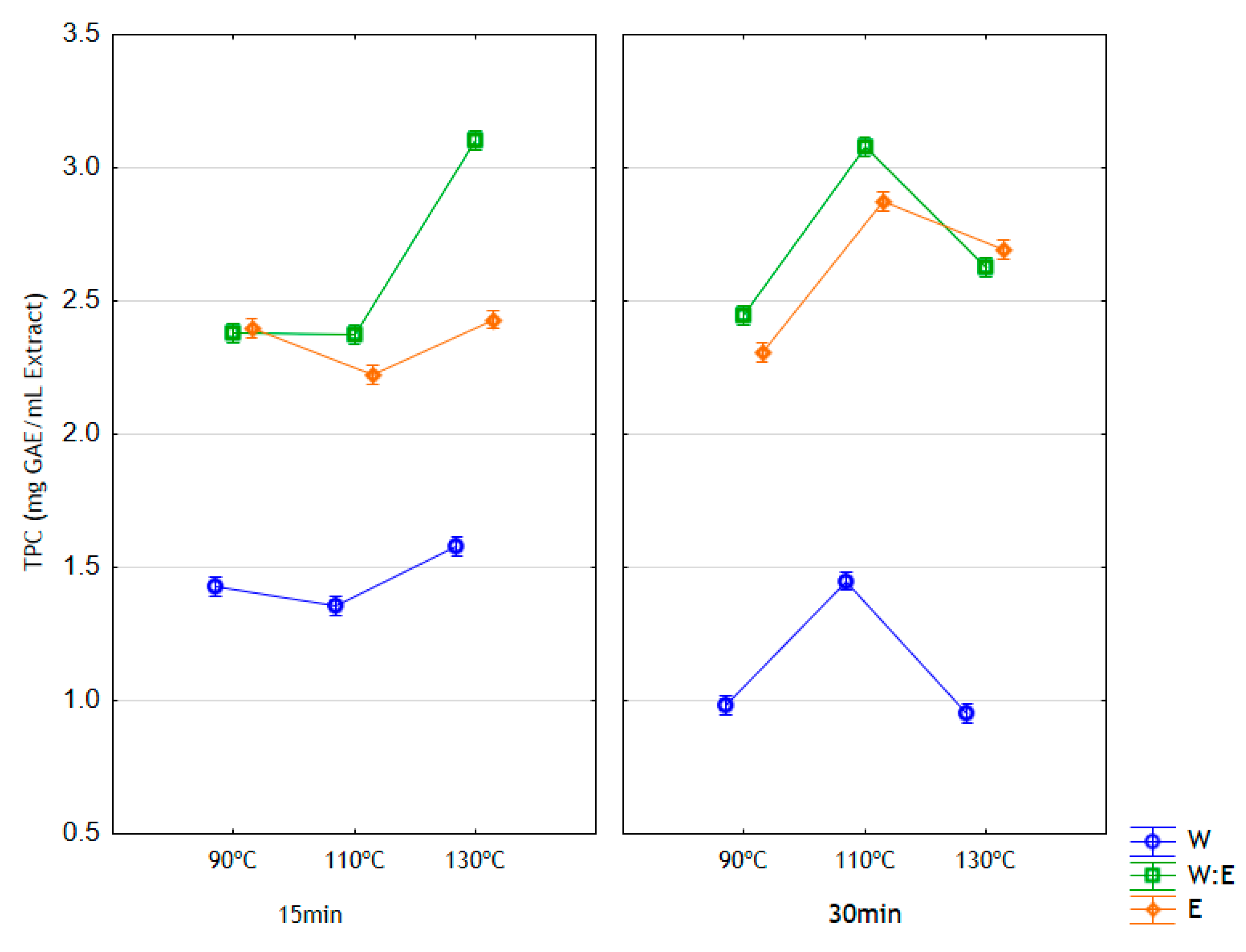
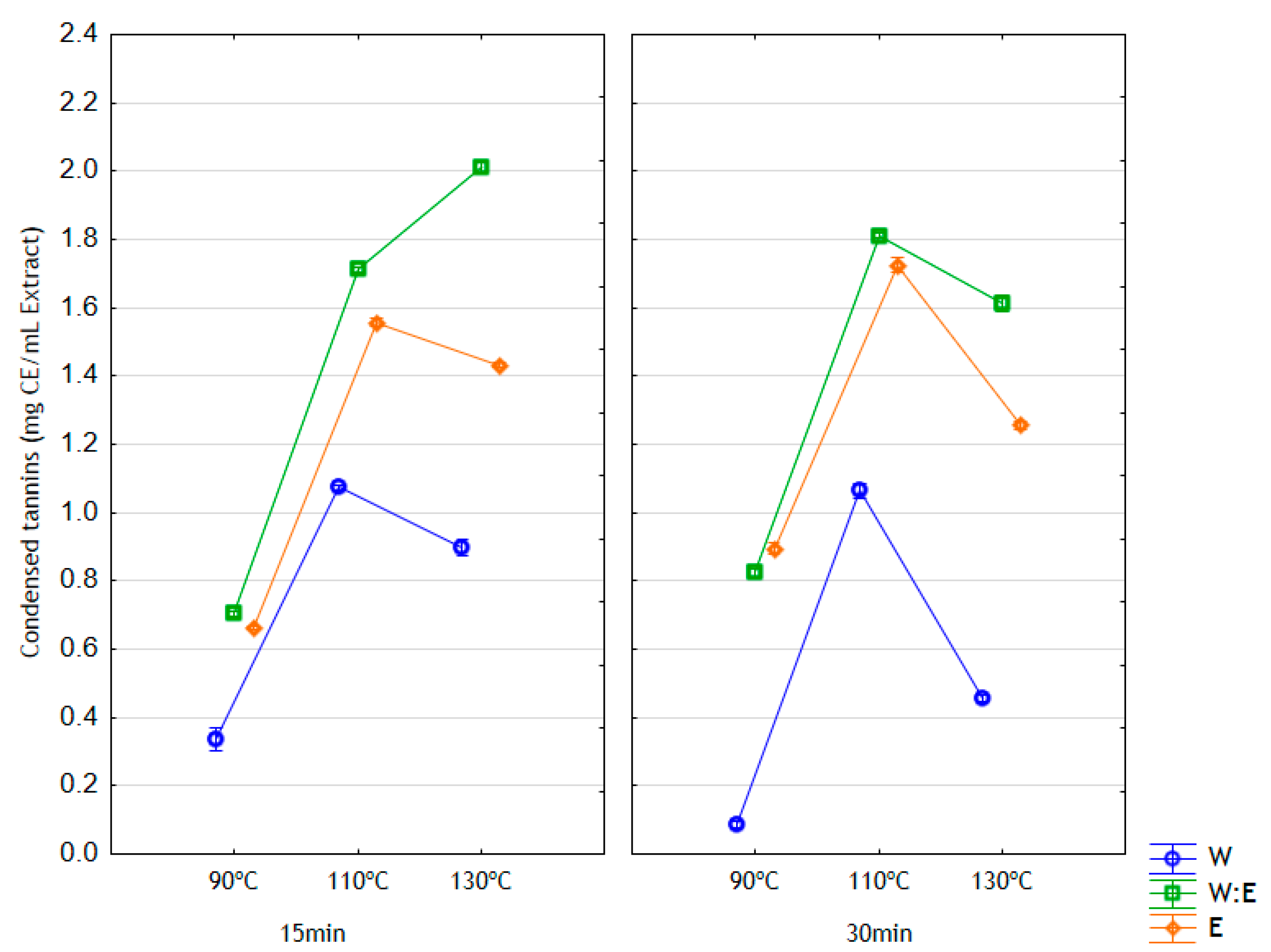
3.3. Total Phenolic Content and Condensed Tannin Content
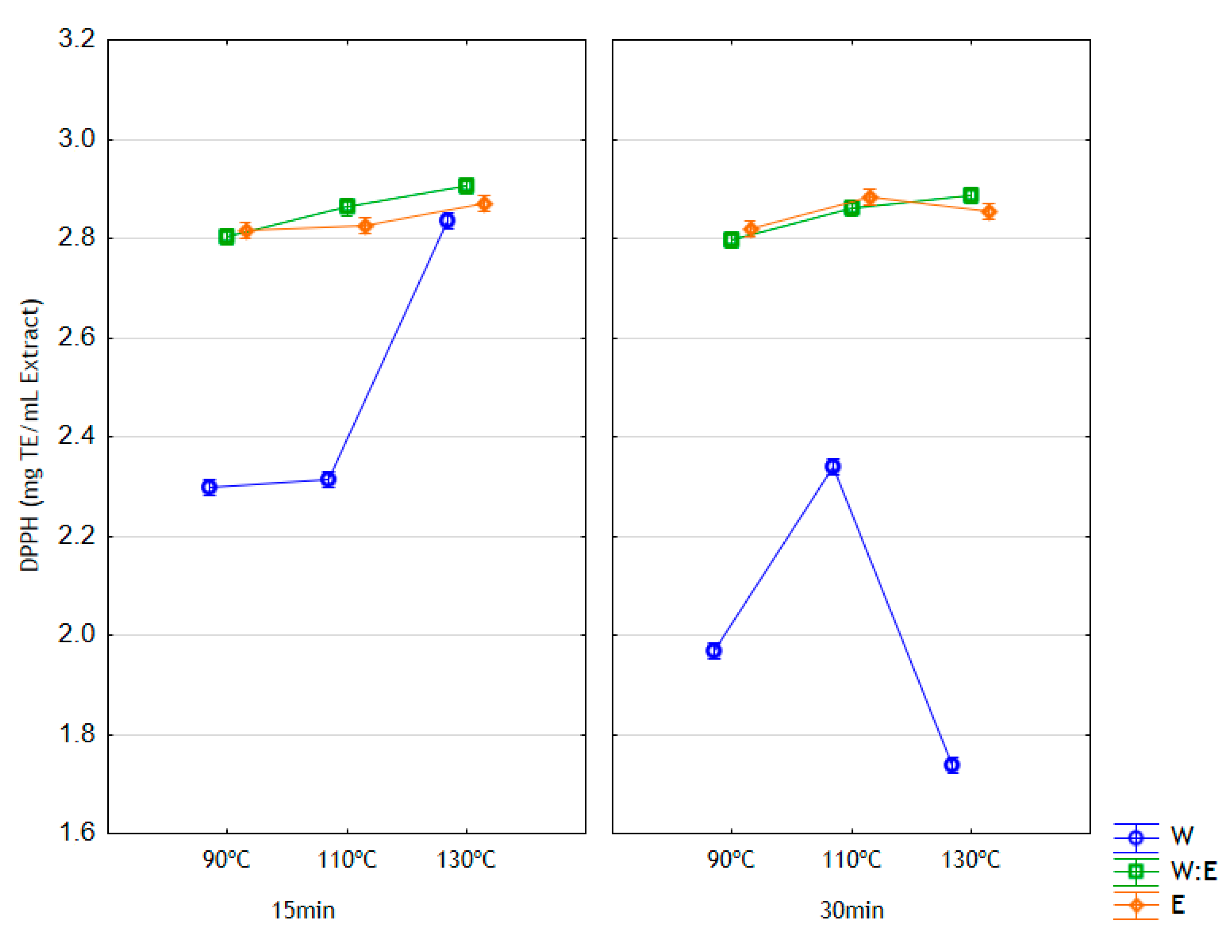
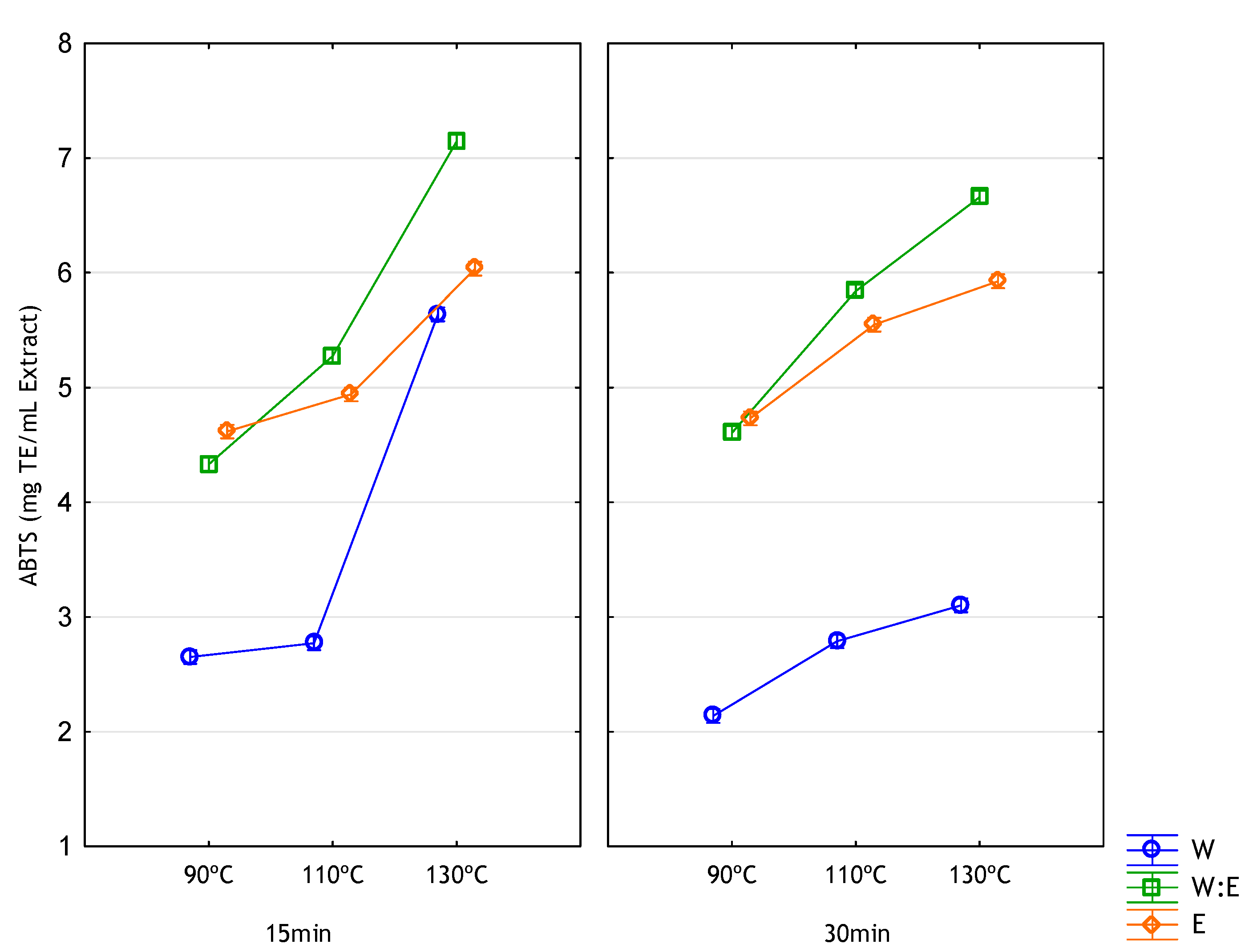
3.4. Antioxidant Activity
3.5. Quantitative Analysis of Extracts by HPLC-DAD Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ku, C.S.; Jang, J.P.; Mun, S.P. Exploitation of polyphenol-rich pine barks for potent antioxidant activity. J. Wood Sci. 2007, 53, 524–528. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Barros, D.; Vieito, C.; Santos, J.; Ramos, C.; Vaz-Velho, M. Inhibitory Effects of Pinus pinaster Aiton Subsp. Atlantica Bark Extracts Against Known Food Pathogens. Chem. Eng. Trans. 2020, 79, 163–168. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Charrier-El Bouhtoury, F. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crops Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. Biomed. Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Carocho, M.; Barros, D.; Velho, M.V.; Heleno, S.; Barros, L. Chemical composition and industrial applications of Maritime pine (Pinus pinaster Ait.) bark and other non-wood parts. Rev. Environ. Sci. Bio/Technol. 2022, 21, 583–633. [Google Scholar] [CrossRef]
- Abad Viñas, R.; Caudullo, G.; Oliveira, S.; de Rigo, D. Pinus pinaster in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office: Luxembourg, 2016. [Google Scholar]
- Conde, E.; Díaz-Reinoso, B.; Moure, A.; Hemming, J.; Willför, S.; Domínguez, H.; Parajó, J.C. Extraction of Phenolic and Lipophilic Compounds from Pinus pinaster Knots and Stemwood by Supercritical CO2. In Proceedings of the IIIrd Iberoamerican Conference on Supercritical Fluids, Cartagena de Indias, Colombia, 1–5 April 2013; Available online: http://www.nupeg.ufrn.br/prosciba/prosciba2013/Papers/T2-19.pdf (accessed on 23 June 2025).
- Caballero-Valdés, E.; Olivares-Miralles, A.; Soto-Maldonado, C.; Zúñiga-Hansen, M.E. Solid–Liquid Extraction of Polyphenols at Low Pressure. In Advances in Technologies for Producing Food-Relevant Polyphenols; Cuevas-Valenzuela, J., Vergara-Salinas, J.R., Pérez-Correa, J.R., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2016; pp. 63–76. [Google Scholar]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef]
- Plaza, M.; Domínguez-Rodríguez, G.; Castro-Puyana, M.; Marina, M. Polyphenols analysis and related challenges. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Cambridge, UK, 2018; pp. 177–232. [Google Scholar] [CrossRef]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Extraction|Microwave-Assisted Extraction. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 67–77. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Chen, J.; Thilakarathna, W.; Astatkie, T.; Rupasinghe, H.P.V. Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction. Biomolecules 2020, 10, 243. [Google Scholar] [CrossRef]
- Pals, M.; Lauberte, L.; Ponomarenko, J.; Lauberts, M.; Arshanitsa, A. Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization. Plants 2022, 11, 1544. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Bharudin, M.A.; Zakaria, S.; Chia, C.H. Condensed tannins from acacia mangium bark: Characterization by spot tests and FTIR. AIP Conf. Proc. 2013, 1571, 153–157. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, D.M.; Neto, C.P.; Evtuguin, D.; Jorge, F.C.; Irle, M.A.; Gil, M.H.; Pedrosa de Jesus, J. Chemical characterisation of bark and of alkaline bark extracts from maritime pine grown in Portugal. Ind. Crops Prod. 2002, 16, 23–32. [Google Scholar] [CrossRef]
- Seabra, I.J.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. High pressure solvent extraction of maritime pine bark: Study of fractionation, solvent flow rate and solvent composition. J. Supercrit. Fluids 2012, 62, 135–148. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive study on the antioxidant capacity and phenolic profiles of black seed and other spices and herbs: Effect of solvent and time of extraction. J. Food Meas. Charact. 2021, 15, 4561–4574. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Ustun, O.; Senol, F.S.; Kurkcuoglu, M.; Orhan, I.E.; Kartal, M.; Baser, K.H.C. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Feria-Reyes, R.; Ramírez-Cruz, S.O.; Ruiz-Aquino, F.; Robledo-Taboada, L.H.; Sánchez-Medina, M.A.; Mijangos-Ricárdez, O.F.; Gabriel-Parra, R.; Suárez-Mota, M.E.; Puc-Kauil, R.; Porcallo-Vargas, J. Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX). Forests 2023, 14, 1433. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Santos, R.M.S.; Seabra, I.J.; Facanali, R.; Marques, M.O.M.; de Sousa, H.C. Fractioned SFE of antioxidants from maritime pine bark. J. Supercrit. Fluids 2008, 47, 37–48. [Google Scholar] [CrossRef]
- Jusoh, Y.; Orsat, V.; Gariepy, Y.; Raghavan, V. Optimisation of Radio Frequency Assisted Extraction of Apple Peel Extract: Total Phenolic Contents and Antioxidant Activity. Chem. Eng. Trans. 2017, 56, 1153–1158. [Google Scholar] [CrossRef]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, W.; Wei, H.; Deng, S.; Yu, X.; Huang, T. The mechanisms and applications of cryoprotectants in aquatic products: An overview. Food Chem. 2023, 408, 135202. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Otto, F.; Parlar, H. A comparative study of flavonoid contents and antioxidant activities of supercritical CO2 extracted pine barks grown in different regions of Turkey and Germany. Eur. Food Res. Technol. 2009, 229, 671–677. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.; Barros, L.; Ferreira, I. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]

| Extraction Temperature (°C) | Solvent | Extraction Time (Minutes) |
|---|---|---|
| 90 | W | 15 |
| 30 | ||
| W:E | 15 | |
| 30 | ||
| E | 15 | |
| 30 | ||
| 110 | W | 15 |
| 30 | ||
| W:E | 15 | |
| 30 | ||
| E | 15 | |
| 30 | ||
| 130 | W | 15 |
| 30 | ||
| W:E | 15 | |
| 30 | ||
| E | 15 | |
| 30 |
| Compound | Wavelength (nm) (1) | R (2) | LoD (3) (mg L−1) |
|---|---|---|---|
| Cinnamic acid | 280 | 0.9993 | 4.5 |
| Catechin | 280 | 0.9910 | 15.8 |
| Quercetin | 320 | 0.9999 | 9.8 |
| Gallic acid | 280 | 0.9997 | 15.5 |
| Syringic acid | 280 | 0.9997 | 7.6 |
| Caffeic acid | 320 | 0.9971 | 9.1 |
| Taxifolin | 280 | 0.9996 | 8.8 |
| Ferulic acid | 320 | 0.9999 | 7.2 |
| Ellagic acid | 280 | 0.9992 | 13.9 |
| Protocatechuic acid | 280 | 0.9995 | 18.9 |
| Rutin | 320 | 0.9978 | 39.8 |
| Tyrosol | 280 | 0.9994 | 8.9 |
| Vanillin | 280 | 0.9999 | 3.7 |
| Resveratrol | 320 | 0.9997 | 1.5 |
| O-coumaric | 280 | 0.9994 | 8.4 |
| Variable | Effect | SS | DF | MS | F | p |
|---|---|---|---|---|---|---|
| Extraction Yield | Temperature | 45.9 | 2 | 22.93 | 7149 | <0.001 |
| Time | 0.4 | 1 | 0.43 | 133 | <0.001 | |
| Solvent | 147.7 | 2 | 73.83 | 230 × 102 | <0.001 | |
| Temperature*Time | 2.6 | 2 | 1.30 | 405 | <0.001 | |
| Temperature*Solvent | 10.3 | 4 | 2.59 | 807 | <0.001 | |
| Time*Solvent | 0.4 | 2 | 0.20 | 61 | <0.001 | |
| Temperature*Time*Solvent | 0.2 | 4 | 0.04 | 13 | <0.001 | |
| TPC | Temperature | 0.00 | 1 | 0.00 | 1 | 0.338 |
| Time | 20.17 | 2 | 10.09 | 2617 | <0.001 | |
| Solvent | 1.51 | 2 | 0.75 | 195 | <0.001 | |
| Temperature*Time | 0.25 | 4 | 0.06 | 16 | <0.001 | |
| Temperature*Solvent | 0.85 | 2 | 0.43 | 111 | <0.001 | |
| Time*Solvent | 0.37 | 4 | 0.09 | 24 | <0.001 | |
| Temperature*Time*Solvent | 0.115 | 2 | 0.057 | 75 | <0.001 | |
| DPPH | Temperature | 0.318 | 1 | 0.318 | 414 | <0.001 |
| Time | 4.328 | 2 | 2.164 | 2815 | <0.001 | |
| Solvent | 0.381 | 2 | 0.191 | 248 | <0.001 | |
| Temperature*Time | 0.046 | 4 | 0.011 | 15 | <0.001 | |
| Temperature*Solvent | 0.659 | 2 | 0.330 | 429 | <0.001 | |
| Time*Solvent | 0.613 | 4 | 0.153 | 199 | <0.001 | |
| Temperature*Time*Solvent | 33.58 | 2 | 16.79 | 1571 | <0.001 | |
| ABTS | Temperature | 0.71 | 1 | 0.71 | 67 | <0.001 |
| Time | 63.98 | 2 | 31.99 | 2993 | <0.001 | |
| Solvent | 4.92 | 2 | 2.46 | 230 | <0.001 | |
| Temperature*Time | 2.59 | 4 | 0.65 | 61 | <0.001 | |
| Temperature*Solvent | 4.13 | 2 | 2.07 | 193 | <0.001 | |
| Time*Solvent | 1.82 | 4 | 0.46 | 43 | <0.001 | |
| Temperature*Time*Solvent | 122.6 | 2 | 61.3 | 1842 | <0.001 | |
| ORAC | Temperature | 2.5 | 1 | 2.5 | 76 | <0.001 |
| Time | 274.2 | 2 | 137.1 | 4118 | <0.001 | |
| Solvent | 5.4 | 2 | 2.7 | 82 | <0.001 | |
| Temperature*Time | 44.7 | 4 | 11.2 | 336 | <0.001 | |
| Temperature*Solvent | 12.7 | 2 | 6.3 | 191 | <0.001 | |
| Time*Solvent | 16.9 | 4 | 4.2 | 127 | <0.001 | |
| Temperature*Time*Solvent | 8.078 | 2 | 4.039 | 5183 | <0.001 | |
| CTC | Temperature | 0.072 | 1 | 0.072 | 93 | <0.001 |
| Time | 6.179 | 2 | 3.089 | 3964 | <0.001 | |
| Solvent | 0.482 | 2 | 0.241 | 309 | <0.001 | |
| Temperature*Time | 0.630 | 4 | 0.158 | 202 | <0.001 | |
| Temperature*Solvent | 0.216 | 2 | 0.108 | 138 | <0.001 | |
| Time*Solvent | 0.062 | 4 | 0.016 | 20 | <0.001 | |
| Temperature*Time*Solvent | 0.00 | 1 | 0.00 | 1 | 0.338 |
| Phenolic Compound (mg/L) | |||||||
|---|---|---|---|---|---|---|---|
| Extraction Temperature (°C) | EtOH (% v/v) | Extraction Time (Minutes) | Protocatechuic | Catechin | Tyrosol | Taxifolin | Total |
| 90 | 0 | 15 | <LoD | 18.84 ± 0.33 i | <LoD | 10.98 ± 0.16 g | 29.83 |
| 30 | <LoD | 34.77 ± 1.34 h | <LoD | 13.70 ± 0.31 f | 48.47 | ||
| 50 | 15 | <LoD | 76.54 ± 0.86 e | <LoD | 37.22 ± 0.08 c | 113.76 | |
| 30 | <LoD | 91.25 ± 0.37 d | <LoD | 37.37 ± 0.05 c | 128.62 | ||
| 100 | 15 | <LoD | 30.16 ± 1.94 h | <LoD | 20.87 ± 0.16 e | 51.03 | |
| 30 | <LoD | <LoD | <LoD | 26.26 ± 0.33 d | 26.26 | ||
| 110 | 0 | 15 | <LoD | 45.53 ± 0.14 g | <LoD | 11.23 ± 0.29 f,g | 56.76 |
| 30 | <LoD | <LoD | <LoD | 13.66 ± 1.25 f | 13.66 | ||
| 50 | 15 | <LoD | 142.82 ± 2.31 c | 13.59 ± 0.19 d | 49.98 ± 1.14 b | 206.39 | |
| 30 | 26.77 ± 0.20 c | 229.94 ± 0.00 b | 41.28 ± 0.33 c | 54.95 ± 0.72 a | 352.94 | ||
| 100 | 15 | <LoD | <LoD | <LoD | 23.46 ± 1.13 d,e | 23.46 | |
| 30 | <LoD | <LoD | <LoD | 23.72 ± 0.61 d,e | 23.72 | ||
| 130 | 0 | 15 | <LoD | 59.16 ± 1.46 f | 10.57 ± 0.39 e | 8.09 ± 7.01 g | 77.83 |
| 30 | <LoD | <LoD | <LoD | 9.98 ± 0.47 g | 9.98 | ||
| 50 | 15 | 29.54 ± 0.83 a | 252.89 ± 1.97 a | 53.26 ± 1.01 a | 58.53 ± 0.91 a | 394.23 | |
| 30 | 27.56 ± 2.16 b | 232.39 ± 6.24 b | 44.73 ± 1.57 b | 55.91 ± 1.75 a | 360.59 | ||
| 100 | 15 | <LoD | <LoD | <LoD | 34.95 ± 0.09 c | 34.95 | |
| 30 | <LoD | <LoD | <LoD | 35.32 ± 3.93 c | 35.32 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, D.; Pereira-Pinto, R.; Fernandes, É.; Pires, P.; Vaz-Velho, M. Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark. Sustain. Chem. 2025, 6, 26. https://doi.org/10.3390/suschem6030026
Barros D, Pereira-Pinto R, Fernandes É, Pires P, Vaz-Velho M. Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark. Sustainable Chemistry. 2025; 6(3):26. https://doi.org/10.3390/suschem6030026
Chicago/Turabian StyleBarros, Diana, Ricardo Pereira-Pinto, Élia Fernandes, Preciosa Pires, and Manuela Vaz-Velho. 2025. "Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark" Sustainable Chemistry 6, no. 3: 26. https://doi.org/10.3390/suschem6030026
APA StyleBarros, D., Pereira-Pinto, R., Fernandes, É., Pires, P., & Vaz-Velho, M. (2025). Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark. Sustainable Chemistry, 6(3), 26. https://doi.org/10.3390/suschem6030026









