Innovative Green Approach for Extraction of Piperine from Black Pepper Based on Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.2.1. Plant Material
2.2.2. Natural Deep Eutectic Solvents
2.2.3. Other Reagents
2.3. Isolation and Purification of Piperine
2.4. Piperine Yield and Experimental Design
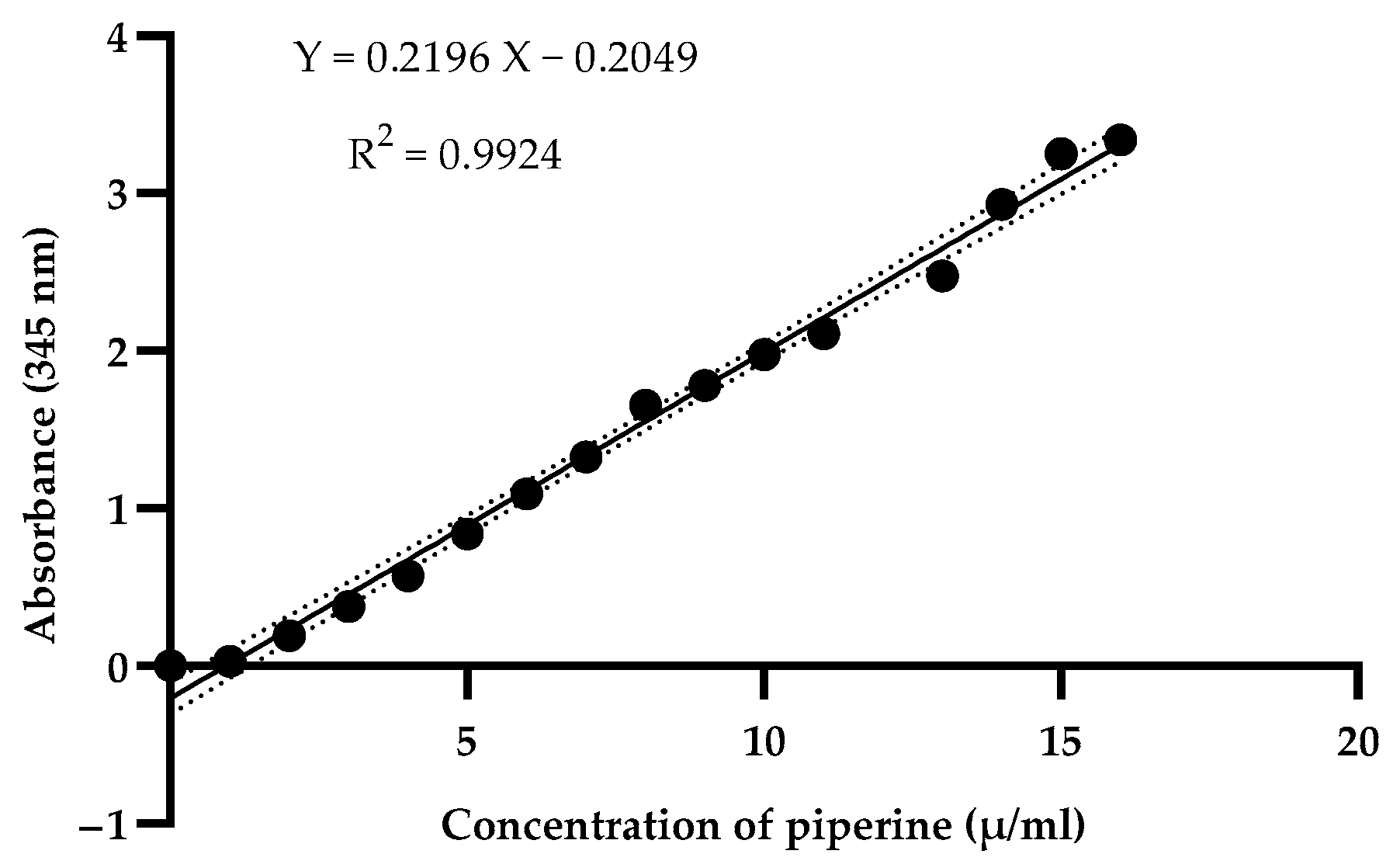
2.4.1. Piperine Yield
2.4.2. Experimental Design
- Extraction time (A): 20–60 min;
- Extraction temperature (B): 25–60 °C;
- Water percent (C): 10–30%;
- Solid–liquid ratio (D): 10–40 mL/g.
2.5. Evaluation of Extraction
2.5.1. Antioxidant Activity (AOA)
2.5.2. Total Polyphenol Content (TPC)
2.5.3. Total Flavonoid Content (TFC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Piperine Using NADES Extraction
3.2. Optimizing the Extraction Conditions by Experimental Design
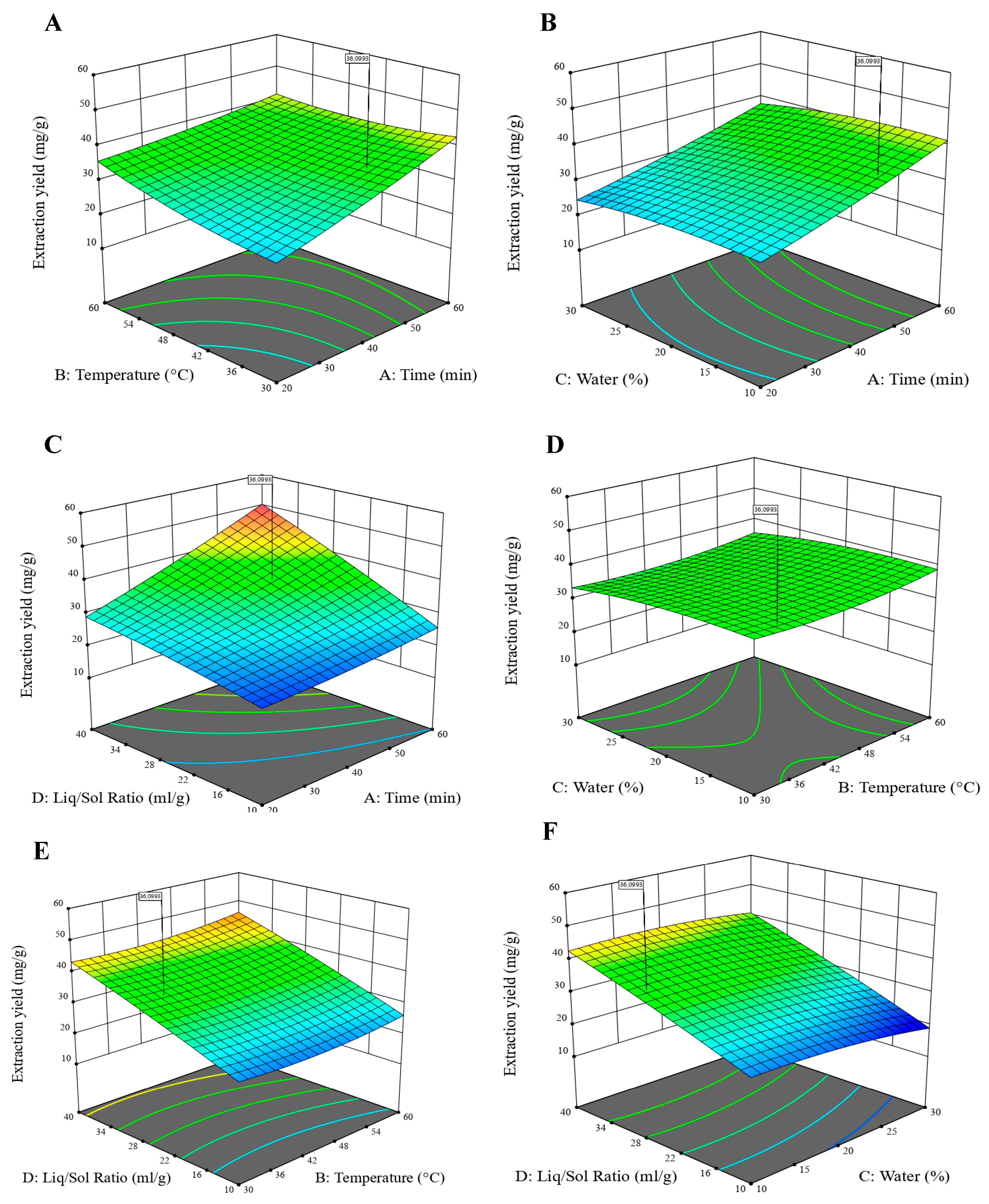
3.2.1. Design of Experiment Methodology and Factor Signification
3.2.2. Determination of the Optimal Conditions by RSM
3.3. Antioxidant, Total Polyphenols, and Total Flavonoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doucette, C.D.; Rodgers, G.; Liwski, R.S.; Hoskin, D.W. Piperine from black pepper inhibits activation-induced proliferation and effector function of T lymphocytes. J. Cell. Biochem. 2015, 116, 2577–2588. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Quijia, C.R.; Chorilli, M. Characteristics, biological properties and analytical methods of piperine: A review. Crit. Rev. Anal. Chem. 2020, 50, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Thangaselvabal, T.; Gailce Leo Justin, C.; Leelamathi, M. Black pepper (Piper nigrum L.)‘the king of spices’–A review. Agric. Rev. 2008, 29, 89–98. [Google Scholar]
- Vasavirama, K.; Upender, M. Piperine: A valuable alkaloid from piper species. Int. J. Pharm. Pharm. Sci. 2014, 6, 34–38. [Google Scholar]
- Chopra, B.; Dhingra, A.K.; Kapoor, R.P.; Prasad, D.N. Piperine and its various physicochemical and biological aspects: A review. Open Chem. J. 2016, 3, 75–96. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (NADES): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents-Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a replacement for conventional organic solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC-Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Washington, T.L.; Yagiz, Y.; Gu, L. Ultrasound-assisted extraction of phenolic acids, flavonols, and flavan-3-ols from muscadine grape skins and seeds using natural deep eutectic solvents and predictive modelling by artificial neural networking. Ultrason. Sonochemistry 2021, 79, 105773. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Corroto, E.; Boussetta, N.; Marina, M.L.; García, M.C.; Vorobiev, E. High voltage electrical discharges followed by deep eutectic solvents extraction for the valorization of pomegranate seeds (Punica granatum L.). Innov. Food Sci. Emerg. Technol. 2022, 79, 103055. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Mostafa, H.; Riaz, A.; Maqsood, S. Utilization of natural deep eutectic solvents and ultrasound-assisted extraction as green extraction technique for the recovery of bioactive compounds from date palm (Phoenix dactylifera L.) seeds: An investigation into optimization of process parameters. Ultrason. Sonochemistry 2022, 91, 106233. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential microwave-ultrasound-assisted extraction for isolation of piperine from black pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Olalere, O.A.; Abdurahman, N.H.; Yunus, R.b.M.; Alara, O.R.; Akbari, S. Evaluation of optimization parameters in microwave reflux extraction of piperine-oleoresin from black pepper (Piper nigrum). Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 626–631. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S.; Fu, D.; Zhang, X.; Liu, H.; Xu, B.; Huang, M. Surfactant-assisted enzymatic extraction of piperine from Piper nigrum L. Int. J. Food Prop. 2020, 23, 52–62. [Google Scholar] [CrossRef]
- Nag, A.; Chowdhury, R.R. Piperine, an alkaloid of black pepper seeds can effectively inhibit the antiviral enzymes of Dengue and Ebola viruses, an in silico molecular docking study. Virusdisease 2020, 31, 308–315. [Google Scholar] [CrossRef]
- Gammoudi, N.; Mabrouk, M.; Bouhemda, T.; Nagaz, K.; Ferchichi, A. Modeling and optimization of capsaicin extraction from Capsicum annuum L. using response surface methodology (RSM), artificial neural network (ANN), and Simulink simulation. Ind. Crops Prod. 2021, 171, 113869. [Google Scholar] [CrossRef]
- Venkata Rao, K.; Murthy, P.B.G.S.N. Modeling and optimization of tool vibration and surface roughness in boring of steel using RSM, ANN and SVM. J. Intell. Manuf. 2018, 29, 1533–1543. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mesbah, M.; Igwegbe, C.A.; Ezeliora, C.D.; Osagie, C.; Khan, N.A.; Dotto, G.L.; Salari, M.; Dehghani, M.H. Sono electro-chemical synthesis of LaFeO3 nanoparticles for the removal of fluoride: Optimization and modeling using RSM, ANN and GA tools. J. Environ. Chem. Eng. 2021, 9, 105320. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid–liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Daghaghele, S.; Kiasat, A.R.; Safieddin Ardebili, S.M.; Mirzajani, R. Intensification of Extraction of Antioxidant Compounds from Moringa Oleifera Leaves Using Ultrasound-Assisted Approach: BBD-RSM Design. Int. J. Fruit Sci. 2021, 21, 693–705. [Google Scholar] [CrossRef]
- Cao, X.; Ye, X.; Lu, Y.; Yu, Y.; Mo, W. Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal. Chim. Acta 2009, 640, 47–51. [Google Scholar] [CrossRef]
- Yu, G.W.; Cheng, Q.; Nie, J.; Wang, P.; Wang, X.J.; Li, Z.G.; Lee, M.R. DES-based microwave hydrodistillation coupled with GC-MS for analysis of essential oil from black pepper (Piper nigrum) and white pepper. Anal. Methods 2017, 9, 6777–6784. [Google Scholar] [CrossRef]
- Yin, X.-s.; Zhong, Z.-f.; Bian, G.-l.; Cheng, X.-j.; Li, D.-q. Ultra-rapid, enhanced and eco-friendly extraction of four main flavonoids from the seeds of Oroxylum indicum by deep eutectic solvents combined with tissue-smashing extraction. Food Chem. 2020, 319, 126555. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Makkliang, F.; Siriwarin, B.; Yusakul, G.; Phaisan, S.; Sakdamas, A.; Chuphol, N.; Putalun, W.; Sakamoto, S. Biocompatible natural deep eutectic solvent-based extraction and cellulolytic enzyme-mediated transformation of Pueraria mirifica isoflavones: A sustainable approach for increasing health-bioactive constituents. Bioresour. Bioprocess. 2021, 8, 76. [Google Scholar] [CrossRef]
- Ozturk, H.; Kolak, U.; Meric, C. Antioxidant, anticholinesterase and antibacterial activities of jurinea consanguinea dc. Rec. Nat. Prod. 2011, 5, 43–51. [Google Scholar]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Huang, R.; Wu, W.; Shen, S.; Fan, J.; Chang, Y.; Chen, S.; Ye, X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods 2018, 10, 2575–2587. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of pomegranate peel waste: Natural deep eutectic solvents as a green strategy to recover valuable phenolic compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Vishvnath, G.; Jain, U. Quantitative analysis of piperine in ayurvedic formulation by UV Spectrophotometry. Int. J. Pharm. Sci. Res. IJPSR 2011, 2, 58–61. [Google Scholar]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Qin, B.; Yang, K.; Cao, R. Synthesis and antioxidative activity of piperine derivatives containing phenolic hydroxyl. J. Chem. 2020, 2020, 2786359. [Google Scholar] [CrossRef]
- Rathod, S.S.; Rathod, V.K. Extraction of piperine from Piper longum using ultrasound. Ind. Crops Prod. 2014, 58, 259–264. [Google Scholar] [CrossRef]
- Park, H.M.; Kim, J.H.; Kim, D.K. Anti-oxidative effect of piperine from Piper nigrum L. In caenorhabditis elegans. Nat. Prod. Sci. 2019, 25, 255–260. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Gülçin, I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin− Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]


| NADES Components | ||||
|---|---|---|---|---|
| HBA * | HBD ** | HBD ** | HBA:HBD:HBD Ratio | |
| 1 | Choline Chloride | Urea | - | 1:1 |
| 2 | Choline Chloride | 1-2 Propylene glycol | - | 1:1 |
| 3 | Choline Chloride | Malic Acid | - | 1:1 |
| 4 | Choline Chloride | Citric Acid | - | 1:1 |
| 5 | L-Proline | Malic Acid | - | 1:1 |
| 6 | L-Proline | Citric Acid | - | 1:1 |
| 7 | Choline Chloride | Glycerin | Urea | 1:1:1 |
| 8 | L-Proline | Glycerin | Malic Acid | 1:2:2 |
| 9 | Choline Chloride | 1-2 Propylene Glycol | Citric Acid | 1:3:1 |
| 10 | Choline Chloride | Urea | Citric Acid | 1:2:1 |
| 11 | Choline Chloride | Malic Acid | Citric Acid | 1:1:1 |
| 12 | Choline Chloride | Glucose | Citric Acid | 1:1:1 |
| 13 | Choline Chloride | 1-2 Propylene Glycol | Citric Acid | 1:2:2 |
| 14 | Choline Chloride | Glycerin | Citric Acid | 1:2:2 |
| 15 | Choline Chloride | Urea | Citric Acid | 1:2:2 |
| 16 | Choline Chloride | Malic Acid | Citric Acid | 1:2:2 |
| Coded Variables | Variables | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | Extraction Time (min) (A) | Extraction Temperature (°C) (B) | Water Content (%) (C) | Solid–Liquid Ratio (mg−1) (D) | Yield (mg/g) | SD | |
| 1 | 0 | 1 | −1 | 0 | 40 | 45 | 20 | 25 | 36.23 | 1.16 |
| 2 | 0 | 1 | 0 | −1 | 20 | 45 | 10 | 25 | 31.10 | 0.46 |
| 3 | 1 | 0 | 1 | 0 | 40 | 60 | 10 | 25 | 37.6 | 3.43 |
| 4 | −1 | 1 | 0 | 0 | 60 | 45 | 20 | 10 | 22.28 | 1.11 |
| 5 | 1 | 1 | 0 | 0 | 20 | 60 | 20 | 25 | 28.63 | 3.08 |
| 6 | 0 | −1 | 1 | 0 | 40 | 45 | 10 | 10 | 25.61 | 1.01 |
| 7 | 1 | 0 | 0 | −1 | 40 | 45 | 10 | 40 | 40.31 | 0.67 |
| 8 | 0 | 0 | −1 | 1 | 20 | 30 | 20 | 25 | 24.44 | 0.16 |
| 9 | 0 | 0 | 0 | 0 | 20 | 45 | 20 | 10 | 22.96 | 2.52 |
| 10 | 0 | −1 | 0 | −1 | 40 | 30 | 30 | 25 | 27.28 | 0.91 |
| 11 | 0 | 0 | 1 | −1 | 20 | 45 | 30 | 25 | 27.87 | 1.27 |
| 12 | 0 | 0 | 0 | 0 | 40 | 30 | 20 | 40 | 38.16 | 1.31 |
| 13 | 1 | 0 | 0 | 1 | 40 | 60 | 20 | 40 | 43.72 | 2.10 |
| 14 | −1 | 0 | 0 | 1 | 40 | 45 | 20 | 25 | 32.29 | 0.08 |
| 15 | 0 | −1 | −1 | 0 | 40 | 45 | 20 | 25 | 28.34 | 4.04 |
| 16 | 1 | 0 | −1 | 0 | 20 | 45 | 20 | 40 | 33.81 | 1.77 |
| 17 | 0 | 0 | 0 | 0 | 40 | 60 | 20 | 10 | 24.85 | 0.50 |
| 18 | −1 | 0 | −1 | 0 | 40 | 45 | 20 | 25 | 36.89 | 0.30 |
| 19 | 0 | 0 | −1 | −1 | 40 | 30 | 10 | 25 | 28.45 | 1.78 |
| 20 | 0 | 0 | 0 | 0 | 40 | 45 | 30 | 40 | 37.91 | 0.81 |
| 21 | −1 | 0 | 0 | −1 | 60 | 45 | 10 | 25 | 33.16 | 2.56 |
| 22 | 1 | −1 | 0 | 0 | 40 | 30 | 20 | 10 | 20.31 | 0.91 |
| 23 | 0 | −1 | 0 | 1 | 60 | 60 | 20 | 25 | 40.36 | 2.02 |
| 24 | 0 | 1 | 1 | 0 | 40 | 60 | 30 | 25 | 36.13 | 2.27 |
| 25 | 0 | 0 | 0 | 0 | 40 | 45 | 30 | 10 | 20.40 | 2.22 |
| 26 | 0 | 0 | 1 | 1 | 40 | 45 | 20 | 25 | 30.57 | 0.08 |
| 27 | 0 | 1 | 0 | 1 | 60 | 45 | 30 | 25 | 28.27 | 5.81 |
| 28 | −1 | −1 | 0 | 0 | 60 | 45 | 20 | 40 | 49.80 | 0.20 |
| 29 | 0 | 0 | 0 | 0 | 60 | 30 | 20 | 25 | 47.35 | 0.86 |
| 30 | −1 | 0 | 1 | 0 | 40 | 45 | 20 | 25 | 27.55 | 0.13 |
| Parameters | A (min) | B (°C) | C (%) | D (mg−1) | Optimum (mg/g) | Desirability |
|---|---|---|---|---|---|---|
| Values | 50 | 30 | 14.5 | 30 | 39.075 | 0.923 |
| AOA (%) | TPC (mmol/L) | TFC (mmol/L) | |
|---|---|---|---|
| H2O | 11.81 ± 1.61 | 3.95 ± 9.31 | 1.22 ± 0.11 |
| EtOH | 21.60 ± 2.47 | 49.42 ± 7.44 | 40.57 ± 4.04 |
| MeOH | 22.66 ± 0.85 | 46.06 ± 11.17 | 39.15 ± 6.06 |
| NADES-13 | 45.34 ± 5.78 | 94.876 ± 1.86 | 82.033 ± 9.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lwamba, C.; Aboushanab, S.A.; Ambati, R.R.; Kovaleva, E.G. Innovative Green Approach for Extraction of Piperine from Black Pepper Based on Response Surface Methodology. Sustain. Chem. 2023, 4, 40-53. https://doi.org/10.3390/suschem4010005
Lwamba C, Aboushanab SA, Ambati RR, Kovaleva EG. Innovative Green Approach for Extraction of Piperine from Black Pepper Based on Response Surface Methodology. Sustainable Chemistry. 2023; 4(1):40-53. https://doi.org/10.3390/suschem4010005
Chicago/Turabian StyleLwamba, Charles, Saied A. Aboushanab, Ranga Rao Ambati, and Elena G. Kovaleva. 2023. "Innovative Green Approach for Extraction of Piperine from Black Pepper Based on Response Surface Methodology" Sustainable Chemistry 4, no. 1: 40-53. https://doi.org/10.3390/suschem4010005
APA StyleLwamba, C., Aboushanab, S. A., Ambati, R. R., & Kovaleva, E. G. (2023). Innovative Green Approach for Extraction of Piperine from Black Pepper Based on Response Surface Methodology. Sustainable Chemistry, 4(1), 40-53. https://doi.org/10.3390/suschem4010005










