Teaching the Nature of Science Through Biodiesel Synthesis from Waste Cooking Oil: A Literature Review with Experimental Insights
Abstract
1. Introduction
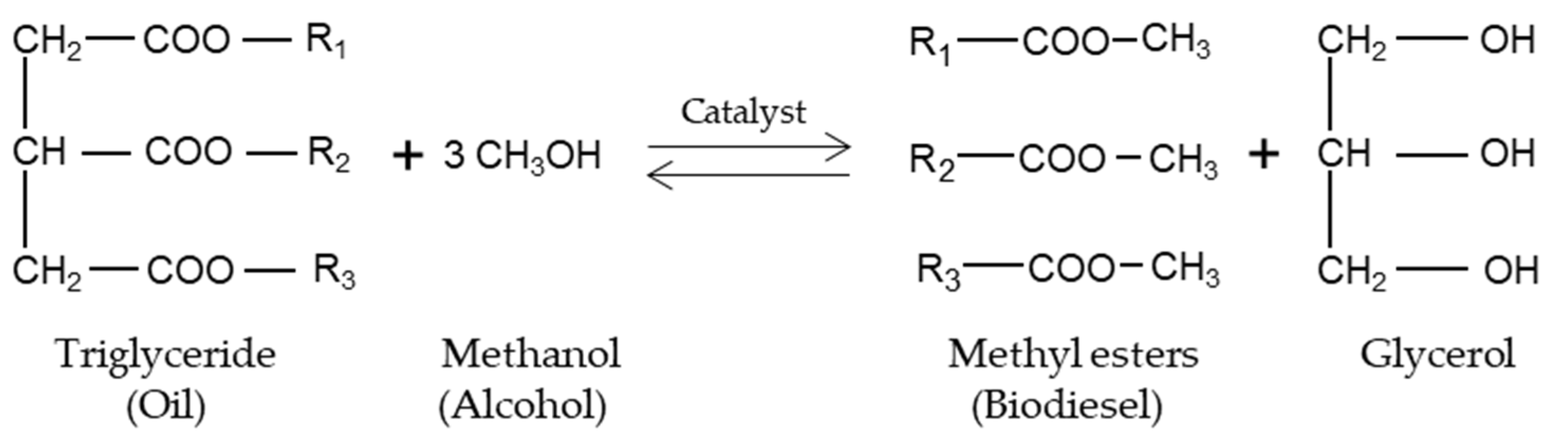
2. Biodiesel and the Nature of Science
2.1. Explicit vs. Implicit NOS Teaching
2.2. Models for Explicit NOS Teaching
2.3. Biodiesel Lab Experiences in Science Education
2.4. Assessment Strategies, Student Feedback and Learning Outcomes
2.5. Practical Considerations for School Implementation
3. Materials and Methods
3.1. Experimental Considerations
3.2. Homogeneous Basic Catalysis Key Conditions
3.3. Design of Experiments (DoE): Hadamard Optimisation Method
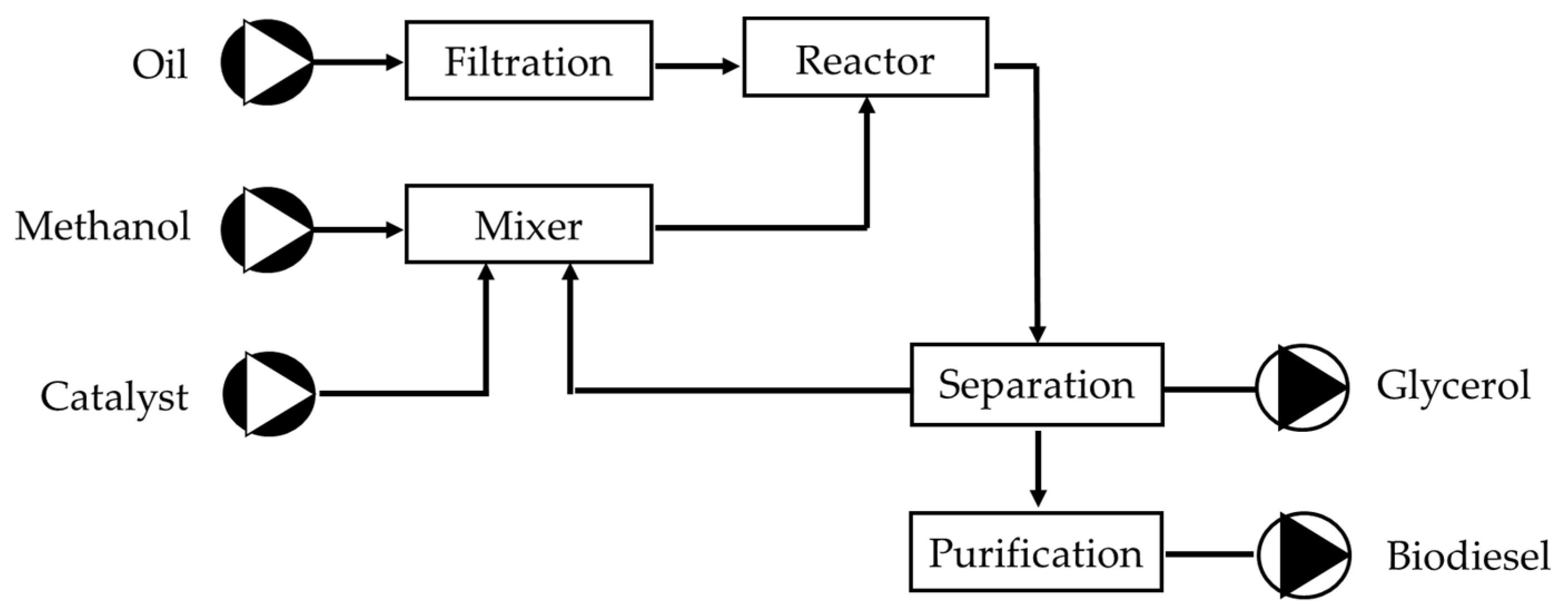
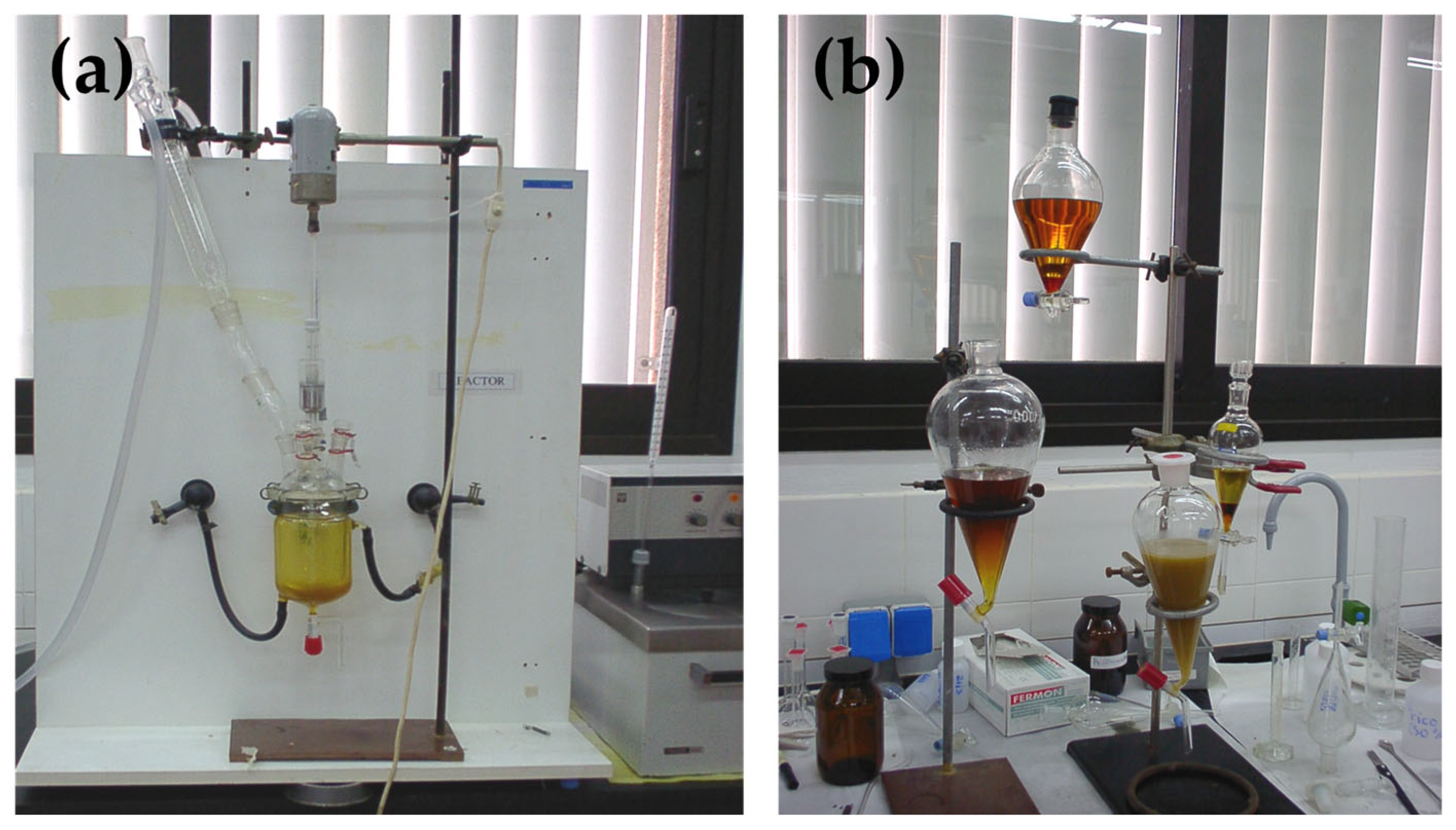
3.4. Experimental Procedure
- Set-up of the isothermal reactor: Start the oil bath and set the desired reaction temperature. Wait for the system to reach a steady-state temperature before proceeding with the reaction.
- Preparation of reagents: Weigh the required amounts of oil and methanol according to the molar ratio specified in the experiment. Weigh the necessary amount of NaOH or KOH regarding the design of experiments. Prepare the corresponding methoxide solution by stirring.
- Introduction of reagents into the reactor: Add the oil into the reactor and wait until it reaches the programmed temperature. Open the water-cooling line of the distillation column to prevent the evaporation of methanol when the reaction temperature exceeds its boiling point, ensuring reflux. Just before adding the remaining reagents, turn on the reactor stirrer, setting it to a speed of 600 rpm or higher for all trials. Then, add the prepared methoxide solution into the corresponding volume of methanol. Start the timer to record the reaction time.
- Reaction completion: Once the reaction time has passed, turn off the stirrer, the oil bath, and the timer. Allow the reactor to cool down sufficiently to prevent the loss of unreacted methanol by evaporation. The methanol can then be recovered by distillation using a rotary evaporator. Close the water inlet (cooling system) and transfer the reactor contents into a separatory funnel. You should observe the formation of two distinct phases: the lower phase containing glycerol and the upper phase containing the methyl esters (biodiesel). If both phases are not visible, it may indicate emulsion formation or that the transesterification did not occur. To break any emulsions, treat the mixture with a rotary evaporator at 60 °C for 30 min, then let the phases settle again.
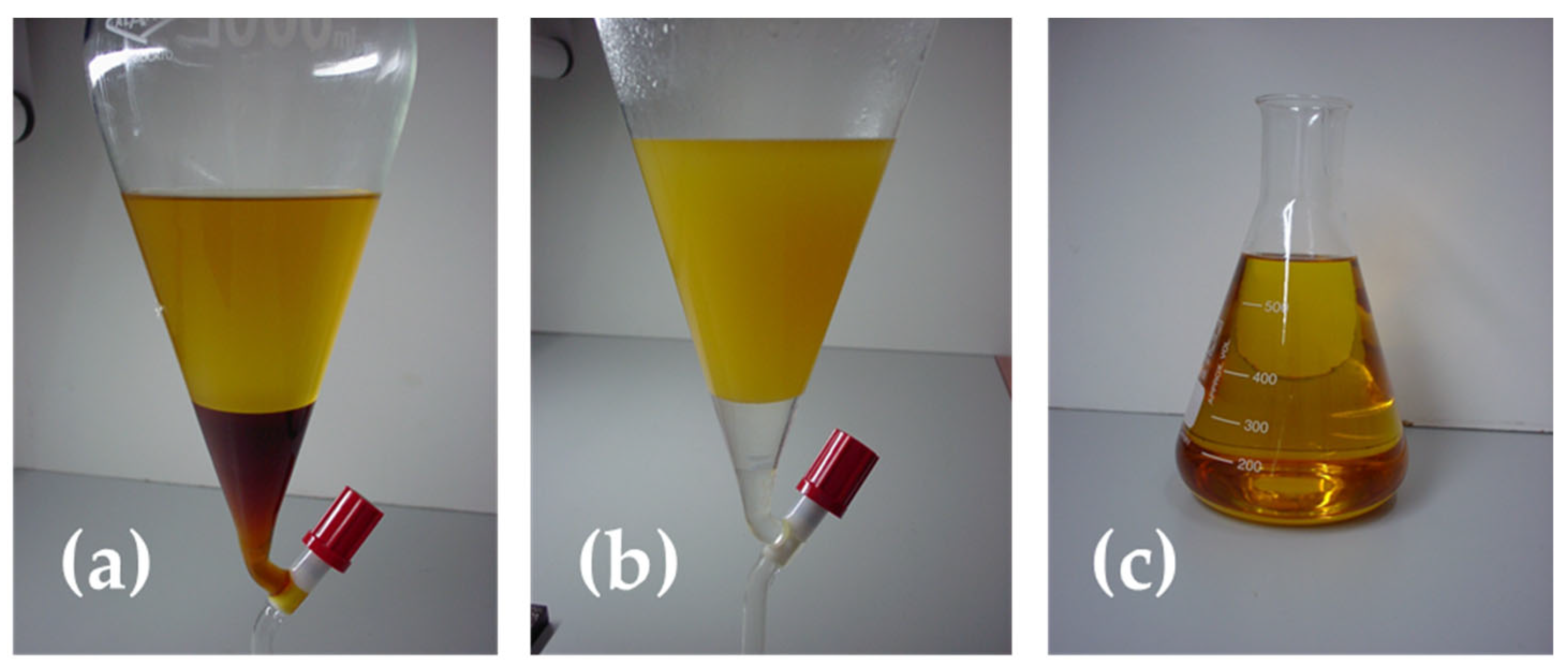
- Purification of the separated phases (Figure 4): Once two distinct phases have formed, collect the lower phase (glycerol) in a beaker and send it to the rotary evaporator to recover any methanol. Weigh and label the glycerol phase with the experiment date and number. For the biodiesel (upper phase), wash it with two solutions: first with an acidified solution to neutralise the biodiesel phase and then with distilled water. In the first wash, add 20 mL of 5% phosphoric acid (by weight) for every 100 g of biodiesel phase. In the second wash, add 20 mL of distilled water. For both washes, agitate the solution, allow phases to separate, and discard the lower layer. Repeat the process for the second wash. After the washes, the biodiesel is separated, stored in an opaque container, and kept for further analysis.
- 6.
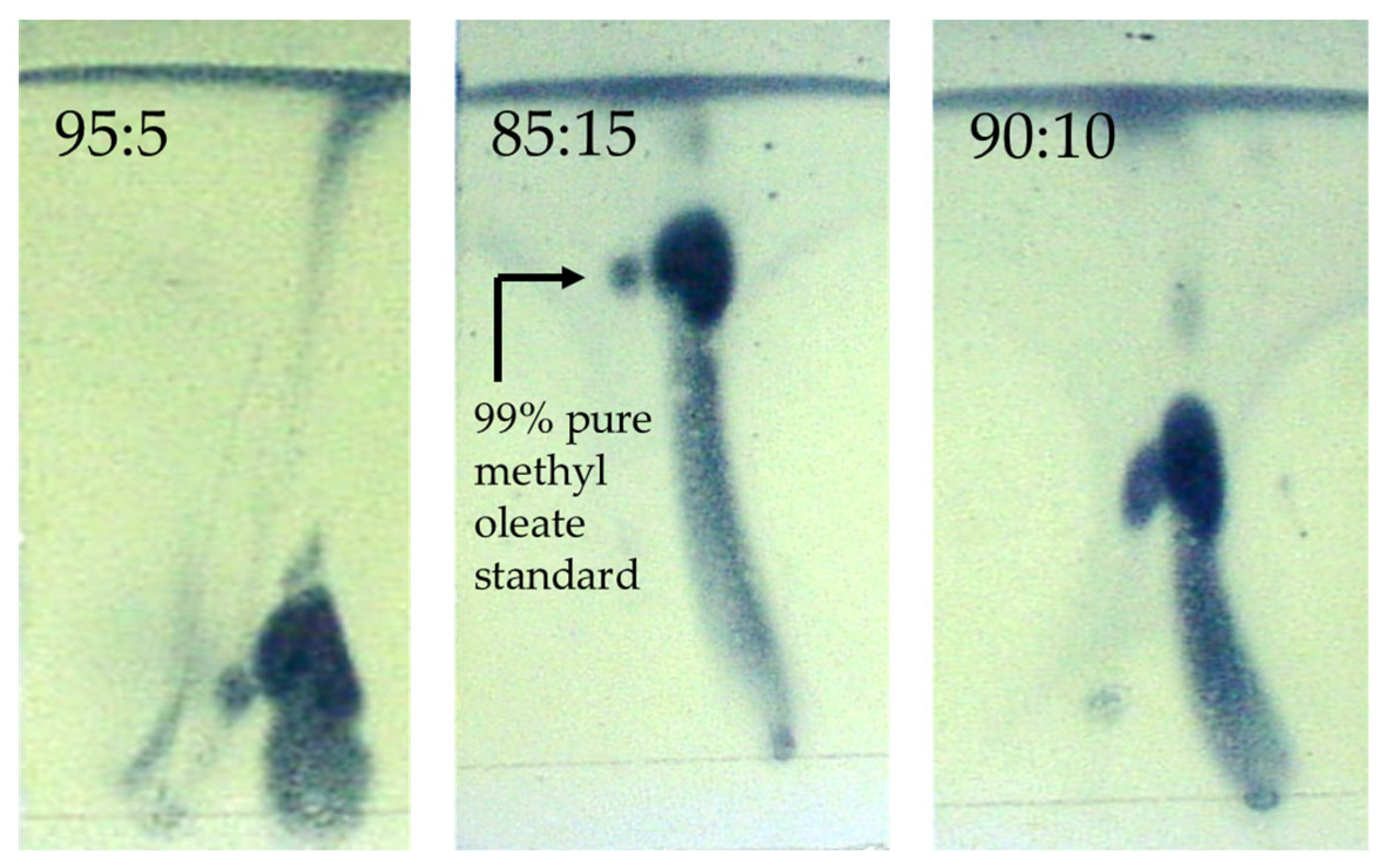
- Characterisation of viscosity and density: The viscosity and density of the biodiesel are measured in some samples. In addition, TLC is performed to confirm the presence of methyl esters.
4. Results and Discussion
4.1. Reaction Yield
4.2. Viscosity and Density Reduction
4.2.1. Influence of Reaction Time
4.2.2. Influence of Catalyst Mass
4.2.3. Influence of Methanol-to-Oil Molar Ratio and Reaction Temperature
4.3. Design of Experiments (DoE)
4.4. Comparison with Quality Standard Biodiesel
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Test | Catalyst | 3 Percentage (%) | Catalyst Mass ±0.01 (g) | Temperature ±0.5 (°C) | 4 Molar Ratio | Reaction Time (min) |
|---|---|---|---|---|---|---|
| 01 1 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 60 |
| 02 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 60 |
| 03 2 | - | - | - | 70.0 | 6/1 | 60 |
| 04 | KOH | 5.00 | 3.30 | 70.0 | 6/1 | 60 |
| 05 | NaOH | 3.33 | 2.20 | 70.0 | 6/1 | 60 |
| 06 | KOH | 1.25 | 0.83 | 70.0 | 6/1 | 40 |
| 07 | KOH | 3.33 | 2.20 | 70.0 | 6/1 | 60 |
| 08 | NaOH | 1.25 | 0.83 | 60.0 | 6/1 | 60 |
| 09 | NaOH | 1.25 | 0.83 | 80.0 | 6/1 | 60 |
| 10 | NaOH | 1.25 | 0.83 | 25.0 | 6/1 | 60 |
| 11 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 30 |
| 12 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 15 |
| 13 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 45 |
| 14 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 75 |
| 15 | NaOH | 1.25 | 0.83 | 70.0 | 6/1 | 60 |
| 16 | KOH | 0.05 | 2.20 | 70.0 | 4/1 | 60 |
| 17 | KOH | 0.03 | 2.20 | 70.0 | 8/1 | 60 |
| 18 | KOH | 1.25 | 0.83 | 70.0 | 6/1 | 60 |
| 19 | KOH | 1.25 | 0.83 | 70.0 | 6/1 | 260 |
| 20 | KOH | 3.33 | 2.20 | 70.0 | 6/1 | 60 |
| 21 | KOH | 0.75 | 0.49 | 70.0 | 6/1 | 60 |
| 22 | KOH | 5.00 | 3.30 | 70.0 | 6/1 | 60 |
| 23 | KOH | 0.01 | 0.49 | 70.0 | 4/1 | 60 |
| 24 | KOH | 0.01 | 0.49 | 70.0 | 8/1 | 60 |
| 25 | KOH | 1.25 | 0.83 | 70.0 | 6/1 | 100 |
| 26 | KOH | 1.25 | 0.83 | 70.0 | 6/1 | 60 |
| 27 | KOH | 1.25 | 0.83 | 70.0 | 6/1 | 60 |
| H-1 | KOH | 0.03 | 2.20 | 70.0 | 8/1 | 100 |
| H-2 | KOH | 0.01 | 0.49 | 70.0 | 4/1 | 100 |
| H-3 | KOH | 0.05 | 2.20 | 70.0 | 4/1 | 40 |
| H-4 | KOH | 0.01 | 0.49 | 70.0 | 8/1 | 40 |
| Test | Viscosity ±0.05 (mm2/s) | Density ±0.5 (kg/m3) | Mass Biodiesel ±0.01 (g) | Mass Glycerol ±0.01 (g) | Yield (%) |
|---|---|---|---|---|---|
| 01 1 | 4.12 | 850.0 | 243.54 | 25.0 | 90.2 |
| 02 | 6.80 | 892.0 | 282.60 | 41.08 | 94.2 |
| 03 2 | 36.45 | 922.0 | - | - | - |
| 04 | 4.85 | 884.0 | 274.33 | 63.92 | 91.4 |
| 05 | 5.60 | 888.0 | 280.70 | 61.30 | 93.6 |
| 06 | 7.77 | 892.5 | 266.18 | 21.80 | - |
| 07 | 5.67 | 888.0 | 283.53 | 30.45 | 94.5 |
| 08 | 6.21 | 888.5 | 268.72 | 31.75 | 89.6 |
| 09 | 6.18 | 888.5 | 272.25 | 25.60 | 90.7 |
| 10 | 8.45 | 895.0 | 285.24 | 28.30 | - |
| 11 | 7.54 | 892.0 | 279.03 | 19.93 | - |
| 12 | 7.87 | 892.5 | 270.44 | 17.40 | - |
| 13 | 7.23 | 891.0 | 280.29 | 20.95 | - |
| 14 | 6.63 | 890.0 | 281.62 | 23.71 | 93.8 |
| 15 | 6.40 | 890.0 | 280.60 | 22.50 | 93.5 |
| 16 | 5.49 | 888.0 | 268.98 | 29.60 | 89.6 |
| 17 | 4.52 | 884.5 | 272.86 | 34.29 | 90.9 |
| 18 | 6.59 | 892.0 | 284.38 | 19.22 | 94.8 |
| 19 | 5.34 | 888.0 | 281.93 | 21.64 | 93.9 |
| 20 | 4.67 | 885.0 | 270.02 | 28.03 | 90.0 |
| 21 | 9.00 | 899.5 | 205.02 | 20.07 | - |
| 22 | 4.53 | 884.0 | 258.07 | 34.13 | 86.0 |
| 23 | 18.14 | 914.5 | 273.34 | - | - |
| 24 | 8.86 | 899.0 | 286.20 | 15.67 | - |
| 25 | 5.91 | 895.0 | 274.10 | 24.26 | 91.4 |
| 26 | 6.63 | 892.0 | 277.48 | 19.30 | 92.5 |
| 27 | 7.79 | 893.0 | 272.37 | 13.32 | - |
| H-1 | 4.57 | 883.5 | 266.38 | 31.95 | 88.8 |
| H-2 | 32.53 | 921.0 | 262.70 | - | - |
| H-3 | 6.85 | 892.0 | 270.20 | 22.43 | 90.1 |
| H-4 | 18.35 | 912.5 | 175.08 | 15.01 | - |
References
- Kumar, A.; Bhayana, S.; Singh, P.K.; Tripathi, A.D.; Paul, V.; Balodi, V.; Agarwal, A. Valorization of used cooking oil: Challenges, current developments, life cycle assessment and future prospects. Discov. Sustain. 2025, 6, 119. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Sheinbaum-Pardo, C.; Calderón-Irazoque, A.; Ramírez-Suárez, M. Potential of biodiesel from waste cooking oil in Mexico. Biomass Bioenergy 2013, 56, 230–238. [Google Scholar] [CrossRef]
- Caporusso, A.; Radice, M.; Biundo, A.; Gorgoglione, R.; Agrimi, G.; Pisano, I. Waste cooking oils as a sustainable feedstock for bio-based application: A systematic review. J. Biotechol. 2025, 400, 48–65. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Patel, K.; Singh, S.K. Sustainable biodiesel from used cooking oil: A comparative life cycle, energy, and uncertainty analysis. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- EN 14214:2003; European Committee for Standardization—Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods. CEN: Brussels, Belgium, 2003.
- ASTM D6751-23a; Standard Specification for Biodiesel Fuel Blendstock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2023.
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, S108–S117. [Google Scholar] [CrossRef]
- Ramos, M.; Dias, A.P.S.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel production processes and sustainable raw materials. Energies 2019, 12, 4408. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Characterization of the key fuel properties of methyl ester–diesel fuel blends. Fuel 2009, 88, 75–80. [Google Scholar] [CrossRef]
- Lopresto, C.G.; De Paola, M.G.; Calabrò, V. Importance of the properties, collection, and storage of waste cooking oils to produce high-quality biodiesel—An overview. Biomass Bioenergy 2024, 189, 107363. [Google Scholar] [CrossRef]
- Knoerzer, T.A.; Hill, E.M.; Davis, T.A.; Iacono, S.T.; Johnson, J.E.; Balaich, G.J. Comparative analysis of fuel composition and physical properties of biodiesel, diesel, kerosene, and jet fuel. J. Chem. Educ. 2018, 95, 1821–1826. [Google Scholar] [CrossRef]
- Fukuda, H.; Kond, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Derobertis, F.; Leone, M.S.; Mesto, E.; Schingaro, E.; Porfido, C.; Ditaranto, N.; Mali, M.; Dell’Anna, M.M.; Mastrorilli, P. Microwave assisted biodiesel production from waste cooking oil using steel slags as catalyst. Eur. J. Inorg. Chem. 2024, 27, e202400375. [Google Scholar] [CrossRef]
- Kerstiens, G.A. The impact of Nature of Science Instruction on the Chemistry Laboratory Experience. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2019. [Google Scholar]
- Lederman, N.G. Nature of Science: Past, present, and future. In Handbook of Research on Science Education; Abell, S.K., Lederman, N.G., Eds.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2007; pp. 831–879. [Google Scholar]
- Khishfe, R. Improving students’ conceptions of Nature of Science: A review of the literature. Sci. Educ. 2023, 32, 1887–1931. [Google Scholar] [CrossRef]
- Bell, R.L.; Matkins, J.J.; Gansneder, G.M. Impacts of contextual and explicit instruction on preservice elementary teachers’ understandings of the nature of science. J. Res. Sci. Teach. 2011, 48, 414–436. [Google Scholar] [CrossRef]
- Hodson, D.; Wong, S.L. From the horse’s mouth: Why scientists’ views are crucial to nature of science understanding. Int. J. Sci. Educ. 2014, 36, 2639–2665. [Google Scholar] [CrossRef]
- Lederman, N.G.; Abd-El-Khalick, F.; Bell, R.L.; Schwartz, R.S. Views of nature of science questionnaire: Toward valid and meaningful assessment of learners’ conceptions of nature of science. J. Res. Sci. Teach. 2002, 39, 497–521. [Google Scholar] [CrossRef]
- Khishfe, R.; Lederman, N. Teaching nature of science within a controversial topic: Integrated versus nonintegrated. J. Res. Sci. Teach. 2006, 43, 395–418. [Google Scholar] [CrossRef]
- McDonald, C.V. The influence of explicit nature of science and argumentation instruction on preservice primary teachers’ views of nature of science. J. Res. Sci. Teach. 2010, 47, 1137–1164. [Google Scholar] [CrossRef]
- Abd-El-Khalick, F.; Bell, R.L.; Lederman, N.G. The nature of science and instructional practice: Making the unnatural natural. Sci. Educ. 1998, 82, 417–436. [Google Scholar] [CrossRef]
- Khishfe, R.; Abd-El-Khalick, F. The influence of explicit and reflective versus implicit inquiry-oriented instruction on sixth graders’ views of nature of science. J. Res. Sci. Teach. 2002, 39, 551–578. [Google Scholar] [CrossRef]
- Rudge, D.W.; Howe, E.M. An explicit and reflective approach to the use of history to promote understanding of the nature of science. Sci. Educ. 2009, 18, 561–580. [Google Scholar] [CrossRef]
- Tytler, R. Socio-Scientific Issues, Sustainability and Science Education. Res. Sci. Educ. 2012, 42, 155–163. [Google Scholar] [CrossRef]
- Clough, M.P. Teaching and Learning About the Nature of Science. Sci. Educ. 2018, 27, 1–5. [Google Scholar] [CrossRef]
- Erduran, S.; Dagher, Z.R.; McDonald, C.V. Contributions of the Family Resemblance Approach to Nature of Science in Science Education. Sci. Educ. 2019, 28, 311–328. [Google Scholar] [CrossRef]
- Allchin, D. Evaluating knowledge of the nature of (whole) science. Sci. Educ. 2011, 95, 518–542. [Google Scholar] [CrossRef]
- Matthews, M.R. Changing the focus: From nature of science to features of science. In Advances in Nature of Science Research; Khine, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–26. [Google Scholar]
- Erduran, S.; Dagher, Z.R. Reconceptualizing the Nature of Science for Science Education: Scientific Knowledge, Practices and Other Family Categories; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Irzik, G.; Nola, R. New directions for nature of science research. In International Handbook of Research in History, Philosophy and Science Teaching; Matthews, M., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 999–1021. [Google Scholar]
- Cheung, K.K.C.; Erduran, S. A systematic review of research on family resemblance approach to Nature of Science in Science Education. Sci. Educ. 2023, 32, 1637–1673. [Google Scholar] [CrossRef]
- Keppeler, N.; Novaki, L.P.; El Seoud, O.A. Teaching the undergraduate laboratory during pandemic time: Using the synthesis of a biodiesel model to demonstrate aspects of green chemistry. J. Chem. Educ. 2021, 98, 3962–3967. [Google Scholar] [CrossRef]
- Leibfarth, F.A.; Russell, M.G.; Langley, D.M.; Seo, H.; Kelly, L.P.; Carney, D.W.; Sello, J.K.; Jamison, T.F. Continuous-flow chemistry in undergraduate education: Sustainable conversion of reclaimed vegetable oil into biodiesel. J. Chem. Educ. 2018, 95, 1371–1375. [Google Scholar] [CrossRef]
- Georgiou, Y.; Kyza, E.A. Fostering chemistry students’ scientific literacy for responsible citizenship through socio-scientific inquiry-based learning (SSIBL). Sustainability 2023, 15, 6442. [Google Scholar] [CrossRef]
- Nida, S.; Marsuki, M.F.; Eilks, I. Palm-oil-based biodiesel in Indonesia: A case study on a socioscientific issue that engages students to learn chemistry and its impact on Society. J. Chem. Educ. 2021, 98, 2536–2548. [Google Scholar] [CrossRef]
- Armstrong, L.B.; Rivas, M.C.; Zhou, Z.; Irie, L.M.; Kerstiens, G.A.; Robak, M.T.; Douskey, M.C.; Baranger, A.M. Developing a green chemistry focused general chemistry laboratory curriculum: What do students understand and value about green chemistry? J. Chem. Educ. 2019, 96, 2410–2419. [Google Scholar] [CrossRef]
- Jin, G.; Bierma, T. Sustainability education and civic engagement through integration of undergraduate research with Service Learning. Sci. Educ. Civ. Engagem. 2023, 15, 17–21. [Google Scholar]
- Neiles, K.Y.; Bowers, G.B.; Chase, D.T.; VerMeulen, A.; Hovland, D.E.; Bresslour-Rashap, E.; Eller, L.; Koch, A.S. Teaching collaborations and scientific practices through a vertically scaffolded biodiesel laboratory experience. J. Chem. Educ. 2019, 96, 1988–1997. [Google Scholar] [CrossRef]
- Hupp, A.M. Incorporating chemometric methods in the undergraduate curriculum: A problem set activity for an upper-level analytical elective course. J. Chem. Educ. 2023, 100, 1377–1381. [Google Scholar] [CrossRef]
- Yang, J.; Xu, C.; Li, B.; Ren, G.; Wang, L. Synthesis and determination of biodiesel: An experiment for high school chemistry laboratory. J. Chem. Educ. 2013, 90, 1362–1364. [Google Scholar] [CrossRef]
- Khamhaengpol, A.; Phewphong, S.; Chuamchaitrakool, P. STEAM activity on biodiesel production: Encouraging creative thinking and basic science process skills of high school students. J. Chem. Educ. 2022, 99, 736–744. [Google Scholar] [CrossRef]
- Bladt, D.; Murray, S.; Gitch, B.; Trout, H.; Liberko, C. Acid-catalyzed preparation of biodiesel from waste vegetable oil: An experiment for the undergraduate organic chemistry laboratory. J. Chem. Educ. 2011, 88, 201–203. [Google Scholar] [CrossRef]
- Clarke, N.R.; Casey, J.P.; Brown, E.D.; Oneyma, E.; Donaghy, K.J. Preparation and viscosity of biodiesel from new and used vegetable oil. An inquiry-based environmental chemistry laboratory. J. Chem. Educ. 2006, 83, 257–259. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Keppeler, N.; Sandrini, D.M.F.; Morselli, G.R. Active learning in the undergraduate chemistry laboratory: Synthesis of biodiesel from an Amazon region oil (Babassu): Keep it simple! J. Chem. Educ. 2023, 100, 2926–2934. [Google Scholar] [CrossRef]
- Kim, J. Production of biodiesel from waste cooking oil: A guided inquiry chemistry laboratory activity at a two-year college. J. Chem. Educ. 2022, 99, 4162–4168. [Google Scholar] [CrossRef]
- ASTM D446-24; Standard Specifications and Operating Instructions for Glass Capillary Kinematic Viscometers. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D1298-12b(2017); Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2017.
- Vicente, G.; Martínez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Coteron, A.; Vicente, G.; Martínez, M.; Aracil, J. Biodiesel production from vegetable oils. Influence of catalysts and operating conditions. Recent. Res. Dev. Oil Chem. 1997, 1, 109–114. [Google Scholar]
- Zhang, Y. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Peña Romero, A.; Costa, J.B.; Castel-Maroteaux, I.; Chulia, D. Statistical optimization of a controlled release formulation obtained by a double compression process: Application of an Hadamard matrix and a factorial design. Drug Dev. Ind. Pharm. 1989, 15, 2419–2440. [Google Scholar] [CrossRef]
- Balasubramanian, K. Characterization of Hadamard matrices. Mol. Phys. 1993, 78, 1309–1329. [Google Scholar] [CrossRef]
- Llabrés, M.; Fariña, J.B. Design and evaluation of sustained-release tablets of Lithium in a fat matrix and its bioavailability in humans. J. Pharm. Sci. 1991, 80, 1012–1016. [Google Scholar] [CrossRef]
- Wehrle, P.; Korner, D.; Benita, S. Sequential statistical optimization of a positively-charged submicron emulsion of miconazole. Pharm. Dev. Technol. 1996, 1, 97–111. [Google Scholar] [CrossRef]
- Baro, M.; Sánchez, E.; Delgado, A.; Perera, A.; Évora, C. In vitro–in vivo characterization of gentamicin bone implants. J. Control. Release 2002, 83, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, D.; Djuris, J.; Jakimenko, S.; Ibric, S. Application of the fractional factorial design in multiple W/O/W emulsions. J. Dispers. Sci. Technol. 2017, 38, 1732–1737. [Google Scholar] [CrossRef][Green Version]
| Students’ Role | Experimental Involvement | Pedagogical Aspects | Refs. |
|---|---|---|---|
| Passive experimenters | Perform synthesis (follow structured protocols), basic tests; no design or decision-making | Observation, empirical basis, properties of materials | [44,45,46] |
| Active experimenters | Modify reaction conditions, conduct synthesis and analysis; guided inquiry | Inquiry, control of variables, scientific methods | [37,47,48] |
| Experimental designers | Literature research, propose variables, validate results with further tests | Modeling, DoE, creative thinking | [42,48,49] |
| Data analysts | Analyze GC-MS data sets using chemometric methods | Data interpretation, pattern recognition, chemometrics | [43] |
| Socio-scientific debaters | Simulations, discussions, role-play, choose biodiesel type (limited lab work) | SSI, ethical reasoning, argumentation | [38,39] |
| Service-based participants | Collect local WCO, synthesise biodiesel for real-world application | Community engagement | [41] |
| Scientific communicators | Write reports, create communications for various audiences, peer collaboration | Communication, argument from evidence, interdisciplinary learning | [42] |
| Variable | High Level | Low Level |
|---|---|---|
| Reaction time (min) | 100 | 40 |
| Catalyst mass (g) | 2.20 | 0.49 |
| Methanol-to-oil molar ratio | 8:1 | 4:1 |
| Test | Response (Y) | Time (min) | Catalyst Mass (g) | Molar Ratio 1 | Viscosity (mm2/s) | Density (kg/m3) |
|---|---|---|---|---|---|---|
| H-1 | Y1 | 100 | 2.20 | 8:1 | 4.57 | 0.8835 |
| H-2 | Y2 | 100 | 0.49 | 4:1 | 32.53 | 0.9210 |
| H-3 | Y3 | 40 | 2.20 | 4:1 | 6.85 | 0.8920 |
| H-4 | Y4 | 40 | 0.49 | 8:1 | 18.35 | 0.9125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Martínez, J.; Beltrán-Martínez, J.; Cano-Ortiz, A.; Rosales-Conrado, N. Teaching the Nature of Science Through Biodiesel Synthesis from Waste Cooking Oil: A Literature Review with Experimental Insights. Sustain. Chem. 2025, 6, 15. https://doi.org/10.3390/suschem6020015
Peña-Martínez J, Beltrán-Martínez J, Cano-Ortiz A, Rosales-Conrado N. Teaching the Nature of Science Through Biodiesel Synthesis from Waste Cooking Oil: A Literature Review with Experimental Insights. Sustainable Chemistry. 2025; 6(2):15. https://doi.org/10.3390/suschem6020015
Chicago/Turabian StylePeña-Martínez, Juan, Jessica Beltrán-Martínez, Ana Cano-Ortiz, and Noelia Rosales-Conrado. 2025. "Teaching the Nature of Science Through Biodiesel Synthesis from Waste Cooking Oil: A Literature Review with Experimental Insights" Sustainable Chemistry 6, no. 2: 15. https://doi.org/10.3390/suschem6020015
APA StylePeña-Martínez, J., Beltrán-Martínez, J., Cano-Ortiz, A., & Rosales-Conrado, N. (2025). Teaching the Nature of Science Through Biodiesel Synthesis from Waste Cooking Oil: A Literature Review with Experimental Insights. Sustainable Chemistry, 6(2), 15. https://doi.org/10.3390/suschem6020015








