Abstract
Sustainable hydrocarbons are one of the main methods of decreasing the use of fossil fuels and derivatives, contributing to the mitigation of environmental impacts and greenhouse gas emissions. Circular economic concepts focus on reusing waste by converting it into new products, which are then input again into industrial production lines, thus decreasing the necessity of fossils. Polypropylene-based plastic waste can be depolymerized into smaller chemical chains, producing a liquid phase rich in hydrocarbons. In the same way, triacylglycerol-based waste biomasses can also be converted into renewable hydrocarbons. Our research studied the co-processing of polypropylene (PP) and cottonseed oil dreg (BASOs) waste from the biodiesel industry using a micropyrolysis system at 550 °C, previously validated to predict the scale-up of the process. PP showed the production of alkanes and alkenes, while BASOs also produced carboxylic acids in addition to the PP products. The main impacts were observed in the conversion yields, reaching the highest values of pyrolytic liquid (64%), gas (14%), and solid product (13%) compared to the co-processing mixture of BASO:PP (1:2). Also, in this mixture, the production of carboxylic acids decreased to the lowest value (~10%), improving the conversion to sustainable hydrocarbons.
1. Introduction
Among the urban solid waste produced, there is a high percentage of non-biodegradable materials, among which plastics represent an environmental risk due to the large amount generated and discarded in nature. Mechanical recycling for waste such as plastic is still limited by the quantity and quality of the product generated [1]. Among chemical treatments, gasification requires a huge amount of thermal energy (~1000 °C) compared to pyrolysis, which operates between 400 °C and 750 °C and does not require high pressures [2]. In addition, pyrolysis is a carbon-neutral process, with a practical destination for the products generated and the potential to add value to the product, making it highly promising [3,4]. In industry, the generation and management of solid waste is also a crucial problem [5]. For example, the biofuel industry, with the production of biodiesel, for example, generates 10 tons of waste for every 100 tons of fuel produced [6]. This waste is made up of glycerols, methanol, soap, oils, salts, and solid organic materials [7,8].
Considering that plastic waste has already been found in all ocean basins and the enormous difficulty in reusing it, a new, more effective route is needed to manage this waste [9]. Polypropylene (PP) is an inexpensive thermoplastic polymer with high thermal distortion temperatures and dimensional fastness [10]. PP is a plastic widely used in industry where it is applied in the production of packaging and labels. Its production represents 16% of the world’s plastics market [11]. In terms of chemical composition, PP is a petrochemical derived from a propylene “olefin monomer” [12]. To recycle post-consumer polypropylene, it is necessary to eliminate impurities from the recycled polymer using additional techniques that cause unwanted environmental impacts. Thus, with current recycling methods, it is much more difficult to obtain a product that can be reintroduced into the economic chain [13].
Biodiesel, defined as the mono-alkyl esters of vegetable oils or animal fats, is an attractive and ecological alternative to petrodiesel due to its biodegradable, renewable, and non-toxic attributes, but attention is needed because its production can generate major environmental impacts such as the use of large amounts of water, the destruction of forests, and increased soil degradation [14,15]. To produce biodiesel from cottonseed oil, the oil needs to go through a basic neutralization pretreatment for the removal of free fatty acids. The fatty acid salts formed are called “SOAPSTOCK” and are separated from the oil by simple decantation or centrifugal separators [16,17]. This material is rich in triacylglycerols and free fatty acids (approximately 65% by weight) and is an excellent source of biomass to produce biofuels [18] or bio-oils by pyrolysis [19].
Pyrolysis consists of the thermal degradation of a raw material in an inert atmosphere, can occur in a temperature range of 350 to 800 °C, and has three products: pyrolytic liquid, charcoal (in some cases called coke), and pyrolytic gas [20]. Pyrolytic products have a viable application in the market; they produce biochar for application in soil or electrolytic cells, pyrolysis gas that can be used as a synthesis gas, and the liquid product can have fuel fractions identical to those derived from petroleum. Such flexibility makes pyrolysis an excellent process for studying new ways to reintroduce waste into the economy [21].
During pyrolysis, long-chain polymers like PP are broken down into smaller fractions, and the resulting oils and gases are highly valuable precursors to fuels and chemicals. Without the use of any catalyst, PP pyrolysis at 500–600 °C mainly produces hydrocarbon chains with more than 20 carbons, which are a solid wax product at room temperature [22]. Thermal cracking of PP produces a wide range of hydrocarbons that are mainly composed of gases (C1–C4), C5–C10 non-aromatic hydrocarbons, monoaromatics, and waxes (>C11) [23]. Depolymerization follows a free radical mechanism (initiation), which is followed by random scission and chain-end scission (secondary decomposition), and then recombination of different chains (termination) [24]. On the other hand, pyrolysis of cotton sludge residue mainly produces alkanes, alkenes, and carboxylic acids, and a way to reduce the amount of acids formed is needed to increase the added value of the product [25]. The cracking of TAGs and FFAs follows three distinct processes: decarboxylation, decarbonylation, and random cleavage [26].
Several studies have reported that introducing a hydrogen-rich waste plastic into co-pyrolysis increases hydrocarbon yield and decreases coke formation. Causing the increase in the H/C ratio and modifying the oxygen removal reaction mechanism, replacing the decarbonylation and decarboxylation reactions with the dehydration reaction [27,28,29,30]. The liquid fraction for PP pyrolysis has properties and composition similar to petroleum-derived fuels and can be used as a raw material for the petrochemical industry and the gaseous fraction can also be used to provide energy for the process [31]. On the other hand, BASO’s liquid pyrolysis product has characteristics, such as high acidity, that prevent its direct application to diesel engines. The pyrolysis of waste plastic with triglyceric waste from biodiesel production can then improve the quality of the liquid fraction by the donor effect of H from the plastic [32].
In this context, the present work evaluated the pyrolysis and co-pyrolysis processes as a sustainable alternative for the management of urban and industrial waste, such as polypropylene and cottonseed oil dregs, in order to propose a waste treatment process, obtain value-added products, and reinsert the products in the production chain aiming at a circular economy.
2. Materials and Methods
2.1. Feedstock
Cottonseed oil dregs (BASOs) were provided by the experimental biodiesel plant of the Northeast Strategic Technologies Center (CETENE, Caetes, Pernambuco, Brazil) and were used in the experiments in their raw form without any previous treatment. Polypropylene waste (PP) was obtained at the waste collector sector from the Federal University of Sergipe. The plastic waste was previously washed and dried at 120 °C in an oven. After drying, the material was finely ground (<2 mm). The samples were named PP (polypropylene), BASOs (cottonseed oil dregs), BASO:PP (1:1), BASO:PP (1:2), e BASO:PP (2:1).
2.2. Immediate and Elemental Analysis
The determination of moisture, ash, fixed carbon content, and elemental analysis (CHN) followed the procedures previously described in the literature [33].
2.3. Thermogravimetric Analysis (TGA)
The thermal profile of the samples was obtained using a TGA-50 thermogravimetric analyzer (Shimadzu, Kyoto, Japan), operating under an atmosphere of N2 at a flow rate of 50 mL min−1. For the analysis, ~8 mg of sample was subjected to heating using a rate of 15 °C min−1. Mass loss was monitored over a temperature range of 30 °C to 1000 °C.
2.4. Micropyrolysis Off-Line
A total of 100 mg of each sample was subjected to pyrolysis in a tandem microreactor with furnace 1 at 550 °C and furnace 2 at 500 °C. The experiments were carried out in triplicate, and the pyrolytic liquid was eluted from the reactor with 5 mL of THF. The resulting solution was submitted directly to GC/MS analysis without any previous derivatization process. The microreactor scheme and analytical conditions are previously described in the literature [33].
2.5. FTIR
Infrared absorption spectroscopy analysis was utilized to determine the functional groups present in the produced wax. As the BASO:PP (1:2) mixture showed the highest yield in the production of the respective product, it was selected for the analysis. A PerkinElmer spectrophotometer, model Spectrum Two, was used, with a scanning range of 4000 to 400 cm−1, a resolution of 4 cm–1, and 32 scans per minute to obtain the infrared spectrum. For the analyses, a blank was prepared using only KBr; then, the sample was prepared by maceration with KBr and pressed to obtain a pellet for subsequent analysis.
2.6. APCI(+)-FT-Orbitrap MS
A Thermo Fisher Scientific Exactive Plus Orbitrap (Bremen, Germany), equipped with an Ion Max API source with an APCI probe operating in positive ionization mode, was used for the analyses. The samples were dissolved in toluene to produce a 300 ppm solution and analyzed by direct infusion at a flow rate of 80 µL min−1. The APCI(+)-FT Orbitrap MS data were collected under the following conditions: sheath gas flow at 20 arbitrary units (AU), auxiliary gas flow at 20 AU, ion source temperatures at 320 °C, spray current at 1 µA and S-Lens RF level:60. The mass spectrum acquisition was performed in full-scan mode over a mass range of 100–1000 Da, with a resolution of 140,000 FWHM (full width at half maximum) at m/z 200. A total of 100 μscans were accumulated for each analysis. The final mass spectrum for each sample was obtained by subtracting the blank spectrum. Molecular formula assignments for the ions were made using Xcalibur 3.0 software, with a maximum error tolerance of 3 ppm between the experimental and theoretical m/z values.
3. Results
3.1. Immediate and Elemental Analysis
The results obtained during the immediate analysis and those obtained in the elemental analysis are available in Table 1.

Table 1.
Immediate and elemental analysis of PP and BASO waste and their mixtures.
3.2. Thermogravimetric Behavior
The thermal degradation profile of individual polypropylene samples (PP) and the cottonseed oil dregs (BASOs) and their mixes (1:1), (1:2), and (2:1) were evaluated by thermogravimetric analysis (TGA). From Figure 1A, it is possible to observe the loss of mass of the individual BASOs and PP samples in relation to the increase in temperature.
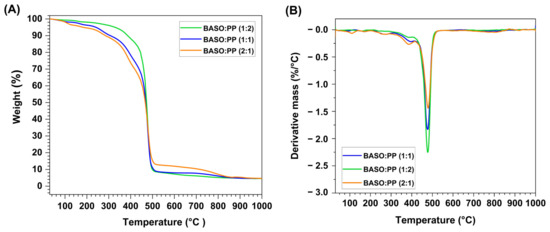
Figure 1.
Thermogram (A) and first derivative curve (B) of PP and BASO materials.
The thermal behavior of the mixtures of BASO and PP, (1:1), (1:2), and (2:1), was also evaluated, as shown in Figure 2.
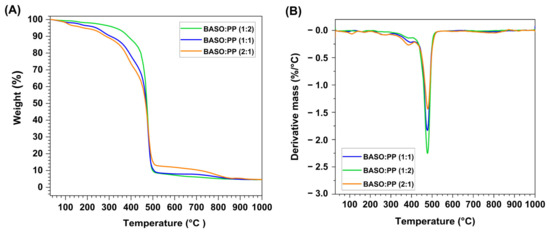
Figure 2.
Thermogram (A) and first derivative curve (B) of BASO:PP (1:1), (1:2), and (2:1) material mixes.
The synergistic effect between the two materials during the co-pyrolysis process was evaluated and is shown in Figure 3, the synergistic effect was calculated using the following formula [34]:
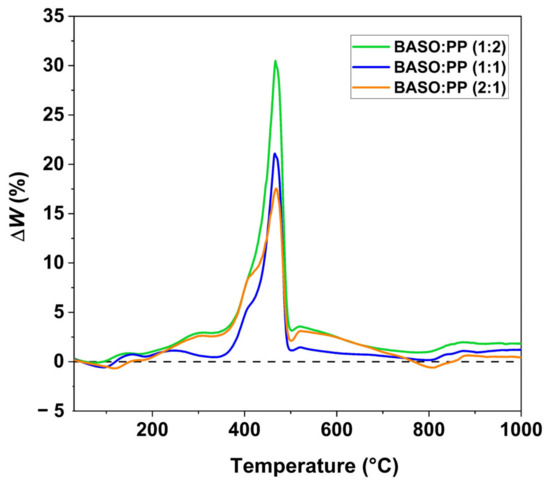
Figure 3.
Synergistic effect of BASO:PP (1:1), (1:2) e (2:1) co-pyrolysis.
The ΔW values are analyzed as a function of temperature. When ΔW < 0, the experimental weight is lower than the calculated value, indicating a greater release of volatiles in the real process and suggesting the presence of a synergistic effect. Conversely, when ΔW > 0, an inhibitory effect is observed.
3.3. Pyrolytic Gravimetry Yields
In order to evaluate the potential of waste conversion into products that can be reintroduced into the chemical industry, conventional pyrolysis and co-pyrolysis experiments were carried out for PP and BASO samples, and in their mixtures. The gravimetric yield of the experiments performed can be shown in Table 2.

Table 2.
Predicted yields of co-pyrolysis obtained by micropyrolysis experiments.
Char is mainly constituted by a solid carbonaceous structure with an involved inorganic fraction represented by alkali metals such as Na+, K+, and Ca2+ in the form of oxides in most cases. These inorganic constituents contribute to the application of char for soil amendment, making nutrients available and increasing soil pH [35]. Inorganics in char are associated with the ash content from the feedstock, as the higher values are found in sewage sludges and lignocellulosic biomasses [35,36]. In our work, PP and BASOs present 0% and 4.06% of the ash content, respectively, contributing to produce chars with a lower inorganic fraction, when compared with other chars from lignocellulosic biomasses [36], which can be addressed as an absorbent material to wastewater treatment for pollutants removal [37]. Biomass with a higher ash content can also produce bio-oils with metal traces in its composition that need to be removed to prevent catalyst poisoning during the upgrading process [38]. For pyrolysis of triacylglycerol-based biomass, the main role of inorganic salts is on the catalytic effect to promote the production of olefins and paraffins [39].
3.4. Chemical Characterization of Pyrolytic Liquid by GC/MS
The volatilizable compounds present in the pyrolytic oils resulting from the pyrolysis and co-pyrolysis process of BASO and PP were characterized by GC/MS using the criterion of peak detection with an area greater than 0.1%, as shown in Supplementary Materials (Figures S1–S6). The identification of the compounds was based on the comparison of the mass spectra with those of the NIST library, considering the compounds with similarity greater than 75%. This resulted in the integration of 66 peaks for the PP sample, 73 for the BASO sample, and 89, 95, and 79 for the BASO:PP (1:1), (2:1), and (1:2), respectively. The identified compounds cover about 90% of the total chromatographic area.
The identified compounds were grouped into different classes according to their main organic functions. The chemical distribution of these compounds is shown in the class histogram in Figure 4.
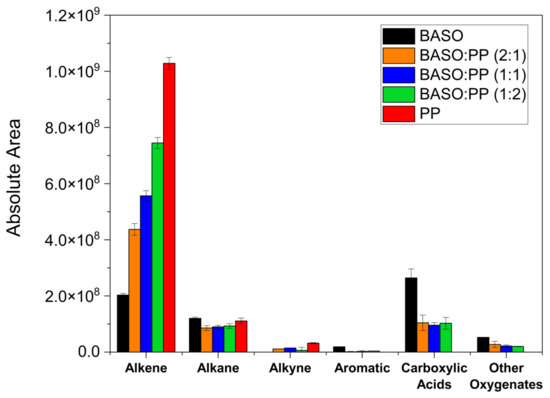
Figure 4.
Histogram of chemical classes of the compounds present in the pyrolytic liquid.
3.5. FTIR of the Pyrolytic Wax
During the pyrolysis experiments on PP and the mixtures containing PP, the production of a solid wax product condensed into the reactor was observed. We performed FTIR analysis to confirm the chemical characteristics of the product, such as the presence of saturated hydrocarbons, as shown in Figure 5.
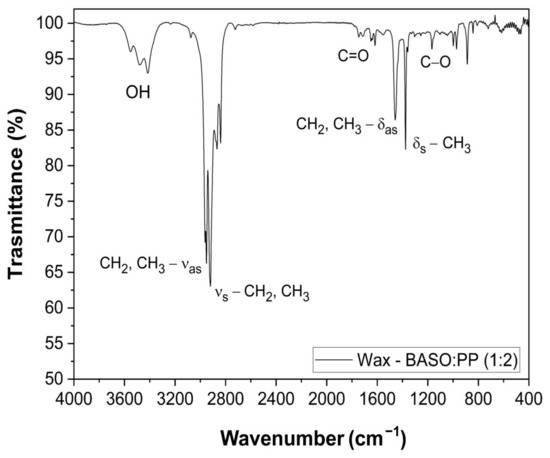
Figure 5.
FTIR spectrum of the pyrolytic wax recovered from the reactor.
3.6. APCI(+)-FT-Orbitrap MS
Non-volatile and medium polar compounds present in the pyrolytic oils were evaluated by APCI(+)-FT-Orbitrap MS. The detected ions that had their formulas assigned were separated according to their heteroatom class and according to their relative intensity, as shown in Figure 6.
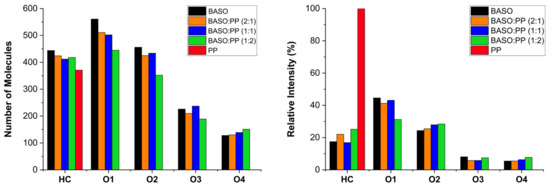
Figure 6.
Histogram by chemical classes of the pyrolytic liquid constituents obtained by APCI(+).
In order to evaluate the characteristics of hydrocarbon class compounds, an iso-abundance contour plot of DBE vs. carbon number was created, as shown in Figure 7.

Figure 7.
Diagrams of the chemical constituents of the pyrolytic liquid obtained by APCI(+).
4. Discussion
4.1. Elemental Analysis
From the results obtained through the elemental analysis, it was observed that the PP and BASO residues have approximately 86.29% and 66.48% of carbon, respectively. This fact is directly related to the high energy potential of the raw material. The hydrogen content in both materials was in the range of 11–15%, while the oxygen contents were 0% for PP and 22.31% for BASOs. The moisture content was ~8% for BASOs and 0% for PP; the ash from the BASO material (~4.06%) is correlated with inorganic salts not degraded at working temperature, and it is attached to the fixed carbon content. The percentage of carbon for the mixtures was 75.33%, 78.28%, and 72.91% for the mixtures (1:1), (1:2), and (2:1), respectively. The values were oxygen: 12.73%, 8.45%, and 14.37%, and hydrogen: 11.94%, 13.27%, and 12.73% for the mixtures (1:1), (1:2), and (2:1), respectively. The variation in the amount of each element occurs proportionally to the amount of each residue in the mixture. The mixture that presented the highest oxygen content was BASO:PP (2:1) due to the higher amount of carboxylic acids and derivatives from the BASOs.
The H/C ratio was ~2 for both samples, which indicates linear or naphthenic structures and low unsaturation content present in the samples [40]. The H/C and O/C ratios have values very close to those observed for liquid fuels such as diesel and gasoline, which have H/C ratios of 1.5 to 2.0 and O/C ratios close to zero, thus indicating that PP, BASO, and their mixtures have a high potential to be converted into liquid hydrocarbons of high commercial value [41]. It is expected that, to facilitate this conversion pathway, the oxygenated compounds present in BASO will be converted, for the most part, into hydrocarbons through deoxygenation reactions. In relation to the molar O/C ratio, BASOs had a ratio of 0.25, while PP does not have oxygen in its composition, which confirms that BASOs are mostly composed of molecules with aliphatic characteristics and low oxygen content, which is characteristic of triacylglycerols that mostly compose the sample. For the mixtures, the H/C ratio remained between 1.9 and 2.1, and O/C between 0.08 and 0.15 [42].
4.2. Thermogravimetric Behavior
The PP sample remained stable up to 360 °C, which reveals the absence of moisture in the sample; after this temperature, the first and only mass loss event begins, where it decomposes completely up to approximately 490 °C. It is observed that the ash content and fixed carbon of this sample are approximately 0%, confirming the data obtained in Topic 4.1. The absence of ash and fixed carbon occurs due to the polymer’s molecular structure undergoing depolymerization and hydrogenation, resulting in lower molecular carbon chains of smaller molecular weight that are fully volatilized [43].
The BASO material is composed of mono-, di-, and/or triacylglycerols (TAGs), free fatty acids (FFA), and organic salts of fatty acids formed in the pre-treatment of cottonseed oil used to remove impurities and decrease the acidity of the oil [44]. The BASO thermogravimetric curve has a total of five mass loss events, as observed in Figure 1B. The first mass loss event occurs between 100 and 150 °C and can be attributed to the moisture present in the material, where it corresponds to ~10% of the total mass. Between 220 and 350 °C, the degradation of FFAs can be attributed, followed by the decomposition of mono-, di-, and/or triacylglycerols, which represent 60.2% of the BASO mass. This decomposition continues until 450 °C [44,45]. It is followed by a degradation event of the surfactant compounds that follows up to ~550 °C, and, finally, the last event that starts at 750 °C is attributed to inorganic salts present in the residue derived from the degradation of the previously decomposed fatty acid salts. At the end of the analysis, a remaining mass of 5.8% was recorded, which corresponds to the percentage of fixed carbon of the sample, together with the ashes formed.
From the thermogram of Figure 1A, it was possible to define the pyrolysis temperature at 550 °C because this is the temperature at which there is a stability of the mass of the samples after their greatest mass loss events. This temperature correlates well with the values found in the literature [46,47].
The thermal degradation curve of the mixture of BASO:PP (1:1) is presented in Figure 2A and showed that the first mass loss corresponds to the water mass of the BASO sample, since PP has no moisture and corresponds to ~5% of the total mass; the second event, from 250 °C to 300 °C, occurs due to the FFAs that come from the BASO sample, and, in this analysis, correspond to 10% of the mass of the sample; the next event begins at ~330 °C where, for BASO, the degradation of TAGs would correspond; however, at this same temperature, there is the beginning of PP degradation; due to this interaction, the TAGs and fatty acid salts degradation events are suppressed in the thermogram. The event visualized at 750 °C in BASO is also not seen in the BASO:PP (1:1) thermogram due to its relatively low percentage of mass loss (~2%).
The BASO:PP (2:1) has a total of five mass loss events, as observed in Figure 2A,B, due to its higher mass percentage coming from BASOs; its profile follows similar characteristics to the individual BASO material. The first event of 100–150 °C is related to the moisture present in the material, where it corresponds to ~10% of the total mass. From 220 to 350 °C, the degradation of the FFAs begins. Soon after that, there is the beginning of the degradation of the mono, di, and triacylglycerols present in the material; this event joins the degradation event of the PP polymer that begins at 430 °C, and this decomposition continues until 550 °C. The degradation of the surfactant compounds is inhibited by the event of polymer degradation. Finally, the last event that begins at 750 °C is attributed to inorganic salts present in the residue derived from the degradation of previously decomposed fatty acid salts. At the end of the analysis, a remaining 5% mass was recorded, which corresponds to the percentage of fixed carbon of the sample, together with the ashes formed.
The BASO:PP (1:2) has its profile closer to the thermogram of the isolated PP because its higher mass percentage comes from the polymer. However, as we can see in Figure 2B, the events arise from the degradation of BASOs. They follow the same order as the previous ones, with the difference in the duration of the PP degradation event. The interactions of the residues can be visualized in the calculation of the synergistic effect for co-pyrolysis. Finally, considering the temperature at which the maximum thermal conversion of both samples occurs, to carry out the subsequent pyrolysis experiments, the temperature adopted for the process was 550 °C.
From Figure 3, it is possible to observe an inhibitory effect during the thermal degradation process, with ΔW > 0. The interaction of the mixture being inhibitory means that the experimental mass loss was less than calculated. This is a result of a greater thermal stability caused by polypropylene. Although the addition of PP helps to form products with higher added value, its interaction with BASOs tends to inhibit the volatilization of volatiles from the cracking of biomass through the change in the physical state of the plastic, which, when it reaches its melting point, becomes “liquid” and changes the way that heat is transferred to the waste as well as traps the vapors produced, which are released after the volatilization of the plastic waste, generating the negative synergistic effect shown in Figure 3 [48]. Although the synergistic effect is negative, the permanence of the compounds in the reactor for a longer time can help in the thermal cracking of the waste, which can generate products with higher added value [49].
4.3. Pyrolysis Yields
It can be seen that BASO’s individual pyrolysis formed 44.45% of pyrolytic liquid product, a difference of ~20% less when compared to PP. According to Melo et al. (2021) [45], pyrolytic oils from triglyceric-based biomasses have a higher amount of oxygenates, demonstrating the need for treatments to add value and increase their yield. Gaseous products accounted for ~30% and ~19% for BASOs and PP, respectively. When coke was evaluated, there was a formation of ~26% for BASO and 14% for PP. The coke is recognized by a structured carbon in the form of coal; the results corroborate the literature [50].
It is possible to observe that there have been significant changes in relation to conventional pyrolysis and co-pyrolysis; the percentage of the pyrolytic liquid obtained from BASO:PP (1:1) is higher by ~8% compared to BASOs and lower by ~8% than PP. For BASO:PP (1:2) and (2:1), we have, for the pyrolytic liquid, a ~64% and 59% yield, respectively. Despite the higher amount of BASOs in the (2:1) mix, its yield in the liquid product remained at the same percentage as the individual PP and less than 4% of the BASO:PP (1:2); this suggests a better interaction of PP radicals with BASO, promoting the breakdown of larger molecules, such as fatty acids and TAGs, producing less coke than BASO: PP (1:1) and more gaseous than the other mixtures.
For the coke formed to BASO:PP (1:1), there was an increase of ~0.7% in relation to the individual BASO; on the other hand, there was a reduction of ~8.8% and ~13.2% of the coke for the BASO:PP (2:1) and (1:2), respectively. The decrease in coke may be linked to the interaction of ●H radicals produced from polypropylene with the ones from BASO radicals, making it impossible to form polyaromatic chains that are precursors of coke [51]. Co-pyrolysis also produced, respectively, 20 to 14% of gas, which is proportional to the pyrolysis of the waste. The percentage of BASO:PP (1:2) liquid product increased in relation to PP, showing that the interaction between these two residues can enable the formation of a higher quality liquid product, along with the reduction in the solid product.
In the individual pyrolysis of PP and in the co-pyrolysis, the production of wax was visualized in the range of 4 to 10%, a solid product from the repolymerization of the radicals formed by the volatilization of PP or partial cracking of the polymer. In the pyrolysis of polypropylene, there was a 6.52% formation of wax; for the mixtures (1:1), (2:1), and (1:2), it produced 4.04%, 3.79%, and 9.61%, respectively. It was found that the percentage of BASO present in the mixture alters the amount of wax formed. Since the melting temperature of PP (160 °C) is lower than the initial degradation temperature of BASO (220 °C), the PP melts on the surface of the biomass, retaining the pyrolytic vapors generated during the initial volatilization of the material. This situation contributes to a longer residence time of BASO and, consequently, to the formation of lower molecular weight radicals, which are released from the initial degradation of the polymer (360 °C). Thus, the radicals from the cleavage of PP tend to interact with the lower molecular weight radicals, promoting their stabilization and inhibiting polymerization reactions [33]. This situation can be seen in Table 2, where the lower amount of BASO in the mixture (1:2) resulted in the highest wax formation; therefore, it is believed that the lower formation of lower molecular weight radicals occurs.
Thus, to evaluate the improvement in the quality of the pyrolytic liquid obtained from co-pyrolysis, the products obtained during the studied processes were characterized using the gas chromatography/mass spectrometry (GC/MS) technique and ultra-high resolution mass spectrometry (APCI(+)-FT- Orbitrap MS).
4.4. Chemical Characterization of Pyrolytic Liquid
When evaluating Figure 4, it was observed that the pyrolysis of PP has, as its only product, the hydrocarbon class; this is due to the composition of the plastic waste, where there are only polymerized chains of isoparaffins. The products obtained in this pyrolysis are precisely the result of the depolymerization of these chains [43]. As for the cottonseed oil dregs, we have the majority production of carboxylic acids that come from the primary degradation of TAGs. Along with this, the amount of alkenes and alkanes can be explained through the cracking of fatty acids through decarboxylation and decarbonylation reactions, which consist of the release of CO2 and CO + H2O, respectively, where these two routes can lead to hydrocarbon production.
The co-pyrolysis process promoted the reduction of carboxylic acids when compared to the BASO’s individual pyrolysis. There was a decrease from ~60%, 64%, and 61% in the area for the BASO:PP (2:1), (1:1), and (1:2) samples, respectively. This reduction can be attributed to the deoxygenation reactions of acids into hydrocarbons, as well as possible repolymerization reactions of alkanes and alkenes, given the formation of waxes in the co-pyrolysis processes. In the co-processing of the mixtures, a selectivity in the decarbonylation of carboxylic acids into alkenes is observed, with a higher formation of these compounds compared to the individual pyrolysis of BASO, where an increase of 115%, 174%, and 267% can be observed in the BASO:PP (2:1), (1:1), and (1:2) mixtures, respectively.
Sustainable aviation fuel (SAF) is mostly made up of renewable hydrocarbons, whose carbon chain length varies between C8 and C16. Among these, the main components present in this biofuel correspond to paraffins, isoparaffins, and naphthenic and aromatic hydrocarbons, which are also the main components of fossil kerosene [52,53].
Comparing the pyrolysis of isolated materials with the mixtures, it was observed that the co-pyrolysis between BASO and PP in all proportions reduced the carboxylic acid content, from the degradation of TAGs from BASO. This decrease refers to the deoxygenation of carboxylic acids through decarboxylation and decarbonylation. In addition, the increase in alkenes in the co-pyrolysis was evidenced when compared to individual pyrolysis of BASO, first by the presence of PP but also by the synergistic effect between both materials that contributes to the degradation of the TAGs, FFAs, and fatty acid salts present in BASO. The presence of sodium and potassium ions in fatty acid salts also contributes to the catalytic cracking process [54], which may have catalyzed the reduction, along with hydrogenation of the acid to alkenes [55,56].
4.5. Chemical Characterization of the Wax
For the FTIR spectrum of solid wax produced during the pyrolysis process, Figure 5, it was observed that the material is mainly composed by paraffinic material, confirmed by the symmetric and asymmetric stretching of 1300–1400 cm−1 and 2800–3000 cm−1, characteristic of CH2 and CH3 in aliphatic chains. Between 3400 and 3600 cm−1, we can see the wax from BASO:PP (1:2), a band characteristic of the OH stretch, in a possible intramolecular interaction, from residual carboxylic acids from the pyrolysis process. The peaks at 1745 cm⁻1 and 1164 cm⁻1 are attributed to C=O and C–O stretching, respectively, reinforcing the hypothesis of the presence of residual carboxylic acids.
4.6. Comprehensive Chemical Characterization of Pyrolytic Liquid by APCI(+)-FT-Orbitrap MS
Ultra-high resolution mass spectrometry can help to evaluate the chemical composition of complex mixtures as well as to understand the mechanisms of thermal cracking of macromolecules during the pyrolysis process. Figure 6 shows that the main pyrolytic liquid constituents are distributed in the HC class for PP, while BASO also includes oxygenated classes as O1, O2, O3, and O4. The number of molecules in the HC class follows the increasing trend from PP to BASO. The main observation was the synergistic effect of PP to help in decreasing the O1 and O2 classes, corroborating the data from GC/MS, producing more alkenes with the greatest impact on the process BASO:PP (1:2).
An in-depth assessment of the class of hydrocarbons present in the pyrolytic liquids produced (Figure 7) revealed distinct profiles between the samples analyzed. BASO, as a triglyceride-based biomass, produced hydrocarbons with DBE (double bond equivalent) characteristics ranging from 2 to 18 and carbon numbers between 8 and 40. The cracking of PP, on the other hand, produced hydrocarbons with lower DBE values (between 2 and 15), which means that, compared to BASO, PP produces fewer aromatic compounds. On the other hand, the hydrocarbons produced by the plastic polymer have a longer carbon chain, with values ranging from 8 to 75, especially the compounds in the homologous series of DBE 2, 3, 4, and 6, which can refer to diolefinic or cyclic hydrocarbons (DBE 2), triolefinic or cycloalkadiene (DBE 3), monoaromatic (DBE 4), and dinaphthenoaromatic (DBE 6) [57].
Analysis of the hydrocarbons from the co-pyrolysis samples revealed the presence of compounds with DBEs ranging from 2 to 15. The formation of structures with DBEs greater than 15 was inhibited during co-processing (region highlighted in green), indicating that the synergistic interaction between the PP polymer matrix and the triglyceride-based biomass reduces the occurrence of condensation mechanisms.
This result may be due to the stabilization process of active radicals derived from the cracking of BASO constituents, with intermediates from the scission of the PP structure, inducing the production of compounds with lower DBE values, i.e., lower aromaticity [33]. According to Ureel et al. (2024) [57], during the depolymerization of PP there is intermolecular hydrogen abstraction, causing the generation of stable alkyl radicals, which can interact with benzyl radicals from the degradation of BASO and, through the alkylation reaction, produce aromatic hydrocarbons, as seen in the co-pyrolysis, especially in the BASO:PP (1:1) sample, the region highlighted in red. In addition, when analyzing the distribution of compounds based on the number of carbons, there was a tendency for the carbon chain to increase as the amount of PP increased in the mixture, from C8 to C52 in the BASO:PP (2:1) sample to C8 to C70 in the BASO:PP (1:2) sample, which corroborates the production of wax in the co-pyrolysis samples as well, as highlighted in the black regions.
5. Conclusions
The results presented in this work showed that the co-pyrolysis process of polypropylene with BASO makes it possible to obtain a less oxygenated oil with an improvement in the alkene content. In addition, co-pyrolysis oil has shown promise to produce fuels and chemical inputs due to the high production of branched and linear alkenes, alkanes, and the synergistic effect among the feedstocks. PP demonstrated an interesting effect to increase the conversion of fatty acids into alkenes. Thus, the results indicate that the conversion of urban plastic waste and industrial triacylglycerol-based waste through the co-pyrolysis process has high potential for energy application, as well as in the fine chemical industry, in addition to being an efficient way to produce sustainable hydrocarbons. In addition, it is still possible to add catalysts to the process, making the conversion of residual acids into higher value-added products even more effective, thus increasing the potential application of the product in industries and energy sectors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/suschem6020012/s1, Figure S1: TICC of pyrolytic product from BASO; Figure S2: TICC of pyrolytic product from PP; Figure S3: TICC of pyrolytic product from BASO:PP (1:1); Figure S4: TICC of pyrolytic product from BASO:PP (1:2); Figure S5: TICC of pyrolytic product from BASO:PP (2:1); Figure S6: TICC of pyrolytic product from BASO:PP (1:2) with the tentative identification of the peaks.
Author Contributions
I.d.C.G.: Writing—original draft, methodology, investigation, formal analysis; M.S.d.S.: Writing—review and editing, validation, methodology, data curation. T.M.: Writing—review and editing, validation, methodology, data curation; A.W.J.: Writing—review and editing, supervision, project administration, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Council for Scientific and Technological Development for research fellowships (125998/2023-6, 163121/2021-4 and 303342/2021-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available as requested.
Acknowledgments
The authors would like to thank the Center of Multi-users Chemistry Laboratories (CLQM) from the Federal University of Sergipe for facilities and analysis support and to the National Council for Scientific and Technological Development (CNPq).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Naderi Kalali, E.; Lotfian, S.; Entezar Shabestari, M.; Khayatzadeh, S.; Zhao, C.; Yazdani Nezhad, H. A Critical Review of the Current Progress of Plastic Waste Recycling Technology in Structural Materials. Curr. Opin. Green Sustain. Chem. 2023, 40, 100763. [Google Scholar] [CrossRef]
- Stiegel, G.J.; Maxwell, R.C. Gasification Technologies: The Path to Clean, Affordable Energy in the 21st Century. Fuel Process. Technol. 2001, 71, 79–97. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic Waste Recycling via Pyrolysis: A Bibliometric Survey and Literature Review. J. Anal. Appl. Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Merrington, A. Recycling of Plastics. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 177–192. [Google Scholar]
- Raut, S.P.; Ralegaonkar, R.V.; Mandavgane, S.A. Development of Sustainable Construction Material Using Industrial and Agricultural Solid Waste: A Review of Waste-Create Bricks. Constr. Build. Mater. 2011, 25, 4037–4042. [Google Scholar] [CrossRef]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biodiesel Industry Waste: A Potential Source of Bioenergy and Biopolymers. Indian J. Microbiol. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol Production and Its Applications as a Raw Material: A Review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Maru, B.T.; Bielen, A.A.M.; Constantí, M.; Medina, F.; Kengen, S.W.M. Glycerol Fermentation to Hydrogen by Thermotoga Maritima: Proposed Pathway and Bioenergetic Considerations. Int. J. Hydrog. Energy 2013, 38, 5563–5572. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in Freshwater Ecosystems: What We Know and What We Need to Know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental Impacts of Polypropylene (PP) Production and Prospects of Its Recycling in the GCC Region. Mater. Today Proc. 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Bora, R.R.; Wang, R.; You, F. Waste Polypropylene Plastic Recycling toward Climate Change Mitigation and Circular Economy: Energy, Environmental, and Technoeconomic Perspectives. ACS Sustain. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef]
- Aliu, I.R.; Ajala, A.O. Intra-City Polarization, Residential Type and Attribute Importance: A Discrete Choice Study of Lagos. Habitat Int. 2014, 42, 11–20. [Google Scholar] [CrossRef]
- Welivita, I.; Wattage, P.; Gunawardena, P. Review of Household Solid Waste Charges for Developing Countries—A Focus on Quantity-Based Charge Methods. Waste Manag. 2015, 46, 637–645. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Khilji, I.A.; Sirohi, R.; Pandey, A.; Baskar, G.; Satyavolu, J. Maximizing the Value of Biodiesel Industry Waste: Exploring Recover, Recycle, and Reuse for Sustainable Environment. Environ. Technol. Innov. 2023, 32, 103447. [Google Scholar] [CrossRef]
- Bhosle, B.M.; Subramanian, R. New Approaches in Deacidification of Edible Oils—A Review. J. Food Eng. 2005, 69, 481–494. [Google Scholar] [CrossRef]
- Daud, N.M.; Sheikh Abdullah, S.R.; Abu Hasan, H.; Yaakob, Z. Production of Biodiesel and Its Wastewater Treatment Technologies: A Review. Process Saf. Environ. Prot. 2015, 94, 487–508. [Google Scholar] [CrossRef]
- Da Silva Almeida, H.; Corrêa, O.A.; Eid, J.G.; Ribeiro, H.J.; de Castro, D.A.R.; Pereira, M.S.; Pereira, L.M.; de Andrade Aâncio, A.; Santos, M.C.; da Mota, S.A.P.; et al. Performance of Thermochemical Conversion of Fat, Oils, and Grease into Kerosene-like Hydrocarbons in Different Production Scales. J. Anal. Appl. Pyrolysis 2016, 120, 126–143. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Hu, K.; Hu, C.; Zhang, Z.; Liu, Q.; Hu, S.; Xiang, J.; Wang, Y.; Zhang, S. Progress in the Reforming of Bio-Oil Derived Carboxylic Acids for Hydrogen Generation. J. Power Sources 2018, 403, 137–156. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Artetxe, M.; Bilbao, J. Product Yields and Compositions in the Continuous Pyrolysis of High-Density Polyethylene in a Conical Spouted Bed Reactor. Ind. Eng. Chem. Res. 2011, 50, 6650–6659. [Google Scholar] [CrossRef]
- Aisien, E.T.; Otuya, I.C.; Aisien, F.A. Thermal and Catalytic Pyrolysis of Waste Polypropylene Plastic Using Spent FCC Catalyst. Environ. Technol. Innov. 2021, 22, 101455. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Idem, R.O.; Katikaneni, S.P.R.; Bakhshi, N.N. Catalytic Conversion of Canola Oil to Fuels and Chemicals: Roles of Catalyst Acidity, Basicity and Shape Selectivity on Product Distribution. Fuel Process. Technol. 1997, 51, 101–125. [Google Scholar] [CrossRef]
- Maher, K.D.; Bressler, D.C. Pyrolysis of Triglyceride Materials for the Production of Renewable Fuels and Chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef]
- Muneer, B.; Zeeshan, M.; Qaisar, S.; Razzaq, M.; Iftikhar, H. Influence of In-Situ and Ex-Situ HZSM-5 Catalyst on Co-Pyrolysis of Corn Stalk and Polystyrene with a Focus on Liquid Yield and Quality. J. Clean. Prod. 2019, 237, 117762. [Google Scholar] [CrossRef]
- Wang, L.; Lei, H.; Liu, J.; Bu, Q. Thermal Decomposition Behavior and Kinetics for Pyrolysis and Catalytic Pyrolysis of Douglas Fir. RSC Adv. 2018, 8, 2196–2202. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic Waste to Liquid Oil through Catalytic Pyrolysis Using Natural and Synthetic Zeolite Catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: A Review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Zulkafli, A.H.; Hassan, H.; Ahmad, M.A.; Mohd Din, A.T.; Wasli, S.M. Co-Pyrolysis of Biomass and Waste Plastics for Production of Chemicals and Liquid Fuel: A Review on the Role of Plastics and Catalyst Types. Arab. J. Chem. 2023, 16, 104389. [Google Scholar] [CrossRef]
- Paradela, F.; Pinto, F.; Gulyurtlu, I.; Cabrita, I.; Lapa, N. Study of the Co-Pyrolysis of Biomass and Plastic Wastes. Clean Technol. Environ. Policy 2009, 11, 115–122. [Google Scholar] [CrossRef]
- Sá, M.S.; Martins, T.; Melo, J.A.; de Carvalho Carregosa, J.; Wisniewski, A. Assessment of Co-Pyrolysis of Polyethylene Terephthalate with Waste Cooking Oil: Kinetic Study and Impact on the Chemical Constituents of the Liquid Product. Waste Manag. 2025, 193, 237–249. [Google Scholar] [CrossRef]
- Alam, M.; Bhavanam, A.; Jana, A.; Viroja, J.k.S.; Peela, N.R. Co-Pyrolysis of Bamboo Sawdust and Plastic: Synergistic Effects and Kinetics. Renew. Energy 2020, 149, 1133–1145. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Li, Y.; Bao, H.; Xing, J.; Zhu, Y.; Nan, J.; Xu, G. Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review. Energies 2022, 16, 410. [Google Scholar] [CrossRef]
- Doumer, M.E.; Arízaga, G.G.C.; da Silva, D.A.; Yamamoto, C.I.; Novotny, E.H.; Santos, J.M.; dos Santos, L.O.; Wisniewski, A.; de Andrade, J.B.; Mangrich, A.S. Slow Pyrolysis of Different Brazilian Waste Biomasses as Sources of Soil Conditioners and Energy, and for Environmental Protection. J. Anal. Appl. Pyrolysis 2015, 113, 434–443. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Olsson Månsson, E.; Achour, A.; Ho, P.H.; Arora, P.; Öhrman, O.; Creaser, D.; Olsson, L. Removal of Inorganic Impurities in the Fast Pyrolysis Bio-Oil Using Sorbents at Ambient Temperature. Energy Fuels 2024, 38, 414–425. [Google Scholar] [CrossRef]
- Long, F.; Zhang, X.; Cao, X.; Zhai, Q.; Song, Y.; Wang, F.; Jiang, J.; Xu, J. Mechanism Investigation on the Formation of Olefins and Paraffin from the Thermochemical Catalytic Conversion of Triglycerides Catalyzed by Alkali Metal Catalysts. Fuel Process. Technol. 2020, 200, 106312. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef]
- Santana, K.V.R.; Apolônio, F.C.S.O.; Wisniewski, A. Valorization of Cattle Manure by Thermoconversion Process in a Rotary Kiln Reactor to Produce Environmentally Friendly Products. Bioenergy Res. 2020, 13, 605–617. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Zhao, J. Thermochemical Conversion of Triglycerides for Production of Drop-in Liquid Fuels. Renew. Sustain. Energy Rev. 2016, 58, 331–340. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, H.; Mao, Q.; Zhu, J.; Zhang, S.; Yang, H.; Chen, H. The Effect of Co-Pyrolysis of Bamboo Waste and Polypropylene on Biomass Deoxygenation and Carbonization Processes. Energy 2024, 291, 130339. [Google Scholar] [CrossRef]
- Dos Santos Souza, T.G.; Santos, B.L.P.; Santos, A.M.A.; de Souza, A.M.G.P.; de Melo, J.C.; Wisniewski, A. Thermal and Catalytic Micropyrolysis for Conversion of Cottonseed Oil Dregs to Produce Biokerosene. J. Anal. Appl. Pyrolysis 2018, 129, 21–28. [Google Scholar] [CrossRef]
- Melo, J.A.; de Sá, M.S.; Moral, A.; Bimbela, F.; Gandía, L.M.; Wisniewski, A. Renewable Hydrocarbon Production from Waste Cottonseed Oil Pyrolysis and Catalytic Upgrading of Vapors with Mo-Co and Mo-Ni Catalysts Supported on γ-Al2O3. Nanomaterials 2021, 11, 1659. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Uzun, B.B.; Önal, E.; Pütün, A.E. Synthetic Fuel Production from Cottonseed: Fast Pyrolysis and a TGA/FT-IR/MS Study. J. Anal. Appl. Pyrolysis 2014, 105, 83–90. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Wang, N.; Hou, W.; Zhang, R.; Liu, J.; Zhou, Q.; Yan, D.; Lu, X. Co-Pyrolysis Mechanism of PP and PET under Steam Atmosphere. J. Anal. Appl. Pyrolysis 2023, 173, 106033. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Kinetics of Synergistic Effects in Co-Pyrolysis of Biomass with Plastic Wastes. Appl. Energy 2018, 220, 408–418. [Google Scholar] [CrossRef]
- Seah, C.C.; Tan, C.H.; Arifin, N.A.; Hafriz, R.S.R.M.; Salmiaton, A.; Nomanbhay, S.; Shamsuddin, A.H. Co-Pyrolysis of Biomass and Plastic: Circularity of Wastes and Comprehensive Review of Synergistic Mechanism. Results Eng. 2023, 17, 100989. [Google Scholar] [CrossRef]
- Jung, S.-H.; Cho, M.-H.; Kang, B.-S.; Kim, J.-S. Pyrolysis of a Fraction of Waste Polypropylene and Polyethylene for the Recovery of BTX Aromatics Using a Fluidized Bed Reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Xu, D.; Xiong, Y.; Zhang, S.; Su, Y. The Synergistic Mechanism between Coke Depositions and Gas for H2 Production from Co-Pyrolysis of Biomass and Plastic Wastes via Char Supported Catalyst. Waste Manag. 2021, 121, 23–32. [Google Scholar] [CrossRef]
- Rumizen, M.A. Qualification of Alternative Jet Fuels. Front. Energy Res. 2021, 9, 760713. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of Jet Fuel and Green Diesel Range Biohydrocarbons by Hydroprocessing of Soybean Oil over Niobium Phosphate Catalyst. Fuel 2019, 245, 458–466. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of Biodiesel Obtained from Cottonseed Oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- Balaraman, E.; Khaskin, E.; Leitus, G.; Milstein, D. Catalytic Transformation of Alcohols to Carboxylic Acid Salts and H2 Using Water as the Oxygen Atom Source. Nat. Chem. 2013, 5, 122–125. [Google Scholar] [CrossRef]
- Karam, L.; Neumann, C.N. Heterogeneously Catalyzed Carboxylic Acid Hydrogenation to Alcohols. ChemCatChem 2022, 14, e202200953. [Google Scholar] [CrossRef]
- Ureel, Y.; Chacón-Patiño, M.L.; Kusenberg, M.; Rodgers, R.P.; Sabbe, M.K.; Van Geem, K.M. Characterization of PP and PE Waste Pyrolysis Oils by Ultrahigh-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2024, 38, 11148–11160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).