Energy Densification of Biomass-Derived Furfurals to Furanic Biofuels by Catalytic Hydrogenation and Hydrodeoxygenation Reactions
Abstract
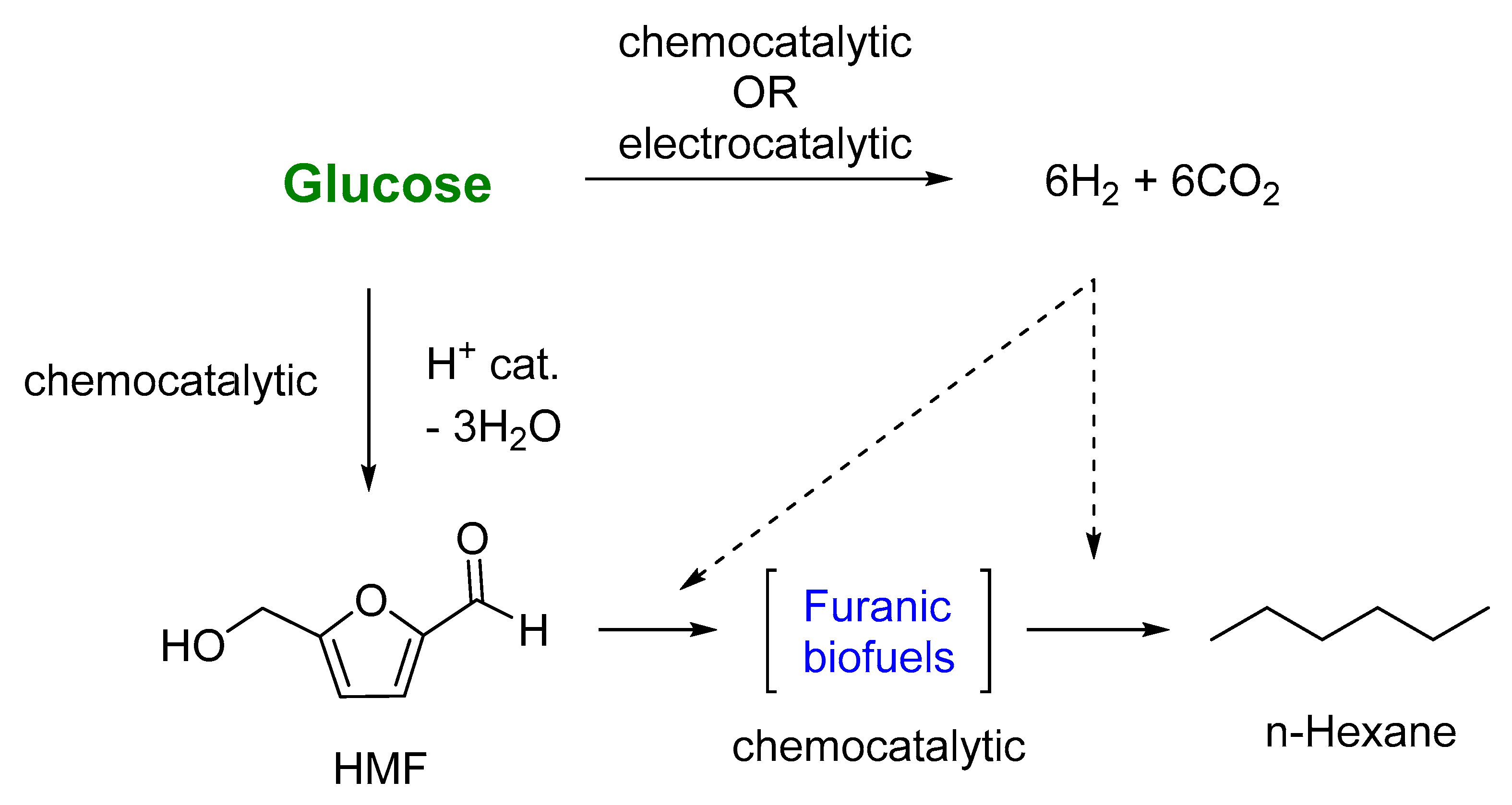
:1. Introduction
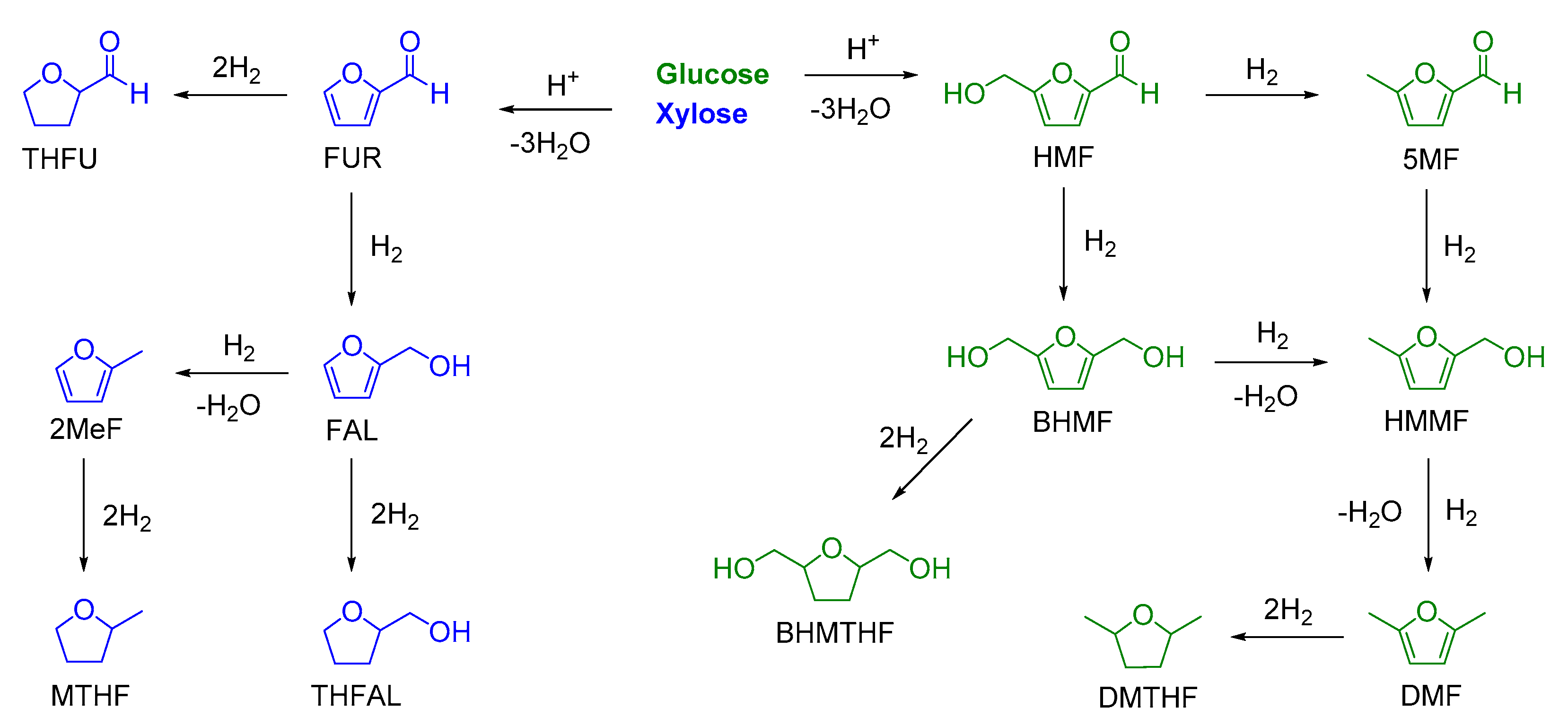
2. Catalytic Reduction of Furfurals to Furanic Compounds
2.1. Catalytic Reduction of FUR to Furanic Biofuels
2.1.1. FUR to FAL and Biofuels Thereof
2.1.2. FUR to 2MeF
2.1.3. FUR to THFAL
2.1.4. FUR to THFU
2.1.5. FUR to MTHF
2.2. Catalytic Reduction of HMF to Furanics
2.2.1. HMF to BHMF
2.2.2. HMF to BHMTHF
2.2.3. HMF to 5MF
2.2.4. HMF to DMF
2.2.5. HMF to DMTHF
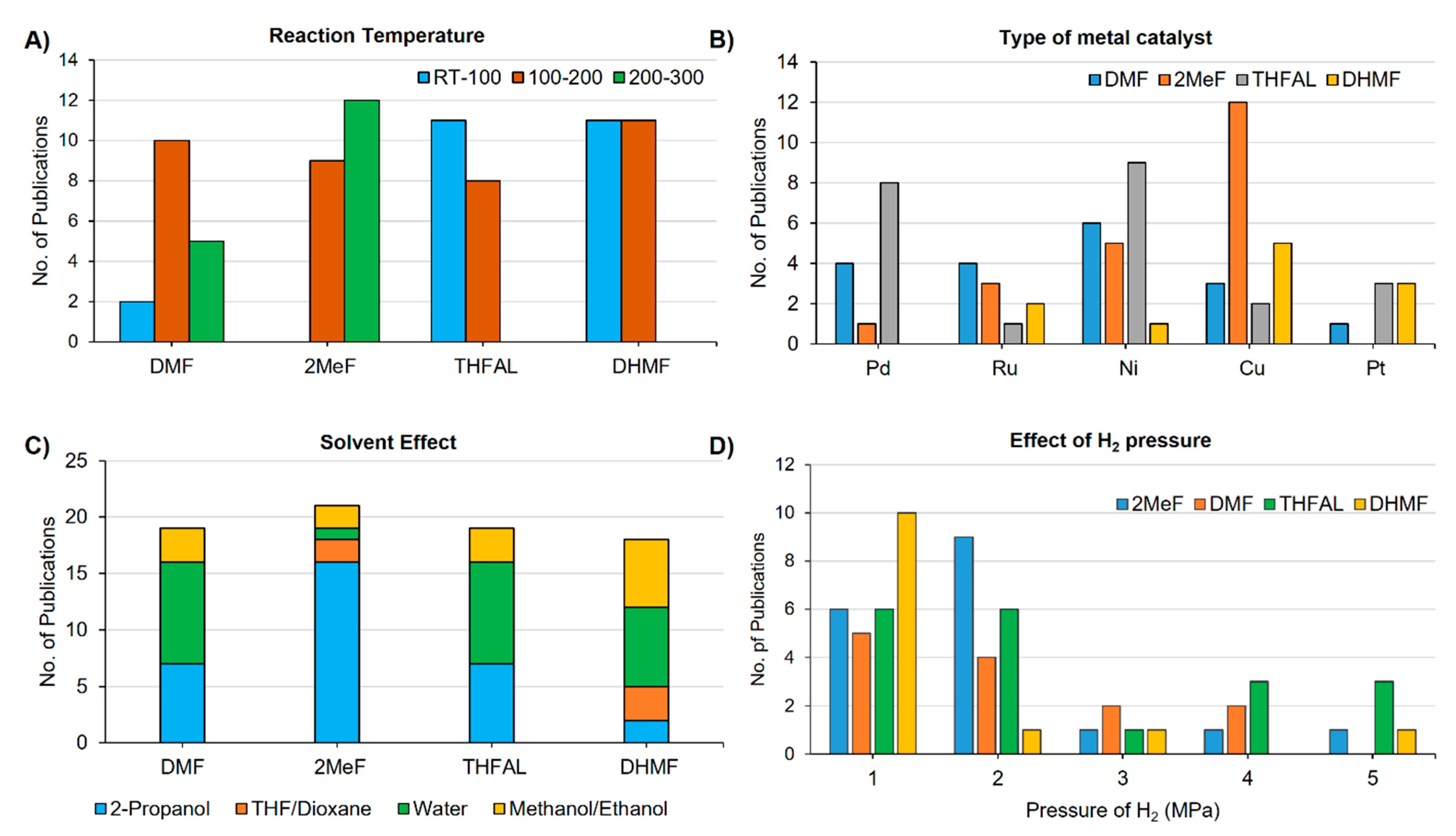
3. Effect of Process Parameters on The Selectivity of Furanics
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from Biomass: Technological versus Environmental Feasibility. A Review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Höfer, R.; Bigorra, J. Biomass-Based Green Chemistry: Sustainable Solutions for Modern Economies. Green Chem. Lett. Rev. 2008, 1, 79–97. [Google Scholar] [CrossRef]
- Philp, J.C.; Ritchie, R.J.; Allan, J.E.M. Biobased Chemicals: The Convergence of Green Chemistry with Industrial Biotechnology. Trends Biotechnol. 2013, 31, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P. The Agricultural Ethics of Biofuels: The Food vs. Fuel Debate. Agriculture 2012, 2, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Matson, T.D.; Barta, K.; Iretskii, A.V.; Ford, P.C. One-Pot Catalytic Conversion of Cellulose and of Woody Biomass Solids to Liquid Fuels. J. Am. Chem. Soc. 2011, 133, 14090–14097. [Google Scholar] [CrossRef] [Green Version]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. Energy, U.D. of 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; ORNL/TM–2016/160; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; p. 448. [Google Scholar]
- Maity, S.K. Opportunities, Recent Trends and Challenges of Integrated Biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445. [Google Scholar] [CrossRef] [Green Version]
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current Status, Challenges, and Future Direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar] [CrossRef]
- Geboers, J.A.; Van de Vyver, S.; Ooms, R.; Op de Beeck, B.; Jacobs, P.A.; Sels, B.F. Chemocatalytic Conversion of Cellulose: Opportunities, Advances and Pitfalls. Catal. Sci. Technol. 2011, 1, 714–726. [Google Scholar] [CrossRef]
- Hara, M.; Nakajima, K.; Kamata, K. Recent Progress in the Development of Solid Catalysts for Biomass Conversion into High Value-Added Chemicals. Sci. Technol. Adv. Mater. 2015, 16, 034903. [Google Scholar] [CrossRef] [PubMed]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on Its Manufacture. Starch 1990, 42, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Liu, S.; Tamura, M.; Nakagawa, Y.; Tomishige, K. One-Pot Conversion of Cellulose into n -Hexane over the Ir-ReOx/SiO2 Catalyst Combined with HZSM-5. ACS Sustain. Chem. Eng. 2014, 2, 1819–1827. [Google Scholar] [CrossRef]
- Caes, B.R.; Teixeira, R.E.; Knapp, K.G.; Raines, R.T. Biomass to Furanics: Renewable Routes to Chemicals and Fuels. ACS Sustain. Chem. Eng. 2015, 3, 2591–2605. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Len, C.; Varma, R.S. Sustainable Pathway to Furanics from Biomass via Heterogeneous Organo-Catalysis. Green Chem. 2017, 19, 164–168. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery-Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Hydrogen Production: Natural Gas Reforming. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming (accessed on 15 April 2021).
- García, L. Hydrogen production by steam reforming of natural gas and other nonrenewable feedstocks. In Compendium of Hydrogen Energy; Woodhead Publishing: Cambridge, UK, 2015; pp. 83–107. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. In Proceedings of the Conference Papers in Energy 2013, Limassol, Cyprus, 19–21 November 2012. [Google Scholar] [CrossRef] [Green Version]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Scott, K. Introduction to Electrolysis, Electrolysers and Hydrogen Production. In Electrochemical Methods for Hydrogen Production; Royal Society of Chemistry: London, UK, 2019; pp. 1–27. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen Production from the Thermochemical Conversion of Biomass: Issues and Challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Moreira, F.S.; Machado, R.G.; Romão, B.B.; Batista, F.R.X.; Ferreira, J.S.; Cardoso, V.L. Improvement of Hydrogen Production by Biological Route Using Repeated Batch Cycles. Process Biochem. 2017, 58, 60–68. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A Review of Catalytic Hydrogen Production Processes from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M.K.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Jin, X.; Yin, B.; Xia, Q.; Fang, T.; Shen, J.; Kuang, L.; Yang, C. Catalytic Transfer Hydrogenation of Biomass-Derived Substrates to Value-Added Chemicals on Dual-Function Catalysts: Opportunities and Challenges. ChemSusChem 2019, 12, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Mechanisms of Chromophore Formation and Cross-Linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Moazzen, K.; Zohuriaan-Mehr, M.J.; Jahanmardi, R.; Kabiri, K. Toward Poly(furfuryl alcohol) Applications Diversification: Novel Self-Healing Network and Toughening Epoxy-Novolac Resin. J. Appl. Polym. Sci. 2018, 135, 45921. [Google Scholar] [CrossRef]
- Wang, H.; Yao, J. Use of Poly(furfuryl alcohol) in the Fabrication of Nanostructured Carbons and Nanocomposites. Ind. Eng. Chem. Res. 2006, 45, 6393–6404. [Google Scholar] [CrossRef]
- Nanni, G.; Heredia-Guerrero, J.A.; Paul, U.C.; Dante, S.; Caputo, G.; Canale, C.; Athanassiou, A.; Fragouli, D.; Bayer, I.S. Poly(furfuryl alcohol)-Polycaprolactone Blends. Polymers 2019, 11, 1069. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R. A Review on Epoxy and Polyester Based Polymer Concrete and Exploration of Polyfurfuryl Alcohol as Polymer Concrete. J. Polym. 2016, 7249743. [Google Scholar] [CrossRef]
- Chaffey, D.R.; Davies, T.; Taylor, S.H.; Graham, A.E. Etherification Reactions of Furfuryl Alcohol in the Presence of Orthoesters and Ketals: Application to the Synthesis Furfuryl Ether Bio-Fuels. ACS Sustain. Chem. Eng. 2018, 6, 4996–5002. [Google Scholar] [CrossRef]
- Onkarappa, S.B.; Bhat, N.S.; Dutta, S. Preparation of Alkyl Levulinates from Biomass-Derived 5-(Halomethyl)furfural (X = Cl, Br), Furfuryl Alcohol, and Angelica Lactone Using Silica-Supported Perchloric Acid as a Heterogeneous Acid Catalyst. Biomass Convers. Biorefin. 2020, 10, 849–856. [Google Scholar] [CrossRef]
- González Maldonado, G.M.; Assary, R.S.; Dumesic, J.; Curtiss, L.A. Experimental and Theoretical Studies of the Acid-Catalyzed Conversion of Furfuryl Alcohol to Levulinic Acid in Aqueous Solution. Energy Environ. Sci. 2012, 5, 6981. [Google Scholar] [CrossRef]
- Merat, N.; Godawa, C.; Gaset, A. High Selective Production of Tetrahydrofurfuryl Alcohol: Catalytic Hydrogenation of Furfural and Furfuryl Alcohol. J. Chem. Technol. Biotechnol. 2007, 48, 145–159. [Google Scholar] [CrossRef]
- Price, B.J.; Clitherow, J.W.; Bradshaw, J. Aminoalkyl Furan Derivatives. U.S. Patent 4128658A, 5 December 1978. [Google Scholar]
- Morozov, E. Furfural Production, 2nd ed.; Forest Industry: Moscow, Russia, 1988; pp. 32–56. [Google Scholar]
- Audemar, M.; Wang, Y.; Zhao, D.; Royer, S.; Jérôme, F.; Len, C.; De Oliveira Vigier, K. Synthesis of Furfuryl Alcohol from Furfural: A Comparison between Batch and Continuous Flow Reactors. Energies 2020, 13, 1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Bonita, Y.; Jain, V.; Geng, F.; O’Connell, T.P.; Wilson, W.N.; Rai, N.; Hicks, J.C. Direct Synthesis of Furfuryl Alcohol from Furfural: Catalytic Performance of Monometallic and Bimetallic Mo and Ru Phosphides. Catal. Sci. Technol. 2019, 9, 3656–3668. [Google Scholar] [CrossRef]
- Ahmed, I. Processes for the Preparation of 2-Methylfuran and 2-Methyltetrahydrofuran. U.S. Patent 6479677B1, 12 November 2002. [Google Scholar]
- Zhu, Y.-L.; Xiang, H.-W.; Li, Y.-W.; Jiao, H.; Wu, G.-S.; Zhong, B.; Guo, G.-Q. A New Strategy for the Efficient Synthesis of 2-Methylfuran and γ-Butyrolactone. New J. Chem. 2003, 27, 208–210. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Yang, J.; Zhu, Y.-L.; Zhao, G.-W. Synthesis of g-Butyrolactone and 2-Methylfuran through the Coupling of Dehydrogenation and Hydrogenation over Copper-Chromite Catalyst. React. Kinet. Catal. Lett. 2004, 82, 263–269. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, H.-Y.; Zhu, Y.-L.; Zhao, G.-W.; Zhang, C.-H.; Teng, B.-T.; Xiang, H.-W.; Li, Y. Effects of Calcination Temperature on Performance of Cu–Zn–Al Catalyst for Synthesizing γ-Butyrolactone and 2-Methylfuran through the Coupling of Dehydrogenation and Hydrogenation. Catal. Commun. 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Vlachos, D.G. Liquid Phase Catalytic Transfer Hydrogenation of Furfural over a Ru/C Catalyst. Appl. Catal. A 2014, 480, 17–24. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Martin, N.; Vlachos, D.G. Effect of Hydrogen Donor on Liquid Phase Catalytic Transfer Hydrogenation of Furfural over a Ru/RuO2/C Catalyst. J. Mol. Catal. A Chem. 2014, 392, 223–228. [Google Scholar] [CrossRef]
- Niu, H.; Luo, J.; Li, C.; Wang, B.; Liang, C. Transfer Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran over CuZnAl Catalysts. Ind. Eng. Chem. Res. 2019, 58, 6298–6308. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef]
- Dong, F.; Ding, G.; Zheng, H.; Xiang, X.; Chen, L.; Zhu, Y.; Li, Y. Highly Dispersed Cu Nanoparticles as an Efficient Catalyst for the Synthesis of the Biofuel 2-Methylfuran. Catal. Sci. Technol. 2016, 6, 767–779. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Sun, H.; Zhao, C. Selective Deoxygenation of Aqueous Furfural to 2-Methylfuran over Cu0/Cu2O·SiO2 Sites via a Copper Phyllosilicate Precursor without Extraneous Gas. ACS Sustain. Chem. Eng. 2018, 6, 12096–12103. [Google Scholar] [CrossRef]
- Cui, J.; Tan, J.; Cui, X.; Zhu, Y.; Deng, T.; Ding, G.; Li, Y. Conversion of Xylose to Furfuryl Alcohol and 2-Methylfuran in a Continuous Fixed-Bed Reactor. ChemSusChem 2016, 9, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiang, X.; Chen, H.; Zheng, H.; Li, Y.-W.; Zhu, Y. Efficient Synthesis of Furfuryl Alcohol and 2-Methylfuran from Furfural over Mineral-Derived Cu/ZnO Catalysts. ChemCatChem 2017, 9, 3023–3030. [Google Scholar] [CrossRef]
- Date, N.S.; Hengne, A.M.; Huang, K.-W.; Chikate, R.C.; Rode, C.V. Single Pot Selective Hydrogenation of Furfural to 2-Methylfuran over Carbon Supported Iridium Catalysts. Green Chem. 2018, 20, 2027–2037. [Google Scholar] [CrossRef] [Green Version]
- Jaatinen, S.K.; Karinen, R.S.; Lehtonen, J.S. Liquid Phase Furfural Hydrotreatment to 2-Methylfuran with Carbon Supported Copper, Nickel, and Iron Catalysts. ChemistrySelect 2017, 2, 51–60. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; He, B.; Qi, J.; Liang, C. Highly Stable and Selective Ru/NiFe2O4 Catalysts for Transfer Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran. J. Energy Chem. 2017, 26, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Selective Production of 2-Methylfuran by Gas-Phase Hydrogenation of Furfural on Copper Incorporated by Complexation in Mesoporous Silica Catalysts. ChemSusChem 2017, 10, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.S.; Luc, W.; Lu, Q.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Nanoporous Cu–Al–Co Alloys for Selective Furfural Hydrodeoxygenation to 2-Methylfuran. Ind. Eng. Chem. Res. 2017, 56, 3866–3872. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, H.; Wang, G.; Zhao, H. Efficient Synthesis of 2-Methylfuran from Bio-Derived Furfural over Supported Copper Catalyst: The Synergistic Effect of CuOx and Cu. ChemistrySelect 2017, 2, 9984–9991. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Yang, S.; Xiao, L.; Wu, W. Influence of Acidity on the Catalytic Performance of Ni2P in Liquid-Phase Hydrodeoxygenation of Furfural to 2-Methylfuran. J. Nanopart. Res. 2020, 22, 67. [Google Scholar] [CrossRef]
- Geng, W.; Li, W.; Liu, L.; Liu, J.; Liu, L.; Kong, X. Facile Assembly of Cu-Cu2O/N-Reduced Graphene Oxide Nanocomposites for Efficient Synthesis of 2-Methylfuran. Fuel 2020, 259, 116267. [Google Scholar] [CrossRef]
- Park, S.; Kannapu, H.P.R.; Jeong, C.; Kim, J.; Suh, Y. Highly Active Mesoporous Cu−Al2O3 Catalyst for the Hydrodeoxygenation of Furfural to 2-methylfuran. ChemCatChem 2020, 12, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-S.; Wang, Z.; Zheng, W.; Vlachos, D.G.; Bhan, A. Vapor Phase Hydrodeoxygenation of Furfural to 2-Methylfuran on Molybdenum Carbide Catalysts. Catal. Sci. Technol. 2014, 4, 2340–2352. [Google Scholar] [CrossRef]
- Xiong, K.; Lee, W.-S.; Bhan, A.; Chen, J.G. Molybdenum Carbide as a Highly Selective Deoxygenation Catalyst for Converting Furfural to 2-Methylfuran. ChemSusChem 2014, 7, 2146–2149. [Google Scholar] [CrossRef]
- Grazia, L.; Bonincontro, D.; Lolli, A.; Tabanelli, T.; Lucarelli, C.; Albonetti, S.; Cavani, F. Exploiting H-Transfer as a Tool for the Catalytic Reduction of Bio-Based Building Blocks: The Gas-Phase Production of 2-Methylfurfural Using a FeVO4 Catalyst. Green Chem. 2017, 19, 4412–4422. [Google Scholar] [CrossRef]
- Mäkelä, E.; Lahti, R.; Jaatinen, S.; Romar, H.; Hu, T.; Puurunen, R.L.; Lassi, U.; Karinen, R. Study of Ni, Pt, and Ru Catalysts on Wood-Based Activated Carbon Supports and Their Activity in Furfural Conversion to 2-Methylfuran. ChemCatChem 2018, 10, 3269–3283. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Zhu, Y.; Zheng, H.; Zhu, Y.; Li, X.; Li, Y. Cr-Free Cu-Catalysts for the Selective Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran: The Synergistic Effect of Metal and Acid Sites. J. Mol. Catal. A Chem. 2015, 398, 140–148. [Google Scholar] [CrossRef]
- Lessard, J.; Morin, J.-F.; Wehrung, J.-F.; Magnin, D.; Chornet, E. High Yield Conversion of Residual Pentoses into Furfural via Zeolite Catalysis and Catalytic Hydrogenation of Furfural to 2-Methylfuran. Top. Catal. 2010, 53, 1231–1234. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, Y.; Luan, P.; Zhang, X.; Yuan, Z.; Guo, S.-X.; Gu, Q.; Johannessen, B.; Mollah, M.; Chaffee, A.L.; et al. Selective Electrochemical Hydrogenation of Furfural to 2-Methylfuran over a Single Atom Cu Catalyst under Mild pH Conditions. Green Chem. 2021, 23, 3028–3038. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. A Versatile Bi-metallic Copper–Cobalt Catalyst for Liquid Phase Hydrogenation of Furfural to 2-Methylfuran. RSC Adv. 2016, 6, 1649–1658. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Lin, W.; Song, W.; Li, S. High Efficient Conversion of Furfural to 2-Methylfuran over Ni-Cu/Al2O3 Catalyst with Formic Acid as a Hydrogen Donor. Appl. Catal. A 2017, 547, 248–255. [Google Scholar] [CrossRef]
- Dohade, M.G.; Dhepe, P.L. One Pot Conversion of Furfural to 2-Methylfuran in the Presence of PtCo Bimetallic Catalyst. Clean Technol. Environ. Policy 2018, 20, 703–713. [Google Scholar] [CrossRef]
- Chang, X.; Liu, A.-F.; Cai, B.; Luo, J.-Y.; Pan, H.; Huang, Y.-B. Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran and 2-Methyltetrahydrofuran over Bimetallic Copper-Palladium Catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef]
- Gandarias, I.; García-Fernández, S.; Obregón, I.; Agirrezabal-Telleria, I.; Arias, P.L. Production of 2-Methylfuran from Biomass through an Integrated Biorefinery Approach. Fuel Process. Technol. 2018, 178, 336–343. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Lin, W.; Song, W. Conversion of Furan Derivatives for Preparation of Biofuels over Ni–Cu/C Catalyst. Energy Sources Part A 2017, 39, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Pei, Z.; Chen, H.; Chen, K.; Hou, Z.; Lu, X.; Ouyang, P.; Fu, J. Catalytic In-Situ Hydrogenation of Furfural over Bimetallic Cu–Ni Alloy Catalysts in Isopropanol. Ind. Eng. Chem. Res. 2018, 57, 4225–4230. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, J.; Cheng, Y.; Chen, Z.; Kang, S.; Cai, Z.; Xu, Y.; Wei, J. Enhanced Catalytic Transfer Hydrogenation of Biomass-Based Furfural into 2-Methylfuran over Multifunctional Cu–Re Bimetallic Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 16624–16636. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over Ni/CNTs and Bimetallic Cu Ni/CNTs Catalysts. Int. J. Hydrogen Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Gao, L.; Wu, Y.; Yang, X.; Sheng, P.; Xiao, G. Short Channeled Ni-Co/SBA-15 Catalysts for Highly Selective Hydrogenation of Biomass-Derived Furfural to Tetrahydrofurfuryl Alcohol. Microporous Mesoporous Mater. 2018, 262, 154–165. [Google Scholar] [CrossRef]
- Yin, D.; Ren, H.; Li, C.; Liu, J.; Liang, C. Highly Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over MIL-101(Cr)-NH2 Supported Pd Catalyst at Low Temperature. Chin. J. Catal. 2018, 39, 319–326. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Liu, X.; Zhang, Y.; Fu, Y. Hydrogenation of Biomass-Derived Furfural to Tetrahydrofurfuryl Alcohol over Hydroxyapatite-Supported Pd Catalyst under Mild Conditions. Ind. Eng. Chem. Res. 2017, 56, 8843–8849. [Google Scholar] [CrossRef]
- Huang, R.; Cui, Q.; Yuan, Q.; Wu, H.; Guan, Y.; Wu, P. Total Hydrogenation of Furfural over Pd/Al2O3 and Ru/ZrO2 Mixture under Mild Conditions: Essential Role of Tetrahydrofurfural as an Intermediate and Support Effect. ACS Sustain. Chem. Eng. 2018, 6, 6957–6964. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.; Yu, Z.; Wang, Y.; Sun, Z.; Kogan, V.M.; Liu, Y.-Y. Aqueous Phase Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over Pd/UiO-66. Catal. Commun. 2021, 148, 106178. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hsu, C.-Y.; Chen, S.S.; Ahamad, T.; Alshehri, S.M.; Tsang, D.C.W.; Wu, K.C.-W. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over a Rh-Loaded Carbon Catalyst in Aqueous Solution under Mild Conditions. Sustainable Energy Fuels 2020, 4, 293–301. [Google Scholar] [CrossRef]
- Sunyol, C.; English Owen, R.; González, M.D.; Salagre, P.; Cesteros, Y. Catalytic Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol Using Competitive Nickel Catalysts Supported on Mesoporous Clays. Appl. Catal. A 2021, 611, 117903. [Google Scholar] [CrossRef]
- Albilali, R.; Douthwaite, M.; He, Q.; Taylor, S.H. The Selective Hydrogenation of Furfural over Supported Palladium Nanoparticle Catalysts Prepared by Sol-Immobilisation: Effect of Catalyst Support and Reaction Conditions. Catal. Sci. Technol. 2018, 8, 252–267. [Google Scholar] [CrossRef]
- Koley, P.; Rao, B.S.; Sabri, Y.M.; Bhargava, S.K.; Tardio, J.; Lingaiah, N. Selective Conversion of Furfural into Tetrahydrofurfuryl Alcohol Using a Heteropoly Acid-Based Material as a Hydrogenation Catalyst. Sustainable Energy Fuels 2020, 4, 4768–4779. [Google Scholar] [CrossRef]
- Pendem, S.; Bolla, S.R.; Morgan, D.J.; Shinde, D.B.; Lai, Z.; Nakka, L.; Mondal, J. Metal–Organic-Framework Derived Co–Pd Bond Is Preferred over Fe–Pd for Reductive Upgrading of Furfural to Tetrahydrofurfuryl Alcohol. Dalton Trans. 2019, 48, 8791–8802. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, C.; Zhu, X.; Zhang, Y.; Gong, W.; Zhang, H.; Zhao, H.; Wang, G. Carbon-Embedded Ni Nanocatalysts Derived from MOFs by a Sacrificial Template Method for Efficient Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. Dalton Trans. 2017, 46, 6358–6365. [Google Scholar] [CrossRef]
- Yang, Y. Aqueous Phase Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol on Alkaline Earth Metal Modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Chen, Q.; Chen, L.; Liu, Q.; Wang, C.; Ma, L. One-Pot Hydrogenation of Furfural into Tetrahydrofurfuryl Alcohol under Ambient Conditions over PtNi Alloy Catalyst. Energy Fuels 2019, 34, 2178–2184. [Google Scholar] [CrossRef]
- Parikh, J.; Srivastava, S.; Jadeja, G.C. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol Using Supported Nickel–Cobalt Catalysts. Ind. Eng. Chem. Res. 2019, 58, 16138–16152. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Liu, K.; Zhang, Q.; Chen, K.-J. Biowaste-Derived Bimetallic Ru–MoOx Catalyst for the Direct Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. ACS Sustain. Chem. Eng. 2019, 7, 12858–12866. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M. A Control over Hydrogenation Selectivity of Furfural via Tuning Exposed Facet of Ni Catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Cocq, A.; Léger, B.; Noël, S.; Bricout, H.; Djedaïni-Pilard, F.; Tilloy, S.; Monflier, E. Anionic Amphiphilic Cyclodextrins Bearing Oleic Grafts for the Stabilization of Ruthenium Nanoparticles Efficient in Aqueous Catalytic Hydrogenation. ChemCatChem 2020, 12, 1013–1018. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Takada, K.; Tamura, M.; Tomishige, K. Total Hydrogenation of Furfural and 5-Hydroxymethylfurfural over Supported Pd–Ir Alloy Catalyst. ACS Catal. 2014, 4, 2718–2726. [Google Scholar] [CrossRef]
- Khokhar, M.D.; Shukla, R.S.; Jasra, R.V. Hydroformylation of Dihydrofurans Catalyzed by Rhodium Complex Encapsulated Hexagonal Mesoporous Silica. J. Mol. Catal. A Chem. 2015, 400, 1–6. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, K.; Zhang, X. Highly Selective Bisphosphine Ligands for Asymmetric Hydroformylation of Heterocyclic Olefins. Tetrahedron Lett. 2015, 56, 1149–1152. [Google Scholar] [CrossRef]
- Chikkali, S.H.; Bellini, R.; de Bruin, B.; van der Vlugt, J.I.; Reek, J.N.H. Highly Selective Asymmetric Rh-Catalyzed Hydroformylation of Heterocyclic Olefins. J. Am. Chem. Soc. 2012, 134, 6607–6616. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, S.; Shen, X.; Chen, B.; Liu, H.; Han, B. Selective Hydrogenation of Aromatic Furfurals into Aliphatic Tetrahydrofurfural Derivatives. Green Chem. 2020, 22, 4937–4942. [Google Scholar] [CrossRef]
- Hu, X.; Kadarwati, S.; Song, Y.; Li, C.-Z. Simultaneous Hydrogenation and Acid-Catalyzed Conversion of the Biomass-Derived Furans in Solvents with Distinct Polarities. RSC Adv. 2016, 6, 4647–4656. [Google Scholar] [CrossRef]
- Dong, F.; Zhu, Y.; Ding, G.; Cui, J.; Li, X.; Li, Y. One-Step Conversion of Furfural into 2-Methyltetrahydrofuran under Mild Conditions. ChemSusChem 2015, 8, 1534–1537. [Google Scholar] [CrossRef]
- Liu, P.; Sun, L.; Jia, X.; Zhang, C.; Zhang, W.; Song, Y.; Wang, H.; Li, C. Efficient One-Pot Conversion of Furfural into 2-Methyltetrahydrofuran Using Non-Precious Metal Catalysts. Mol. Catal. 2020, 490, 110951. [Google Scholar] [CrossRef]
- Date, N.S.; Hengne, A.M.; Huang, K.-W.; Chikate, R.C.; Rode, C.V. One Pot Hydrogenation of Furfural to 2-Methyl tetrahydrofuran over Supported Mono- and Bi-metallic Catalysts. ChemistrySelect 2020, 5, 9590–9600. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient Route to Hydroxymethylfurans from Sugars via Transfer Hydrogenation. ChemSusChem 2010, 3, 1139–1141. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Etherification and Reductive Etherification of 5-(Hydroxymethyl)furfural: 5-(Alkoxymethyl)furfurals and 2,5-Bis(alkoxymethyl)furans as Potential Bio-Diesel Candidates. Green Chem. 2012, 14, 1626–1634. [Google Scholar] [CrossRef]
- Ohyama, J.; Esaki, A.; Yamamoto, Y.; Arai, S.; Satsuma, A. Selective Hydrogenation of 2-Hydroxymethyl-5-furfural to 2,5-Bis(hydroxymethyl)furan over Gold Sub-Nano Clusters. RSC Adv. 2013, 3, 1033–1036. [Google Scholar] [CrossRef]
- Chen, J.; Lu, F.; Zhang, J.; Yu, W.; Wang, F.; Gao, J.; Xu, J. Immobilized Ru Clusters in Nanosized Mesoporous Zirconium Silica for the Aqueous Hydrogenation of Furan Derivatives at Room Temperature. ChemCatChem 2013, 5, 2822–2826. [Google Scholar] [CrossRef]
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Rapid Synthesis of Unsaturated Alcohols under Mild Conditions by Highly Selective Hydrogenation. Chem. Commun. 2013, 49, 7034–7036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kong, X.; Zheng, H.; Ding, G.; Zhu, Y.; Li, Y.-W. Efficient Synthesis of 2,5-Dihydroxymethylfuran and 2,5-Dimethylfuran from 5-Hydroxymethylfurfural Using Mineral-Derived Cu Catalysts as Versatile Catalysts. Catal. Sci. Technol. 2015, 5, 4208–4217. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Bis-(hydroxymethyl)furan Using Pt/MCM-41 in an Aqueous Medium: A Simple Approach. Green Chem. 2014, 16, 4734–4739. [Google Scholar] [CrossRef]
- Hao, W.; Li, W.; Tang, X.; Zeng, X.; Sun, Y.; Liu, S.; Lin, L. Catalytic Transfer Hydrogenation of Biomass-Derived 5-Hydroxymethyl Furfural to the Building Block 2,5-Bishydroxymethyl Furan. Green Chem. 2016, 18, 1080–1088. [Google Scholar] [CrossRef]
- Hu, L.; Yang, M.; Xu, N.; Xu, J.; Zhou, S.; Chu, X.; Zhao, Y. Selective Transformation of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Dihydroxymethylfuran via Catalytic Transfer Hydrogenation over Magnetic Zirconium Hydroxides. Korean J. Chem. Eng. 2018, 35, 99–109. [Google Scholar] [CrossRef]
- Xiang, X.; Cui, J.; Ding, G.; Zheng, H.; Zhu, Y.; Li, Y. One-Step Continuous Conversion of Fructose to 2,5-Dihydroxymethylfuran and 2,5-Dimethylfuran. ACS Sustain. Chem. Eng. 2016, 4, 4506–4510. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, Y.K.; Hwang, D.W. An Integrated Process for the Production of 2,5-Dihydroxymethylfuran and Its Polymer from Fructose. Green Chem. 2018, 20, 879–885. [Google Scholar] [CrossRef]
- Hu, L.; Li, T.; Xu, J.; He, A.; Tang, X.; Chu, X.; Xu, J. Catalytic Transfer Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Dihydroxymethylfuran over Magnetic Zirconium-Based Coordination Polymer. Chem. Eng. J. 2018, 352, 110–119. [Google Scholar] [CrossRef]
- Wei, J.; Cao, X.; Wang, T.; Liu, H.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Liu, S.; Lin, L. Catalytic Transfer Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)Furan over Tunable Zr-Based Bimetallic Catalysts. Catal. Sci. Technol. 2018, 8, 4474–4484. [Google Scholar] [CrossRef]
- Hu, D.; Hu, H.; Zhou, H.; Li, G.; Chen, C.; Zhang, J.; Yang, Y.; Hu, Y.; Zhang, Y.; Wang, L. The Effect of Potassium on Cu/Al2O3 Catalysts for the Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan in a Fixed-Bed Reactor. Catal. Sci. Technol. 2018, 8, 6091–6099. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, W.; Li, H.; Fang, C.; Yang, T.; Wang, Z.; He, C.; Yang, S. Quantitative Synthesis of 2,5-Bis(hydroxymethyl)Furan from Biomass-Derived 5-Hydroxymethylfurfural and Sugars over Reusable Solid Catalysts at Low Temperatures. Fuel 2018, 217, 365–369. [Google Scholar] [CrossRef]
- Jain, A.B.; Vaidya, P.D. Kinetics of Catalytic Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Bis-Hydroxymethylfuran in Aqueous Solution over Ru/C. Int. J. Chem. Kinet. 2016, 48, 318–328. [Google Scholar] [CrossRef]
- Pasini, T.; Lolli, A.; Albonetti, S.; Cavani, F.; Mella, M. Methanol as a Clean and Efficient H-Transfer Reactant for Carbonyl Reduction: Scope, Limitations, and Reaction Mechanism. J. Catal. 2014, 317, 206–219. [Google Scholar] [CrossRef]
- Liu, Y.; Mellmer, M.A.; Alonso, D.M.; Dumesic, J.A. Effects of Water on the Copper-Catalyzed Conversion of Hydroxymethylfurfural in Tetrahydrofuran. ChemSusChem 2015, 8, 3983–3986. [Google Scholar] [CrossRef]
- Vikanova, K.; Redina, E.; Kapustin, G.; Chernova, M.; Tkachenko, O.; Nissenbaum, V.; Kustov, L. Advanced Room-Temperature Synthesis of 2,5-Bis(hydroxymethyl)furan—A Monomer for Biopolymers—from 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2021, 9, 1161–1171. [Google Scholar] [CrossRef]
- Hu, L.; Liu, S.; Song, J.; Jiang, Y.; He, A.; Xu, J. Zirconium-Containing Organic–Inorganic Nanohybrid as a Highly Efficient Catalyst for the Selective Synthesis of Biomass-Derived 2,5-Dihydroxymethylfuran in Isopropanol. Waste Biomass Valorization 2020, 11, 3485–3499. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Jin, B.; Liang, X.; Wang, Q.; Zhao, Z.; Li, Q. Pt–Carbon Interaction-Determined Reaction Pathway and Selectivity for Hydrogenation of 5-Hydroxymethylfurfural over Carbon Supported Pt Catalysts. Catal. Sci. Technol. 2021, 11, 1298–1310. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Hu, Y.; Yuan, Z.; Zhang, Y.; Xu, C.C. Green Synthesis of Heterogeneous Copper-Alumina Catalyst for Selective Hydrogenation of Pure and Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan. Appl. Catal. A 2021, 609, 117892. [Google Scholar] [CrossRef]
- Silva, W.R.; Matsubara, E.Y.; Rosolen, J.M.; Donate, P.M.; Gunnella, R. Pd Catalysts Supported on Different Hydrophilic or Hydrophobic Carbonaceous Substrate for Furfural and 5-(Hydroxymethyl)-furfural Hydrogenation in Water. Mol. Catal. 2021, 504, 111496. [Google Scholar] [CrossRef]
- Kim, J.; Bathula, H.B.; Yun, S.; Jo, Y.; Lee, S.; Baik, J.H.; Suh, Y.-W. Hydrogenation of 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)furan over Mesoporous Cu–Al2O3 Catalyst: From Batch to Continuous Processing. J. Ind. Eng. Chem. 2021, 102, 186–194. [Google Scholar] [CrossRef]
- Roylance, J.J.; Kim, T.W.; Choi, K.-S. Efficient and Selective Electrochemical and Photoelectrochemical Reduction of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan Using Water as the Hydrogen Source. ACS Catal. 2016, 6, 1840–1847. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.; de Jong, E.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in the Absence and Presence of Glucose. ChemSusChem 2013, 6, 1659–1667. [Google Scholar] [CrossRef]
- Kwon, Y.; Birdja, Y.Y.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in Acidic Solution. ChemSusChem 2015, 8, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, S.; Simeonov, S.P.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Direct Transformation of 5-Hydroxymethylfurfural to the Building Blocks 2,5-Dihydroxymethylfurfural (DHMF) and 5-Hydroxymethyl furanoic acid (HMFA) via Cannizzaro Reaction. Green Chem. 2013, 15, 2849–2853. [Google Scholar] [CrossRef]
- Kang, E.-S.; Chae, D.W.; Kim, B.; Kim, Y.G. Efficient Preparation of DHMF and HMFA from Biomass-Derived HMF via a Cannizzaro Reaction in Ionic Liquids. J. Ind. Eng. Chem. 2012, 18, 174–177. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Guo, Y.; Chen, L.; Gao, H. Selective Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural over Catalyst of Palladium Immobilized on Amine-Functionalized Metal–Organic Frameworks. ACS Catal. 2015, 5, 722–733. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Zheng, H.; Dong, F.; Zhu, Y.; Li, Y.-W. Switchable Synthesis of 2,5-Dimethylfuran and 2,5-Dihydroxymethyltetrahydrofuran from 5-Hydroxymethylfurfural over Raney Ni Catalyst. RSC Adv. 2014, 4, 60467–60472. [Google Scholar] [CrossRef]
- Lima, S.; Chadwick, D.; Hellgardt, K. Towards Sustainable Hydrogenation of 5-(Hydroxymethyl)Furfural: A Two-Stage Continuous Process in Aqueous Media over RANEY® Catalysts. RSC Adv. 2017, 7, 31401–31407. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.-W. Rational Design of Ni-Based Catalysts Derived from Hydrotalcite for Selective Hydrogenation of 5-Hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Perret, N.; Grigoropoulos, A.; Zanella, M.; Manning, T.D.; Claridge, J.B.; Rosseinsky, M.J. Catalytic Response and Stability of Nickel/Alumina for the Hydrogenation of 5-Hydroxymethylfurfural in Water. ChemSusChem 2016, 9, 521–531. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Ma, J.; Lu, F.; Zhang, J.; Xu, J. Biphasic Catalytic Conversion of Fructose by Continuous Hydrogenation of HMF over a Hydrophobic Ruthenium Catalyst. ChemSusChem 2014, 7, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagán-Torres, Y.; Dumesic, J. The Selective Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural Using Heterogeneous Catalysts. Green Chem. 2012, 14, 1413. [Google Scholar] [CrossRef]
- Tan, J.; Cui, J.; Zhu, Y.; Cui, X.; Shi, Y.; Yan, W.; Zhao, Y. Complete Aqueous Hydrogenation of 5-Hydroxymethylfurfural at Room Temperature over Bimetallic RuPd/Graphene Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10670–10678. [Google Scholar] [CrossRef]
- Cadu, A.; Sekine, K.; Mormul, J.; Ohlmann, D.M.; Schaub, T.; Hashmi, A.S.K. Homogeneous Catalysed Hydrogenation of HMF. Green Chem. 2018, 20, 3386–3393. [Google Scholar] [CrossRef]
- Hirota, M. Process for Preparing 5-Methyl-2-Furfural. U.S. Patent 200770078273A1, 5 April 2007. [Google Scholar]
- Hamada, K.; Suzukamo, G.; Fujisawa, K. Process for Producing 5-Methylfurfural. U.S. Patent 4335049A, 15 June 1982. [Google Scholar]
- Gowda, A.S.; Parkin, S.; Ladipo, F.T. Hydrogenation and Hydrogenolysis of Furfural and Furfuryl Alcohol Catalyzed by Ruthenium(II) Bis(Diimine) Complexes. Appl. Organomet. Chem. 2012, 26, 86–93. [Google Scholar] [CrossRef]
- Peng, Y.; Li, X.; Gao, T.; Li, T.; Yang, W. Preparation of 5-Methylfurfural from Starch in One Step by Iodide Mediated Metal-Free Hydrogenolysis. Green Chem. 2019, 21, 4169–4177. [Google Scholar] [CrossRef]
- Sun, G.; An, J.; Hu, H.; Li, C.; Zuo, S.; Xia, H. Green Catalytic Synthesis of 5-Methylfurfural by Selective Hydrogenolysis of 5-Hydroxymethylfurfural over Size-Controlled Pd Nanoparticle Catalysts. Catal. Sci. Technol. 2019, 9, 1238–1244. [Google Scholar] [CrossRef]
- Yang, W.; Sen, A. Direct Catalytic Synthesis of 5-Methylfurfural from Biomass-Derived Carbohydrates. ChemSusChem 2011, 4, 349–352. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Long, S.; Sun, Y.; Tang, X.; Zeng, X.; Lin, L. Direct Conversion of Biomass Derived l-Rhamnose to 5-Methylfurfural in Water in High Yield. Green Chem. 2020, 22, 5984–5988. [Google Scholar] [CrossRef]
- Li, S.; Dong, M.; Yang, J.; Cheng, X.; Shen, X.; Liu, S.; Wang, Z.-Q.; Gong, X.-Q.; Liu, H.; Han, B. Selective Hydrogenation of 5-(Hydroxymethyl)Furfural to 5-Methylfurfural over Single Atomic Metals Anchored on Nb2O5. Nat. Commun. 2021, 12, 584. [Google Scholar] [CrossRef]
- Qian, Y. Recent Progress in the Development of Biofuel 2,5-Dimethylfuran. Renew. Sustain. Energy Rev. 2015, 41, 633–646. [Google Scholar] [CrossRef]
- Wang, X. Catalytic Hydrogenolysis of Biomass-Derived 5-Hydroxymethylfurfural to Biofuel 2, 5-Dimethylfuran. Appl. Catal. A 2019, 576, 85–95. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Ruiz, D.; Murzin, D.Y. Catalytic Hydrogenation/Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. ChemSusChem 2021, 14, 150–168. [Google Scholar] [CrossRef]
- Boot, M. (Ed.) Biofuels from Lignocellulosic Biomass: Innovations beyond Bioethanol; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; ISBN 978-3-527-68531-8. [Google Scholar]
- Li, Q.; Man, P.; Yuan, L.; Zhang, P.; Li, Y.; Ai, S. Ruthenium Supported on CoFe Layered Double Oxide for Selective Hydrogenation of 5-Hydroxymethylfurfural. Mol. Catal. 2017, 431, 32–38. [Google Scholar] [CrossRef]
- Solanki, B.S.; Rode, C.V. Selective Hydrogenolysis of 5-(Hydroxymethyl)Furfural over Pd/C Catalyst to 2,5-Dimethylfuran. J. Saudi Chem. Soc. 2019, 23, 439–451. [Google Scholar] [CrossRef]
- Goyal, R.; Sarkar, B.; Bag, A.; Siddiqui, N.; Dumbre, D.; Lucas, N.; Bhargava, S.K.; Bordoloi, A. Studies of Synergy between Metal–Support Interfaces and Selective Hydrogenation of HMF to DMF in Water. J. Catal. 2016, 340, 248–260. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Lin, Y.; Liang, Y.; Chen, Y.; Zhou, W. Catalytic Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Ru Based Catalyst: Effects of Process Parameters on Conversion and Products Selectivity. Renew. Energy 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Li, S.; Chen, C.; Wu, T.; Mei, Q.; Wang, Y.; Chen, B.; Liu, H.; Han, B. Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran under Mild Conditions without Any Additive. ACS Sustain. Chem. Eng. 2019, 7, 5711–5716. [Google Scholar] [CrossRef]
- Ma, N.; Song, Y.; Han, F.; Waterhouse, G.I.N.; Li, Y.; Ai, S. Highly Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran at Low Temperature over a Co–N–C/NiAl-MMO Catalyst. Catal. Sci. Technol. 2020, 10, 4010–4018. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Fan, G.; Yang, L.; Li, F. Nitrogen-Doped Carbon-Decorated Copper Catalyst for Highly Efficient Transfer Hydrogenolysis of 5-Hydroxymethylfurfural to Convertibly Produce 2,5-Dimethylfuran or 2,5-Dimethyltetrahydrofuran. Appl. Catal. B 2018, 226, 523–533. [Google Scholar] [CrossRef]
- Nishimura, S.; Ikeda, N.; Ebitani, K. Selective Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF) under Atmospheric Hydrogen Pressure over Carbon Supported PdAu Bimetallic Catalyst. Catal. Today 2014, 232, 89–98. [Google Scholar] [CrossRef]
- Wang, G.-H.; Hilgert, J.; Richter, F.H.; Wang, F.; Bongard, H.-J.; Spliethoff, B.; Weidenthaler, C.; Schüth, F. Platinum–Cobalt Bimetallic Nanoparticles in Hollow Carbon Nanospheres for Hydrogenolysis of 5-Hydroxymethylfurfural. Nat. Mater. 2014, 13, 293–300. [Google Scholar] [CrossRef]
- Esen, M.; Akmaz, S.; Koç, S.N.; Gürkaynak, M.A. The Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF) with Sol–Gel Ru-Co/SiO2 Catalyst. J. Sol-Gel Sci. Technol. 2019, 91, 664–672. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, G.; Liu, M.; Yang, L.; Li, F. Dandelion-like Cobalt Oxide Microsphere-Supported RuCo Bimetallic Catalyst for Highly Efficient Hydrogenolysis of 5-Hydroxymethylfurfural. Appl. Catal. B 2018, 237, 649–659. [Google Scholar] [CrossRef]
- Yang, P.; Xia, Q.; Liu, X.; Wang, Y. High-Yield Production of 2,5-Dimethylfuran from 5-Hydroxymethylfurfural over Carbon Supported Ni–Co Bimetallic Catalyst. J. Energy Chem. 2016, 25, 1015–1020. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.; Yang, L.; Li, F. Highly Efficient Synchronized Production of Phenol and 2,5-Dimethylfuran through a Bimetallic Ni–Cu Catalyzed Dehydrogenation–Hydrogenation Coupling Process without Any External Hydrogen and Oxygen Supply. Green Chem. 2017, 19, 4353–4363. [Google Scholar] [CrossRef]
- Mhadmhan, S.; Franco, A.; Pineda, A.; Reubroycharoen, P.; Luque, R. Continuous Flow Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran Using Highly Active and Stable Cu–Pd/Reduced Graphene Oxide. ACS Sustain. Chem. Eng. 2019, 7, 14210–14216. [Google Scholar] [CrossRef]
- Sarkar, C.; Koley, P.; Shown, I.; Lee, J.; Liao, Y.-F.; An, K.; Tardio, J.; Nakka, L.; Chen, K.-H.; Mondal, J. Integration of Interfacial and Alloy Effects to Modulate Catalytic Performance of Metal–Organic-Framework-Derived Cu–Pd Nanocrystals toward Hydrogenolysis of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 10349–10362. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Carbon-Coated Cu-Co Bimetallic Nanoparticles as Selective and Recyclable Catalysts for Production of Biofuel 2,5-Dimethylfuran. Appl. Catal. B 2017, 200, 192–199. [Google Scholar] [CrossRef]
- Han, W.; Tang, M.; Li, J.; Li, X.; Wang, J.; Zhou, L.; Yang, Y.; Wang, Y.; Ge, H. Selective Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran Catalyzed by Ordered Mesoporous Alumina Supported Nickel-Molybdenum Sulfide Catalysts. Appl. Catal. B 2020, 268, 118748. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Chen, M.-Y.; Yan, L.; Guo, Q.-X.; Fu, Y. Nickel-Tungsten Carbide Catalysts for the Production of 2,5-Dimethylfuran from Biomass-Derived Molecules. ChemSusChem 2014, 7, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Przydacz, M.; Jędrzejczyk, M.; Rogowski, J.; Szynkowska-Jóźwik, M.; Ruppert, A.M. Highly Efficient Production of DMF from Biomass-Derived HMF on Recyclable Ni-Fe/TiO2 Catalysts. Energies 2020, 13, 4660. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Lu, Y.; Cao, Q.; Fang, W. Efficient Hydrogenation of 5-Hydroxymethylfurfural Using a Synergistically Bimetallic Ru–Ir/C Catalyst. Chem. Commun. 2021, 57, 1742–1745. [Google Scholar] [CrossRef]
- Pisal, D.S.; Yadav, G.D. Production of Biofuel 2,5-Dimethylfuran Using Highly Efficient Single-Step Selective Hydrogenation of 5-Hydroxymethylfurfural over Novel Pd-Co/Al-Zr Mixed Oxide Catalyst. Fuel 2021, 290, 119947. [Google Scholar] [CrossRef]
- Grochowski, M.R.; Yang, W.; Sen, A. Mechanistic Study of a One-Step Catalytic Conversion of Fructose to 2,5-Dimethyltetrahydrofuran. Chem. Eur. J. 2012, 18, 12363–12371. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Appell, M.; Blackburn, J.A. Hydrodeoxygenation of Fructose to 2,5-Dimethyltetrahydrofuran Using a Sulfur Poisoned Pt/C Catalyst. Ind. Eng. Chem. Res. 2015, 54, 7059–7066. [Google Scholar] [CrossRef]
- Zhou, H.; Song, J.; Meng, Q.; He, Z.; Jiang, Z.; Zhou, B.; Liu, H.; Han, B. Cooperative Catalysis of Pt/C and Acid Resin for the Production of 2,5-Dimethyltetrahydrofuran from Biomass Derived 2,5-Hexanedione under Mild Conditions. Green Chem. 2016, 18, 220–225. [Google Scholar] [CrossRef]
- Chen, S.; Ciotonea, C.; De Oliveira Vigier, K.; Jérôme, F.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. Hydroconversion of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran and 2,5-Dimethyltetrahydrofuran over Non-promoted Ni/SBA-15. ChemCatChem 2020, 12, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Bottari, G.; Kumalaputri, A.J.; Krawczyk, K.K.; Feringa, B.L.; Heeres, H.J.; Barta, K. Copper-Zinc Alloy Nanopowder: A Robust Precious-Metal-Free Catalyst for the Conversion of 5-Hydroxymethylfurfural. ChemSusChem 2015, 8, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
| S/N | Reaction Conditions | Catalyst | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | 170 °C, 4 h, 3 MPa H2, 2-propanol | 17.16 wt.% Cu/AC-400/2 | 100 | [63] |
| 2 | 252 °C, 3 min (residence time), water-toluene | 50Cu/50Fe-SiO2 | 98 | [72] |
| 3 | 210 °C, 4.7 s (residence time), BDO | 1.3Cu-Zn | 96.5 | [47] |
| 4 | 240 °C, 4 h, 1.5 MPa H2, 1,4-dioxane | 15 wt.% Cu-Cu2O/N-RGO | 95.5 | [65] |
| 5 | 210 °C, 5 h, H2 flow rate 10 mL/min, CPME | 10Cu-MS | 95 | [61] |
| 6 | 200 °C, 9.5 h, 0.1 MPa H2 | 39.3 wt.% AC-CZ | 94.5 | [57] |
| 7 | 220 °C, 5 h, 0.69 MPa H2, 2-propanol | 5% Ir/C | 94 | [58] |
| 8 | 220 °C, 4 h, 2-propanol | 5Cu-3Re/Al2O3 | 94 | [81] |
| 9 | 225 °C, LHSV 0.7 h−1, BDO | Cu-Zn-Al | ~93 | [49] |
| 10 | 210 °C, 4 h, formic acid, 2-propanol | 10%Ni-10%Cu/Al2O3 | 92 | [75] |
| 11 | 260 °C, 3 h, 1.5 MPa H2, 2-propanol | 17.2 wt.% Ni2P | 91.2 | [64] |
| 12 | 200 °C, LHSV 0.2 h−1,0.1 MPa H2, BDO | Cu2Cr2O5 | 91.1 | [48] |
| 13 | 200 °C, 8 h, formic acid, 2-propanol | 10 wt.% Ni-Cu/C | 91 | [79] |
| 14 | 220 °C, 2 h, methanol | 10 wt.% Cu/SiO2-HT | 90 | [55] |
| 15 | 220 °C, WHSV 0.5 h−1,0.1 MPa H2 | Cu/SiO2 | 89.5 | [71] |
| 16 | 190 °C, WHSV 0.02 h−1, 0.1 MPa H2, GBL/water | Hβ/CuO/ZnO/Al2O3 | 86.8 | [56] |
| 17 | 200 °C, 0.1 MPa H2, WHSV 2.0 h−1 | 24 wt.% AE-Cu/SiO2 | 84.5 | [54] |
| 18 | 180 °C, 4 h, 2.1 MPa N2, 2-propanol | 8 wt.% Ru/NiFe2O4 | 83 | [60] |
| 19 | 320 °C, 1 h, 0.1 MPa N2, methanol | FeVO4 | 80 | [69] |
| 20 | 220 °C, 4 h, 3 MPa H2, DMTHF | 23Cu-12Co/γ-Al2O3 | 80 | [78] |
| 21 | 220 °C, 4 h, 4 MPa H2, 2-propanol | 10 wt.% Cu–Co/γ-Al2O3 | 78 | [74] |
| 22 | 180 °C, 10 h, 2.04 MPa N2, 2-butanol/2-pentanol | 41 wt.% Ru/RuO2/C | 76 | [51] |
| 23 | 250 °C, WHSV 0.4 h−1, H2 flow rate 80 mL/min, CPME | 21.3 wt.% Cu/Al2O3 | ~75.9 | [66] |
| 24 | 180 °C, 4 h, 0.1 MPa N2, 2-propanol | 25 wt.% Cu2.5Zn-Al-600 | 72 | [52] |
| 25 | 240 °C, 7 h, H2 flow rate 60 mL/min | Cu95-Al-Co5 | 64.8 | [62] |
| 26 | 230 °C, 4 h, 2-propanol | 25 wt.% CuNi2Al | 64.8 | [80] |
| 27 | 220 °C, 4 h, 2-propanol | 10Cu-3Pd/ZrO2 | 61.9 | [77] |
| 28 | 180 °C, 10 h, 2.04 MPa N2, 2-propanol | 5% Ru/C | 61 | [50] |
| 29 | 180 °C, 7.5 h, 2.5 MPa N2, 2-propanol | 2% Pd/Fe2O3 | 60 | [53] |
| 30 | 180 °C, 8 h, 0.5 MPa H2, 2-propanol | 22 wt.% Pt-Co/C | 59 | [76] |
| 31 | 240 °C, 2 h, 4 MPa H2, 2-propanol | 3Pt/AC-S | 50 | [70] |
| 32 | 230 °C, 2 h, 4 MPa H2, 2-propanol | 10 wt.% Ni/C | 48.9 | [59] |
| S/N | Reaction Conditions | Catalyst | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | 40 °C, 3 h, 1 MPa H2, 2-propanol | 1% Pd-HAP | 100 | [85] |
| 2 | 30 °C, 3 h, 0.5 MPa H2, water | 1.1Pd/Al2O3 and 1.7Ru/ZrO2 | 100 | [86] |
| 3 | 60 °C, 4 h, 1 MPa H2, water | 1.2 wt.% Pd/UiO-66 | 100 | [88] |
| 4 | 120 °C, 2 h, 1 MPa H2, 2-propanol | 100 wt.% Ni/C | 100 | [94] |
| 5 | 30 °C, 14 h, 1 MPa H2, water | Ru_OS-β-CD | 100 | [100] |
| 6 | 40 °C, 4 h, 2 MPa H2, water | 5.4 wt.% Pd@MIL- 101(Cr)-NH2 | 99.5 | [84] |
| 7 | 110 °C, 3 h, 3 MPa H2, 2-propanol | 17.2 wt.% Ni/MMO-CO3 | 99 | [99] |
| 8 | 140 °C, 4 h, 4 MPa H2, water | 40 wt.% Ni/Ba-Al2O3 | 98 | [95] |
| 9 | 130 °C, 3 h, 4 MPa H2, methanol | Ni+Cu | 97 | [40] |
| 10 | 150 °C, 6 h, 2 MPa H2, 2-propanol | 70 wt.% PdCo3O4@NC | 95 | [93] |
| 11 | 150 °C, 6 h, 2.5 MPa H2, 2-propanol | 0.5 wt.% PdMPAV2/Al2O3 | 95 | [92] |
| 12 | 30 °C, 2 h, 0.3 MPa H2, 2-propanol | 0.97% Pd-Pt/TiO2 | 95 | [91] |
| 13 | 210 °C, 6 h, 7 MPa H2, 2-propanol | 10Ni-10Co/MS | 94.6 | [97] |
| 14 | 140 °C, 0.5 h, H2 flow rate 30 mL/min | 10 wt.% Ni/SiO2 | 94 | [87] |
| 15 | 2 °C, 1 h, 8 MPa H2, water | 2 wt.% Pd-Ir/SiO2 | 94 | [101] |
| 16 | 35 °C, 12 h, 2 MPa H2, water | 50 wt.% PtNi/C | 93 | [96] |
| 17 | 30 °C, 12 h, 1 MPa H2, water | 5 wt.% Rh/C | 92 | [89] |
| 18 | 90 °C, 2 h, 5 MPa H2, ethanol | 13.8 wt.% Ni-Co/SBA-15 | 92.1 | [83] |
| 19 | 130 °C, 10 h, 4 MPa H2, ethanol | 10 wt.% Ni/CNT | 84.3 | [82] |
| Cu-Ni/CNT | 90.3 | |||
| 20 | 140 °C, 4 h, 4 MPa H2, water | Ni(40)/MgO(30)-M | 81 | [90] |
| 21 | 100 °C, 1 h, 2 MPa H2, water | 40 wt.% Ru-MoOx/CN | 43 | [98] |
| S/N | Reaction Conditions | Catalyst | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | 160 °C, 3 h, methanol | MgO | 100 | [126] |
| 2 | 0 °C, 1 h, [EMIm]TFSI | NaOH | 100 | [138] |
| 3 | 100 °C, 1 h, 1.5 MPa H2, 1,4-dioxane | 33 wt.% Cu-ZnO | 99.1 | [115] |
| 4 | 40 °C, 2 h, formic acid, THF, NaOH/NEt3 | Cp*Ir(TsDPEN) | 99 | [110] |
| 5 | 35 °C, 2 h, 0.8 MPa H2, water | 20 wt.% Pt/MCM-41 | 98.9 | [116] |
| 6 | 130 °C, 1 h, 3 MPa H2, methanol | Cu/Al2O3 | 93 | [123] |
| 120 °C, WHSV 1 h−1, 2 MPa H2, ethanol | 1.5K-Cu/Al2O3 | 98.9 | ||
| 7 | 30 °C, 6 h, 0.8 MPa H2, water | Ir-ReOx/SiO2 | 98 | [114] |
| 8 | 100 °C, 4 h, 1.5 MPa H2, 1-butanol | Cu(50)SiO2 | >97 | [120] |
| 9 | 140 °C, 2 h, 3.8 MPa H2, water | Au/Al2O3 | >96 | [112] |
| 10 | 25 °C, 6 h, PMHS, DMSO | KF | 95 | [124] |
| 11 | 20 °C, 2 h, 0.1 MPa H2, ethanol | 1% Pt/CeO2-ZrO2 | 95 | [128] |
| 12 | 140 °C, 5 h, 2-butanol, N2 atmosphere | MZCCP | 93.4 | [121] |
| 13 | 130 °C, 1 h, 3 MPa H2, methanol | 20 mol% Cu/Al2O3 | 93 | [131] |
| 14 | 100 ℃, 5 MPa H2, WHSV 0.2 h−1, ethanol | Cu-Al2O3 | >90 | [133] |
| 15 | 150 °C, 2.5 h, 2-propanol | 20 wt.% ZrBa/SBA | 90.6 | [122] |
| 16 | 25 °C, 4 h, 0.5 MPa H2, water | 5 wt.% Ru/MSN-Zr | ~90.4 | [113] |
| 17 | 160 °C, 10 h, 1 MPa H2, 1-butanol | 10 wt.% Pt/BC-IM | 90.1 | [130] |
| 18 | 150 °C, 5 h, 2-butanol | MZH | 89.6 | [118] |
| 19 | 23 °C, 18 h, 1.37 MPa H2, ethanol | Pt/Al2O3 | 85 | [111] |
| 20 | 150 °C, 2.5 h, ethanol | 100 wt.% ZrO(OH)2 | 83.6 | [117] |
| 21 | 140 °C, 4 h, 2-propanol | 40 wt.% Zr-DTPA | 80 | [129] |
| 22 | RT, 18 h, water | NaOH | 80 | [137] |
| 23 | 110 °C, 2 h, 4.8 MPa H2, water | 2% Pd/CSCNT-AC | ~75 | [132] |
| 24 | 175 °C, 0.5 h, 2.06 MPa H2, THF/water | Cu/γ-Al2O3 | ~68 | [127] |
| 25 | 140 °C, 10 h, 0.1 MPa H2, GBL/water | HT-Cu/ZnO/Al2O3 | 48.2 | [119] |
| 26 | 55 °C, 1 h, 0.69 MPa H2, water | 5% Ru/C | 43 | [125] |
| S/N | Reaction Conditions | Catalyst | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | 120 °C, 8 h, 1.5 MPa H2, THF | 40 wt.% Ru-Co/SiO2 | >99.9 | [170] |
| 2 | 170 °C, 6 h, 1.5 MPa H2, THF | 40 wt.% Co-N-C/NiAl-MMO | 99.9 | [166] |
| 3 | 180 °C, 8 h, 5 MPa H2, ethanol | 8 wt.% Cu-Co/C | 99.4 | [176] |
| 4 | 120 °C, 1 h, 1 MPa H2, THF | 36 wt.% Ru-Ir/C | 99 | [180] |
| 5 | 200 °C, 6 h, 3 MPa H2, water | 2.5 wt.% Ni-OMD3 | 98.7 | [163] |
| 6 | 170 °C, 4 h, 2 MPa H2, 2-propanol | 3%Pd/C | 98.5 | [162] |
| 7 | 180 °C, 6 h, 1 MPa H2, THF | 40 wt.% Ru/CoFe-LDO | 98.2 | [161] |
| 8 | 180 °C, 2 h, 1 MPa H2, butanol | 20 wt.% Pt-Co/HCS | 98 | [169] |
| 9 | 240 °C, 4 h, 0.1 MPa H2, 1,4-dioxane | Ni2-Cu1 | >97 | [173] |
| 10 | 220 °C, 3 h, 3 MPa H2, 1,4-dioxane | 15 wt.% NiFe/PC500 | 97 | [179] |
| 11 | 100 °C, 2 h, 1 MPa H2, THF | 2%Pd-5%Co/AZMOCP | 97 | [181] |
| 12 | 120 °C, 7 h, 1.5 MPa H2, THF | 47 wt.% Cu-28 wt.% Pd@C | 96.5 | [175] |
| 13 | 200 °C, 2 h, 0.5 MPa H2, 1,4-dioxane | 20 wt.% RuCo/CoOx | 96.5 | [171] |
| 14 | 220 °C, 0.5 h, cyclohexanol | 20 wt.% NC-Cu/MgAlO | 96.1 | [167] |
| 15 | 60 °C, 12 h, 0.1 MPa H2, THF | Pd50Au50/C | 96 | [168] |
| 16 | 180 °C, 3 h, 4 MPa H2, THF | 10 wt.% Ni-47 wt.% W2C/AC | 96 | [178] |
| 17 | 80 °C, 24 h, 2 MPa H2, THF | 8 wt.% Pd-GVL/C | 95.6 | [165] |
| 18 | 180 °C, 3 h, 1.7 MPa H2, ethanol | 50 wt.% Ru/ZSM-5 | 95 | [164] |
| 19 | 130 °C, 24 h, 1 MPa H2, THF | 2%Ni-20%Co/C | 95 | [172] |
| 20 | 180 °C, 2 h, 0.3 MPa H2, 2-propanol | 10Cu-1Pd/RGO | 95 | [174] |
| 21 | 130 °C, 7 h, 1 MPa H2, 2-propanol | 5Ni-7MoS2/mAl2O3 | 95 | [177] |
| Properties | DMF | DMTHF | 2MeF | MTHF |
|---|---|---|---|---|
| Molecular formula/Molar mass | C6H8O/96.13 | C6H12O/100.16 | C5H6O/82.10 | C5H10O/86.13 |
| Density (g/cc) | 0.89 | 0.83 | 0.91 | 0.85 |
| Flash point (°C) | −1 | −26.6 | −22 | −10 |
| Lower calorific value (LCV) (MJ/kg) | 33.8 | 32.8 | 31.2 | 32.8 |
| Research octane number | 119 | 92 | 103 | 86 |
| Solubility in water (g/L) | 1.47 | 6.70 | 3 | 150 |
| (C + H)/O (weight ratio) [a] | 5.00 | 5.25 | 4.12 | 4.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinod, N.; Dutta, S. Energy Densification of Biomass-Derived Furfurals to Furanic Biofuels by Catalytic Hydrogenation and Hydrodeoxygenation Reactions. Sustain. Chem. 2021, 2, 521-549. https://doi.org/10.3390/suschem2030029
Vinod N, Dutta S. Energy Densification of Biomass-Derived Furfurals to Furanic Biofuels by Catalytic Hydrogenation and Hydrodeoxygenation Reactions. Sustainable Chemistry. 2021; 2(3):521-549. https://doi.org/10.3390/suschem2030029
Chicago/Turabian StyleVinod, Nivedha, and Saikat Dutta. 2021. "Energy Densification of Biomass-Derived Furfurals to Furanic Biofuels by Catalytic Hydrogenation and Hydrodeoxygenation Reactions" Sustainable Chemistry 2, no. 3: 521-549. https://doi.org/10.3390/suschem2030029
APA StyleVinod, N., & Dutta, S. (2021). Energy Densification of Biomass-Derived Furfurals to Furanic Biofuels by Catalytic Hydrogenation and Hydrodeoxygenation Reactions. Sustainable Chemistry, 2(3), 521-549. https://doi.org/10.3390/suschem2030029






