Abstract
Rambutan peel is a great source of bioactive compounds, the same that, over the years, has been extracted using conventional technologies which have been proven to be harmful to the environment and potentially toxic to human beings. This study aimed to extract the same compounds using a hybridization of ultrasound/microwave extraction. The results were promising, as a total of 378.48 ± 9.19 mg/g of polyphenols were recovered from this procedure, and the most important molecules (geraniin, corilagin, and ellagic acid) were identified, giving this much more relevance. Furthermore, treatment with rambutan peel extract recovered with green technologies significantly reduced cell viability in HCV-infected liver cells. Notably, higher concentrations (4000 and 5000 ppm) led to more pronounced cell death in huh7 cells. The treatment also led to a significant reduction in viral protein and RNA expression in HCV-infected cells. These findings suggest that rambutan peel extract obtained from the combination of ultrasound and microwave extraction, particularly the ellagitannins present, have potential antiviral properties. Further research is needed to explore its mechanism of action and its potential as a therapeutic agent for Hepatitis C.
1. Introduction
In recent years, green technologies have revolutionized extraction processes, offering more sustainable and environmentally friendly alternatives to traditional methods [1]. Conventional solvent-based techniques, which rely on petrochemical solvents, contribute to environmental pollution and generate harmful by-products. In contrast, green technologies focus on reducing environmental footprints by utilizing renewable resources and improving energy efficiency. Innovative methods like microwave and ultrasound extraction (MAE/UAE) have gained prominence for their ability to extract bioactive compounds using only water, creating heat, vibrations, and cavitation bubbles to aid the process, all without the need for toxic solvents; the use of this technologies simultaneously can also increase the yield of the bioactive compounds obtained [2]. The application of ultrasound and microwaves has gained significant importance in the extraction of bioactive compounds from various natural matrices, such as hulls, seeds, and the pomaces of specific plant sources. Ultrasound offers several advantages, including reduced energy consumption and the ability to operate at lower temperatures, which is beneficial for the extraction of thermolabile compounds like polyphenols. The underlying principle of ultrasound technology is acoustic cavitation, a process in which cavitation bubbles are generated. These bubbles facilitate the fragmentation, erosion, pore formation, and swelling of plant cellular matrices. Upon collapsing, the bubbles generate a substantial amount of heat (reaching temperatures as high as 5000 K under pressures of up to 1000 atm), which enhances the solubilization of bioactive compounds into the solvent being used [3,4].
On the other hand, microwave-assisted extraction primarily relies on microwaves operating at frequencies ranging from 300 MHz to 300 GHz. This technology involves two key phenomena: ionic conduction and dipole rotation, both of which contribute to the generation of microwave-induced heating. The heating effect is driven by friction resulting from the movement of ions. In many instances, water-based solvents or pure water are preferred to enhance heating efficiency. This heating process can lead to the overheating of the medium, causing vaporization, which in turn induces rupturing of the cell walls, facilitating the release of bioactive compounds [5,6,7].
The usage of these technologies allows for the shift toward using green solvents such as ethanol and water, which are defined as non-toxic and biodegradable and, in some cases, can be derived from renewable sources, allowing for efficient extractions, minimizing the harmful environmental impacts associated with traditional solvents like hexane and chloroform [8].
These green extraction technologies not only reduce environmental impacts, but also tend to require less energy, making them more efficient and sustainable. As consumer demand for eco-friendly products rises, industries like pharmaceuticals, food, and cosmetics are increasingly adopting these advanced extraction techniques to align with sustainability goals. One example is the production of bioactive compounds from rambutan (Nephelium lappaceum L.), an exotic fruit native to Malaysia and introduced to Mexico in the 1960s. With a national production of nearly 9887 tons in 2022 [9], according to data from SAGARPA, rambutan is now widely cultivated in regions like Chiapas, Tabasco, Nayarit, and Oaxaca [10].
The extraction of valuable compounds from rambutan, particularly its husk, benefits greatly from these greener technologies, which also support more sustainable practices in the agriculture and food industries. Notably, rambutan’s husk accounts for about 45.7% of the fruit’s total weight and is typically discarded as waste [11]. In Mexico, this means that approximately 5458 tons of husk and seed are left behind annually. However, these waste materials have untapped potential, as research has shown that rambutan husk is rich in polyphenolic compounds, particularly ellagitannins like geraniin and corilagin [12,13,14,15], which are known for their antioxidant, anti-inflammatory, antiproliferative, anticarcinogenic, prebiotic, and antiviral properties [13,16,17,18,19].
Recent studies have highlighted the potential of rambutan husk as a source of natural antioxidants with antiproliferative and antiviral activities, particularly in the context of combating diseases like Hepatitis C (HCV). With an estimated 58 million people worldwide suffering from chronic HCV, and the virus contributing to approximately 290,000 deaths annually due to complications like cirrhosis and liver cancer, the need for new antiviral agents has never been more urgent [20]. Current antiviral treatments for HCV are limited and often ineffective due to the virus’s rapid mutation, underscoring the importance of developing new compounds [21]. Phenolic compounds like geraniin, ellagic acid, and corilagin, found in rambutan husk, have demonstrated hepatoprotective properties [22,23,24], which, while not curing HCV, could help delay or mitigate the symptoms of liver damage and inflammation associated with chronic infections and hepatocarcinoma [25].
The objective of this study is to evaluate the inherent nutraceutical properties of rambutan peel extract (Nephelium lappaceum L.), which is constituted mainly of ellagitannins, to evidence a new potential source of natural antioxidants with antiproliferative and antiviral activities, which may, in the long term, have potential applications in the pharmaceutical and food industries.
2. Materials and Methods
2.1. Extraction Procedures
2.1.1. Preparation of Materials and Extraction Conditions
Rambutan (Nephelium lappaceum L.) samples were sourced from the Soconusco region in Chiapas, Mexico. Upon receipt, the peels were dried for preservation in a conventional oven at 50 °C for 48 h. Following the drying process, the peels were ground into a fine powder and homogenized to achieve a particle size of 2 mm.
2.1.2. Hybrid Extraction
The extract was obtained utilizing two emergent technologies such as ultrasound and microwave; these techniques were performed simultaneously, making a hybridization of both. For the extraction water was used as a solvent using a 1:16 m/v ratio (62.5 g of rambutan peel in 1 L of water). For the UAE, the equipment was operated at a frequency of 25 kHz for 20 min. For MAE, the machine was set to 2450 MHz with a 5 min extraction time until it reached a target temperature of 70 °C. This short exposure to high temperature ensured that the polyphenolic compounds remained intact, as prolonged high temperatures can degrade the compounds, leading to loss of yield and quality. The extraction of polyphenolic content was carried out using a hybrid technology system that combined ultrasound and microwave-assisted extraction, specifically the Ultrasound/Microwave Cooperative Workstation (Nanjing ATPIO Instruments Manufacture Co., Ltd., Nanjing, China) [26].
After extraction, all the resulting extracts were filtered in a dark room to protect the phenolic compounds from external environmental factors and remove any remains of rambutan peel which could alter the results. Once they were filtered, the extracts were placed in a glass bottle covered with aluminum foil for further protection from light and then were stored at −18 °C.
2.1.3. Column Fractionation
The resulting extract underwent chromatographic fractionation using a glass column (60 cm height and 2.5 cm diameter) packed with Amberlite XAD-16 resin to selectively recover the compounds of interest. To carry out this process, the raw extract was passed through the column in batches of 30 mL to not overcharge the filling of Amberlite; once all the extract had gone through the column, an elusion of water was made to remove the undesired compounds present in the filling. Once the water elusion was made, the next step was to recover the compounds using an ethanol elusion; the recovered ethanolic fraction was then stored in a glass bottle covered with aluminum foil to prevent decay caused from external factors.
The ethanolic fraction was then left to dry in a conventional oven for 12 h at a temperature of 50 °C; this was performed to obtain a polyphenolic powder, which can later be used in known concentrations. The drying of the compounds was performed on glass Petri dishes [13]. The resulting powder was weighed to calculate the yield of the extraction with the following formula:
2.1.4. HPLC/ESI/MS Analysis
To confirm the presence and identity of the target compounds, a HPLC coupled with mass spectrometry (MS) and electrospray ionization (ESI) analysis was performed. Identification of polyphenolic compounds obtained from rambutan peel extract was carried out with the method described by Hernandez-Hernandez et al. (2020) [26]. Mexican rambutan extract powder (300 mg) containing polyphenols was prepared in a 2 mL solution of 50% ethanol. Then, the volume was filtered using 0.45 μm nylon membranes. Samples were analyzed using reversed-phase high-performance liquid chromatography in a Varian HPLC system, including an auto-sampler (ProStar 410, Varian, Palo Alto, CA, USA), a ternary pump (ProStar 230I, Varian, Palo Alto, CA, USA), and a PDA (Photo Diode Array) detector (ProStar 330, Varian, Atlanta, GA, USA). A chromatography ion trap mass spectrometer (Varian 500-MS I.T. Mass Spectrometer, Palo Alto, CA, USA) equipped with an electrospray ion source, coupled with the HPLC system, was also used. Samples (5 μL) were injected into a preequilibrated Denali C18 column (150 mm × 2.1 mm, 3 μm, Grace, Albany, OR, USA), and the oven temperature was maintained at 30 °C. The eluents were formic acid (0.2%, v/v; solvent A) and acetonitrile (solvent B). The following gradient was applied: 3% B from 0 to 5 min, 9% B linear from 5 to 15 min, 16% B linear from 15 to 45 min, and 50% B linear from 45 to 60 min. Then, the column was washed and reconditioned. The flow rate was maintained at 0.2 mL/min, and elution was monitored at 245, 280, 320, and 550 nm for the compounds of interest. The whole effluent (0.2 mL/min) was subsequently injected into the source of the mass spectrometer (MS) without splitting. All MS experiments were carried out in negative mode. [M-H]-nitrogen was used as nebulizing gas and helium as damping gas. The ion source parameters were as follows: 5.0 kV of spray voltage, 90.0 V of capillary voltage, and 350 °C of temperature. Data were collected and processed using MS Workstation software (V 6.9). Samples were firstly analyzed in full-scan mode acquired in the m/z range 50–2000. MS/MS analyzes were performed on a series of selected precursor ions. Finally, the compounds were compared using a database of bioactive compounds (WorkStation version 2.0 database, VARIAN, Palo Alto, CA, USA) [26].
2.1.5. Polyphenol Quantification
Hydrolyzable polyphenols were measured with the Folin–Ciocalteu method. Briefly, 400 μL of Folin–Ciocalteu reagent was added to 400 μL of the sample. After 5 min, 400 μL of Na2CO3 was added to the mixture. After 1 min, 2.5 mL of distilled water was added. Samples absorbance was measured at 790 nm by using a 1 cm cell in a Biomate 3 spectrophotometer (Thermo Spectronic, Madison, WI, USA). This process was carried out in triplicate, and the results are expressed as the mean mg of gallic acid equivalents per g of dry rambutan peel. Gallic acid was used as a standard in a range of 0–500 ppm. Condensed polyphenols were assayed using the HCl butanol method. Sample volumes of 500 µL were transferred into screw cap test tubes with a maximum of 10 mL capacity. Then, 3 mL of HCl-Butanol 1:10 solution was added to the mixture. Subsequently, 100 μL of a 1:9 ferric solution was added. Then, tubes were mixed and placed in a water bath at 100 °C for 1 h. Next, solutions were cooled down at room temperature, and finally the absorbance of the samples and catechin standards (0–1000 ppm) was read at 460 nm. The process was carried out in triplicate [13].
2.2. Antiproliferative Assays
2.2.1. Cell Culture
Huh7 hepatocarcinoma cells and genotype 1b hepatitis C virus (HCV) subgenomic Huh7-replicon cells were obtained from the collection of the Biochemistry and Molecular Medicine Department in the School of Medicine from the Autonomous University of Nuevo Leon [27]; the cells were cultured in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin solution, 1% non-essential amino acids, and 1% glutamine. The cells were cultured in 5 mL flasks of 25 cm2 at 37 °C in a humidified atmosphere with 5% CO2. The cultured cells were trypsinized with 2.5 mL when they had an 85–90% confluence for later use [28].
2.2.2. RPE Treatment and Cytotoxic Assay
The purified extracts of the rambutan peel (RPE) were used for the evaluation of cell viability with an MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyltetrazol bromide) assay to Huh7 parental and HCV replicon cells. In total, 100 μL of culture medium were added to 96-well plates (~2 × 104 cells/well) and cultured for 24 h. Afterward, the medium was changed and the samples of RPE with the following concentrations were placed in each well: 2000, 3000, 4000, and 5000 ppm. No extracts were added to the controls. Cells were incubated with the extracts at 37 °C and 5% CO2 for 24, 36, and 72 h to evaluate the antiproliferative effect.
After the 24 h incubation period, 10 μL of MTT reagent (final concentration 0.5 mg/mL) was added to each well, including controls. After 4 h, 100 μL of the solubilization solution was added to each well. Plates were incubated overnight; afterward, the complete solubilization of the purple formazan crystals was verified, and the spectrometric absorbance of the samples was measured using a microplate reader at a wavelength of 600 nm to measure the absorbance of the formazan product. The reference wavelength was 670 nm [29,30,31].
2.3. Antiviral Activity
2.3.1. RNA Extraction
Total RNA was extracted from Huh7 HCV replicon cells (~2 × 105 cells/well) using Trizol solution according to the manufacturer’s instructions (Ambion; Thermo Fisher Scientific, Inc., Austin, TX, USA). After obtaining the RNA precipitate, they were washed with 75% ethanol three times until suspended with free RNAasa water (12 μL). The extracted RNA was kept in deep freezing for later use [32].
2.3.2. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
After extracting the total RNA, retro-transcription (RT-PCR) was performed to obtain complementary DNA (cDNA) using an MMLV RT according to the manufacturer’s specifications (Applied Biosystems; Thermo Fisher Scientific, Inc., Foster City, CA, USA). Two mixtures were made in two different tubes with the legend: tube 1 (mix 1) and tube 2 (mix 2). For mix 1, 5.5 μL of H2O DEPC, 1 μL of Random Primers, and 5 μL of the extracted RNA were added. This mixture was incubated at 72 °C for 10 min in the thermocycler. For mix 2, added 4 μL of Buffer RT 5× 2 μL of DTT reagent (0.1 M), 0.5 μL of RNAasa out, 1 μL of dNTP’s, and 1 μL of M-MLV. Subsequently, mix 1 was put on ice for 3 min and 8.5 μL of mix 2 was added. The mixture was then placed in the thermocycler under the following conditions: 10 min at 25 °C, 1 h at 37 °C, 5 min at 94 °C, and 30 min at 4 °C. Finally, the cDNA obtained was stored at −20 °C until use [33]. Expression levels of HCV-RNA were measured using RT-qPCR (2−ΔΔCt). Huh7 HCV replicon cells (~2 × 105 cells) were incubated with 1000 and 2000 RPE or without RPE for 24, 48, and 72 h. Total cell RNA was extracted at each time point, and HCV-RNA levels were quantified using RT-qPCR. RNA viral levels were normalized based on the ratio of HCV/GAPDH-RNA using the 2−ΔΔCt method that was amplified in the same plate.
2.3.3. Quantification of HCV-RNA
Quantification of HCV-RNA using quantitative PCR (qPCR) was performed. In total, 200 ng of cDNA were used to quantify HCV and GAPDH levels using TaqMan probes (Applied Biosystems) A mixture of Master mix (10 μL) and probe (1 μL) using an ABI-7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.), with the following cycle conditions: 50 °C at 2 min, 95 °C at 10 min, 95 °C at 15 s, and 60 °C at 1 min, followed by 40 equal cycles. The relative quantification method 2−ΔΔCt was used to calculate the relative changes in gene expression [34].
2.3.4. Protein Extraction
The Huh7 HCV replicon cells (~2 × 105 cells/well) were seeded onto 6-well plates, cultured for 24 h, and treated with RPE, later cultivated for 24 h, and treated with a concentration of extracts of 2000, 3000, 4000, 5000 ppm at times of 24, 48, and 72 h. Subsequently, extraction of these proteins was performed for each point in time. They were washed twice with 1 mL of ice-cold 1× PBS/0.5 M EDTA, scraped with a sterile plastic scraper, and the proteins were extracted with 1× lysis buffer containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 2 mM MgCl2, 1% Triton X-100, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 3 µg/mL aprotinin, 1 µg/mL leupeptin, and 1 µg/mL pepstatin. Cell lysates were centrifuged for 5 min at 13,000 rpm at 4 °C to obtain the total proteins in the supernatant, which was recovered, and the protein concentration was measured using the Bradford method with a Bio-Rad Protein Assay kit (500-0006; Bio-Rad Laboratories, Inc., Hercules, CA, USA). A standard curve was obtained using bovine serum albumin (Amresco LLC, Solon, OH, USA). The proteins were stored in deep freeze until use [33]. HCV NS3 protein levels in Huh7 HCV replicon cells treated with polyphenols from rambutan peel extract. Huh7 HCV replicon cells (~2 × 105 cells) were incubated in the presence and absence of concentrations of 2000, 3000, 4000, and 5000 ppm of extract for different times (0, 24, 48, and 72 h).
2.3.5. Western Blot Analysis
Total cellular protein extracts were analyzed and conducted using electrophoresis in a polyacrylamide gel at a concentration of 12%. Afterward, the samples were transferred to Hybond-P vinylidene Polyfluoride (PVDF) membranes. Electro-transference was performed at 4 °C with 120 V for 2 h, then the membrane was blocked with 5% fat-free milk for one hour, then the membrane was incubated overnight with nonstructural protein 3 (NS3) monoclonal antibody (MAb; 1:1000; Biodesign-International, Saco, ME, USA) and anti-actin MAb (β-actin 1:1000; MP-Biomedicals, Aurora, OH, USA). A chemiluminescence assay (Santa Cruz Biotechnology, Dallas, TX, USA) was performed to detect immunocomplexes in the membranes. With the ImageJ program (version 1.51), the NS3 protein expression was quantified together with the actin protein [35].
3. Results
3.1. Mexican Rambutan Peel Extract
The hybrid technology allowed us to recover a total yield of 7.6 g from the 62.5 g of rambutan peel, translated into a percentage. A total yield of 12.16% was obtained from the extraction method employed.
The extraction method allowed us to obtain a raw extract and a semi purified phenolic extract, which both were analyzed in search of polyphenolic content; HCl-Butanol and Folin–Ciocalteu methodology was employed to quantify the polyphenols present in both extracts. The results can be seen in Table 1.

Table 1.
Polyphenol content in the raw and purified extract.
The HPLC analysis revealed the presence of ellagitannins and a derivative, which are part of the hydrolyzable polyphenolic family. Figure 1 exhibits the results of the partial purification with Amberlite XAD-16. The main compounds found in this extract can be seen in Table 2 and the chromatogram in Figure 1, geraniin being the most abundant, followed by corilagin and ellagic acid (Figure 2 shows the chemical structures found in the extraction).
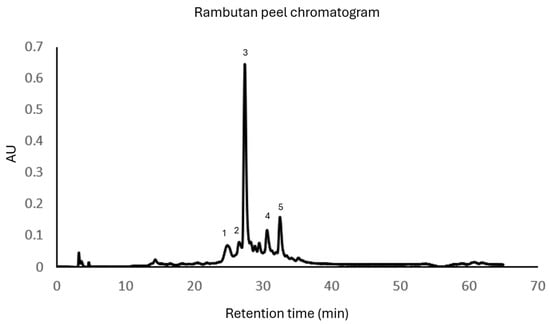
Figure 1.
Chromatogram exhibiting the main compounds found in the rambutan peel extract: (1) dimmers of tergallagic-O-hexoside, (2) Corilagin, (3) Geraniin, (4) Ellagic Acid pentoside, (5) Ellagic Acid. The chemical structures represent (1) Corilagin, (2) Geraniin, and (3) Ellagic Acid.

Table 2.
Identified compounds with retention time, mass, and the group they belong to.
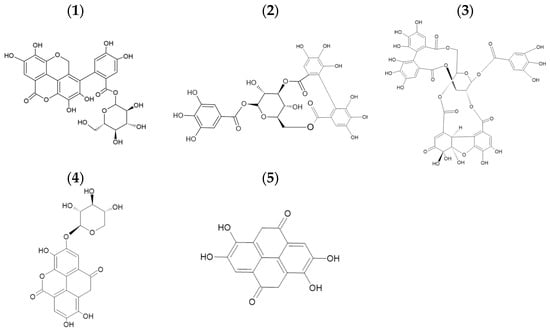
Figure 2.
Chemical structures of the compounds found in the extraction: (1) Dimmers of tergallagic-O-hexoside, (2) Corilagin, (3) Geraniin, (4) Ellagic Acid pentoside, (5) Ellagic Acid.
3.2. Antiproliferative Activity MTT Assay with Rambutan Peel Extracts
Concentrations between 4000 and 5000 ppm of rambutan peel extract were able to decrease the viability of Huh7 parental and Huh7 HCV replicon cells at 72 h (Figure 3). Parental cells were the most affected by the treatment of rambutan polyphenols, resulting in a decrease in cell viability to values of 31.65 ± 3.01% at a concentration of 4000 ppm and 23.19 ± 1.35% at a concentration of 5000 ppm. Rambutan’s peel extract effect on Huh7 HCV replicon cells was lower with cell viability values of 61.52 ± 5.04% at concentrations of 4000 ppm and 68.37 ± 3.39%.
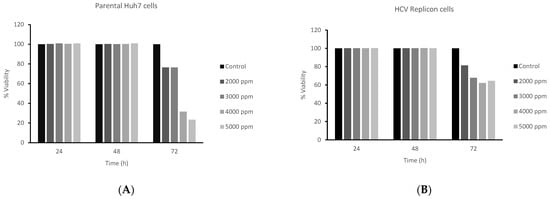
Figure 3.
Percentage of cell viability with RPE treatment. Effects of RPE on the growth of (A) Huh7 parental and (B) Huh7 HCV replicon cells. Both cell lines were treated with different concentrations of RPE (2000–5000 ppm; 2 × 104 cells/well) and were incubated for 24–72 h. Cells were assessed using an MTT assay.
3.3. Antiviral Effect of Rambutan Peel Extract
3.3.1. Effect of Rambutan Peel Extract on HCV Replication
The effect of rambutan peel extract (ellagitannins) on HCV-RNA expression was evaluated using RT-qPCR on HCV Huh7 replicon cells that were treated with RPE or that were untreated (Figure 4). The treated cells were incubated with 0, 1000, and 2000 ppm rambutan peel extract (ellagitannins) at different times of 24, 48, and 72 h. Subsequently, quantification of cells using RT-qPCR showed that RPE inhibits the expression of HCV-RNA at a concentration of 2000 ppm.
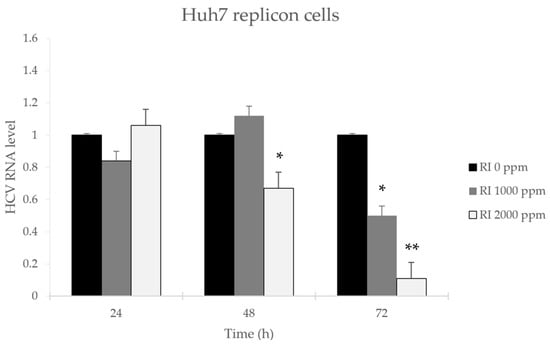
Figure 4.
HCV-RNA expression was measured using RT-qPCR (2−ΔΔCt). Huh7 HCV replicon cells (~2 × 105) were treated with 1000 or 2000 ppm RPE or without RPE for 24–72 h. Total RNA was extracted at each time point and HCV-RNA levels quantified, * p < 0.05 vs. control ** p < 0.01 vs. control.
3.3.2. HCV-NS3 Protein Levels Are Downregulated by Polyphenols from Rambutan Peel Extract in Huh7 HCV Replicon Cells
The RPE compounds reduced NS3 protein expression in a dose-dependent manner, mainly from 48 and 72 h, with concentrations from 2000 ppm to 5000 ppm compared to the untreated control value. The polyphenols of RPE tested inhibited the expression of NS3 protein by 94.67, 94.92, 98.3%, and almost 99% in concentrations of 2000, 3000, 4000, and 5000 ppm, respectively, as shown in Figure 5A.
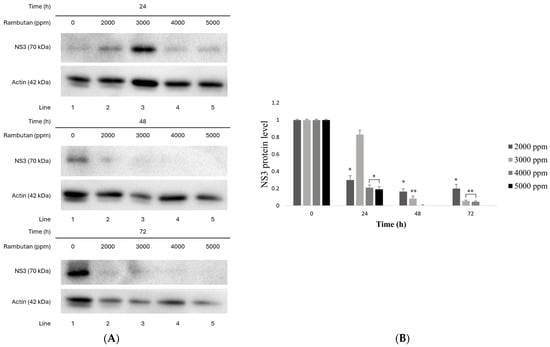
Figure 5.
Huh7 HCV replicon cells (~2 × 105) were treated with 2000–5000 ppm extract for 0, 24, 48, and 72 h. (A) Cell lysates (50 µg) were analyzed using Western blot for NS3 and actin levels. (B) Quantified NS3/actin ratios from immunoblot. * p < 0.05 vs. control ** p < 0.01 vs. control.
The results from the densitometric analysis of the Western blot are shown in Figure 5B. The highest inhibition of protein expression was obtained at 48 and 72 h with 4000 and 5000 ppm, as shown in Figure 5B. In addition, from 24 h, with concentrations of 2000, 4000, and 5000 ppm, a decrease in the expression of the NS3 protein is observed, which continues in a time-dependent manner until 72 h, when the protein expression of NS3 decreases by up to 90%, compared to the untreated control (p < 0.05).
4. Discussion
The use of emerging technologies has facilitated the recovery of various bioactive compounds from different agro-industrial wastes, such as bagasse, seeds, and peels of certain food products. Specifically, ultrasound–microwave hybridization has been successfully applied in the recovery of bioactive compounds from matrices other than rambutan, including mango, red corn, seeds from various oilseeds, and pomegranate. Previous studies have shown that these matrices also yield significant amounts of hydrolyzable and condensed polyphenols [36,37,38,39].
The advantage of these extraction methods, compared to conventional techniques such as maceration, Soxhlet extraction, percolation, and hydro distillation, is that ultrasound generates cavitation bubbles which aid in releasing secondary metabolites into the aqueous medium by causing the sudden rupture of plant cell walls. This method, when combined with microwaves that induce water molecule vibration, enhances the release of secondary metabolites within the plant cells, resulting in higher yields compared to traditional extraction methods [13,40,41].
The use of conventional technologies for extracting polyphenolic compounds from rambutan peel has yielded varied results depending on the solvents employed. For example, in the study by Phuong et al. (2020) [42], maceration with methanol, ethanol, and water resulted in yields of 17.11, 12.25, and 9.17 mg GAE/g, respectively. Conventional extraction methods have also achieved yields like those found in this study, where Phuong et al. (2020) [43] obtained 310 mg GAE/g; however, this was performed using 80% ethanol solutions, which are highly polluting and toxic to human health. Emerging extraction methods have shown promising results in avoiding the use of harmful solvents while effectively recovering high-value bioactive compounds that are of interest to the food and pharmaceutical industries.
The compounds found in rambutan’s peel such as geraniin, corilagin, and ellagic acid, possess a great deal of biological activities that allow for this extract to possess antiviral and antiproliferative activities. Geraniin was used previously in a study by Xu et al., 2020 [44], who determined that, at a concentration of 10 µM, it was able to reduce cell viability in 54.3% to inhibit bladder cancer cells. Geraniin has also been found to possess antiviral capacities, as it can completely suppress the expression of the DENV-2 E gene for dengue infection in a dose of 26.30 µM [45]. Ellagic acid can possess antiproliferative activity, as a previous study has demonstrated that, at different concentrations, it can inhibit cell proliferation in PANC-1, AsPC-1, and MIA PaCA-2 pancreatic cell lines [46]. Antiviral activity present in ellagic acid is capable of inhibiting NS3/4A protease and NS3 helicase in vitro for hepatitis C virus with an IC50 value of 40.37 ± 5.47 nM and 6.58 ± 0.99 µM [47].
Hepatitis C virus is a parenterally transmitted virus associated with the unsafe use of needles and high-risk sexual behavior. HCV evades innate and adaptive immunity and can lead to chronic infection in 70% of the cases of infection. When HCV is untreated, it can lead to hepatocellular carcinoma. Antivirals target NS3 protease, which is needed to process the viral polyprotein and the viral RNA-dependent polymerase which catalyzes genome replication [48].
Ellagitannins can act as an inhibitor of the NS3 protease needed for HCV to replicate; ellagic acid, which is a derivative of ellagitannins, can also block the replication of HCV in different stages of the life of the virus [49]. An study on ellagic acid antiviral activity on HCV determined that pure compounds extracted from natural sources such as pomegranate blocked the HCV/NS3/4A protease activity in vitro; furthermore, the structural analysis of this molecule identified that ligand molecules can interact with the catalytic and substrate-binding residues of NS3/4A protease, which leads to the inhibition of this enzyme; this study also demonstrated that up to 5000 mg/kg are safe for consumption in the diet of the mice, and that levels of up to 5000 ppm could be safe for consumption also [50].
The importance of using new compounds as antiviral agents relies on the fact that medicine is becoming more and more expensive each year. The research and development of new antiviral treatments takes more time, as viruses are one of the most adaptable pathogens that plage humankind; antiviral agents that can already be found in plants, etc., and agro-industrial wastes that represent a by-product of the process of certain products such as juices, jams, and canned fruits, already do possess antiviral agents that can be exploited for the greater good. This study emphasizes the use of rambutan peel which, by itself, possesses three compounds that can already be antiviral, geraniin, corilagin, and ellagic acid; ellagic acid has already proven to be an effective treatment for HCV, as one of the most important factors in the cycle of replication of the virus is the NS3 protease which allows for the virus to replicate in host cells. The results of this study suggest that almost all of the activity of the NS3 protease is inhibited by the compounds present in rambutan peel; and, even more so, by preventing the appearance of hepatitis C, hepatocarcinoma can be prevented.
The use of new compounds in the treatment of viral diseases can be overwhelming at first due to all of the work that is needed in the background to finally make a viable treatment. This study marks the starting point of a possible new way to treat patients with HCV; more research on murine trials and even clinical trials are needed in order to set this compound apart as a new way to treat HCV, but just knowing the underlying potential of the compounds analyzed in this study can open up new research possibilities.
5. Conclusions
The use of emerging technologies such as microwaves and ultrasound have significant advantages over conventional technologies; it is currently of vital importance to do everything possible to care for the environment and to grow technologically. Emerging technologies allow us to have the same or better performance than conventional technologies without harming the environment. The compounds recovered through these techniques are also able to provide new alternatives in the treatment of some diseases, as in the case of rambutan husk polyphenols, which have demonstrated their antiviral and antiproliferative effect on hepatitis C virus and huh7 cells, respectively, reducing their cell viability, opening the door to possible new treatments that use the ellagitannins of rambutan husk to obtain benefits.
Author Contributions
Conceptualization, J.A.A.-V.; methodology, C.H.-H., L.E.E.-G., S.A.L.-S. and M.G.-S.; formal analysis, A.M.R.-E., J.M.-C. and C.N.A.; investigation, J.A.A.-V., C.N.A. and A.M.R.-E.; resources, J.M.-C., S.A.L.-S. and A.M.R.-E.; data curation, M.G.-S. and S.A.L.-S.; writing—original draft preparation, C.H.-H. and L.E.E.-G.; writing—review and editing, C.H.-H., L.E.E.-G., S.A.L.-S. and M.G.-S.; supervision, J.A.A.-V., A.M.R.-E. and C.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the National Council of Science, Humanities, and Technology for their support and the National Laboratory BIOBANCO CONAHCYT with site in UAdeC-UANL.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maroun, R.G.; Rajha, H.N.; El Darra, N.; El Kantar, S.; Chacar, S.; Debs, E.; Vorobiev, E.; Louka, N. Emerging technologies for the extraction of polyphenols from natural sources. In Polyphenols: Properties, Recovery, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 265–293. [Google Scholar]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Woodhouse, I.H. Introduction to Microwave Remote Sensing; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-assisted extraction of functional compounds from plants: A Review. BioResources 2023, 18, 6614. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- SIAP. Produccion Agricola. Anuario Estadístico de la Producción Agrícola. 2023. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 2 August 2024).
- INIFAP. ¿Qué es el Rambután? 2022. Available online: https://www.gob.mx/inifap/articulos/que-es-el-rambutan?idiom=es (accessed on 2 August 2024).
- Hernández-Hernández, C.; Aguilar, C.; Rodríguez-Herrera, R.; Flores-Gallegos, A.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J. Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Mahmood, K.; Fazilah, A.; Yang, T.A.; Sulaiman, S.; Kamilah, H. Valorization of rambutan (Nephelium lappaceum) by-products: Food and non-food perspectives. Int. Food Res. J. 2018, 25, 890–902. [Google Scholar]
- Estrada-Gil, L.; Contreras-Esquivel, J.C.; Flores-Gallegos, C.; Zugasti-Cruz, A.; Govea-Salas, M.; Mata-Gómez, M.A.; Rodríguez-Herrera, R.; Ascacio-Valdés, J.A. Recovery of Bioactive Ellagitannins by Ultrasound/Microwave-Assisted Extraction from Mexican Rambutan Peel (Nephelium lappaceum L.). Molecules 2022, 27, 1592. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda-Torre, L.; Torres-León, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Solid-State Fermentation for the Recovery of Phenolic Compounds from Agro-Wastes. Resources 2023, 12, 36. [Google Scholar] [CrossRef]
- De La Rosa-Esteban, K.; Sepúlveda, L.; Chávez-González, M.L.; Torres-León, C.; Estrada-Gil, L.E.; Aguilar, C.N.; Ascacio-Valdés, J.A. Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast. Fermentation 2023, 9, 723. [Google Scholar] [CrossRef]
- Cheng, H.S.; Ton, S.H.; Kadir, K.A. Ellagitannin geraniin: A review of the natural sources, biosynthesis, pharmacokinetics and biological effects. Phytochem. Rev. 2017, 16, 159–193. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sharma, G.; Bag, P.; Roy, C.L.; Sudha, G.; Tandon, H.; Dave, P.; Shukla, A.; et al. A natural small molecule inhibitor corilagin blocks HCV replication and modulates oxidative stress to reduce liver damage. Antivir. Res. 2018, 150, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dong, X.; Wang, B.; Liu, Y.; Li, S. Geraniin Attenuates Lipopolysaccharide-Induced Cognitive Impairment in Mice by Inhibiting Toll-Like Receptor 4 Activation. J. Agric. Food Chem. 2019, 67, 10079–10088. [Google Scholar] [CrossRef] [PubMed]
- Yoganathan, S.; Alagaratnam, A.; Acharekar, N.; Kong, J. Ellagic acid and schisandrins: Natural biaryl polyphenols with therapeutic potential to overcome multidrug resistance in cancer. Cells 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- WHO. Hepatitis C. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 2 August 2024).
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef]
- Aishwarya, V.; Solaipriya, S.; Sivaramakrishnan, V. Role of ellagic acid for the prevention and treatment of liver diseases. Phytother. Res. 2021, 35, 2925–2944. [Google Scholar] [CrossRef]
- Santhi, V.P.; Masilamani, P.; Sriramavaratharajan, V.; Murugan, R.; Gurav, S.S.; Sarasu, V.P.; Parthiban, S.; Ayyanar, M. Therapeutic potential of phytoconstituents of edible fruits in combating emerging viral infections. J. Food Biochem. 2021, 45, e13851. [Google Scholar] [CrossRef]
- Yan, F.; Cheng, D.; Wang, H.; Gao, M.; Zhang, J.; Cheng, H.; Wang, C.; Zhang, H.; Xiong, H. Corilagin Ameliorates Con A-Induced Hepatic Injury by Restricting M1 Macrophage Polarization. Front. Immunol. 2022, 12, 807509. [Google Scholar] [CrossRef]
- Fredsgaard, M.; Kaniki, S.E.K.; Antonopoulou, I.; Chaturvedi, T.; Thomsen, M.H. Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules 2023, 28, 5312. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Aguilar, C.N.; Flores-Gallegos, A.C.; Sepúlveda, L.; Rodríguez-Herrera, R.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J. Preliminary testing of ultrasound/microwave-assisted extraction (U/M-AE) for the isolation of Geraniin from Nephelium lappaceum L. (Mexican Variety) peel. Processes 2020, 8, 572. [Google Scholar] [CrossRef]
- Govea-Salas, M.; Rivas-Estilla, A.M.; Rodríguez-Herrera, R.; Lozano-Sepúlveda, S.A.; Aguilar-Gonzalez, C.N.; Zugasti-Cruz, A.; Salas-Villalobos, T.B.; Morlett-Chávez, J.A. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp. Ther. Med. 2015, 11, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.K.; Nandakumar, N.; Devasagayam, T.P.A. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line, and possible mechanisms. J. Clin. Biochem. Nutr. 2010, 48, 85–90. [Google Scholar] [CrossRef]
- Mitra, I.; Mukherjee, S.; Reddy, B.V.P.; Dasgupta, S.; Bose, K.J.C.; Mukherjee, S.; Linert, W.; Moi, S.C. Benzimidazole based Pt(II) complexes with better normal cell viability than cisplatin: Synthesis, substitution behavior, cytotoxicity, DNA binding and DFT study. RSC Adv. 2016, 6, 76600–76613. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Tchounwou, P.B. In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoresis (Comet) assays. Mol. Cell. Biochem. 2007, 301, 123–130. [Google Scholar] [CrossRef]
- Rios-Ibarra, C.P.; Verduzco-Garza, B.; Ortiz-Lopez, R.; Grondin, Y.; Salinas-Santander, M.; Arvizu-Gutierrez, L.A.; Sanchez-Salazar, M.G.; Cervantes-Astorga, E.; Orozco-Nunnelly, D.A.; Rivas-Estilla, A.M. Transcriptional Profile of HCV Replicon Cells after Treatment with Acetylsalicylic Acid. Ann. Clin. Lab. Sci. 2022, 52, 222–229. [Google Scholar]
- Kopff, M.; Kopff, A.; Kowalczyk, E. The effect of nonsteroidal anti-inflammatory drugs on oxidative/antioxidative balance. Pol. Merkur. Lek. 2007, 23, 184–187. [Google Scholar]
- Lee, H.-K.; Yoon, H.; Jang, K.L. All-trans retinoic acid inhibits HCV replication by downregulating core levels via E6AP-mediated proteasomal degradation. Biochem. Biophys. Res. Commun. 2022, 594, 15–21. [Google Scholar] [CrossRef]
- Arakawa, M.; Tabata, K.; Ishida, K.; Kobayashi, M.; Arai, A.; Ishikawa, T.; Suzuki, R.; Takeuchi, H.; Tripathi, L.P.; Mizuguchi, K.; et al. Flavivirus recruits the valosin-containing protein–NPL4 complex to induce stress granule disassembly for efficient viral genome replication. J. Biol. Chem. 2022, 298, 101597. [Google Scholar] [CrossRef]
- Kullappan, M.; Benedict, B.A.; Rajajagadeesan, A.; Baskaran, P.; Periadurai, N.D.; Ambrose, J.M.; Gandhamaneni, S.H.; Nakkella, A.K.; Agarwal, A.; Veeraraghavan, V.P.; et al. Ellagic Acid as a Potential Inhibitor against the Nonstructural Protein NS3 Helicase of Zika Virus: A Molecular Modelling Study. BioMed Res. Int. 2022, 2022, 2044577. [Google Scholar] [CrossRef]
- García-Ortíz, J.; Ascacio-Valdés, J.; Nery-Flores, S.; Sáenz-Galindo, A.; Flores-Gallegos, A.; Rodríguez-Herrera, R. Microwave-ultrasound hybrid technology assisted extraction of pigments with antioxidant potential from red corn. Appl. Food Res. 2023, 3, 100350. [Google Scholar] [CrossRef]
- González-González, G.M.; Esparza-González, S.C.; Nery-Flores, S.D.; Morlett-Chávez, J.A.; Ascacio-Valdés, J.A.; Flores-Gallegos, A.C.; Saenz-Galindo, A.; Rodríguez-Herrera, R. Anticancer activity of polyphenolic Punica granatum peel extracts obtained by hybrid ultrasound-microwave assisted extraction: Evaluation on HeLa and HepG2 cells. Environ. Qual. Manag. 2024, 33, 295–304. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Serna-Cock, L.; Belmares-Cerda, R.; Aguilar, C.N. Extraction of antioxidants from mango seed kernel: Optimization assisted by microwave. Food Bioprod. Process. 2017, 105, 188–196. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.-K.; Lee, Y.Y. A review on application of ultrasound and ultrasound assisted technology for seed oil extraction. J. Food Sci. Technol. 2023, 60, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Martina, K.; Tagliapietra, S.; Barge, A.; Cravotto, G. Combined Microwaves/Ultrasound, a Hybrid Technology. Top. Curr. Chem. 2016, 374, 175–201. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.; Fabiano-Tixier, A.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le, T.T.; Dang, M.Q.; Van Camp, J.; Raes, K. Selection of Extraction Conditions of Phenolic Compounds from Rambutan (Nephelium lappaceum L.) Peel. Food Bioprod. Process. 2020, 122, 222–229. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le, T.T.; Van Camp, J.; Raes, K. Evaluation of Antimicrobial Activity of Rambutan (Nephelium lappaceum L.) Peel Extracts. Int. J. Food Microbiol. 2020, 321, 108539. [Google Scholar] [CrossRef]
- Xu, J.; Qin, N.; Yao, Y.; Chen, T.; Jiang, W. Geraniin inhibits bladder cancer cell growth via regulation of PI3K/AKT signaling pathways. Trop. J. Pharm. Res. 2020, 19, 253–257. [Google Scholar] [CrossRef]
- Ahmad, S.A.A.; Palanisamy, U.D.; Khoo, J.J.; Dhanoa, A.; Hassan, S.S. Efficacy of geraniin on dengue virus type-2 infected BALB/c mice. Virol. J. 2019, 16, 26. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, Y.J.; Kim, H.-J. Determining the effect of ellagic acid on the proliferation and migration of pancreatic cancer cell lines. Transl. Cancer Res. 2021, 10, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Othman, R.; Yusof, R.; Heh, C.H. Rational drug discovery: Ellagic acid as a potent dual-target inhibitor against hepatitis C virus genotype 3 (HCV G3) NS3 enzymes. Chem. Biol. Drug Des. 2021, 97, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, T.; Brown, R.J. Hepatitis C Virus. Trends Microbiol. 2019, 27, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.J. Preventing Future Emerging Viral Infections Using broad-spectrum Antivirals. Future Virol. 2018, 13, 229–232. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sudha, G.; Srinivasan, N.; Das, S. Small molecule inhibitors of HCV replication from Pomegranate. Sci. Rep. 2014, 4, 5411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).