Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives
Abstract
:1. Introduction
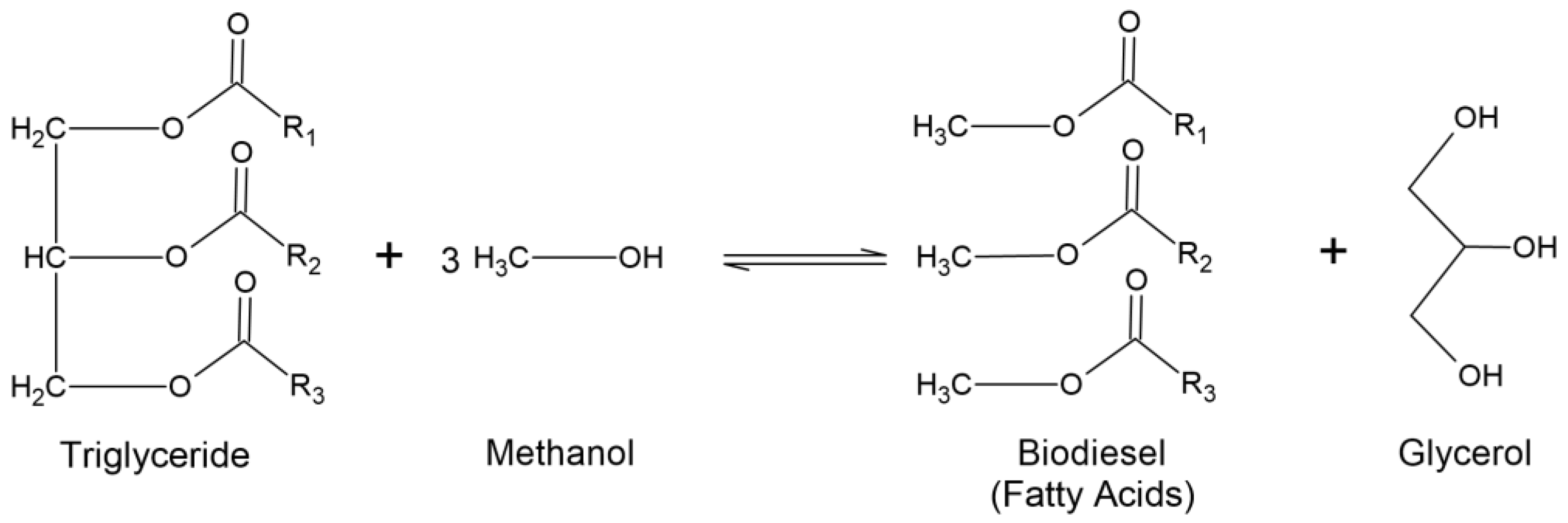
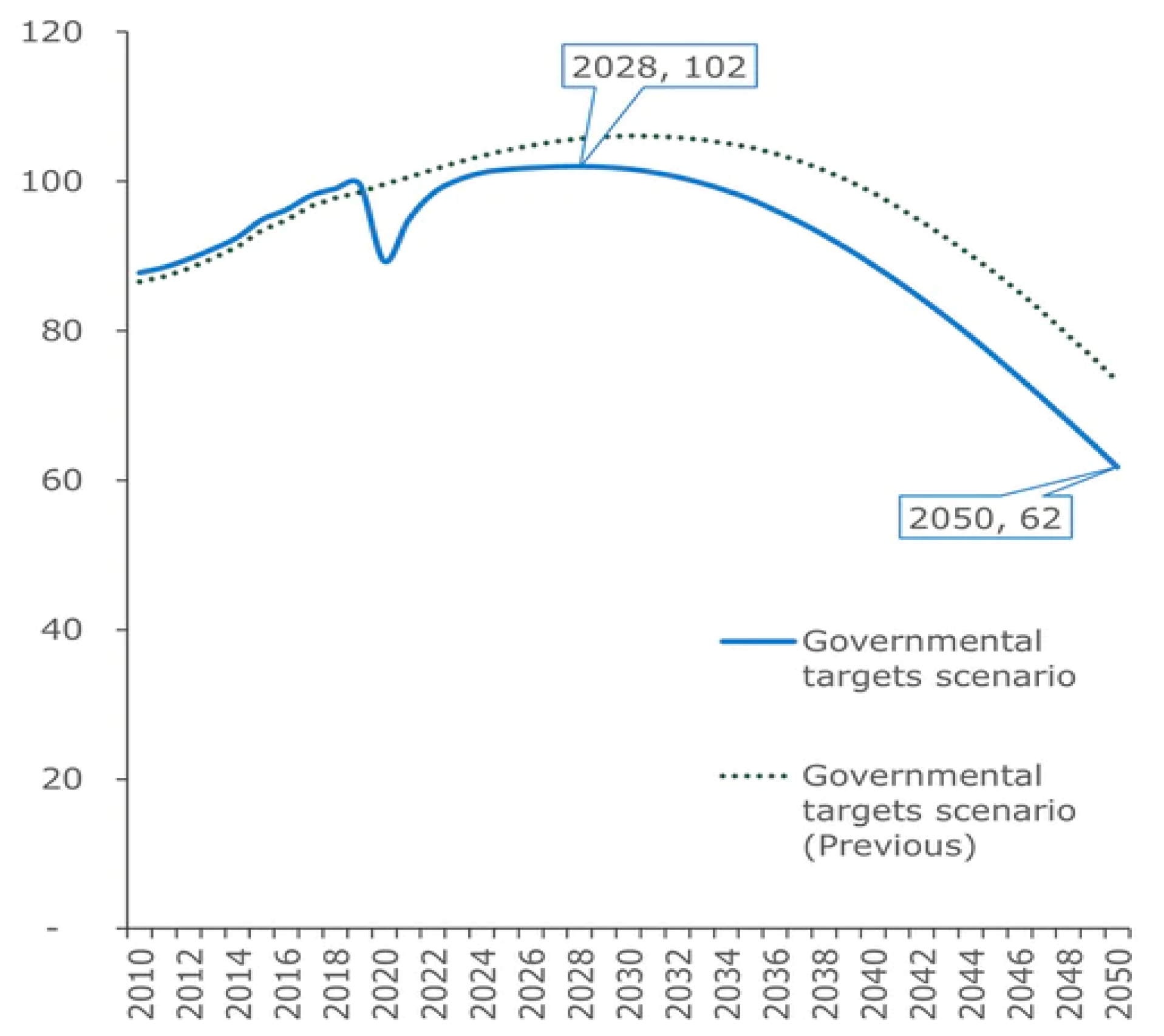
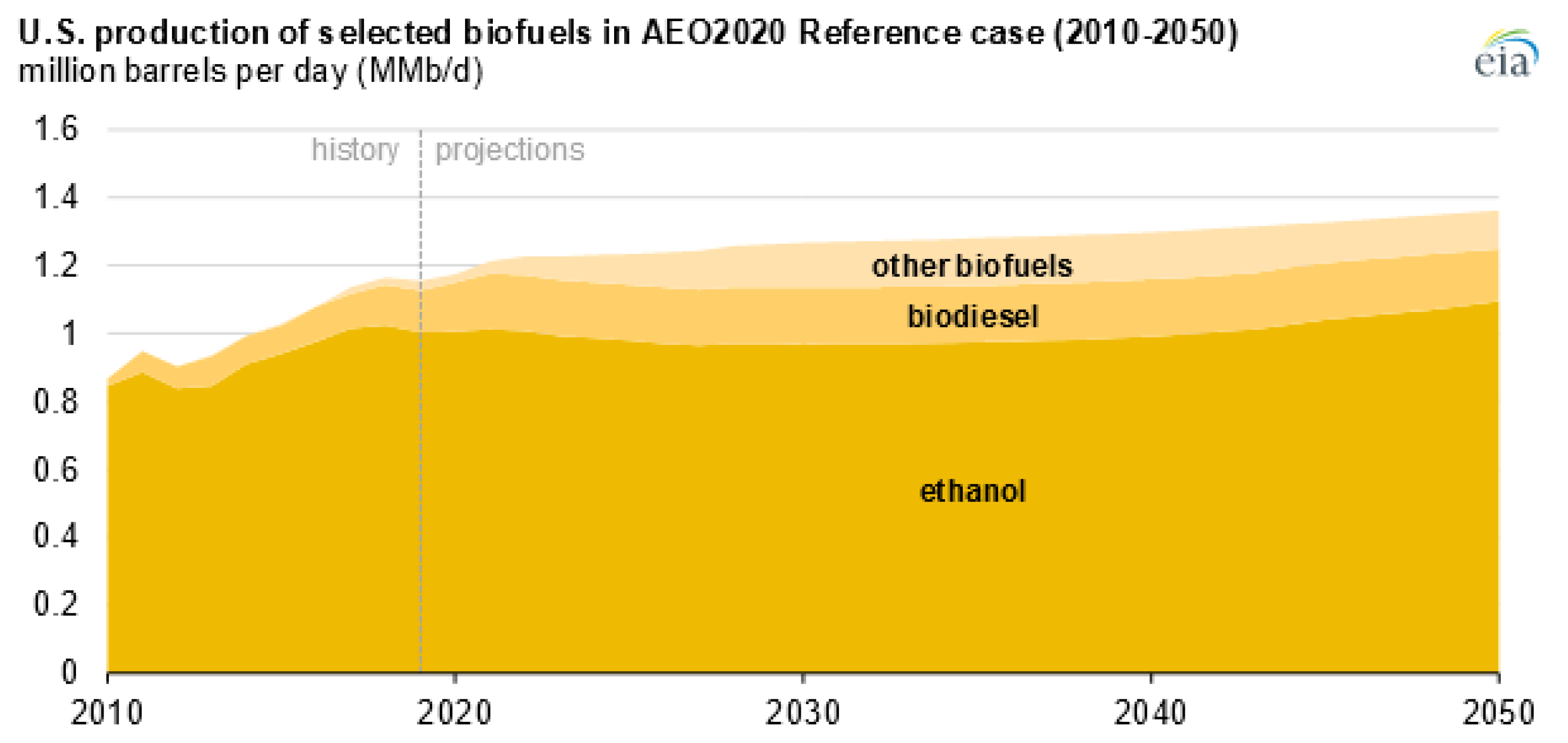
2. Biodiesel Production and Glycerol Market
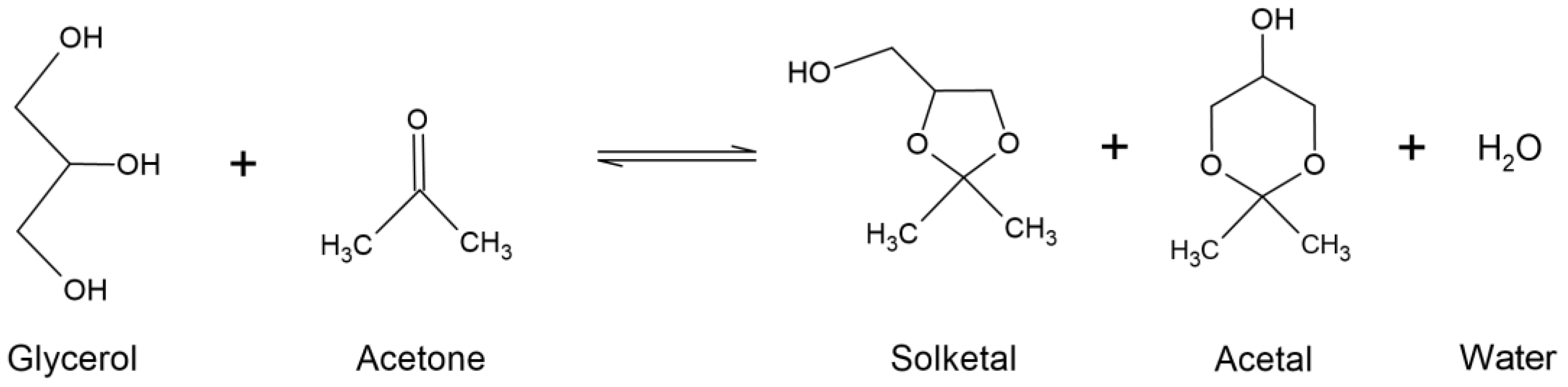
3. Solketal
3.1. Solketal Properties
3.2. Solketal Uses
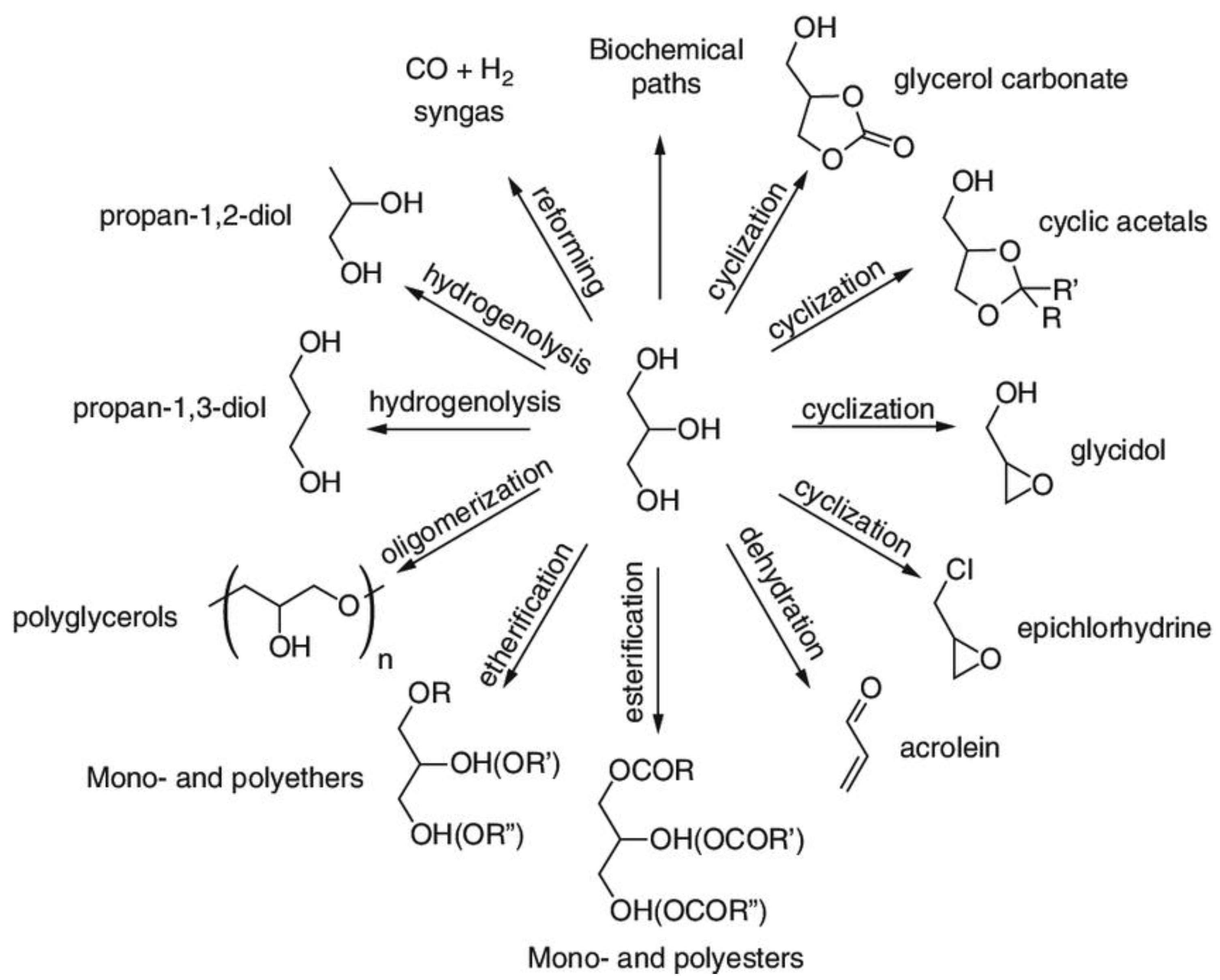
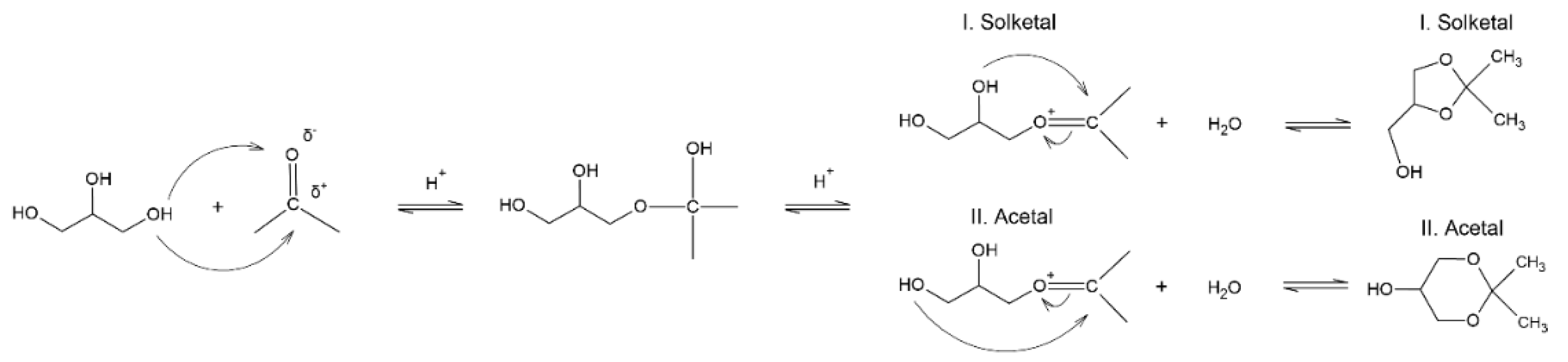
4. Solketal Synthesis
4.1. History
4.2. Catalyst Study
4.2.1. Recent Advances on Catalysts
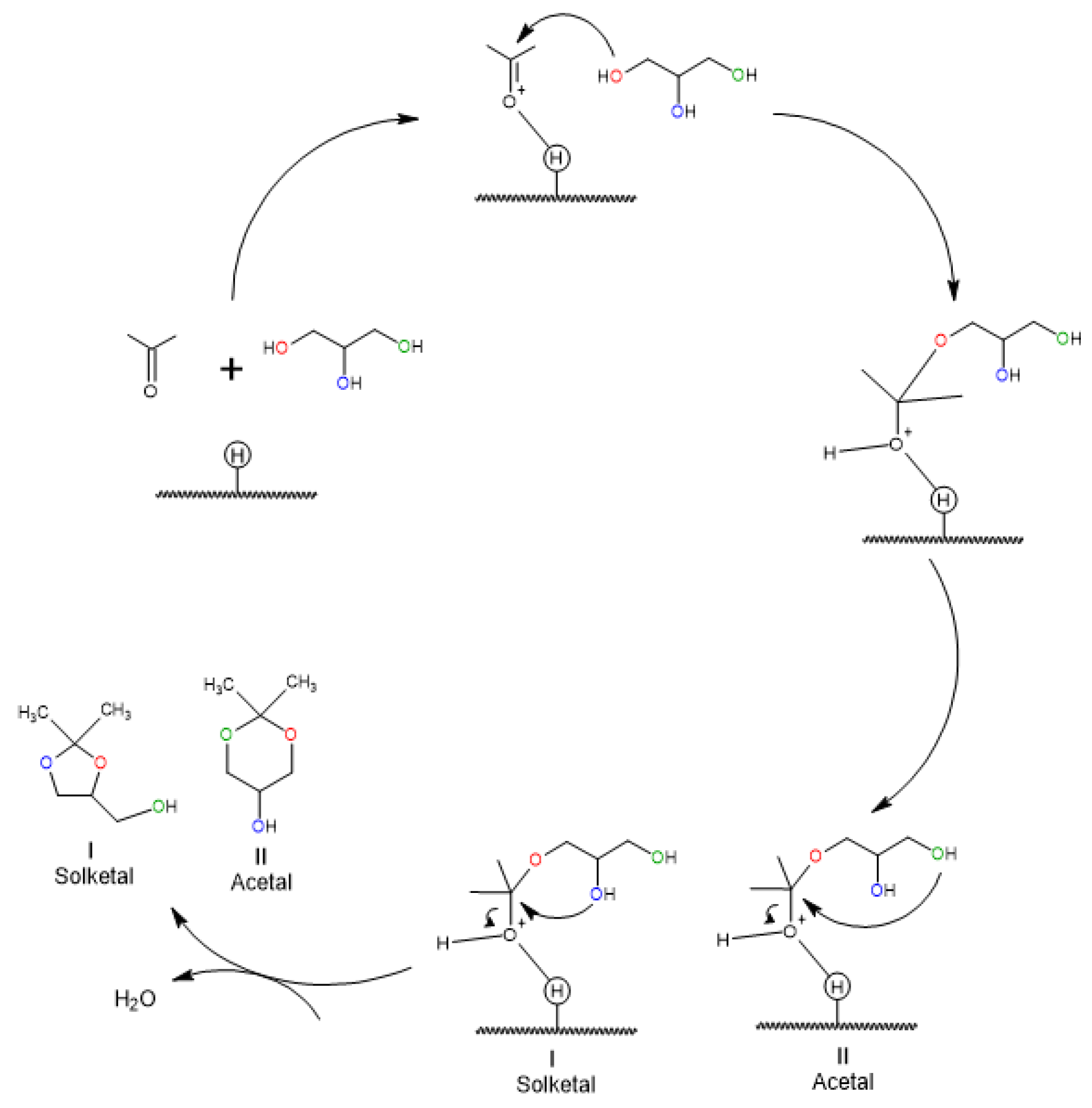
4.2.2. Kinetics and Mechanisms of Reaction
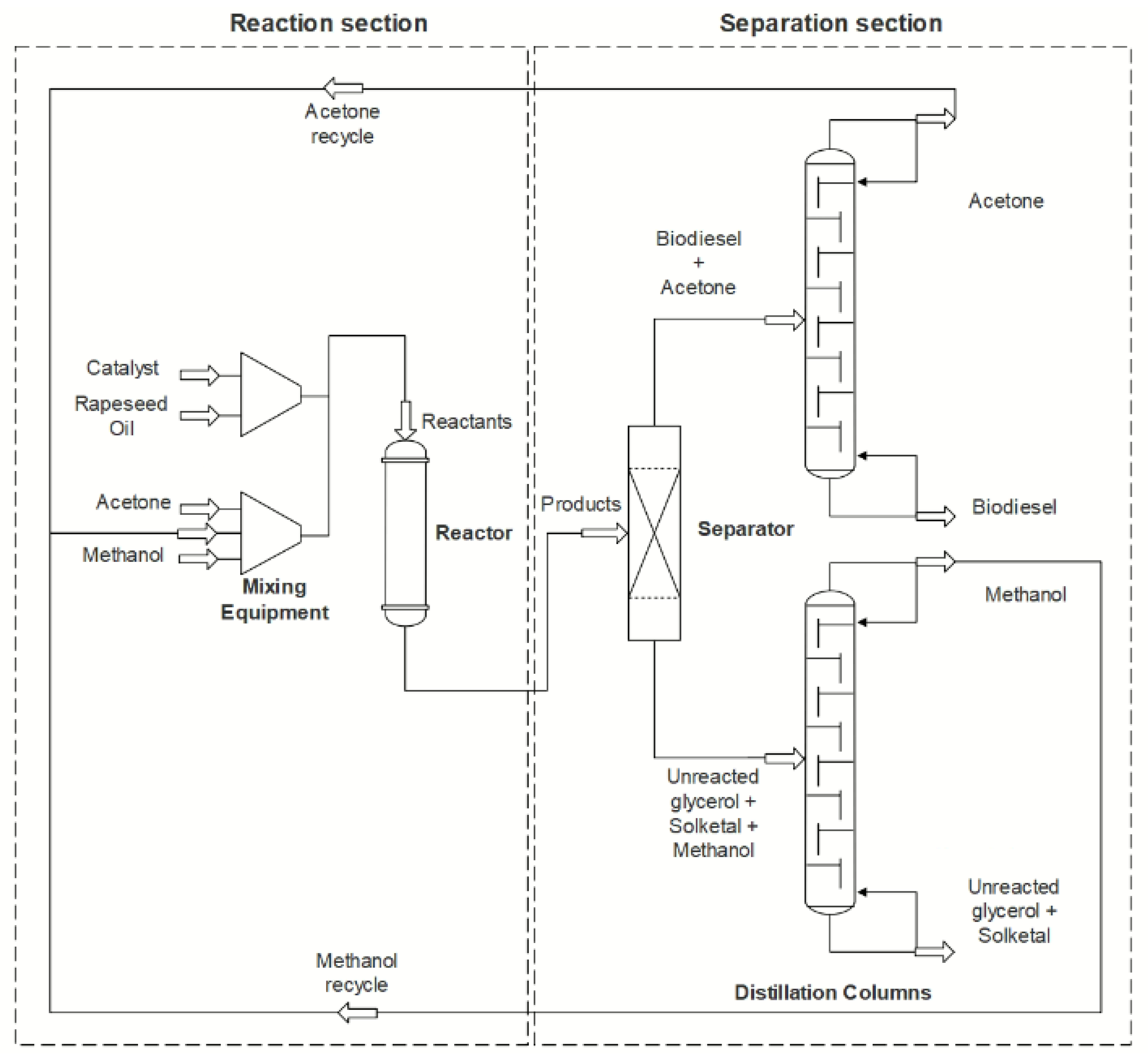
4.3. Recent Advances on the Continuous Process
Process Intensification
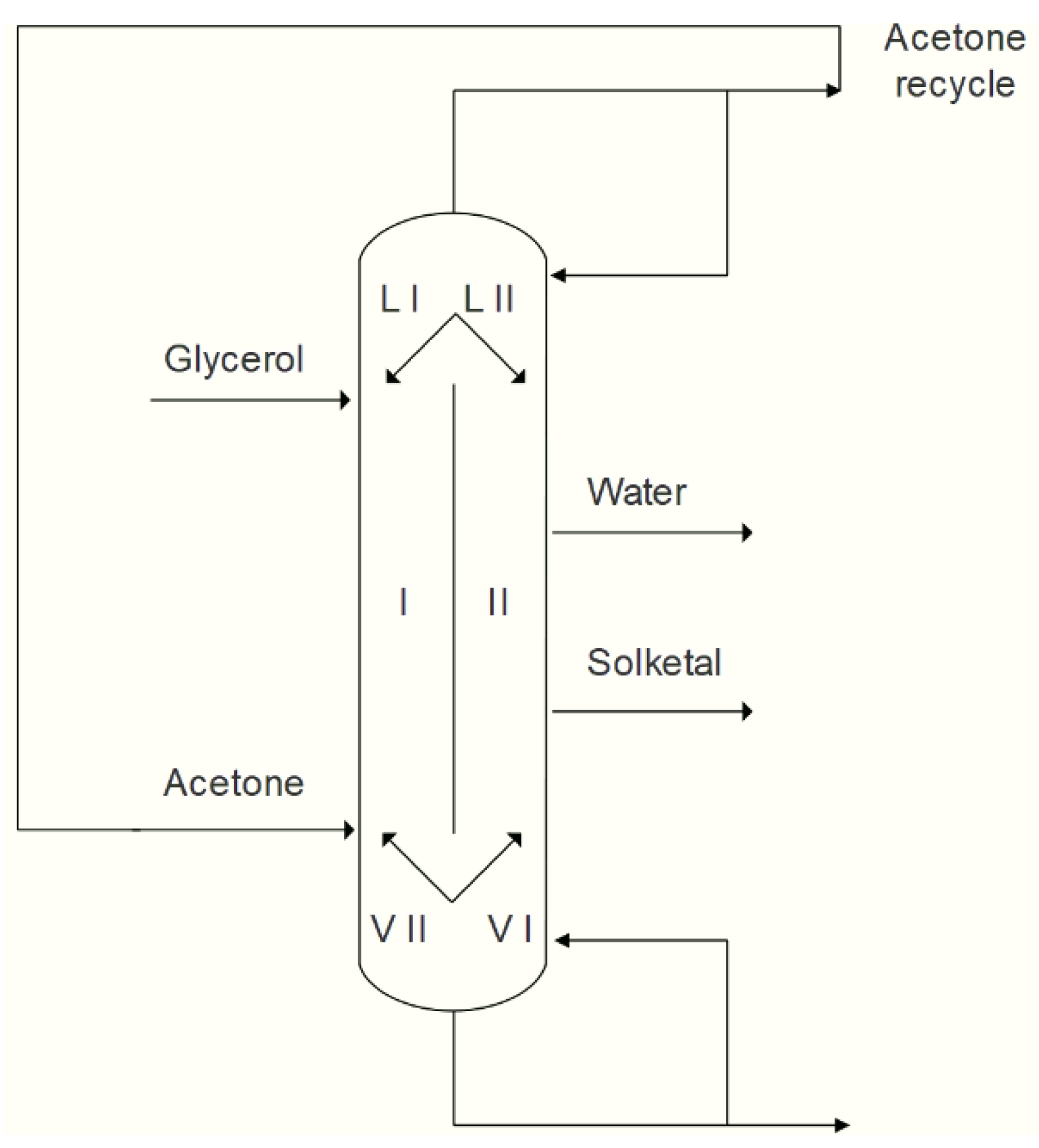
5. Solketal Production
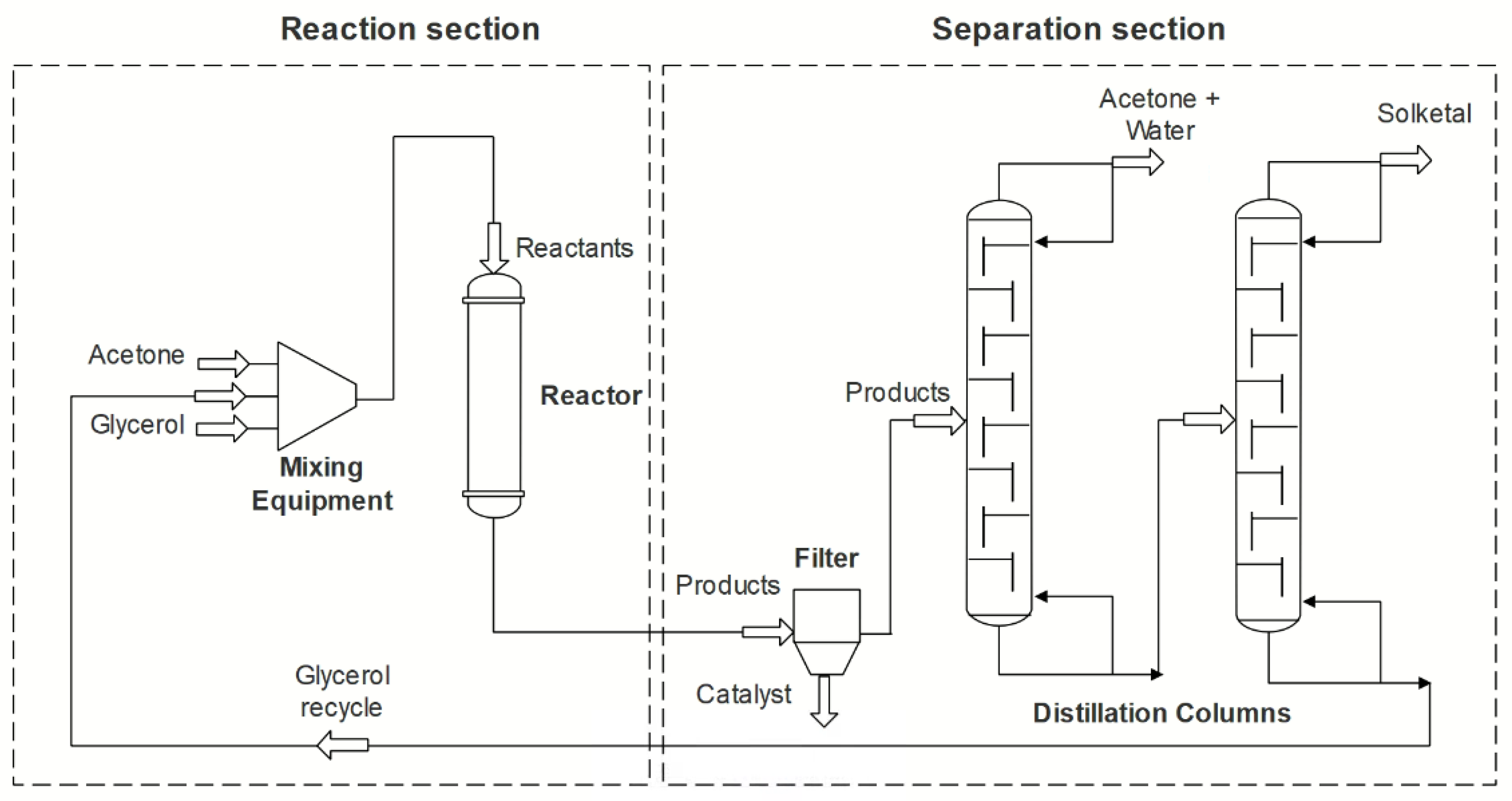
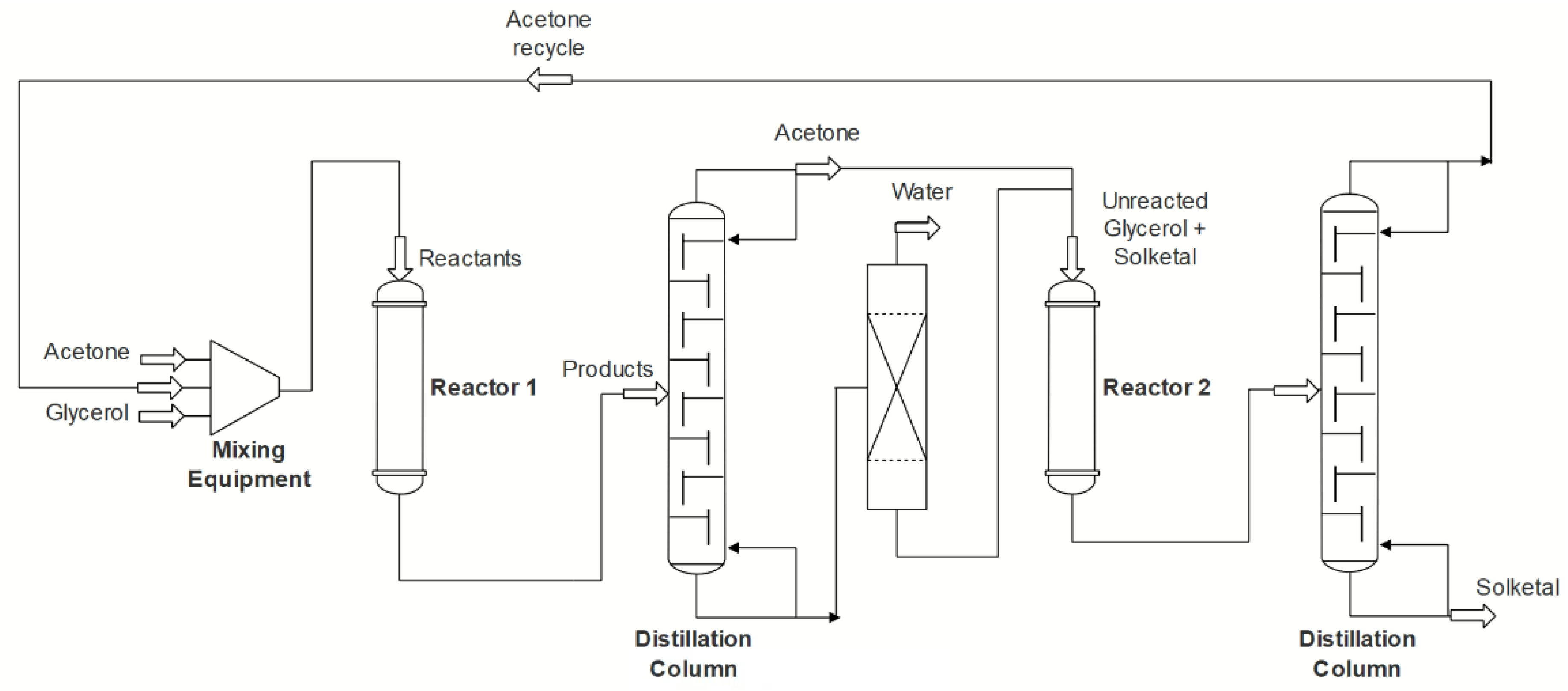
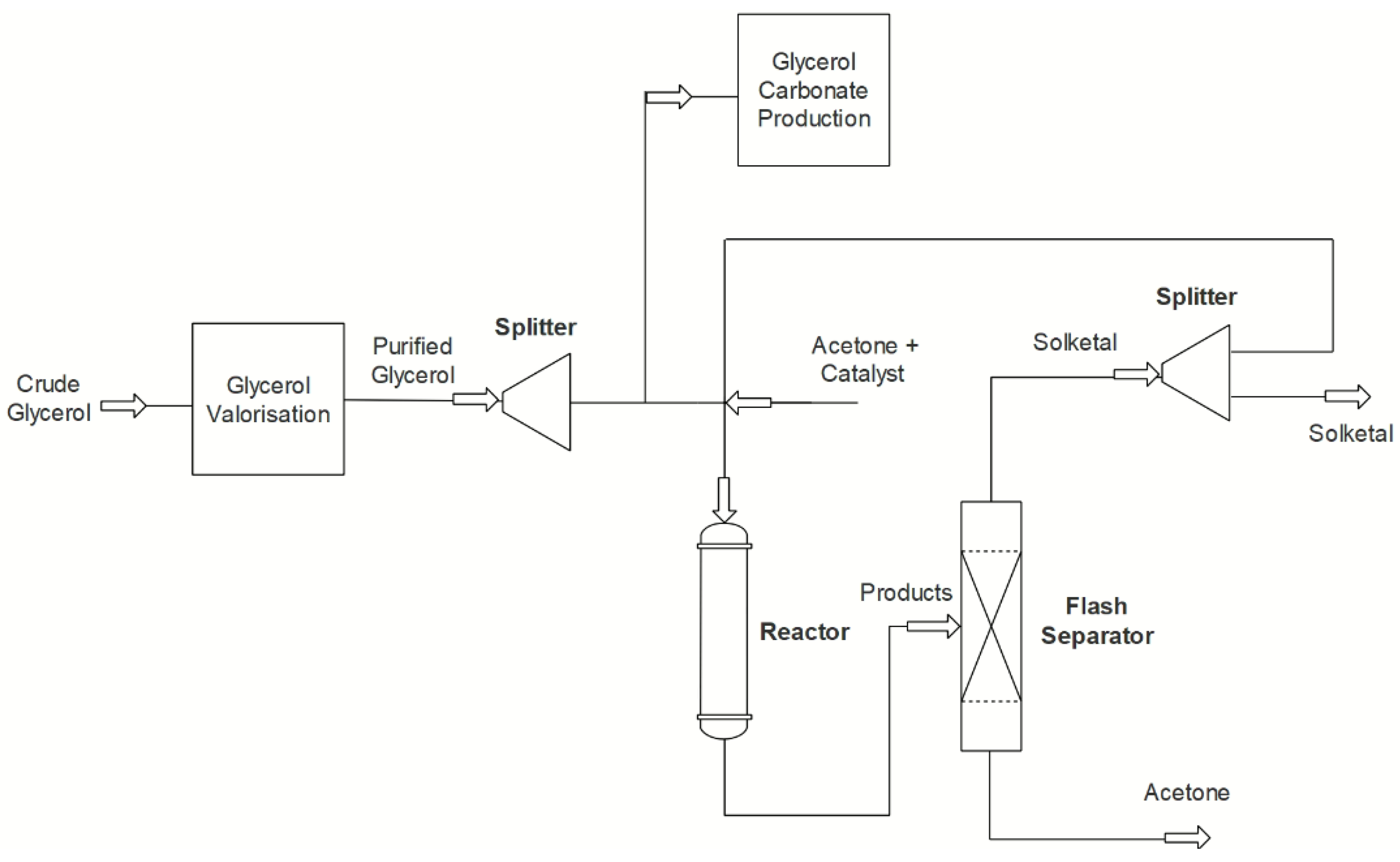
5.1. Processes
5.2. Patents
6. Conclusions and Future Prospects
Funding
Conflicts of Interest
References
- Anastas, P.T.; Beach, E.S. Changing the course of chemistry. In Green Chemistry Education; American Chemical Society: Washington, DC, USA, 2009; Volume 1011, pp. 1–18. ISBN 9780841274471. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK; New York, NY, USA, 1998; ISBN 9780198506980. [Google Scholar]
- Global Energy Review 2020; International Energy Agency: Paris, France, 2020.
- Tracking Transport 2020; International Energy Agency: Paris, France, 2020.
- Pousa, G.P.A.G.; Santos, A.L.F.; Suarez, P.A.Z. History and policy of biodiesel in Brazil. Energy Policy 2007, 35, 5393–5398. [Google Scholar] [CrossRef]
- Rouhany, M.; Montgomery, H. Global biodiesel production: The state of the art and impact on climate change. In Biodiesel: From Production to Combustion; Tabatabaei, M., Aghbashlo, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–14. ISBN 978-3-030-00985-4. [Google Scholar]
- Trifoi, A.R.; Agachi, P.Ş.; Pap, T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew. Sustain. Energy Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Moreira, M.N.; Faria, R.P.V.; Ribeiro, A.M.; Rodrigues, A.E. Solketal production from glycerol ketalization with acetone: Catalyst selection and thermodynamic and kinetic reaction study. Ind. Eng. Chem. Res. 2019, 58, 17746–17759. [Google Scholar] [CrossRef]
- Moreira, M.N.; Corrêa, I.; Ribeiro, A.M.; Rodrigues, A.E.; Faria, R.P.V. Solketal production in a fixed bed adsorptive reactor through the ketalization of glycerol. Ind. Eng. Chem. Res. 2020, 59, 2805–2816. [Google Scholar] [CrossRef]
- Analysis and Forecasts of the Glycerine Market; LMC: Oxford, UK, 2020.
- Glycerine Market Size, Share & COVID-19 Impact Analysis, By Grade (USP Grade, and Technical Grade), by Application (Personal Care, Food & Beverages, Pharmaceuticals, Polyether Polyols, Chemical Intermediate, Tobacco, and Others), and Regional Forecast 2020–2027; Fortune Business Insights: Pune, Maharashtra, India, 2020.
- INSIGHT: Spot Glycerine Soars on Biodiesel Cut Fears; Independent Commodity Intelligence Services: London, UK, 2020.
- Pagliaro, M. Chapter 1—Properties, applications, history, and market. In Glycerol; Pagliaro, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–21. ISBN 978-0-12-812205-1. [Google Scholar]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts 2018, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Glycerol Market Size, Share & Trends Analysis Report By Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), by Type (Crude, Refined) By End Use (Food & Beverage, Pharmaceutical), By Region, And Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020.
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Mota, C.J.A.; da Silva, C.X.A.; Rosenbach, N.; Costa, J.; da Silva, F. Glycerin derivatives as fuel additives: The addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Mushrush, G.W.; Stalick, W.M.; Beal, E.J.; Basu, S.C.; Slone, J.E.; Cummings, J. The synthesis of acetals and ketals of the reduced sugar mannose as fuel system icing inhibitors. Pet. Sci. Technol. 1997, 15, 237–244. [Google Scholar] [CrossRef]
- Aviation: Benefits Beyond Borders; Air Transport Action Group: Geneva, Switzerland, 2018.
- Mushrush, G. Fuel System Icing Inhibitor and Deicing Composition. U.S. Patent No. US5705087, 6 January 1998. [Google Scholar]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Al-Saadi, L.S.; Eze, V.C.; Harvey, A.P. Techno-economic analysis of glycerol valorization via catalytic applications of sulphonic acid-functionalized copolymer beads. Front. Chem. 2020, 7. [Google Scholar] [CrossRef]
- World Energy Outlook 2020; International Energy Agency: Paris, France, 2020.
- The Covid-19 Crisis and Clean Energy Progress; International Energy Agency: Paris, France, 2020.
- Browning, N. Factbox: Pandemic Brings Forward Predictions for Peak Oil Demand. Available online: https://www.reuters.com/article/oil-demand/factbox-pandemic-brings-forward-predictions-for-peak-oil-demand-idUSL8N2IB5EG (accessed on 15 January 2020).
- Offshore. Analyst Revises Long-Term Oil Demand Forecast. Available online: https://www.offshore-mag.com/production/article/14186589/analyst-revises-longterm-oil-demand-forecast (accessed on 20 January 2021).
- International Energy Agency. Renewable Energy Market Update; International Energy Agency: Paris, France, 2020.
- Liquid Biofuels Market Size, Share & Trends Analysis Report by Product (Biodiesel, Bioethanol), By Application (Transportation Fuel, Power Generation, Thermal Heating), and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020.
- Giraldo, S.Y.; Rios, L.A.; Suárez, N. Comparison of glycerol ketals, glycerol acetates and branched alcohol-derived fatty esters as cold-flow improvers for palm biodiesel. Fuel 2013, 108, 709–714. [Google Scholar] [CrossRef]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Iodice, P.; Senatore, A.; Langella, G.; Amoresano, A. Advantages of ethanol–gasoline blends as fuel substitute for last generation Si engines. Environ. Prog. Sustain. Energy 2017, 36, 1173–1179. [Google Scholar] [CrossRef]
- Transport Biofuels; International Energy Agency: Paris, France, 2020.
- Shi, E.; Hanson, S.; EIA Projects U.S. Biofuel Production to Slowly Increase Through 2050. Available online: https://www.eia.gov/todayinenergy/detail.php?id=43096 (accessed on 15 January 2020).
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García Sancho, C.; Maireles-Torres, P.; Luque, R. Industrial food waste valorization: A general overview. In Biorefinery; Springer: Cham, Switzerland, 2019; pp. 253–277. ISBN 978-3-030-10960-8. [Google Scholar]
- Garlapati, V.K.; Shankar, U.; Budhiraja, A. Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. 2016, 9, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monbaliu, J.-C.M.; Winter, M.; Chevalier, B.; Schmidt, F.; Jiang, Y.; Hoogendoorn, R.; Kousemaker, M.A.; Stevens, C.V. Effective production of the biodiesel additive STBE by a continuous flow process. Bioresour. Technol. 2011, 102, 9304–9307. [Google Scholar] [CrossRef]
- Solketal: An EHS Friendly and Sustainable Solvent; IMPAG Switzerland: Zürich, Switzerland, 2017.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7528, Solketal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Solketal (accessed on 13 December 2020).
- Esteban, J.; García-Ochoa, F.; Ladero, M. Solventless synthesis of solketal with commercially available sulfonic acid based ion exchange resins and their catalytic performance. Green Process. Synth. 2017, 6, 79–89. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Isac-García, J.; Dobado, J.A.; Calvo-Flores, F.G.; Martínez-García, H. Chapter 5—Determining physical and spectroscopic properties. In Experimental Organic Chemistry; Isac-García, J., Dobado, J.A., Calvo-Flores, F.G., Martínez-García, H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 145–175. ISBN 978-0-12-803893-2. [Google Scholar]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Ilgen, O.; Yerlikaya, S.; Akyurek, F.O. Synthesis of solketal from glycerol and acetone over amberlyst-46 to produce an oxygenated fuel additive. Period. Polytech. Chem. Eng. 2016. [Google Scholar] [CrossRef] [Green Version]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Fischer, E. Ueber die Verbindungen der Zucker mit den Alkoholen und Ketonen. Ber. Dtsch. Chem. Ges. 1895, 28, 1145–1167. [Google Scholar] [CrossRef] [Green Version]
- Newman, M.S.; Renoll, M. Improved preparation of isopropylidene glycerol. J. Am. Chem. Soc. 1945, 67, 1621. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C. Catalytic conversion of glycerol to oxygenated fuel additive in a continuous flow reactor: Process optimization. Fuel 2014, 128, 113–119. [Google Scholar] [CrossRef]
- García, E.; Laca, M.; Pérez, E.; Garrido, A.; Peinado, J. New class of acetal derived from glycerin as a biodiesel fuel component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Roldán, L.; Mallada, R.; Fraile, J.M.; Mayoral, J.A.; Menéndez, M. Glycerol upgrading by ketalization in a zeolite membrane reactor. Asia-Pac. J. Chem. Eng. 2009, 4, 279–284. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Nekhaev, A.I.; Ramazanov, D.N.; Arinicheva, Y.A.; Dzyubenko, A.A.; Khadzhiev, S.N. Preparation of high-octane oxygenate fuel components from plant-derived polyols. Pet. Chem. 2011, 51, 61–69. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites as catalysts. Chem. Eng. J. 2011, 178, 291–296. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Velty, A.; Santos, L.L.; Leyva-Pérez, A.; Sabater, M.J.; Iborra, S.; Corma, A. Gold catalysts and solid catalysts for biomass transformations: Valorization of glycerol and glycerol–water mixtures through formation of cyclic acetals. J. Catal. 2010, 271, 351–357. [Google Scholar] [CrossRef]
- Da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Madeira, L.M.; Wu, Y.-J.; Faria, R. Sorption Enhanced Reaction Processes; World Scientific Publishing Co Pte Ltd.: Singapore, 2017; ISBN 978-1-78634-356-7. [Google Scholar]
- De Torres, M.; Jiménez-osés, G.; Mayoral, J.A.; Pires, E.; de los Santos, M. Glycerol ketals: Synthesis and profits in biodiesel blends. Fuel 2012, 94, 614–616. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Glycerol valorization as biofuel: Thermodynamic and kinetic study of the acetalization of glycerol with acetaldehyde. Ind. Eng. Chem. Res. 2013, 52, 1538–1547. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Glycerol valorisation as biofuels: Selection of a suitable solvent for an innovative process for the synthesis of GEA. Chem. Eng. J. 2013, 233, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Bruchmann, B.; Haberle, K.; Gruner, H.; Hirn, M. Preparation of Cyclic Acetals or Ketals. U.S. Patent No. US5917059A, 29 June 1999. [Google Scholar]
- Royon, D.; Locatelli, S.; Gonzo, E.E. Ketalization of glycerol to solketal in supercritical acetone. J. Supercrit. Fluids 2011, 58, 88–92. [Google Scholar] [CrossRef]
- Eigenberger, G.; Ruppel, W. Catalytic fixed-Bed reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-Vch: Weinheim, Germany, 2012; ISBN 9783527303854. [Google Scholar]
- Clarkson, J.S.; Walker, A.J.; Wood, M.A. Continuous reactor technology for ketal formation: An improved synthesis of solketal. Org. Process Res. Dev. 2001, 5, 630–635. [Google Scholar] [CrossRef]
- Cablewski, T.; Faux, A.F.; Strauss, C.R. Development and application of a continuous microwave reactor for organic synthesis. J. Org. Chem. 1994, 59, 3408–3412. [Google Scholar] [CrossRef]
- Kowalska-Kus, J.; Held, A.; Frankowski, M.; Nowinska, K. Solketal formation from glycerol and acetone over hierarchical zeolites of different structure as catalysts. J. Mol. Catal. A Chem. 2017, 426, 205–212. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; García-Ochoa, F. Kinetic modelling of the solventless synthesis of solketal with a sulphonic ion exchange resin. Chem. Eng. J. 2015, 269, 194–202. [Google Scholar] [CrossRef]
- Cornejo, A.; Campoy, M.; Barrio, I.; Navarrete, B.; Lázaro, J. Solketal production in a solvent-free continuous flow process: Scaling from laboratory to bench size. React. Chem. Eng. 2019, 4, 1803–1813. [Google Scholar] [CrossRef]
- Zahid, I.; Ayoub, M.; Abdullah, B.B.; Nazir, M.H.; Ameen, M.; Zulqarnain; Mohd Yusoff, M.H.; Inayat, A.; Danish, M. Production of fuel additive solketal via catalytic conversion of biodiesel-derived glycerol. Ind. Eng. Chem. Res. 2020, 59, 20961–20978. [Google Scholar] [CrossRef]
- Esposito, R.; Cucciolito, M.E.; D’Amora, A.; Di Guida, R.; Montagnaro, F.; Ruffo, F. Highly efficient iron(III) molecular catalysts for solketal production. Fuel Process. Technol. 2017, 167, 670–673. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Rodrigues, A.A.; Pinheiro, P.F. Solketal synthesis from glycerol and acetone in the presence of metal salts: A Lewis or Brønsted acid catalyzed reaction? Fuel 2020, 276, 118164. [Google Scholar] [CrossRef]
- Manjunathan, P.; Maradur, S.P.; Halgeri, A.B.; Shanbhag, G.V. Room temperature synthesis of solketal from acetalization of glycerol with acetone: Effect of crystallite size and the role of acidity of beta zeolite. J. Mol. Catal. A Chem. 2015, 396, 47–54. [Google Scholar] [CrossRef]
- Rossa, V.; Pessanha, Y.d.S.P.; Díaz, G.C.; Câmara, L.D.T.; Pergher, S.B.C.; Aranda, D.A.G. Reaction kinetic study of solketal production from glycerol ketalization with acetone. Ind. Eng. Chem. Res. 2017, 56, 479–488. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Tarighi, S. Hierarchical faujasite zeolite-supported heteropoly acid catalyst for acetalization of crude-glycerol to fuel additives. J. Ind. Eng. Chem. 2019, 79, 452–464. [Google Scholar] [CrossRef]
- Sulistyo, H.; Priadana, D.P.; Fitriandini, Y.W.; Ariyanto, T.; Azis, M.M. Utilization of glycerol by ketalization reactions with acetone to produce solketal using indion 225 Na as catalyst. Int. J. Technol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, M.N.; Panchenko, V.N.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Effect of nitric acid modification of montmorillonite clay on synthesis of solketal from glycerol and acetone. Catal. Commun. 2017, 90, 65–69. [Google Scholar] [CrossRef]
- Amri, S.; Gómez, J.; Balea, A.; Merayo, N.; Srasra, E.; Besbes, N.; Ladero, M. Green production of glycerol ketals with a clay-based heterogeneous acid catalyst. Appl. Sci. 2019, 9, 4488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhao, Z.; Ao, Y. Design of highly efficient Zn-, Cu-, Ni- and Co-promoted M-AlPO4 solid acids: The acetalization of glycerol with acetone. Appl. Catal. A 2015, 496, 32–39. [Google Scholar] [CrossRef]
- Gadamsetti, S.; Rajan, N.P.; Rao, G.S.; Chary, K.V. Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts. J. Mol. Catal. A Chem. 2015, 410, 49–57. [Google Scholar] [CrossRef]
- Rodrigues, R.; Mandelli, D.; Gonçalves, N.S.; Pescarmona, P.P.; Carvalho, W.A. Acetalization of acetone with glycerol catalyzed by niobium-aluminum mixed oxides synthesized by a sol–gel process. J. Mol. Catal. A Chem. 2016, 422, 122–130. [Google Scholar] [CrossRef]
- Gui, Z.; Zahrtmann, N.; Saravanamurugan, S.; Reyero, I.; Qi, Z.; Bañares, M.A.; Riisager, A.; Garcia-Suarez, E.J. Brønsted Acid Ionic Liquids (BAILs) as efficient and recyclable catalysts in the conversion of glycerol to solketal at room temperature. ChemistrySelect 2016, 1, 5869–5873. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Zhang, T.; Gui, X.; Shi, H.; Yun, Z. Solventless ketalization of glycerol to solketal with acetone over the ionic liquid [P(C4H9)3C14H29][TsO]. Chin. J. Chem. Eng. 2020, 28, 158–164. [Google Scholar] [CrossRef]
- Gonçalves, M.; Rodrigues, R.; Galhardo, T.S.; Carvalho, W.A. Highly selective acetalization of glycerol with acetone to solketal over acidic carbon-based catalysts from biodiesel waste. Fuel 2016, 181, 46–54. [Google Scholar] [CrossRef]
- Fernández, P.; Fraile, J.M.; García-Bordejé, E.; Pires, E. Sulfonated hydrothermal carbons from cellulose and glucose as catalysts for glycerol ketalization. Catalysts 2019, 9, 804. [Google Scholar] [CrossRef] [Green Version]
- Carvalho Ballotin, F.; da Silva, M.J.; Paula de Carvalho Teixeira, A.; Montero Lago, R. Amphiphilic acid carbon catalysts produced by bio-oil sulfonation for solvent-free glycerol ketalization. Fuel 2020, 274, 117799. [Google Scholar] [CrossRef]
- Sandesh, S.; Halgeri, A.B.; Shanbhag, G.V. Utilization of renewable resources: Condensation of glycerol with acetone at room temperature catalyzed by organic–inorganic hybrid catalyst. J. Mol. Catal. A Chem. 2015, 401, 73–80. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhou, R.; Hou, Z. Layered α-zirconium phosphate: An efficient catalyst for the synthesis of solketal from glycerol. Appl. Clay Sci. 2019, 174, 120–126. [Google Scholar] [CrossRef]
- Vannucci, J.A.; Nichio, N.N.; Pompeo, F. Solketal synthesis from ketalization of glycerol with acetone: A kinetic study over a sulfated zirconia catalyst. Catal. Today 2020. [Google Scholar] [CrossRef]
- Sulistyo, H.; Perdana, I.; Pratiwi, F.T.; Hartati, I. Kinetics and thermodynamics studies of ketalization of glycerol and acetone in the presence of Basolite F300 as catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2020, 742, 012007. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Teixeira, M.G.; Chaves, D.M.; Siqueira, L. An efficient process to synthesize solketal from glycerol over tin (II) silicotungstate catalyst. Fuel 2020, 281, 118724. [Google Scholar] [CrossRef]
- Podolean, I.; Zhang, J.; Shamzhy, M.; Pârvulescu, V.I.; Čejka, J. Solvent-free ketalization of polyols over germanosilicate zeolites: The role of the nature and strength of acid sites. Catal. Sci. Technol. 2020, 10, 8254–8264. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhou, R.; Hou, Z. Acetalization of glycerol with acetone over appropriately-hydrophobic zirconium organophosphonates. Appl. Clay Sci. 2020, 189, 105555. [Google Scholar] [CrossRef]
- Hussein, H.; Vivian, A.; Fusaro, L.; Devillers, M.; Aprile, C. Synthesis of highly accessible gallosilicates via impregnation procedure: Enhanced catalytic performances in the conversion of glycerol into solketal. ChemCatChem 2020, 12, 5966–5976. [Google Scholar] [CrossRef]
- Vivian, A.; Soumoy, L.; Fusaro, L.; Fiorilli, S.; Debecker, D.P.; Aprile, C. Surface-functionalized mesoporous gallosilicate catalysts for the efficient and sustainable upgrading of glycerol to solketal. Green Chem. 2021, 23, 354–366. [Google Scholar] [CrossRef]
- Esposito, R.; Raucci, U.; Cucciolito, M.E.; Di Guida, R.; Scamardella, C.; Rega, N.; Ruffo, F. Iron(III) complexes for highly efficient and sustainable ketalization of glycerol: A combined experimental and theoretical study. ACS Omega 2019, 4, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-W.; Park, Y.-M.; Lee, K.-Y. Heterogeneous base catalysts for transesterification in biodiesel synthesis. Catal. Surv. Asia 2009, 13, 63–77. [Google Scholar] [CrossRef]
- Gomes, I.S.; de Carvalho, D.C.; Oliveira, A.C.; Rodríguez-Castellón, E.; Tehuacanero-Cuapa, S.; Freire, P.T.C.; Filho, J.M.; Saraiva, G.D.; de Sousa, F.F.; Lang, R. On the reasons for deactivation of titanate nanotubes with metals catalysts in the acetalization of glycerol with acetone. Chem. Eng. J. 2018, 334, 1927–1942. [Google Scholar] [CrossRef]
- Pinheiro, A.L.G.; do Carmo, J.V.C.; Carvalho, D.C.; Oliveira, A.C.; Rodríguez-Castellón, E.; Tehuacanero-Cuapa, S.; Otubo, L.; Lang, R. Bio-additive fuels from glycerol acetalization over metals-containing vanadium oxide nanotubes (MeVOx-NT in which, Me = Ni, Co, or Pt). Fuel Process. Technol. 2019, 184, 45–56. [Google Scholar] [CrossRef]
- Dmitriev, G.S.; Terekhov, A.V.; Zanaveskin, L.N.; Maksimov, A.L.; Khadzhiev, S.N. Kinetics of the formation of solketal in the presence of sulfuric acid. Kinet. Catal. 2018, 59, 504–508. [Google Scholar] [CrossRef]
- Taddeo, F.; Esposito, R.; Russo, V.; Di Serio, M. Kinetic modeling of solketal synthesis from glycerol and acetone catalyzed by an iron(III) complex. Catalysts 2021, 11, 83. [Google Scholar] [CrossRef]
- Li, X.; Lai, J.; Cong, H.; Shu, C.; Zhao, R.; Wang, Y.; Li, H.; Gao, X. Toward sustainable and eco-efficient novel catalytic distillation process for production of solketal using seepage catalytic packing internal. Catal. Today 2020. [Google Scholar] [CrossRef]
- Dmitriev, G.S.; Terekhov, A.V.; Khadzhiev, S.N.; Zanaveskin, L.N. Specific features of solketal synthesis on KU-2-8 cation-exchange resin. Russ. J. Appl. Chem. 2016, 89, 45–50. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Souza, R.O.M.A.; Mota, C.J.A. Atmospheric pressure continuous production of solketal from the acid-catalyzed reaction of glycerol with acetone. J. Braz. Chem. Soc. 2016, 27, 1832–1837. [Google Scholar] [CrossRef]
- Guidi, S.; Noè, M.; Riello, P.; Perosa, A.; Selva, M. Towards a rational design of a continuous-flow method for the acetalization of crude glycerol: Scope and limitations of commercial amberlyst 36 and AlF3·3H2O as model catalysts. Molecules 2016, 21, 657. [Google Scholar] [CrossRef] [Green Version]
- Konwar, L.J.; Samikannu, A.; Mäki-Arvela, P.; Boström, D.; Mikkola, J.-P. Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives. Appl. Catal. B 2018, 220, 314–323. [Google Scholar] [CrossRef]
- Domínguez-Barroso, V.; Herrera, C.; Larrubia, M.Á.; González-Gil, R.; Cortés-Reyes, M.; Alemany, L.J. Continuous-flow process for glycerol conversion to solketal using a brönsted acid functionalized carbon-based catalyst. Catalysts 2019, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K. Solketal formation in a continuous flow process over hierarchical zeolites. ChemCatChem 2020, 12, 510–519. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K. A continuous-flow process for the acetalization of crude glycerol with acetone on zeolite catalysts. Chem. Eng. J. 2020, 401, 126143. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Zhang, L.; Zhang, Q. Continuous flow synthesis of a ZSM-5 film in capillary microchannel for efficient production of solketal. ACS Omega 2020, 5, 20784–20791. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, L.; Wang, X.; Chen, A.; Zhang, Q. Microfluidic processing of HZSM-5 films in a capillary microreactor for the continuous acetalisation reaction of glycerol with acetone. React. Chem. Eng. 2020, 5, 539–546. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Moulijn, J.A. Process intensification: Transforming chemical engineering. Chem. Eng. Prog. 2000, 96, 22–33. [Google Scholar]
- Stankiewicz, A.; Moulijn, J.A. Chapter 2—Process intensification—An overview. In Process Intensification, 2nd ed.; Reay, D., Ramshaw, C., Harvey, A., Eds.; Butterworth-Heinemann: Oxford, UK, 2008; pp. 21–45. ISBN 978-0-7506-8941-0. [Google Scholar]
- Priya, S.S.; Selvakannan, P.R.; Chary, K.V.R.; Kantam, M.L.; Bhargava, S.K. Solvent-free microwave-assisted synthesis of solketal from glycerol using transition metal ions promoted mordenite solid acid catalysts. Mol. Catal. 2017, 434, 184–193. [Google Scholar] [CrossRef]
- Eze, V.C.; Harvey, A.P. Continuous reactive coupling of glycerol and acetone—A strategy for triglyceride transesterification and in-situ valorisation of glycerol by-product. Chem. Eng. J. 2018, 347, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Al-Saadi, L.S.; Eze, V.C.; Harvey, A.P. A reactive coupling process for co-production of solketal and biodiesel. Green Process. Synth. 2019, 8, 516–524. [Google Scholar] [CrossRef]
- Qing, W.; Chen, J.; Shi, X.; Wu, J.; Hu, J.; Zhang, W. Conversion enhancement for acetalization using a catalytically active membrane in a pervaporation membrane reactor. Chem. Eng. J. 2017, 313, 1396–1405. [Google Scholar] [CrossRef]
- Zaharia, E.; Bildea, C.; Muntean, O. Design, economic evaluation and plantwide control of glycerol ketalization plant. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2015, 77, 41–52. [Google Scholar]
- Dmitriev, G.S.; Terekhov, A.V.; Zanaveskin, L.N.; Khadzhiev, S.N.; Zanaveskin, K.L.; Maksimov, A.L. Choice of a catalyst and technological scheme for synthesis of solketal. Russ. J. Appl. Chem. 2017, 89, 1619–1624. [Google Scholar] [CrossRef]
- Da Silva, M.J.; de Ávila Rodrigues, F.; Júlio, A.A. SnF2-catalyzed glycerol ketalization: A friendly environmentally process to synthesize solketal at room temperature over on solid and reusable Lewis acid. Chem. Eng. J. 2017, 307, 828–835. [Google Scholar] [CrossRef]
- Al-Saadi, L.S.; Eze, V.C.; Harvey, A.P. Techno-economic analysis of processes for biodiesel production with integrated co-production of higher added value products from glycerol. Biofuels 2020, 1–8. [Google Scholar] [CrossRef]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Clarke, N.S. The basics of patent searching. World Pat. Inf. 2018, 54, S4–S10. [Google Scholar] [CrossRef]
- Frietsch, R.; Schmoch, U.; Van Looy, B.; Walsh, J.P.; Devroede, R.; Du Plessis, M.; Jung, T.; Meng, Y.; Neuhäusler, P.; Peeters, B. The Value and Indicator Function of Patents; Studien zum Deutschen Innovations System: Berlin, Germany, 2010. [Google Scholar]
- Boesch, V.; Herguijuela, J.R. Process of Manufacturing Equipment for Preparing Acetals and Ketals. U.S. Patent No. US 6528025 B1, 4 March 2003. [Google Scholar]
- Winkler, H.; Poll, H.-G.; Neumann, M.; Koehler, G.; Bauer, F. Process for Preparing Acetals and Ketals with the Aid of Multistage Pervaporation or Vapor Permeation. U.S. Patent No. US20040024260A1, 5 February 2004. [Google Scholar]
- Dubois, J.L. Synthesis of Cyclic Acetals by Reaction of Aldehyde and/or Ketone with a Polyol, in a Simulated Mobile bed Reactor Having Garnished Columns of Acidic Solid Bed Able to Selectively Adsorb the Reaction Products. French Patent No. FR2906246A1, 6 September 2008. [Google Scholar]
- Dubois, J.L.; Iborra, C.S.; Corma, C.A.; Velty, A. Process for the Synthesis of Cyclic Acetals by Reactive Extraction of a Polyol in a Concentrated Solution. French Patent No. FR2906807A1, 2008. Available online: https://patents.google.com/patent/US20100099894A1/en (accessed on 11 March 2021).
- Haiyu, Y.; Chunlong, H.; Peng, J.; Qingyan, C.; Liqing, X. A Kind of Method That Molecular Film Reactor Prepares Solketal. Chinese Patent No. CN107698552A, 16 February 2018. [Google Scholar]
- Haiyu, Y.; Chunlong, H.; Peng, J.; Qingyan, C.; Liqing, X. Industrial Continuous Method for Preparing Solketal by Catalyst Distillation Device. Chinese Patent No. CN107652263A, 2 February 2018. [Google Scholar]
- Yujia, L.; Changjiu, X.; Yamin, W.; Yongjia, Y.; Bin, Z.; Xinxin, P.; Min, L.; Yibin, L.; Xingtian, S. Method for Preparing Ketal Glycerine and/or Acetal Glycerine. Chinese Patent No. CN111253359A, 9 June 2020. [Google Scholar]
- Yujia, L.; Yamin, W.; Changjiu, X.; Bin, Z.; Min, L.; Yibin, L.; Xingtian, S. Method for Preparing Ketal Glycerine and/or Acetal Glycerine. Chinese Patent No. CN111253363A, 9 June 2020. [Google Scholar]
- Yujia, L.; Changjiu, X.; Xinxin, P.; Bin, Z.; Min, L.; Yibin, L.; Xingtian, S. Method for Preparing Ketal Glycerine and/or Acetal Glycerine. Chinese Patent No. CN111253362A, 9 June 2020. [Google Scholar]
- Yujia, L.; Changjiu, X.; Yongjia, Y.; Bin, Z.; Xinxin, P.; Min, L.; Yibin, L.; Xingtian, S. Method for Preparing Ketal Glycerine and/or Acetal Glycerine by Catalyzing Glycerine. Chinese Patent No. CN111253364A, 9 June 2020. [Google Scholar]
- Abe, T.; Kawakami, N. Method for Producing Dioxolane Compound. Japanese Patent No. JP2004059435A, 26 February 2004. [Google Scholar]
- Wimmer, T.; Ertl, C.; Kalb, R. Process for Preparing Cyclic Glycerol Acetals or Cyclic Glycerol Ketals or Mixtures Thereof. European Patent No. EP2183238B1, 2 March 2011. [Google Scholar]
- Terrill, D.L.; McMurray, B.D.; Billodeaux, D.R.; Little, J.L.; Howard, A.S. Production of Cyclic Acetals or Ketals Using Liquid-Phase Acid Catalysts. U.S. Patent No. US20120330033A1, 3 March 2012. [Google Scholar]
- Mastroianni, S. Process for Producing Dioxolane. U.S. Patent No. US20130178638A, 13 July 2013. [Google Scholar]
- Rodrigues, E.; Malheiro, A. Process for the Production of a Dioxolane Compound from Crude Glycerol Including a Liquid-Liquid Extraction Step. World Patent No. WO2013045967Al, 11 July 2013. [Google Scholar]
- Rodrigues, E.; Malheiro, A. Process for the Production of a Dioxolane Compound from Crude Glycerol. U.S. Patent No. US20140235878A1, 8 September 2014. [Google Scholar]
- Terrill, D.L.; McMurray, B.D.; Billodeaux, D.R.; Little, J.L.; Howard, A.S. Production of Cyclic Acetals or Ketals Using Solid Acid Catalysts. U.S. Patent No. US8829206B2, 9 September 2014. [Google Scholar]
- Terrill, D.L.; McMurray, B.D.; Billodeaux, D.R.; Little, J.L.; Howard, A.S. Production of Cyclic Acetals or Ketals Using Solid Acid Catalysts. U.S. Patent No. US9440944B2, 13 September 2016. [Google Scholar]
- Varfolomeev, S.D.; Makarov, G.G.; Volyeva, V.B.; Komissarova, N.L.; Malkova, A.V.; Ovsyannikova, M.N.; Koverzanova, E.V.; Usachev, S.V.; Aynullov, T.S.; Shamsullin, A.I.; et al. Method of Producing Solketal. Russian Patent No. RU2668987C1, 5 October 2018. [Google Scholar]
- Constantino, D.S.M.; Faria, R.P.V.; Pereira, C.S.M.; Loureiro, J.M.; Rodrigues, A.E. Enhanced simulated moving bed reactor process for butyl acrylate synthesis: Process analysis and optimization. Ind. Eng. Chem. Res. 2016, 55, 10735–10743. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Sorption enhanced reactive process for the synthesis of glycerol ethyl acetal. Chem. Eng. J. 2014, 258, 229–239. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Gomes, P.S.; Gandi, G.K.; Silva, V.M.T.M.; Rodrigues, A.E. Multifunctional reactor for the synthesis of dimethylacetal. Ind. Eng. Chem. Res. 2008, 47, 3515–3524. [Google Scholar] [CrossRef]
- Minceva, M.; Gomes, P.S.; Meshko, V.; Rodrigues, A.E. Simulated moving bed reactor for isomerization and separation of p-xylene. Chem. Eng. J. 2008, 140, 305–323. [Google Scholar] [CrossRef]
- Ströhlein, G.; Mazzotti, M.; Morbidelli, M. Optimal operation of simulated-moving-bed reactors for nonlinear adsorption isotherms and equilibrium reactions. Chem. Eng. Sci. 2005, 60, 1525–1533. [Google Scholar] [CrossRef]
- Kawase, M.; Suzuki, T.B.; Inoue, K.; Yoshimoto, K.; Hashimoto, K. Increased esterification conversion by application of the simulated moving-bed reactor. Chem. Eng. Sci. 1996, 51, 2971–2976. [Google Scholar] [CrossRef]
- Dubé, M.A.; Tremblay, A.Y.; Liu, J. Biodiesel production using a membrane reactor. Bioresour. Technol. 2007, 98, 639–647. [Google Scholar] [CrossRef]
- Itoh, N. A membrane reactor using palladium. AIChE J. 1987, 33, 1576–1578. [Google Scholar] [CrossRef]
- De Falco, M.; Capocelli, M.; Giannattasio, A. Membrane reactor for one-step DME synthesis process: Industrial plant simulation and optimization. J. CO2 Util. 2017, 22, 33–43. [Google Scholar] [CrossRef]
- Deshayes, A.L.; Miró, E.E.; Horowitz, G.I. Xylene isomerization in a membrane reactor: Part II. Simulation of an industrial reactor. Chem. Eng. J. 2006, 122, 149–157. [Google Scholar] [CrossRef]


| Author | Catalyst | Acidity (mmol/gcat) | Reaction Conditions | Results (%) | Reusability | Ref. |
|---|---|---|---|---|---|---|
| Homogeneous Catalysts | ||||||
| Esposito, 2019 | Iron (III) Complex FeCl3 (1–NO2) 0.05 mol% (glycerol) | N.A. 1 | G:A = 1:4 | Xgly > 99% Sel ≥ 98.5% | N.A. 1 | [73] |
| T = – | ||||||
| t = 90 min | ||||||
| Da Silva, 2020 | Fe (NO3)3 9⋅H2O 0.30 mol% 2 | N.A. 1 | G:A = 1:20 | Xgly = 98% Sel = 97% | Four cycles without significant activity loss. | [74] |
| T = 298 K | ||||||
| t = 60 min | ||||||
| Zeolites | ||||||
| Manjunathan, 2015 | H–Beta 5 wt% (glycerol) | 1.51 | G:A = 1:2 | Xgly = 86% Sel = 98.5% | One cycle without significant activity loss. | [75] |
| T = 301 K | ||||||
| t = 60 min | ||||||
| Rossa, 2017 | H–Beta 5 wt% (glycerol) | 0.094 | G:A = 1:4 | Xgly = 72.62% Sel = 98.3% | A 22% decrease on the activity after the first use. Activity remained until the fourth cycle. | [76] |
| T = 333 K | ||||||
| t = 60 min | ||||||
| Kowalska–Kus, 2017 | MFI Beta MOR 1 wt% (glycerol) | 0.369 | G:A = 1:1 | Xgly = 85, 85, 80% Sel < 99% Ysolk ≈ 80% | N.A. 1 | [69] |
| 0.388 | T = 343 K | |||||
| 0.55 | t = – | |||||
| Talebian–Kiakalaieh, 2019 | HR/Y–W20 10 wt% (glycerol) | 1.806 | G:A = 1:10 | Xgly = 100% Sel = 97.9% Ysolk = 97.9% | Four cycles without significant activity loss. | [77] |
| T = 313 K | ||||||
| t = 90 min | ||||||
| Ion Exchange Resins | ||||||
| Esteban, 2015 | Lewatit GF101 0.5 wt% 2 | 5.11 | G:A = 1:12 | Xgly = 96% Ysolk ≈ 80% | N.A. 1 | [70] |
| T = 313 K | ||||||
| T = 240 min | ||||||
| Cornejo, 2019 | Purolite CT275 5 wt% (glycerol) | 5.2 | G:A = 1:12 | Xgly = 93% | N.A. 1 | [71] |
| T = 323 K | ||||||
| T = 300 min | ||||||
| Moreira, 2019 | Amberlyst–35 0.5 wt% (reactants) | 5.08 | G:A = 1:2 | Xgly = 70% | N.A. 1 | [10] |
| T = 303 K | ||||||
| T = 480 min | ||||||
| Solv. = Ethanol | ||||||
| Sulistyo, 2020 | Indion 225 Na 5 wt% 2 | N.A. 1 | G:A = 1:5 | Xgly = 31.9% | N.A. 1 | [78] |
| T = 328 K | ||||||
| T = 180 min | ||||||
| Clays | ||||||
| Timofeeva, 2017 | HNO3 Modified montmorillonite clay 5 wt% (glycerol) | 0.015 3 | G:A = 1:2.5 | Xgly = 94% Sel = 95.4% | Three cycles without significant activity loss. | [79] |
| T = 298 K | ||||||
| T = 15 min | ||||||
| Solv. = acetonitrile | ||||||
| Amri, 2019 | HCl activated clay 5 w/v% (reactants) | 0.0065 3 | G:A = 1:6 | Ysolk = 69.3% | Significant activity loss in the second cycle. | [80] |
| T = 298 K | ||||||
| T = 60 min | ||||||
| Solv. = Isopropanol | ||||||
| Metal Oxides | ||||||
| Zhang, 2015 | M–NiAlPO4 4 wt% (glycerol) | 0.12 | G:A = 1:8 | Sel = 75.1% Ysolk = 75.4% | Three cycles without significant activity loss. Activity decreased from the fourth cycle on. | [81] |
| T = 353 K | ||||||
| T = 60 min | ||||||
| Gadamsetti, 2015 | MoPO/SBA–15 2.7 wt% (reactants) | ≈ 1 | G:A = 1:2 | Xgly = 100% Sel = 98% | A 30% activity loss after the first cycle. Activity remained until de fourth cycle (Xgly = 100, 70, 68, and 62%). | [82] |
| T = 301 K | ||||||
| T = 60 min | ||||||
| Rodrigues, 2016 | 1Nb:0.05Al 2.7 wt% (glycerol) | 0.094 | G:A = 1:4 | Xgly = 84% Sel = 98% | Four cycles without significant activity loss. | [83] |
| T = 323 K | ||||||
| T = 360 min | ||||||
| Ionic Liquid | ||||||
| Gui, 2016 | 1–(4–sulfonylbutyl)triphenylphosphonium methanesulfonate 2.7 mol% (glycerol) | N.A. 1 | G:A = 1:15 | Xgly = 96% Sel = 98.5% | Four cycles without significant activity loss. | [84] |
| T = 298 K | ||||||
| T = 30 min | ||||||
| Ji, 2020 | [P (C4H9)3C14H29] [TsO] 5 wt% (glycerol) | N.A. 1 | G:A = 1:6 | Ysolk = 86% | Ten cycles without significant activity loss. | [85] |
| T = 303 K | ||||||
| t = 30 min | ||||||
| Sulfonated Carbon based | ||||||
| Gonçalves, 2016 | GC–1:2 3 wt% (glycerol) | 3.8 | G:A = 1:4 | Xgly = 82% Sel = 95% | A 10% activity loss after five cycles. | [86] |
| T = 298 K | ||||||
| t = 240 min | ||||||
| Fernández, 2019 | Cel–215–2 M–20 h–S | G:A = 1:7 | ||||
| Glu–195–20 h–S | 5.43 | T = 298 K | Ysolk = 80 − 86% | Stable for 60 h of operation. | [87] | |
| No information on the recycle. | ||||||
| 1 wt% (glycerol) | 3.42 | t = 120–240 min | Ysolk = 80 − 86% | |||
| Ballotin, 2020 | BS9.20.6 wt% 2 | 0.3 | G:A = 1:10 | Xgly = 93% Sel = 98% | A 4% activity loss after four cycles. | [88] |
| T = 298 K | ||||||
| t = 120 min | ||||||
| Others | ||||||
| Sandesh, 2015 | Heteropoly acids (C3H7)4N+/PWA 3 wt% (reactants) | 0.6 | G:A = 1:6 | Xgly = 94% Sel = 98% Ysolk = 93% | A 5% activity loss after three cycles. | [89] |
| T = 303 K | ||||||
| t = 120 min | ||||||
| Li, 2019 | Layered crystalline α–zirconium phosphate 5 wt% (glycerol) | 1.3 | G:A = 1:10 | Xgly = 85.7% Sel = 98.3% | Four cycles without significant activity loss. Activity decreased until the fifth cycle. | [90] |
| T = 323 K | ||||||
| t = 180 min | ||||||
| Vannucci, 2020 | Sulfated zirconium oxide 0.3 wt% (glycerol) | 0.09 | G:A = 1:8 | Xgly ≈ 80% | A 16% activity loss after four cycles. | [91] |
| T = 313 K | ||||||
| t = 280 min | ||||||
| Sulistyio, 2020 | Basolite F300 1 wt% (glycerol) | N.A. 1 | G:A = 1:4 | Xgly ≈ 84.3% | N.A. 2 | [92] |
| T = 323 K | ||||||
| t = 60 min | ||||||
| Da Silva, 2020 | Tin (II) silicotungstate acid salt 0.01 mol% 2 | 1.3 | G:A = 1:4 | Xgly ≈ 74% Sel > 98% Ysolk = 73% | “No decrease in the catalytic activity was found.” | [93] |
| T = 298 K | ||||||
| t = 120 min | ||||||
| Podolean, 2020 | Germanosilicate zeolite 5 wt% (glycerol) | N.A. 1 | G:A = 1:5 | Xgly = 56% Sel = 98% | Six cycles without significant activity loss. Stable up to 12 h on stream. | [94] |
| T = 298 K | ||||||
| t = 180 min | ||||||
| Li, 2020 | Zirconium organophosphonate 5 wt% 2 | 1.12 | G:A = 1:10 | Xgly = 90.2% Sel = 98.5% | A 2.7% activity loss after five cycles. Stable up to 10 h on stream. | [95] |
| T = 313 K | ||||||
| t = 360 min | ||||||
| Hussein, 2020 | Gallosilicate 10 mg | 0.39 | G:A = 1:4 | Xgly = 34% Sel > 95% | Seven cycles without significant activity loss. Stable up to 6 h on stream. | [96] |
| T = 353 K | ||||||
| t = 180 min | ||||||
| Vivian, 2021 | Gallosilicate 3.2 wt% (glycerol) | N.A. 1 | G:A = 1:4 | Xgly = 43% Sel = 93% | Four cycles without significant activity loss. | [97] |
| T = 323 K | ||||||
| t = 60 min | ||||||
| Author | Catalyst | EA (kJ·mol–1) | Other Parameters 1,2,3,4,5 | Ref. |
|---|---|---|---|---|
| Pseudo-homogeneous (PH) | ||||
| Rossa, 2017 | Zeolite H-Beta | 44.77 ± 1.2 | EA-1 = 41.40 ± 1.8 kJ·mol−1 | [76] |
| Keq = 0.5159 (313 K) | ||||
| Dmitriev, 2018 | Sulfuric acid | 87.1 | EA-1 = 101.67 kJ·mol−1 | [102] |
| Keq = 0.77 (303 K) | ||||
| Cornejo, 2019 | Purolite® CT275 | 39.78 ± 0.34 | k = (4.800 ± 0.030). 10−3 L2 mol−1·gcat−1·min−1 (298 K) | [71] |
| ∆H = − 6.605 ± 0.168 kJ·mol−1 | ||||
| Amri, 2019 | HCl activated clay | 65.4 | EA-1 = 70.65 kJ·mol−1 | [80] |
| Keq = 0.3931 (313 K) | ||||
| Ji, 2020 | Ionic Liquid [P(C4H9) 3C14H29][TsO] | 28.2 | Keq = 0.4703 (298 K) | [85] |
| Vannucci, 2020 | Sulfated zirconium oxide | 88.1 ± 8.9 | k = 0.11516 ± 0.0093 mol·gcat−1·min−1 | [91] |
| ∆H = −11.6 ± 1.1 kJ·mol−1 | ||||
| ∆G = 4.0 ± 0.1 kJ·mol−1 | ||||
| Taddeo, 2021 | Iron(III) Complex FeCl3(1–NO2) | 13 | EA-1 = 64 | [103] |
| Langmuir-Hinshelwood–Hougen–Watson (LHHW) | ||||
| Moreira, 2019 | Sulphonic Ion Exchange Resin Amberlyst-35 | 69.0 ± 6.6 | k = 0.492 ± 0.093 mol·gcat–1·s–1 | [10] |
| Kwater = 14.4 ± 3.1 | ||||
| ∆H = − 20.1 ± 1.1 kJ·mol−1 | ||||
| ∆G = 1.4 ± 0.3 kJ·mol−1 | ||||
| Sulistyo, 2020 | Metal Organic Framework Basolite F300 | 15.7 | Keq = 6.345 (at 303 K) | [92] |
| Kwater = 1.029 | ||||
| ∆H = −29.7176 kJ·mol−1 | ||||
| ∆G = −4.8675 kJ·mol−1 | ||||
| Li, 2020 | Cation Exchange Resin NKC–9 | 44.3 | EA-1 = 47.23 kJ·mol−1 | [104] |
| Keq = 0.9690 (323 K) | ||||
| Kwater = 0.7511 | ||||
| Eley–Rideal (ER) | ||||
| Esteban, 2015 | Ion Exchange Resin Lewatit GF101 | 124.0 ± 12.9 | EA-1 = 127.3 ± 12.6 kJ·mol−1 | [70] |
| Keq = 0.367879441 Kwater = 128.0 ± 21.4 | ||||
| Sulistyo, 2020 | Ion Exchange Resin Indion 225 Na | 21.2 | Kacetone = 0.62 | [78] |
| Ksolketal = 0.03 | ||||
| Author | Catalyst | Catalyst Loading | Reaction Conditions | Results | Ref |
|---|---|---|---|---|---|
| Fixed Bed | |||||
| Dmitriev, 2016 | KU–2–8 Cation–Exchange Resin | 205 g | G:A = 1:5 | Xgly = 65% | [105] |
| T = 308 K | |||||
| P = N.A. | |||||
| Solv. = Ethanol | |||||
| Space time = 1.45 h | |||||
| Oliveira, 2016 | Amberlyst–15 | 7 g | G:A = 1:20 | Xgly = 96% Sel = 94% | [106] |
| T = 323 K | |||||
| P = 1 bar | |||||
| Solv. = DMF | |||||
| Space time = 0.2 h | |||||
| Guidi, 2016 | Amberlyst–36 | 0.09 gcat/ggly | G:A = 1:4 | Xgly = 95% Sel > 99% | [107] |
| T = 298 K; 373 K | |||||
| P = 10 bar | |||||
| AlF3·3H2O | 0.067 gcat/ggly | Solv. = Methanol | Xgly = 80% Sel > 99% | ||
| WHSV 1 = 2 h−1 | |||||
| Konwar, 2018 | Lignosulfonate–based macro/mesoporous solid protonic acids | 0.5 g | G:A = 1:8 | Xgly = 92% Sel = 99.5% | [108] |
| T = 313 K | |||||
| P = 1 bar | |||||
| Solv. = Ethanol | |||||
| Space time = 0.25 h | |||||
| Cornejo, 2019 | Purolite CT275 | N.A. 2 | G:A = – | Xgly = 91% Sel > 99.5% | [71] |
| T = 323 K | |||||
| P = High pressure 2 | |||||
| Solvent Free | |||||
| Space time = – | |||||
| Domínguez-Barroso, 2019 | Monolithic structured carbon-based functionalized with sulfonic acid | 0.345 gcat/ggly | G:A = 1:8 | Xgly = 99% Sel > 99% | [109] |
| T = 330 K | |||||
| P = N.A. | |||||
| Solvent Free | |||||
| WHSV 1 = 2.9 h−1 | |||||
| Kowalska-Kus, 2019 | ZSM5(P) ZSM5(H) | 0.083 gcat/ggly | G:A = 1:3 T = 323 K P = 1 bar Solv. = Methanol WHSV 1 = 3.4 h−1 | Xgly = 35% Xgly = 90% | [110] |
| Beta(P) | Xgly = 90% Sel > 97% | ||||
| Beta(H) | Xgly = 90% Sel > 97% | ||||
| Mordenite(P) | Xgly = 86% Sel = 97% | ||||
| Mordenite(H) | Xgly = 86% Sel = 97% | ||||
| Fernández, 2019 | Sulfonated hydrothermal carbons (SHTC) | 0.00085 gcat/ggly | G:A = 1:9 | Prod = 2048 mmolsolketal gcatalyst−1 h−1 3 | [87] |
| T = 298 K | |||||
| P = 1 bar | |||||
| Solv. = Ethanol | |||||
| Space time = 1 min | |||||
| Moreira, 2019 | Amberlyst–35 | 25 g | G:A = 1:2 | Xgly = 81% | [11] |
| T = 313 K | |||||
| P = N.A. | |||||
| Solv. = Ethanol | |||||
| Space time = 0.25 h | |||||
| Kowalska-Kus, 2020 | ZSM5 (H) | 0.083 gcat/ggly | G:A = 1:3 | Xgly = 85% Sel > 95% | [111] |
| Beta(P) | T = 323 K | ||||
| P = 1 bar | Xgly = 69% Sel > 95% | ||||
| Solv. = Methanol | Xgly = 85% Sel > 95% | ||||
| USY(P) | Space time = 5 min | ||||
| Capillary Microreactor | |||||
| Huang, 2020 | ZSM5 Film | 0.050 gcat/ggly | G:A = 1:2 | Ysolk = 30% | [112] |
| T = 323 K | |||||
| P = N.A. | |||||
| Solvent Free | |||||
| Space time = 2.86 min | |||||
| Zhang, 2020 | ZSM5 Film | 4.7 μm film thickness | G:A = 1:8 | Xgly = 62% | [113] |
| T = 323 K | |||||
| P = N.A. | |||||
| Solvent Free | |||||
| Space time = 2.86 min | |||||
| Continuously Stirred Tank Reactor | |||||
| Dmitriev, 2018 | Sulfuric Acid | 0.00066 gcat/ggly | G:A = 1:5 | Xgly = 68.4% | [102] |
| T = 308 K | |||||
| P = N.A. | |||||
| Solvent Free | |||||
| Space time = 1.73 h | |||||
| Author | Catalyst | Catalyst Loading | Reaction Conditions | Results | Observations | Ref |
|---|---|---|---|---|---|---|
| Microwave Reactor | ||||||
| Cablewski, 1994 | pTSA | 0.05 gcat/ggly | G:A = 1:13.5 | Ysolk = 84% | Continuous process. | [68] |
| T = 405 K | ||||||
| P = 1 bar | ||||||
| Space time = 1.2 h | ||||||
| Priya, 2017 | Copper metal suported on Mordenite | 0.43 gcat/ggly | G:A = 1:3 | Xgly = 95% Sel > 98% | Three cycles without significant activity loss. Activity decreased slightly from the fourth cycle on. | [116] |
| T = 373 K | ||||||
| P = 1 bar | ||||||
| Space time = 0.25 h | ||||||
| Reactive Distillation | ||||||
| Clarkson, 2001 | Amberlyst DPT–1 | 0.05 gcat/ggly | G:A = 1:23.6 | Xgly = 70% Sel > 99% | “Only a slight loss of catalytic activity was observed.” | [67] |
| T = 343 K | ||||||
| Reactive Stages = 10 | ||||||
| Space time = 0.5 h/stage | ||||||
| Li, 2020 | Seepage packing internals filled with cation Exchange ResinNKC–9 | NA | G:A = 1:3 | Xgly = 85.9% | [104] | |
| T = 323 K | ||||||
| P ≈ 1 bar | ||||||
| Reactive Stages = 20 1 | ||||||
| Space time = 20 s | ||||||
| Reflux ratio = 2.5 | ||||||
| Membrane Reactor | ||||||
| Roldán, 2009 | K10 montmorillonite | 0.1 gcat/ggly | G:A = 1:2 | Xgly > 90% | Vapor permeation. | [54] |
| T = 333 K | ||||||
| P = 1 bar | ||||||
| Vaccum = 2 mbar | ||||||
| Adsorptive Reactor | ||||||
| Moreira, 2020 | Amberlyst–35 | 25 g | G:A = 1:2 | Xgly = 81% | [11] | |
| T = 313 K | ||||||
| P = N.A. | ||||||
| Solv. = Ethanol | ||||||
| Space time = 0.25 h | ||||||
| Reactive coupling | ||||||
| Eze, 2018 | Amberlyst 70–SO3H Amberlyst 70–SO3H Amberlyst A26–OH | 9 g | 1 glycerol:4 acetone 2; 30 methanol:1 triacetin:4 acetone 3; 6 methanol:1 triacetin:4 acetone 4 | Xgly = 80.6% 2 Xgly = 48.5% 3 Xgly = 80.6% 4 | Mesoscale oscillatory baffled reactors (meso-OBRs). | [117] |
| T = 323 K | ||||||
| P = N.A. | ||||||
| Sol = Methanol | ||||||
| Space time = 0.5 h | ||||||
| Al–Saadi, 2019 | DBSA (p–Dodecylbenzenesulfonic acid) | 0.5 mol | 10 methanol: 1 triacetin: 7 acetone | Ysolk = 82% | Silica gel added as dehydrating agent to overcome the thermodynamic equilibrium limitation. | [118] |
| T = 323 K | ||||||
| P = N.A. | ||||||
| Space time = 8 h | ||||||
| First Inventor, Publication Date | Patent | Assignee | Catalyst | Reaction | Separation | Ref |
|---|---|---|---|---|---|---|
| Process Intensification Strategies/Non-conventional operation | ||||||
| Bruchmann, 1999 | US5917059A | BASF SE | Homo- or heterogeneous | Reaction vessel with continuous distillation | Not necessary | [64] |
| Boesch, 2003 | US6528025B1 | Roche Vitamins Inc. | Heterogeneous | Not specified | Pervaporation unit | [127] |
| Winkler, 2004 | US20040024260A1 | Evonik Degussa GmbH | Homo- or heterogeneous | Not specified | At least two-stage pervaporation or vapor permeation | [128] |
| Dubois, 2008 | FR2906246A1 | Arkema France SA | Heterogeneous catalyst | Simulated Moving Bed Reactor | Not specified | [129] |
| Dubois, 2008 | FR2906807A1 | Arkema France SA | Homo- or heterogeneous | Reactive extraction | Vacuum evaporation | [130] |
| Haiyu, 2018 | CN107698552A | Guangzhou Yintian New Material Co., LTD | Heterogeneous catalyst | Membrane reactor | Not specified | [131] |
| Haiyu, 2018 | CN107652263A | Guangzhou Yintian New Material Co., LTD | Heterogeneous catalyst | Reactive distillation on a shell and tube or column plate device | Not specified | [132] |
| Yujia, 2020 | CN111253359A | China Petroleum & Chemical Corporation | Heterogeneous catalyst | Tank, fixed bed, moving bed, suspended bed, or slurry bed reactor with tin-titanium-silicon molecular sieve as catalyst | Not specified | [133] |
| Yujia, 2020 | CN111253363A | China Petroleum & Chemical Corporation | Heterogeneous catalyst | Tank, fixed bed, moving bed, suspended bed, or slurry bed reactor with a mixture of a titanium silicalite and a tin silicalite molecular sieves | Not specified | [134] |
| Yujia, 2020 | CN111253362A CN111253364A | China Petroleum & Chemical Corporation | Heterogeneous catalyst | Tank, fixed bed, moving bed, suspended bed, or slurry bed reactor with tin-silicon molecular sieve | Not specified | [135,136] |
| Traditonal production methods | ||||||
| Abe, 2004 | JP2006273750A | Nippon Oil & Fat Co. Ltd. | Homogeneous catalyst | Reactor with stirring equipment | Distillation column(s) | [137] |
| Wimmer, 2011 | EP2183238A1 | Christof International Management GmbH | Heterogeneous catalyst | Plug-flow reactor | Distillation column(s) | [138] |
| Terrill, 2012 | US20120330033A1 | Eastman Chemical Co | Homogeneous catalyst | Reaction vessel | Distillation column(s) | [139] |
| Mastroianni, 2013 | US20130178638A1 | Rhodia Operations SAS | Homo- or heterogeneous | Reaction vessel or fixed-bed reactor | Distillation column(s) | [140] |
| Rodrigues, 2013 | WO2013045967A1 | Rhodia Poliamida e Especialidades Ltd.a | Homogeneous catalyst | Reaction vessel | Decantation, filtration, or centrifugation, followed by liquid-liquid extraction and distillation(s) | [141] |
| Rodrigues, 2014 | US20140235878A1 | Rhodia Poliamida e Especialidades Ltd.a | Homogeneous catalyst | Reaction vessel | Distillation column(s) | [142] |
| Terrill, 2014 | US8829206B2 | Eastman Chemical Co | Heterogeneous catalyst | Fixed-bed reactor | Distillation column(s) | [143] |
| Terrill, 2016 | US9440944B2 | Eastman Chemical Co | Heterogeneous catalyst | Fixed-bed reactor | Distillation column(s) | [144] |
| Varfolomeev, 2018 | RU2625318C2 | Institute of Biochemical Physics, Russian Academy of Sciences, in association with Company Tatneft | Homogeneous catalyst | Reaction vessel | Distillation column(s) | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustain. Chem. 2021, 2, 286-324. https://doi.org/10.3390/suschem2020017
Corrêa I, Faria RPV, Rodrigues AE. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustainable Chemistry. 2021; 2(2):286-324. https://doi.org/10.3390/suschem2020017
Chicago/Turabian StyleCorrêa, Isabella, Rui P. V. Faria, and Alírio E. Rodrigues. 2021. "Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives" Sustainable Chemistry 2, no. 2: 286-324. https://doi.org/10.3390/suschem2020017
APA StyleCorrêa, I., Faria, R. P. V., & Rodrigues, A. E. (2021). Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustainable Chemistry, 2(2), 286-324. https://doi.org/10.3390/suschem2020017







