Cerium-Doped Strontium Ferrate Perovskite Oxides: Sustainable Materials to Face Energy and Environmental Challenges
Abstract
1. Introduction

2. Background
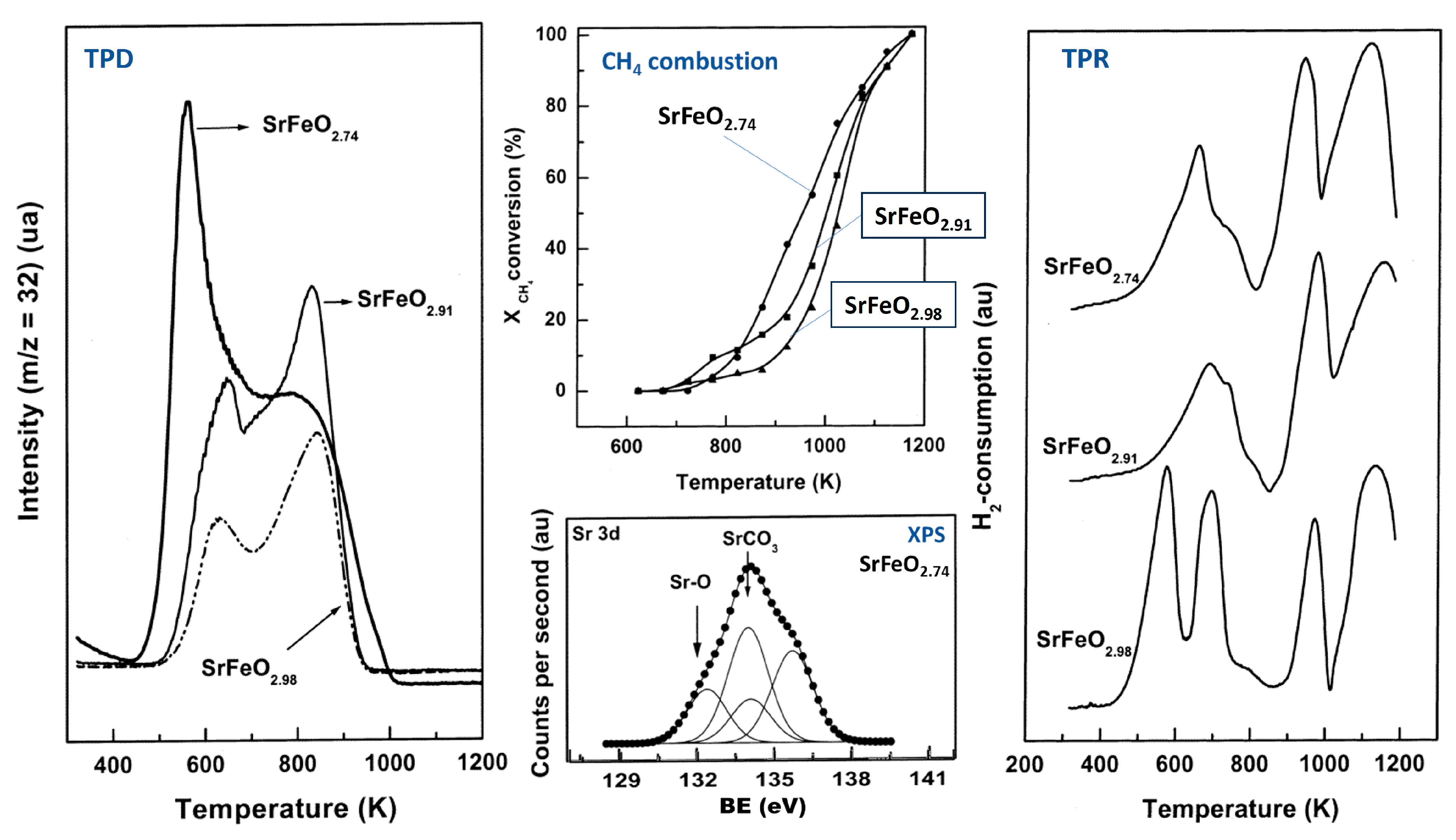
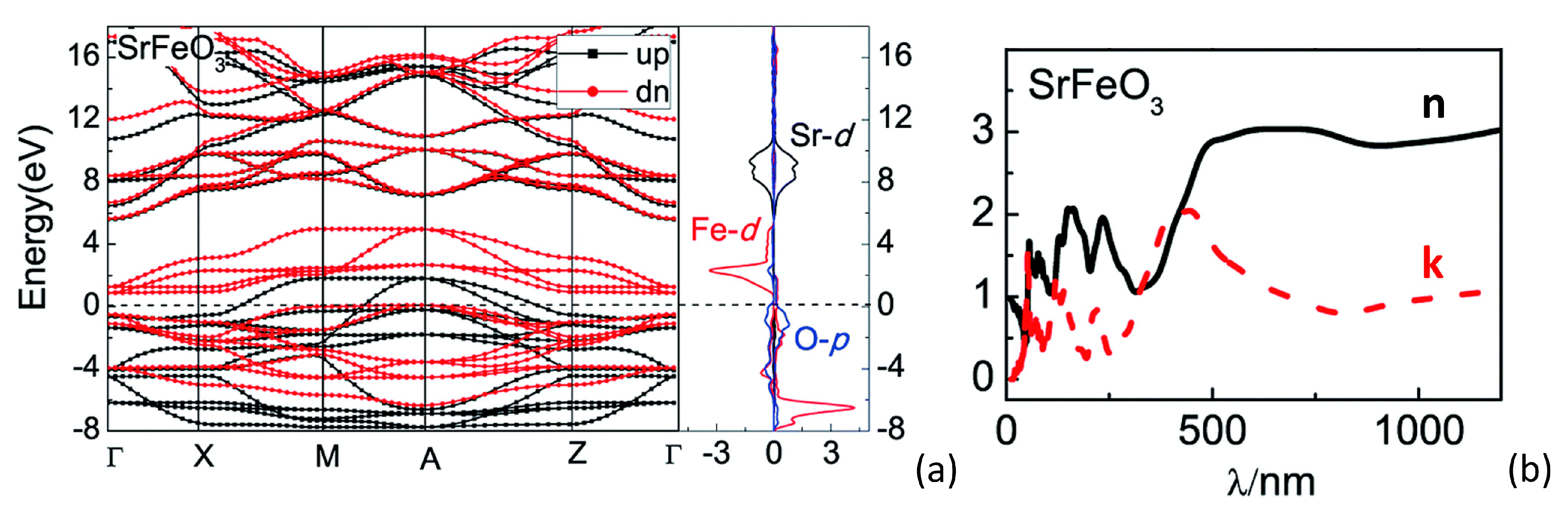
2.1. Chemical–Physical Properties of Undoped Strontium Ferrates
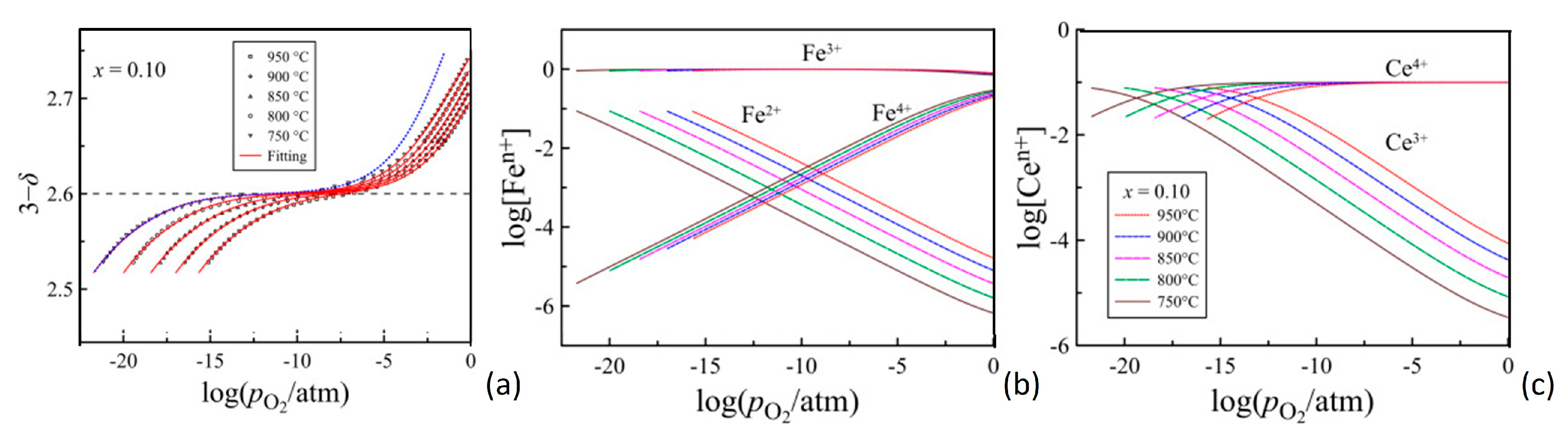
2.2. Chemical–Physical Properties of Cerium-Doped Strontium Ferrates
2.3. Preparation Procedures of Doped SrFeO3 Perovskite Oxides
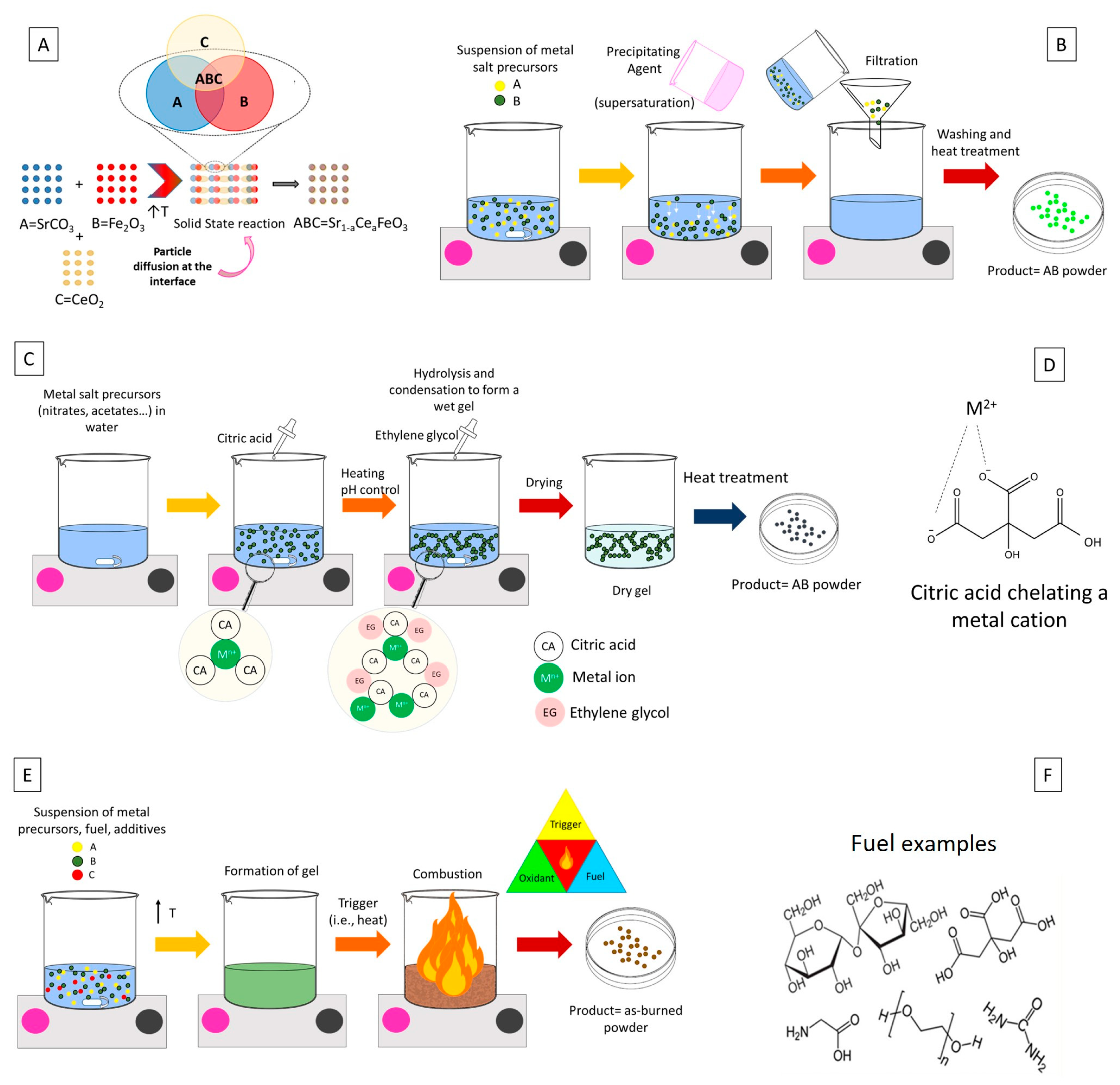
2.3.1. Solid-State Methods
2.3.2. Co-Precipitation
2.3.3. Strategies Based on Sol–Gel: Pechini Method and Solution Combustion Synthesis
2.3.4. Other Synthetic Methods and Post-Treatments
2.3.5. Synthesis of Doped SrFeO3 Perovskite Oxides: Important Aspects and General Guidelines
3. Applications of Ce-Doped SrFeO3 Perovskite Materials
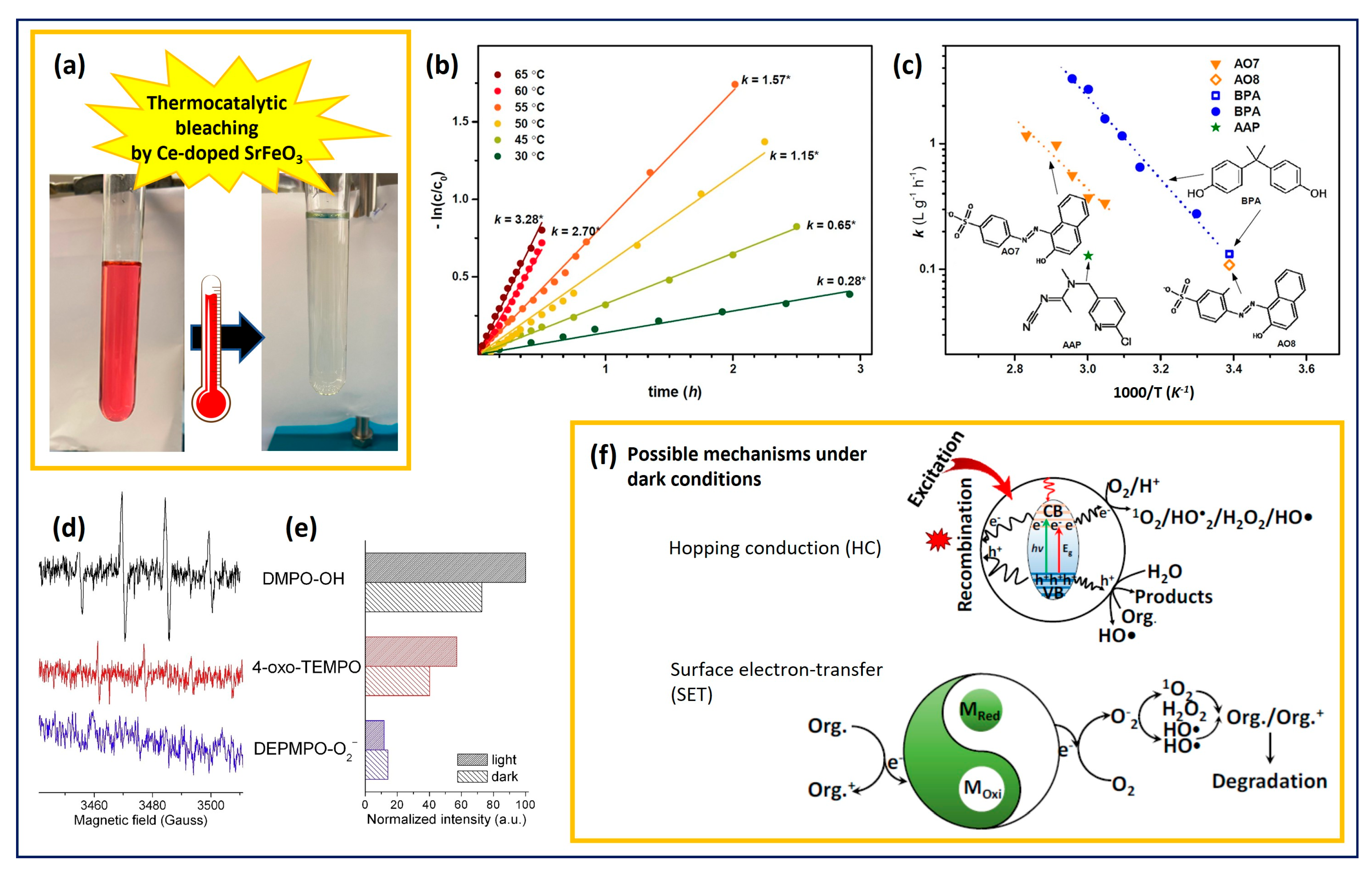
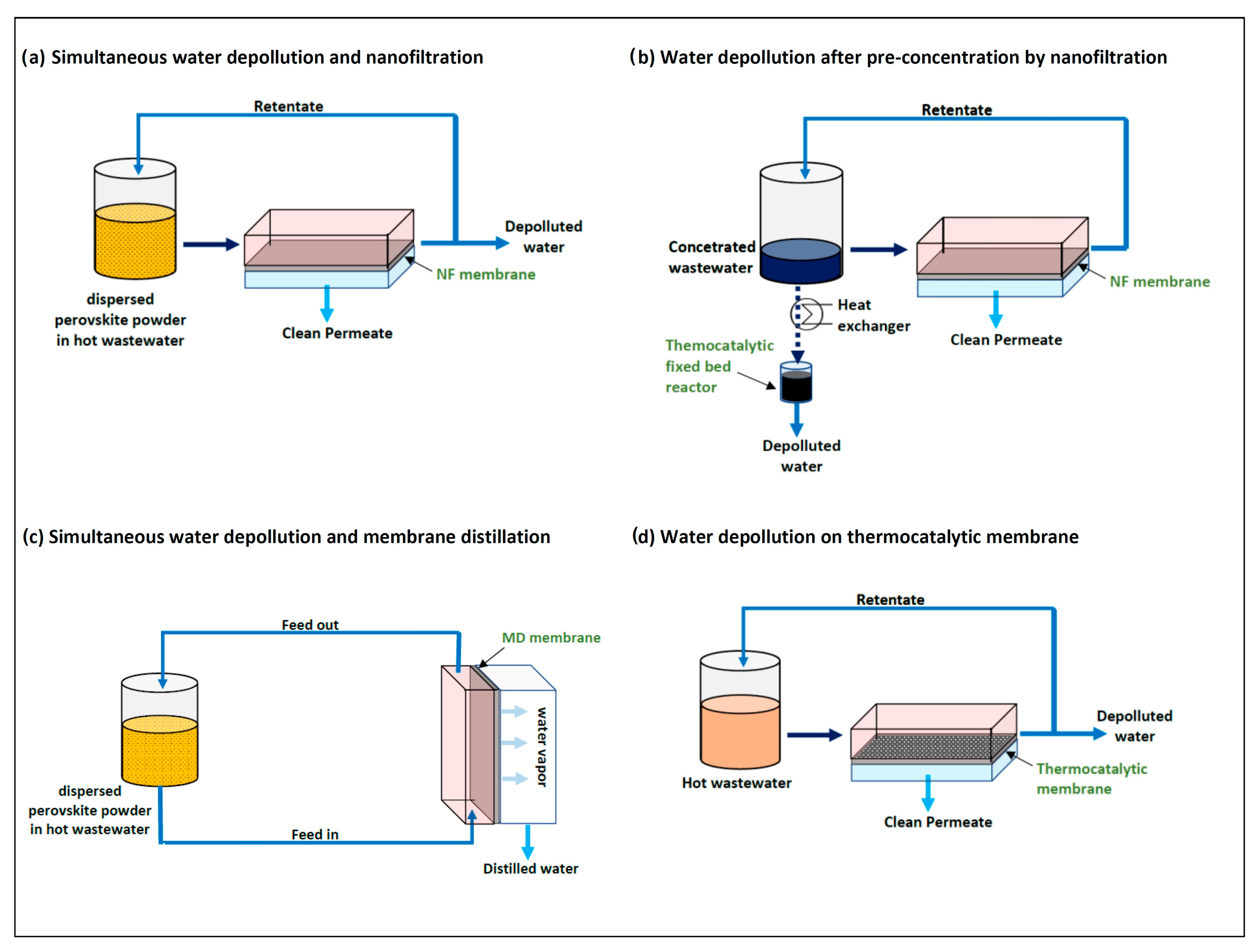
3.1. Thermocatalysts for Wastewater Cleaning
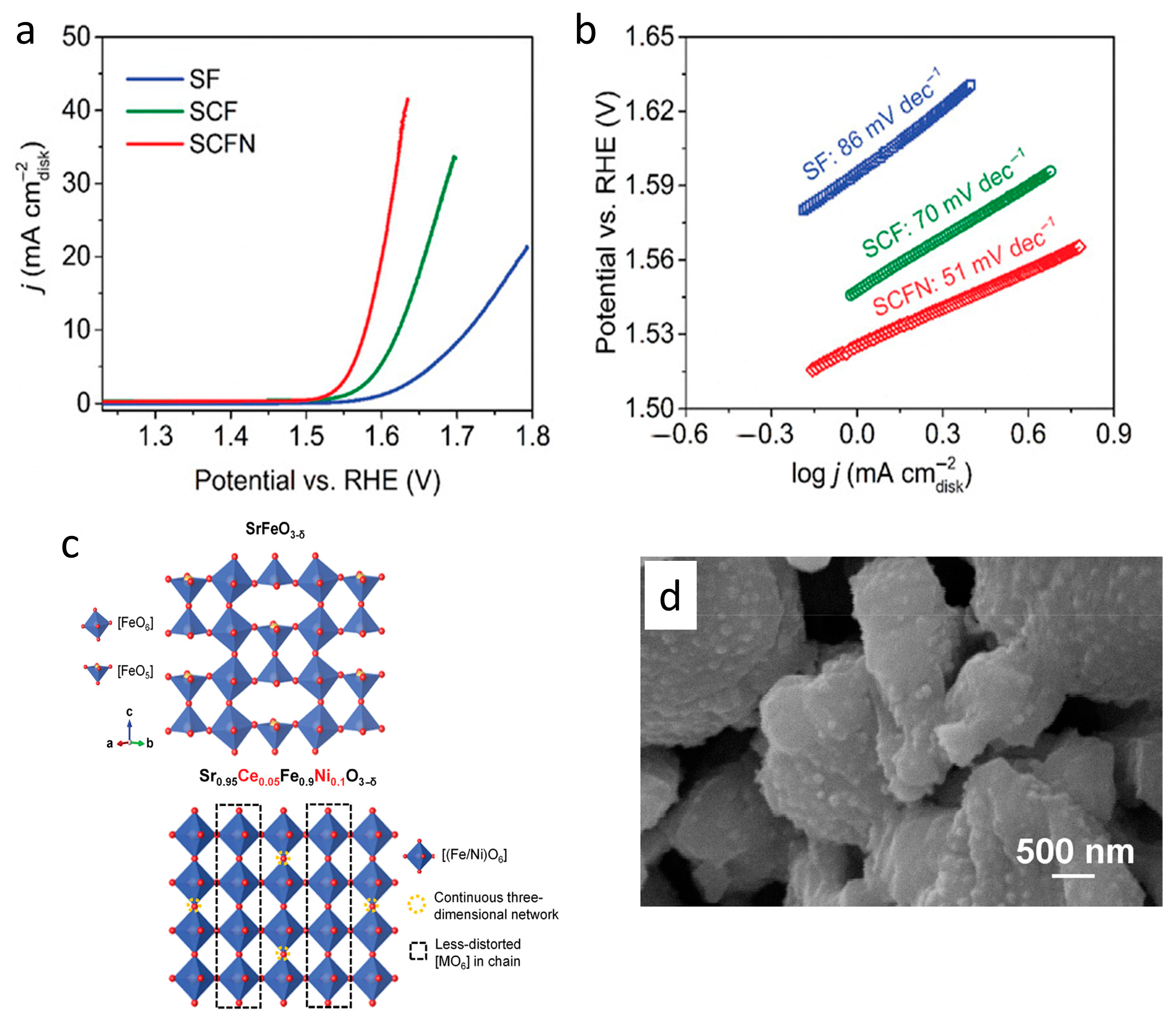
3.2. Electrocatalysts for Energy Production
| Compound | Application | Main Results | References |
|---|---|---|---|
| Sr0.94Ce0.06FeO3−δ | SOFCs | Cubic structure; Ce solubility < 0.15; Ce optimal amount (0.06) improved structural and redox properties with the lowest ASR. | [77] |
| Sr0.9Ce0.1FeO3 | Electrochemical cell | Structure: a mixture of tetragonal and orthorhombic phases; Ce solubility ≤ 0.15; Ce doping caused high oxygen exchange and electrical conductivity. | [91,101] |
| Sr0.5Sm0.5FeO3−δ | SSOFCs | Cubic structure (stable under reducing conditions up to 750 °C); Sm solubility(max) reported = 0.5; the material exhibited good structural stability and catalytic activity in a redox atmosphere at IT and a semiconductor behavior both in cathode and anode atmospheres. | [184] |
| Sr0.5Bi0.5FeO3−δ | IT-SOFCs | Cubic structure; Bi solubility(max) reported = 0.5; Bi-doping promoted TEC compatibility, oxygen ionic mobility and low ASR. | [185] |
| Sr0.7Ba0.3FeO3−δ | MIEC systems | Cubic structure (orthorhombic after reduction); Ba solubility = 0.5; Ba optimal amount (0.3) enhanced the oxygen permeability and reduction tolerance. | [186] |
| Sr10.8Gd0.2FeO3−δ | SSOFCs | 20% Gd improved electrocatalytic activity compared with recognized perovskite oxides like La0.75Sr0.25Cr0.5Mn0.5O3−δ and Sr2Fe1.5Mo0.5O6−δ. | [113] |
| Sr0.35Pr0.65FeO3−δ | SSOFCs | (Sr1−xPrx)0.95FeO3−δ series had a progressive phase transition up to the orthorhombic phase for x ≥0.6. The total electrical conductivity and the polarization resistance in air were comparable across the series, while the highest conductivity and lowest polarization resistance under reducing conditions were for Pr0.6, which was identified as optimal, with moderate thermal expansion coefficients and improved electrical properties and stability. | [132] |
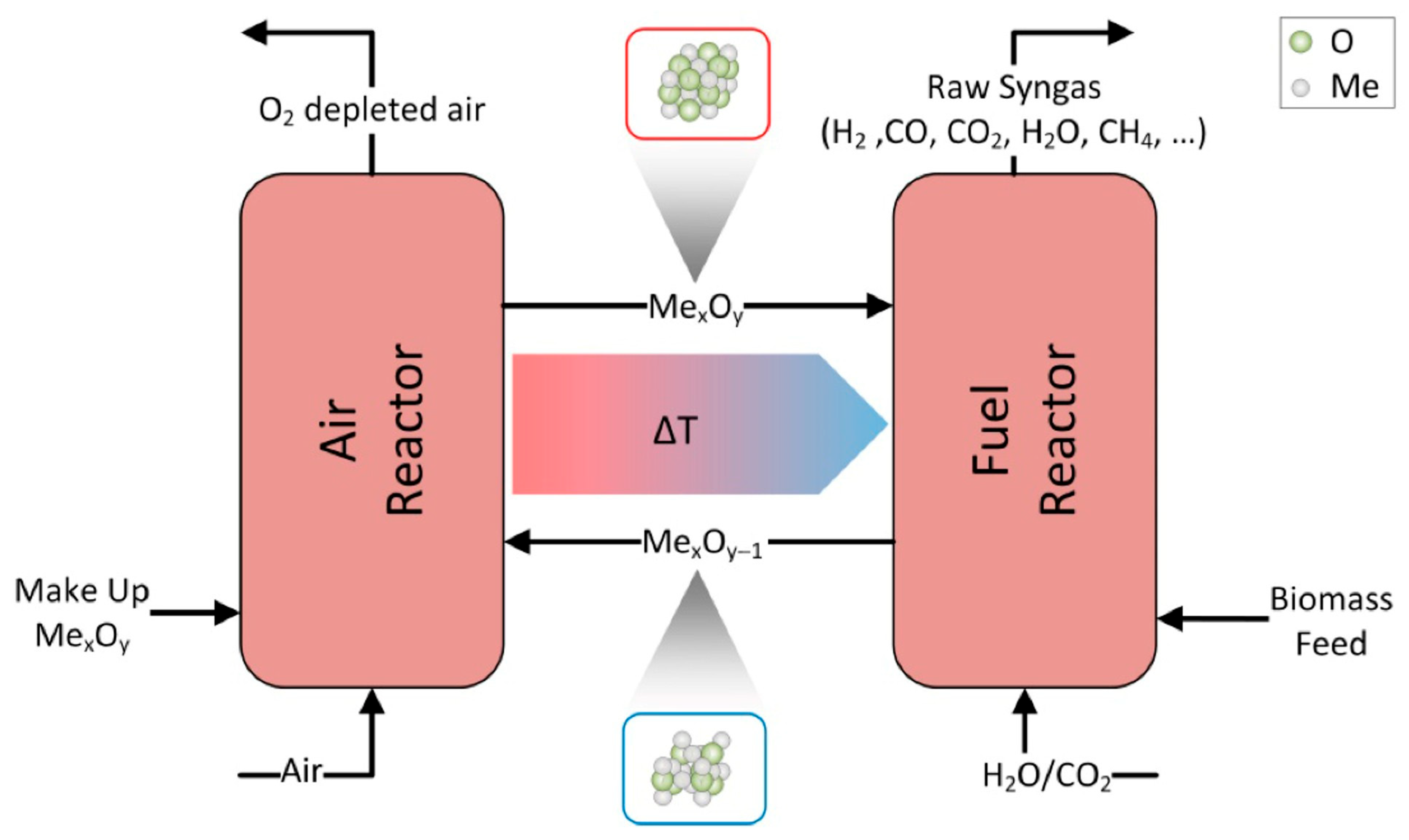
3.3. Oxygen Carriers for Chemical Looping Technologies
3.4. Other Applications
4. Limitations and Challenges for Technological Applications of Ce-Doped SrFeO3
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michalak, A.M.; Xia, J.; Brdjanovic, D.; Mbiyozo, A.-N.; Sedlak, D.; Pradeep, T.; Lall, U.; Rao, N.; Gupta, J. The Frontiers of Water and Sanitation. Nat. Water 2023, 1, 10–18. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Circular Economy Strategies for Combating Climate Change and Other Environmental Issues. Environ. Chem. Lett. 2022, 21, 55–80. [Google Scholar] [CrossRef]
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A.; Tahir, A.A.; Tseng, K.J.; Sayed, E.T.; et al. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Baraneedharan, P.; Vadivel, S.; A, A.C.; Mohamed, S.B.; Rajendran, S. Advances in Preparation, Mechanism and Applications of Various Carbon Materials in Environmental Applications: A Review. Chemosphere 2022, 300, 134596. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, H.; Lin, N.; Gong, Y.; Jiang, H.; Chen, J.; Wang, Y.; Zhang, X. Oxygen-Deficient Engineering for Perovskite Oxides in the Application of AOPs: Regulation, Detection, and Reduction Mechanism. Catalysts 2023, 13, 148. [Google Scholar] [CrossRef]
- Tummino, M.L.; Varesano, A.; Copani, G.; Vineis, C. A Glance at Novel Materials, from the Textile World to Environmental Remediation. J. Polym. Environ. 2023, 31, 2826–2854. [Google Scholar] [CrossRef]
- He, Z.; Ong, J.H.; Bao, Y.; Hu, X. Chemocatalytic Ceramic Membranes for Removing Organic Pollutants in Wastewater: A Review. J. Environ. Chem. Eng. 2023, 11, 109548. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Boumeriame, H.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Boosting Carbon Nitride Photoactivity by Metal-Free Functionalization for Selective H2O2 Synthesis under Visible Light. ACS Sustain. Chem. Eng. 2023, 11, 894–909. [Google Scholar] [CrossRef]
- von Vacano, B.; Mangold, H.; Vandermeulen, G.W.M.; Battagliarin, G.; Hofmann, M.; Bean, J.; Künkel, A. Sustainable Design of Structural and Functional Polymers for a Circular Economy. Angew. Chem. Int. Ed. 2023, 62, e202210823. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmad, T.; Naaz, F.; Islam, S.u. Review on Metals and Metal Oxides in Sustainable Energy Production: Progress and Perspectives. Energy Fuels 2023, 37, 1577–1632. [Google Scholar] [CrossRef]
- Dahlan, I.; Keat, O.H.; Aziz, H.A.; Hung, Y.T. Synthesis and Characterization of MOF-5 Incorporated Waste-Derived Siliceous Materials for the Removal of Malachite Green Dye from Aqueous Solution. Sustain. Chem. Pharm. 2023, 31, 100954. [Google Scholar] [CrossRef]
- Qin, B.; Wu, Y.; Qiu, R.; Ruan, J. Preparing La-Doped LiAl5O8 from the Electrode Materials of Waste Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2023, 11, 1386–1393. [Google Scholar] [CrossRef]
- Boukayouht, K.; Bazzi, L.; El Hankari, S. Sustainable Synthesis of Metal-Organic Frameworks and Their Derived Materials from Organic and Inorganic Wastes. Coord. Chem. Rev. 2023, 478, 214986. [Google Scholar] [CrossRef]
- Çelik, B.; Kandemir, D.; Luleburgaz, S.; Çakmakçi, E.; Saim Gunay, U.; Kumbaraci, V.; Durmaz, H. Propiolated Castor Oil: A Novel and Highly Versatile Bio-Based Platform for Extremely Fast, Catalyst-, and Solvent-Free Amino-Yne Click Reactions. ACS Sustain. Chem. Eng. 2023, 11, 831–841. [Google Scholar] [CrossRef]
- Quinson, J.; Kunz, S.; Arenz, M. Surfactant-Free Colloidal Syntheses of Precious Metal Nanoparticles for Improved Catalysts. ACS Catal. 2023, 13, 4903–4937. [Google Scholar] [CrossRef]
- Souri, M.; Salar Amoli, H. Gas Sensing Mechanisms in ABO3 Perovskite Materials at Room Temperature: A Review. Mater. Sci. Semicond. Process. 2023, 156, 107271. [Google Scholar] [CrossRef]
- Ning, Y.; Pu, Y.; Zhang, Q.; Zhou, S.; Wu, C.; Zhang, L.; Shi, Y.; Sun, Z. Achieving High Energy Storage Properties in Perovskite Oxide via High-Entropy Design. Ceram. Int. 2023, 49, 12214–12223. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Sun, Z. Metal Exsolution Engineering on Perovskites for Electrocatalysis: A Perspective. Mater. Today Energy 2023, 31, 101216. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite Oxides: Materials Science in Catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Li, Z.; Israr, M.; Khan, A.; Luo, W.; Wang, C.; Shao, Z. Perovskite Type ABO3 Oxides in Photocatalysis, Electrocatalysis, and Solid Oxide Fuel Cells: State of the Art and Future Prospects. Chem. Rev. 2025, 125, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, Y.; An, L.; Wu, S.; Zhang, N.; Jin, J.; Wang, R.; Xi, P. Regulation of Perovskite Oxides Composition for the Efficient Electrocatalytic Reactions. Smart Mol. 2023, 1, e20220005. [Google Scholar] [CrossRef]
- Katz, E.A. Perovskite: Name Puzzle and German-Russian Odyssey of Discovery. Helv. Chim. Acta 2020, 103, e2000061. [Google Scholar] [CrossRef]
- Ubic, R.; Subodh, G. The Prediction of Lattice Constants in Orthorhombic Perovskites. J. Alloys Compd. 2009, 488, 374–379. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze Der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Huang, C.Y.; Li, H.; Wu, Y.; Lin, C.H.; Guan, X.; Hu, L.; Kim, J.; Zhu, X.; Zeng, H.; Wu, T. Inorganic Halide Perovskite Quantum Dots: A Versatile Nanomaterial Platform for Electronic Applications. Nano-Micro Lett. 2022, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, W.; Gu, H.; Fu, Y.; Wang, B.; Cai, H.; Xia, J.; Zhang, N.; Liang, C.; Xing, G.; et al. Perovskite Solar Cell Powered Integrated Fuel Conversion and Energy Storage Devices. Adv. Mater. 2023, 35, 2300383. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Gholamrezaei, S.; Salavati-Niasari, M. Sonochemical Synthesis of SrMnO3 Nanoparticles as an Efficient and New Catalyst for O2 Evolution from Water Splitting Reaction. Ultrason. Sonochemistry 2018, 40, 651–663. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L. Nanocrystalline SrMnO3 Perovskite Prepared by Sol–Gel Self-Combustion Method for Sensor Applications. J. Sol-Gel Sci. Technol. 2021, 97, 146–154. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Cao, H.; Chi, Z.; Chen, C.; Duan, X.; Xie, Y.; Qi, F.; Song, W.; Liu, J.; et al. Role of Oxygen Vacancies and Mn Sites in Hierarchical Mn2O3/LaMnO3-δ Perovskite Composites for Aqueous Organic Pollutants Decontamination. Appl. Catal. B Environ. 2019, 245, 546–554. [Google Scholar] [CrossRef]
- Luo, K.; Zheng, Q.; Yu, Y.; Wang, C.; Jiang, S.; Zhang, H.; Liu, Y.; Luo, K.; Zheng, Q.; Yu, Y.; et al. Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery. Batteries 2023, 9, 90. [Google Scholar] [CrossRef]
- Deganello, F.; Oko, D.N.; Testa, M.L.; La Parola, V.; Tummino, M.L.; Soares, C.O.; Rivera, J.G.; Orozco, G.; Guay, D.; Tavares, A.C. Perovskite-Type Catalysts Prepared by Nanocasting: Effect of Metal Silicates on the Electrocatalytic Activity toward Oxygen Evolution and Reduction Reactions. ACS Appl. Energy Mater. 2018, 1, 2565–2575. [Google Scholar] [CrossRef]
- Li, H.; Li, P.; Lin, M.; Zhu, X. Effects of LaFeO3 Morphology on Oxygen Species and Chemical Looping Partial Oxidation of Methane. Chem. Mater. 2025, 37, 2931–2942. [Google Scholar] [CrossRef]
- Christensen, B.H.; Deganello, F.; La Parola, V.; Jørgensen, M.K.; Boffa, V.; Østergaard, M.B. Thermocatalytic Performance of LaCo1−xNixO3−δ Perovskites in the Degradation of Rhodamine B. Catalysts 2023, 13, 325. [Google Scholar] [CrossRef]
- Dragan, M.; Enache, S.; Varlam, M.; Petrov, K.; Dragan, M.; Enache, S.; Varlam, M.; Petrov, K. Perovskite-Type Lanthanum Cobaltite LaCoO3: Aspects of Processing Route toward Practical Applications. In Cobalt Compounds and Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Dou, B.; Ma, Y.; Liang, Y. The Investigation of SrCoO3-δ Perovskite Doping Cobalt Oxide Thermochemical Energy Storage System. J. Taiwan Inst. Chem. Eng. 2022, 136, 104406. [Google Scholar] [CrossRef]
- Zeng, P.; Ran, R.; Chen, Z.; Zhou, W.; Gu, H.; Shao, Z.; Liu, S. Efficient Stabilization of Cubic Perovskite SrCoO3−δ by B-Site Low Concentration Scandium Doping Combined with Sol–Gel Synthesis. J. Alloys Compd. 2008, 455, 465–470. [Google Scholar] [CrossRef]
- Shi, K.; Yin, Y.; Tang, Z.; Yu, S.; Zhang, Q. Ba-Deficiency in BaCoO3 Cathode Allows High Performance for Proton-Conducting Solid Oxide Fuel Cells. Ceram. Int. 2022, 48, 13024–13031. [Google Scholar] [CrossRef]
- Hu, X.; Lu, P.; Fu, M.; Chen, Z.; He, Y.; Bai, J.; Zhou, X. Simple Synthesis of the Novel Adsorbent BaCO3/g-C3N4 for Rapid and High-Efficient Selective Removal of Crystal Violet. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124948. [Google Scholar] [CrossRef]
- Grisolia, M.N.; Varignon, J.; Sanchez-Santolino, G.; Arora, A.; Valencia, S.; Varela, M.; Abrudan, R.; Weschke, E.; Schierle, E.; Rault, J.E.; et al. Hybridization-Controlled Charge Transfer and Induced Magnetism at Correlated Oxide Interfaces. Nat. Phys. 2016, 12, 484–492. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zuo, F.; Li, F.; Chan, H.; Wu, Q.; Zhang, Z.; Narayanan, B.; Ramadoss, K.; Chakraborty, I.; Saha, G.; et al. Perovskite Nickelates as Bio-Electronic Interfaces. Nat. Commun. 2019, 10, 1651. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Ma, C.; Lu, L.; Han, C.Y.; Liu, M. Unravelling the Role of Oxygen Vacancies on the Current Transport Mechanisms in All-Perovskite Nickelate/Titanate Heterojunctions for Nonvolatile Memory Applications. J. Appl. Phys. 2022, 132, 135303. [Google Scholar] [CrossRef]
- Bin Adnan, M.A.; Arifin, K.; Minggu, L.J.; Kassim, M.B. Titanate-Based Perovskites for Photochemical and Photoelectrochemical Water Splitting Applications: A Review. Int. J. Hydrogen Energy 2018, 43, 23209–23220. [Google Scholar] [CrossRef]
- Paramanik, L.; Subudhi, S.; Parida, K.M. Visible Light Active Titanate Perovskites: An Overview on Its Synthesis, Characterization and Photocatalytic Applications. Mater. Res. Bull. 2022, 155, 111965. [Google Scholar] [CrossRef]
- Porta, P.; Cimino, S.; De Rossi, S.; Faticanti, M.; Minelli, G.; Pettiti, I. AFeO3 (A=La, Nd, Sm) and LaFe1−xMgxO3 Perovskites: Structural and Redox Properties. Mater. Chem. Phys. 2001, 71, 165–173. [Google Scholar] [CrossRef]
- Tummino, M.L. SrFeO3 Peculiarities and Exploitation in Decontamination Processes and Environmentally-Friendly Energy Applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100339. [Google Scholar] [CrossRef]
- Kumar, S.; Das, A.; Omar, S. Electrochemical Performance of SrFeO3−δ for Application as a Symmetric Electrode in Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2023, 6, 2049–2062. [Google Scholar] [CrossRef]
- Liu, X.; Pan, S.-k.; Zhong, X.; Cheng, L.-c. Microwave-Absorbing Properties of Strontium Ferrites Prepared via Sol-Gel Method. Cryst. Res. Technol. 2017, 52, 1700057. [Google Scholar] [CrossRef]
- Ferreira, S.; Carvalho, R.; Ferreira, J.; Teixeira, S.S.; Ferreira, R.; Carvalho, J.; Ferreira, N.M. Unveiling the Synthesis of Strontium Ferrites by Sol-Gel and Laser Floating Zone Methods for Energy Application. Crystals 2024, 14, 550. [Google Scholar] [CrossRef]
- Kokarovtseva, I.G.; Belyaev, I.N.; Semenyakova, L.V. Oxygen Compounds of Iron(VI, V, IV). Russ. Chem. Rev. 1972, 41, 929–937. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T.; Currie, D.B. The Synthesis and Structure of Sr2FeO4. J. Solid State Chem. 1991, 92, 237–240. [Google Scholar] [CrossRef]
- Dar, A.A.; Pan, B.; Qin, J.; Zhu, Q.; Lichtfouse, E.; Usman, M.; Wang, C. Sustainable Ferrate Oxidation: Reaction Chemistry, Mechanisms and Removal of Pollutants in Wastewater. Environ. Pollut. 2021, 290, 117957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K. Oxidation of Inorganic Contaminants by Ferrates (VI, V, and IV)–Kinetics and Mechanisms: A Review. J. Environ. Manag. 2011, 92, 1051–1073. [Google Scholar] [CrossRef] [PubMed]
- Homonnay, Z.; Stichleutner, S.; Kuzmann, E.; Kuti, M.; Láng, G.G.; Béres, K.A.; Trif, L.; Nagy, D.J.; Záray, G.; Lendvai, J. Quantitative Analysis of Ferrate(VI) and Its Degradation Products in Electrochemically Produced Potassium Ferrate for Waste Water Treatment. Appl. Sci. 2024, 14, 9144. [Google Scholar] [CrossRef]
- Hosaka, Y.; Ichikawa, N.; Saito, T.; Manuel, P.; Khalyavin, D.; Attfield, J.P.; Shimakawa, Y. Two-Dimensional Charge Disproportionation of the Unusual High Valence State Fe4+ in a Layered Double Perovskite. J. Am. Chem. Soc. 2015, 137, 7468–7473. [Google Scholar] [CrossRef]
- Ahmed, R.; Wang, S.T.; Sun, J.; Wang, J.; Li, T.Y.; Yu, Y.; Li, Q.J.; Wang, C.C. Colossal Dielectric Behavior in BaFeO3-δ Ceramics. Ceram. Int. 2019, 45, 13484–13487. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Motawea, M.A.; Aboelnasr, M.A.; El-Bahnasawy, H.H. Study the Oxygen Vacancies and Fe Oxidation States in CaFeO3-δ Perovskite Nanomaterial. Phys. B Condens. Matter 2022, 624, 413415. [Google Scholar] [CrossRef]
- Hamdad, N.; Rozale, H.; Lakdja, A.; Chahed, A.; Benhelal, O. New Theoretical Investigation on the Electronic Structure and Magnetic Interaction for Both Cubic SrFeO3 and CaFeO3 Oxides: Comparison between GGA and GGA+U Approaches. Superlattices Microstruct. 2013, 63, 182–196. [Google Scholar] [CrossRef]
- Yuan, N.; Han, Z.; Guo, Q.; Jian, H.; Ma, J.; Bai, H. Chemical Looping Combustion Characteristics and Kinetic Behaviour of Sr-Doped Perovskite-Type CaFeO3 Oxygen Carriers: Theoretical and Experimental Investigations. Can. J. Chem. Eng. 2023, 101, 1577–1590. [Google Scholar] [CrossRef]
- Hombo, J.; Matsumoto, Y.; Kawano, T. Electrical Conductivities of SrFeO3−δ and BaFeO3−δ Perovskites. J. Solid State Chem. 1990, 84, 138–143. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, H.; Liu, Y.; Sun, Q.; Lu, X.; Li, S. CO Oxidation Mechanism on Surfaces of B-Site Doped SrFeO3-δ-Based Perovskite Materials for Thermochemical Water Splitting. Comput. Theor. Chem. 2023, 1224, 114109. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, L.; Dou, Y.; Li, Q.; Li, N.; Huo, L.; Zhao, H. Insights into the Oxygen Reduction Reaction on Cu-Doped SrFeO3-δ Cathode for Solid Oxide Fuel Cells. J. Power Sources 2021, 497, 229877. [Google Scholar] [CrossRef]
- Huan, D.; Zhang, L.; Zhu, K.; Li, X.; Zhang, B.; Shi, J.; Peng, R.; Xia, C. Tailoring the Structural Stability, Electrochemical Performance and CO2 Tolerance of Aluminum Doped SrFeO3. Sep. Purif. Technol. 2022, 290, 120843. [Google Scholar] [CrossRef]
- Su, T.; Zhang, T.; Xie, H.; Zhong, J.; Xia, C. Investigation into Structure and Property of W and Ti Co-Doped SrFeO3 Perovskite as Electrode of Symmetrical Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2022, 47, 16272–16282. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Tian, Y.; Wang, Z.; Pu, J.; Ciucci, F.; Chi, B. Electrochemical Performance of Symmetric Solid Oxide Cells Employing a Sc-Doped SrFeO3-δ-Based Electrode. Chem. Eng. J. 2024, 485, 149970. [Google Scholar] [CrossRef]
- Su, T.; Li, Y.; Yang, Y.; Xu, Z.; Shi, N.; Wan, Y.; Xie, Y.; Huan, D.; Xue, S.; Xia, C. Effect of Tungsten Doping on Strontium Ferrite Electrode for Symmetrical Solid Oxide Electrochemical Cell. Int. J. Hydrogen Energy 2020, 45, 23401–23410. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; He, W.; Zhao, P.; Zhang, D.; He, T.; Wang, Y.; Liu, T. Pr and Mo Co-Doped SrFeO3–δ as an Efficient Cathode for Pure CO2 Reduction Reaction in a Solid Oxide Electrolysis Cell. Energy Technol. 2020, 8, 2000539. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the Catalytic Activity of a Doped SrFeO3 for Water Pollutants Removal: Effect of Light and Temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Strunck, A.B.; Jørgensen, M.K.; Boffa, V. Abatement of Oil Residues from Produced Water Using a Thermocatalytic Packed Bed Reactor. J. Environ. Chem. Eng. 2021, 9, 106749. [Google Scholar] [CrossRef]
- Janowska, K.; Boffa, V.; Jørgensen, M.K.; Quist-Jensen, C.A.; Hubac, F.; Deganello, F.; Coelho, F.E.B.; Magnacca, G. Thermocatalytic Membrane Distillation for Clean Water Production. NPJ Clean Water 2020, 3, 34. [Google Scholar] [CrossRef]
- Dou, J.; Krzystowczyk, E.; Wang, X.; Robbins, T.; Ma, L.; Liu, X.; Li, F. A- and B-Site Codoped SrFeO3 Oxygen Sorbents for Enhanced Chemical Looping Air Separation. ChemSusChem 2020, 13, 385–393. [Google Scholar] [CrossRef]
- Wang, X.; Krzystowczyk, E.; Dou, J.; Li, F. Net Electronic Charge as an Effective Electronic Descriptor for Oxygen Release and Transport Properties of SrFeO3-Based Oxygen Sorbents. Chem. Mater. 2021, 33, 2446–2456. [Google Scholar] [CrossRef]
- Hu, S.; Yuan, J.; Tang, S.; Luo, D.; Shen, Q.; Qin, Y.; Zhou, J.; Tang, Q.; Chen, S.; Luo, X.; et al. Perovskite-Type SrFeO3/g-C3N4 S-Scheme Photocatalyst for Enhanced Degradation of Acid Red B. Opt. Mater. 2022, 132, 112760. [Google Scholar] [CrossRef]
- Zheng, K.; Lach, J.; Czaja, P.; Gogacz, M.; Czach, P.; Brzoza-Kos, A.; Winiarz, P.; Luo, J. Designing High-Performance Quasi-Symmetrical Solid Oxide Cells with a Facile Chemical Modification Strategy for Sr2Fe2−xWxO6−δ Ferrites Electrodes with in Situ Exsolution of Nanoparticles. J. Power Sources 2023, 587, 233707. [Google Scholar] [CrossRef]
- Li, Y.; Mushtaq, N.; Chen, Y.; Ye, W.; Zhuang, Z.; Singh, M.; Jing, Y.; Fan, L. Revisiting Mo-Doped SrFeO3-δ Perovskite: The Origination of Cathodic Activity and Longevity for Intermediate-Temperature Solid Oxide Fuel Cells. Adv. Funct. Mater. 2025, 35, 2411025. [Google Scholar] [CrossRef]
- Tummino, M.L.; Liotta, L.F.; Magnacca, G.; Faro, M.L.; Trocino, S.; Zignani, S.C.; Aricò, A.S.; Deganello, F. Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel. Catalysts 2020, 10, 134. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Longo, A.; Casaletto, M.P.; Scopelliti, M. Cerium Effect on the Phase Structure, Phase Stability and Redox Properties of Ce-Doped Strontium Ferrates. J. Solid State Chem. 2006, 179, 3406–3419. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Leonardi, S.G.; Neri, G. Electrochemical Properties of Ce-Doped SrFeO3 Perovskites-Modified Electrodes towards Hydrogen Peroxide Oxidation. Electrochim. Acta 2016, 190, 939–947. [Google Scholar] [CrossRef]
- Cui, J.; Sun, Y.; Yin, C.; Wang, H.; Liu, Z.; Zhou, Z.; Wu, K.; Zhou, J. The Investigation of Ni-Doped SrFeO3−δ Perovskite for a Symmetrical Electrode in Proton Ceramic Fuel Cells. Materials 2025, 18, 1460. [Google Scholar] [CrossRef]
- Hodges, J.P.; Short, S.; Jorgensen, J.D.; Xiong, X.; Dabrowski, B.; Mini, S.M.; Kimball, C.W. Evolution of Oxygen-Vacancy Ordered Crystal Structures in the Perovskite Series SrnFenO3n−1 (n=2, 4, 8, and ∞), and the Relationship to Electronic and Magnetic Properties. J. Solid State Chem. 2000, 151, 190–209. [Google Scholar] [CrossRef]
- Ikeda, H.; Nikata, S.; Hirakawa, E.; Tsuchida, A.; Miura, N. Oxygen Sorption/Desorption Behavior and Crystal Structural Change for SrFeO3−δ. Chem. Eng. Sci. 2016, 147, 166–172. [Google Scholar] [CrossRef]
- Mizusaki, J.; Okayasu, M.; Yamauchi, S.; Fueki, K. Nonstoichiometry and Phase Relationship of the SrFeO2.5—SrFeO3 System at High Temperature. J. Solid State Chem. 1992, 99, 166–172. [Google Scholar] [CrossRef]
- Fossdal, A.; Einarsrud, M.A.; Grande, T. Phase Equilibria in the Pseudo-Binary System SrO–Fe2O3. J. Solid State Chem. 2004, 177, 2933–2942. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.A.; Grande, T. Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite. Materials 2016, 9, 154. [Google Scholar] [CrossRef]
- Han, H.; Sharma, A.; Meyerheim, H.L.; Yoon, J.; Deniz, H.; Jeon, K.R.; Sharma, A.K.; Mohseni, K.; Guillemard, C.; Valvidares, M.; et al. Control of Oxygen Vacancy Ordering in Brownmillerite Thin Films via Ionic Liquid Gating. ACS Nano 2022, 16, 6206–6214. [Google Scholar] [CrossRef]
- Heifets, E.; Kotomin, E.A.; Bagaturyants, A.A.; Maier, J. Thermodynamic Stability of Non-Stoichiometric SrFeO3−δ: A Hybrid DFT Study. Phys. Chem. Chem. Phys. 2019, 21, 3918–3931. [Google Scholar] [CrossRef]
- Falcón, H.; Barbero, J.A.; Alonso, J.A.; Martínez-Lope, M.J.; Fierro, J.L.G. SrFeO3-δ Perovskite Oxides: Chemical Features and Performance for Methane Combustion. Chem. Mater. 2002, 14, 2325–2333. [Google Scholar] [CrossRef]
- Jia, T.; Zeng, Z.; Lin, H.Q.; Duan, Y.; Ohodnicki, P. First-Principles Study on the Electronic, Optical and Thermodynamic Properties of ABO3 (A = La,Sr, B = Fe,Co) Perovskites. RSC Adv. 2017, 7, 38798–38804. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, K.; Li, S.E.; Zhang, Q.; Wang, J.O.; Guo, E.J.; Qian, H.; Gu, L.; Qian, T.; Ibrahim, K.; et al. Electronic-Structure Evolution of SrFeO3–x during Topotactic Phase Transformation. J. Phys. Condens. Matter 2021, 34, 064001. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zheng, C.; Zhao, H. Ce-Modified SrFeO3-δ for Ethane Oxidative Dehydrogenation Coupled with CO2 Splitting via a Chemical Looping Scheme. Appl. Catal. B Environ. 2022, 303, 120894. [Google Scholar] [CrossRef]
- Trofimenko, N.E.; Ullmann, H. Oxygen Stoichiometry and Mixed Ionic-Electronic Conductivity of Sr1−aCeaFe1−bCobO3−x Perovskite-Type Oxides. J. Eur. Ceram. Soc. 2000, 20, 1241–1250. [Google Scholar] [CrossRef]
- Markov, A.A.; Nikitin, S.S.; Politov, B.V.; Shalaeva, E.V.; Tyutyunnik, A.P.; Leonidov, I.A.; Patrakeev, M.V. The Impact of Cerium Content on Oxygen Stoichiometry, Defect Equilibrium, and Thermodynamic Quantities of Sr1−xCexFeO3−δ. J. Alloys Compd. 2021, 875, 160051. [Google Scholar] [CrossRef]
- D’angelo, A.M.; Chaffee, A.L. Correlations between Oxygen Uptake and Vacancy Concentration in Pr-Doped CeO2. ACS Omega 2017, 2, 2544–2551. [Google Scholar] [CrossRef]
- Yang, N.; Orgiani, P.; Di Bartolomeo, E.; Foglietti, V.; Torelli, P.; Ievlev, A.V.; Rossi, G.; Licoccia, S.; Balestrino, G.; Kalinin, S.V.; et al. Effects of Dopant Ionic Radius on Cerium Reduction in Epitaxial Cerium Oxide Thin Films. J. Phys. Chem. C 2017, 121, 8841–8849. [Google Scholar] [CrossRef]
- Chauhan, S.; Jaiswal, S.K. Investigation on Structure and Stability of Cerium Doped (Ba0.5Sr0.5)(Fe1-XCex)O3-δ (x = 0–1.0) Oxides by Rietveld Refinement. Solid State Commun. 2021, 334–335, 114343. [Google Scholar] [CrossRef]
- Penwell, W.D.; Giorgi, J.B. Conductivity of Cerium Doped BaFeO3−δ and Applications for the Detection of Oxygen. Sens. Actuators B Chem. 2014, 191, 171–177. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Yang, W. Structural Stability and Oxygen Permeability of Cerium Lightly Doped BaFeO3−δ Ceramic Membranes. Solid State Ion. 2006, 177, 2917–2921. [Google Scholar] [CrossRef]
- Choi, H.; Fuller, A.; Davis, J.; Wielgus, C.; Ozkan, U.S. Ce-Doped Strontium Cobalt Ferrite Perovskites as Cathode Catalysts for Solid Oxide Fuel Cells: Effect of Dopant Concentration. Appl. Catal. B Environ. 2012, 127, 336–341. [Google Scholar] [CrossRef]
- Tummino, M.L.; Vineis, C.; Varesano, A.; Liotta, L.F.; Rigoletto, M.; Laurenti, E.; Deganello, F.; Frontistis, Z.; Ozkara-Aydinoglu, S.; Dhainaut, J. Sr0.85Ce0.15Fe0.67Co0.33-XCuxO3 Perovskite Oxides: Effect of B-Site Copper Codoping on the Physicochemical, Catalytic and Antibacterial Properties upon UV or Thermal Activation. Front. Environ. Eng. 2023, 2, 1249931. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Deganello, F.; La Parola, V.; Liotta, L.F.; Boffa, V.; Jørgensen, M.K. Beneficial Effect of Cerium Excess on in Situ Grown Sr0.86Ce0.14FeO3–CeO2 Thermocatalysts for the Degradation of Bisphenol A. RSC Adv. 2023, 13, 21459–21470. [Google Scholar] [CrossRef]
- Trofimenko, N.E.; Ullmann, H.; Paulsen, J.; Müller, R. Structure, Oxygen Stoichiometry and Electrical Conductivity in the System Sr-Ce-Fe-O. Solid State Ion. 1997, 99, 201–214. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Yu, X.; Li, Z.; Zhao, Y.; Chai, J. Study on Ce and Y Co-Doped BaFeO3-δ Cubic Perovskite as Free-Cobalt Cathode for Proton-Conducting Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 23868–23878. [Google Scholar] [CrossRef]
- Safe and Sustainable by Design Chemicals and Materials—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/eb0a62f3-031b-11ed-acce-01aa75ed71a1/language-en (accessed on 10 June 2023).
- Meng, Y.; Sun, L.; Gao, J.; Tan, W.; Chen, C.; Yi, J.; Bouwmeester, H.J.M.; Sun, Z.; Brinkman, K.S. Insights into the CO2 Stability-Performance Trade-Off of Antimony-Doped SrFeO3-δ Perovskite Cathode for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 11498–11506. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, G.; Xu, R.; Hu, R.; Liu, L.; Jiang, G.; Demir, M.; Ma, P. SrFe1-XZrxO3-δ Perovskite Oxides as Negative Electrodes for Supercapacitors. Electrochim. Acta 2023, 437, 141527. [Google Scholar] [CrossRef]
- Ghaffari, M.; Huang, H.; Tan, P.Y.; Tan, O.K. Synthesis and Visible Light Photocatalytic Properties of SrTi(1−x)FexO(3−δ) Powder for Indoor Decontamination. Powder Technol. 2012, 225, 221–226. [Google Scholar] [CrossRef]
- Duell, B.A.; Ramazani, A.; Natesakhawat, S.; Popczun, E.J.; Lekse, J.W.; Duan, Y. Targeted Chemical Looping Materials Discovery by an Inverse Design. Adv. Intell. Syst. 2025, 7, 2401118. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, H.; Xu, S.; Xu, X.; Jiang, J.; Xiao, B.; Julião, P.S.B.; Su, C.; Chen, D.; Zhou, W. Investigation and Optimization of High-Valent Ta-Doped SrFeO3−δ as Air Electrode for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Miner. Metall. Mater. 2024, 31, 2102–2109. [Google Scholar] [CrossRef]
- Dai, H.; Du, H.; Boulfrad, S.; Yu, S.; Bi, L.; Zhang, Q. Manipulating Nb-Doped SrFeO3−δ with Excellent Performance for Proton-Conducting Solid Oxide Fuel Cells. J. Adv. Ceram. 2024, 13, 579–589. [Google Scholar] [CrossRef]
- Athayde, D.D.; Souza, D.F.; Silva, A.M.A.; Vasconcelos, D.; Nunes, E.H.M.; Da Costa, J.C.D.; Vasconcelos, W.L. Review of Perovskite Ceramic Synthesis and Membrane Preparation Methods. Ceram. Int. 2016, 42, 6555–6571. [Google Scholar] [CrossRef]
- Krzystowczyk, E.; Wang, X.; Dou, J.; Haribal, V.; Li, F. Substituted SrFeO3 as Robust Oxygen Sorbents for Thermochemical Air Separation: Correlating Redox Performance with Compositional and Structural Properties. Phys. Chem. Chem. Phys. 2020, 22, 8924–8932. [Google Scholar] [CrossRef]
- Luongo, G.; Donat, F.; Müller, C.R. Structural and Thermodynamic Study of Ca A- or Co B-Site Substituted SrFeO3−δ Perovskites for Low Temperature Chemical Looping Applications. Phys. Chem. Chem. Phys. 2020, 22, 9272–9282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, G.; Zhou, X.; Zhang, H.; Jiang, H.; Jin, Y.; Chu, L.; Huang, M. Gadolinium-Doped SrFeO3 as a Highly Active and Stable Electrode for Symmetrical Solid Oxide Fuel Cells. Mater. Today Energy 2024, 44, 101615. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Compana, J.M.; Bruque, S.; Losilla, E.R.; Marrero-López, D. Symmetric Electrodes for Solid Oxide Fuel Cells Based on Zr-Doped SrFeO3-δ. J. Power Sources 2015, 279, 419–427. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, M.; Zhang, X.; Si, C.; Tai, C.; Lu, Q.; Wei, M.; Han, X.; Ma, J.; Chen, S.; et al. Boosting Oxygen/Hydrogen Evolution Catalysis via Ruthenium Doping in Perovskite Oxide for Efficient Alkaline Water Splitting. Appl. Surf. Sci. 2024, 664, 160278. [Google Scholar] [CrossRef]
- Deganello, F.; Marcì, G.; Deganello, G. Citrate-Nitrate Auto-Combustion Synthesis of Perovskite-Type Nanopowders: A Systematic Approach. J. Eur. Ceram. Soc. 2009, 29, 439–450. [Google Scholar] [CrossRef]
- Siddique, F.; Gonzalez-Cortes, S.; Mirzaei, A.; Xiao, T.; Rafiq, M.A.; Zhang, X. Solution Combustion Synthesis: The Relevant Metrics for Producing Advanced and Nanostructured Photocatalysts. Nanoscale 2022, 14, 11806–11868. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, E.; Kelly, J.P.; Bhaduri, S.; Graeve, O.A. A Review of Solution Combustion Synthesis: An Analysis of Parameters Controlling Powder Characteristics. Int. Mater. Rev. 2021, 66, 188–214. [Google Scholar] [CrossRef]
- Parauha, Y.R.; Sahu, V.; Dhoble, S.J. Prospective of Combustion Method for Preparation of Nanomaterials: A Challenge. Mater. Sci. Eng. B 2021, 267, 115054. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution Combustion Synthesis, Energy and Environment: Best Parameters for Better Materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Wang, S.; Komvokis, V.G.; Amiridis, M.D.; Heyden, A.; Ma, S.; Chen, F. Synthesis and Characterization of Mo-Doped SrFeO3−δ as Cathode Materials for Solid Oxide Fuel Cells. J. Power Sources 2012, 202, 63–69. [Google Scholar] [CrossRef]
- Nunocha, P.; Bongkarn, T.; Eiad-ua, A.; Channei, D.; Suriwong, T. Efficient and Novel Synthesis of Nb-Doped SrTiO3 Nanoparticles via Microwave-Assisted Sol-Gel Auto-Combustion. Opt. Mater. 2025, 159, 116665. [Google Scholar] [CrossRef]
- Sindhu, T.; Ravichandran, A.T.; Xavier, A.R.; Sofiya, K.; Kumaresavanji, M. Impact of Gd Doping on Structural and Magnetic Characteristics of SrFeO3 Perovskite Nanomaterial. J. Phys. Condens. Matter 2024, 36, 505809. [Google Scholar] [CrossRef]
- Irshad, M.; Aslam, M.Z.; Butt, M.S.; Babar, Z.U.D.; Hanif, M.B.; Ahsan, M.; Khan, M.Z.; Zheng, K.; Rafique, M.; Ghaffar, A.; et al. Evaluation of Highly Conductive Cermet Cathodes Synthesized with Organic Chelating Agents and Sintered at Low Temperatures for IT-SOFCs. Energy Convers. Manag. X 2024, 23, 100609. [Google Scholar] [CrossRef]
- Aliotta, C.; Liotta, L.F.; La Parola, V.; Martorana, A.; Muccillo, E.N.S.; Muccillo, R.; Deganello, F. Ceria-Based Electrolytes Prepared by Solution Combustion Synthesis: The Role of Fuel on the Materials Properties. Appl. Catal. B Environ. 2016, 197, 14–22. [Google Scholar] [CrossRef]
- Deganello, F.; Testa, M.L.; La Parola, V.; Longo, A.; Tavares, A.C. LaFeO3-Based Nanopowders Prepared by a Soft-Hard Templating Approach: The Effect of Silica Texture. J. Mater. Chem. A 2014, 2, 8438–8447. [Google Scholar] [CrossRef]
- Manukyan, K.V. Template-Assisted Solution Combustion Synthesis. In Concise Encyclopedia of Self-Propagating High-Temperature Synthesis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 376–378. [Google Scholar] [CrossRef]
- Tay, S.W.; Hong, L.; Liu, Z. The Role of Metallo-Organic Chelating Ligand in the Preparation of Ferromagnetic La2O3–SrO–Co3O4 Nano Composite. Mater. Chem. Phys. 2009, 113, 994–1002. [Google Scholar] [CrossRef]
- De Sloovere, D.; Marchal, W.; Ulu, F.; Vranken, T.; Verheijen, M.; Van Bael, M.K.; Hardy, A. Combustion Synthesis as a Low Temperature Route to Li4Ti5O12 Based Powders for Lithium Ion Battery Anodes. RSC Adv. 2017, 7, 18745–18754. [Google Scholar] [CrossRef]
- Palma, D.; Deganello, F.; Liotta, L.F.; La Parola, V.; Prevot, A.B.; Malandrino, M.; Laurenti, E.; Boffa, V.; Magnacca, G. Main Issues in the Synthesis and Testing of Thermocatalytic Ce-Doped SrFeO3 Perovskites for Wastewater Pollutant Removal. Inorganics 2023, 11, 85. [Google Scholar] [CrossRef]
- Bhavisha, M.; Balamurugan, S.; Ashika, S.A.; Venkatesha, N.J.; Maiyalagan, T.; Sakthivel, A. Combustion Synthesis of Copper-Doped Perovskite SrFe1-XCuxO3-δ Nanomaterials and Its Potential Application on Hydroxylation of Anisole, a Biomass Model Component. Mater. Today Sustain. 2023, 21, 100266. [Google Scholar] [CrossRef]
- Sánchez-Caballero, A.; Zamudio-García, J.; dos Santos-Gómez, L.; da Silva, I.; Pérez-Coll, D.; Porras-Vázquez, J.M.; Marrero-López, D. Reduced Thermal Expansion and Improved Electrochemical Performance in Pr-Substituted SrFeO3 as Symmetrical Electrode for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2025, 17, 21380–21391. [Google Scholar] [CrossRef]
- Zapata-Ramírez, V.; Mather, G.C.; Pérez-Coll, D. Optimisation of Electrochemical Performance of Sr(Fe,Sb)O3-δ Air Electrodes for Intermediate-Temperature Solid Oxide Cells through Spray-Pyrolysis Processing. J. Power Sources 2024, 600, 234243. [Google Scholar] [CrossRef]
- Shabbir, S.; Khalid, B.; Sehrish, H.; Iqbal, M.T.; Morley, N.; Anwar, H. Exploring the Structural, Morphological, Optical, and Dielectric Properties, along with Photocatalytic Performance of La-Doped SrFeO3 Nanofibers. Mater. Res. Bull. 2024, 179, 112970. [Google Scholar] [CrossRef]
- Darwish, E.; Mansouri, M.; Yilmaz, D.; Leion, H. Effect of Mn and Cu Substitution on the SrFeO3 Perovskite for Potential Thermochemical Energy Storage Applications. Processes 2021, 9, 1817. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Veis, A.; Deganello, F.; Boffa, V.; Jørgensen, M.K. A Thermocatalytic Ceramic Membrane by Perovskite Incorporation in the Alumina Framework. Adv. Mate.r Interfaces 2023, 10, 2300435. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future Global Urban Water Scarcity and Potential Solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.; Martinez, G.S.; Kendrovski, V.; Diaz, J. A New Integrative Perspective on Early Warning Systems for Health in the Context of Climate Change. Environ. Res. 2020, 187, 109623. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Ghaffari, M.; Tan, P.Y.; Oruc, M.E.; Tan, O.K.; Tse, M.S.; Shannon, M. Effect of Ball Milling on the Characteristics of Nano Structure SrFeO3 Powder for Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation and Its Reaction Kinetics. Catal. Today 2011, 161, 70–77. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Saraf, R.; Shivakumara, C. Effective Degradation of Aqueous Nitrobenzene Using the SrFeO3-δ Photocatalyst under UV Illumination and Its Kinetics and Mechanistic Studies. Ind. Eng. Chem. Res. 2015, 54, 7800–7810. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Z.; Jiang, Y.; Liu, L.; Sun, Y. Photoinduced Structural Transformation of SrFeO3 and Ca2Fe2O5 during Photodegradation of Methyl Orange. Mater. Sci. Eng. B 2006, 132, 311–314. [Google Scholar] [CrossRef]
- Jia, L.; Ding, T.; Li, Q.; Tang, Y. Study of Photocatalytic Performance of SrFeO3−x by Ultrasonic Radiation. Catal. Commun. 2007, 8, 963–966. [Google Scholar] [CrossRef]
- Leiw, M.Y.; Guai, G.H.; Wang, X.; Tse, M.S.; Ng, C.M.; Tan, O.K. Dark Ambient Degradation of Bisphenol A and Acid Orange 8 as Organic Pollutants by Perovskite SrFeO3-δ Metal Oxide. J. Hazard. Mater. 2013, 260, 1–8. [Google Scholar] [CrossRef]
- Manzoor, S.; Imtiaz, Q. Role of Perovskite Non-Stoichiometry on Catalytic Oxygen Dark Activation for the Removal of Azo Dyes from Wastewater. Heliyon 2024, 10, e40157. [Google Scholar] [CrossRef]
- da Silva Júnior, A.H.; de Oliveira, C.R.S.; Pellenz, L.; Moraes, P.A.D.; Marques, W.P.; Mazur, L.P.; Costa, T.G.; Horn, A., Jr.; Guelli Ulson de Souza, S.M.d.A.; de Souza, A.A.U.; et al. Perovskite-Type Strontium Ferrite-Based Catalyst: Characterization and Antibiotic Degradation Approach. Process Saf. Environ. Prot. 2024, 187, 1403–1421. [Google Scholar] [CrossRef]
- Chen, H.; Ku, J.; Wang, L. Thermal Catalysis under Dark Ambient Conditions in Environmental Remediation: Fundamental Principles, Development, and Challenges. Chin. J. Catal. 2019, 40, 1117–1134. [Google Scholar] [CrossRef]
- Dodd, N.J.F.; Jha, A.N. Photoexcitation of Aqueous Suspensions of Titanium Dioxide Nanoparticles: An Electron Spin Resonance Spin Trapping Study of Potentially Oxidative Reactions. Photochem. Photobiol. 2011, 87, 632–640. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Zuki, F.M.; Jamari, N.L.A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef]
- Du, Y.; Pramanik, B.K.; Zhang, Y.; Dumée, L.; Jegatheesan, V. Recent Advances in the Theory and Application of Nanofiltration: A Review. Curr. Pollut. Rep. 2022, 8, 51–80. [Google Scholar] [CrossRef]
- Janowska, K.; Ma, X.; Boffa, V.; Jørgensen, M.K.; Candelario, V.M. Combined Nanofiltration and Thermocatalysis for the Simultaneous Degradation of Micropollutants, Fouling Mitigation and Water Purification. Membranes 2021, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Ayala, L.I.M.; Paquet, M.; Janowska, K.; Jamard, P.; Quist-Jensen, C.A.; Bosio, G.N.; Mártire, D.O.; Fabbri, D.; Boffa, V. Water Defluoridation: Nanofiltration vs Membrane Distillation. Ind. Eng. Chem. Res. 2018, 57, 14740–14748. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Nurisso, F.; Boffa, V.; Ma, X.; Rasse-Suriani, F.A.O.; Roslev, P.; Magnacca, G.; Candelario, V.; Deganello, F.; La Parola, V. A Thermocatalytic Perovskite-Graphene Oxide Nanofiltration Membrane for Water Depollution. J. Water Process Eng. 2022, 49, 102941. [Google Scholar] [CrossRef]
- Song, J.; Zhu, T.; Chen, X.; Ni, W.; Zhong, Q. Cobalt and Titanium Substituted SrFeO3 Based Perovskite as Efficient Symmetrical Electrode for Solid Oxide Fuel Cell. J. Mater. 2020, 6, 377–384. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Chen, Y.; Dong, F.; Tade, M.O.; Shao, Z. Cobalt-Free SrFe0.9Ti0.1O3-δ as a High-Performance Electrode Material for Oxygen Reduction Reaction on Doped Ceria Electrolyte with Favorable CO2 Tolerance. J. Eur. Ceram. Soc. 2015, 35, 2531–2539. [Google Scholar] [CrossRef]
- Zapata-Ramírez, V.; Rosendo-Santos, P.; Amador, U.; Ritter, C.; Mather, G.C.; Pérez-Coll, D. Optimisation of High-Performance, Cobalt-Free SrFe1-XMoxO3-δ Cathodes for Solid Oxide Fuel Cells Prepared by Spray Pyrolysis. Renew Energy 2022, 185, 1167–1176. [Google Scholar] [CrossRef]
- Li, B.; He, S.; Li, J.; Yue, X.; Irvine, J.T.S.; Xie, D.; Ni, J.; Ni, C. A Ce/Ru Codoped SrFeO3−δ Perovskite for a Coke-Resistant Anode of a Symmetrical Solid Oxide Fuel Cell. ACS Catal. 2020, 10, 14398–14409. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Ti-Doped SrFeO3 Nanostructured Electrodes for Symmetric Solid Oxide Fuel Cells. RSC Adv. 2015, 5, 107889–107895. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Antonova, E.P.; Porotnikova, N.M.; Bogdanovich, N.M. Features of the Electrochemical Reaction of Hydrogen Oxidation on the Composite SrFeO3-Based Anode for a Protonic Ceramic Fuel Cell. Int. J. Energy Res. 2022, 46, 12597–12607. [Google Scholar] [CrossRef]
- Ling, Y.; Wu, Y.; Tian, Y.; Wang, X.; Shen, S.; Ou, X.; Wang, S. Stable Solid Oxide Electrolysis Cells with SSF-Based Symmetrical Electrode for Direct High-Temperature Steam Electrolysis. Ceram. Int. 2022, 48, 981–991. [Google Scholar] [CrossRef]
- Liu, D.; Dou, Y.; Xia, T.; Li, Q.; Sun, L.; Huo, L.; Zhao, H. B-Site La, Ce, and Pr-Doped Ba0.5Sr0.5Co0.7Fe0.3O3-δ Perovskite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Effectively Promoted Oxygen Reduction Activity and Operating Stability. J. Power Sources 2021, 494, 229778. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Zhong, Y.; Shao, Z. Oxide-Based Precious Metal-Free Electrocatalysts for Anion Exchange Membrane Fuel Cells: From Material Design to Cell Applications. J. Mater. Chem. A 2021, 9, 3151–3179. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.G.; Zhou, W.; Jiang, S.; Zou, J.; Shao, Z. An A-Site-Deficient Perovskite Offers High Activity and Stability for Low-Temperature Solid-Oxide Fuel Cells. ChemSusChem 2013, 6, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- El-Ads, E.H.; Galal, A.; Atta, N.F. The Effect of A-Site Doping in a Strontium Palladium Perovskite and Its Applications for Non-Enzymatic Glucose Sensing. RSC Adv. 2016, 6, 16183–16196. [Google Scholar] [CrossRef]
- Barros Julião, P.S. A-Site Cation Influences on Performance, Structure and Conductivity of a Lanthanide-Based Perovskite Electrode for Symmetrical Solid Oxide Fuel Cells. J. Power Sources 2020, 450, 227723. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Antipov, E.V. Cathode Materials Based on Perovskite-like Transition Metal Oxides for Intermediate Temperature Solid Oxide Fuel Cells. Russ. Chem. Rev. 2013, 82, 686–700. [Google Scholar] [CrossRef]
- Li, Z.; Peng, M.; Zhao, Y.; Li, J.; Sun, Y. Minimized Thermal Expansion Mismatch of Cobalt-Based Perovskite Air Electrodes for Solid Oxide Cells. Nanoscale 2021, 13, 20299–20308. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Muchtar, A.; Somalu, M.R. Short Review on Cobalt-Free Cathodes for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 9149–9155. [Google Scholar] [CrossRef]
- Zhou, X.D.; Cai, Q.; Yang, J.; Kim, M.; Yelon, W.B.; James, W.J.; Shin, Y.W.; Scarfino, B.J.; Anderson, H.U. Coupled Electrical and Magnetic Properties in (La,Sr)FeO3-δ. J. Appl. Phys. 2005, 97, 10C314. [Google Scholar] [CrossRef]
- Patrakeev, M.V.; Kharton, V.V.; Bakhteeva, Y.A.; Shaula, A.L.; Leonidov, I.A.; Kozhevnikov, V.L.; Naumovich, E.N.; Yaremchenko, A.A.; Marques, F.M.B. Oxygen Nonstoichiometry and Mixed Conductivity of SrFe1−xMxO3−δ (M=Al, Ga): Effects of B-Site Doping. Solid State Sci. 2006, 8, 476–487. [Google Scholar] [CrossRef]
- Colomer, M.T.; Steele, B.C.H.; Kilner, J.A. Structural and Electrochemical Properties of the Sr0.8Ce0.1Fe0.7Co0.3O3–δ Perovskite as Cathode Material for ITSOFCs. Solid State Ion. 2002, 147, 41–48. [Google Scholar] [CrossRef]
- Wu, M.; Ni, J.; Ni, C. Achieving High-Efficiency CO2 Electrolysis for SrFeO3-δ-Based Symmetric Electrodes. ACS Sustain. Chem. Eng. 2023, 11, 10717–10726. [Google Scholar] [CrossRef]
- Tummino, M.L.; Liotta, L.F.; Lo Faro, M.; Campagna Zignani, S.; Deganello, F. Effects of B-Site Doping and Stoichiometry on Strontium Ferrate Sr0.85Ce0.15FexCoyO3 Perovskites as Electrocatalysts. J. Am. Ceram. Soc. 2025, 108, e20528. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, J.; Deng, Q.; Yang, J.; Li, Q.; Zhu, Z.; Wu, K. Interfacial Electron Transfer in Heterojunction Nanofibers for Highly Efficient Oxygen Evolution Reaction. Nanoscale 2023, 15, 677–686. [Google Scholar] [CrossRef]
- She, S.; Zhu, Y.; Wu, X.; Hu, Z.; Shelke, A.; Pong, W.F.; Chen, Y.; Song, Y.; Liang, M.; Chen, C.T.; et al. Realizing High and Stable Electrocatalytic Oxygen Evolution for Iron-Based Perovskites by Co-Doping-Induced Structural and Electronic Modulation. Adv. Funct. Mater. 2022, 32, 2111091. [Google Scholar] [CrossRef]
- Li, B.; Irvine, J.T.S.; Ni, J.; Ni, C. High-Performance and Durable Alcohol-Fueled Symmetrical Solid Oxide Fuel Cell Based on Ferrite Perovskite Electrode. Appl. Energy 2022, 306, 118117. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; Zhu, L.; O’Hayre, R.; Chou, K.C. Electrochemical Performance and Stability of La0.5Sr0.5Fe0.9Nb0.1O3-δ Symmetric Electrode for Solid Oxide Fuel Cells. J. Power Sources 2018, 399, 398–405. [Google Scholar] [CrossRef]
- Wu, M.; Cai, H.; Jin, F.; Sun, N.; Xu, J.; Zhang, L.; Han, X.; Wang, S.; Su, X.; Long, W.; et al. Assessment of Cobalt–Free Ferrite–Based Perovskite Ln0.5Sr0.5Fe0.9Mo0.1O3–δ (Ln = lanthanide) as Cathodes for IT-SOFCs. J. Eur. Ceram. Soc. 2021, 41, 2682–2690. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.; Jin, C.; Xiao, G.; Chen, F.; Han, M. Sulfur-Tolerant Redox-Reversible Anode Material for Direct Hydrocarbon Solid Oxide Fuel Cells. Adv. Mater. 2012, 24, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zhao, L.; Lin, B.; Dong, Y.; Zhang, X.; Meng, G.; Liu, X. Investigation of Cobalt-Free Cathode Material Sm0.5Sr0.5Fe0.8Cu0.2O3−δ for Intermediate Temperature Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2010, 35, 6905–6910. [Google Scholar] [CrossRef]
- Ling, Y.; Yu, J.; Lin, B.; Zhang, X.; Zhao, L.; Liu, X. A Cobalt-Free Sm0.5Sr0.5Fe0.8Cu0.2O3−δ–Ce0.8Sm0.2O2−δ Composite Cathode for Proton-Conducting Solid Oxide Fuel Cells. J. Power Sources 2011, 196, 2631–2634. [Google Scholar] [CrossRef]
- Chen, X.; Ni, W.; Wang, J.; Zhong, Q.; Han, M.; Zhu, T. Exploration of Co-Fe Alloy Precipitation and Electrochemical Behavior Hysteresis Using Lanthanum and Cobalt Co-Substituted SrFeO3-δ SOFC Anode. Electrochim. Acta 2018, 277, 226–234. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhou, S.; Zhu, W.; Song, W.; Bao, H.; Chen, H.; Ou, X.; Khan, M.; Ling, Y. Enhanced Redox-Stable Sm0.5Sr0.5FeO3-δ Electrode Material for Symmetric Solid Oxide Fuel Cells at Reduced Temperatures. Ceram. Int. 2020, 46, 6714–6722. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, W.; Sunarso, J.; Ge, L.; Zhu, Z.; Shao, Z. High Performance Cobalt-Free Perovskite Cathode for Intermediate Temperature Solid Oxide Fuel Cells. J. Mater. Chem. 2010, 20, 9619–9622. [Google Scholar] [CrossRef]
- Teraoka, Y.; Shimokawa, H.; Kang, C.Y.; Kusaba, H.; Sasaki, K. Fe-Based Perovskite-Type Oxides as Excellent Oxygen-Permeable and Reduction-Tolerant Materials. Solid State Ion. 2006, 177, 2245–2248. [Google Scholar] [CrossRef]
- Gabra, S.; Marek, E.J.; Poulston, S.; Williams, G.; Dennis, J.S. The Use of Strontium Ferrite Perovskite as an Oxygen Carrier in the Chemical Looping Epoxidation of Ethylene. Appl. Catal. B Environ. 2021, 286, 119821. [Google Scholar] [CrossRef]
- Marek, E.; Hu, W.; Gaultois, M.; Grey, C.P.; Scott, S.A. The Use of Strontium Ferrite in Chemical Looping Systems. Appl. Energy 2018, 223, 369–382. [Google Scholar] [CrossRef]
- Chein, R.Y.; Lu, C.Y.; Chen, W.H. Syngas Production via Chemical Looping Reforming Using Methane-Based Feed and NiO/Al2O3 Oxygen Carrier. Energy 2022, 250, 123815. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, R.; Zeng, D.; Zhao, Y.; Zhang, H.; Shen, D. High H2/CO Ratio Syngas Production from Chemical Looping Gasification of Sawdust in a Dual Fluidized Bed Gasifier. Energy Fuels 2016, 30, 1764–1770. [Google Scholar] [CrossRef]
- Dieringer, P.; Marx, F.; Alobaid, F.; Ströhle, J.; Epple, B. Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Appl. Sci. 2020, 10, 4271. [Google Scholar] [CrossRef]
- Chan, M.S.C.; Marek, E.; Scott, S.A.; Dennis, J.S. Chemical Looping Epoxidation. J. Catal. 2018, 359, 1–7. [Google Scholar] [CrossRef]
- Jia, T.; Popczun, E.J.; Lekse, J.W.; Duan, Y. The Optimal Co-Doping of SrFe1-xCoxO3-δ oxygen Carriers in Redox Applications. Phys. Chem. Chem. Phys. 2020, 22, 16721–16726. [Google Scholar] [CrossRef]
- Marek, E.J.; García-Calvo Conde, E. Effect of Catalyst Preparation and Storage on Chemical Looping Epoxidation of Ethylene. Chem. Eng. J. 2021, 417, 127981. [Google Scholar] [CrossRef]
- Harrison, A.R.P.; Kwong, K.Y.; Zheng, Y.; Balkrishna, A.; Dyson, A.; Marek, E.J. Kinetic and Thermodynamic Enhancement of Low-Temperature Oxygen Release from Strontium Ferrite Perovskites Modified with Ag and CeO2. Energy Fuels 2023, 37, 9487–9499. [Google Scholar] [CrossRef]
- Ahangari, M.; Mahmoodi, E.; Delibaş, N.; Mostafaei, J.; Asghari, E.; Niaei, A. Application of SrFeO3 Perovskite as Electrode Material for Supercapacitor and Investigation of Co-Doping Effect on the B-Site. Turk. J. Chem. 2022, 46, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Avcıoğlu, C.; Avcıoğlu, S. Recent Advances in Iron-Containing Perovskites for Supercapacitors. Adv. Energy Sustain. Res. 2025, 6, 2400289. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Rayan, D.A.; Askar, A.A.; Maksoud, M.I.A.A.; El-Bahnasawy, H.H. Synthesis and Characterization of SrFeO3-δ Nanoparticles as Antimicrobial Agent. J. Sol-Gel Sci. Technol. 2021, 97, 27–38. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, P.Y.; Chow, C.L.; Lim, C.K.; Tan, O.K.; Tse, M.S.; Sze, C.C. Antibacterial Activities of Mechanochemically Synthesized Perovskite Strontium Titanate Ferrite Metal Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 169–175. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Strunck, A.B.; Boffa, V.; Jørgensen, M.K. Kinetics of Strontium Carbonate Formation on a Ce-Doped SrFeO3 Perovskite. Catalysts 2022, 12, 265. [Google Scholar] [CrossRef]
- Koo, B.; Kim, K.; Kim, J.K.; Kwon, H.; Han, J.W.; Jung, W.C. Sr Segregation in Perovskite Oxides: Why It Happens and How It Exists. Joule 2018, 2, 1476–1499. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, G.; Li, J.; Ma, J.; Duan, T.; Sun, Q.; Chen, S.; Lu, X.; Cheng, H. Structure Stability and CO2 Absorption Mechanism on Surfaces of B-Site Doped SrFeO3-δ Perovskite Ceramic Membrane. Ceram. Int. 2024, 50, 48384–48390. [Google Scholar] [CrossRef]
- Yu, D.; He, J.; Xie, T.; Xu, Q.; Zhu, Q.; Yang, J.; An, J.; Ye, F.; Wang, J.; Xiang, B. New Insights into Sr-O Bonds Enhances Co/Fe Catalytic Activity in SrCoFe Perovskite for Boosted Peroxymonosulfate Activation. Chem. Eng. J. 2021, 426, 131525. [Google Scholar] [CrossRef]
- Margellou, A.; Manos, D.; Petrakis, D.; Konstantinou, I. Activation of Persulfate by LaFe1-xCoxO3 Perovskite Catalysts for the Degradation of Phenolics: Effect of Synthetic Method and Metal Substitution. Sci. Total Environ. 2022, 832, 155063. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Jia, S.; Tang, J.; Wu, H.; Wang, X.; Gao, S.; Li, H.; Lu, H.; Fu, G.; et al. Ultrahigh Specific Surface Area Mesoporous Perovskite Oxide Nanosheets with Rare-Earth-Enhanced Lattice Oxygen Participation for Superior Water Oxidation. J. Mater. Sci. Technol. 2025, 227, 255–261. [Google Scholar] [CrossRef]
- Yu, J.; Ran, R.; Zhong, Y.; Zhou, W.; Ni, M.; Shao, Z. Advances in Porous Perovskites: Synthesis and Electrocatalytic Performance in Fuel Cells and Metal–Air Batteries. Energy Environ. Mater. 2020, 3, 121–145. [Google Scholar] [CrossRef]
- Otaguro, H.; Takeno, T.; Sugimoto, R.; Hosokawa, S.; Akamatsu, H.; Yamamoto, T.; Nakanishi, K.; Hayashi, K.; Hasegawa, G. Hydrogarnet-Derived Porous Polyhedral Particles of SrFeO3-δ Perovskite. Chem. Mater. 2023, 35, 6423–6436. [Google Scholar] [CrossRef]
- Shih, D.P.C.; Aguadero, A.; Skinner, S.J. A-Site Acceptor-Doping Strategy to Enhance Oxygen Transport in Sodium–Bismuth–Titanate Perovskite. J. Am. Ceram. Soc. 2023, 106, 100–108. [Google Scholar] [CrossRef]
- Li, M.; Feng, M.; Guo, C.; Qiu, S.; Zhang, L.; Zhao, D.; Guo, H.; Zhang, K.; Wang, F. Green and Efficient Al-Doped LaFexAl1-xO3 Perovskite Oxide for Enhanced Phosphate Adsorption with Creation of Oxygen Vacancies. ACS Appl. Mater. Interfaces 2022, 15, 16942–16952. [Google Scholar] [CrossRef]
- Li, S.F.; Zheng, J.; Hu, L.; Ma, Y.; Yan, D. Facile Surface Defect Engineering on Perovskite Oxides for Enhanced OER Performance. Dalton Trans. 2023, 52, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Bian, L.; Ting, T.; Liu, C.; Yang, L.; Xu, Y.; Peng, J.; Song, X.; An, S. Boosting Electrochemical CO2 Directly Electrolysis by Tuning the Surface Oxygen Defect of Perovskite. J. Power Sources 2023, 570, 233032. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Gong, Y.; Lin, N.; Yang, Q.; Zhang, X.; Wang, Y. Perovskite Catalysts with Different Dimensionalities for Environmental and Energy Applications: A Review. Sep. Purif. Technol. 2023, 307, 122716. [Google Scholar] [CrossRef]


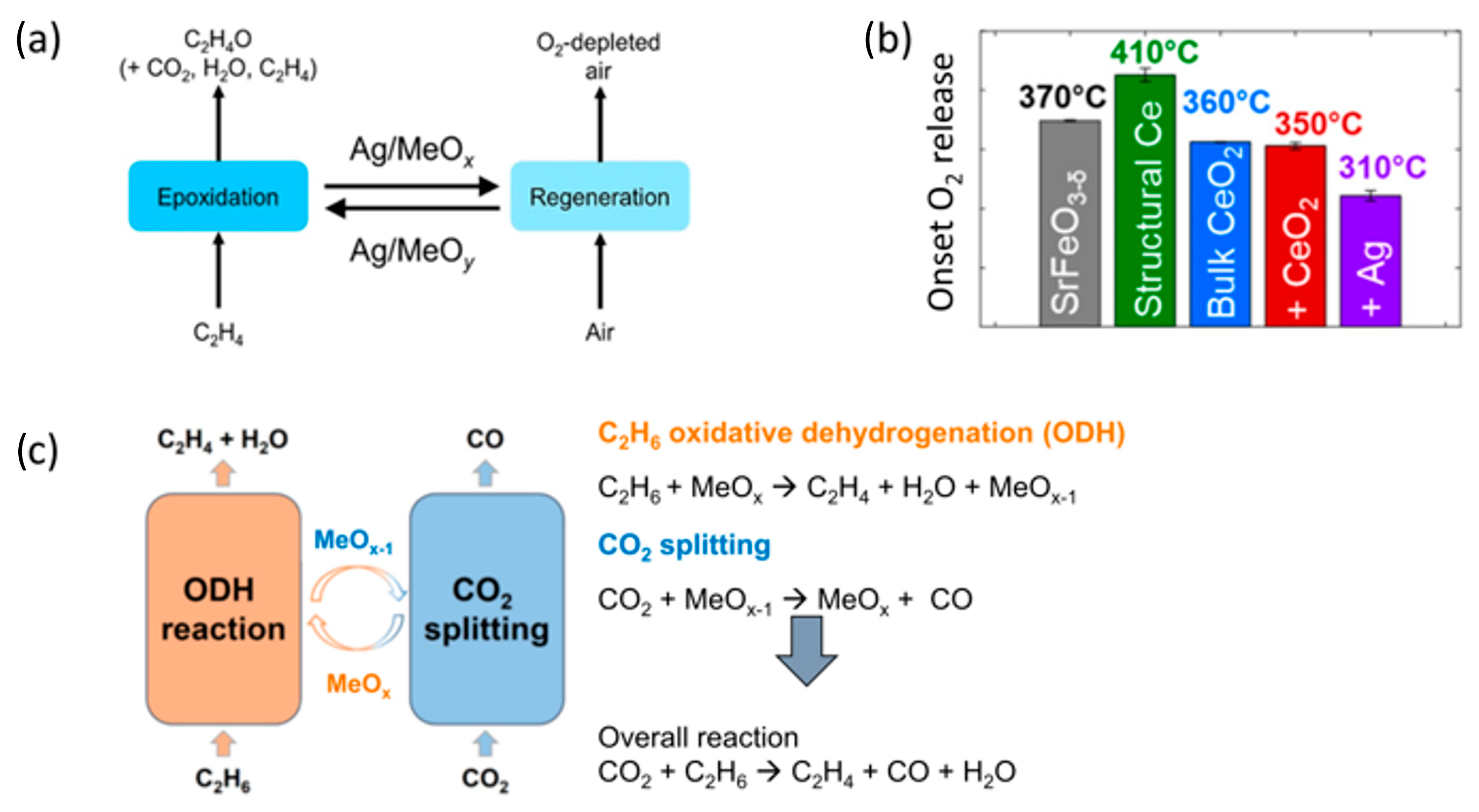
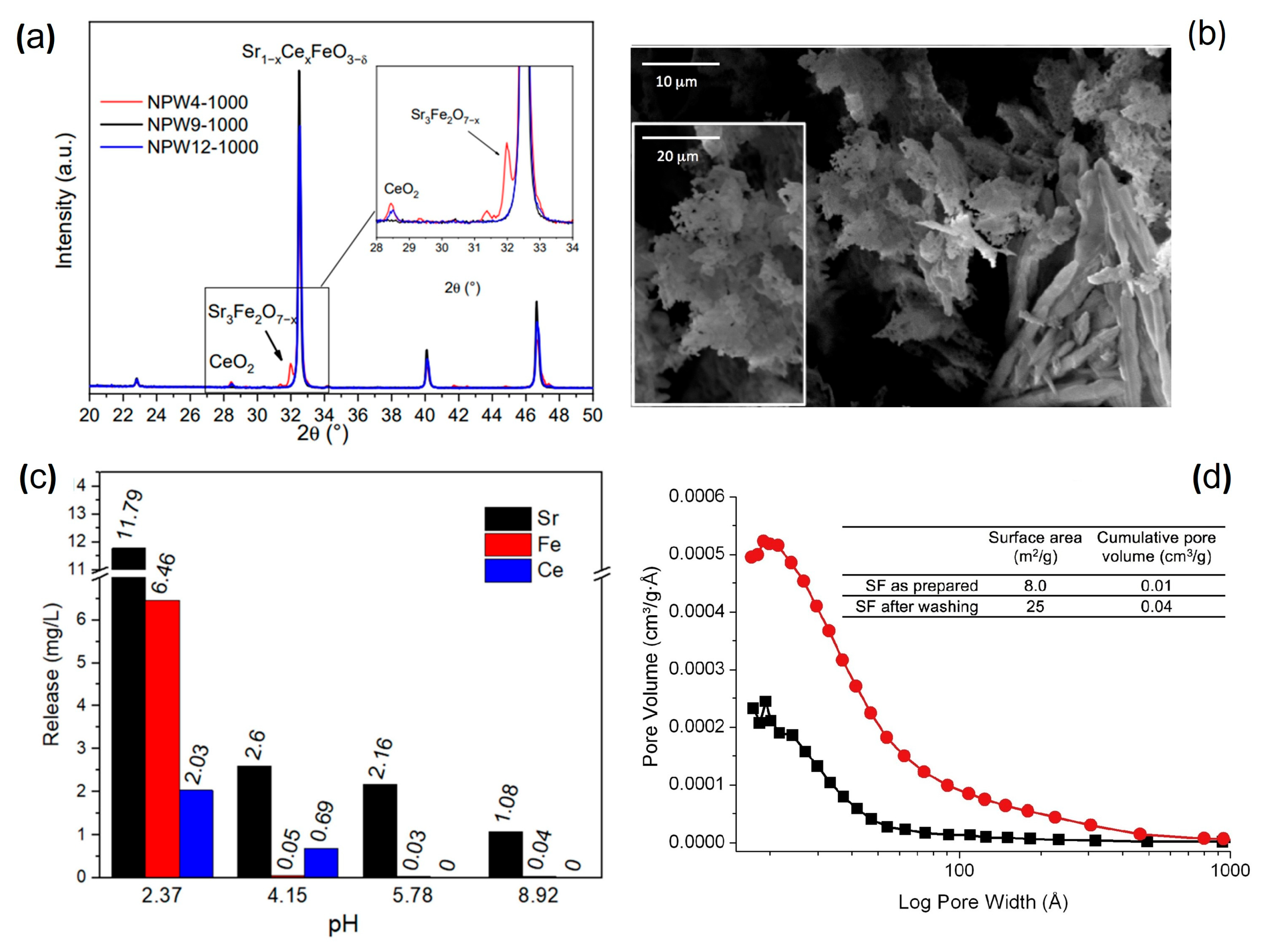
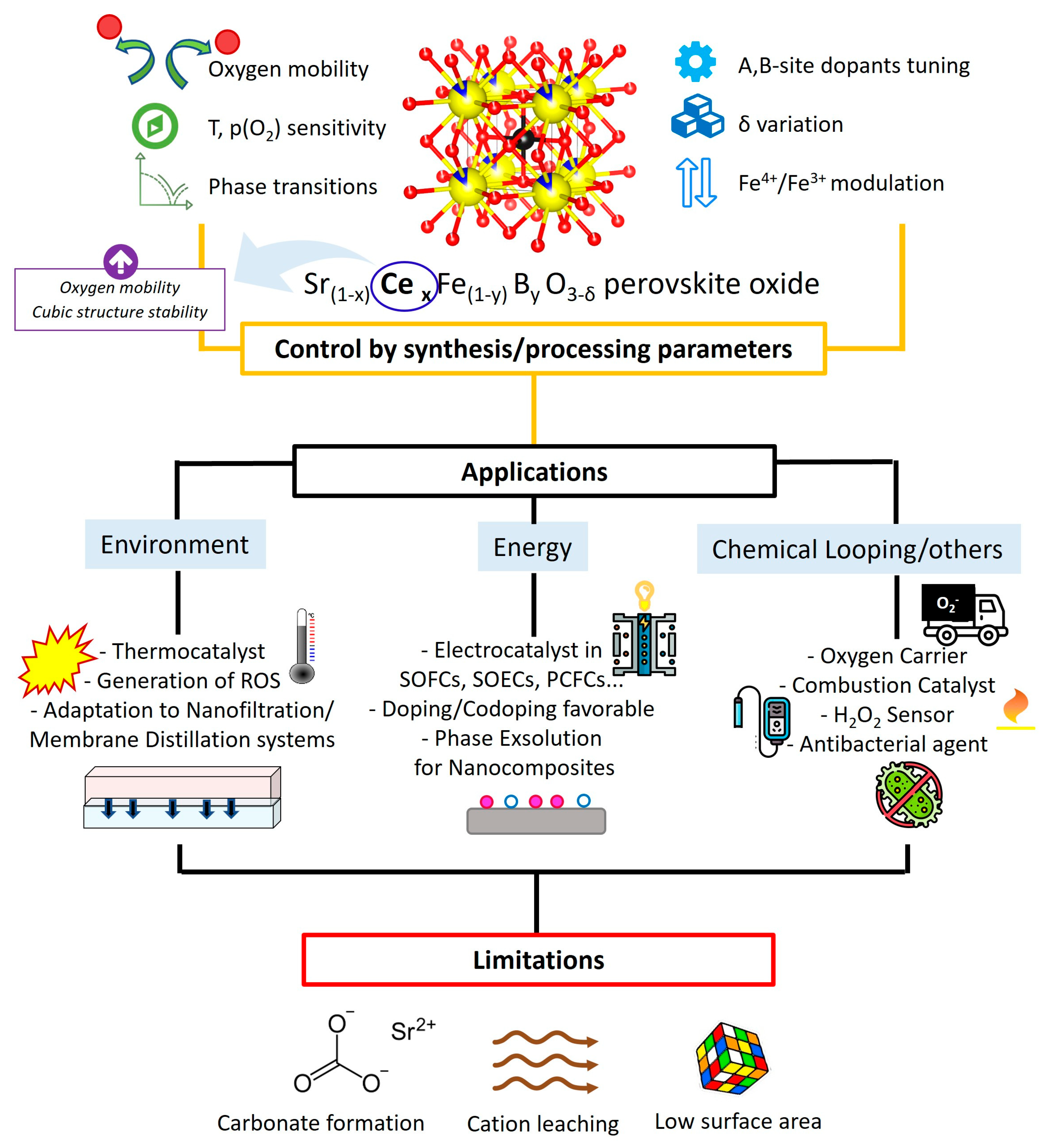
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummino, M.L.; Deganello, F.; Boffa, V. Cerium-Doped Strontium Ferrate Perovskite Oxides: Sustainable Materials to Face Energy and Environmental Challenges. Sustain. Chem. 2025, 6, 24. https://doi.org/10.3390/suschem6030024
Tummino ML, Deganello F, Boffa V. Cerium-Doped Strontium Ferrate Perovskite Oxides: Sustainable Materials to Face Energy and Environmental Challenges. Sustainable Chemistry. 2025; 6(3):24. https://doi.org/10.3390/suschem6030024
Chicago/Turabian StyleTummino, Maria Laura, Francesca Deganello, and Vittorio Boffa. 2025. "Cerium-Doped Strontium Ferrate Perovskite Oxides: Sustainable Materials to Face Energy and Environmental Challenges" Sustainable Chemistry 6, no. 3: 24. https://doi.org/10.3390/suschem6030024
APA StyleTummino, M. L., Deganello, F., & Boffa, V. (2025). Cerium-Doped Strontium Ferrate Perovskite Oxides: Sustainable Materials to Face Energy and Environmental Challenges. Sustainable Chemistry, 6(3), 24. https://doi.org/10.3390/suschem6030024









