Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Chlorella sp. Cultivation and Experimental Design
2.2. Cultivation of Chlorella sp. with 98% CO2 (v/v) Injection and Simulated Gas at Concentrations of 15% CO2, 73% N2, and 12% O2
3. Results and Discussion
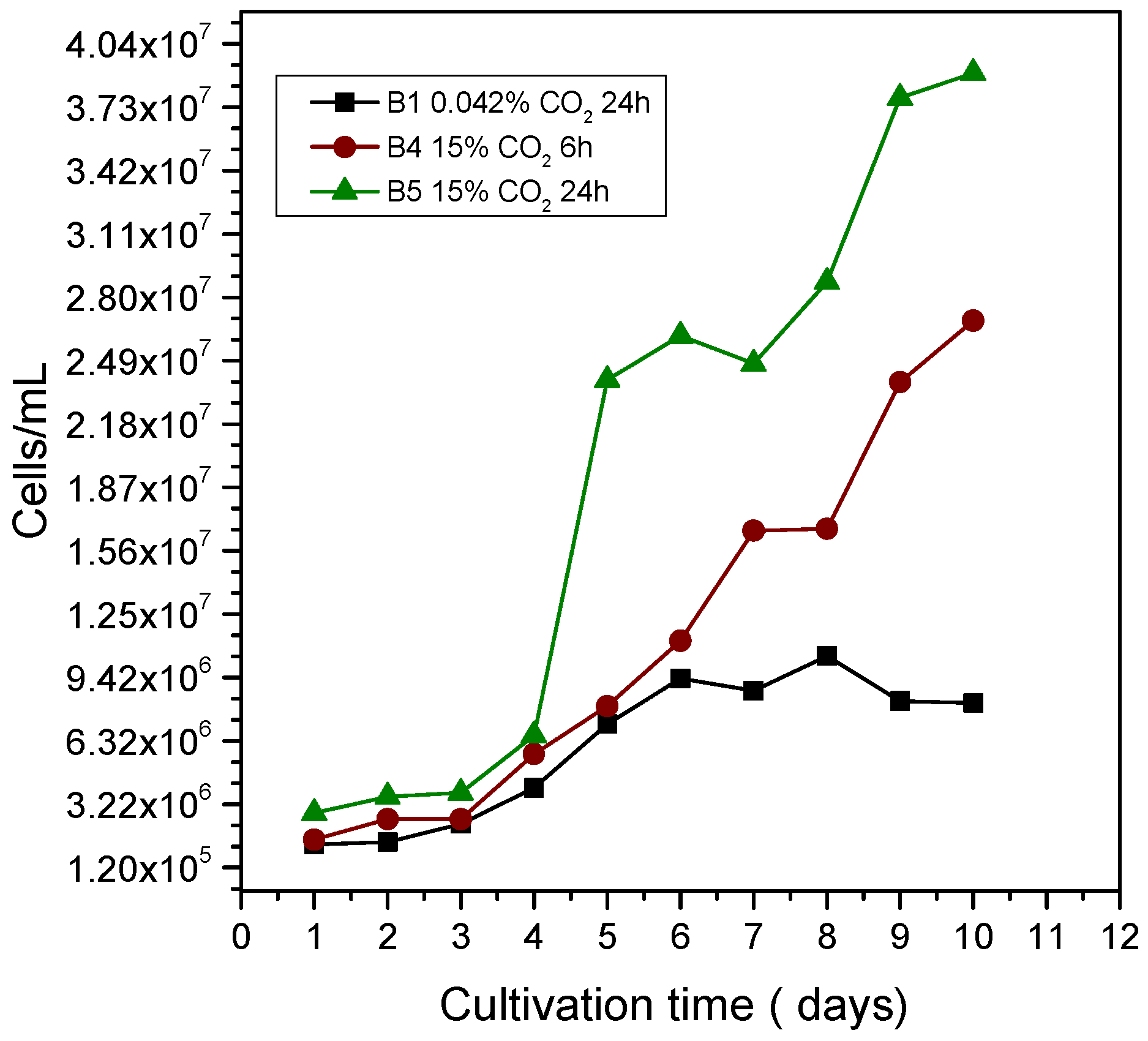
3.1. Growth Curve of Chlorella sp. and pH Control Parameters When Adding 98% CO2
3.2. Growth Curve of Chlorella sp. and pH Control Parameters When Adding Simulated CO2 at Concentrations of 15% CO2, 73% N2, and 12% O2
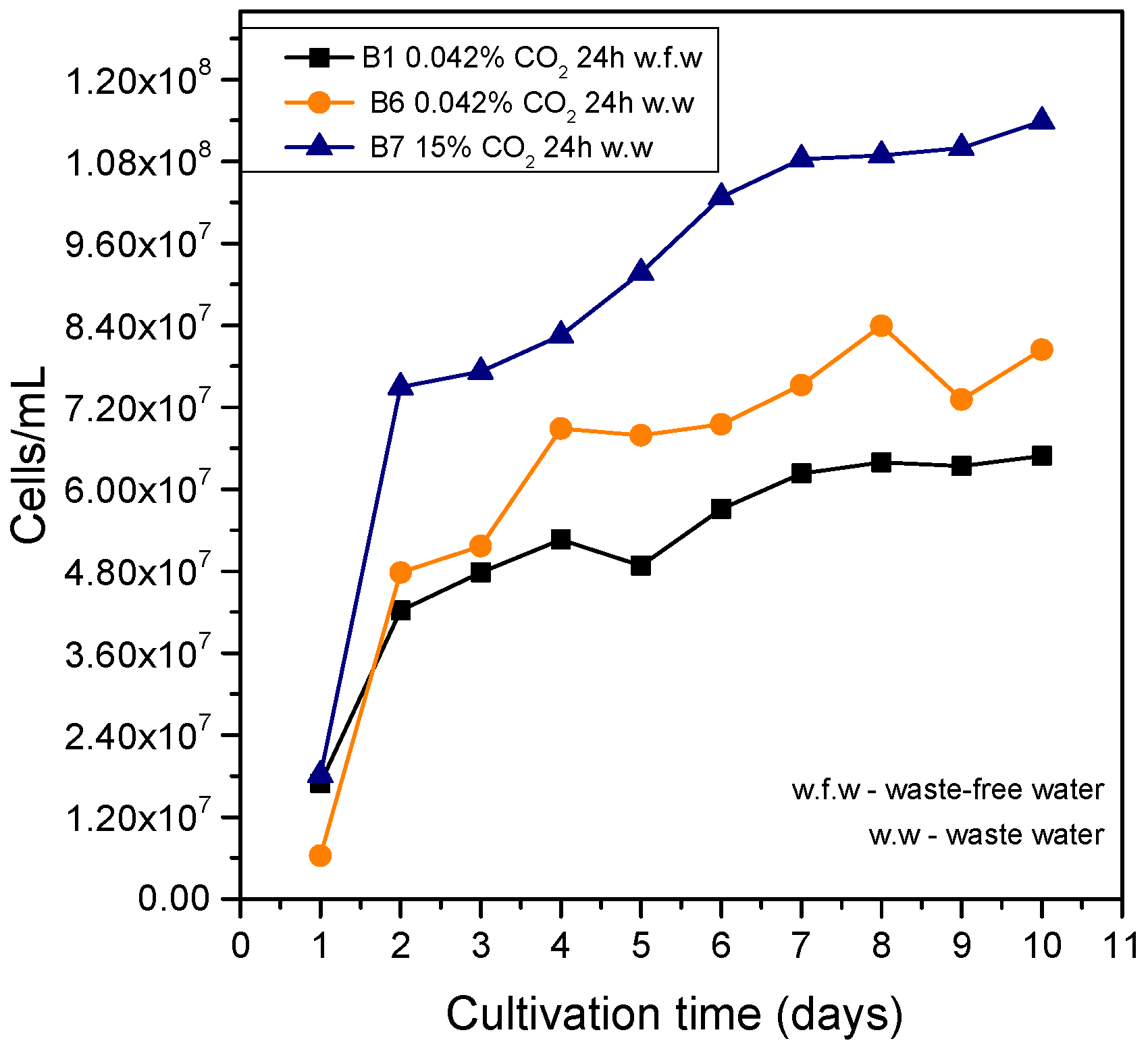
3.3. Use of Wastewater in the Cultivation of Chlorella sp.
3.4. Production and CO2 Fixation Rate
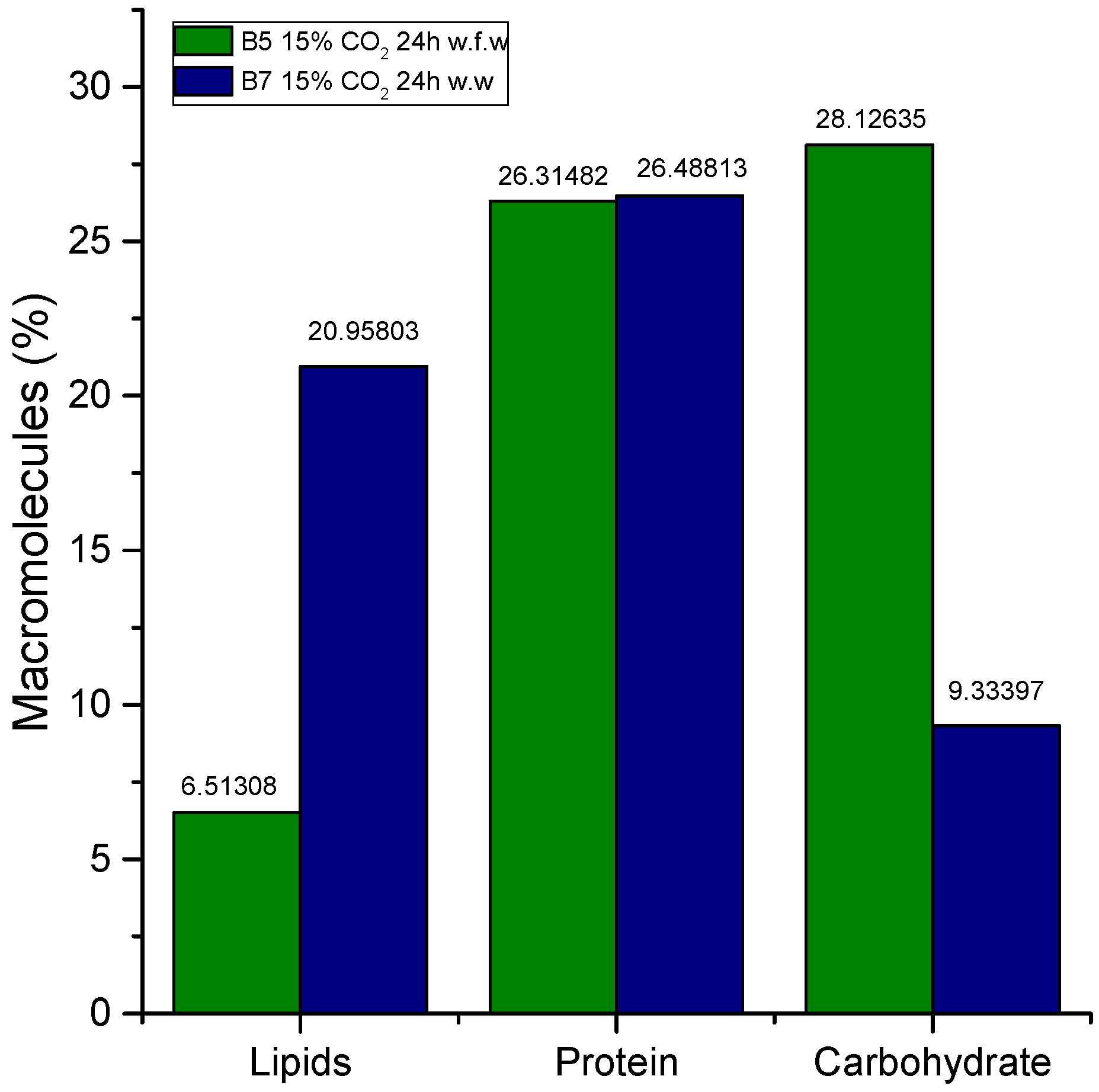
3.5. Biochemical Composition (Carbohydrates, Proteins, and Lipids)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Lin, G.; Zeng, J.; Yang, Z.; Wang, L. Construction of algal-bacterial consortia using green microalgae Chlorella vulgaris and As(III)-oxidizing bacteria: As tolerance and metabolomic profiling. J. Environ. Sci. 2024, 139, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Larki, I.; Zahedi, A.; Asadi, M.; Forootan, M.M.; Farajollahi, M.; Ahmadi, R.; Ahmadi, A. Mitigation approaches and techniques for combustion power plants flue gas emissions: A comprehensive review. Sci. Total Environ. 2023, 903, 166108. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Ho, S.H. Microalgae as a solution of third world energy crisis for biofuels production from wastewater toward carbon neutrality: An updated review. Chemosphere 2022, 291, 132863. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yuan, H.; Yahong, W.; Xu, Q. Pathways to carbon neutrality in G7 economies: The role of technology-innovation and R&D in reducing CO₂ emissions. Gondwana Res. 2024, 128, 55–68. [Google Scholar] [CrossRef]
- Xu, P.; Shao, S.; Qian, J.; Li, J.; Xu, R.; Liu, J.; Zhou, W. Scale-up of microalgal systems for decarbonization and bioproducts: Challenges and opportunities. Bioresour. Technol. 2024, 398, 130528. [Google Scholar] [CrossRef] [PubMed]
- Aditya, L.; Vu, H.P.; Johir, M.A.H.; Mahlia, T.M.I.; Silitonga, A.S.; Zhang, X.; Liu, Q.; Tra, V.T.; Ngo, H.H.; Nghiem, L.D. Role of culture solution pH in balancing CO₂ input and light intensity for maximising microalgae growth rate. Chemosphere 2023, 343, 140255. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Tsiplakou, E.; Zerva, A.; Pantiora, P.D.; Georgakis, N.D.; Tsintzou, G.P.; Madesis, P.; Labrou, N.E. Microalgae as a Sustainable Source of Antioxidants in Animal Nutrition, Health and Livestock Development. Antioxidants 2023, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Farooq, W.; Naqvi, S.R.; Sajid, M.; Shrivastav, A.; Kumar, K. Monitoring lipids profile, CO₂ fixation, and water recyclability for the economic viability of microalgae Chlorella vulgaris cultivation at different initial nitrogen. J. Biotechnol. 2022, 345, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Cruz, Y.R.; Leonett, A.Z.F.; Díaz, G.C.; Carliz, R.G.; Rossa, V.; Galindo, M.; Aranda, D.A.G.; Carliz, R.G. Biofixação de CO2 pela microalga Monoraphidium sp. Acta Sci. Tech. 2018, 6. [Google Scholar]
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 854–858, 1–9. [Google Scholar] [CrossRef]
- Galvani, F.; Gaertner, E. Adequação da Metodologia Kjeldahl para determinação de Nitrogênio Total e Proteína Bruta. Circ. Técnica 2006, 4, 1–9. [Google Scholar]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas. Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, W.M.; Chang, J.S. Scenedesmus obliquus CNW-N as a potential candidate for CO₂ mitigation and biodiesel production. Bioresour. Technol. 2010, 101, 8725–8730. [Google Scholar] [CrossRef] [PubMed]
- Mutaf-Kılıc, T.; Demir, A.; Elibol, M.; Oncel, S.S. Microalgae pigments as a sustainable approach to textile dyeing: A critical review. Algal Res. 2023, 76, 103291. [Google Scholar] [CrossRef]
- Rinaldi, K.L.; Senhorinho, G.N.A.; Laamanen, C.A.; Scott, J.A. A review of extremophilic microalgae: Impacts of experimental cultivation conditions for the production of antimicrobials. Algal Res. 2024, 78, 103427. [Google Scholar] [CrossRef]
- Adamczyk, M.; Lasek, J.; Skawińska, A. CO2 Biofixation and Growth Kinetics of Chlorella vulgaris and Nannochloropsis gaditana. Appl. Biochem. Biotechnol. 2016, 179, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.R.; Palmieri, M.; Altieri, S.; Ciniglia, C.; Lubritto, C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. Int. J. Environ. Res. Public Health 2021, 18, 2291. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Subashchandrabose, S.R.; Panneerselvan, L.; Venkateswarlu, K.; Megharaj, M. Potential of acid-tolerant microalgae, Desmodesmus sp. MAS1 and Heterochlorella sp. MAS3, in heavy metal removal and biodiesel production at acidic pH. Bioresour. Technol. 2019, 278, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Oh, Y.-K.; Lee, J.; Chang, Y.K.; Park, W.-K. Development of dual strain microalgae cultivation system for the direct carbon dioxide utilization of power plant flue gas. Bioresour. Technol. 2024, 393, 130051. [Google Scholar] [CrossRef]


| Gas | Calcination Oven Output | Slender Filter Chimney |
|---|---|---|
| O2 (%) | 10.0 | 15.0 |
| CO2 (%) | 15.8 | 9.3 |
| CO (ppm) | 2550 | 1500 |
| NOx (ppm) | 65 | 38 |
| SO2 (ppm) | <20 | <20 |
| SO3 (ppm) | <3.6 | <2.1 |
| H2O (%) | 4.8 | 2.9 |
| N2 (%) | 73.4 | 75.7 |
| Particulate Material (mg/Nm3) | 60 | 35 |
| Temperature (°C) | 349 | - |
| Solution | Concentration | Used Amount |
|---|---|---|
| Solution 1 (sodium nitrate) | 300 g/L | 5 mL/L |
| Solution 2 (potassium phosphate) | 80 g/L | 5 mL/L |
| Solution 3 (magnesium sulfate) | 15 g/L | 5 mL/L |
| Modified Solution 4 (calcium chloride) | 7.2 g/L | 5 mL/L |
| Solution 5 (citric acid) | 1.2 g/L | 5 mL/L |
| Modified Solution 6 (ammonium ferric sulfate) | 1.2 g/L | 5 mL/L |
| Solution 7 (EDTA) | 0.2 g/L | 5 mL/L |
| Solution 8 (sodium bicarbonate) | 40 g/L | 5 mL/L |
| Solution 9 (trace metals) | 4.849 g/L | 1 mL/L |
| Boric acid | 2.8 g/L | |
| Manganese chloride | 1.8 g/L | |
| Zinc sulfate | 0.22 g/L | |
| Sodium molybdate | 0.021 g/L | |
| Copper sulfate | 0.008 g/L |
| Sample | Medium | Aeration Rate (vvm) | CO2 Concentration (%) | Aeration Time (CO2/h) |
|---|---|---|---|---|
| B1 Control | Well water | Air | 0.042 | 24 |
| B2 | Well water | 0.03 | 98 | 0.5 |
| B3 | Well water | 0.03 | 98 | 1 |
| B4 | Well water | 0.01 | 15 | 6 |
| B5 | Well water | 0.01 | 15 | 24 |
| B6 | Wastewater | Air | 0.048 | 24 |
| B7 | Wastewater | 0.01 | 15 | 24 |
| Treatment | %C | Production (g/L·d) | Fixation Rate (g/L·d) | %CO2 Fixed in Biomass (Simplified Method) [17] | %CO2 Fixed in Biomass (Advanced Method) [17] |
|---|---|---|---|---|---|
| Treatment B6 | 25.04 | 0.26 | 0.23 | --- | --- |
| Treatment B7 | 30.64 | 0.81 | 0.91 | 27 | 17.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, T.J.T.; Calixto, G.Q.; Câmara, F.R.d.A.; Teixeira, D.I.A.; Braga, R.M.; Pergher, S.B.C. Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential. Sustain. Chem. 2025, 6, 11. https://doi.org/10.3390/suschem6020011
Cruz TJT, Calixto GQ, Câmara FRdA, Teixeira DIA, Braga RM, Pergher SBC. Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential. Sustainable Chemistry. 2025; 6(2):11. https://doi.org/10.3390/suschem6020011
Chicago/Turabian StyleCruz, Thiago J. T., Guilherme Q. Calixto, Fabiana R. de A. Câmara, Dárlio I. A. Teixeira, Renata M. Braga, and Sibele B. C. Pergher. 2025. "Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential" Sustainable Chemistry 6, no. 2: 11. https://doi.org/10.3390/suschem6020011
APA StyleCruz, T. J. T., Calixto, G. Q., Câmara, F. R. d. A., Teixeira, D. I. A., Braga, R. M., & Pergher, S. B. C. (2025). Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential. Sustainable Chemistry, 6(2), 11. https://doi.org/10.3390/suschem6020011








