A Perspective on Solar-Driven Electrochemical Routes for Sustainable Methanol Production
Abstract
1. Introduction
2. Methods
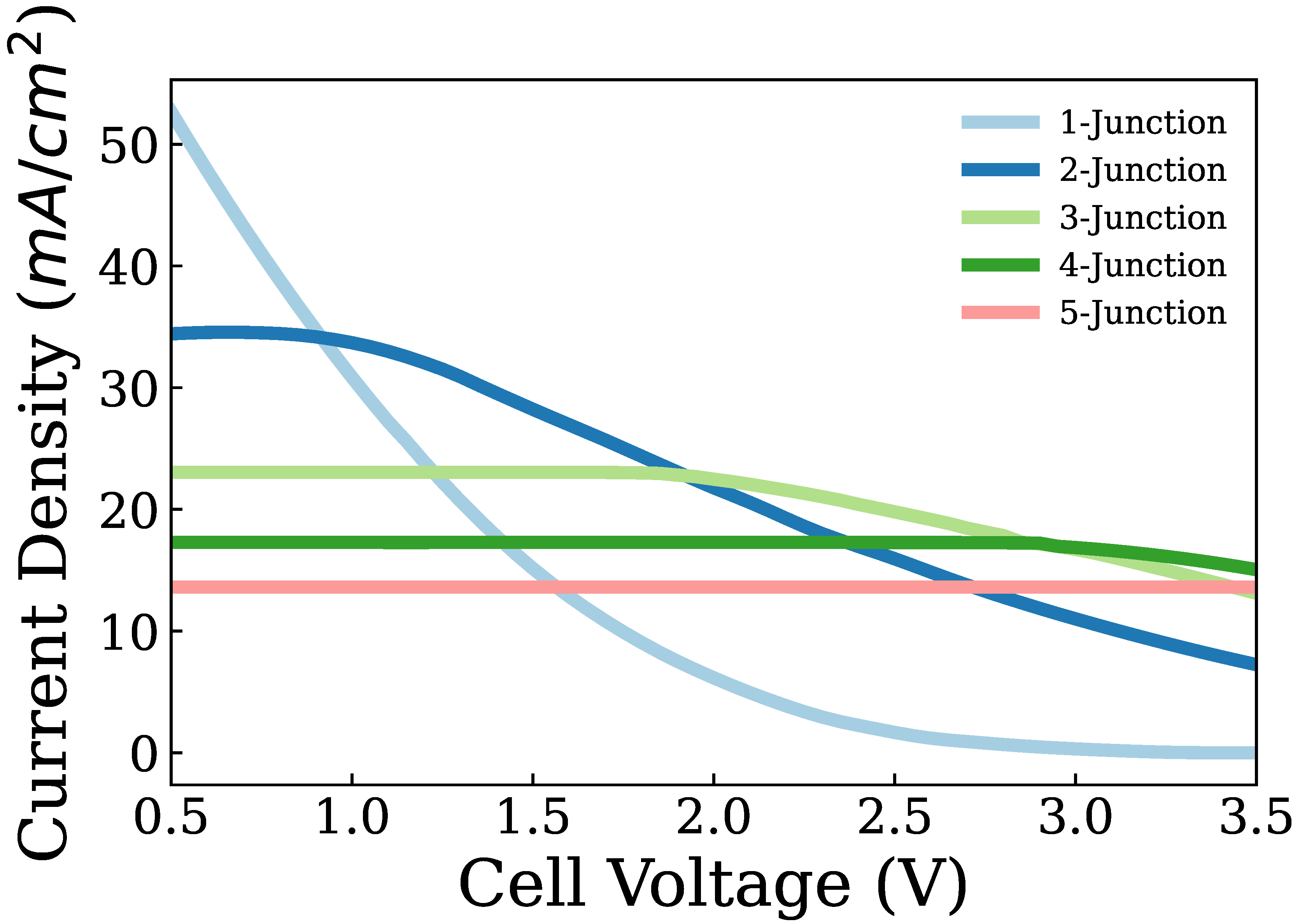
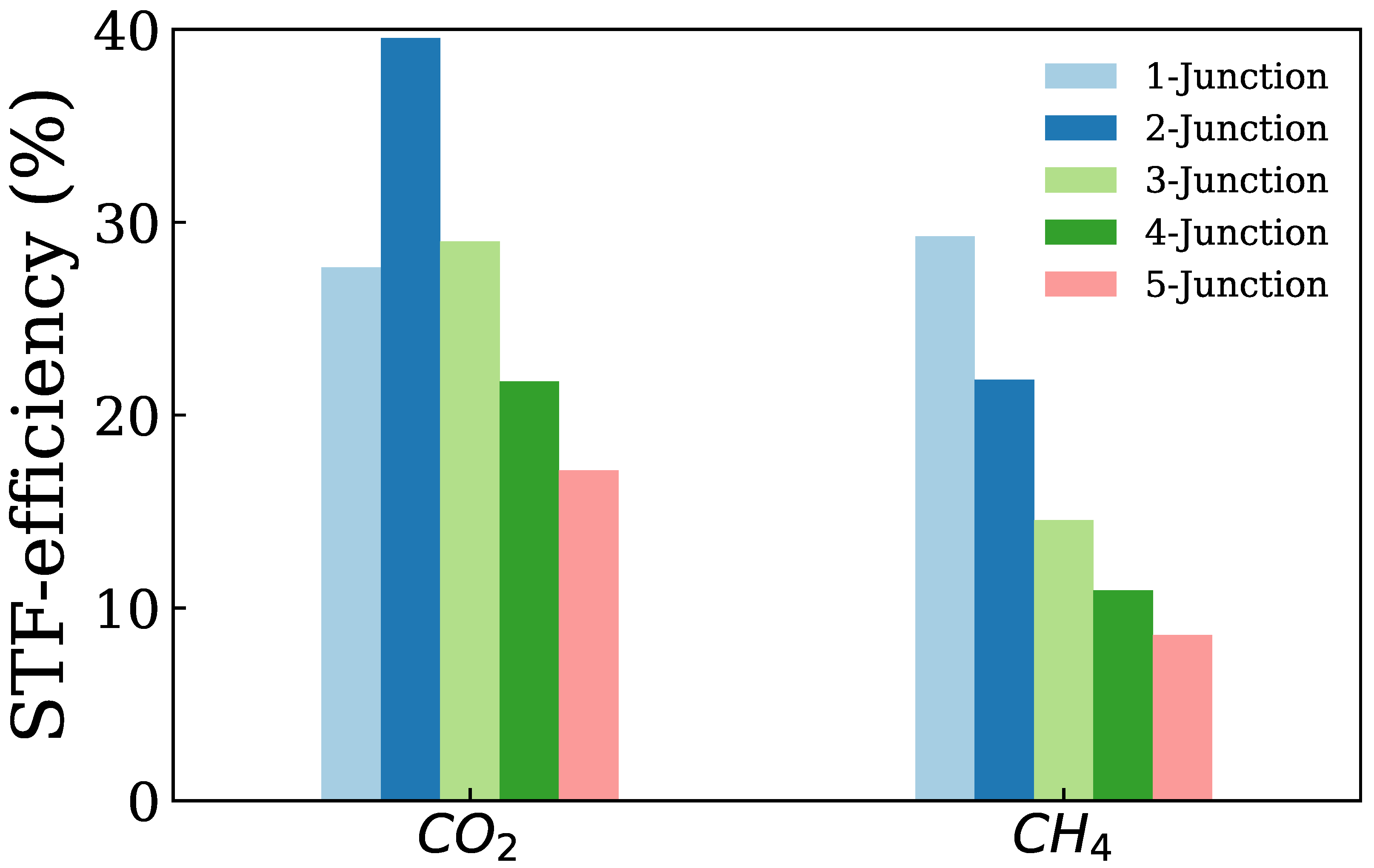
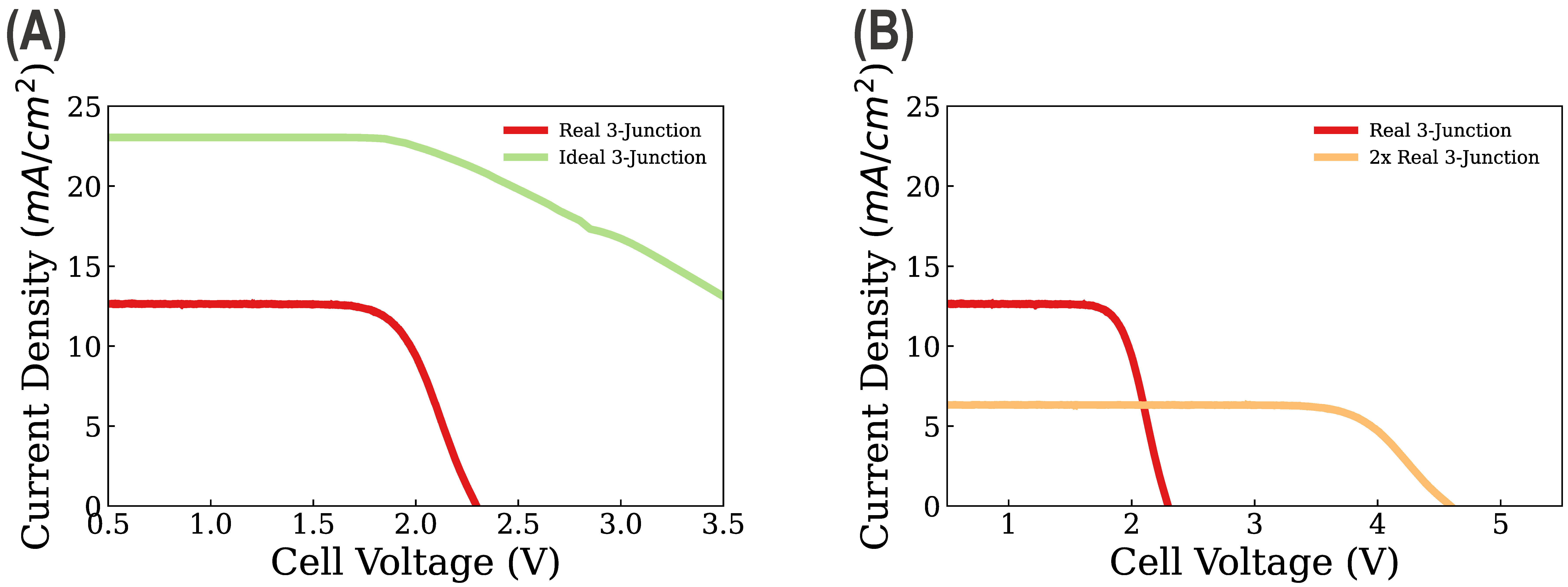
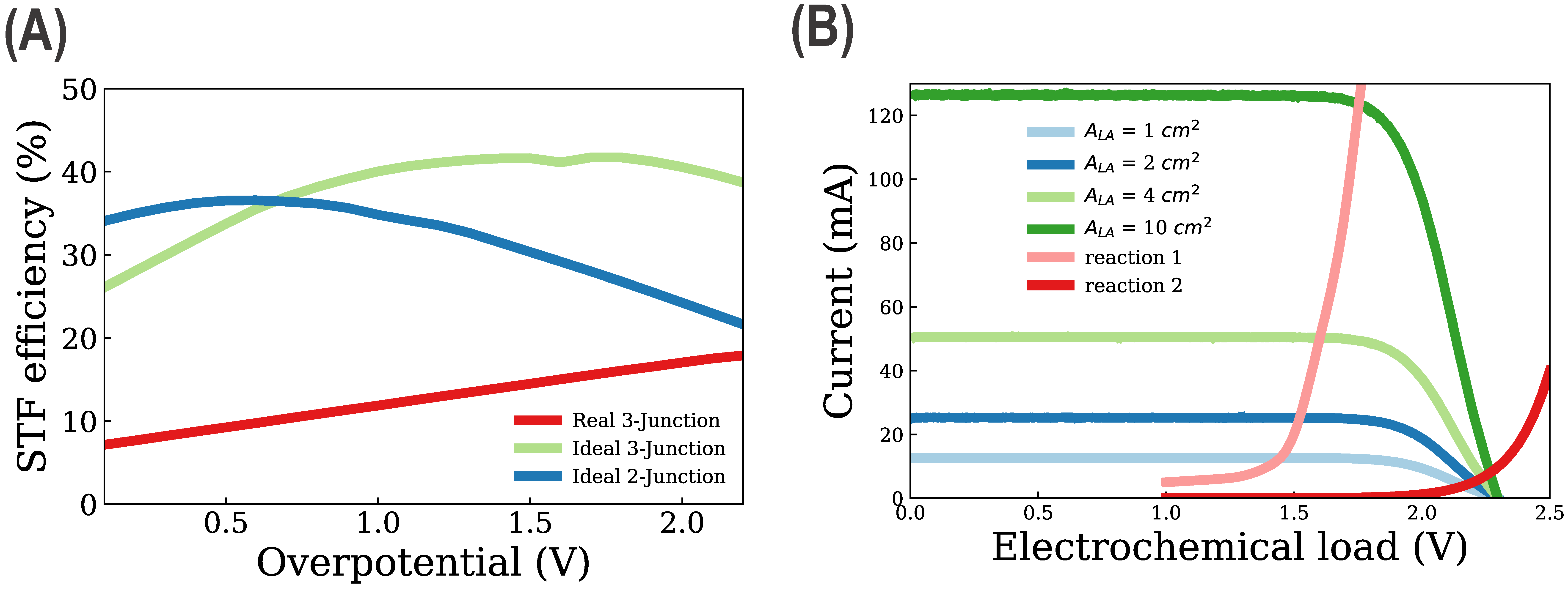
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, W.U.H.; Hao, G.; Yan, H.; Zhu, N.; Yasmeen, R.; Dincă, G. Role of renewable, non-renewable energy consumption and carbon emission in energy efficiency and productivity change: Evidence from G20 economies. Geosci. Front. 2023, 101631. [Google Scholar] [CrossRef]
- Bp Statistical Review of World Energy, 71st ed.; Pureprint Group Limited: London, UK, 2022.
- Sonthalia, A.; Kumar, N.; Tomar, M.; Edwin Geo, V.; Thiyagarajan, S.; Pugazhendhi, A. Moving ahead from hydrogen to methanol economy: Scope and challenges. Clean Technol. Environ. Policy 2021, 25, 551–575. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Djilali, N.; Yang, Y.; Ye, D.; Chen, R.; Liao, Q. Comparative well-to-pump assessment of fueling pathways for zero-carbon transportation in China: Hydrogen economy or methanol economy? Renew. Sustain. Energy Rev. 2022, 169, 112935. [Google Scholar] [CrossRef]
- Filosa, C.; Gong, X.; Bavykina, A.; Chowdhury, A.D.; Gallo, J.M.R.; Gascon, J. Enabling the Methanol Economy: Opportunities and Challenges for Heterogeneous Catalysis in the Production of Liquid Fuels via Methanol. Acc. Chem. Res. 2023, 56, 3492–3503. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.S. Beyond Oil and Gas: The Methanol Economy; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol production and applications: An overview. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. [Google Scholar]
- Alias, M.; Kamarudin, S.; Zainoodin, A.; Masdar, M. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Cox, C.R.; Lee, J.Z.; Nocera, D.G.; Buonassisi, T. Ten-percent solar-to-fuel conversion with nonprecious materials. Proc. Natl. Acad. Sci. USA 2014, 111, 14057–14061. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, I.J.; Lee, M.G.; Kwon, K.C.; Hong, S.-P.; Kim, D.H.; Lee, S.A.; Lee, T.H.; Kim, C.; Moon, C.W. Water splitting exceeding 17% solar-to-hydrogen conversion efficiency using solution-processed Ni-based electrocatalysts and perovskite/Si tandem solar cell. ACS Appl. Mater. Interfaces 2019, 11, 33835–33843. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sato, N.; Orita, M.; Kuang, Y.; Kaneko, H.; Minegishi, T.; Yamada, T.; Domen, K. Development of highly efficient CuIn0.5Ga0.5Se2-based photocathode and application to overall solar driven water splitting. Energy Environ. Sci. 2018, 11, 3003–3009. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Kato, N.; Mizuno, S.; Shiozawa, M.; Nojiri, N.; Kawai, Y.; Fukumoto, K.; Morikawa, T.; Takeda, Y. A large-sized cell for solar-driven CO2 conversion with a solar-to-formate conversion efficiency of 7.2%. Joule 2021, 5, 687–705. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R. Nanostructured photocatalysts for the production of methanol from methane and water. ChemSusChem 2021, 14, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Tang, Z.; Wu, X.; Huang, A.; Luo, X.; Xu, G.Q.; Zhu, Y.; Wang, S.L. Photocatalytic oxidation of methane to methanol by tungsten trioxide-supported atomic gold at room temperature. Appl. Catal. B Environ. 2022, 306, 120919. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Tomanec, O.; Petr, M.; Zhu Chen, J.; Miller, J.T.; Varma, R.S.; Gawande, M.B.; Zbořil, R. Carbon nitride-based ruthenium single atom photocatalyst for CO2 reduction to methanol. Small 2021, 17, 2006478. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Q.; Fu, Y.; Zhang, Y.; Hu, J.; Li, T.; Zhong, Q.; Fan, M.; Kung, H.H. Core–shell covalently linked graphitic carbon nitride–melamine–resorcinol–formaldehyde microsphere polymers for efficient photocatalytic CO2 reduction to methanol. J. Am. Chem. Soc. 2022, 144, 9576–9585. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.H.; Ambrosini, A.; Coker, E.N.; Miller, J.E.; Chueh, W.C.; O’Hayre, R.; Tong, J. Nonstoichiometric perovskite oxides for solar thermochemical H2 and CO production. Energy Procedia 2014, 49, 2009–2018. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: A review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Frese, K.; Leach, S. Electrochemical reduction of carbon dioxide to methane, methanol, and CO on Ru electrodes. J. Electrochem. Soc. 1985, 132, 259. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Electrochemical reduction of CO2 on Cu electrode in methanol at low temperature. In Utilization of Greenhouse Gases; ACS Publications: Washington, DC, USA, 2003. [Google Scholar]
- Chang, X.; Wang, T.; Zhang, P.; Wei, Y.; Zhao, J.; Gong, J. Stable aqueous photoelectrochemical CO2 reduction by a Cu2O dark cathode with improved selectivity for carbonaceous products. Angew. Chem. Int. Ed. 2016, 55, 8840–8845. [Google Scholar] [CrossRef]
- Kong, Q.; Kim, D.; Liu, C.; Yu, Y.; Su, Y.; Li, Y.; Yang, P. Directed assembly of nanoparticle catalysts on nanowire photoelectrodes for photoelectrochemical CO2 reduction. Nano Lett. 2016, 16, 5675–5680. [Google Scholar] [CrossRef]
- Nakada, A.; Koike, K.; Nakashima, T.; Morimoto, T.; Ishitani, O. Photocatalytic CO2 reduction to formic acid using a Ru (II)–Re (I) supramolecular complex in an aqueous solution. Inorg. Chem. 2015, 54, 1800–1807. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.; Chan, K.; Hahn, C. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Jouny, M.; Luc, W.; Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Shi, C.; Chan, K.; Yoo, J.S.; Nørskov, J.K. Barriers of electrochemical CO2 reduction on transition metals. Org. Process Res. Dev. 2016, 20, 1424–1430. [Google Scholar] [CrossRef]
- Frese, K.; Canfield, D. Reduction of CO2 on n-GaAs electrodes and selective methanol synthesis. J. Electrochem. Soc. 1984, 131, 2518. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Barton, E.E.; Rampulla, D.M.; Bocarsly, A.B. Selective solar-driven reduction of CO2 to methanol using a catalyzed p-GaP based photoelectrochemical cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. [Google Scholar] [CrossRef]
- Rajeshwar, K.; de Tacconi, N.R.; Ghadimkhani, G.; Chanmanee, W.; Janáky, C. Tailoring copper oxide semiconductor nanorod arrays for photoelectrochemical reduction of carbon dioxide to methanol. ChemPhysChem 2013, 14, 2251–2259. [Google Scholar] [CrossRef]
- Yuan, J.; Hao, C. Solar-driven photoelectrochemical reduction of carbon dioxide to methanol at CuInS2 thin film photocathode. Sol. Energy Mater. Sol. Cells 2013, 108, 170–174. [Google Scholar] [CrossRef]
- World’s Largest CO2-To-Methanol Plant Starts Production. Available online: https://www.carbonrecycling.is/news-media/worlds-largest-co2-to-methanol-plant-starts-production (accessed on 25 February 2024).
- Habibic, A. Yale-Based Start-Up Converts Captured CO2 into Methanol. Available online: https://www.offshore-energy.biz/yale-based-start-up-converts-captured-co2-into-methanol/ (accessed on 25 February 2024).
- Prajapati, A.; Collins, B.A.; Goodpaster, J.D.; Singh, M.R. Fundamental insight into electrochemical oxidation of methane towards methanol on transition metal oxides. Proc. Natl. Acad. Sci. USA 2021, 118, e2023233118. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.; Sartape, R.; Kani, N.C.; Gauthier, J.A.; Singh, M.R. Chloride-Promoted High-Rate Ambient Electrooxidation of Methane to Methanol on Patterned Cu–Ti Bimetallic Oxides. ACS Catal. 2022, 12, 14321–14329. [Google Scholar] [CrossRef]
- Spinner, N.; Mustain, W.E. Electrochemical methane activation and conversion to oxygenates at room temperature. ECS Trans. 2013, 53, 1. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Y.; Nan, G.; Chen, W.; Guo, Z.; Li, S.; Tang, Z.; Wei, W.; Sun, Y. Electrocatalytic oxidation of methane to ethanol via NiO/Ni interface. Appl. Catal. B Environ. 2020, 270, 118888. [Google Scholar] [CrossRef]
- Kim, R.S.; Surendranath, Y. Electrochemical reoxidation enables continuous methane-to-methanol catalysis with aqueous Pt salts. ACS Cent. Sci. 2019, 5, 1179–1186. [Google Scholar] [CrossRef]
- Ma, M.; Jin, B.J.; Li, P.; Jung, M.S.; Kim, J.I.; Cho, Y.; Kim, S.; Moon, J.H.; Park, J.H. Ultrahigh electrocatalytic conversion of methane at room temperature. Adv. Sci. 2017, 4, 1700379. [Google Scholar] [CrossRef]
- Omasta, T.J.; Rigdon, W.A.; Lewis, C.A.; Stanis, R.J.; Liu, R.; Fan, C.Q.; Mustain, W.E. Two pathways for near room temperature electrochemical conversion of methane to methanol. ECS Trans. 2015, 66, 129. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Moon, J.H. Solar cell-powered electrochemical methane-to-methanol conversion with CuO/CeO2 catalysts. ACS Energy Lett. 2021, 6, 893–899. [Google Scholar] [CrossRef]
- Bonchio, M.; Bonin, J.; Ishitani, O.; Lu, T.-B.; Morikawa, T.; Morris, A.J.; Reisner, E.; Sarkar, D.; Toma, F.M.; Robert, M. Best practices for experiments and reporting in photocatalytic CO2 reduction. Nat. Catal. 2023, 6, 657–665. [Google Scholar] [CrossRef]
- Seger, B.; Robert, M.; Jiao, F. Best practices for electrochemical reduction of carbon dioxide. Nat. Sustain. 2023, 6, 236–238. [Google Scholar] [CrossRef]
- Prajapati, A. Electrochemical Routes for Upgrading Carbon-Based Greenhouse Gases. Ph.D. Thesis, University of Illinois, Chicago, IL, USA, 2022. [Google Scholar]
- Sahara, G.; Kumagai, H.; Maeda, K.; Kaeffer, N.; Artero, V.; Higashi, M.; Abe, R.; Ishitani, O. Photoelectrochemical reduction of CO2 coupled to water oxidation using a photocathode with a Ru (II)–Re (I) complex photocatalyst and a CoOx/TaON photoanode. J. Am. Chem. Soc. 2016, 138, 14152–14158. [Google Scholar] [CrossRef]
- Schreier, M.; Curvat, L.; Giordano, F.; Steier, L.; Abate, A.; Zakeeruddin, S.M.; Luo, J.; Mayer, M.T.; Grätzel, M. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 2015, 6, 7326. [Google Scholar] [CrossRef]
- Sekimoto, T.; Shinagawa, S.; Uetake, Y.; Noda, K.; Deguchi, M.; Yotsuhashi, S.; Ohkawa, K. Tandem photo-electrode of InGaN with two Si pn junctions for CO2 conversion to HCOOH with the efficiency greater than biological photosynthesis. Appl. Phys. Lett. 2015, 106, 073902. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, R.; Sun, K.; Chen, Y.; Verlage, E.; Francis, S.A.; Lewis, N.S.; Xiang, C. Solar-driven reduction of 1 atm of CO2 to formate at 10% energy-conversion efficiency by use of a TiO2-protected III–V tandem photoanode in conjunction with a bipolar membrane and a Pd/C cathode. ACS Energy Lett. 2016, 1, 764–770. [Google Scholar] [CrossRef]
- He, J.; Janaky, C. Recent advances in solar-driven carbon dioxide conversion: Expectations versus reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Quek, W.K.; Kim, B.; Sugiarto, S.; Oh, J.; Kai, D. Pitfalls and protocols: Evaluating catalysts for CO2 reduction in electrolyzers based on gas diffusion electrodes. ACS Energy Lett. 2022, 7, 2012–2023. [Google Scholar] [CrossRef]
- Tan, J.; Kang, B.; Kim, K.; Kang, D.; Lee, H.; Ma, S.; Jang, G.; Lee, H.; Moon, J. Hydrogel protection strategy to stabilize water-splitting photoelectrodes. Nat. Energy 2022, 7, 537–547. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Maeda, K.; Liu, G.; Wang, L. Addressing the stability challenge of photo (electro) catalysts towards solar water splitting. Chem. Sci. 2023, 14, 3415–3427. [Google Scholar] [CrossRef]
- Garg, S.; Li, M.; Weber, A.Z.; Ge, L.; Li, L.; Rudolph, V.; Wang, G.; Rufford, T.E. Advances and challenges in electrochemical CO2 reduction processes: An engineering and design perspective looking beyond new catalyst materials. J. Mater. Chem. A 2020, 8, 1511–1544. [Google Scholar] [CrossRef]
- Liang, S.; Altaf, N.; Huang, L.; Gao, Y.; Wang, Q. Electrolytic cell design for electrochemical CO2 reduction. J. CO2 Util. 2020, 35, 90–105. [Google Scholar] [CrossRef]
- Garg, S.; Xie, Z.; Chen, J.G. Tandem reactors and reactions for CO2 conversion. Nat. Chem. Eng. 2024, 1, 139–148. [Google Scholar] [CrossRef]
- Kim, B.; Tan, Y.C.; Ryu, Y.; Jang, K.; Abbas, H.G.; Kang, T.; Choi, H.; Lee, K.-S.; Park, S.; Kim, W. Trace-level cobalt dopants enhance CO2 electroreduction and ethylene formation on copper. ACS Energy Lett. 2023, 8, 3356–3364. [Google Scholar] [CrossRef]
- Pan, F.; Yang, Y. Designing CO2 reduction electrode materials by morphology and interface engineering. Energy Environ. Sci. 2020, 13, 2275–2309. [Google Scholar] [CrossRef]
- Prajapati, A.; Singh, M.R. Assessment of artificial photosynthetic systems for integrated carbon capture and conversion. ACS Sustain. Chem. Eng. 2019, 7, 5993–6003. [Google Scholar] [CrossRef]
- Kim, B.; Seong, H.; Song, J.T.; Kwak, K.; Song, H.; Tan, Y.C.; Park, G.; Lee, D.; Oh, J. Over a 15.9% solar-to-CO conversion from dilute CO2 streams catalyzed by gold nanoclusters exhibiting a high CO2 binding affinity. ACS Energy Lett. 2019, 5, 749–757. [Google Scholar] [CrossRef]
- Bagchi, D.; Raj, J.; Singh, A.K.; Cherevotan, A.; Roy, S.; Manoj, K.S.; Vinod, C.; Peter, S.C. Structure-Tailored Surface Oxide on Cu–Ga Intermetallics Enhances CO2 Reduction Selectivity to Methanol at Ultralow Potential. Adv. Mater. 2022, 34, 2109426. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, X.; Wang, Y.; Xie, C. Electrochemical reduction of CO2 on RuO2/TiO2 nanotubes composite modified Pt electrode. Electrochim. Acta 2005, 50, 3576–3580. [Google Scholar] [CrossRef]
- Payra, S.; Shenoy, S.; Chakraborty, C.; Tarafder, K.; Roy, S. Structure-sensitive electrocatalytic reduction of CO2 to methanol over carbon-supported intermetallic PtZn nano-alloys. ACS Appl. Mater. Interfaces 2020, 12, 19402–19414. [Google Scholar] [CrossRef]
- Canfield, D.; Frese, K., Jr. Reduction of carbon dioxide to methanol on n-and p-GaAs and p-InP. Effect of crystal face, electrolyte and current density. J. Electrochem. Soc. 1983, 130, 1772–1773. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly efficient electroreduction of CO2 to methanol on palladium–copper bimetallic aerogels. Angew. Chem. 2018, 130, 14345–14349. [Google Scholar] [CrossRef]
- Frese, K.W. Electrochemical reduction of CO2 at intentionally oxidized copper electrodes. J. Electrochem. Soc. 1991, 138, 3338. [Google Scholar] [CrossRef]
- Bandi, A. Electrochemical reduction of carbon dioxide on conductive metallic oxides. J. Electrochem. Soc. 1990, 137, 2157. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.S.; Camargo, L.M.; Lanza, M.R.V.; Bertazzoli, R. A Feasibility Study of the Electro-recycling of Greenhouse Gases: Design and Characterization of a (TiO2/RuO2)/PTFE Gas Diffusion Electrode for the Electrosynthesis of Methanol from Methane. Electrocatalysis 2010, 1, 224–229. [Google Scholar] [CrossRef]
- Lee, B.; Hibino, T. Efficient and selective formation of methanol from methane in a fuel cell-type reactor. J. Catal. 2011, 279, 233–240. [Google Scholar] [CrossRef]
- Rocha, R.S.; Reis, R.M.; Lanza, M.R.; Bertazzoli, R. Electrosynthesis of methanol from methane: The role of V2O5 in the reaction selectivity for methanol of a TiO2/RuO2/V2O5 gas diffusion electrode. Electrochim. Acta 2013, 87, 606–610. [Google Scholar] [CrossRef]
- Kani, N.C.; Gauthier, J.A.; Prajapati, A.; Edgington, J.; Bordawekar, I.; Shields, W.; Shields, M.; Seitz, L.C.; Singh, A.R.; Singh, M.R. Solar-driven electrochemical synthesis of ammonia using nitrate with 11% solar-to-fuel efficiency at ambient conditions. Energy Environ. Sci. 2021, 14, 6349–6359. [Google Scholar] [CrossRef]
- Prajapati, A.; Kani, N.C.; Gauthier, J.A.; Sartape, R.; Xie, J.; Bessa, I.; Galante, M.T.; Leung, S.L.; Andrade, M.H.; Somich, R.T. CO2-free high-purity ethylene from electroreduction of CO2 with 4% solar-to-ethylene and 10% solar-to-carbon efficiencies. Cell Rep. Phys. Sci. 2022, 3, 101053. [Google Scholar] [CrossRef]
- Goldman, M.; Prajapati, A.; Duoss, E.; Baker, S.; Hahn, C. Bridging Fundamental Science and Applied Science to Accelerate CO2 Electrolyzer Scale up. Curr. Opin. Electrochem. 2023, 39, 101248. [Google Scholar] [CrossRef]

| Reaction | ||||
|---|---|---|---|---|
| ECR | 6 | 728.74 | 1.259 | |
| MOR | 2 | 121.94 | 0.632 |
| Catalyst | Electrolyte | Applied Potential (V) | Methanol Current Density (mA/cm2) | Faradaic Efficiency (%) | References | |
|---|---|---|---|---|---|---|
| CO2 | CuGa2 (GDE) | CO2 gas with 1 M KOH | −0.3 vs. RHE | 21.4 | 77.26 | [65] |
| RuO2/TiO2 nanotubes (NTs) | 0.5 M NaHCO3 | −0.8 vs. SCE | 1.2 | 60.5 | [66] | |
| PtZn nano-alloys | 0.1 M NaHCO3 | −0.90 vs. RHE | 3.75 | 81.4 | [67] | |
| n-GaAs-crystal-(111)As | 0.2 M Na2SO4 | −1.20 to −1.40 vs. SCE | 0.16–0.2 | 100 | [68] | |
| Pd83Cu17 bimetallic aerogel | 25 mol% [Bmim]BF4 and 75 mol% water | −2.1 vs. Ag/Ag+ | 31.8 | 80 | [69] | |
| Pre-oxidized Cu foil (1 h, 130 °C) | 0.5 M KHCO3 | −0.9 vs. SCE | 0.069 | 33.36 | [70] | |
| RuO2:TiO2 (35:65) | 0.05 M H2SO4 | −0.05 vs. SCE | 0.061 | 76 | [71] | |
| Cu1.63Se0.33 | [Bmim]PF6 (30 wt %)/CH3CN/H2O | −2.1 V vs. Ag/Ag+ | 30 | 80 | [72] | |
| CH4 | TiO2-RuO2 | 0.1 M Na2SO4 | 2.1 vs. SCE | 13 | 30 | [73] |
| V2O5-SnO2 | Sn0.9In0.1P2O7 | 0.9 | 4 | 61.4 | [74] | |
| TiO2/RuO2/V2O5 | 0.1 M Na2SO4 | 2.0 V vs. SCE | - | 56 | [75] | |
| 3.0 NiO/Ni | 0.1 M NaOH | 1.40 V vs. RHE | 3.0 | 14 | [42] | |
| Cu-Ti | 1 M KCl | 3.07 V vs. RHE | 6.24 | 16 | [40] | |
| Cu-Ti | 0.1 M KH2PO4-K2HPO4 | 2.15 V vs. RHE | 0.3 | 7 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendse, A.; Prajapati, A. A Perspective on Solar-Driven Electrochemical Routes for Sustainable Methanol Production. Sustain. Chem. 2024, 5, 13-26. https://doi.org/10.3390/suschem5010002
Pendse A, Prajapati A. A Perspective on Solar-Driven Electrochemical Routes for Sustainable Methanol Production. Sustainable Chemistry. 2024; 5(1):13-26. https://doi.org/10.3390/suschem5010002
Chicago/Turabian StylePendse, Aaditya, and Aditya Prajapati. 2024. "A Perspective on Solar-Driven Electrochemical Routes for Sustainable Methanol Production" Sustainable Chemistry 5, no. 1: 13-26. https://doi.org/10.3390/suschem5010002
APA StylePendse, A., & Prajapati, A. (2024). A Perspective on Solar-Driven Electrochemical Routes for Sustainable Methanol Production. Sustainable Chemistry, 5(1), 13-26. https://doi.org/10.3390/suschem5010002








