Natural Dyes and Pigments: Sustainable Applications and Future Scope
Abstract
1. Introduction
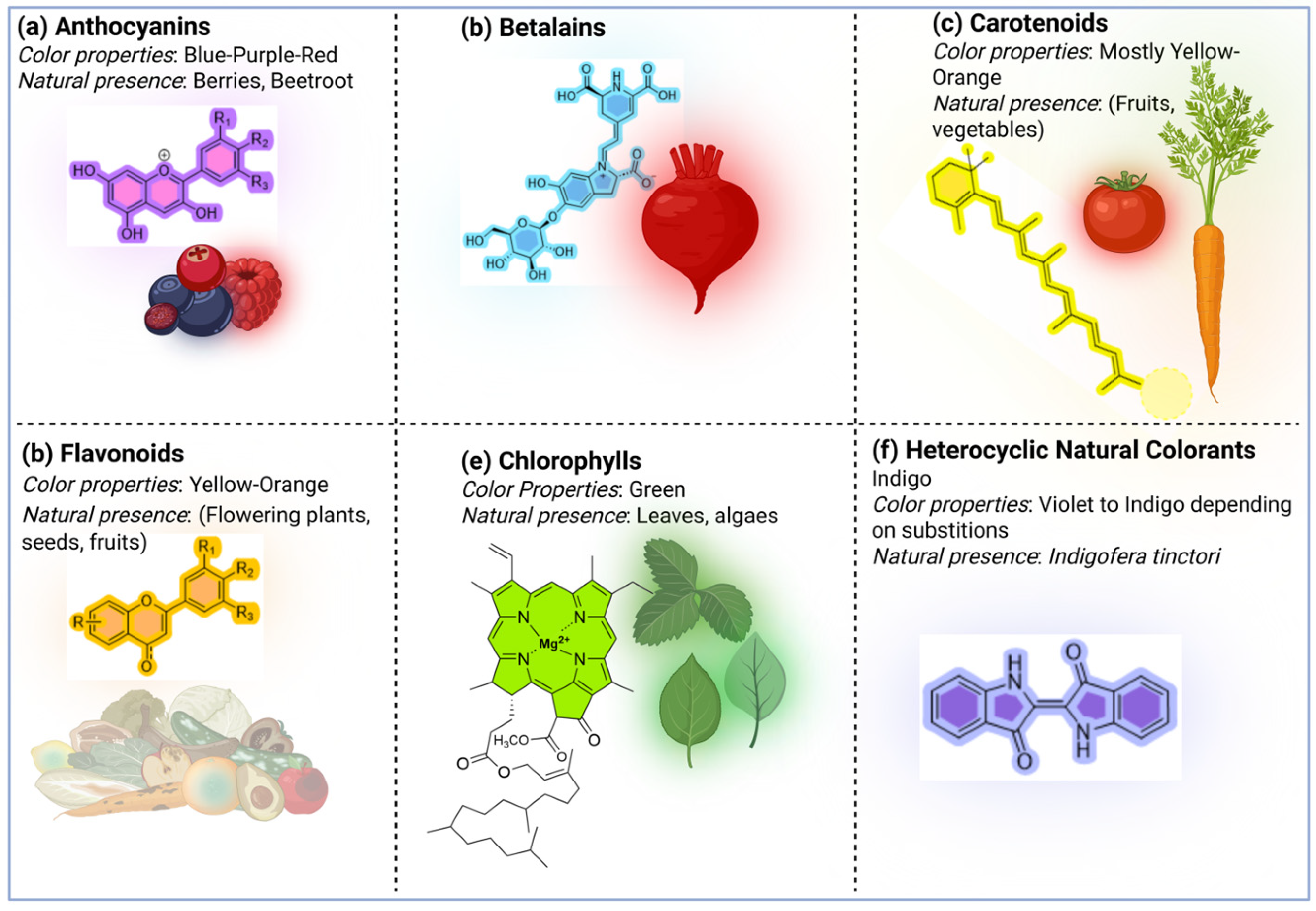
2. Natural Dyes and Pigments
2.1. Color Properties of Natural Dyes and Pigments
2.2. UV-Vis Absorption Spectrum of Natural Dyes and Pigments
2.3. Examples of Commercial Success of Natural Dyes and Pigments
3. Classification of Natural Dyes and Pigments
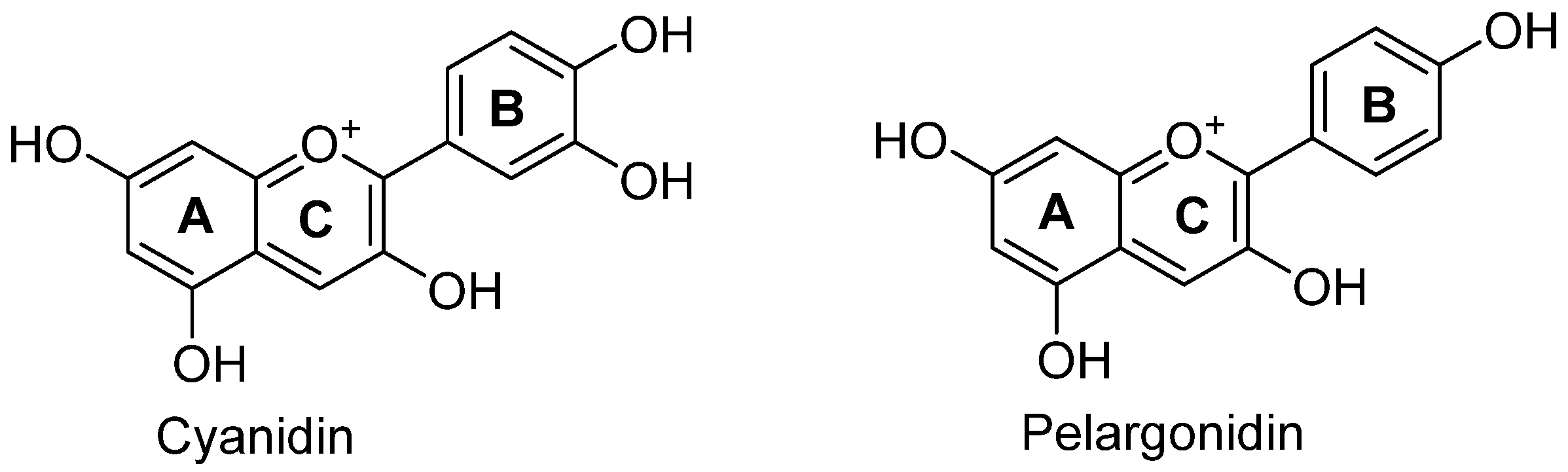
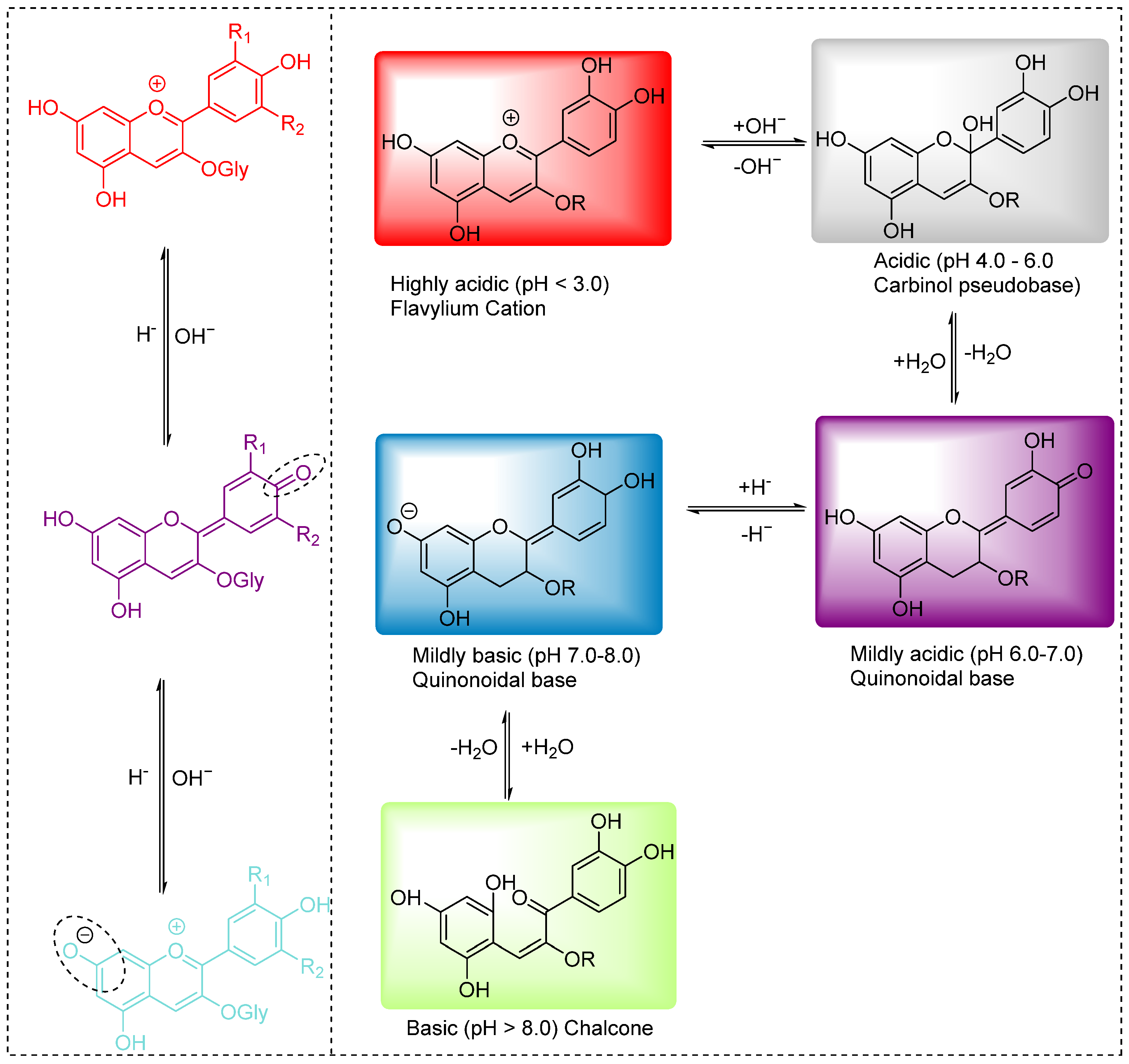
3.1. Anthocyanin-Based Natural Dyes and Pigments
- (i)
- Presence of metal ions: Anthocyanins contain electronegative groups, such as polyphenolic hydroxyls, which can coordinate with metal ions like iron or copper to form metal-anthocyanin complexes. While some organic–metal complexes are known for their bright color properties, in the case of anthocyanins, these interactions often result in undesirable color changes, such as browning or dulling of the original hue.
- (ii)
- Presence of other natural compounds: Anthocyanins may interact with tannins, proteins, and other polyphenols present in their natural sources or extracts [77]. These interactions enhance intermolecular bonding, leading to the formation of insoluble complexes that can precipitate out of solution. As a result, the color intensity and solubility of anthocyanins are reduced.
- (iii)
- Presence of glycosyl units: Glycosylation generally stabilizes anthocyanins by increasing their water solubility and structural integrity. However, the presence of multiple glycosyl (sugar) units can increase the overall polarity of the molecule and, in some cases, cause positional isomerization. This equilibrium is sensitive to changes in environmental conditions (such as pH and temperature), leading to inconsistent color intensity and sometimes visible color shifts.
3.2. Betalain-Based Natural Dyes and Pigments
3.3. Carotenoids-Based Natural Dyes and Pigments
3.4. Flavonoids-Based Natural Dyes and Pigments
3.5. Chlorophyll-Based Natural Dyes and Pigments
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Color. Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and dyes: Developments and applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef]
- Patel, M.J.; Tandel, R.; Sonera, S.A.; Bairwa, S.K. Trends in the synthesis and application of some reactive dyes: A review. Braz. J. Sci. 2023, 2, 14–29. [Google Scholar] [CrossRef]
- Faisal, S.; Lin, L. Green synthesis of reactive dye for ink-jet printing. Color. Technol. 2020, 136, 110–119. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Kaczorowska, M.A.; Bożejewicz, D.; Witt, K. The application of polymer Inclusion membranes for the removal of emerging contaminants and synthetic dyes from aqueous solutions—A mini review. Membranes 2023, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of technical textiles and synthetic nanofibers on environmental pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Negi, A. Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation. Polymers 2025, 17, 871. [Google Scholar] [CrossRef]
- Léonard, E.; Fayeulle, A. Azo-dyes-grafted oligosaccharides—From synthesis to applications. Molecules 2021, 26, 3063. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- De Filippis, B.; Della Valle, A.; Ammazzalorso, A.; Maccallini, C.; Tesse, G.; Amoroso, R.; Mollica, A.; Giampietro, L. Azobenzene as Multi-Targeted Scaffold in Medicinal Chemistry. Molecules 2024, 29, 5872. [Google Scholar] [CrossRef]
- Negi, A.; Kieffer, C.; Voisin-Chiret, A.S. Azobenzene photoswitches in proteolysis targeting chimeras: Photochemical control strategies and therapeutic benefits. ChemistrySelect 2022, 7, e202200981. [Google Scholar] [CrossRef]
- Londoño-Berrío, M.; Pérez-Buitrago, S.; Ortiz-Trujillo, I.C.; Hoyos-Palacio, L.M.; Orozco, L.Y.; López, L.; Zárate-Triviño, D.G.; Capobianco, J.A.; Mena-Giraldo, P. Cytotoxicity and genotoxicity of azobenzene-based polymeric nanocarriers for phototriggered drug release and biomedical applications. Polymers 2022, 14, 3119. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Tang, S.; Wei, S.; He, H.; Ma, M.; Shi, Y.; Zhu, Y.; Chen, S.; Wang, X. Light-Responsive Smart Nanoliposomes: Harnessing the Azobenzene Moiety for Controlled Drug Release under Near-Infrared Irradiation. ACS Appl. Mater. Interfaces 2024, 16, 56850–56861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Q.; Liu, Y. Recent Advances of Light/Hypoxia-Responsive Azobenzene in Nanomedicine Design. ChemBioChem 2024, 25, e202400635. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Yu, J.; Huang, C. Research Progress of Cyanine-Based Near-Infrared Fluorescent Probes for Biological Application. ChemBioChem 2024, 25, e202400467. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Pandit, P.; Maity, S.; Sharma, S. Chapter 11—Harmful 535 environmental effects for textile chemical dyeing practice. In Green Chemistry for Sustainable Textiles; Woodhead Publishing: Sawston, UK, 2021; pp. 153–164. [Google Scholar]
- Roy Choudhury, A. Environmental impacts of the textile industry and its assessment through life cycle assessment. In Roadmap to Sustainable Textiles and Clothing: Environmental and Social Aspects of Textiles and Clothing Supply Chain; Springer: Singapore, 2014; pp. 1–39. [Google Scholar]
- Anliker, R.; Clarke, E.A. International Regulation of Chemicals—Implications for Organic Colorants. J. Soc. Dye. Colour. 1982, 98, 42–55. [Google Scholar] [CrossRef]
- de Oliveira, G.A.R. Textile dyes: Dyeing process and environmental impact. In Eco-Friendly Textile Dyeing and Finishing; IntechOpen: London, UK, 2013; p. 151. [Google Scholar]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Caldwell, A.; Brander, S.; Wiedenmann, J.; Clucas, G.; Craig, E. Incidence of microplastic fiber ingestion by common terns (Sterna hirundo) and roseate terns (S. dougallii) breeding in the northwestern atlantic. Mar. Pollut. Bull. 2022, 177, 113560. [Google Scholar] [CrossRef]
- Vazquez-Cruz, V.A.; Vázquez-Morillas, A.; Cruz-Salas, A.A.; Hernández-Soriano, A.I.; Cervantes-Cabrera, G.; Ballesteros-López, M.E.; Alvarez-Zeferino, J.C. Microplastics in Urban Bird Feces: A Methodological Approach and Case Study in Mexico City. Microplastics 2025, 4, 6. [Google Scholar] [CrossRef]
- Carrasco, L.; Jiménez-Mora, E.; Utrilla, M.J.; Pizarro, I.T.; Reglero, M.M.; Román, R.-S.; Martin-Maldonado, B. Birds as Bioindicators: Revealing the Widespread Impact of Microplastics. Birds 2025, 6, 10. [Google Scholar] [CrossRef]
- Senes, G.P.; Barboza, L.G.A.; Nunes, L.M.; Otero, X.L. Microplastics in feces and pellets from yellow-legged gull (Larus michahellis) in the Atlantic Islands National Park of Galicia (NW Spain). Mar. Pollut. Bull. 2023, 195, 115531. [Google Scholar] [CrossRef]
- Mota, I.G.C.; Neves, R.A.M.D.; Nascimento, S.S.D.C.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Artificial dyes: Health risks and the need for revision of international regulations. Food Rev. Int. 2023, 39, 1578–1593. [Google Scholar] [CrossRef]
- Wirtu, Y.D.; Godana, U.A.; Tucho, G.T. Impact of Synthetic Cosmetic Ingredients on the Human Respiratory System: A Mechanistic Insight. Preprints 2025, 2025030756. [Google Scholar] [CrossRef]
- Wargala, E.; Sławska, M.; Zalewska, A.; Toporowska, M. Health effects of dyes, minerals, and vitamins used in cosmetics. Women 2021, 1, 223–237. [Google Scholar] [CrossRef]
- John, A.; Yang, H.-H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U.F. Cross talk between synthetic food colors (azo dyes), oral flora, and cardiovascular disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- Kiki, M.J. Biopigments of microbial origin and their application in the cosmetic industry. Cosmetics 2023, 10, 47. [Google Scholar] [CrossRef]
- Elsahida, K.; Fauzi, A.; Sailah, I.; Siregar, I. Sustainable production of natural textile dyes industry. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012036. [Google Scholar]
- Jain, K.; Madamwar, D.; Tiwari, O. Mapping of Research Outcome on Remediation of Dyes, Dye Intermediates and Textile Industrial Waste; Department of Biotechnology, Ministry of Science & Technology, Government of India: New Delhi, India, 2019. [Google Scholar]
- Tena, N.; Asuero, A.G. Up-to-date analysis of the extraction methods for anthocyanins: Principles of the techniques, optimization, technical progress, and industrial application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Rosales-Murillo, S.; Sánchez-Bodón, J.; Hernández Olmos, S.; Ibarra-Vázquez, M.; Guerrero-Ramírez, L.; Pérez-Álvarez, L.; Vilas-Vilela, J. Anthocyanin-loaded polymers as promising nature-based, responsive, and bioactive materials. Polymers 2024, 16, 163. [Google Scholar] [CrossRef]
- Vo, T.-V.; Dang, T.-H.; Chen, B.-H. Synthesis of intelligent pH indicative films from chitosan/poly (vinyl alcohol)/anthocyanin extracted from red cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef]
- Kossyvaki, D.; Contardi, M.; Athanassiou, A.; Fragouli, D. Colorimetric indicators based on anthocyanin polymer composites: A review. Polymers 2022, 14, 4129. [Google Scholar] [CrossRef]
- Ekrami, M.; Roshani-Dehlaghi, N.; Ekrami, A.; Shakouri, M.; Emam-Djomeh, Z. pH-responsive color indicator of saffron (Crocus sativus L.) anthocyanin-activated salep mucilage edible film for real-time monitoring of fish fillet freshness. Chemistry 2022, 4, 1360–1381. [Google Scholar] [CrossRef]
- Che Hamzah, N.H.; Khairuddin, N.; Muhamad, I.I.; Hassan, M.A.; Ngaini, Z.; Sarbini, S.R. Characterisation and colour response of smart sago starch-based packaging films incorporated with Brassica oleracea anthocyanin. Membranes 2022, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Goiana, M.L.; Rosa, M.d.F.; Mattos, A.L.A.; Fernandes, F.A.N. Development of Plasma-Treated Corn-Starch-Based Film Incorporated with Acerola and Grape Pomace Extract Possessing pH-Sensing Capability. Polymers 2025, 17, 938. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Cerino, M.C. Chemical Characterization of Malpighia emarginata DC. Bioresidues and Exploration of Their Colourant Potential; Instituto Politecnico de Braganca (Portugal): Bragança, Portugal, 2021. [Google Scholar]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Nile, S.H.; Kim, D.H.; Keum, Y.-S. Determination of anthocyanin content and antioxidant capacity of different grape varieties. Ciênc. Téc. Vitiviníc. 2015, 30, 60–68. [Google Scholar] [CrossRef]
- Silva, J.T.d.P.; Borges, M.H.; de Souza, C.A.C.; Fávaro-Trindade, C.S.; Sobral, P.J.d.A.; de Oliveira, A.L.; Martelli-Tosi, M. Grape pomace rich-phenolics and anthocyanins extract: Production by pressurized liquid extraction in intermittent process and encapsulation by spray-drying. Foods 2024, 13, 279. [Google Scholar] [CrossRef]
- Trentin, J.; Mussagy, C.U.; Arantes, M.S.; Pedro, A.C.; Mafra, M.R.; Farias, F.O. Antioxidant ready-to-use grape pomace extracts recovered with natural eutectic mixtures for Formulation of Color-Rich gummies. Foods 2024, 13, 2840. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Valorization of grape pomace: A review of phenolic composition, bioactivity, and therapeutic potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- De Sales, N.F.; Silva da Costa, L.; Carneiro, T.I.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.; Torres, A.G.; El-Bacha, T. Anthocyanin-rich grape pomace extract (Vitis vinifera L.) from wine industry affects mitochondrial bioenergetics and glucose metabolism in human hepatocarcinoma HepG2 cells. Molecules 2018, 23, 611. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Ciorba, R.; Ceccarelli, D.; Amoriello, M.; Amoriello, T. Phytochemical and Functional Properties of Fruit and Vegetable Processing By-Products. Appl. Sci. 2024, 14, 9172. [Google Scholar] [CrossRef]
- Zuñiga-Miranda, J.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Heredia-Moya, J.; Vizuete, K.; Debut, A.; Barba-Ostria, C.; Coyago-Cruz, E. Phytosynthesis of silver nanoparticles using Mansoa alliacea (Lam.) AH gentry (Bignoniaceae) leaf extract: Characterization and their biological activities. Pharmaceutics 2024, 16, 1247. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, M.; Mirpoor, S.F.; Esposito, S.; Merola, G.; Mariniello, L.; Patanè, G.T.; Barreca, D.; Giosafatto, C.V.L. Manufacture of bioplastics prepared from chitosan functionalized with callistemon citrinus extract. Polymers 2024, 16, 2693. [Google Scholar] [CrossRef] [PubMed]
- Tama, A.; Karaś, M. The Health-Promoting Potential of Fruit Pomace and Its Application in the Confectionery Industry. Appl. Sci. 2025, 15, 5790. [Google Scholar] [CrossRef]
- Wang, H. Medical Benefits and Polymer Applications of Grapes. Polymers 2025, 17, 750. [Google Scholar] [CrossRef]
- Jang, B.-K.; Shin, S.J.; Park, H.H.; Kumar, V.; Park, Y.H.; Kim, J.-Y.; Kang, H.-Y.; Park, S.; Kwon, Y.; Shin, S.-E. Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation. Pharmaceutics 2024, 17, 13. [Google Scholar] [CrossRef]
- Neves, C.M.; Fogeiro, É.; Cardoso, S.M.; Gonçalves, F.; Pinto, A.; Wessel, D.F. Towards the Valorization of Elderberry By-Product: Recovery and Use of Natural Ingredients for Sorbet Formulations. Appl. Sci. 2024, 14, 10328. [Google Scholar] [CrossRef]
- Petrov Ivanković, A.; Ćorović, M.; Milivojević, A.; Blagojević, S.; Radulović, A.; Pjanović, R.; Bezbradica, D. Assessment of enzymatically derived blackcurrant extract as cosmetic ingredient—Antioxidant properties determination and in vitro diffusion study. Pharmaceutics 2024, 16, 1209. [Google Scholar] [CrossRef]
- Khalifa, I.; Xia, D.; Dutta, K.; Peng, J.; Jia, Y.; Li, C. Mulberry anthocyanins exert anti-AGEs effects by selectively trapping glyoxal and structural-dependently blocking the lysyl residues of β-lactoglobulins. Bioorganic Chem. 2020, 96, 103615. [Google Scholar] [CrossRef]
- Zheng, F.; Ke, J.; Lin, S.; Ye, W.; Wu, Z.; Xu, Y.; Mai, S.; Chen, Y.; Guo, Z.; Hu, H. Discovery of cyanidin-3-O-galactoside as a novel CNT2 inhibitor for the treatment of hyperuricemia. Bioorganic Chem. 2025, 154, 108108. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Garavand, F. Application of red cabbage anthocyanins as pH-sensitive pigments in smart food packaging and sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Yang, K.-M.; Chiang, P.-Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [PubMed]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of anthocyanin associated purple sweet potato starch and peel-based pH indicator films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, C.; Chen, M.-H.; Chiang, P.-Y. Stability and quality of anthocyanin in purple sweet potato extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef]
- Çoruh, O.; Gündüz, G.; Çolak, Ü.; Maviş, B. pH-Dependent Coloring of combination effect pigments with anthocyanins from Brassica oleracea var. capitata F. rubra. Colorants 2022, 1, 149–164. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Liu, L.; Cui, Z.; Zhong, Y. Recent advances in pH-responsive freshness indicators using natural food colorants to monitor food freshness. Foods 2022, 11, 1884. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of anthocyanins and their degradation products from cabernet sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef]
- Kang, H.-J.; Ko, M.-J.; Chung, M.-S. Anthocyanin structure and pH dependent extraction characteristics from blueberries (Vaccinium corymbosum) and chokeberries (Aronia melanocarpa) in subcritical water state. Foods 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Dubrović, I.; Herceg, Z.; Režek Jambrak, A.; Badanjak, M.; Dragović-Uzelac, V. Effect of high intensity ultrasound and pasteurization on anthocyanin content in strawberry juice. Food Technol. Biotechnol. 2011, 49, 196–204. [Google Scholar]
- Howard, L.R.; Prior, R.L.; Liyanage, R.; Lay, J.O. Processing and storage effect on berry polyphenols: Challenges and implications for bioactive properties. J. Agric. Food Chem. 2012, 60, 6678–6693. [Google Scholar] [CrossRef]
- Dobson, G.; McDougall, G.J.; Stewart, D.; Cubero, M.Á.; Karjalainen, R.O. Effects of juice matrix and pasteurization on stability of black currant anthocyanins during storage. J. Food Sci. 2017, 82, 44–52. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Hać-Szymańczuk, E.; Kruszewski, B. Impact of Conventional Pasteurization, High Temperature Short Time, Ultra-High Temperature, and Storage Time on Physicochemical Characteristics, Bioactive Compounds, Antioxidant Activity, and Microbiological Quality of Fruit Nectars. Foods 2024, 13, 3963. [Google Scholar] [CrossRef]
- Puzovic, A.; Pelacci, M.; Simkova, K.; Hudina, M.; Rusjan, D.; Veberic, R.; Mikulic-Petkovsek, M. Effect of Heat Pasteurization and Enzymatic Maceration on Yield, Color, Sugars, Organic Acids, and Phenolic Content in the ‘Merlot Kanthus’ Grape Juice. Beverages 2024, 10, 66. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Martìnez Garcìa, L.; Landi, M.; Guidi, L.; Massai, R.; Remorini, D. Influences of postharvest storage and processing techniques on antioxidant and nutraceutical properties of Rubus idaeus L.: A mini-review. Horticulturae 2020, 6, 105. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Kruszewski, B. High-Temperature Short-Time and Ultra-High-Temperature Processing of Juices, Nectars and Beverages: Influences on Enzyme, Microbial Inactivation and Retention of Bioactive Compounds. Appl. Sci. 2024, 14, 8978. [Google Scholar] [CrossRef]
- Salleh, N.; Goh, K.K.; Waterland, M.R.; Huffman, L.M.; Weeks, M.; Matia-Merino, L. Complexation of anthocyanin-bound blackcurrant pectin and whey protein: Effect of pH and heat treatment. Molecules 2022, 27, 4202. [Google Scholar] [CrossRef]
- Yoshida, K.; Okuno, R.; Kameda, K.; Mori, M.; Kondo, T. Influence of E, Z-isomerization and stability of acylated anthocyanins under the UV irradiation. Biochem. Eng. J. 2003, 14, 163–169. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.; González-Manzano, S.; Escribano-Bailón, M.T.; Heredia, F.J.; Santos-Buelga, C. Influence of different phenolic copigments on the color of malvidin 3-glucoside. J. Agric. Food Chem. 2006, 54, 5422–5429. [Google Scholar] [CrossRef]
- Torres-Rochera, B.; Brás, N.F.; García-Estévez, I.; Escribano-Bailón, M.T. Chemical and colorimetric study of the influence of grape soluble polysaccharides on the color and stability of malvidin 3-O-glucoside solutions. LWT 2023, 188, 115420. [Google Scholar] [CrossRef]
- Gombau, J.; Vignault, A.; Pascual, O.; Gómez-Alonso, S.; Gracía-Romero, E.; Hermosín, I.; Canals, J.M.; Teissedre, P.-L.; Zamora, F. Influence of oenological tannins on malvidin-3-O-monoglucoside copigmentation in a model wine solution. Oeno One 2019, 53, 531–547. [Google Scholar] [CrossRef]
- Adams, J.; Brown, H. Discoloration in raw and processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2007, 47, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and synthetic flavylium-based dyes: The chemistry behind the color. Chem. Rev. 2021, 122, 1416–1481. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- de Ancos, B.; Gonzalez, E.; Cano, M.P. Differentiation of raspberry varieties according to anthocyanin composition. Z. Leb. und-Forsch. A 1999, 208, 33–38. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F. Quantitative methods for anthocyanins. 4. Determination of individual anthocyanins in cranberry and cranberry products. J. Food Sci. 1968, 33, 471–478. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Parandi, E.; Heydari, M.; Kolahdouz-Nasiri, A.; Bahraminejad, M.; Mohammadi, R.; Rouhi, M.; Garavand, F. Betalains as promising natural colorants in smart/active food packaging. Food Chem. 2023, 424, 136408. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic application of betalains: A review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The influence of different pretreatment methods on color and pigment change in beetroot products. Molecules 2021, 26, 3683. [Google Scholar] [CrossRef]
- Lazăr, S.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Optimization of betalain pigments extraction using beetroot by-products as a valuable source. Inventions 2021, 6, 50. [Google Scholar] [CrossRef]
- Chapman, G.; Solomon, I.; Patonay, G.; Henary, M. Synthesis and pH-Dependent Spectroscopic Behavior of 2, 4, 6-Trisubstituted Pyridine Derivatives. J. Heterocycl. Chem. 2015, 52, 861–872. [Google Scholar] [CrossRef]
- Tydlitát, J.; Achelle, S.; Rodríguez-López, J.; Pytela, O.; Mikýsek, T.; Cabon, N.; Robin-le Guen, F.; Miklík, D.; Růžičková, Z.; Bureš, F. Photophysical properties of acid-responsive triphenylamine derivatives bearing pyridine fragments: Towards white light emission. Dye. Pigment. 2017, 146, 467–478. [Google Scholar] [CrossRef]
- Lamelas, R.; Garcia, V.; Linares, A.; Bastida, R.; Labisbal, E.; Fernandez-Lodeiro, A.; Lodeiro, C.; Nunez, C.; Valencia, L. Novel trans-disubstituted hexaaza-macrocyclic ligands containing pyridine head units: Synthesis, disubstitution and colorimetric properties. Sens. Actuators B Chem. 2016, 225, 481–491. [Google Scholar] [CrossRef]
- Negi, A.; Mirallai, S.I.; Konda, S.; Murphy, P.V. An improved method for synthesis of non-symmetric triarylpyridines. Tetrahedron 2022, 121, 132930. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; García-Viguera, C.; Gil, J.I.; Gil-Izquierdo, A. Betalains in the era of global agri-food science, technology and nutritional health. Phytochem. Rev. 2008, 7, 261–280. [Google Scholar] [CrossRef]
- Coy-Barrera, E. Analysis of betalains (betacyanins and betaxanthins). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar]
- Devadiga, D.; Ahipa, T. A Red-Violet Pigment—Chemistry and Applications. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; IntechOpen: London, UK, 2020; p. 173. [Google Scholar] [CrossRef]
- Hussain, E.A.; Sadiq, Z.; Zia-Ul-Haq, M. Betalains: Biomolecular Aspects; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M. Anthocyanins and Betalains. In Food Analysis by HPLC; CRC Press: Boca Raton, FL, USA, 2012; pp. 757–775. [Google Scholar]
- Prabhakaran, G.Y.S.; Yoganandan, M.; Verma, P. Food Pigments And Color Measurement Techniques. In Non-Thermal Food Processing Technologies; Apple Academic Press: Cambridge, MA, USA, 2024; pp. 1–33. [Google Scholar]
- Kanner, J.; Harel, S.; Granit, R. Betalains a new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Morilla, M.J.; Ghosal, K.; Romero, E.L. More than pigments: The potential of astaxanthin and bacterioruberin-based nanomedicines. Pharmaceutics 2023, 15, 1828. [Google Scholar] [CrossRef]
- Shi, G.; Gu, L.; Jung, H.; Chung, W.-J.; Koo, S. Apocarotenals of phenolic carotenoids for superior antioxidant activities. ACS Omega 2021, 6, 25096–25108. [Google Scholar] [CrossRef]
- Peng, X.; Zeng, Z.; Hassan, S.; Xue, Y. The potential of marine natural Products: Recent Advances in the discovery of Anti-Tuberculosis agents. Bioorganic Chem. 2024, 151, 107699. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, occurrence, properties, applications, and encapsulation of carotenoids—A review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D. Carotenoids in cancer apoptosis—The road from bench to bedside and back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Amengual, J. Bioactive properties of carotenoids in human health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Focsan, A.L.; Gao, Y.; Kispert, L.D. The endless world of carotenoids—Structural, chemical and biological aspects of some rare carotenoids. Int. J. Mol. Sci. 2023, 24, 9885. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The benefits and risks of certain dietary carotenoids that exhibit both anti-and pro-oxidative mechanisms—A comprehensive review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A narrative review: The effect and importance of carotenoids on aging and aging-related diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in drug discovery and medicine: Pathways and molecular targets implicated in human diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Morales, A.; Medina-Garcia, M.; Martinez-Peinado, P.; Pascual-Garcia, S.; Pujalte-Satorre, C.; Lopez-Jaen, A.B.; Martinez-Espinosa, R.M.; Sempere-Ortells, J.M. The antitumour mechanisms of carotenoids: A comprehensive review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Nakano, T.; Wiegertjes, G. Properties of carotenoids in fish fitness: A review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O. Carotenoids in cancer metastasis—Status quo and outlook. Biomolecules 2020, 10, 1653. [Google Scholar] [CrossRef]
- Papapostolou, H.; Kachrimanidou, V.; Alexandri, M.; Plessas, S.; Papadaki, A.; Kopsahelis, N. Natural carotenoids: Recent advances on separation from microbial biomass and methods of analysis. Antioxidants 2023, 12, 1030. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.d.A.; Lopes, A.S.; Chisté, R.C. Biotechnological production of carotenoids using low cost-substrates is influenced by cultivation parameters: A review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef]
- Raposo, M.F.d.J.; Morais, A.M.M.B.d.; Morais, R.M.S.C.d. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Eroglu, A.; Al’Abri, I.S.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and their health benefits as derived via their interactions with gut microbiota. Adv. Nutr. 2023, 14, 238–255. [Google Scholar] [CrossRef]
- Chandra, R.D.; Prihastyanti, M.N.U.; Lukitasari, D.M. Effects of pH, high pressure processing, and ultraviolet light on carotenoids, chlorophylls, and anthocyanins of fresh fruit and vegetable juices. EFood 2021, 2, 113–124. [Google Scholar] [CrossRef]
- Atencio, S.; Verkempinck, S.H.; Reineke, K.; Hendrickx, M.; Van Loey, A. Heat and light stability of pumpkin-based carotenoids in a photosensitive food: A carotenoid-coloured beverage. Foods 2022, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Molner, J.V.; Pérez-González, R.; Sòria-Perpinyà, X.; Soria, J. Climatic Influence on the Carotenoids Concentration in a Mediterranean Coastal Lagoon Through Remote Sensing. Remote Sens. 2024, 16, 4067. [Google Scholar] [CrossRef]
- Molina, A.K.; Corrêa, R.C.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive natural pigments’ extraction, isolation, and stability in food applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Sam, J.H.; Jeevanandam, J.; Chan, Y.S.; Nandong, J. Thermal degradation of antioxidant compounds: Effects of parameters, thermal degradation kinetics, and formulation strategies. Food Bioprocess Technol. 2022, 15, 1919–1935. [Google Scholar] [CrossRef]
- Moura, J.d.S.; Sousa, R.P.E.; Martins, L.H.d.S.; Costa, C.E.F.d.; Chisté, R.C.; Lopes, A.S. Thermal Degradation of Carotenoids from Jambu Leaves (Acmella oleracea) during Convective Drying. Foods 2023, 12, 1452. [Google Scholar] [CrossRef]
- Ferrando, B.O.; Baenas, N.; Periago, M.J. Changes in Carotenoids and Quality Parameters of Sweet Paprika (Capsicum annuum) After an Accelerated Heat Treatment. Antioxidants 2024, 13, 1492. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant protection from UV-and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- van het Hof, K.H.; Brouwer, I.A.; West, C.E.; Haddeman, E.; Steegers-Theunissen, R.P.; van Dusseldorp, M.; Weststrate, J.A.; Eskes, T.K.; Hautvast, J.G. Bioavailability of lutein from vegetables is 5 times higher than that of β-carotene. Am. J. Clin. Nutr. 1999, 70, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by entrye addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zununi Vahed, S.; Zuluaga Tamayo, M.; Rodriguez-Ruiz, V.; Thibaudeau, O.; Aboulhassanzadeh, S.; Abdolalizadeh, J.; Meddahi-Pellé, A.; Gueguen, V.; Barzegari, A.; Pavon-Djavid, G. Functional mechanisms of dietary crocin protection in cardiovascular models under oxidative stress. Pharmaceutics 2024, 16, 840. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Hsieh, Y.-S.; Ko, H.-H.; Wu, Y.-T. Formulation approaches to crystalline status modification for carotenoids: Impacts on dissolution, stability, bioavailability, and bioactivities. Pharmaceutics 2023, 15, 485. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.-Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants 2018, 7, 98. [Google Scholar] [CrossRef]
- Sadighara, P.; Gharibi, S.; Jafari, A.M.; Khaniki, G.J.; Salari, S. The antioxidant and Flavonoids contents of Althaea officinalis L. flowers based on their color. Avicenna J. Phytomed. 2012, 2, 113. [Google Scholar]
- Pecorini, G.; Ferraro, E.; Puppi, D. Polymeric systems for the controlled release of flavonoids. Pharmaceutics 2023, 15, 628. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C. Flavonoid-Based Nanogels: A Comprehensive Overview. Gels 2025, 11, 267. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, M.; Shang, Z.; Xu, J.; Chen, X.; Zhu, Z.; Wang, W.; Wei, X.; Zhou, X.; Bai, Y. Research Progress on the Antibacterial Activity of Natural Flavonoids. Antibiotics 2025, 14, 334. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, Z.; Zhi, Y.; Mei, C.; Wang, H. Flavonoids as Promising Natural Compounds for Combating Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 2455. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The roles of flavonols/flavonoids in neurodegeneration and neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Kerboeuf, D.; Riou, M.; Guégnard, F. Flavonoids and related compounds in parasitic disease control. Mini Rev. Med. Chem. 2008, 8, 116–128. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Li, Y.; Fang, H.; Xu, W. Recent advance in the research of flavonoids as anticancer agents. Mini Rev. Med. Chem. 2007, 7, 663–678. [Google Scholar]
- Xiao, Z.-P.; Peng, Z.-Y.; Peng, M.-J.; Yan, W.-B.; Ouyang, Y.-Z.; Zhu, H.-L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Feliciano, A.S. Flavonoids: From structure to health issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.H.G.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.; Prado, C.M. Structure-activity association of flavonoids in lung diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Zhevlakova, A.; Selivanova, I. Insights into the pharmacological effects of flavonoids: The systematic review of computer modeling. Int. J. Mol. Sci. 2022, 23, 6023. [Google Scholar] [CrossRef]
- Drețcanu, G.; Știrbu, I.; Leoplold, N.; Cruceriu, D.; Danciu, C.; Stănilă, A.; Fărcaș, A.; Borda, I.M.; Iuhas, C.; Diaconeasa, Z. Chemical structure, sources and role of bioactive flavonoids in cancer prevention: A review. Plants 2022, 11, 1117. [Google Scholar] [CrossRef]
- Masek, A. Flavonoids as natural stabilizers and color indicators of ageing for polymeric materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Yoshida, K.; Oyama, K.i.; Kondo, T. Chemistry of flavonoids in color development. Recent Adv. Polyphen. Res. 2012, 3, 99–129. [Google Scholar]
- Deveoğlu, O.; Karadağ, R. A review on the flavonoids—A dye source. Int. J. Adv. Eng. Pure Sci. 2019, 31, 188–200. [Google Scholar] [CrossRef]
- Ennaceur, S.; Bouaziz, A.; Gargoubi, S.; Mnif, W.; Dridi, D. Enhanced natural dyeing and antibacterial properties of cotton by physical and chemical pretreatments. Processes 2022, 10, 2263. [Google Scholar] [CrossRef]
- Shafiq, F.; Siddique, A.; Pervez, M.N.; Hassan, M.M.; Naddeo, V.; Cai, Y.; Hou, A.; Xie, K.; Khan, M.Q.; Kim, I.-S. Extraction of natural dye from aerial parts of argy wormwood based on optimized taguchi approach and functional finishing of cotton fabric. Materials 2021, 14, 5850. [Google Scholar] [CrossRef]
- Kovačević, Z.; Sutlović, A.; Matin, A.; Bischof, S. Natural dyeing of cellulose and protein fibers with the flower extract of Spartium junceum L. plant. Materials 2021, 14, 4091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, C.; Lin, Y.; Yang, C. Dyeing and performance testing of chitosan-modified cotton fabrics with tea polyphenols. Fibres Text. East. Eur. 2024, 32, 22–30. [Google Scholar] [CrossRef]
- Afonso, T.B.; Bonifácio-Lopes, T.; Costa, E.M.; Pintado, M.E. Phenolic compounds from by-products for functional textiles. Materials 2023, 16, 7248. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Liu, H.; Wang, X. Surface modification of cellulose with triazine derivative to improve printability with reactive dyes. Carbohydr. Polym. 2009, 78, 538–542. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Negi, A. Alkoxide-based solvent dyeing: A feasible strategy for pollution minimization and sustainable approach for the reactive dyeing of cellulosic materials. Cellulose 2024, 31, 7765–7791. [Google Scholar] [CrossRef]
- Negi, A.; Tehrani-Bagha, A.R. Cellulose functionalization using N-heterocyclic-based leaving group chemistry. Polymers 2024, 16, 149. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Liu, X.; Shu, D. Eco-friendly sustainable adsorption dyeing of MOF-modified carboxymethyl cellulose fiber fabric using acid dyes. Cellulose 2024, 31, 7727–7748. [Google Scholar] [CrossRef]
- Negi, A. Cationized Cellulose Materials: Enhancing Surface Adsorption Properties Towards Synthetic and Natural Dyes. Polymers 2024, 17, 36. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, P.; Wang, S.; Li, M.; Hao, Z.; Guan, W.; Pan, J.; Wu, J.; Zhang, Y.; Li, H.; et al. Recent advances in the natural product analogues for the treatment of neurodegenerative diseases. Bioorganic Chem. 2024, 153, 107819. [Google Scholar] [CrossRef]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorganic Chem. 2021, 108, 104681. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-based delivery systems towards cancer therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–based silica particles as flavonoid carrier for drug delivery applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.-M.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Co-microencapsulation of flavonoids from yellow onion skins and lactic acid bacteria lead to multifunctional ingredient for nutraceutical and pharmaceutics applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef]
- Jannat, K.; Paul, A.K.; Bondhon, T.A.; Hasan, A.; Nawaz, M.; Jahan, R.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Pereira, M.d.L. Nanotechnology applications of flavonoids for viral diseases. Pharmaceutics 2021, 13, 1895. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M. Lipid-based delivery systems for flavonoids and flavonolignans: Liposomes, nanoemulsions, and solid lipid nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Zhao, M.; Wang, Y.; Meng, X.; Yan, T.; Ho, C.T. Effect of heat-treating methods on components, instrumental evaluation of color and taste, and antioxidant properties of sea buckthorn pulp flavonoids. J. Food Sci. 2022, 87, 5442–5454. [Google Scholar] [CrossRef]
- Szabłowska, E.; Tańska, M. Effect of Microwave-Assisted Processing of Acorn Flour on Muffin Quality. Appl. Sci. 2025, 15, 6204. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I.; Pankiewicz, U. The Impact of Drying Method on the Physicochemical Bioactive Compounds and Antioxidant Properties of Common Quince Fruit (Cydonia oblonga Mill.). Appl. Sci. 2025, 15, 6122. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; Van der Kooi, C.J. Coloration of flowers by flavonoids and consequences of pH dependent absorption. Front. Plant Sci. 2021, 11, 600124. [Google Scholar] [CrossRef] [PubMed]
- Latva-Mäenpää, H.; Wufu, R.; Mulat, D.; Sarjala, T.; Saranpää, P.; Wähälä, K. Stability and photoisomerization of stilbenes isolated from the bark of Norway spruce roots. Molecules 2021, 26, 1036. [Google Scholar] [CrossRef] [PubMed]
- Mbedzi, D.T.; Mathomu, L.M.; Mhlongo, M.I.; Madala, N.E. Ultraviolet (UV) light-induced geometrical isomerization of cinnamic acid containing molecules: A plausible, non-enzymatic approach to modify metabolite composition of plant extracts. S. Afr. J. Bot. 2022, 150, 845–850. [Google Scholar] [CrossRef]
- Wilhelm, A. Photochemistry of (+)-Catechin and (−)-Epicatechin. 2008. Available online: https://scholar.ufs.ac.za/items/a87b3f67-a791-4ac8-8bb7-7e3bf085628e (accessed on 30 July 2025).
- Ishikawa, H.; Uemura, N.; Yagishita, F.; Baba, N.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Asymmetric synthesis involving reversible photodimerization of a prochiral flavonoid followed by crystallization. Eur. J. Org. Chem. 2017, 2017, 6878–6881. [Google Scholar] [CrossRef]
- Chinh, N.T.; Le Anh, N.T.; Thao, P.T.; Quang, D.D. Photoprotective properties of natural antioxidant flavonoids: A DFT and TD-DFT study on acridone derivatives. Vietnam. J. Chem. 2020, 58, 157–161. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van der Westhuizen, J.H. Photochemistry of flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Ahsan, F. An overview on genistein and its various formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Phytoestrogens (resveratrol and equol) for estrogen-deficient skin—Controversies/misinformation versus anti-aging in vitro and clinical evidence via nutraceutical-cosmetics. Int. J. Mol. Sci. 2021, 22, 11218. [Google Scholar] [CrossRef] [PubMed]
- Qasem, R.J. The estrogenic activity of resveratrol: A comprehensive review of in vitro and in vivo evidence and the potential for endocrine disruption. Crit. Rev. Toxicol. 2020, 50, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary phytoestrogens and their metabolites as epigenetic modulators with impact on human health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serbian Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Walencik, P.K.; Choińska, R.; Gołębiewska, E.; Kalinowska, M. Metal–Flavonoid Interactions—From Simple Complexes to Advanced Systems. Molecules 2024, 29, 2573. [Google Scholar] [CrossRef]
- Zangade, S.B.; Dhulshette, B.S.; Patil, P.B. Flavonoid-metal ion complexes as potent anticancer metallodrugs: A comprehensive review. Mini Rev. Med. Chem. 2024, 24, 1046–1060. [Google Scholar] [CrossRef]
- Matsia, S.; Tsave, O.; Hatzidimitriou, A.; Salifoglou, A. Chromium flavonoid complexation in an antioxidant capacity role. Int. J. Mol. Sci. 2022, 23, 7171. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P. Iron complexes of flavonoids-antioxidant capacity and beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Tyubaeva, P.M.; Gasparyan, K.G.; Romanov, R.R.; Kolesnikov, E.A.; Martirosyan, L.Y.; Larkina, E.A.; Tyubaev, M.A. Biomimetic Materials Based on Poly-3-Hydroxybutyrate and Chlorophyll Derivatives. Polymers 2023, 16, 101. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Ramos, M.; Luzi, F.; Dominici, F.; Viqueira, V.; Torre, L.; Jiménez, A.; Puglia, D.; Garrigós, M.C. Effect of chlorophyll hybrid nanopigments from broccoli waste on thermomechanical and colour behaviour of polyester-based bionanocomposites. Polymers 2020, 12, 2508. [Google Scholar] [CrossRef]
- Bosca, F.; Foglietta, F.; Gimenez, A.; Canaparo, R.; Durando, G.; Andreana, I.; Barge, A.; Peira, E.; Arpicco, S.; Serpe, L. Exploiting lipid and polymer nanocarriers to improve the anticancer sonodynamic activity of chlorophyll. Pharmaceutics 2020, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, A. Chlorophyll as a color and functional ingredient. J. Food Sci. 2004, 69, C422–C425. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological applications of natural colorants in food systems: A review. Foods 2021, 10, 634. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.-E.; Bita, B.; Negut, I. From nature to technology: Exploring the potential of plant-based materials and modified plants in biomimetics, bionics, and green innovations. Biomimetics 2024, 9, 390. [Google Scholar] [CrossRef]
- Arifin, Z.; Soeparman, S.; Widhiyanuriyawan, D.; Suyitno, S.; Setyaji, A.T. Improving stability of chlorophyll as natural dye for dye-sensitized solar cells. J. Teknol. (Sci. Eng.) 2018, 80, 27–33. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, S.-Y.; Lim, D.-H.; Lim, S.-Y.; Choi, J.; Koo, H.-J. Eco-friendly dye-sensitized solar cells based on water-electrolytes and chlorophyll. Materials 2021, 14, 2150. [Google Scholar] [CrossRef]
- Cai, J.-Q.; Liu, X.-M.; Gao, Z.-J.; Li, L.-L.; Wang, H. Chlorophylls derivatives: Photophysical properties, assemblies, nanostructures and biomedical applications. Mater. Today 2021, 45, 77–92. [Google Scholar] [CrossRef]
- Mazzocchi, C.; Benucci, I.; Lombardelli, C.; Esti, M. Enzyme-assisted extraction for the recovery of food-grade chlorophyll-based green colorant. Foods 2023, 12, 3440. [Google Scholar] [CrossRef]
- Al-Tameemi, K.; Nassour, R.; Hamad, A. The medical importance of chlorophylls and their derivatives. SEA J. Islam. Financ. 2022, 8, 4–8. [Google Scholar]
- Vohra, V. Natural dyes and their derivatives integrated into organic solar cells. Materials 2018, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Ramanarayanan, R.; Nijisha, P.; Niveditha, C.; Sindhu, S. Natural dyes from red amaranth leaves as light-harvesting pigments for dye-sensitized solar cells. Mater. Res. Bull. 2017, 90, 156–161. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Liu, X.; Wang, L.; Sun, X. Kinetics of chlorophyll degradation and color loss in heated kiwifruit puree. Trans. Chin. Soc. Agric. Eng. 2012, 28, 289–292. [Google Scholar]
- Ryan-Stoneham, T.; Tong, C. Degradation kinetics of chlorophyll in peas as a function of pH. J. Food Sci. 2000, 65, 1296–1302. [Google Scholar] [CrossRef]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of chlorophyll degradation and color loss in heated broccoli juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef]
- Shen, S.-C.; Hsu, H.-Y.; Huang, C.-N.; Wu, J.S.-B. Color loss in ethanolic solutions of chlorophyll a. J. Agric. Food Chem. 2010, 58, 8056–8060. [Google Scholar] [CrossRef]
- Kramar, A.; Kostic, M.M. Bacterial secondary metabolites as biopigments for textile dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial pigments: A colorful palette reservoir for biotechnological applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef]
- Gomes, M.; Felgueiras, H.P.; Leite, B.R.; Soares, G.M. Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications. Antibiotics 2025, 14, 520. [Google Scholar] [CrossRef]
- Barreto, J.V.d.O.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial pigments: Major groups and industrial applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Celedón, R.S.; Díaz, L.B. Natural pigments of bacterial origin and their possible biomedical applications. Microorganisms 2021, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Guryanov, I.; Naumenko, E. Bacterial Pigment Prodigiosin as Multifaceted Compound for Medical and Industrial Application. Appl. Microbiol. 2024, 4, 1702–1728. [Google Scholar] [CrossRef]
- Lyakhovchenko, N.S.; Abashina, T.N.; Polivtseva, V.N.; Senchenkov, V.Y.; Pribylov, D.A.; Chepurina, A.A.; Nikishin, I.A.; Avakova, A.A.; Goyanov, M.A.; Gubina, E.D. A blue-purple pigment-producing bacterium isolated from the Vezelka river in the city of Belgorod. Microorganisms 2021, 9, 102. [Google Scholar] [CrossRef]
- Guryanov, I.; Karamova, N.; Yusupova, D.; Gnezdilov, O.; Koshkarova, L. Bacterial pigment prodigiosin and its genotoxic effect. Russ. J. Bioorganic Chem. 2013, 39, 106–111. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Renuka Devi, P.; Veera Ravi, A. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef]
- Dufossé, L. Current and potential natural pigments from microorganisms (bacteria, yeasts, fungi, and microalgae). In Handbook on Natural Pigments in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2024; pp. 419–436. [Google Scholar]
- Pavlović, J.; Farkas, Z.; Kraková, L.; Pangallo, D. Color stains on paper: Fungal pigments, synthetic dyes and their hypothetical removal by enzymatic approaches. Appl. Sci. 2022, 12, 9991. [Google Scholar] [CrossRef]
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 1–25. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites; Springer: Cham, Switzerland, 2017; pp. 499–568. [Google Scholar]
- Palomino Agurto, M.E.; Vega Gutierrez, S.M.; Chen, H.-L.; Robinson, S.C. Wood-rotting fungal pigments as colorant coatings on oil-based textile dyes. Coatings 2017, 7, 152. [Google Scholar] [CrossRef]
- Elkhateeb, W.; Daba, G. Fungal pigments: Their diversity, chemistry, food and non-food applications. Appl. Microbiol. 2023, 3, 735–751. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–132. [Google Scholar]
- Lin, L.; Xu, J. Fungal pigments and their roles associated with human health. J. Fungi 2020, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Vega Gutierrez, S.M.; Garcia, R.A.C.; Iroume, N.; Vorland, N.R.; Andersen, C.; de Oliveira Xaxa, I.D.; Kramer, O.E.; Huber, M.E. Potential for fungal dyes as colorants in oil and acrylic paints. J. Coat. Technol. Res. 2018, 15, 845–849. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Oliveira, J.; Sousa-Gallagher, M.; Méndez-Zavala, A.; Montañez, J.C. Assessment of the Dyeing Properties of the Pigments Produced by Talaromyces spp. J. Fungi 2017, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Magalon, H.; Dufossé, L.; Fouillaud, M. Production of pigments from the tropical marine-derived fungi Talaromyces albobiverticillius: New resources for natural red-colored metabolites. J. Food Compos. Anal. 2018, 70, 35–48. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ogbonna, C.N.; Ishika, T.; Vadiveloo, A. Microalgal pigments: A source of natural food colors. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 81–123. [Google Scholar]
- Orona-Navar, A.; Aguilar-Hernández, I.; Nigam, K.; Cerdán-Pasarán, A.; Ornelas-Soto, N. Alternative sources of natural pigments for dye-sensitized solar cells: Algae, cyanobacteria, bacteria, archaea and fungi. J. Biotechnol. 2021, 332, 29–53. [Google Scholar] [CrossRef]
- Armendáriz-Mireles, E.N.; Calles-Arriaga, C.A.; Pech-Rodríguez, W.; Castillo-Robles, A.; Rocha-Rangel, E. Alternative sources of natural photosensitizers: Role of algae in dye-sensitized solar cell. Colorants 2023, 2, 137–150. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red seaweed pigments from a biotechnological perspective. Phycology 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Perales Romero, E.; Bermejo, R.; Jordán-Núñez, J.; Viqueira, V.; Pérez, J. Using laminar nanoclays for phycocyanin and phycoerythrin stabilization as new natural hybrid pigments from microalgae extraction. Appl. Sci. 2021, 11, 11992. [Google Scholar] [CrossRef]
- Blanckart, L.; Munasinghe, E.A.; Bendt, E.; Rahaman, A.; Abomohra, A.; Mahltig, B. Algae-Based Coatings for Fully Bio-Based and Colored Textile Products. Textiles 2025, 5, 3. [Google Scholar] [CrossRef]
- Nunes, A.N.; Monte, J.; Rodríguez-Rojo, S.; Nogueira, I.D.; Gouveia, L.F.; Brazinha, C.; Matias, A.A. Development of a carotenoid-rich microalgae colorant by microencapsulation. Colorants 2024, 3, 39–52. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Minei, P.; Contiero, L.; Labat, F.d.r.; Ciofini, I.; Adamo, C.; Bellina, F.; Pucci, A. Aggregation effects on pigment coatings: Pigment red 179 as a case study. ACS Omega 2019, 4, 20315–20323. [Google Scholar] [CrossRef]
- Prazukin, A.V.; Firsov, Y.K.; Gureeva, E.V.; Kapranov, S.V.; Zheleznova, S.N.; Maoka, T.; Nekhoroshev, M.V. Biomass of green filamentous alga Cladophora (Chlorophyta) from a hypersaline lake in Crimea as a prospective source of lutein and other pigments. Algal Res. 2021, 54, 102195. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, W.; Wen, Y.; Zhang, L.; Wu, Y.; Farid, M.S.; El-Seedi, H.R.; Capanoglu, E.; Zhao, C. Recent advances of natural pigments from algae. Food Prod. Process. Nutr. 2023, 5, 39. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Torres, F.A.E.; Zaccarim, B.R.; de Lencastre Novaes, L.C.; Jozala, A.F.; Santos, C.A.d.; Teixeira, M.F.S.; Santos-Ebinuma, V.C. Natural colorants from filamentous fungi. Appl. Microbiol. Biotechnol. 2016, 100, 2511–2521. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Chitosan nanoparticle encapsulation of antibacterial essential oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- de Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194. [Google Scholar] [CrossRef]
- Agarry, I.E.; Wang, Z.; Cai, T.; Kan, J.; Chen, K. Chlorophyll encapsulation by complex coacervation and vibration nozzle technology: Characterization and stability study. Innov. Food Sci. Emerg. Technol. 2022, 78, 103017. [Google Scholar] [CrossRef]

| Anthocyanin | Source | Structural Specificity | Applications | Biological Activities |
|---|---|---|---|---|
| 6-Hydroxycyanidin | corn, berries | C6-Hydroxylated anthocyanin | Nutraceuticals, food | Antioxidant, anti-aging |
| 6-Hydroxypeonidin | corn, grapes | C6-Hydroxylated anthocyanin | Beverages, textiles, cosmetics | Antioxidant, anticancer |
| Acylated cyanidin | Red cabbage, radishes | Cyanidin with acyl groups | Food, textiles, cosmetics | Antioxidant, UV protection |
| Acylated delphinidin | Eggplants, violets | Delphinidin with acyl groups | Cosmetics, food | Antioxidant, neuroprotective |
| Acylated pelargonidin | Strawberries, red currants | Pelargonidin with acyl groups | Food, pharmaceuticals, textiles | Antioxidant, anti-inflammatory |
| Apigeninidin | Sorghum, millet | Flavylium cation, flavone derivative | Food, cosmetics, beverages | Antioxidant, cardiovascular health |
| Caffeoylated cyanidin | Red cabbage, black rice | Cyanidin with caffeoyl group | Food, cosmetics | Antioxidant, cardiovascular health |
| Caffeoylated delphinidin | Grapes, eggplants | Delphinidin with caffeoyl group | Food, beverages | Antioxidant, anticancer |
| Coumaroylated cyanidin | Raspberries, grapes | Cyanidin with coumaroyl group | Beverages, cosmetics, textiles | Antioxidant, UV protection |
| Coumaroylated delphinidin | Plums, berries | Delphinidin with coumaroyl group | Food, pharmaceuticals | Antioxidant, antimicrobial |
| Cyanidin | Blackberries, cherries, apples | Flavylium cation, hydroxyl groups | Food, cosmetics, textiles | Antioxidant, anti-inflammatory |
| Cyanidin-3-rutinoside | Black rice, red cabbage | Cyanidin with rutin sugar (O-linkage) | Nutraceuticals, cosmetics | Antioxidant, supports circulation |
| Delphinidin | Blueberries, eggplants | Flavylium cation, multiple hydroxyl groups | Food, nutraceuticals, textiles | Antioxidant, cardiovascular health |
| Delphinidin-3-rutinoside | Blueberries, violets | Delphinidin with rutin sugar | Cosmetics, food | Antioxidant, anti-aging |
| Europinidin | Flowers (Ajuga species) | Flavylium cation, hydroxylated | Food, cosmetics, textiles | Antioxidant, anti-inflammatory |
| Europinidin-3-glucoside | Ajuga flowers, grapes | Europinidin with glucose (O-linkage) | Cosmetics, textiles | Antioxidant, neuroprotection |
| Feruloylated malvidin | Wine grapes, bilberries | Malvidin with feruloyl group | Wine, textiles | Antioxidant, neuroprotection |
| Feruloylated peonidin | Red grapes, plums | Peonidin with feruloyl group | Food, supplements | Antioxidant, anticancer |
| Glucoside cyanidin | Blackcurrants, cherries | Cyanidin with glucose moiety | Food, cosmetics | Antioxidant, neuroprotective |
| Glucoside delphinidin | Blue corn, blackberries | Delphinidin with glucose moiety | Textiles, cosmetics | Antioxidant, antimicrobial |
| Glucoside pelargonidin | Strawberries, roses | Pelargonidin with glucose moiety | Food, beverages | Antioxidant, anti-inflammatory |
| Glycosylated cyanidin | Blackberries, cherries | Cyanidin with sugar moieties | Beverages, textiles, cosmetics | Antioxidant, improves gut health |
| Glycosylated delphinidin | Blueberries, plums | Delphinidin with sugar moieties | Food, textiles | Antioxidant, UV protection |
| Glycosylated pelargonidin | Strawberries, raspberries | Pelargonidin with sugar moieties | Food, cosmetics | Antioxidant, anti-inflammatory |
| Hirsutidin | Cranberries, grapes | Flavylium cation, methoxy groups | Cosmetics, food colorant | Antioxidant, neuroprotective |
| Luteolinidin | Sorghum, millet | Flavylium cation, hydroxyflavone | Food, beverages, textiles | Antioxidant, anti-diabetic |
| Malonyl cyanidin | Black rice, black currants | Cyanidin with malonyl group | Food, textiles, pharmaceuticals | Antioxidant, anti-diabetic |
| Malonyl pelargonidin | Cherries, strawberries | Pelargonidin with malonyl group | Food, nutraceuticals | Antioxidant, anti-inflammatory |
| Malvidin | Grapes, red wine | Flavylium cation, methoxy groups | Wine, textiles, food | Antioxidant, neuroprotective |
| Malvidin-3-glucoside | Grapes, red wine | Malvidin with glucose moiety | Wine, food, cosmetics | Antioxidant, cardiovascular health |
| Methylated cyanidin | Black carrots, berries | Methylated anthocyanin | Food, beverages, nutraceuticals | Antioxidant, cardiovascular support |
| Methylated delphinidin | Petunias, blue flowers | Delphinidin with methyl groups | Textiles, food, pharmaceuticals | Antioxidant, UV protection |
| Pelargonidin | Strawberries, raspberries | Flavylium cation, fewer hydroxyl groups | Food, cosmetics, beverages | Antioxidant, anticancer |
| Pelargonidin-3-rutinoside | Strawberries, pomegranates | Pelargonidin with rutin sugar | Food, beverages | Antioxidant, UV protection |
| Peonidin | Cranberries, plums | Flavylium cation, methylated hydroxyl groups | Food, supplements, cosmetics | Anti-inflammatory, neuroprotective |
| Peonidin-3-glucoside | Cranberries, plums | Peonidin with glucose moiety | Food, beverages, pharmaceuticals | Antioxidant, anti-inflammatory |
| Petunidin | Grapes, bilberries | Flavylium cation, additional methoxy groups | Food, beverages, textiles | Antioxidant, anticancer properties |
| Rosinidin | Catharanthus roseus | Flavylium cation, methyl groups | Food, cosmetics | Antioxidant, antimicrobial |
| Rosinidin-3-glucoside | Carnations, Catharanthus roseus | Rosinidin with glucose | Food, textiles, research | Antioxidant, antimicrobial |
| Rutinose cyanidin | Red cabbage, radishes | Cyanidin with rutinose sugar | Food, supplements | Antioxidant, vascular support |
| Rutinose delphinidin | Blue grapes, plums | Delphinidin with rutinose sugar | Food, textiles | Antioxidant, UV protection |
| Sophoroside cyanidin | Black rice, grapes | Cyanidin with sophoroside sugar | Food, cosmetics | Antioxidant, anti-diabetic |
| Sophoroside delphinidin | Blue grapes, eggplants | Delphinidin with sophoroside sugar | Textiles, food | Antioxidant, cardiovascular support |
| Carotenoid | Source | Structural Specificity | Applications | Biological Activities |
|---|---|---|---|---|
| Antheraxanthin | Green plants, algae | Xanthophyll, epoxide groups | Food, cosmetics | Antioxidant, photoprotection |
| Astaxanthin | Microalgae, salmon, shrimp | Xanthophyll, keto groups | Aquaculture, cosmetics | Antioxidant, skin protection |
| Bixin | Annatto seeds | Carotenoid, apocarotenoid | Dairy, snacks, cosmetics | Antioxidant, anti-inflammatory |
| Canthaxanthin | Crustaceans, fungi, flamingos | Xanthophyll, keto groups | Poultry feed, aquaculture | Antioxidant, skin pigment enhancer |
| Capsanthin | Red peppers, paprika | Xanthophyll, keto groups | Food colorant, cosmetics | Anti-inflammatory, antioxidant |
| Capsorubin | Red peppers, paprika | Xanthophyll, keto groups | Food, cosmetics | Anti-inflammatory, UV protection |
| Deoxyxanthin | Marine bacteria | Xanthophyll, deoxygenated | Biotechnology, food | Antioxidant, anti-aging |
| Diadinoxanthin | Diatoms, algae | Xanthophyll, epoxide group | Nutraceuticals, aquaculture | Antioxidant, photoprotection |
| Diatoxanthin | Diatoms, algae | Xanthophyll, epoxide group | Aquaculture, research | Photoprotection, antioxidant |
| Echinenone | Cyanobacteria, microalgae | Xanthophyll, keto group | Food, supplements | Antioxidant, anti-inflammatory |
| Echinenone | Cyanobacteria, algae | Xanthophyll, ketone group | Aquaculture, research | Antioxidant, supports photosynthesis |
| Flexixanthin | Marine bacteria | Xanthophyll, ketone group | Research, pharmaceuticals | Antioxidant, antimicrobial |
| Fucoxanthin | Brown seaweed, diatoms | Xanthophyll, epoxide groups | Nutraceuticals, food | Anti-obesity, anti-inflammatory |
| Lutein | Marigold, spinach, kale | Xanthophyll, hydroxyl groups | Eye health supplements, food | Antioxidant, eye health |
| Luteoxanthin | Carrots, plants | Xanthophyll, hydroxyl groups | Food, dietary supplements | Antioxidant, UV protection |
| Lycopene | Tomatoes, watermelon | Acyclic carotenoid | Food, cosmetics, supplements | Antioxidant, anti-inflammatory |
| Methylhexacosahexaenoate | Algae, bacteria | Carotenoid, esterified | Nutraceuticals, pharma | Anti-inflammatory, neuroprotective |
| Mutatochrome | Mushrooms, bacteria | Carotene derivative | Research, food colorant | Antioxidant |
| Myxoxanthophyll | Cyanobacteria | Xanthophyll, glycosylated | Biotechnology | Antioxidant, photoprotection |
| Neoxanthin | Green leafy vegetables | Xanthophyll, epoxide groups | Food, cosmetics | Antioxidant, neuroprotective |
| Norbixin | Annatto seeds | Carotenoid, apocarotenoid | Food, dairy products | Antioxidant, neuroprotective |
| Oscillaxanthin | Cyanobacteria | Xanthophyll, oxygenated | Research, biotechnology | Antioxidant, anti-inflammatory |
| Peridinin | Dinoflagellates (algae) | Xanthophyll, lactone group | Fluorescent dyes, research | Antioxidant, photoprotection |
| Phytoene | Tomatoes, carrots, algae | Precursor carotenoid, colorless | Food, cosmetics | Antioxidant, anti-aging |
| Phytofluene | Tomatoes, carrots, oranges | Precursor carotenoid, colorless | Food, skincare | Anti-inflammatory, UV protection |
| Rhodoxanthin | Conifers, flamingos | Xanthophyll, oxygenated | Food, bird pigmentation | Antioxidant, pigmentation |
| Rubixanthin | Rose hips, red berries | Xanthophyll, hydroxyl groups | Food, cosmetics | Antioxidant, UV protection |
| Salinixanthin | Halophilic bacteria | Xanthophyll, glycosylated | Biotechnology | Antioxidant, membrane stabilizer |
| Saproxanthin | Bacteria (Sphingomonas) | Xanthophyll, hydroxyl groups | Biotechnology | Antioxidant, anti-UV effects |
| Spheroidene | Purple bacteria, plants | Carotene, cyclized chain | Research, food | Antioxidant, photoprotection |
| Spheroidenone | Photosynthetic bacteria | Carotene, oxygenated chain | Biotechnology | Antioxidant, photoprotective |
| Spirilloxanthin | Photosynthetic bacteria | Xanthophyll, conjugated chain | Biotechnology, research | Antioxidant, UV protection |
| Synechoxanthin | Cyanobacteria | Xanthophyll, keto group | Research, biotechnology | Antioxidant, photoprotection |
| Tetradehydrolycopene | Algae, bacteria | Carotenoid, acyclic | Research, nutraceuticals | Antioxidant |
| Torularhodin | Yeast (Rhodotorula) | Xanthophyll, hydroxyl groups | Pharmaceuticals, food | Antioxidant, immune-boosting |
| Torulene | Yeast (Rhodotorula) | Carotenoid, hydrocarbon | Food, cosmetics | Antioxidant, antimicrobial |
| Trollichrome | Fungi, lichens | Carotenoid-like structure | Food, pharmaceuticals | Antioxidant, antibacterial |
| Violaxanthin | Yellow flowers, paprika | Xanthophyll, epoxide groups | Food, nutraceuticals | Antioxidant, anti-inflammatory |
| Zeaxanthin | Corn, paprika, egg yolk | Xanthophyll, hydroxyl groups | Vision supplements, food | Antioxidant, prevents macular degeneration |
| Zeaxanthin-diglucoside | Maize, bacteria | Xanthophyll, glycosylated | Nutraceuticals, food | Antioxidant, eye health |
| α-Carotene | Pumpkins, carrots | Tetraterpene, hydrocarbon | Food, nutraceuticals | Pro-vitamin A, antioxidant |
| β-Carotene | Carrots, sweet potatoes, spinach | Tetraterpene, hydrocarbon | Food, cosmetics, supplements | Pro-vitamin A, antioxidant |
| β-Cryptoxanthin | Oranges, papaya, red peppers | Xanthophyll, hydroxyl groups | Pro-vitamin A, food | Antioxidant, supports immune function |
| β-Isorenieratene | Bacteria, fungi | Xanthophyll, methoxy group | Food, pharmaceuticals | Antioxidant, anticancer properties |
| γ-Carotene | Oranges, pumpkins | Carotene, single cyclization | Food, supplements | Antioxidant, vitamin A precursor |
| Name | Source | Structural Specificity | Applications | Biological Activities |
|---|---|---|---|---|
| Amentoflavone | Selaginella species (Spikemoss) | Biflavonoid | Potential pharmaceuticals | Antioxidant; antiviral; neuroprotective |
| Apigenin | Matricaria chamomilla (Chamomile) | Flavone | Textile dye; food colorant; cosmetic applications | Antioxidant; anti-inflammatory; anticancer |
| Baicalein | Scutellaria baicalensis (Baikal skullcap) | Flavone | Traditional medicine; potential cosmetic applications | Antioxidant; anti-inflammatory; neuroprotective |
| Baohuoside I | Epimedium species (Horny Goat Weed) | Flavonol glycoside | Traditional Chinese medicine | Antioxidant; vasodilatory; neuroprotective |
| Bilobetin | Ginkgo biloba | Biflavonoid | Herbal medicine; supplements | Antioxidant; neuroprotective |
| Biochanin A | Red clover (Trifolium pratense) | Isoflavone | Dietary supplement; potential cosmetic applications | Phytoestrogenic activity; antioxidant; anticancer |
| Butin | Dalbergia sissoo (Indian Rosewood) | Flavanone | Traditional medicine | Antioxidant; anti-inflammatory |
| Carlinoside | Phlomis umbrosa | Flavone glycoside | Traditional medicine | Antioxidant; hepatoprotective |
| Chrysin | Passiflora caerulea (Blue passionflower) | Flavone | Dietary supplement; potential cosmetic applications | Antioxidant; anti-inflammatory; anxiolytic |
| Corylifolinin | Psoralea corylifolia (Babchi) | Flavone | Traditional medicine | Antioxidant; antifungal |
| Cryptostrobin | Cryptocarya species | Flavonoid | Traditional medicine | Antioxidant; antimicrobial |
| Daidzein | Soybeans (Glycine max) | Isoflavone | Food colorant; dietary supplement; cosmetic applications | Phytoestrogenic activity; antioxidant; anticancer |
| Derrone | Derris scandens (Climbing Derris) | Flavone | Traditional medicine | Antioxidant; anti-inflammatory |
| Echinatin | Glycyrrhiza glabra (Licorice) | Chalcone | Traditional medicine; cosmetics | Antioxidant; antimicrobial |
| Engeletin | Engelhardtia roxburghiana | Flavanone glycoside | Traditional medicine | Antioxidant; anti-inflammatory |
| Eriodictyol | Citrus fruits, Yerba Santa | Flavanone | Food additive; bitter blocker in flavors | Antioxidant; anti-inflammatory; neuroprotective |
| Eriodictyol-7-O-glucoside | Eriodictyon californicum (Yerba Santa) | Flavanone | Herbal medicine | Antioxidant; expectorant |
| Eupatilin | Artemisia asiatica (Mugwort) | Flavone | Traditional medicine; pharmaceuticals | Antioxidant; anti-inflammatory; gastroprotective |
| Eupatorin | Eupatorium perfoliatum (Boneset) | Flavone | Herbal medicine | Antioxidant; anticancer |
| Farrerol | Rhododendron spp. | Flavanone | Traditional Chinese medicine | Antioxidant; anti-inflammatory; antibacterial |
| Fisetin | Rhus cotinus (Smoke tree) | Flavonol | Textile dye; food colorant; cosmetic applications | Antioxidant; neuroprotective; anticancer |
| Formononetin | Red clover (Trifolium pratense) | Isoflavone | Dietary supplement; potential cosmetic applications | Phytoestrogenic activity; antioxidant; anticancer |
| Genistein | Soybeans (Glycine max) | Isoflavone | Food colorant; dietary supplement; cosmetic applications | Phytoestrogenic activity; antioxidant; anticancer |
| Gossypetin | Hibiscus flowers (Hibiscus sabdariffa) | Flavonol | Natural textile dye; food colorant | Antioxidant; antimicrobial; anti-inflammatory |
| Helichrysetin | Helichrysum species | Chalcone | Traditional medicine | Antioxidant; antimicrobial |
| Hesperidin | Citrus fruits (e.g., oranges, lemons) | Flavanone glycoside | Food colorant; dietary supplement; cosmetic applications | Antioxidant; anti-inflammatory; cardioprotective |
| Hinokiflavone | Hinokitiol (Japanese Cypress) | Biflavonoid | Traditional medicine | Antioxidant; antibacterial |
| Hispidulin | Salvia species (Sage) | Flavone | Traditional medicine; potential dietary supplement | Antioxidant; neuroprotective; anticonvulsant |
| Hispidulin | Salvia miltiorrhiza (Red Sage) | Flavone | Traditional medicine; supplements | Antioxidant; neuroprotective; anticonvulsant |
| Hispidulin | Marrubium vulgare (White Horehound) | Flavone | Herbal medicine; potential pharmaceutical use | Antioxidant; neuroprotective |
| Isobavachalcone | Psoralea corylifolia (Babchi) | Chalcone | Traditional medicine | Antioxidant; antimicrobial |
| Isoliquiritigenin | Glycyrrhiza uralensis (Chinese Licorice) | Chalcone | Traditional medicine; potential food coloring | Antioxidant; hepatoprotective |
| Isookanin | Achillea millefolium (Yarrow) | Flavonol | Herbal medicine | Antioxidant; antimicrobial |
| Isorhamnetin | Hippophae rhamnoides (Sea buckthorn) | Flavonol | Food colorant; dietary supplement; cosmetic applications | Antioxidant; anti-inflammatory; anticancer |
| Kaempferol | Ginkgo biloba leaves | Flavonol | Textile dye; food colorant; cosmetic applications | Antioxidant; anti-inflammatory; cardioprotective |
| Karanjin | Pongamia pinnata (Indian Beech) | Flavonol | Pesticide; medicine | Antioxidant; antimicrobial |
| Licarin A | Myristica fragrans (Nutmeg) | Flavonoid | Herbal medicine | Antioxidant; neuroprotective |
| Licoflavone A | Glycyrrhiza glabra (Licorice) | Flavone | Herbal medicine; cosmetics | Antioxidant; anti-inflammatory |
| Lippiflavone | Lippia species | Flavone | Herbal medicine | Antioxidant; antimicrobial |
| Lonchocarpol A | Lonchocarpus species | Flavonoid | Traditional medicine | Antioxidant; anti-inflammatory |
| Luteolin | Reseda luteola (Weld) | Flavone | Textile dye for silk and wool; painting pigment; manuscript illumination | Antioxidant; anti-inflammatory; antimicrobial |
| Methylnaringenin | Citrus species | Flavanone | Food additive; supplements | Antioxidant; anti-inflammatory |
| Morin | Maclura pomifera (Osage orange) | Flavonol | Textile dye; food colorant; wood staining | Antioxidant; anti-inflammatory; anticancer |
| Myricetin | Myrica rubra (Bayberry) | Flavonol | Food colorant; dietary supplement; cosmetic applications | Antioxidant; anti-inflammatory; neuroprotective |
| Naringenin | Grapefruit, tomatoes | Flavanone | Food colorant; dietary supplement | Antioxidant; cardioprotective |
| Naringin | Citrus paradisi (Grapefruit) | Flavanone glycoside | Food colorant; dietary supplement; cosmetic applications | Antioxidant; cholesterol-lowering; anticancer |
| Neobavaisoflavone | Psoralea corylifolia (Babchi) | Isoflavone | Traditional medicine | Antioxidant; anti-inflammatory |
| Nobiletin | Citrus peel (Citrus reticulata) | Flavone | Functional food ingredient; dietary supplement | Antioxidant; anti-inflammatory; neuroprotective |
| Pectolinarigenin | Cirsium japonicum (Japanese Thistle) | Flavone | Traditional medicine | Antioxidant; anti-inflammatory |
| Pinocembrin | Eucalyptus spp., propolis | Flavanone | Cosmetic applications; pharmaceutical potential | Antioxidant; antibacterial; neuroprotective |
| Pinostrobin | Boesenbergia rotunda (Fingerroot) | Flavanone | Traditional medicine | Antioxidant; anti-inflammatory |
| Quercetin | Tagetes spp. (Marigold petals) | Flavonol | Textile dye; food colorant; dietary supplement | Antioxidant; antiviral; anticancer |
| Retusin | Alnus glutinosa (Black Alder) | Flavonol | Natural dye; herbal medicine | Antioxidant; antimicrobial |
| Robinetin | Robinia pseudoacacia (Black Locust) | Flavonol | Natural dye; cosmetics | Antioxidant; antimicrobial |
| Rutin | Sophora japonica (Japanese pagoda tree) | Flavonol glycoside | Textile dye; food colorant; dietary supplement | Antioxidant; vascular protection; anti-inflammatory |
| Sakuranetin | Prunus species (Cherry blossoms) | Flavanone | Potential pharmaceutical and cosmetic applications | Antioxidant; anti-inflammatory; antifungal |
| Sappanchalcone | Caesalpinia sappan (Sappanwood) | Chalcone | Natural dye; herbal medicine | Antioxidant; antimicrobial |
| Scutellarein | Scutellaria lateriflora (Skullcap) | Flavone | Herbal medicine; supplements | Antioxidant; neuroprotective |
| Sibiricaxanthone B | Hypericum perforatum (St. John’s Wort) | Flavone | Traditional medicine | Antioxidant; antidepressant |
| Silymarin | Silybum marianum (Milk thistle) | Flavonolignan complex | Liver health supplement; potential cosmetic applications | Hepatoprotective; antioxidant; anti-inflammatory |
| Sophoranone | Sophora flavescens (Shrubby Sophora) | Flavonoid | Herbal medicine | Antioxidant; anti-inflammatory |
| Sophoricoside | Sophora japonica (Japanese Pagoda Tree) | Flavonol glycoside | Traditional medicine; dietary supplements | Antioxidant; anti-inflammatory; anticancer |
| Sotetsuflavone | Cycas revoluta (Sago Palm) | Biflavonoid | Traditional medicine | Antioxidant; anticancer |
| Tangeretin | Tangerine peel (Citrus tangerina) | Flavone | Food colorant; dietary supplement | Antioxidant; anticancer; neuroprotective |
| Taxifolin | Siberian larch (Larix sibirica) | Flavanonol | Food colorant; dietary supplement; cosmetic applications | Antioxidant; anti-inflammatory; cardioprotective |
| Tectochrysin | Tectona grandis (Teak) | Flavone | Traditional medicine | Antioxidant; anti-inflammatory |
| Tricetin | Eucalyptus globulus (Eucalyptus leaves) | Flavone | Textile dye; potential cosmetic applications | Antioxidant; anti-inflammatory; antiviral |
| Wogonin | Scutellaria baicalensis (Baikal skullcap) | Flavone | Traditional medicine; potential cosmetic applications | Antioxidant; anti-inflammatory; anticancer |
| Xanthohumol | Humulus lupulus (Hops) | Chalcone | Beer additive; dietary supplement | Antioxidant; anticancer |
| Name | Source | Structural Specificity | Applications | Biological Activities |
|---|---|---|---|---|
| Bacteriochlorophyll a | Purple bacteria | Similar to chlorophyll a but modified | Bacterial photosynthesis | Helps anoxygenic photosynthesis |
| Bacteriochlorophyll b | Green sulfur bacteria | Similar to chlorophyll b | Bacterial photosynthesis | Photosynthetic function |
| Bacteriochlorophyll c | Green bacteria | Modified porphyrin ring | Bacterial photosynthesis | Light-harvesting role |
| Bacteriochlorophyll d | Green bacteria | Structural variant | Bacterial photosynthesis | Energy conversion |
| Bacteriochlorophyll e | Green bacteria | Highly modified porphyrin structure | Light-harvesting | Alternative to chlorophyll in bacteria |
| Bacteriochlorophyll f | Green bacteria | Modified bacteriochlorophyll c | Bacterial photosynthesis | Supports light absorption |
| Chlorin e6 | Semi-synthetic from chlorophyll | Partially hydrogenated porphyrin ring | Photodynamic therapy | Antimicrobial, anticancer |
| Chlorophyll a | Green plants, algae | Methyl group at C7, phytyl ester at C17, Mg center | Photosynthesis | Antioxidant properties |
| Chlorophyll b | Green plants, algae | Formyl group at C7, phytyl ester at C17, Mg center | Photosynthesis | Antioxidant properties |
| Chlorophyll c | Diatoms, brown algae | Lacks phytyl chain, porphyrin ring | Light absorption in algae | Supports photosynthesis |
| Chlorophyll d | Red algae, cyanobacteria | Formyl group at C3 | Far-red light absorption | Photosynthetic function |
| Chlorophyll f | Cyanobacteria | Formyl group at C2 | Absorbs infrared light | Alternative light-harvesting pigment |
| Chlorophyllide a | Enzymatic breakdown of chlorophyll a | Lacks phytyl chain | Intermediate in metabolism | Participates in biosynthesis |
| Chlorophyllide b | Enzymatic breakdown of chlorophyll b | Lacks phytyl chain | Chlorophyll metabolism studies | Involved in plant biochemistry |
| Chlorophyllin | Alkaline hydrolysis of chlorophyll | Water-soluble derivative | Food colorant, health supplements | Detoxifying, anti-inflammatory |
| Co-Chlorophyllin | Cobalt-substituted chlorophyllin | Co replaces Mg | Industrial colorants | Used in synthetic chemistry |
| Copper Chlorophyllin | Chlorophyllin with Cu substitution | Copper replaces Mg | Natural food colorant | Antioxidant, potential anticancer |
| Dihydro-Chlorophyll | Reduced chlorophyll derivative | Hydrogenation at C7–C8 | Biomedical research | Potential therapeutic applications |
| Ethyl Chlorophyllide | Oxidized chlorophyll derivative | Ethyl ester modification | Biochemical research | Intermediate in metabolism |
| Fe-Chlorophyllin | Iron-substituted chlorophyllin | Fe replaces Mg | Biomedical research | Possible enzyme mimic |
| Hydroxy-Chlorophyll | Hydroxylated chlorophyll derivative | Hydroxylation at various positions | Research on chlorophyll modifications | Antioxidant potential |
| Methyl Chlorophyllide | Oxidized chlorophyll derivative | Methyl ester modification | Plant biochemistry studies | Intermediate in biosynthesis |
| Mg-Chlorin | Modified chlorophyll structure | Mg at center, hydrogenated porphyrin | Phototherapy | Photosensitizer |
| Ni-Chlorophyllin | Nickel-substituted chlorophyllin | Ni replaces Mg | Synthetic pigment research | Potential catalytic applications |
| Pheophorbide a | Chlorophyll a breakdown product | Lacks phytyl chain, Mg replaced by hydrogen | Photodynamic therapy | Photosensitizer |
| Pheophorbide b | Chlorophyll b breakdown product | Lacks phytyl chain, Mg replaced by hydrogen | Cancer treatment research | Photosensitizer |
| Pheophytin a | Chlorophyll a degradation | Mg replaced by hydrogen | Food colorant | Antioxidant properties |
| Pheophytin b | Chlorophyll b degradation | Mg replaced by hydrogen | Food colorant | Antioxidant properties |
| Phytyl Chlorophyllide | Partial hydrolysis product | Retains phytyl chain | Chlorophyll metabolism studies | Biosynthesis intermediate |
| Protochlorophyllide | Chlorophyll precursor | Lacks phytol chain | Essential in chlorophyll biosynthesis | Involved in plant development |
| Pyropheophorbide a | Thermal degradation of pheophorbide a | Decarboxylation at C132 | Medical applications | Photosensitizing properties |
| Pyropheophorbide b | Thermal degradation of pheophorbide b | Decarboxylation at C132 | Cancer research | Photosensitizer |
| Pyropheophytin a | Heat-treated chlorophyll a | Decarboxylation at C132 | Indicator of food processing | Antioxidant potential |
| Pyropheophytin b | Heat-treated chlorophyll b | Decarboxylation at C132 | Food quality assessment | Antioxidant properties |
| Zn-Chlorophyllin | Zinc-replaced chlorophyllin | Zn replaces Mg | Research in metal-substituted pigments | Potential antimicrobial |
| Name | Source | Microorganism Name | Structural Specificity | Applications | Biological Activities |
|---|---|---|---|---|---|
| Astaxanthin | Microalgae | Haematococcus pluvialis | Carotenoid, xanthophyll | Food colorant, cosmetics, supplements | Antioxidant, anti-inflammatory, anticancer |
| Beta-Carotene | Fungi, Yeast | Blakeslea trispora | Carotenoid, provitamin A | Food colorant, supplements, cosmetics | Antioxidant, immune system booster, eye health |
| Lycopene | Bacteria, Fungi | Brevundimonas sp. | Carotenoid, unsaturated hydrocarbon | Food coloring, supplements, cosmetics | Antioxidant, anticancer, cardiovascular health |
| Canthaxanthin | Bacteria | Xanthomonadaceae | Carotenoid, xanthophyll | Food coloring, dietary supplements | Antioxidant, anti-inflammatory, immune system booster |
| Lutein | Algae | Asteraceae family | Carotenoid, xanthophyll | Food colorant, supplements, eye health | Antioxidant, anti-inflammatory, eye protection |
| Spirulina Blue | Cyanobacteria | Arthrospira platensis | Phycocyanin, protein-pigment complex | Food colorant, cosmetics, health supplements | Antioxidant, anti-inflammatory, neuroprotective |
| Phycocyanin | Cyanobacteria | Spirulina | Phycobiliprotein, protein-pigment complex | Food coloring, supplements, cosmetics | Antioxidant, immune-boosting, anti-inflammatory |
| Violacein | Bacteria | Chromobacterium violaceum | Indole-derived, violacein | Bio-dye, antimicrobial, anticancer research | Antimicrobial, anticancer, anti-inflammatory |
| Prodigiosin | Bacteria | Serratia marcescens | Indole-derived pigment, prodigiosin | Cosmetics, research, antibacterial products | Antimicrobial, anticancer, anti-inflammatory |
| Xanthophylls | Yeast | Xanthophyllomyces dendrorhous | Carotenoid, oxygenated carotenoid | Food colorant, supplements, cosmetics | Antioxidant, anti-inflammatory |
| Astaxanthin | Yeast | Phaffia rhodozyma | Carotenoid, xanthophyll | Food colorant, aquaculture feed, cosmetics | Antioxidant, anti-inflammatory, neuroprotective |
| Carotenoids | Fungi | Mucor spp. | Carotenoid, unsaturated hydrocarbon | Food coloring, pharmaceuticals | Antioxidant, anticancer, immune-boosting |
| Curcumin | Microorganisms (Fermented) | Curcuma longa | Phenolic compound, diarylheptanoid | Food coloring, cosmetics, supplements | Antioxidant, anti-inflammatory, anticancer |
| Rutin | Fungi | Fusarium oxysporum | Flavonoid glycoside | Food and beverage colorant, health supplement | Antioxidant, anti-inflammatory, cardiovascular health |
| Betanin | Beetroot (Fermented) | Beta vulgaris | Betacyanin, betalain | Food coloring, pharmaceuticals | Antioxidant, anti-inflammatory, liver health |
| Safflor Yellow | Plants (fermented) | Carthamus tinctorius | Flavonoid-based, safflor yellow | Food and beverage coloring, dye industry | Antioxidant, anti-inflammatory |
| Carminic Acid | Insects (via fermentation) | Dactylopius coccus | Anthraquinone derivative | Food coloring, cosmetics | Antimicrobial, anti-inflammatory |
| Rubropunctatin | Fungus | Monascus purpureus | Anthraquinone derivative | Food and beverage coloring, medicinal products | Antioxidant, anticancer, anti-inflammatory |
| Rhamnetin | Fungi | Fusarium graminearum | Flavonoid-derived | Cosmetics, supplements | Antioxidant, anti-inflammatory |
| Indirubin | Plant-derived bacteria | Indigofera tinctoria | Indole-derived, derivative of indigo | Textile and food colorant | Anticancer, anti-inflammatory |
| Alizarin | Plants, Fermented Bacteria | Rubia tinctorum | Anthraquinone derivative | Textile and food coloring | Antioxidant, anti-inflammatory |
| Emodin | Plants, Fungi | Rheum spp. | Anthraquinone derivative | Food coloring, medicinal products, textiles | Anticancer, anti-inflammatory |
| Melanin | Fungi | Aspergillus niger | Polymorphic pigment | Cosmetics, medical applications, hair products | Anti-inflammatory, skin protection |
| Carmines | Insect-derived | Cochineal | Anthraquinone derivative | Food and cosmetics coloring | Antimicrobial, anti-inflammatory |
| Lichens Yellow | Lichens | Usnea | Lichen-derived, pulvinic acid | Textile coloring, potential use in food colorants | Antimicrobial, antifungal |
| Pseudomonas Yellow | Bacteria | Pseudomonas spp. | Quinone-based pigment | Food and industrial colorant | Antimicrobial, anti-inflammatory |
| Algal Red | Algae | Chlorella | Carotenoid, xanthophyll | Food, beverages, cosmetics, supplements | Antioxidant, immune-boosting |
| Rhodamine B | Bacteria | Rhodococcus | Xanthene dye (organic compound) | Food coloring, textile dyeing | Antimicrobial, anti-inflammatory |
| Riboflavin (B2) | Fungi | Aspergillus oryzae | Vitamin B2, flavin compound | Food and pharmaceutical industry, dietary supplements | Antioxidant, anticancer, anti-inflammatory |
| Methyl red | Bacteria | Micrococcus luteus | Azobenzene derivative | pH indicator, dye industry | Antimicrobial, diagnostic applications |
| Tyrosinase Inhibitors | Fungi | Cunninghamella elegans | Fungal-based inhibitors of tyrosinase | Cosmetics, skincare, food coloring | Skin whitening, anti-inflammatory |
| Xanthine | Penicillium | Penicillium | Purine derivative | Industrial colorant | Antioxidant, potential anticancer |
| Quinones | Bacillus | Bacillus spp. | Quinone pigments | Food and textile dyeing | Antioxidant, antimicrobial |
| Septempyrone | Actinomycetes | Streptomyces spp. | Pyrone-based pigment | Research, medical applications | Anticancer, antimicrobial |
| Curvularia Yellow | Fungi | Curvularia spp. | Polyketide-derived pigment | Textile, food colorant | Antimicrobial, antifungal |
| Anthraquinone | Plant-related bacteria | Averrhoa carambola | Quinone derivative | Textile, food coloring | Anticancer, anti-inflammatory |
| Helianthin | Sunflower | Helianthus annuus | Carotenoid-based, xanthophyll | Food and beverage coloring, industrial use | Antioxidant, immune-boosting |
| Torulene | Yeast | Torula yeast | Carotenoid, red pigment | Food, beverages, cosmetics | Antioxidant, anti-inflammatory, immune-boosting |
| Capsanthin | Capsicum | Capsicum annuum | Carotenoid, xanthophyll | Food coloring, supplements | Anti-inflammatory, antioxidant |
| Rhizobium Yellow | Bacteria | Rhizobium spp. | Bacterially derived yellow pigment | Food and textile dyeing | Antioxidant, antimicrobial |
| Aureusidin | Fungus | Penicillium aureus | Flavonoid derivative | Textile, food colorant | Antioxidant, anti-inflammatory |
| Bacterioruberin | Halophilic bacteria | Halobacterium | Red carotenoid derivative | Food and industrial coloring | Antioxidant, anti-inflammatory |
| Marinol | Yeast | Phaffia rhodozyma | Carotenoid, xanthophyll | Food coloring, nutritional supplements | Antioxidant, anti-inflammatory, neuroprotective |
| β-Damascenone | Bacteria | Bacillus spp. | Terpenoid-derived pigment | Industrial coloring, research | Anticancer, anti-inflammatory |
| Carotene | Yeast | Saccharomyces cerevisiae | Carotenoid, beta-carotene | Food coloring, dietary supplements | Antioxidant, immune boosting |
| Astaxanthin | Microalgae | Chlorophyta | Carotenoid, xanthophyll | Aquaculture feed, food colorant | Antioxidant, anti-inflammatory, neuroprotective |
| Phaeophytin | Microalgae | Chlorella vulgaris | Chlorophyll derivative | Food colorant, cosmetics | Antioxidant, detoxifying |
| Torulin | Yeast | Torulaspora delbrueckii | Carotenoid derivative, torulene | Food coloring, dietary supplements | Antioxidant, immune system booster |
| Annatto | Plant-derived bacteria | Bixa orellana | Carotenoid, bixin | Food coloring, cosmetics | Antioxidant, anti-inflammatory |
| Astaxanthin | Yeast | Xanthophyllomyces dendrorhous | Carotenoid, xanthophyll | Food colorant, cosmetics | Antioxidant, anti-inflammatory, immune-boosting |
| Alpha-carotene | Bacteria | Brevundimonas sp. | Carotenoid derivative | Food coloring, supplements | Antioxidant, anticancer |
| Fumonisin | Fungus | Fusarium verticillioides | Mycotoxin-derived pigment | Food colorant, agricultural use | Antifungal, anti-inflammatory |
| Lentinan | Fungus | Lentinula edodes | Polysaccharide, non-carotenoid | Food coloring, supplements, pharmaceuticals | Immunomodulatory, anti-inflammatory |
| Pterin | Bacteria | Brevundimonas spp. | Pteridine-derived pigment | Bio-dye, food coloring, textile dyeing | Antioxidant, anti-inflammatory |
| Biliverdin | Bacteria | Pseudomonas spp. | Porphyrin-derived pigment | Bio-dye, research | Anticancer, anti-inflammatory |
| Phycourobilin | Cyanobacteria | Spirulina platensis | Phycobiliprotein, biliverdin-like | Food colorant, supplements, cosmetics | Antioxidant, neuroprotective, anti-inflammatory |
| Indole Red | Bacteria | Bacillus subtilis | Indole-derived pigment | Food coloring, research | Anticancer, anti-inflammatory |
| Erythrosine | Bacteria | Erythrobacter spp. | Xanthene dye (organic compound) | Food coloring, textile dyeing | Antioxidant, anti-inflammatory |
| Rhizobium Red | Bacteria | Rhizobium spp. | Quinone derivative | Food and textile dyeing | Antioxidant, anti-inflammatory |
| Fungal Melanin | Fungi | Aspergillus spp. | Polymorphic pigment | Medical applications, cosmetics, dyeing | Anti-inflammatory, anticancer |
| Neosartoryamine | Fungi | Aspergillus niger | Alkaloid-derived pigment | Food and textile coloring | Antioxidant, anti-inflammatory |
| Laccase | Fungi | Trametes versicolor | Enzyme-derived pigment | Food and textile coloring, bioremediation | Antimicrobial, antioxidant |
| Violacein | Bacteria | Chromobacterium violaceum | Indole-derived pigment | Bio-dye, antimicrobial, anticancer | Antimicrobial, anticancer, anti-inflammatory |
| Prodigiosin | Bacteria | Serratia marcescens | Indole-derived pigment, prodigiosin | Antimicrobial, research | Antimicrobial, anticancer, anti-inflammatory |
| Monascus Red | Fungus | Monascus purpureus | Anthraquinone derivative | Food and beverage coloring, medicinal products | Anticancer, anti-inflammatory |
| Carotenoids | Fungi | Aspergillus spp. | Carotenoid derivatives | Food coloring, pharmaceutical | Antioxidant, immune boosting |
| Chlorophyll a | Algae | Chlorella vulgaris | Chlorophyll derivative | Food colorant, supplements | Antioxidant, detoxifying |
| Bacteriochlorophyll | Bacteria | Rhodopseudomonas spp. | Chlorophyll derivative | Bioremediation, food coloring | Anti-inflammatory, antioxidant |
| Mycosporine-like Amino Acids | Fungi | Cladosporium spp. | Amino acid derivatives, UV-protective pigments | Cosmetics, pharmaceutical | UV-protection, antioxidant |
| Cytochrome C | Fungi | Fusarium spp. | Protein-derived pigment | Medical and research | Antioxidant, anticancer |
| Luteoxanthin | Algae | Chlamydomonas spp. | Carotenoid, xanthophyll | Food and beverage colorant | Antioxidant, anti-inflammatory |
| Echinocandin B | Fungus | Glarea lozoyensis | Terpenoid-derived pigment | Pharmaceutical use, medical colorants | Antifungal, antimicrobial |
| Rhamnose-pyranose | Bacteria | Bacillus spp. | Sugar-based pigment | Food and industrial coloring | Antioxidant, anti-inflammatory |
| Polyketide Pigments | Fungi | Aspergillus nidulans | Polyketide-derived pigment | Food coloring, medicinal products | Anti-inflammatory, antioxidant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, A. Natural Dyes and Pigments: Sustainable Applications and Future Scope. Sustain. Chem. 2025, 6, 23. https://doi.org/10.3390/suschem6030023
Negi A. Natural Dyes and Pigments: Sustainable Applications and Future Scope. Sustainable Chemistry. 2025; 6(3):23. https://doi.org/10.3390/suschem6030023
Chicago/Turabian StyleNegi, Arvind. 2025. "Natural Dyes and Pigments: Sustainable Applications and Future Scope" Sustainable Chemistry 6, no. 3: 23. https://doi.org/10.3390/suschem6030023
APA StyleNegi, A. (2025). Natural Dyes and Pigments: Sustainable Applications and Future Scope. Sustainable Chemistry, 6(3), 23. https://doi.org/10.3390/suschem6030023






