1. Introduction
As fossil resource reserves are being depleted and the risks of climate change are increasing, new renewable energy sources are needed. The most popular renewable energy sources are biomass, hydropower, solar panels, and wind turbines [
1]. Waste heat is a form of thermal energy and is abundant but often less prevalent and neglected. In manufacturing industries, temperatures exceeding 1000 °C are frequently achieved. It has been reported that up to 24% of waste heat energy can be recovered during the steelmaking process [
2]. However, most wasted heat energy occurs below 150 °C [
3]. Even low-temperature sources like the human body could be used to generate electrical energy [
4]. There are many different sources of high and low temperature waste heat thermal energy. Given the growing energy demand, it is crucial to utilize all available renewable energy sources to ensure opportunities for sustainable development. The thermoelectric (TE) effect can be used to convert thermal energy to electricity without any moving parts. The efficiency of TE materials is characterized by the figure of merit, as in Equation (1).
where
S is the Seebeck coefficient,
σ is the specific electrical conductivity,
is the thermal conductivity, and
T is the temperature. The multiplication of
S2 and
σ defines the power factor (PF), a common parameter for assessing thermoelectric efficiency in thin films, especially since measuring thermal conductivity in thin films can be challenging. A high
S, high
σ, but low
k, is desired to obtain an effective TE material.
The specific electrical conductivity
σ can be expressed as a product of three components, as in Equation (2).
where
e is the elementary charge,
is the charge carrier mobility, and
n is the charge carrier concentration, which also affects the
S. As the charge carrier concentration increases, the
S decreases [
5]. Therefore, the optimal charge carrier concentration for TE materials is in the range from 10
18 to 10
20 cm
−3 [
6,
7]. Consequently, achieving the highest possible charge carrier mobility is essential for maximizing both the figure of merit and the specific electrical conductivity. Several factors affect the mobility of charge carriers, such as material composition, doping level, temperature, nano-structuring, and defects and impurities. For organic materials, molecular structure, material purity, and the concentration of defects and traps play significant roles [
8,
9]. For example, oxygen in polymers can lead to a reduction in charge carrier mobility [
10]. Additionally, oxidation-induced oxide layers on the metal powder particle surface can significantly reduce electrical conductivity [
11].
There is active research being conducted on hybrid TE materials [
3]. TE composites consist of TE nanoparticles within an organic matrix. Various organic materials, including conducting or insulating polymers and charge transport materials, can be used. For this purpose, various polymers have been studied, such as PANI (polyaniline) [
12], P3HT (poly(3-hexylthiophene-2,5-diyl)) [
13], PEDOT (poly(3,4-ethylenedioxythiophene)) [
14], and PMMA (poly(methyl methacrylate)) [
15,
16,
17]. Hybrid materials have several advantages over traditional TE materials, such as mechanical flexibility, solution processability [
18], reduced thermal conductivity, and increased
S [
19,
20,
21]. Additionally, composite materials possess reduced density, decreased cost, and environmental impact [
22]. While composite materials offer numerous advantages, their performance can be hindered by certain challenges. These materials, composed of NPs, contain numerous interfaces between components. Consequently, optimizing interfacial transport is essential to maintain charge carrier mobility within the nanostructure of composite materials. Recent studies have highlighted the critical role of nanoparticle morphology and structure in determining thermoelectric performance. Baghdadi et al. demonstrated that ZnO nanorods exhibit superior thermoelectric properties compared to ZnO nanoparticles at elevated temperatures, primarily due to reduced interface barriers and enhanced electron mean free path [
23]. Additionally, the nanoparticle packaging density and order significantly influence charge carrier transport, with more ordered structures yielding improved thermoelectric efficiency [
24]. As the packaging density increases, the distance between nanoparticles decreases. As a result, the charge carriers are able to take the shortest path through the material with the least amount of energetic barriers.
Furthermore, particle size plays a dual role: larger nanoparticles tend to have higher
σ, while smaller ones offer increased
S and reduced
k due to enhanced grain boundary scattering [
25].
A small barrier at the NP and polymer matrix interface can potentially enhance the
S by filtering low-energy charge carriers. Ideally, the barrier height should be within the range of 0.04 to 0.10 eV to achieve high electrical conductivity [
26]. Nonetheless, empirical observations indicate that the energy filtering effect is minimal [
27]. The oxidation of TE NPs may introduce an interfacial barrier impacting charge transfer [
26]. Effective control of oxidation in hybrid materials is crucial for improving interfacial transport within these materials [
28]. Consequently, increased charge carrier mobility has led to significant improvements in both the
S and electrical conductivity
σ [
29].
Sb
2Te
3 is one of the best p-type TE materials with a high
S and
σ at room temperature. Sb
2Te
3 NPs can be synthesized by various methods, such as thermal decomposition, solvothermal, and hydrothermal synthesis [
19,
30,
31,
32]. With these methods, it is possible to fine-tune the morphology of the NPs; however, the synthesis times are lengthy, taking up to 12 h. An alternative method is microwave-assisted heating, which can produce NPs within minutes [
33,
34,
35,
36,
37,
38]. During the preparation of the hybrid material and the synthesis of the TE material, the NPs are exposed to various solvents and high temperatures. This may lead to oxide formation on the surface of the nanostructures, which can impact the properties of the composite materials.
A detailed study on the bulk Sb
2Te
3 oxidation process was conducted by Volykhhov et al. [
39]. The authors have shown that oxidation is a 3-step process. It starts with molecular adsorption, followed by surface reaction and oxide layer growth. Upon oxidation, the Sb-to-Te ratio changes to approximately 1:1 at the surface, resulting in the formation of a Sb
2Te
2O
7 layer. While oxygen is directly responsible for the oxidation of Sb
2Te
3, a separate effect is caused by water. Water may cause Sb leaching and enrich the surface with Te. Additionally, it may accelerate the rate of oxidation [
39]. Oxidation has also been observed for Sb
2Te
3 NPs synthesized with microwave-assisted thermolysis. The porous films have a high interface surface between the TE material and voids, which causes charge carrier scattering and trapping [
40]. A study by Fang et al. [
41] has demonstrated a promising technique for removing surface oxides on Sb
2Te
3 thin films. Results show that the material can be restored to its original condition by using a 1% hydrochloric acid (HCl) solution etch to remove oxides, followed by annealing at 150 °C to reduce the increased Te concentration at the surface [
41].
Hybrid TE materials are environmentally friendly, but their manufacturing often involves toxic solvents. There is a lack of research on eco-friendly, water-based alternatives. This study presents a hybrid TE material made from a water-soluble polymer, specifically PEO (polyethylene oxide), combined with Sb2Te3 NPs produced through solution chemistry using microwave-assisted heating.
2. Materials and Methods
2.1. Synthesis of Sb2Te3 Nanoparticles
The chemicals used in the typical synthesis are SbCl3 (99.95%), Te powder (99.8%), ethylene glycol (EG, 99%), thioglycolic acid (TGA, 98%), and tri-octylphosphine (TOP, 90%), thioglycolic acid (TGA, 98%). All the chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) and used directly without any purification.
The synthesis process was carried out using microwave-assisted thermolysis in a SynthWAVE (Milestone, Sorisole, Italy) system (1500 watts) equipped with a high-pressure rotor capable of handling multiple vessels. This method was conducted in a 70 mL Teflon container across four parallel reaction setups, all of which were run simultaneously. The synthesis began with the creation of two precursor solutions. First, a Te precursor was prepared by heating a mixture consisting of 3.7 mmol of Te and 10 mL of TOP on a hot plate for 10 min at 200 °C, with continuous stirring. The Te-TOP complex exhibited an olive-yellowish color before being allowed to cool. A total of 2.5 mmol of SbCl3 was dissolved in 20 mL of ethylene glycol for the Sb precursor. The ratio of the binary components can be readily adjusted by changing SbCl3 with an equivalent quantity of Te precursors. Next, 1 mL of TGA was added as a directing agent into the dissolved precursors and stirred for 10 min to achieve a uniform mixture. The Te-TOP complex and the Sb-EG solutions were then combined and placed in a 70 mL Teflon vessel, which underwent microwave processing for 2 min at 220 °C under 40 mbar of nitrogen gas and with constant stirring. The particles were washed with isopropanol and acetone several times and dried in an oven at 80 °C overnight.
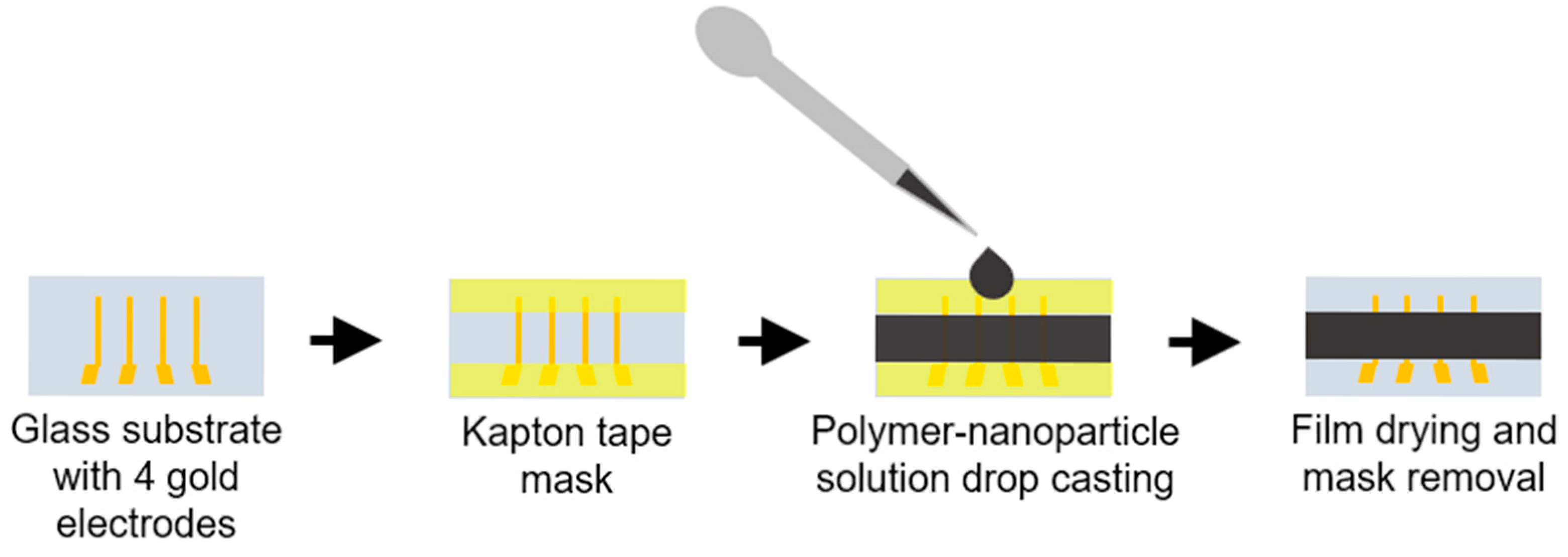
2.2. Sample Preparation
The samples were made on 25 × 13 mm glass substrates. Substrates were cleaned with acetone, detergent (2% Hellmanex® III, Hellma GmbH, Müllheim, Germany) in deionized water (DW)), and isopropanol, followed by plasma cleaning in plasma asher GIGAbatch 360M (PVA Metrology & Plasma Solutions GmbH, Wettenberg, Germany) (800 W for 10 min, O2 flow 1000 sccm). Next, 10 nm of the chrome adhesion layer and 100 nm of the gold layer were thermally evaporated in a vacuum with Edwards Auto 306 (Edwards High Vacuum International, Crawley, UK) to create 4 gold electrodes 2 mm apart. Substrates were stored in isopropanol after electrode deposition.
Three different sample types, with 80% nanoparticle and 20% polymer concentrations, were made using a mortar. A total of 7.5 mg of PEO (Polysciences, Warrington, PA, USA, MW = 1,000,000) was dissolved in 200 µL of DW for the first sample. Afterwards, 30 mg of Sb
2Te
3 NPs were added and mixed until a thick, homogeneous paste was obtained. For the second type of samples, 37% HCl (Sigma Aldrich, 1003172500) was diluted to a 5% concentration and used instead of the DW. For the third type of samples, nanoparticles were ultrasonicated in the 5% HCl solution for 5 min. The treated NPs were rinsed with isopropanol 3 times after removing the excess solvent, and then the particles were dried on a hot plate at 60 °C for 30 min. Slurry preparation was then repeated the same way as for the first type samples. The TE slurry was drop-cast onto glass substrates with electrodes as shown in
Figure 1. The samples were dried on a hot plate at 50 °C and then annealed at 90 °C for 10 min. The two-stage drying process was used to prevent the formation of cracks and defects in the films. In samples prepared this way, the nanoparticles are encapsulated in the polymer, forming a particle–polymer percolation network structure (see
Figure 2).
2.3. Sample Characterization
X-ray powder diffraction (XRD) analysis was performed using a MiniFlex X-ray diffractometer (Rigaku Corporation, Tokyo, Japan), equipped with a copper anode (Cu-Kα1 radiation, λ = 1.5406 Å), to identify the crystalline phase of the synthesized material. A step size of (2θ) = 0.2° and a scan speed of 0.1°/s were set in the continuous scan mode. The crystalline phases of the samples were determined via High-score Pro software (Version 3.0.5, produced by PANalytica B.V, Almelo, The Netherlands).
The sample morphology and microstructure were studied using a scanning electron microscope SEM/FIB Tescan Lyra 3 (TESCAN ORSAY HOLDING, Brno, Czech Republic) at an electron accelerating voltage of 20 kV. A secondary electron detector was used.
Current-voltage characteristic measurements were carried out using the 4-point probe method with a Keithley SMU 2450 (Keithley Instruments, Beaverton, OR, USA) source meter unit. Silver paste was applied between the spring-loaded probes and gold electrodes to improve electrical contact. Measurement currents were set in the range of 10–100 µA. Each measurement was repeated 3 times. Thin film thicknesses were determined with a profilometer Dektak 150 (Veeco Instruments Inc., Plainview, NY, USA), by measuring the complete thin film profile between the two central electrodes. Thin film thicknesses were in the range from 137 µm to 238 µm. Specific electrical conductivities were calculated from the obtained data.
Seebeck coefficient was measured by placing the samples on 2 Peltier elements at an initial temperature of 20 °C. Both Peltier elements were gradually heated and cooled, reaching a maximum temperature difference of 4 °C. Heating and cooling were then reversed 3 times, resulting in 4 Seebeck coefficient measurements. The slope factors from measured ΔT/U slopes correspond to the Seebeck coefficients. The Peltier elements were controlled with a temperature controller (Stanford Research Systems PTC10, Stanford Research Systems (SRS), Sunnyvale, CA, USA), and the TE voltage was measured with a nanovoltmeter (Keithley 2182A, Keithley Instruments, Beaverton, OR, USA). Two external K-type thermocouples were used to measure the actual temperature difference between electrodes. A more detailed setup description is given in [
42].
Electrical conductivities and Seebeck coefficients were remeasured once a week for 8 weeks to study the aging effect of the materials.
X-ray photoelectron spectroscopy (XPS) was used to study sample oxidation and HCl content. The measurements were carried out with the photoelectron spectrometer ThermoFisher ESCLAB Xi (Thermo Fisher Scientific, East Grinstead, UK). The first measurement was performed on the unaffected sample surface. Sample surfaces were then etched using a monatomic argon ion beam with an energy of 2000 eV for 30 s. The etch and measurement cycle was repeated five times for each sample. The samples were measured immediately after fabrication and eight weeks later.
3. Results
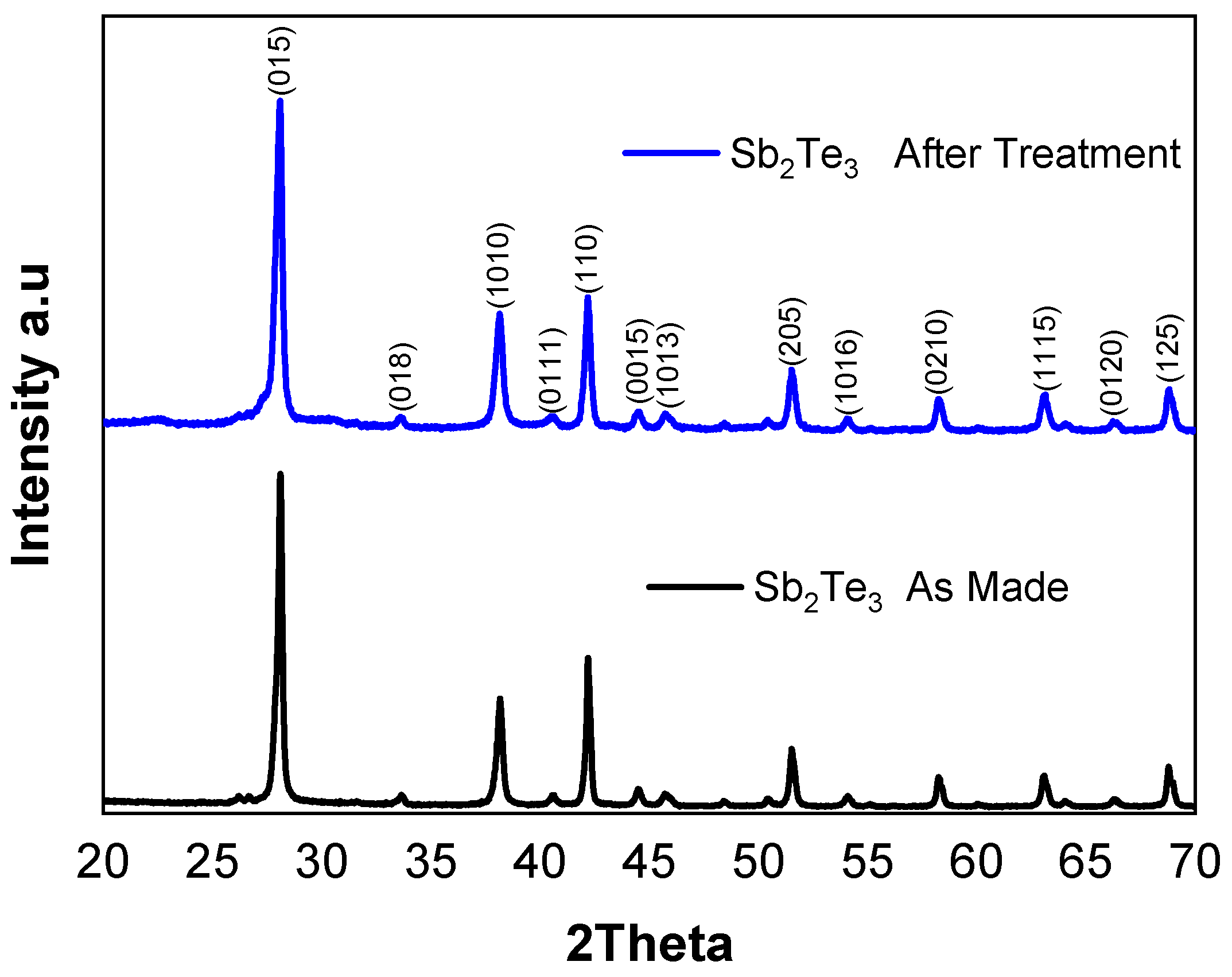
The phase purity of the synthesized Sb
2Te
3 particles was examined using X-ray diffraction (XRD), with the diffraction patterns indicating high phase purity for Sb
2Te
3, as illustrated in
Figure 3. The main crystalline phases are indexed to Sb
2Te
3 (ICDD 03-065-3678), which has a rhombohedral crystal structure.
Figure A1 in
Appendix A.1 compares treated particles with the original ones.
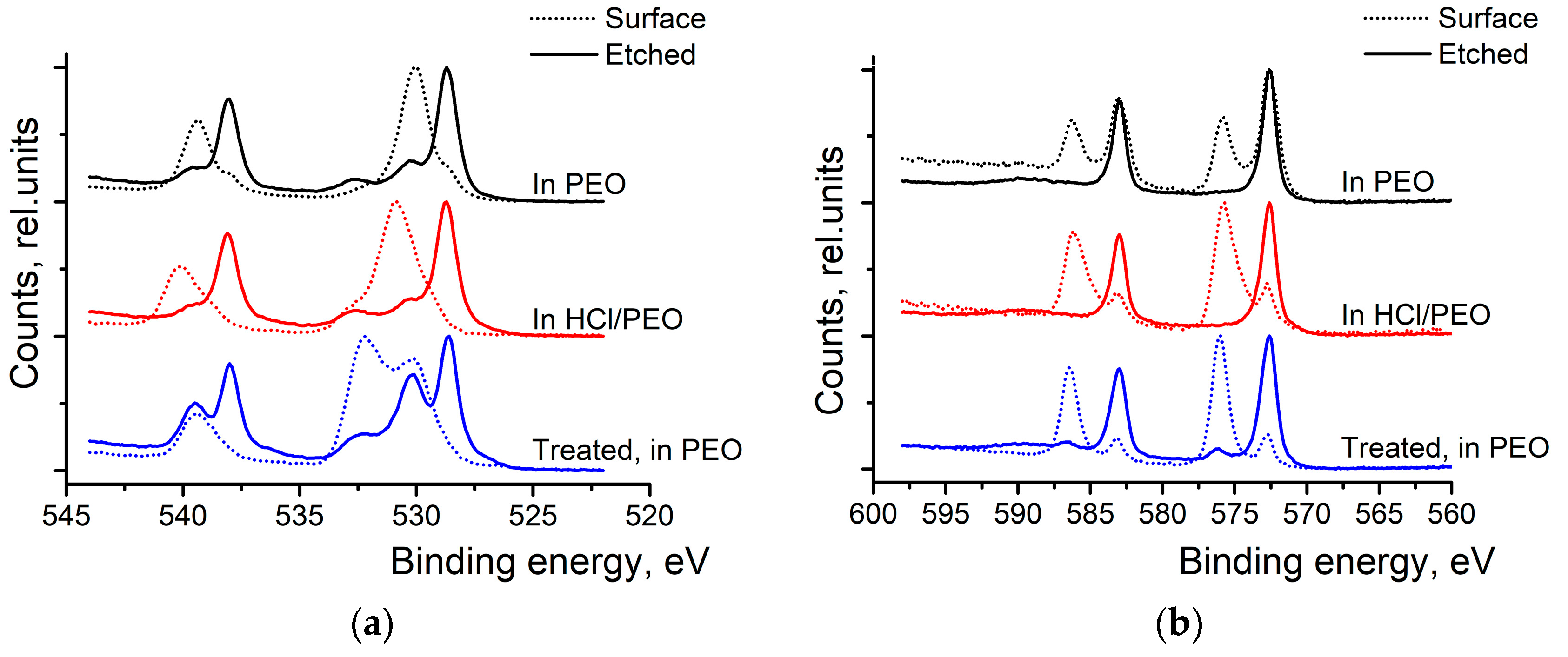
High-resolution XPS scans of Sb 3d and Te 3d were conducted to investigate surface oxidation (see
Figure 4). At first, bare Sb
2Te
3 NPs were studied. In the Sb spectra (top spectra of
Figure 4a), we can see the two peaks at 528.7 and 530.0 eV, which correspond to the metallic Sb and the oxidized antimony in the Sb
2O
3, respectively. After the surface etching, the oxide peak diminished while the peak corresponding to the metallic Sb grew. The Te 3d scans (top spectra of
Figure 4b) also show two peaks—at 572.7 eV and 576.1 eV—corresponding to the metallic and oxidized Te, respectively. The peak corresponding to TeO
3 (576.1 eV) disappears after surface etching. After 8 weeks in the air, the peaks corresponding to the oxidized states of Sb and Te have grown and remain visible even after the surface etching.
Bottom spectra of
Figure 4a,b show that washing nanoparticles in (HCl) and rinsing them in IP is an efficient way to remove the oxide layer at the surface of the particles. Only a tiny amount of Sb
2O
3 can be detected at the surface of the particles, and it completely disappears after the surface etching. In the Te 3d spectra (
Figure 4b), only metallic Te can be observed after washing the NPs with HCl and rinsing them in isopropyl alcohol.
SEM micrographs reveal that most NPs are configured as two-dimensional nanosheets. These NPs exhibit sizes with lengths extending up to 2 µm. The thickness of these nanosheets ranges from 29 nm to 121 nm, with the majority being approximately 60 nm thick for the Sb
2Te
3 in PEO sample and the treated Sb
2Te
3 in PEO sample (
Figure 5). However, the median NP thickness for Sb
2Te
3 in HCl/PEO is 46 nm. NPs embedded in pure polymer exhibit a smooth surface, while HCl/PEO and treated NP have a rough surface. SEM images show that the surface of Sb
2Te
3 NPs has been etched, but the thickness remains unchanged. NPs in HCl/PEO have been exposed to more aggressive etching, which has resulted in an additional reduction in NP thickness. The NPs predominantly orient with their narrower edges protruding from the block or wire-like structures.
To investigate the optimal composition of the TE composite film based on water-based inks containing Sb
2Te
3 as the nanocarrier and PEO as the matrix, we performed a study of the percolation curve of Sb
2Te
3 nanoparticles within the PEO matrix, monitoring the TE properties of the films (
Figure 6).
In a typical percolation curve, the electrical conductivity of the composite film is related to the filler concentration introduced into the insulating host polymer matrix, following the principles of Percolation Theory as described in [
43] and Equation (3)
where
is the concentration of filler introduced,
is the percolation threshold,
is the scaling factor, and
is the critical exponent. Percolation Theory is frequently used to describe insulating-to-conductive transitions in materials composed of conductive fillers embedded in an insulating matrix. Initially, the electrical conductivity of the composite film is practically negligible until the percolation threshold is reached at a nanoparticle concentration of 40%. Generally, a low percolation threshold indicates a homogeneous distribution of the conductive phase within the insulating host matrix [
16,
44,
45]. So, this percolation threshold value indicates that the particles are not completely homogeneous distributed along the film. From this point onward, conductivity progressively increases. The highest observed conductivity was at the highest tested nanoparticle concentration of 80%. Adding nanoparticles above 80% concentration resulted in a high viscosity paste with poor processability and thin film quality. The Seebeck coefficient remains constant during the incorporation of Sb
2Te
3 nanoparticles into the polymer matrix, as the carrier concentration does not change, and the increase in conductivity is attributed to the improved percolation network. Since the Seebeck coefficient remains stable, the power factor (PF) follows the same trend as the electrical conductivity. Therefore, an 80 wt% Sb
2Te
3/PEO concentration was chosen for detailed study due to its superior properties. Various NP treatment methods were tested to maintain these properties over time.
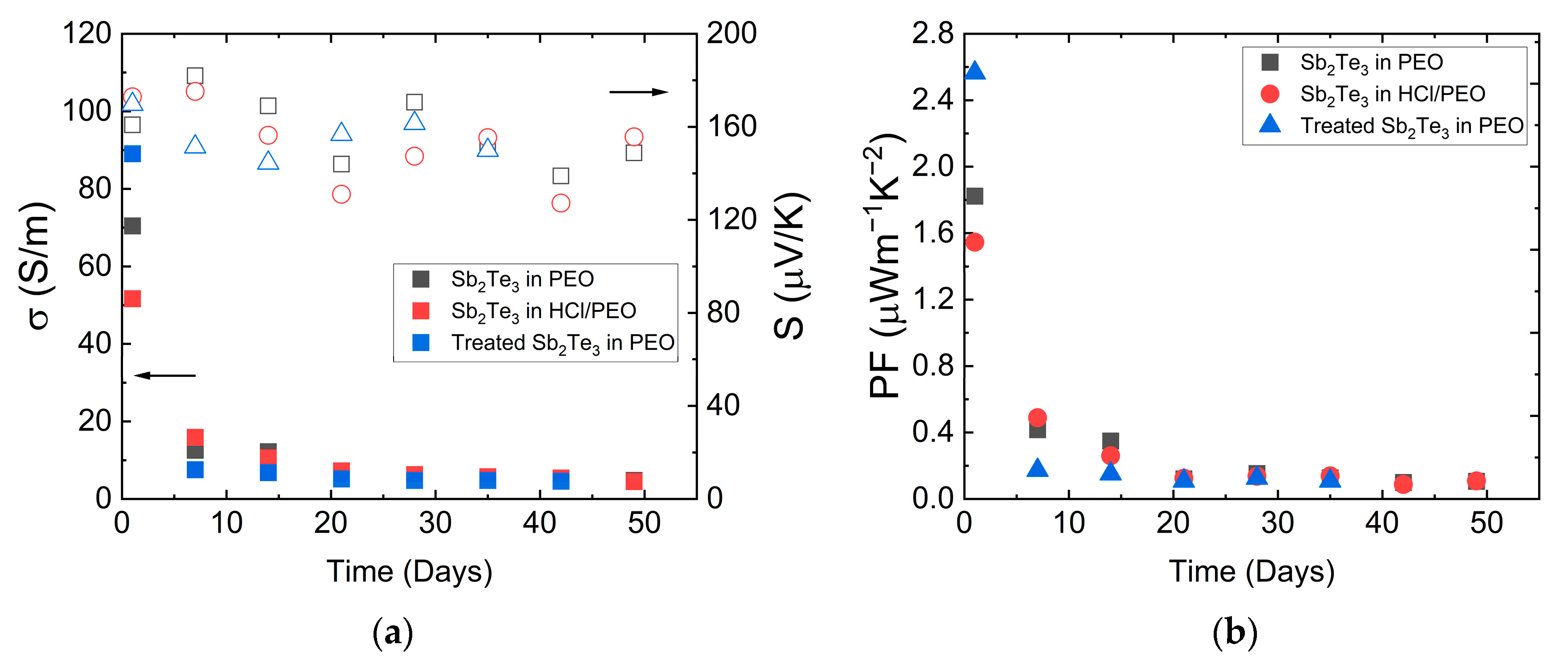
For stability studies, the time dependence of the specific electrical conductivity and Seebeck coefficient of the samples was measured. The results are presented in
Figure 7. The initial measurement was performed immediately after sample fabrication. Subsequently, the samples were stored in the air at room temperature and remeasured weekly for 8 weeks. The highest
σ observed was 89 ± 1 S/m for the sample with HCl-treated NPs. However, it shows the highest decrease in
σ over time. This may be due to HCl treatment removing the protective TGA shell, which encapsulated the particles to prevent oxidation. The second most conductive sample, containing pristine NPs, exhibited an
σ value of 70 ± 1 S/m. The sample treated with a 5% HCl solution shows an initial
σ of 52 ± 1 S/m. The
S for pristine and HCl sonicated NP samples were similar within the margin of error, with measured values of 170 ± 2 µV/K and 173 ± 3 µV/K, respectively.
Over 8 weeks, the
σ of all samples degraded exponentially (see
Figure 6a). Initially, conductivity varied among fresh samples, but all of them converged to the value of around 5 S/m because of Sb
2Te
3 re-oxidation. The HCl-treated NP sample, initially the most conductive, showed rapid
σ loss but then exhibited the slowest decrease in
σ after the second week. Despite starting with the lowest
σ, the HCl/PEO sample remained the most conductive post-second week due to the presence of HCl which dissolves the oxide layer of Sb
2Te
3 particles in the polymer. The overall trend of the
S dependence on time shows a slight decrease but remains close within the margin of measurement errors for all the samples. The
σ values for all samples tend to converge to a single value after 7 weeks, indicating that the tested particle treatment methods do not provide a long-term effect on stability, although different methods affect the initial properties and their changes in the first weeks differently.
The power factor values are shown in
Figure 7b. The sample with HCl treated NPs has shown the highest PF value of 2.56 ± 0.05 µ Wm
−1 K
−2, which is attributed to the highest
σ. The sample with pristine NPs and the HCl-treated sample show similar power factor values of 1.82 ± 0.04 µ Wm
−1 K
−2 and 1.55 ± 0.05 µ Wm
−1 K
−2, respectively. After 8 weeks, the power factors are significantly lower at ≈ 0.1 µ Wm
−1 K
−2.
XPS studies reveal that mixing the NPs in polymer (PEO) introduces an additional peak in Sb high-resolution scan spectra at 532 eV (see
Figure 8a). This peak corresponds to C-O bond peak from PEO polymer. The XPS spectra of O 1s peak of pure PEO polymer is shown at
Figure A2. Another peak at 530 eV represents Sb
2O
3, due to the partial oxidation of the NPs by oxygen, most likely originating from water. When the NPs are mixed with HCl and PEO, the Sb
2O
3 peak decreases and a new peak appears at 530.9 eV, corresponding to SbCl
5, indicating that the oxide on the surface of Sb
2Te
3 has partially dissolved and SbCl
5 has formed (see middle spectra of
Figure 8a). After deep profile XPS etching, the peak at 528.8 eV corresponding to the Sb–Te bond becomes more clearly visible. Meanwhile, only metallic Te peaks at 572.7 eV could be observed (
Figure 8b). HCl treatment before mixing in the PEO does not hinder the oxidation of the Sb
2Te
3 NPs. Before the etching (deep profile XPS), most of the obtained signal comes from the oxidized states of the Sb and Te (see bottom spectra of
Figure 8a,b).
After 8 weeks in air, the XPS spectra for Sb
2Te
3 mixed in PEO are similar to the initial samples, but with larger peaks indicating more oxidation of metals (
Figure A3). This suggests that particles from aqueous solutions continue to oxidize, leading to low conductivity after 8 weeks, and causing conductivity values to converge over time despite different treatments.
It is known that 1,6-hexanedithiol (HDT) improves the performance of Sb
2Te
3 PMMA composites [
40]. The stability of water-based solution composite treatment with HDT was tested. In this case, old particles were used and treated with HCl to remove the oxide layer, as previously performed. Additionally, in the final rinsing cycle with isopropanol (IP), HDT was added to IP. This method binds the HDT to the Te in the particles.
Figure 9 presents the long-term stability comparison between standard-treated samples and HDT-treated samples over 50 days. Untreated old particle composite displays low conductivity values, indicating the significance of oxide removal from the surface of particles. The low
σ of the untreated samples does not allow us to perform reliable
S measurements. The addition of HDT as a surface modification agent demonstrates remarkable effects on the transport performance of Sb
2Te
3 NPs. The HDT-treated samples consistently outperform other treatments, maintaining higher PF (above 0.12 μW m
−1 K
−2) and more stable s throughout the testing period. This superior performance is attributed to the strong chemical bonding between the thiol groups and the NPs surface, which is not influenced by further possible oxidation of the nanoparticles’ surface.
XPS analysis reveals that HDT treatment leads to significant changes in the surface chemistry, with the appearance of new peaks and shifts in binding energies that indicate successful chemical bonding between the dithiol groups and the NP surface.
Figure 10 shows XPS spectra of sulfur (S 2p) peaks of nanoparticles with and without the added HDT during the treatment process. The top spectra show that without the addition of HDT, there is no detectable amount of sulfur in the nanoparticles. In the spectra of pure nanoparticles and when the nanoparticles are mixed in PEO, the sulfur peak is centered at 163.5 eV which corresponds to the thiol (R-S-H) chemical group (see middle and bottom spectra in
Figure 10). After the surface etching, the peak widens and extra shoulder at 161.7 eV can be seen. This extra signal shows that the HDT has been attached to the nanoparticle surface, forming a metal sulfide.
Figure 11 shows the XPS spectra comparison between old NPs in PEO, treated samples in PEO, and HDT-treated samples.
The top spectra of
Figure 11a shows 1 year old Sb
2Te
3 particles mixed in PEO. Here, before the etching, almost all the signal comes from the Sb
2O
3 (peak at 530.0 eV) and oxygen in the PEO (peak at 532.5 eV)—the signal of metallic Sb is small. Similarly, the top spectra of
Figure 11b show only the oxidized states of Te before the surface etching. The asymmetric nature of the peak suggests that there is a mixture of two oxides on the surface of the particles- TeO
2 and TeO
3. This is even more evident when the NPs are treated with HCl and washed with IP. This treatment has only partially removed the metal oxides from the surface of the particles (see middle spectra of
Figure 11a,b).
4. Discussion
The results of this study confirm the high purity and quality of the synthesized Sb2Te3 nanoparticles, as evidenced by X-ray diffraction (XRD) analysis. The S, σ, PF, and long-term stability of the Sb2Te3/PEO hybrid material were thoroughly evaluated. These findings align with previous studies that have highlighted the potential of Sb2Te3 as a high-performance TE material due to its high S and σ at room temperature.
X-ray photoelectron spectroscopy (XPS) measurements revealed that exposure to water and oxygen leads to oxidation of the NPs, which can be partially mitigated by a 5% hydrochloric acid (HCl) solution. However, this treatment does not completely prevent the ongoing oxidation process. This observation is consistent with the findings of Volykhhov et al. [
39], who described the oxidation of Sb
2Te
3 as a multi-step process involving molecular adsorption, surface reaction, and oxide layer growth. The study by Fang et al. [
41] also supports the effectiveness of HCl treatment in removing surface oxides, although it does not halt oxidation entirely. XPS analysis indicated that incorporating the nanoparticles into PEO generates an additional peak in the high-resolution antimony spectra at 532 eV. This peak is associated with the oxygen atom in the polymer’s C–O bond. Adding HCl to the polymer facilitates the formation of SbCl
5, as determined by the peak at 530.9 eV. Meanwhile, only metallic Te peaks at 572.7 eV were observed. HCl treatment before mixing in the PEO does not hinder the oxidation of the Sb
2Te
3 nanoparticles.
The TE transport properties of the Sb
2Te
3/PEO hybrid material investigated in this study are consistent with those reported by Batili et al. [
40] for electrophoretically deposited films; however, a long-term stability assessment is not provided.
This study shows that the initial PF of the Sb
2Te
3/PEO hybrid materials are close to those of plastic ductile SnSe
1.95Br
0.05 single crystals and outperform AgCySe-based plastic TE materials [
46,
47]. Hybrid films enable flexible TE materials, and optimizing their porous morphology, as observed in SEM images, could further enhance the power factor.
The utilization of single or small organic molecules as components in electronic devices are currently under investigation for the development of functional materials using semiconductor and metal nanoparticles [
48]. There are reports on thiolated molecules positively influencing the electronic properties of nanoparticle films. This was demonstrated earlier by ethane dithiol treatment of semiconductor PbS films leading to nearly a tenfold increase in mobility [
49]. Recently, a similar trend of reduction in the resistance of organic- inorganic hybrid TE films was demonstrated by the use of dodecanethiol [
17]. The interaction of thiolated surfactants was suggested to be redox type, causing the reduction in surface oxide [
50], which may also contribute to the reduction in interfacial resistance between the particles.
The following chemical equations illustrate the interaction of 1,6-hexanedithiol (HS–(CH2)6–SH) with a generic metal oxide (M2O3, MO2), where M could be Te or Sb. These reactions are presented in both single-thiol and double-thiol surface bonds.
In single-thiol interaction reaction, only one thiol group of 1,6-hexanedithiol reacts with the metal oxide, forming a single M–S bond. The other thiol group remains unreacted.
In the double-thiol interaction (bridging) reaction, both thiol groups of HDT react with two metal oxide units, forming a bridge between the two metal atoms belonging to different nanoparticles.