The Multifaceted Perspective on the Role of Green Synthesis of Nanoparticles in Promoting a Sustainable Green Economy
Abstract
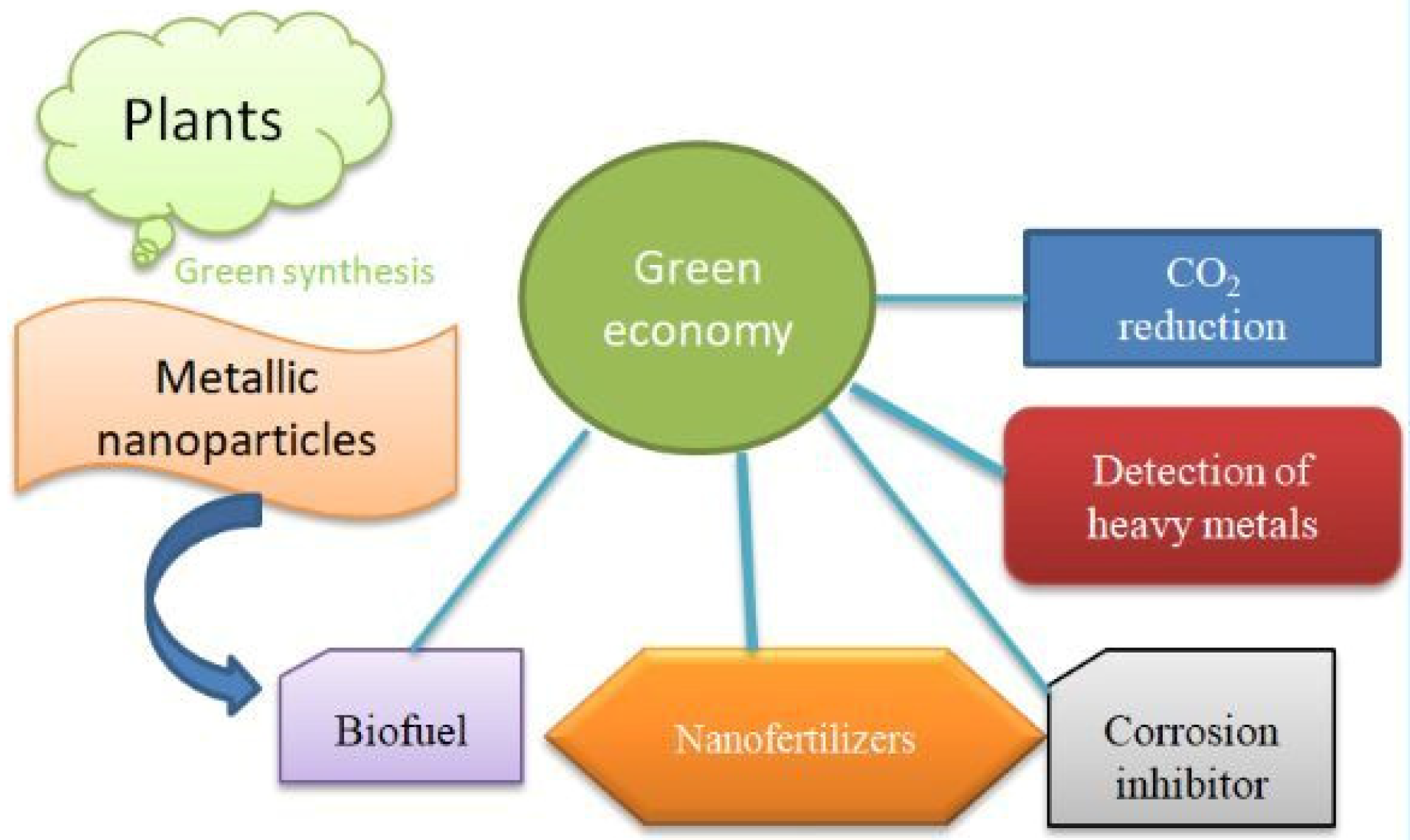
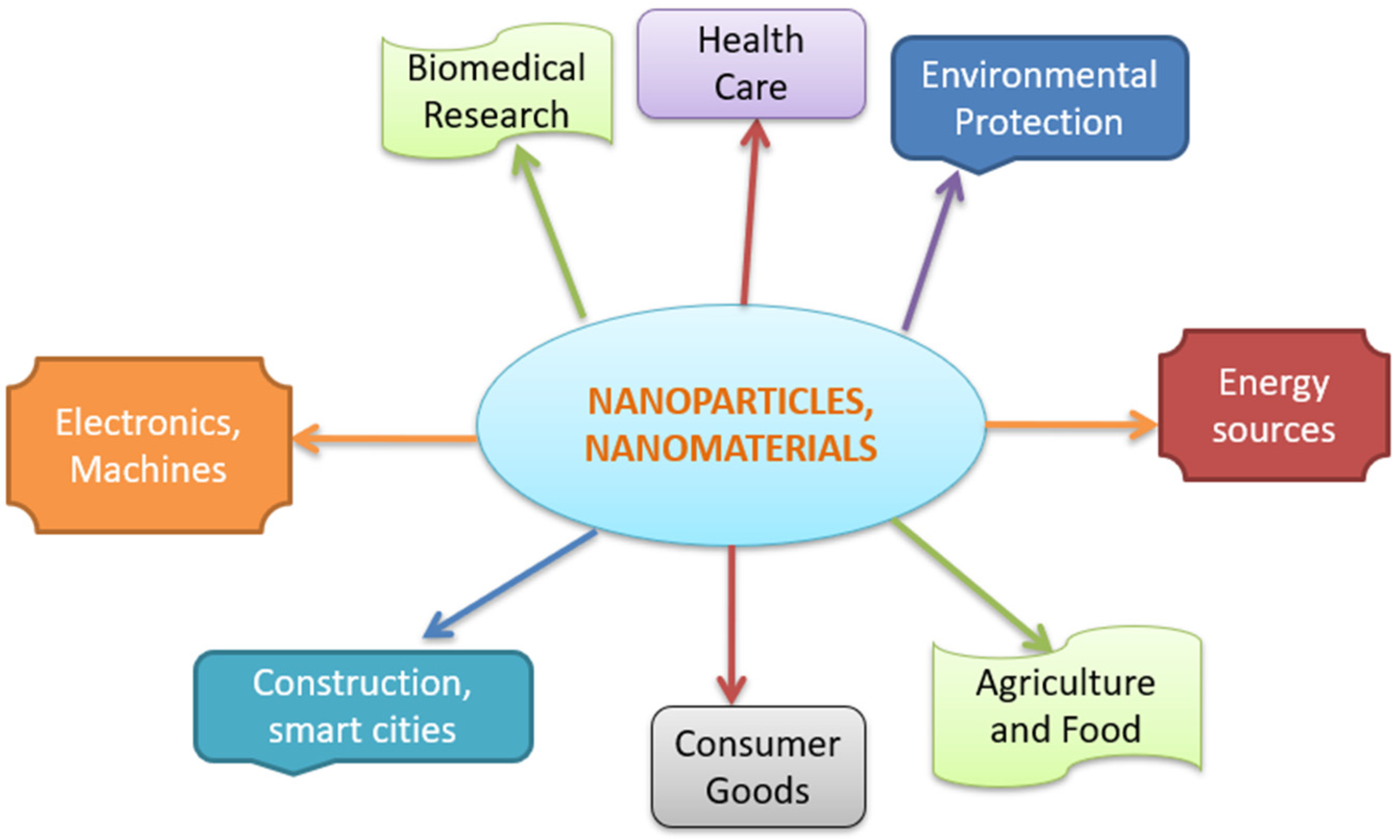
1. Introduction
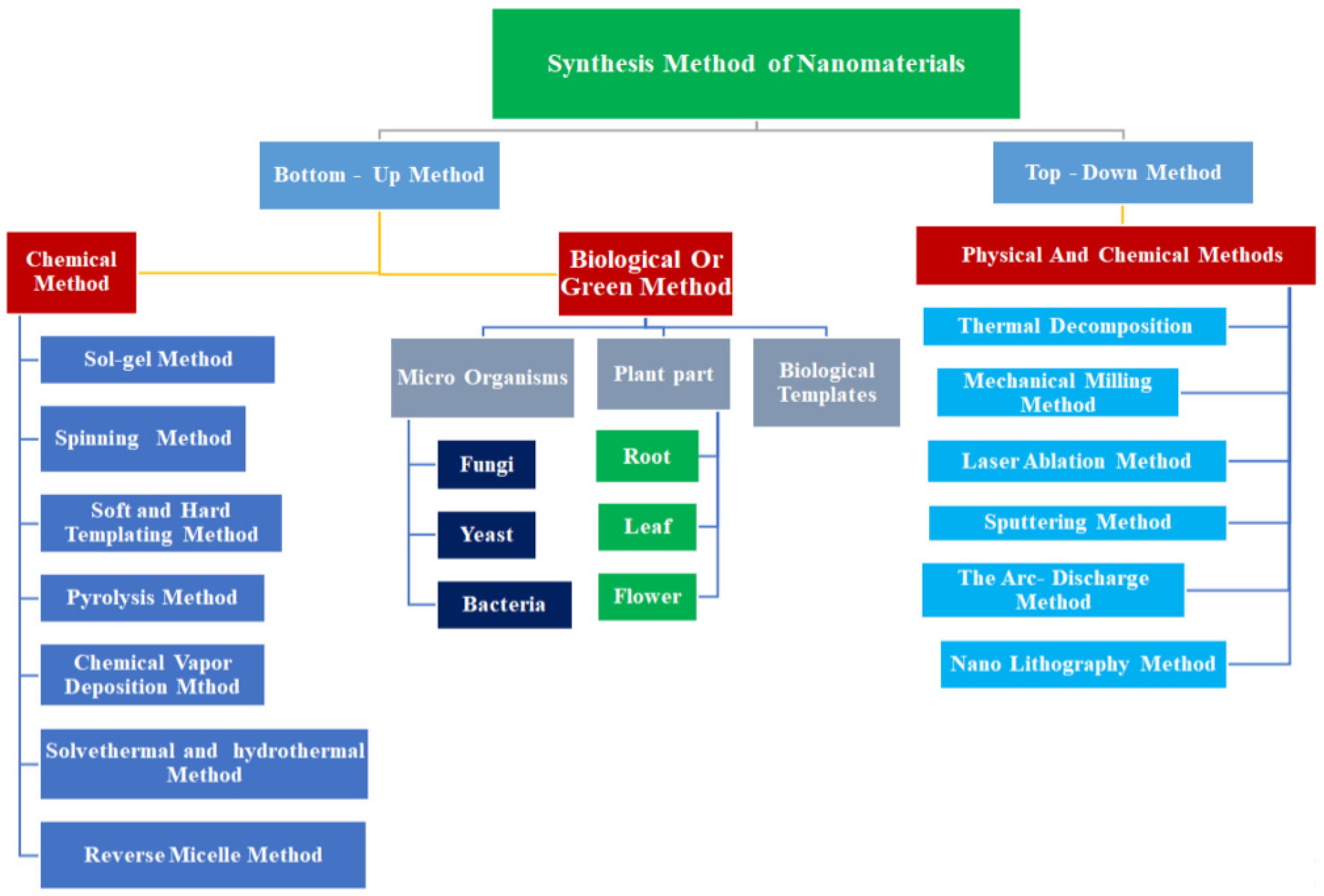
2. Green Synthesis
3. Applications
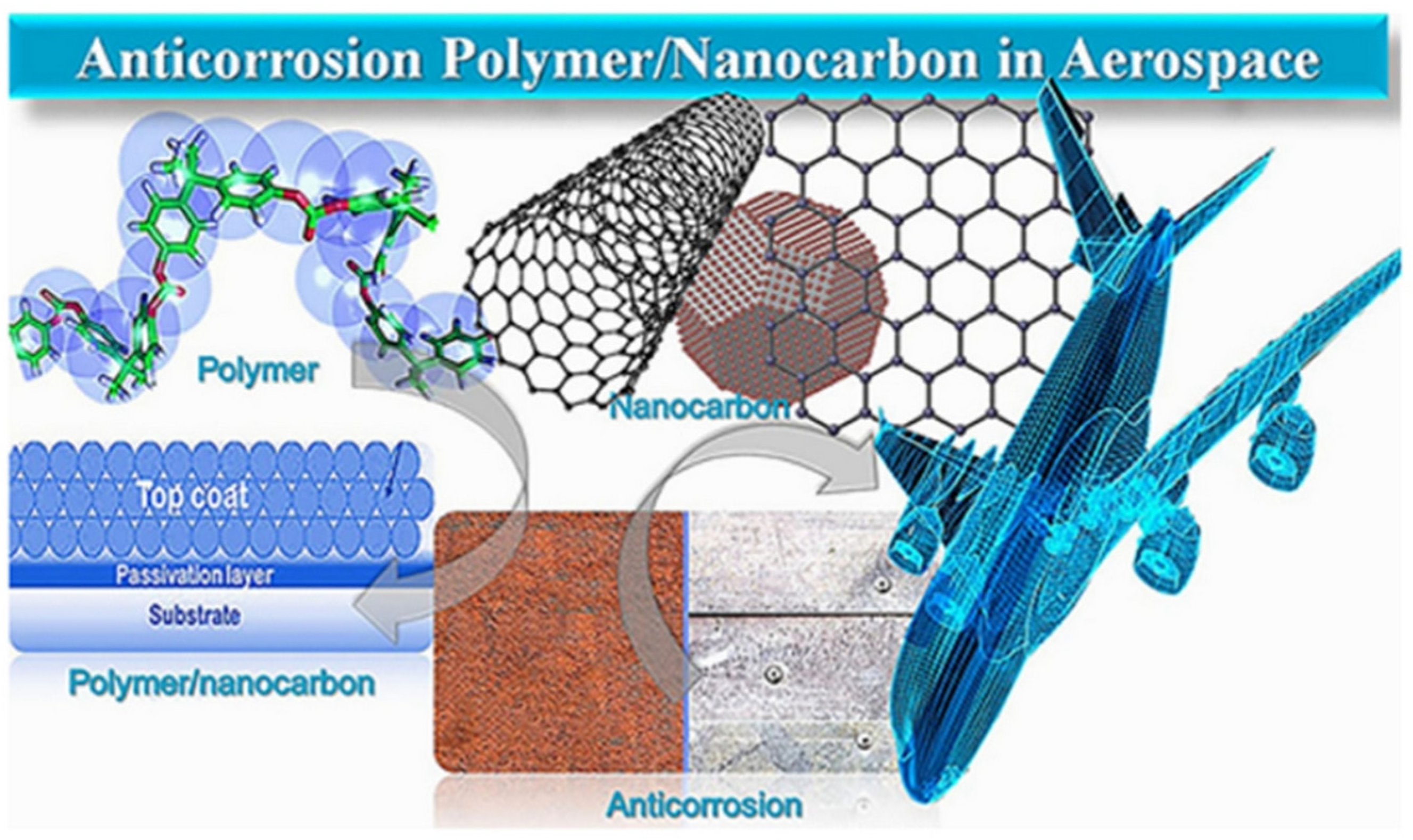
3.1. Corrosion Inhibitor
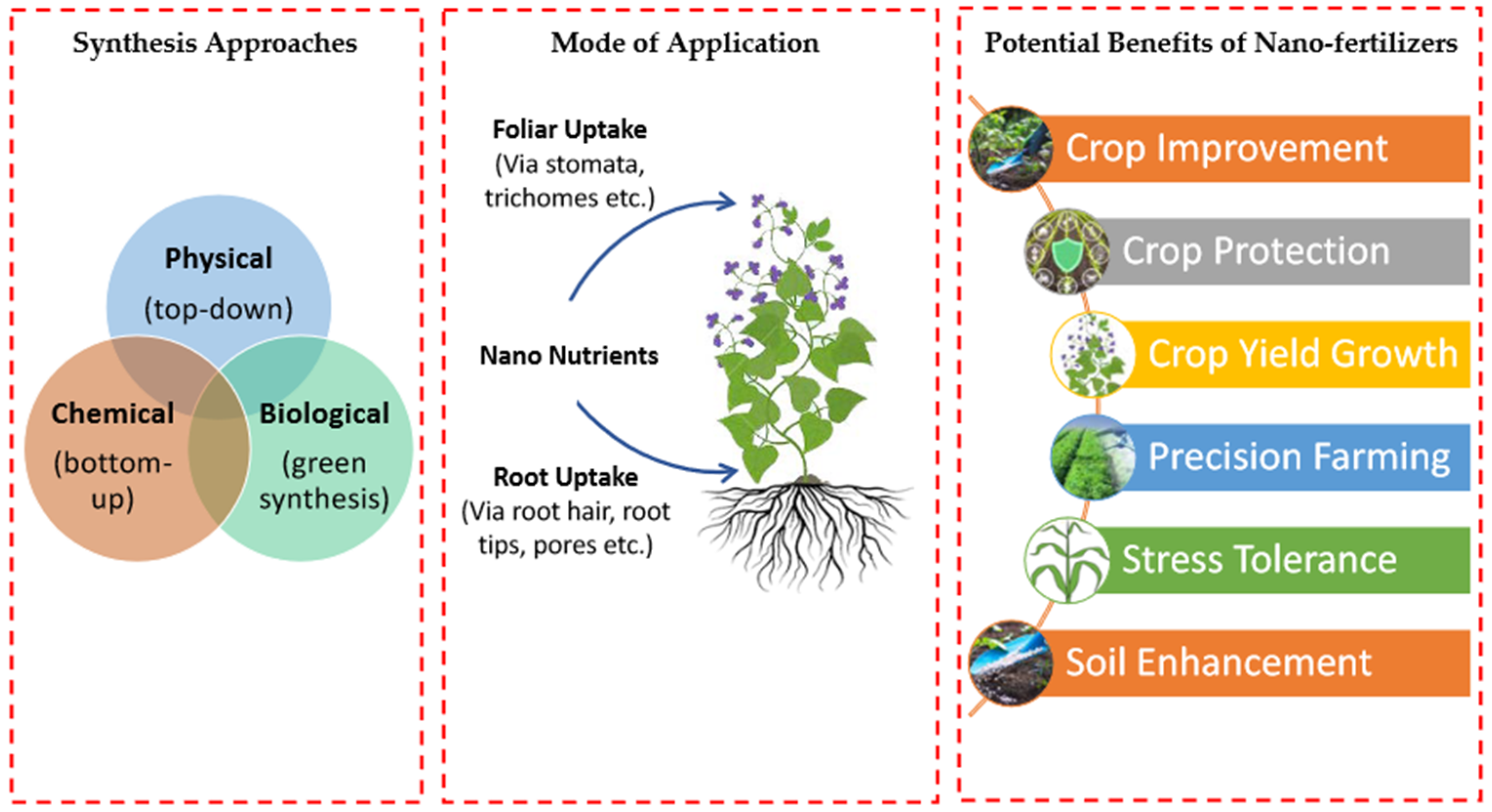
3.2. Nanofertilizers
3.3. Heavy Metal Detection
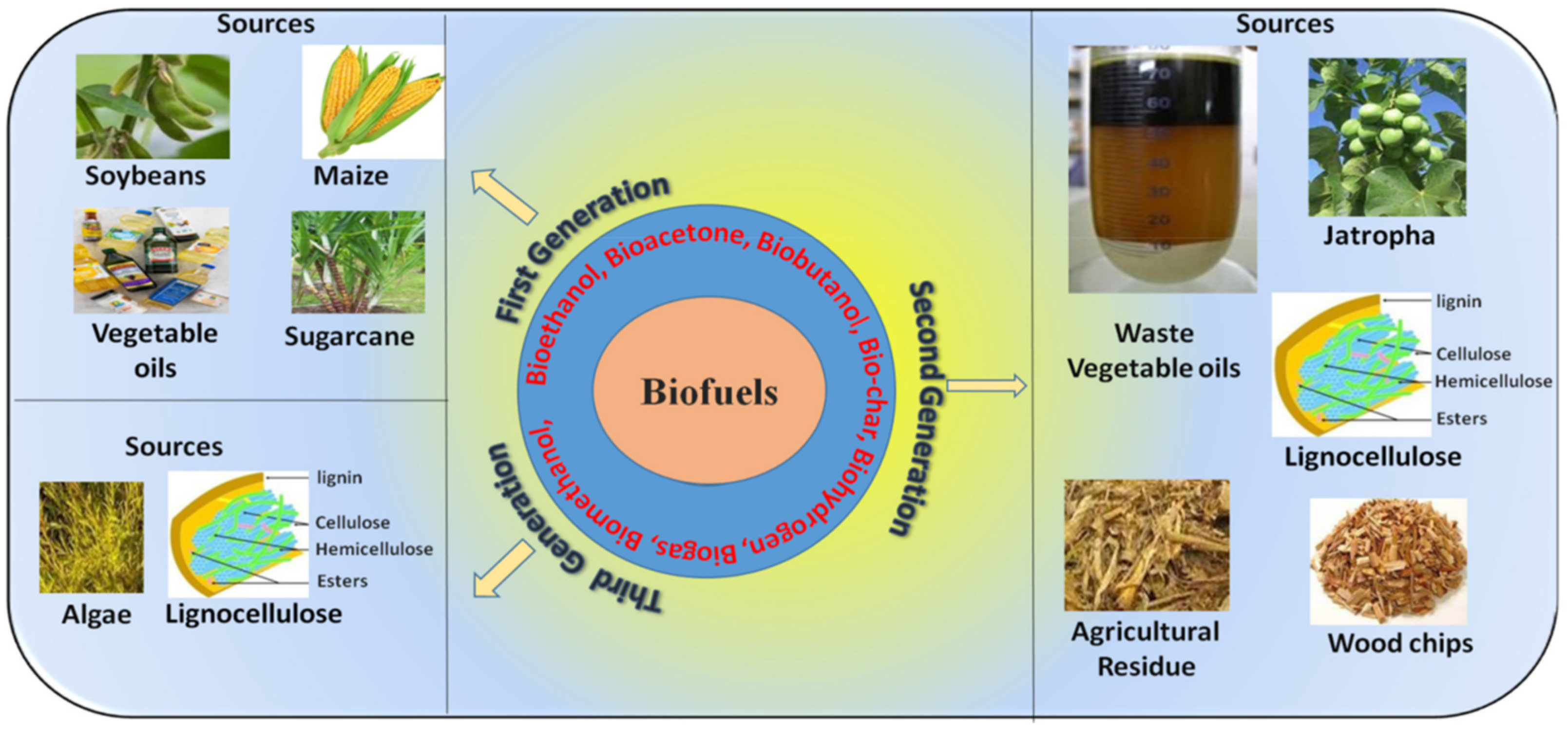
3.4. Biofuel
3.5. Catalytic Reduction of CO2
3.6. Insecticides and Pesticides
4. Conclusions
4.1. Future Perspectives
- Ongoing investigation into novel environmentally friendly nanoparticle production techniques.
- Researching materials for biodegradable nanoparticles to lessen their influence on the environment.
- The creation of intelligent nanofertilizers to minimize chemical usage and enable precision farming.
- Using nanoremediation methods to remediate pollution in water and soil.
- Improving the biodegradation processes based on nanoparticles to manage waste effectively.
- Progress in heavy metal identification technology to enhance environmental surveillance.
- Using catalysts made of nanoparticles to increase the generation of biofuels for sustainable energy.
- Research how nanoparticles affect ecosystems and microbial communities.
- Improving and extending the application of nanoparticles in herbicides and insecticides to manage pests.
- Research on environmentally acceptable and sustainable substitutes for conventional chemical pesticides.
- Using nanoparticles and catalytic reduction of CO2 to fight climate change.
- Examining the possibility of using nanoparticles for carbon collection and usage.
- Developing rules and policies for the safe and responsible use of nanoparticles.
- Public awareness initiatives to inform people about the advantages of green nanoparticles as well as any possible hazards.
- Cooperation to hasten the adoption of green nanoparticles for a greener economy among businesses, academic institutions, and governments.
4.2. Ethical Approval
- The manuscript is not submitted to more than one journal for simultaneous consideration.
- The submitted work is original and should not have been published elsewhere in any form or language (partially or in full).
- A single study has not been split up.
- Results are presented, honestly, and without fabrication, falsification, or inappropriate data manipulation (including image-based manipulation). Authors adhere to discipline-specific rules for acquiring, selecting, and processing data.
- No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”). Proper acknowledgements of other works are given.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| ZnO | Zinc Oxide |
| NPs | Nanoparticles |
| NFs | Nanofertilizers |
References
- Pearce, D.W.; Markandya, A.; Barbier, E.R. Blueprint for a Green Economy; Earthscan Publications Ltd.: London, UK, 1989. [Google Scholar]
- Yu, Y.; Li, C.; Fu, Y.; Yang, W. A Group Decision-Making Method to Measure National Energy Architecture Performance: A Case Study of the International Energy Agency. Appl. Energy 2023, 330, 120285. [Google Scholar] [CrossRef]
- Abbas, S.; Saqib, N.; Shahzad, U. Global Export Flow of Chilean Copper: The Role of Environmental Innovation and Renewable Energy Transition. Geosci. Front. 2023, 101697. [Google Scholar] [CrossRef]
- Mentes, M. Sustainable Development Economy and the Development of Green Economy in the European Union. Energy Sustain. Soc. 2023, 13, 32. [Google Scholar] [CrossRef]
- De Almeida, S.J.; Esperidião, F.; de Moura, F.R. The Impact of Institutions on Economic Growth: Evidence for Advanced Economies and Latin America and the Caribbean Using a Panel VAR Approach. Int. Econ. 2024, 178, 100480. [Google Scholar] [CrossRef]
- Zaki, B.L.; Dupont, C. Understanding Political Learning by Scientific Experts: A Case of EU Climate Policy. J. Eur. Public Policy 2023, 1–33. [Google Scholar] [CrossRef]
- Cui, S.; Wang, Z. The Impact and Transmission Mechanisms of Financial Agglomeration on Eco-Efficiency: Evidence from the Organization for Economic Co-Operation and Development Economies. J. Clean. Prod. 2023, 392, 136219. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Nanotechnology as a Sustainable Approach for Combating the Environmental Effects of Climate Change. J. Agric. Food Res. 2023, 12, 100541. [Google Scholar] [CrossRef]
- Omran, B.A.; Baek, K.-H. Valorization of Agro-Industrial Biowaste to Green Nanomaterials for Wastewater Treatment: Approaching Green Chemistry and Circular Economy Principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P.; et al. The “End Life” of the Grape Pomace Waste Become the New Beginning: The Development of a Virtuous Cycle for the Green Synthesis of Gold Nanoparticles and Removal of Emerging Contaminants from Water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.-Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2023, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in Green Synthesis of Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical Properties of Nanocrystalline Materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green Biosynthesis of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using the Aqueous Extracts of Food Processing Wastes under Photo-Catalyzed Condition and Investigation of Their Antimicrobial and Antioxidant Activity. J. Photochem. Photobiol. B Biol. 2017, 173, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, K.R.; Verma, R.; Singh, J.; Singh, R.P. Efficient Electro-Optical Characteristics of Bioinspired Iron Oxide Nanoparticles Synthesized by Terminalia Chebula Dried Seed Extract. Mater. Lett. 2022, 307, 131053. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Alhuthal, N.A.; Albukhaty, S. Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nanofertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials 2022, 12, 1527. [Google Scholar] [CrossRef]
- Ali, T.; Warsi, M.F.; Zulfiqar, S.; Sami, A.; Ullah, S.; Rasheed, A.; Alsafari, I.A.; Agboola, P.O.; Shakir, I.; Baig, M.M. Green Nickel/Nickel Oxide Nanoparticles for Prospective Antibacterial and Environmental Remediation Applications. Ceram. Int. 2022, 48, 8331–8340. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized Silver Nanoparticles Using Bacillus Amyloliquefaciens; Application for Cytotoxicity Effect on A549 Cell Line and Photocatalytic Degradation of p-Nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar] [CrossRef]
- Jain, P.; Patidar, B.; Bhawsar, J. Potential of Nanoparticles as a Corrosion Inhibitor: A Review. J. Bio- Tribo-Corros. 2020, 6, 43. [Google Scholar] [CrossRef]
- Rathish, R.J.; Joany RD, R.; Pandiarajan, M.; Rajendran, S. Corrosion resistance of nanoparticle-incorporated nano coatings. Eur. Chem. Bull. 2013, 2, 965–970. [Google Scholar]
- Ituen, E.; Ekemini, E.; Yuanhua, L.; Singh, A. Green Synthesis of Citrus Reticulata Peels Extract Silver Nanoparticles and Characterization of Structural, Biocide and Anticorrosion Properties. J. Mol. Struct. 2020, 1207, 127819. [Google Scholar] [CrossRef]
- Fetouh, H.A.; Hefnawy, A.; Attia, A.M.; Ali, E. Facile and Low-Cost Green Synthesis of Eco-Friendly Chitosan-Silver Nanocomposite as Novel and Promising Corrosion Inhibitor for Mild Steel in Chilled Water Circuits. J. Mol. Liq. 2020, 319, 114355. [Google Scholar] [CrossRef]
- Liao, B.; Cen, H.; Xiang, T.; Dai, H.; Wu, H.; Wan, S.; Guo, X. Functionalized Nanocomposites as Corrosion Inhibitors. In ACS Symposium Series; ACS: Washington, DC, USA, 2022; pp. 213–229. [Google Scholar] [CrossRef]
- Picchio, M.L.; Minudri, D.; Mantione, D.; Criado-Gonzalez, M.; Guzmán-González, G.; Schmarsow, R.; Müller, A.J.; Tomé, L.C.; Minari, R.J.; Mecerreyes, D. Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors. ACS Sustain. Chem. Eng. 2022, 10, 8135–8142. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Lu, H.; Xu, L. Surfactant-Modified Silica Nanoparticles-Stabilized Magnetic Polydimethylsiloxane-in-Water Pickering Emulsions for Lubrication and Anticorrosion. ACS Sustain. Chem. Eng. 2022, 10, 10816–10826. [Google Scholar] [CrossRef]
- Su, W.; Tang, B.; Fu, F.; Huang, S.; Zhao, S.; Bin, L.; Ding, J.; Chen, C. A New Insight into Resource Recovery of Excess Sewage Sludge: Feasibility of Extracting Mixed Amino Acids as an Environment-Friendly Corrosion Inhibitor for Industrial Pickling. J. Hazard. Mater. 2014, 279, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Chen, W.-J.; Chen, S. Nanoparticle-Mediated Bioremediation as a Powerful Weapon in the Removal of Environmental Pollutants. J. Environ. Chem. Eng. 2023, 11, 109591. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, X.; Huang, M.; Zheng, M.; Yue, O.; Hao, D.; Wang, Y.; Zou, X.; Cui, B.; Xie, L.; et al. Versatile Nano–Micro Collagen Fiber-Based Wearable Electronics for Health Monitoring and Thermal Management. J. Mater. Chem. A 2023, 11, 726–741. [Google Scholar] [CrossRef]
- Grisolia, A.; Dell’Olio, G.; Spadafora, A.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. Hybrid Polymer-Silica Nanostructured Materials for Environmental Remediation. Molecules 2023, 28, 5105. [Google Scholar] [CrossRef]
- Soni, A.; Bhandari, M.P.; Tripathi, G.K.; Bundela, P.; Khiriya, P.K.; Khare, P.S.; Kashyap, M.K.; Dey, A.; Vellingiri, B.; Sundaramurthy, S.; et al. Nano-biotechnology in Tumour and Cancerous Disease: A Perspective Review. J. Cell. Mol. Med. 2023, 27, 737–762. [Google Scholar] [CrossRef]
- Singh, A.K.; Rathod, V.; Singh, D.; Ninganagouda, S.; Kulkarni, P.; Mathew, J.; Haq, M.U. Bioactive silver nanoparticles from endophytic fungus Fusarium sp. isolated from an ethanomedicinal plant Withania somnifera (Ashwagandha) and its antibacterial activity. Int. J. Nanomater. Biostruct 2015, 5, 15–19. [Google Scholar]
- Kokura, S.; Handa, O.; Takagi, T.; Ishikawa, T.; Naito, Y.; Yoshikawa, T. Silver Nanoparticles as a Safe Preservative for Use in Cosmetics. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Samberg, M.E.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Evaluation of Silver Nanoparticle Toxicity in Skin in Vivo and Keratinocytes in Vitro. Environ. Health Perspect. 2010, 118, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Report ILZSG. World Directory of Continuous Galvanizing Lines; ILZSG: New York, NY, USA, 2017. [Google Scholar]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention Application and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2008. [Google Scholar]
- Koch, G. Cost of Corrosion. In Woodhead Publishing Series in Energy, Trends in Oil and Gas Corrosion Research and Technologies; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–30. [Google Scholar]
- Nishimura, R.; Maeda, Y. Stress Corrosion Cracking of Type 304 Austenitic Stainless Steel in Sulphuric Acid Solution Including Sodium Chloride and Chromate. Corros. Sci. 2004, 46, 343–360. [Google Scholar] [CrossRef]
- Sanyal, B. Organic Compounds as Corrosion Inhibitors in Different Environments—A Review. Prog. Org. Coat. 1981, 9, 165–236. [Google Scholar] [CrossRef]
- Trabanelli, G. Corrosion inhibitors. In Corrosion Mechanisms; CRC Press: Boca Raton, FL, USA, 2020; pp. 119–163. [Google Scholar]
- Oguzie, E.E. Corrosion Inhibition of Aluminium in Acidic and Alkaline Media by Sansevieria Trifasciata Extract. Corros. Sci. 2007, 49, 1527–1539. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A.; Ebenso, E.E. Synergistic and antagonistic effects of anions and Ipomoea invulcrata as green corrosion inhibitor for aluminium dissolution in acidic medium. Int. J. Electrochem. Sci. 2010, 5, 994–1007. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brandán, S.A.; Gervasi, C.A. Rollinia Occidentalis Extract as Green Corrosion Inhibitor for Carbon Steel in HCl Solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Chetouani, A.; Hammouti, B. Corrosion inhibition of iron in hydrochloric acid solutions by naturally henna. Bull. Electrochem. 2003, 19, 23–25. [Google Scholar]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Extraction and Experimental Studies of Citrus Aurantifolia as an Economical and Green Corrosion Inhibitor for Mild Steel in Acidic Media. J. Adhes. Sci. Technol. 2019, 33, 1169–1183. [Google Scholar] [CrossRef]
- Akkalatham, W.; Taghipour, A.; Yongsiri, P.; Ali, S.M. Circular Economy in Materials to Decarbonize Mobility. In Renewable Energy in Circular Economy; Springer: Cham, Switzerland, 2023; pp. 89–112. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Anti-Corrosive Potential of the Sustainable Corrosion Inhibitors Based on Biomass Waste: A Review on Preceding and Perspective Research. J. Phys. Conf. Ser. 2022, 2267, 012079. [Google Scholar] [CrossRef]
- Lateef, A. Cola Nitida: Milestones in Catalysis, Biotechnology and Nanotechnology for Circular Economy and Sustainable Development. Biocatal. Agric. Biotechnol. 2023, 53, 102856. [Google Scholar] [CrossRef]
- de Souza, F.S.; Spinelli, A. Caffeic Acid as a Green Corrosion Inhibitor for Mild Steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Raja, P.B.; Rahim, A.A.; Osman, H.; Awang, K. Inhibitive Effect of XylopiaFerruginea Extract on the Corrosion of Mild Steel in 1M HCl Medium. Int. J. Miner. Metall. Mater. 2011, 18, 413–418. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Zhao, T. Corrosion-Resisting Nanocarbon Nanocomposites for Aerospace Application: An Up-to-Date Account. Appl. Nano 2023, 4, 138–158. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Gouda, M. Novel Nanocomposites of Nickel and Copper Oxide Nanoparticles Embedded in a Melamine Framework Containing Cellulose Nanocrystals: Material Features and Corrosion Protection Applications. J. Mol. Liq. 2021, 342, 116960. [Google Scholar] [CrossRef]
- Mrunal, V.K.; Vishnu, A.K.; Momin, N.; Manjanna, J. Cu2O Nanoparticles for Adsorption and Photocatalytic Degradation of Methylene Blue Dye from Aqueous Medium. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100265. [Google Scholar] [CrossRef]
- Ardekani, P.S.; Karimi, H.; Ghaedi, M.; Asfaram, A.; Purkait, M.K. Ultrasonic Assisted Removal of Methylene Blue on Ultrasonically Synthesized Zinc Hydroxide Nanoparticles on Activated Carbon Prepared from Wood of Cherry Tree: Experimental Design Methodology and Artificial Neural Network. J. Mol. Liq. 2017, 229, 114–124. [Google Scholar] [CrossRef]
- Razali, S.Z.; Aziz, M.Y.; Edinur, H.A.; Razali Ishak, A. Adsorption of Methylene Blue onto Iron Oxide Magnetic Nanoparticles Coated with Sugarcane Bagasse. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012052. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Rama Devi, D.; Mohan Botsa, S.; Basavaiah, K. Facile Green Synthesis of Fe3O4 Nanoparticles Using Aqueous Leaf Extract of Zanthoxylum armatum DC. for Efficient Adsorption of Methylene Blue. J. Asian Ceram. Soc. 2018, 6, 145–155. [Google Scholar] [CrossRef]
- Kamaraj, M.; Srinivasan, N.R.; Assefa, G.; Adugna, A.T.; Kebede, M. Facile Development of Sunlit ZnO Nanoparticles-Activated Carbon Hybrid from Pernicious Weed as an Operative Nano-Adsorbent for Removal of Methylene Blue and Chromium from Aqueous Solution: Extended Application in Tannery Industrial Wastewater. Environ. Technol. Innov. 2020, 17, 100540. [Google Scholar] [CrossRef]
- Xu, Z.; He, P.; Yin, X.; Huang, Q.; Ding, W.; Xu, X.; Struik, P.C. Can the Advisory System Nutrient Expert® Balance Productivity, Profitability and Sustainability for Rice Production Systems in China? Agric. Syst. 2023, 205, 103575. [Google Scholar] [CrossRef]
- Řezbová, H.; Slaboch, J.; Mach, J. Emissions from Managed Agricultural Soils in Context of Consumption of Inorganic Nitrogen Fertilisers in Selected EU Countries. Agronomy 2023, 13, 159. [Google Scholar] [CrossRef]
- Smerald, A.; Kraus, D.; Rahimi, J.; Fuchs, K.; Kiese, R.; Butterbach-Bahl, K.; Scheer, C. A Redistribution of Nitrogen Fertiliser across Global Croplands Can Help Achieve Food Security within Environmental Boundaries. Commun. Earth Environ. 2023, 4, 315. [Google Scholar] [CrossRef]
- Higgins, S.; Keesstra, S.D.; Kadziuliene, Ž.; Jordan-Meille, L.; Wall, D.; Trinchera, A.; Spiegel, H.; Sandén, T.; Baumgarten, A.; Jensen, J.L.; et al. Stocktake Study of Current Fertilisation Recommendations across Europe and Discussion towards a More Harmonised Approach. Eur. J. Soil Sci. 2023, 74, e13422. [Google Scholar] [CrossRef]
- Głodniok, M.; Deska, M.; Kaszycki, P. Impact of the Stabilized Sewage Sludge-Based Granulated Fertilizer on Sinapis alba Growth and Biomass Chemical Characteristics. Biol. Life Sci. Forum 2021, 3, 35. [Google Scholar]
- Kechasov, D.; Verheul, M.J.; Paponov, M.; Panosyan, A.; Paponov, I.A. Organic Waste-Based Fertilizer in Hydroponics Increases Tomato Fruit Size but Reduces Fruit Quality. Front. Plant Sci. 2021, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Piersa, P.; Szufa, S.; Czerwinska, J.; Uenyay, H.; Adrian, L.; Wielgosinski, G.; Obraniak, A.; Lewandowska, W.; Marczak-Grzesik, M.; Dzikuc, M.; et al. Pine Wood and Sewage Sludge Torrefaction Process for Production Renewable Solid Biofuels and Biochar as Carbon Carrier for Fertilizers. Energies 2021, 14, 8176. [Google Scholar] [CrossRef]
- Escribà-Gelonch, M.; Butler, G.D.; Goswami, A.; Tran, N.N.; Hessel, V. Definition of Agronomic Circular Economy Metrics and Use for Assessment for a Nanofertilizer Case Study. Plant Physiol. Biochem. 2023, 196, 917–924. [Google Scholar] [CrossRef]
- Rabalao, T.M.; Ndaba, B.; Roopnarain, A.; Vatsha, B. Towards a Circular Economy: The Influence of Extraction Methods on Phytosynthesis of Metallic Nanoparticles and Their Impact on Crop Growth and Protection. JSFA Rep. 2022, 2, 208–221. [Google Scholar] [CrossRef]
- Jeet, K.; Kumar, V.; Anushree; Devi, R. Valorization of Agricultural Wastes: A Step Toward Adoption of Smart Green Materials with Additional Benefit of Circular Economy. In Handbook of Biomass Valorization for Industrial Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 343–367. [Google Scholar] [CrossRef]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.F.E.; Imran, M. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostructures (DJNB) 2018, 13, 315. [Google Scholar]
- Khodakovskaya, M.V.; Kim, B.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2012, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison Study of Zinc Nanoparticles and Zinc Sulphate on Wheat Growth: From Toxicity and Zinc Biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar forCucurbitaPepoL. J. Nanomater. 2019, 2019, 3476347. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent Advances in Nano-Enabled Fertilizers and Pesticides: A Critical Review of Mechanisms of Action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T. Molecular Mechanism of Nanofertilizer in Plant Growth and Development: A Recent Account. In Advances in Nanofertilizers and Nano-Pesticides in Agriculture; Woodhead Publishing: New Delhi, India, 2021; pp. 535–560. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nanofertilizers and Their Smart Delivery System. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Zahra, Z.; Habib, Z.; Hyun, H.; Shahzad, H.M.A. Overview on Recent Developments in the Design, Application, and Impacts of Nanofertilizers in Agriculture. Sustainability 2022, 14, 9397. [Google Scholar] [CrossRef]
- Singla, R.; Kumari, A.; Yadav, S.K. Impact of Nanomaterials on Plant Physiology and Functions. In Nanomaterials and Plant Potential; Springer: Cham, Swizerland, 2019; pp. 349–377. [Google Scholar] [CrossRef]
- Masarovičová, E.; Kráľová, K. Metal Nanoparticles and Plants/NanocząstkiMetaliczne I Rośliny. Ecol. Chem. Eng. S 2013, 20, 9–22. [Google Scholar] [CrossRef]
- Salama, D.M.; Abd El-aziz, M.E.; El-naggar, M.E.; Shaaban, E.A.; Abd El-Wahed, M.S. Synthesis of an Eco-Friendly Nanocomposite Fertilizer for Common Bean Based on Carbon Nanoparticles from Agricultural Waste Biochar. Pedosphere 2021, 31, 923–933. [Google Scholar] [CrossRef]
- Hussein, H.S.; Shaarawy, H.H.; Hussien, N.H.; Hawash, S.I. Preparation of Nanofertilizer Blend from Banana Peels. Bull. Natl. Res. Cent. 2019, 43, 26. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Soliman, M.I.; Abo Al-Saoud, A.M.; El-Sherbeny, G.A. Waste-Derived NPK Nanofertilizer Enhances Growth and Productivity of Capsicum annuum L. Plants 2021, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.; George, S.D.; Rao, P. Glycogen Nanoparticles as a Potential Corrosion Inhibitor. Int. J. Biol. Macromol. 2021, 182, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, S.; Gowthambabu, V.; Balamurugan, A.; Sudha, M.; Senthil Kumar, V.B.; Suresh, K.C. Rapid Green Synthesis of CuO Nanoparticles and Evaluation of Its Photocatalytic and Electrochemical Corrosion Inhibition Performance. Mater. Today Proc. 2021, 47, 1011–1016. [Google Scholar] [CrossRef]
- Syed Khadar, Y.A.; Surendhiran, S.; Gowthambabu, V.; Halimabi Alias Shakila Banu, S.; Devabharathi, V.; Balamurugan, A. Enhancement of Corrosion Inhibition of Mild Steel in Acidic Media by Green-Synthesized Nano-Manganese Oxide. Mater. Today Proc. 2021, 47, 889–893. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.T.; Zgârian, R.G.; Fierascu, I.; Baroi, A.M.; Răileanu, S.; Fierăscu, R.C. Metallic and Metal Oxides Nanoparticles for Sensing Food Pathogens—An Overview of Recent Findings and Future Prospects. Materials 2022, 15, 5374. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Adessi, A. Cyanoremediation and Phyconanotechnology: Cyanobacteria for Metal Biosorption toward a Circular Economy. Front. Microbiol. 2023, 14, 1166612. [Google Scholar] [CrossRef]
- Maddaloni, M.; Alessandri, I.; Vassalini, I. Food-Waste Enables Carboxylated Gold Nanoparticles to Completely Abat Hexavalent Chromium in Drinking Water. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100686. [Google Scholar] [CrossRef]
- Lokhande, R.S.; Singare, P.U.; Pimple, D.S. Toxicity study of heavy metals pollutants in wastewater effluent samples collected from Taloja industrial estate of Mumbai, India. Resour. Environ. 2011, 1, 13–19. [Google Scholar]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of Water-Stable Metal-Organic Frameworks in the Removal of Water Pollutants: A Review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Salazar Sandoval, S.; Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N.; Rojas-Romo, C.; González-Casanova, J.; Gómez, D.R.; Yutronic, N.; Urzúa, M.; et al. Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications. Coatings 2023, 13, 2085. [Google Scholar] [CrossRef]
- Fu, L.; Li, X.; Yu, J.; Ye, J. Facile and Simultaneous Stripping Determination of Zinc, Cadmium and Lead on Disposable Multiwalled Carbon Nanotubes Modified Screen-Printed Electrode. Electroanalysis 2013, 25, 567–572. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive Electrochemical Detection of Arsenic (III) Using Gold Nanoparticle Modified Carbon Nanotubes via Anodic Stripping Voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.; Jin, H.; Zhuang, J.; Zhang, W.; Wang, S.; Wang, J. Synthesis of Au-Decorated Tripod-Shaped Te Hybrids for Applications in the Ultrasensitive Detection of Arsenic. ACS Appl. Mater. Interfaces 2013, 5, 5733–5740. [Google Scholar] [CrossRef]
- Yu, C.-J.; Tseng, W.-L. Colorimetric Detection of Mercury(II) in a High-Salinity Solution Using Gold Nanoparticles Capped with 3-Mercaptopropionate Acid and Adenosine Monophosphate. Langmuir 2008, 24, 12717–12722. [Google Scholar] [CrossRef]
- Hong, S.; Park, S.; Lee, S.; Yang, Y.I.; Song, H.D.; Yi, J. The Sensitive, Anion-Selective Detection of Arsenate with Poly(Allylamine Hydrochloride) by Single Particle Plasmon-Based Spectroscopy. Anal. Chim. Acta 2011, 694, 136–141. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, Y.; Zhou, K.; Yu, Z. Screen Graphene-Printed Electrode for Trace Cadmium Detection in Rice Samples Combing with Portable Potentiostat. Int. J. Electrochem. Sci. 2018, 13, 6347–6357. [Google Scholar] [CrossRef]
- Ruengpirasiri, P.; Punrat, E.; Chailapakul, O.; Chuanuwatanakul, S. Graphene Oxide-Modified Electrode Coated with In-situ Antimony Film for the Simultaneous Determination of Heavy Metals by Sequential Injection-Anodic Stripping Voltammetry. Electroanalysis 2016, 29, 1022–1030. [Google Scholar] [CrossRef]
- Chen, K.; Lu, G.; Chang, J.; Mao, S.; Yu, K.; Cui, S.; Chen, J. Hg(II) Ion Detection Using Thermally Reduced Graphene Oxide Decorated with Functionalized Gold Nanoparticles. Anal. Chem. 2012, 84, 4057–4062. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. A Screen-Printed Voltammetric Electronic Tongue for the Analysis of Complex Mixtures of Metal Ions. Sens. Actuators B Chem. 2017, 250, 393–401. [Google Scholar] [CrossRef]
- Deshmukh, M.K.G.; Sameeroddin, M.; Abdul, D.; Sattar, M.A. Renewable energy in the 21st century: A review. Mater. Today Proc. 2023, 80, 1756–1759. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F. Future Role of Bioenergy. In The Role of Bioenergy in the Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 435–547. [Google Scholar]
- Duarah, P.; Haldar, D.; Patel, A.K.; Dong, C.-D.; Singhania, R.R.; Purkait, M.K. A review on global perspectives of sustainable development in bioenergy generation. Bioresour. Technol. 2022, 348, 126791. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R.A. Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C.; International Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Ripple, W.J.; Wolf, C.; Newsome, T.W.; Gregg, J.W.; Lenton, M.; Barnard, P.; Moomaw, W.R. World scientists warning of climate emergency 2021. BioScience 2021, 71, 894–898. [Google Scholar] [CrossRef]
- World Population Clock. Available online: https://www.worldometers.info/world-population/ (accessed on 8 April 2023).
- Berahab, R. Global Trends in the Energy Sector and Their Implication on Energy Security in NATO’s Southern Neighbourhood; Elcano Royal Institute: Madrid, Spain, 2020. [Google Scholar]
- European Commission. ‘Fit for 55′ Delivering the EU’s 2030 Climate Target on the Way to Climate Neutrality; Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions Empty; European Commision: Brussels, Berlgium, 2021. [Google Scholar]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Arya, I.; Poona, A.; Dikshit, P.K.; Pandit, S.; Kumar, J.; Singh, H.N.; Jha, N.K.; Rudayni, H.A.; Chaudhary, A.A.; Kumar, S. Current Trends and Future Prospects of Nanotechnology in Biofuel Production. Catalysts 2021, 11, 1308. [Google Scholar] [CrossRef]
- Mena-Cervantes, V.Y.; Hernández-Altamirano, R.; García-Solares, S.M.; Arreola-Valerio, E. Biodiesel in Circular Economy. In Biofuels in Circular Economy; Springer: Singapore, 2022; pp. 251–278. [Google Scholar] [CrossRef]
- Rozina; Chia, S.R.; Ahmad, M.; Sultana, S.; Zafar, M.; Asif, S.; Bokhari, A.; Nomanbhay, S.; Mubashir, M.; Khoo, K.S.; et al. Green Synthesis of Biodiesel from Citrus Medica Seed Oil Using Green Nanoparticles of Copper Oxide. Fuel 2022, 323, 124285. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, F.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.-H. Enhanced Hydrogen Production in a UASB Reactor by Retaining Microbial Consortium onto Carbon Nanotubes (CNTs). Int. J. Hydrogen Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic Role of Selected Noble Metal Nanoparticles in Medicine. Crit. Rev. Microbiol. 2015, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, N.; Ahamed, M.I.; Ahmed, A.; Inamuddin; Rahman, M.; Asiri, A.M. Functionalized Magnetic Nanoparticle-Reduced Graphene Oxide Nanocomposite for Enzymatic Biofuel Cell Applications. Int. J. Hydrogen Energy 2019, 44, 28294–28304. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.B.; Cornejo, D.R.; Costa, A.C.F.M. Magnetic Nanocatalysts of Ni0.5Zn0.5Fe2O4 Doped with Cu and Performance Evaluation in Transesterification Reaction for Biodiesel Production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]
- Cherian, E.; Dharmendirakumar, M.; Baskar, G. Immobilization of Cellulase onto MnO2 Nanoparticles for Bioethanol Production by Enhanced Hydrolysis of Agricultural Waste. Chin. J. Catal. 2015, 36, 1223–1229. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Fe Nanoparticles Using Eucalyptus Leaf Extracts for Treatment of Eutrophic Wastewater. Sci. Total Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, Y.; Wang, P.; Wang, C.; Miao, L.; Wang, X.; Lv, B.; You, G.; Liu, Z. Effects of CeO2, CuO, and ZnO Nanoparticles on Physiological Features of Microcystis Aeruginosa and the Production and Composition of Extracellular Polymeric Substances. Environ. Sci. Pollut. Res. 2016, 24, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sugsaisakon, S.; Kittipongvises, S. Impacts of the Nationally Determined Contribution to the 1.5 °C Climate Goal and Net-Zero Target on Citywide Greenhouse Gas Emissions: A Case Study on Bangkok, Thailand. Environ. Dev. Sustain. 2024; 1–18. [Google Scholar] [CrossRef]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the Treatment of Heavy Metal Contaminated Water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef]
- Sai Ram, M.; Singh, L.; Suryanarayana, M.V.S.; Alam, S.I. Effect of Iron, Nickel and Cobalt on Bacterial Activity and Dynamics During Anaerobic Oxidation of Organic Matter. Water Air Soil Pollut. 2000, 117, 305–312. [Google Scholar] [CrossRef]
- Hart, A. Circular Economy: Closing the Catalyst Loop with Metal Reclamation from Spent Catalysts, Industrial Waste, Waste Shells and Animal Bones. Biomass Convers. Biorefinery 2021, 13, 11483–11498. [Google Scholar] [CrossRef]
- Wrasman, C.J.; Wilson, A.N.; Mante, O.D.; Iisa, K.; Dutta, A.; Talmadge, M.S.; Dayton, D.C.; Uppili, S.; Watson, M.J.; Xu, X.; et al. Catalytic Pyrolysis as a Platform Technology for Supporting the Circular Carbon Economy. Nat. Catal. 2023, 6, 563–573. [Google Scholar] [CrossRef]
- Chen, Z.; Yun, S.; Wu, L.; Zhang, J.; Shi, X.; Wei, W.; Liu, Y.; Zheng, R.; Han, N.; Ni, B.-J. Waste-Derived Catalysts for Water Electrolysis: Circular Economy-Driven Sustainable Green Hydrogen Energy. Nano-Micro Lett. 2022, 15, 4. [Google Scholar] [CrossRef]
- Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Understanding the Role of Nanomaterials in Agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 271–288. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Ishizaki, T.; Kosaka, S.; Takahashi, N.; Isomura, N.; Seki, J.; Matsuoka, Y.; Oh-ishi, K.; Oshima, A.; Kitazumi, K.; et al. Electrochemical CO2 Reduction over Nanoparticles Derived from an Oxidized Cu–Ni Intermetallic Alloy. Chem. Commun. 2020, 56, 15008–15011. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A Review of the Use of Engineered Nanomaterials to Suppress Plant Disease and Enhance Crop Yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Chen, P.; Fang, X.; Du, T. Synthesis of La and Ce Modified X Zeolite from Rice Husk Ash for Carbon Dioxide Capture. J. Mater. Res. Technol. 2020, 9, 4368–4378. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Li, F.-Y.; Lin, P.-Y.; Beck, D.E.; Kirankumar, R.; Wang, G.-J.; Hsieh, S. CaO Recovered from Eggshell Waste as a Potential Adsorbent for Greenhouse Gas CO2. J. Environ. Manag. 2021, 297, 113430. [Google Scholar] [CrossRef]
- Eurostat. 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/AEI_FM_SALPEST09/default/table?lang=en (accessed on 20 May 2021).
- Kim, Y.; Tanaka, K.; Matsuoka, S. Environmental and economic effectiveness of the Kyoto Protocol. PLoS ONE 2020, 15, e0236299. [Google Scholar] [CrossRef] [PubMed]
- Gross, M. Food security in the times of climate change. Curr. Biol. 2013, 23, R1–R4. [Google Scholar] [CrossRef] [PubMed]
- Vanathi, P.; Rajiv, P.; Sivaraj, R. Synthesis and Characterization of Eichhornia-Mediated Copper Oxide Nanoparticles and Assessing Their Antifungal Activity against Plant Pathogens. Bull. Mater. Sci. 2016, 39, 1165–1170. [Google Scholar] [CrossRef]
- Hassan, S.; Karaila, G.K.; Singh, P.; Meenatchi, R.; Venkateswaran, A.S.; Ahmed, T.; Bansal, S.; Kamalraj, R.; Kiran, G.S.; Selvin, J. Implications of Fungal Nanotechnology for Sustainable Agriculture- Applications and Future Perspectives. Biocatal. Agric. Biotechnol. 2024, 103110. [Google Scholar] [CrossRef]
- Intisar, A.; Ramzan, A.; Sawaira, T.; Kareem, A.T.; Hussain, N.; Din, M.I.; Bilal, M.; Iqbal, H.M.N. Occurrence, Toxic Effects, and Mitigation of Pesticides as Emerging Environmental Pollutants Using Robust Nanomaterials—A Review. Chemosphere 2022, 293, 133538. [Google Scholar] [CrossRef]
- Bindra, H.S.; Singh, B. Nanofertilizers and Nanopesticides: Future of Plant Protection. In Advances in Nanofertilizers and Nano-Pesticides in Agriculture; Woodhead Publishing: New Delhi, India, 2021; pp. 57–84. [Google Scholar] [CrossRef]
- Moorthi, P.V.; Balasubramanian, C.; Mohan, S. An Improved Insecticidal Activity of Silver Nanoparticle Synthesized by Using SargassumMuticum. Appl. Biochem. Biotechnol. 2014, 175, 135–140. [Google Scholar] [CrossRef]
- Devi, G.D.; Murugan, K.; Selvam, C.P. Green synthesis of silver nanoparticles using Euphorbia hirta (Euphorbiaceae) leaf extract against crop pest of cotton bollworm, Helicoverpaarmigera (Lepidoptera: Noctuidae). J. Biopestic. 2014, 7, 54. [Google Scholar]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Dinesh, D.; Panneerselvam, C.; Subramaniam, J.; Kumar, P.M.; Suresh, U.; Roni, M.; et al. S argassum muticum-Synthesized Silver Nanoparticles: An Effective Control Tool against Mosquito Vectors and Bacterial Pathogens. Parasitol. Res. 2015, 114, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Kamil, D.; Prameeladevi, T.; Ganesh, S.; Prabhakaran, N.; Nareshkumar, R.; Thomas, S.P. Green Synthesis of Silver Nanoparticles by Entomopathogenic Fungus Beauveria bassiana and Their Bioefficacy against Mustard Aphid (Lipaphis erysimi Kalt.); NISCAIR-CSIR: New Delhi, India, 2017. [Google Scholar]
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Nicoletti, M.; Madhiyazhagan, P.; Dinesh, D.; Suresh, U.; Khater, H.F.; Wei, H.; et al. Characterization and Biotoxicity of HypneaMusciformis-Synthesized Silver Nanoparticles as Potential Eco-Friendly Control Tool against Aedes Aegypti and PlutellaXylostella. Ecotoxicol. Environ. Saf. 2015, 121, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Siva, C.; Kumar, M.S.; Nagar, G.; Nadu, T.; Nagar, G.; Nadu, T. The pesticidal activity of eco-friendly synthesized silver nanoparticles using Aristolochiaindica extracts against HelicoverpaarmigeraHubner (Lepidoptera: Noctuidae). Int. J. Adv. Sci. Tech. Res. 2015, 2, 197–226. [Google Scholar]
- Zahir, A.A.; Bagavan, A.; Kamaraj, C.; Elango, G.; Rahuman, A.A. Efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae. J. Biopestic. 2012, 5, 95. [Google Scholar]
- Chandrashekharaiah, M.; Kandakoor, S.B.; Basana Gowda, G.; Kammar, V.; Chakravarthy, A.K. Nanomaterials: A Review of Their Action and Application in Pest Management and Evaluation of DNA-Tagged Particles. In New Horizons in Insect Science: Towards Sustainable Pest Management; Springer: New Delhi, India, 2015; pp. 113–126. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Madasamy, M.; Anbu Radhika, S. Insecticidal activity of bio-silver and gold nanoparticles against Pericallia ricini fab. (Lepidaptera: Archidae). J. Biopestic. 2016, 9, 63–72. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Taha, A.E.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Elshaer, N. Ecofriendly Synthesis and Insecticidal Application of Copper Nanoparticles against the Storage Pest TriboliumCastaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Muthamil Selvan, S.; Vijai Anand, K.; Govindaraju, K.; Tamilselvan, S.; Kumar, V.G.; Subramanian, K.S.; Kannan, M.; Raja, K. Green Synthesis of Copper Oxide Nanoparticles and Mosquito Larvicidal Activity against Dengue, Zika and Chikungunya Causing Vector Aedes Aegypti. IET Nanobiotechnol. 2018, 12, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Stadler, T.; Buteler, M.; Weaver, D.K. Novel Use of Nanostructured Alumina as an Insecticide. Pest Manag. Sci. 2010, 66, 577–579. [Google Scholar] [CrossRef]
- Hamza, R.Z.M.M. Larvicidal, antioxidant activities and perturbation of transaminases activities of titanium dioxide nanoparticles synthesized using Moringa oleifera leaves extract against the red palm WEEVIL (Rhynchophorusferrugineus). Innovare Acad. Sci. 2015, 49–54. [Google Scholar]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate Material Delivery to Plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Santiago, E.F.; Pontes, M.S.; Arruda, G.J.; Caires, A.R.L.; Colbeck, I.; Maldonado-Rodriguez, R.; Grillo, R. Understanding the Interaction of Nanopesticides with Plants. In Nanopesticides; Springer: Cham, Switzerland, 2020; pp. 69–109. [Google Scholar] [CrossRef]
- Patil, C.D.; Borase, H.P.; Suryawanshi, R.K.; Patil, S.V. Trypsin Inactivation by Latex Fabricated Gold Nanoparticles: A New Strategy towards Insect Control. Enzym. Microb. Technol. 2016, 92, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hák, T.; Janoušková, S.; Moldan, B. Sustainable Development Goals: A Need for Relevant Indicators. Ecol. Indic. 2016, 60, 565–573. [Google Scholar] [CrossRef]


| Plant | Nanoparticles | Applications | Reference |
|---|---|---|---|
| Ficuscarica | Fe3O4 | Antioxidant | [16] |
| Azadirachtaindica | CuO | Anticancer | [16] |
| Peltophorumpterocarpum | Fe3O4 | Degradation of rhodomine | [17] |
| Terminalia chebula | Fe3O4 | Degradation of MB | [17] |
| Punicagranatum | ZnO | Antibacterial | [18] |
| Lactucaserriols | NiO | Dye degradation | [19] |
| Vitisrotundifolia | CoO | Acid blue dye degradation | [20] |
| Ziziphus spina-christi | ZnO-SeO | Antimicrobial/antioxidant activity | [21] |
| Seriphidiumoliverianum | CuO | Photocatalytic dye degradation from water | [22] |
| Punicagranatum | Ag2O | Antibiotic removal from wastewater | [23] |
| Jacaranda mimosaefolia | Cu | Corrosion inhibition | [24] |
| Scallion’s peel | ZnO | Nanofertilizer | [25] |
| FicusBenjamina | TiO2 | Heavy metal detection | [26] |
| watermelon | CaO | The catalyst for biofuel production | [27] |
| Cola nitida | FeO | Absorption of MB/MO dye from wastewater | [28] |
| Nanoparticle | Plant | Effect | Efficacy | Reference |
|---|---|---|---|---|
| Glycogen NP | Biogenic sources | Controlled the corrosion of zinc in sulfamic acid (NH2SO3H) | 92% for 0.02 gL−1 | [23] |
| CuO | Moringa oleifera leaf extract | Improved overall anticorrosive activity | 56% | [23] |
| Manganese oxide | Rose petal (RP) and lotus petal (LP) | Overall anticorrosion behaviour of mild steel increased | 72.63% | [23] |
| Ag | Citrus reticulata peels extract | Inhibited steel corrosion from HCl | 93.9% at 303 K and 90.3% at 333 K | [54] |
| Ag | Palm oil leaf extracts | A protective film formed, which protected the steel from acid attack | 94.1% | [55] |
| Ag nanocomposite | Red onion peels | A surface protection layer formed against corrosion | 86% | [56] |
| Cellulose nanocrystal | Organic product | Protected AISI360-steel from corrosion in petroleum manufacturing | 85.3% at 300 mgL−1 | [57] |
| CuO/melamine/cellulose nanocrystals nanocomposite | Organic product | Protected AISI360-steel from corrosion in petroleum manufacturing | 96.8% at 300 mgL−1 | [58] |
| NiO/melamine/cellulose nanocrystals nanocomposite | Organic product | Protected AISI360 steel from corrosion in petroleum manufacturing | 98.3% at 300 mgL−1 | [59] |
| Nanoparticle | Plant Affected | Effect | Reference |
|---|---|---|---|
| Hydroxylapatite (Ca5(PO4)3OH) | Soybean (Glycine max) | Increase of 33% growth rate and 20% seed yield | [79] |
| AgNPs | Red ginseng shoot | Ginsenoside content increased | [80] |
| TiO2 | Aged spinach seeds | Increased germination rate due to increase in nitrogen assimilation | [81] |
| Iron oxide | Soybean | 48% increase in grain yield | [82] |
| Ag | Fusarium solani | Reduced fungal infection | [83] |
| C nanoparticle | Phaseolus vulgaris L. | Improved the quality and constituents of leaves and seeds | [84] |
| K+, Fe, tryptophan, urea, amino acids | Tomato, fenugreek | Increased germination percentage of tomato from 14% to 97% and fenugreek from 25% to 93.14% | [85] |
| Nano-NPK | Capsicum annuum leaves | Resulted in better fruit quality and increased the yield | [85] |
| Nanoparticles | Heavy Metal Detected | Limit of Detection | Reference |
|---|---|---|---|
| Multiwalled carbon nanotube | Zn (II) | 0.3 μgL−1 | [93] |
| Multiwalled carbon nanotube | Pb (II) | 0.07 μgL−1 | [93] |
| Multiwalled carbon nanotube | Cd (II) | 0.1 μgL−1 | [93] |
| CNT/Pt | As (III) | - | [94] |
| Au-decorated Te hybrids | As (III) | 0.0026 ppb | [95] |
| AuNP | Hg (II) | - | [96] |
| AuNP | As | 0.01 μM | [97] |
| Graphene | Cd (II) | 10−7 M | [98] |
| Graphene oxide | Cd (II) | 0.1–1.5 μM | [99] |
| Graphene oxide | Hg (II) | 2.5 × 10−8 M | [100] |
| AuNP | Cr | 0.01 μM | [100] |
| Carbon nanofibers | Bi (III) | 16.8 μgL−1 | [101] |
| Carbon nanofibers | In (III) | 3 μgL−1 | [101] |
| Nanoparticles | Effect | Reference |
|---|---|---|
| Carbon nanotubes | Their use in biosensors and microbial fuel cell fabrication as well as a catalyst in biofuel production raise the overall concentration of enzymes in biofuel generation, as well as help in enzyme mobilization | [114,115] |
| Aniline incorporated with Fe3O4-NH2 and reduced graphene oxide nanocomposites | Enhances the process of bio-electrocatalysis of glucose oxidase | [116] |
| Magnetic nanoferrites doped with calcium | Raises biodiesel production yield | [117] |
| MnO2 with sugarcane leaf | Increases bioethanol synthesis | [118] |
| Nano zero-valent iron (nZVI) and Fe2O3 | Improves the production of biogas like methane | [119] |
| CeO2 | Improves the production of biogas | [120] |
| Pt and silica | Raises methane production yield | [121] |
| Ni and silica | Raises methane production yield | [121] |
| Co and silica | Raises methane production yield | [121] |
| Fe and silica | Raises methane production yield | [121] |
| Nanoparticle | Treated along with | Period of Experiment | Temperature | Reference |
|---|---|---|---|---|
| CoNP-treated cocoa shell | Cocoa shell and 3-aminopropyltriethoxysilane | 25 °C | [130] | |
| Magnetite nanocapsule nanocomposites | Polyaniline | 90 min | 28 °C | [130] |
| Porous silica nanoparticles | Polyethyleneimine | 30 min | 75 °C | [131] |
| La and Ce | Zeolite | - | 0, 30, 60 °C | [131] |
| CaO | Egg shell waste | 23 min | 700–900 °C | [132] |
| MgO | Graphene oxide | - | 60–120 °C | [132] |
| Nanoparticles | Activity | Pests Affected | Reference |
|---|---|---|---|
| ZnO | Blocks the organism | Fusarium graminearum, Penicillium expansum, Alternaria alternate, F. oxysporum, Rhizopus stolonifer, Mucorplumbeus, Pseudomonas aeruginosa and Aspergillus flavus | [127,128,136] |
| MO | Stops fungal conidiophores and conidia growth on vegetative parts of fungi | Conidia and conidiophores of fungi | [137] |
| C nanotubes | Raises the nutrients and elemental uptake by plants and is also involved in ameliorating the development of plants | [138,139] | |
| Ag | Used to control agricultural pests and organisms | Helicoverpaarmigera, Ariadne merione, Pediculushumanus, Aedesstephensi, Aedes aegypti, Culex quinquefasciatus, Lipaphiserysimiwas, Plutellaxylostella, Helicoverpaarmigera and Sitophilus oryzae. | [140,141,142,143,144,145,146] |
| Cd | Causes larval death of 93.79% at 2400 ppm | Spodopteralitura | [147] |
| TiO2 | Causes larval death of 73.79% at 2400 ppm | Spodopteralitura | [147] |
| Pungam oil-based AuNPs | Causes high mortality of pests | Pericalliaricini larvae | [148] |
| Cu | Causes toxicity against pests | Triboliumcastaneum, Spodopteralittoralis larvae, Aedes aegypti larvae | [149,150] |
| Nanostructured alumina (Al2O3) | Causes mortality when exposed to wheat pests | Sitophilus oryzae, and Rhizopertha dominica | [151] |
| Al | Kills the pest | S. oryzae | [152] |
| TiO2 | Destroys the pest | S. oryzae | [152] |
| Nanosilica | Enters inside the pest from the cuticle, thus destroying the pest | Different pests | [153] |
| Nanosphere of silica | Helps bactericides to enter into plant cell sap | - | [154] |
| Bioactive silver | Lags the action of trypsin, hence, makes the pest harmless | Different pests | [155] |
| AuNPs with protein | Improves catalytic inhibition | - | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, M.K.; Thakuri, B.S.; Pant, J.; Gardas, R.L.; Bhattarai, A. The Multifaceted Perspective on the Role of Green Synthesis of Nanoparticles in Promoting a Sustainable Green Economy. Sustain. Chem. 2024, 5, 40-59. https://doi.org/10.3390/suschem5020004
Sah MK, Thakuri BS, Pant J, Gardas RL, Bhattarai A. The Multifaceted Perspective on the Role of Green Synthesis of Nanoparticles in Promoting a Sustainable Green Economy. Sustainable Chemistry. 2024; 5(2):40-59. https://doi.org/10.3390/suschem5020004
Chicago/Turabian StyleSah, Manish Kumar, Biraj Shah Thakuri, Jyoti Pant, Ramesh L. Gardas, and Ajaya Bhattarai. 2024. "The Multifaceted Perspective on the Role of Green Synthesis of Nanoparticles in Promoting a Sustainable Green Economy" Sustainable Chemistry 5, no. 2: 40-59. https://doi.org/10.3390/suschem5020004
APA StyleSah, M. K., Thakuri, B. S., Pant, J., Gardas, R. L., & Bhattarai, A. (2024). The Multifaceted Perspective on the Role of Green Synthesis of Nanoparticles in Promoting a Sustainable Green Economy. Sustainable Chemistry, 5(2), 40-59. https://doi.org/10.3390/suschem5020004








