Marine Mycosilver Nanoparticles: Screening, Evaluation of Their Antimicrobial Properties, and Synthesis Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Marine-Derived Fungal Strains
2.2. Screening
2.3. Myconanoparticles’ Characterization
2.4. Antimicrobial Activity
2.4.1. Bacteria
2.4.2. Fungi
3. Results
3.1. Marine-Derived Fungi Screening and Silver Nanoparticles’ Characterization
3.2. Antimicrobial Activity of Mycogenic Silver Nanoparticles
3.3. Optimization of Mycogenic Silver Nanoparticles’ Synthesis
3.3.1. AgNO3 Concentration
3.3.2. Fungal Biomass Concentration
3.3.3. Agitation
3.3.4. Temperature
3.3.5. pH
4. Discussion
4.1. Marine-Derived Fungi as a New Resource for Silver Nanoparticles’ Synthesis and Their Antimicrobial Activity
4.2. Mycogenic Synthesis Optimization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green synthesis of silver nanoparticles: A comprehensive review of methods, influencing factors, and applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Simões, M.F.; Ottoni, C.A.; Antunes, A. Mycogenic metal nanoparticles for the treatment of mycobacterioses. Antibiotics 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed]
- Bellingeri, A.; Bono, N.; Venditti, I.; Bertelà, F.; Burrati, L.; Faleri, C.; Protano, G.; Paccagnini, E.; Lupetti, P.; Candiani, G.; et al. Capping drives the behavior, dissolution and (eco)toxicity of silver nanoparticles towards microorganisms and mammalian cells. Environ. Sci. Nano 2024, 11, 2049–2060. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nooshkam, M.; Zargar, M.; Garavand, F.; Ghosh, S.; Hadidi, M.; Forough, M. Green synthesis of nanomaterials for smart biopolymer packaging: Challenges and outlooks. J. Nanostruct Chem. 2024, 14, 113–136. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Danagoudar, A.; Pratap, G.K.; Shantaram, M.; Ghosh, K.; Kanade, S.R.; Joshi, C.G. Characterization, cytotoxic and antioxidant potential of silver nanoparticles biosynthesised using endophytic fungus (Penicillium citrinum CGJ-C1). Mater. Today Commun. 2020, 25, 101385. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Amaral, M.V.M.V.; Carraro, C.B.; Antoniêto, A.C.C.; Costa, M.N.; Fraga-Silva, T.F.C.; Cipriano, U.G.; Abuná, R.P.F.; Rodrigues, T.S.; Martins, R.B.; Luzenti, A.M.; et al. Biogenic silver nanoparticles produced by Trichoderma reesei inhibit SARS-CoV-2 infection, reduce lung viral load and ameliorate acute pulmonary inflammation. Curr. Res. Biotechnol. 2025, 9, 100277. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Alam, M.J.; Park, S.; Alam, M. Biosynthesis of silver nanoparticles and its application against phytopathogenic bacterium and fungus. Int. J. Environ. Anal. Chem. 2020, 12, 1390–1401. [Google Scholar] [CrossRef]
- Ribeiro, L.G.; Rezende, K.F.O.; Barbieri, E.; de Souza, A.O. Study of routine metabolism and acute toxicity of mycogenic silver nanoparticles on Palaemon pandaliformis (shrimp). Environ. Sci. Nano 2023, 10, 1715–1729. [Google Scholar] [CrossRef]
- Mistry, H.; Thakor, R.; Patil, C.; Trivedi, J.; Bariya, H. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnol. Lett. 2021, 43, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Osorio-Echavarría, J.; Ossa-Orozco, C.P. Synthesis of silver nanoparticles using white-rot fungus anamorphous Bjerkandera sp. R1: Influence of silver nitrate concentration and fungus growth time. Sci. Rep. 2021, 11, 3842. [Google Scholar] [CrossRef]
- Al-Limoun, M.; Qaralleh, H.N.; Khleifat, K.M.; Al-Anber, M.; Al-Tarawneh, A.; Al-sharafa, K.; Kailani, M.H.; Zaitoun, M.A.; Matar, S.A.; Al-soub, T. Culture media composition and reduction potential optimization of mycelia-free filtrate for the biosynthesis of silver nanoparticles using the fungus Tritirachium oryzae W5H. Curr. Nanosci. 2020, 16, 757–769. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef]
- Rajput, S.; Werezuk, R.; Lange, R.M.; McDermott, M.T. Fungal isolate optimized for biogenesis of silver nanoparticles with enhanced colloidal stability. Langmuir 2016, 32, 8688–8697. [Google Scholar] [CrossRef]
- Assis da Silva, C.; Ribeiro, B.M.; Trotta, C.V.; Perina, F.C.; Martins, R.; Abessa, D.M.S.; Barbieri, E.; Simões, M.F.; Ottoni, C.A. Effects of mycogenic silver nanoparticles on organisms of different trophic levels. Chemosphere 2022, 308, 136540. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Ramos, C.E.D.; de Souza, R.F.B.; da Silva, S.G.; Spinace, E.V.; Neto, A.O. Glycerol and ethanol oxidation in alkaline medium using PtCu/C electrocatalysts. Int. J. Electrochem. Sci. 2018, 13, 1893–1904. [Google Scholar] [CrossRef]
- Chavez-Esquivel, G.; Cervantes-Cuevas, H.; Ybieta-Olvera, L.F.; Castañeda Briones, M.T.; Acosta, D.; Cabello, J. Antimicrobial activity of graphite oxide doped with silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by agar well diffusion test: Synthesis and characterization. Mater. Sci. Eng. C 2021, 123, 111934. [Google Scholar] [CrossRef] [PubMed]
- Zwar, I.P.; Trotta, C.V.; Ziotti, A.B.S.; Neto, M.L.; Araújo, W.L.; Melo, I.S.; Ottoni, C.A.; Souza, A.O. Biosynthesis of silver nanoparticles using actinomycetes, phytotoxicity on rice seeds, and potential application in the biocontrol of phytopathogens. J. Basic Microbiol. 2023, 63, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ding, B.; Zhang, M.; Dong, T.; Fu, Y.; Lv, Q.; Ding, W.; Wang, X. Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus flavus and Aspergillus fumigatus. Carbohydr. Polym. 2022, 275, 118673. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.; Souza, E.J.D.; Radünz, M.; Gandra, E.A.; Zavareze, E.R.; Dias, A.R.G. Suitability of starch/carvacrol nanofibers as biopreservatives for minimizing the fungal spoilage of bread. Carbohydr. Polym. 2021, 252, 117166. [Google Scholar] [CrossRef]
- Aguiar, A.P.; Ottoni, C.A.; Aquaroli, C.L.R.; Mendes, E.C.V.; Araújo, A.L.S.; Simões, M.F.; Barbieri, E. Mycogenic silver nanoparticles from Penicillium citrinum IB-CLP11-their antimicrobial activity and potential toxicity effects on freshwater organisms. Environ. Sci. Nano 2024, 11, 2229–2238. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison-Wesley: Boston, MA, USA, 1967; p. 262. [Google Scholar]
- Jiang, J.-P.; Leng, S.; Liao, Y.-F.; Liu, X.; Li, D.-X.; Chu, C.; Yu, X.-Y.; Liu, C.-H. The potential role of subseafloor fungi in driving the biogeochemical cycle of nitrogen under anaerobic conditions. Sci. Total Environ. 2023, 897, 165374. [Google Scholar] [CrossRef]
- Nogueira, O.M.N.; Bernal, S.P.F.; Peres, C.K.; Boroski, M.; Passarini, M.R.Z. Isolation of marine-derived filamentous fungi and their potential application for bioremediation process. Braz. J. Microbiol. 2024, 55, 3403–3412. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNAdhari, S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl. Nanosci. 2023, 13, 623–630. [Google Scholar] [CrossRef]
- Basheer, M.A.; Abutaleb, K.; Abed, N.N.; Mekawey, A.A.I. Mycosynthesis of silver nanoparticles using marine fungi and their antimicrobial activity against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2023, 21, 127. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochae techrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Malik, M.A.; Wani, A.H.; Bhat, M.Y. Biogenic silver nanoparticles from fungal sources: Synthesis, characterization, and antifungal potential. Microb. Pathog. 2024, 193, 106742. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, T.; Cui, X.; Tao, R.; Gao, Z. Antifungal mechanism of nanosilver biosynthesized with Trichoderma longibrachiatum and its potential to control muskmelon Fusarium wilt. Sci. Rep. 2024, 14, 20242. [Google Scholar] [CrossRef] [PubMed]
- Abd Elghaffar, R.Y.; Emam, A.M.; Taher, E.S.; Baz, M.M.; Nayel, H.; Abdeen, A.; El-Nablaway, M.; Alwutayd, K.M.; Mihaela, O.; Ioan, B.-D.; et al. The potential biological activities of Aspergillus luchuensis-aided green synthesis of silver nanoparticles. Front. Microbiol. 2024, 15, 1381302. [Google Scholar] [CrossRef]
- Saxena, J.; Sharma, P.K.; Sharma, M.M.; Sharma, M.M.; Singh, A. Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. SpringerPlus 2016, 5, 861. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Balashanmugam, P.; Mukeshkumar, D.J.; Kalaichelvan, P.T. Mycosynthesis of silver and gold nanoparticles: Optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016, 182, 8–20. [Google Scholar] [CrossRef]
- Cui, X.; Zhong, Z.; Xia, R.; Liu, X.; Qin, L. Biosynthesis Optimization of Silver Nanoparticles (AgNPs) Using Trichoderma longibranchiatum and Biosafety Assessment with Silkworm (Bombyx mori). Arab. J. Chem. 2022, 15, 104142. [Google Scholar] [CrossRef]
- Elshafei, A.M.; Othman, A.M.; Elsayed, M.A.; Al-Balakocy, N.G.; Hassan, M.M. Green synthesis of silver nanoparticles using Aspergillus oryzae NRRL447 exogenous proteins: Optimization via central composite design, characterization and biological applications. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100553. [Google Scholar] [CrossRef]
- Cruz, J.N.; Muzammil, S.; Ashraf, A.; Ijaz, M.U.; Siddique, M.H.; Abbas, R.; Sadia, M.; Saba; Hayat, S.; Lima, R.R. A review on mycogenic metallic nanoparticles and their potential role as antioxidant, antibiofilm and quorum quenching agents. Heliyon 2024, 10, e29500. [Google Scholar] [CrossRef]
- Velu, M.; Lee, J.-H.; Chang, W.-S.; Lovanh, N.; Park, Y.-J.; Jayanthi, P.; Palanivel, V.; Oh, B.-T. Fabrication, optimization, and characterization of noble silver nanoparticles from sugarcane leaf (Saccharum officinarum) extract for antifungal application. 3 Biotech. 2017, 7, 147. [Google Scholar] [CrossRef]




| AgNP | SPR (nm) | DLS (nm) | Pζ (mV) | PDI | TEM (nm) |
|---|---|---|---|---|---|
| IBCLP06 | 417 | 685.90 ± 9.36 | −16.04 ± 0.37 | 0.263 | 723.01 ± 22.34 |
| IBCLP08 | 423 | 746.30 ± 22.03 | −18.02 ± 1.91 | 0.456 | 623.3 ± 11.09 |
| IBCLP11 | 421 | 52.00 ± 10.45 | −41.91 ± 0.09 | 0.207 | 57.50 ± 0.92 |
| IBCLP13 | 407 | 88.44 ± 3.30 | −47.58 ± 0.23 | 0.383 | 82.52 ± 10.89 |
| IBCLP15 | 419 | 62.82 ± 1.27 | −23.19 ± 1.07 | 0.273 | 93.21 ± 20.24 |
| IBCLP16 | 410 | 262.91± 8.77 | −31.70 ± 1.16 | 0.187 | 287.11 ± 18.09 |
| IBCLP17 | 423 | 42.72 ± 8.77 | −39.55 ± 0.92 | 0.273 | 52.37 ± 0.02 |
| IBCLP20 | 420 | 80.50 ± 8.77 | −38.56 ± 0.68 | 0.215 | 66.48 ± 5.31 |
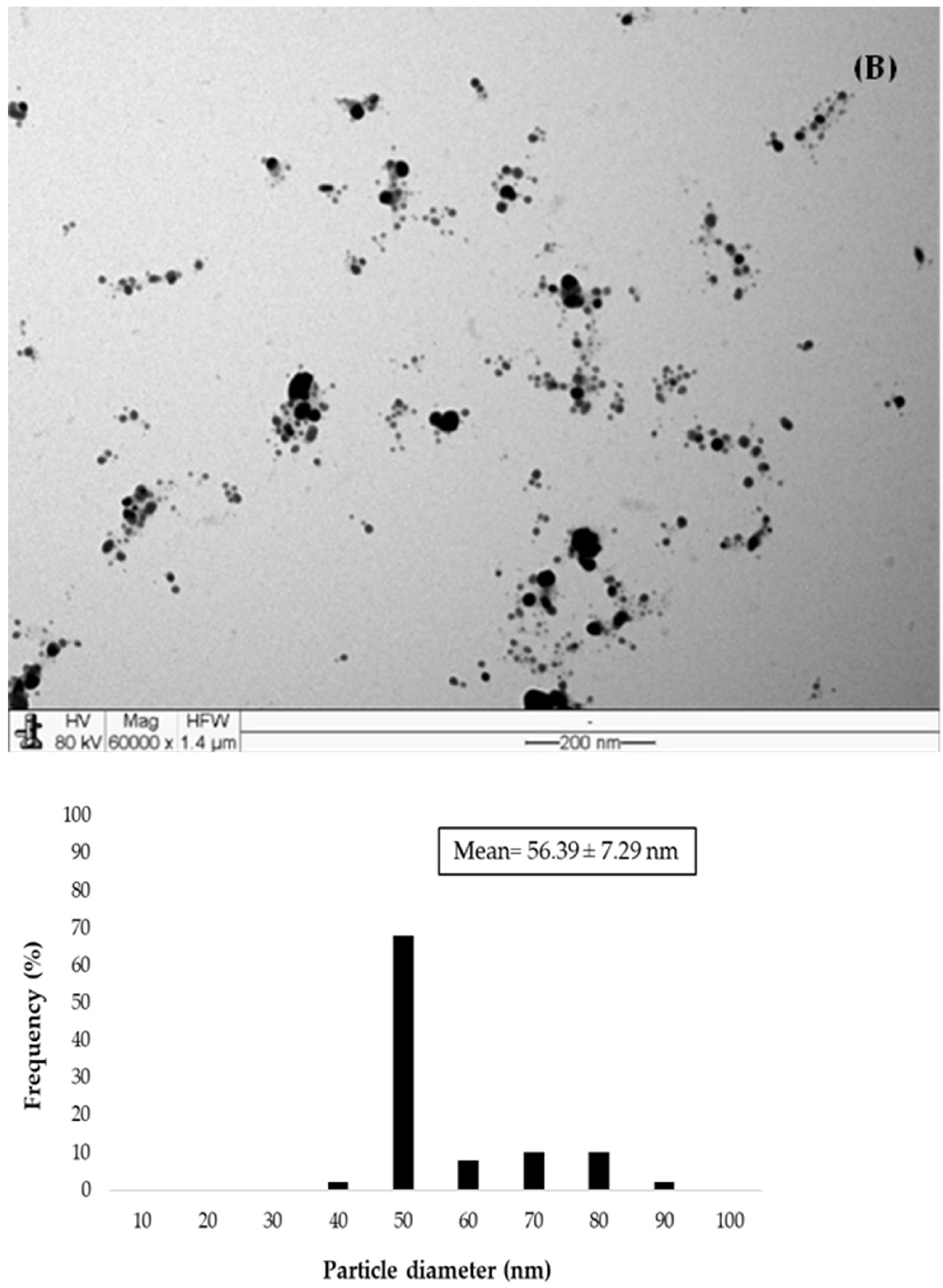
| IBCLP22 | 426 | 89.03 ± 0.33 | −30.92 ± 0.03 | 0.211 | 56.39 ± 7.29 |
| IBCLP30 | 419 | 167.37 ± 11.45 | −22.42 ± 1.52 | 0.424 | 122.64 ± 12.56 |
| IBCLP40 | 424 | 643.89 ± 9.27 | −21.73 ± 1.59 | 0.358 | 525.60 ± 22.09 |
| Antifungal [µg/mL] | IPT295 | IPT423 | IPT330 | IPT728 | IPT729 | IPT311 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| AgNPIBCLP11 | 25 | 50 | 50 | 75 | 25 | 50 | 25 | 50 | 50 | 50 | 50 | 100 |
| AgNPIBCLP17 | 25 | 75 | 75 | 100 | 75 | 125 | 75 | 125 | 75 | 125 | 75 | 125 |
| AgNPIBCLP20 | 10 | 25 | 50 | 75 | 25 | 75 | 25 | 75 | 10 | 25 | 50 | 100 |
| AgNPIBCLP22 | 50 | 125 | 75 | 100 | 50 | 100 | 75 | 125 | 100 | 125 | 75 | 125 |
| FLU | 25 | 25 | 50 | 75 | 25 | 75 | 25 | 75 | 10 | 25 | 50 | 100 |
| FE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Strain | [AgNO3] (mM) | SPR (nm) | DLS (nm) | Pζ (mV) | PDI |
|---|---|---|---|---|---|
| IBCLP11 | 0.25 | nd | nd | nd | nd |
| 0.50 | 415 | 60.1 ± 9.37 | −41.01 ± 0.13 | 0.18 | |
| 0.75 | 427 | 66.9 ± 8.55 | −41.32 ± 0.22 | 0.18 | |
| 1.00 | 427 | 71.0 ± 0.20 | −41.91 ± 0.34 | 0.20 | |
| 1.25 | 427 | 84.5 ± 1.33 | −39.60 ± 0.45 | 0.22 | |
| 1.50 | 427 | 88.2 ± 7.09 | −27.78 ± 1.22 | 0.29 | |
| 2.00 | 427 | 102.3 ± 10.33 | −27.31 ± 1.35 | 0.30 | |
| IBCLP20 | 0.25 | nd | nd | nd | nd |
| 0.50 | 430 | 40.1 ± 0.12 | −38.33 ± 0.23 | 0.20 | |
| 0.75 | 428 | 56.6 ± 0.25 | −38.36 ± 0.05 | 0.21 | |
| 1.00 | 430 | 80.5 ± 0.08 | −38.56 ± 0.05 | 0.21 | |
| 1.25 | 430 | 82.5 ± 1.34 | −30.99 ± 1.44 | 0.23 | |
| 1.50 | 430 | 89.9 ± 6.33 | −29.81 ± 1.25 | 0.25 | |
| 2.00 | 430 | 90.7 ± 10.34 | −28.22 ± 3.33 | 0.34 |
| Strain | [Biomass] (g/L) | SPR (nm) | DLS (nm) | Pζ (mV) | PDI |
|---|---|---|---|---|---|
| IBCLP11 | 75 | 430 | 70.2 ± 0.08 | −41.23 ± 0.69 | 0.18 |
| 100 | 429 | 71.5 ± 0.12 | −41.91 ± 0.03 | 0.20 | |
| 125 | 427 | 70.4 ± 0.03 | −40.56 ± 0.10 | 0.20 | |
| 150 | 430 | 71.6 ± 0.09 | −41.22 ± 0.10 | 0.32 | |
| 200 | 430 | 70.9 ± 0.04 | −41.33 ± 0.10 | 0.39 | |
| IBCLP20 | 75 | 416 | 70.6 ± 0.02 | −36.55 ± 0.33 | 0.20 |
| 100 | 416 | 80.5 ± 0.02 | −38.56 ± 0.04 | 0.21 | |
| 125 | 416 | 82.5 ± 0.01 | −38.55 ± 0.04 | 0.23 | |
| 150 | 416 | 82.3 ± 0.02 | −39.11 ± 0.01 | 0.33 | |
| 200 | 416 | 83.3 ± 0.02 | −39.12 ± 0.03 | 0.37 |
| Strain | Agitation (rpm) | SPR (nm) | DLS (nm) | Pζ (mV) | PDI |
|---|---|---|---|---|---|
| IBCLP11 | 50 | 415 | 72.3 ± 0.08 | −40.33 ± 0.08 | 0.32 |
| 100 | 415 | 75.4 ± 0.06 | −40.99 ± 0.10 | 0.27 | |
| 150 | 415 | 70.2 ± 0.97 | −41.23 ± 0.10 | 0.18 | |
| 200 | 424 | 99.1 ± 2.01 | −40.01 ± 0.34 | 0.50 | |
| IBCLP20 | 50 | 425 | 70.6 ± 0.04 | −33.45 ± 0.01 | 0.34 |
| 100 | 422 | 69.8 ± 0.12 | −32.45 ± 0.08 | 0.26 | |
| 150 | 433 | 80.5 ± 0.48 | −38.56 ± 0.10 | 0.21 | |
| 200 | 449 | 89.3 ± 2.89 | −27.45 ± 2.27 | 0.54 |
| Strain | Temperature (°C) | SPR (nm) | DLS (nm) | Pζ (mV) | PDI |
|---|---|---|---|---|---|
| IBCLP11 | 25 | nd | nd | nd | nd |
| 30 | 413 | 70.3 ± 0.08 | −42.98 ± 1.08 | 0.27 | |
| 35 | 412 | 60.2 ± 0.12 | −43.99 ± 1.55 | 0.15 | |
| IBCLP20 | 25 | 422 | 80.3 ± 0.09 | −31.29 ± 1.44 | 0.23 |
| 30 | 420 | 80.5 ± 0.03 | −38.56 ± 1.28 | 0.21 | |
| 35 | 420 | 82.1 ± 0.03 | −33.33 ± 1.01 | 0.20 |
| Strain | pH | SPR (nm) | DLS (nm) | Pζ (mV) | PDI |
|---|---|---|---|---|---|
| IBCLP11 | 6.0 | 415 | 58.7 ± 0.12 | −41.03 ± 0.22 | 0.20 |
| 6.5 | 437 | 60.4 ± 0.08 | −41.99± 0.10 | 0.23 | |
| 7.0 | 437 | 86.3 ± 0.02 | −21.33± 0.08 | 0.38 | |
| 7.5 | 437 | 92.1 ± 0.08 | −22.45 ± 0.08 | 0.42 | |
| IBCLP20 | 6.0 | 419 | 80.3± 0.43 | −31.29± 0.45 | 0.23 |
| 6.5 | 420 | 71.2 ± 0.27 | −40.05± 0.33 | 0.19 | |
| 7.0 | 420 | 83.2 ± 0.04 | −41.29± 0.06 | 0.22 | |
| 7.5 | 420 | 97.4 ± 0.06 | −38.66 ± 0.06 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotta, C.; Alves, A.L.; Cardoso, M.; da Silva, C.; Léo, P.; de Castro, L.; Domínguez, Y.; Simões, M.F.; Ottoni, C.A. Marine Mycosilver Nanoparticles: Screening, Evaluation of Their Antimicrobial Properties, and Synthesis Optimization. Sustain. Chem. 2025, 6, 10. https://doi.org/10.3390/suschem6010010
Trotta C, Alves AL, Cardoso M, da Silva C, Léo P, de Castro L, Domínguez Y, Simões MF, Ottoni CA. Marine Mycosilver Nanoparticles: Screening, Evaluation of Their Antimicrobial Properties, and Synthesis Optimization. Sustainable Chemistry. 2025; 6(1):10. https://doi.org/10.3390/suschem6010010
Chicago/Turabian StyleTrotta, Caterina, Ana Laura Alves, Mariana Cardoso, Carolina da Silva, Patrícia Léo, Leandro de Castro, Yoannis Domínguez, Marta Filipa Simões, and Cristiane Angélica Ottoni. 2025. "Marine Mycosilver Nanoparticles: Screening, Evaluation of Their Antimicrobial Properties, and Synthesis Optimization" Sustainable Chemistry 6, no. 1: 10. https://doi.org/10.3390/suschem6010010
APA StyleTrotta, C., Alves, A. L., Cardoso, M., da Silva, C., Léo, P., de Castro, L., Domínguez, Y., Simões, M. F., & Ottoni, C. A. (2025). Marine Mycosilver Nanoparticles: Screening, Evaluation of Their Antimicrobial Properties, and Synthesis Optimization. Sustainable Chemistry, 6(1), 10. https://doi.org/10.3390/suschem6010010








